Abstract
BACKGROUND.
There is an urgent need to develop new agents for treating metastatic prostate cancer to overcome multiple drug resistance to the current standard targeted cancer therapy. Emetine is a highly cytotoxic natural product protein synthesis inhibitor, which is toxic to all cell types. Its cytotoxicity can be blocked by derivatizing its N-2′ position. Thus emetine can be selectively delivered to cancer cells in the region of metastatic cancer as a prodrug that will be activated by an enzyme selectively overexpressed within the metastatic tumor microenvironment. In this work, we convert emetine to a prodrug activatable by the fibroblast activation protein (FAP), a serine protease overexpressed by the carcinoma associated fibroblasts.
METHOD.
By using an iterative structure-activity relationship strategy, several peptidyl emetine prodrug analogs (1–11) were synthesized by chemical derivatization of emetine at its N-2′ position and tested for in-vitro activation by FAP. The lead prodrug 11 is made up of a DPPIV activatable prodrug precursor 10 (Ala-Pro-PABC-Emetine) coupled to FAP substrate (Ala-Ser-Gly-Pro-Ala-Gly-Pro). Activation assays of the prodrugs were performed in purified FAP, DPPIV, FBS, and human serum and were analyzed by LCMS. In vitro cytotoxicity assays of these prodrugs are carried out in prostate (LNCaP, PC3) and breast (MCF7 and MDA-MB-231) cancer cell lines. The prodrugs are also tested in normal immortalized human prostatic epithelial cell line (PrEC).
RESULTS.
The lead FAP activated emetine prodrug 11 is activated to emetine in tandem by FAP and DPPIV in about 70% conversion within 24 hr. In prostate and breast cancer cell lines treated with prodrug 11, it is found to be equipotent with emetine in the presence of FAP and DPPIV. However, in the PrEC cell line grown in serum free media, prodrug 11 is more than 200-fold less cytotoxic than emetine in the absence of FAP and DPPIV.
CONCLUSION.
This FAP activated prodrug of cytotoxic agent emetine further shows the crucial role of the N-2′ position of emetine in controlling its cytotoxicity. Significantly reduced toxicity observed in the PrEC cell line in the absence of FAP and DPPIV shows that prodrug 11 could be systemically delivered to regions of metastatic prostate cancer or other solid tumor for activation by cancer selective enzymes within the cancer microenvironment, such as FAP that is overexpressed by the carcinoma-associated fibroblasts. The two-step tandem enzymatic activation of prodrug 11 by FAP and DPPIV is a strategy for overcoming steric hindrance.
Keywords: emetine, prodrug, fibroblast activation protein (FAP), dipeptidyl peptidase IV (DPPIV), para-aminobenzyloxycarbonyl (PABC), linker
INTRODUCTION
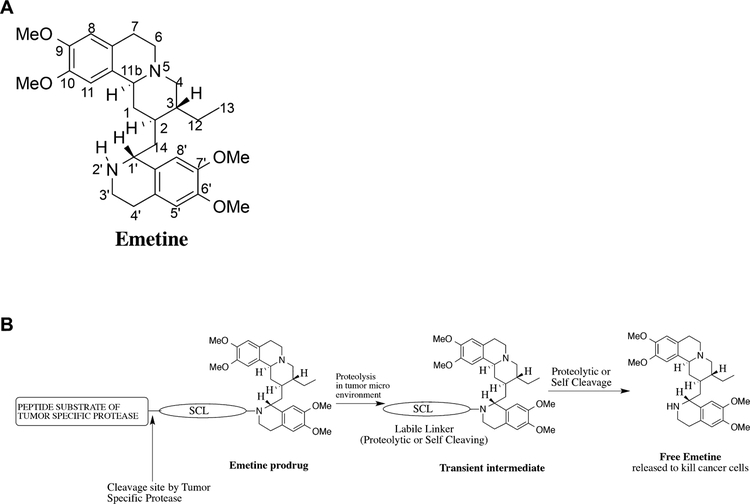
The ability to identify tumor specific protein targets has led to the development of a growing portfolio of “targeted” cancer therapies. These targeted agents have produced modest improvements in survival in some tumor types. However, heterogeneous expression of the target proteins within individual tumor sites eventually leads to the development of “resistance” to these therapeutic inhibitors due to the selection of cancer cells that lack or downregulate expression of the target protein. One strategy to overcome this heterogeneity problem is to identify a protein whose continued expression is critical to the survival of all cell types and develop a strategy to selectively inhibit the target in cancer cells but not in normal cells. An example of such a strategy is to selectively target a compound that is toxic to all cell types to cancer region. Such a cytotoxic compound is the natural product alkaloid emetine (Fig. 1A). Emetine is found in the root of psychotria ipecacuanha [1]. It is the main active agent in ipecac syrup used as an emetic and expectorant [2]. Emetine binds to the 40S ribosomal subunit to inhibit protein synthesis [3]. Based on this mechanism, emetine has been reported to cause the upregulation of pro-apoptotic and down-regulation of anti-apoptotic gene products [4]. As expected, emetine is a potent and non-specific cellular toxin with a potent activity in the NCI 60 cancer cell screen (GI50 of 27 nM).
Fig. 1.
(A) Structure of emetine showing the numbering of the atoms. (B) Schematic of emetine prodrug tandem protease activation strategy to release free emetine in the tumor microenvironment.
Based on reports of therapeutic activity of extracts of the ipecac plant, emetine was found to have specific activity against amoebic dysentery and became the standard therapy for this disease up until the 1950s [5]. Emetine has also been used as an antihelminthic and in the treatment of malaria and trypanosome disease [6]. In the 1970s, emetine was tested in phase I and II clinical trials as an anticancer agent either alone or in combination with other chemotherapies [7–12]. These studies were performed in patients with advanced refractory disease and partial and occasional complete responses were reported in these small studies in heavily pretreated patients. However, the therapeutic index of emetine was found to be very narrow and dose escalation was limited by occurrence of muscle fatigue, cardiac toxicity, and EKG abnormalities [8–10]. Unlike oral emetine, parenteral emetine did not produce significant nausea [8–10]. In addition, the drug had no effects on kidney or liver function and was not myelosuppressive. However, the dose-limiting cardiac and muscular toxicity appear to have inhibited further development of emetine as cancer therapy.
These studies suggest that emetine could be a useful anticancer agent if its therapeutic index could be improved. Previously, structure-activity relationship (SAR) studies revealed that for emetine to be biologically active, its N-2′ position must be a secondary amine [2,13]. Recently, the cryo-EM structure at 3.2 A resolution of emetine bound to the 80S ribosome of plasmodium falciparum further highlighted the importance of the N-2′ secondary amine in stabilizing emetine–ribosome binding by forming a hydrogen bond between the NH group of the isoquinoline ring in emetine and an oxygen atom on the backbone of the protein (U2061) [14]. As part of our ongoing effort to identify emetine analogs and prodrugs with potential for reduced systemic toxicity, we have synthesized N-2′ derived analogs of emetine that are significantly less cytotoxic (i.e., ~300-fold) than emetine [15,16]. These results suggest that an N-2′ derivatized emetine prodrug could be developed. It will function as a “molecular switch” that would be in the “OFF” position in the prodrug form and then switched to the “ON” position when proteolytically activated by tumor specific proteases to release emetine into the tumor microenvironment (Fig. 1B).
Based on this structural understanding, we have developed a prodrug strategy that takes advantage of the selective production of the serine protease fibroblast activation protein (FAP) by cancer-associated fibroblasts present within the tumor microenvironment. FAP is a type II integral membrane bound serine protease of the dipeptidyl peptidase IV family characterized by post-prolyl cleavage specificity (uniquely known for cleaving Pro-Xaa bond) [17]. FAP is selectively overexpressed in the tumor microenvironment by fibroblasts and mesenchymal stem cells within the stroma surrounding more than 90% of epithelia–derived cancers and their metastases examined, but not expressed in normal healthy tissue [18–24]. We have previously identified FAP-selective peptide substrates [25] that were coupled to an amino acid containing analog of the natural product thapsigargin and to the lytic peptide melittin to generate cancer-specific prodrugs [26–28]. For these two compounds, coupling of the FAP peptide to the toxic analog modulated the cytotoxicity sufficiently to generate an improved therapeutic index. In contrast, preliminary results demonstrating a large difference in cytotoxicity between emetine and N-2′ functionalized emetine analogs suggests that emetine represents a more ideal “warhead” for this protease-activated pro-drug strategy with the potential to greatly broaden the therapeutic index of this potent cytotoxin.
Unlike previous studies with prodrugs of thapsigargin and doxorubicin where an amino acid analog of the parent compound was liberated from the peptide carrier by the target protease [29–31], a successful emetine prodrug strategy requires proteolytic release of free emetine, unmodified in the N-2′ position for cytotoxicity in the tumor microenvironment. Here, we describe the iterative development of a sequential, tandem protease-cleavage strategy whose end result is the selective hydrolysis of an inactive emetine prodrug to release active emetine within the tumor microenvironment.
MATERIALS AND METHODS
General Information for Chemical Synthesis
The reaction schemes, synthetic procedures, and compound characterizations are provided as the supporting information. All solvents and reagents used were bought from commercial sources and used without further purification. Peptide synthesis was done as solid-phase synthesis using AAPPTec Apex 396 40-well peptide synthesizer. The 1H- and 13C-NMR spectra were obtained on a Bruker Avance III 500 MHz NMR spectrometer at 500 MHz and 125 MHz, respectively, in deuterated chloroform (CDCl3) or deuterated methanol (CD3OD). Chemical shifts are in δ units (ppm) with TMS (0.00 ppm), CHCl3 (7.27 ppm), or CH3OH (3.34 ppm) as the internal standard for 1H-NMR, and CDCl3 (77.00 ppm) or CD3OD (49.90 ppm) for 13C-NMR. Mass spectra were obtained on Bruker Esquire 3000 Mass Spectrometer equipped with ESI. Analytical thin-layer chromatography was performed using 0.25 mm precoated silica gel 60 F254 plates (Analtech Uniplates). Flash column chromatography was performed using silica gel 60 (200×400 mesh, Sorbent Technologies) with the indicated solvent. Purity of the compounds was determined with reverse phase-HPLC. The purity of all the compounds was determined to be >95%.
Experimental Methods for Biological Studies
Human recombinant CD26 (DPPIV) expressed in sf9 cells was purchased from Sigma–Aldrich (St. Louis, MO). Fibroblast activation protein (FAP) was recombinantly expressed using Schneider’s S2 cells and purified from the culture supernatant after induction with 500 μM CuSO4 using Ni-NTA resin (Qiagen, Valencia, CA) as previously described [25]. The human androgen receptor positive (LNCaP), androgen receptor negative PC3 prostate cancer cell lines, the human breast cancer cell lines MCF7 and MDA-MB-231 were all purchased from the American Type Culture Collection (Manassas, VA). Dulbecco’s modified Eagle medium (DMEM), Roswell Park Memorial Institute (RPMI) 1640 medium, l-glutamine, penicillin-streptomycin were all obtained from Invitrogen (Carlsbad, CA); and the fetal bovine serum (FBS) from Thermo Scientific (Waltham, MA). Both LNCaP and PC3 cells were grown in cell culture flasks in RPMI culture medium and MCF-7 and MDA-MB-231 were grown in DMEM with phenol red (GIBCO) supplemented with only 10% fetal bovine serum, 1% L-glutamine, and 1% penicillin -streptomycin. Normal prostatic epithelial cell lines were grown in keratinocytes serum free media (SFM) was obtained from Dr. John T. Isaac’s laboratory. Cells were cultured in a humidified atmosphere of 95% air and 5% carbon dioxide at 37°C. To sub-culture cells for experiments, cells growing as monolayer cultures were released from the tissue culture flasks by treatment with 0.05% trypson/EDTA. Cell population density was determined with the aid of hemocytometer. For all the in vitro experiments, cell-growth was maintained in the log phase and the cells were used while still in this logarithmic growth phase.
Stability of Test Compounds in Buffer
Solutions of tested compounds (10 mM in DMSO) were added to PBS buffer without any enzyme to make a final concentration of 200 mM and 2% DMSO (v/v). The mixtures were incubated at 37°C for 48 hr in a shaking incubator. To measure the stability of the compounds 50 μl of cold acetonitrile was added and the mixture was then centrifuged at 13,000 rpm for 6 min. The supernatant was analyzed by the HPLC (using method 1, supporting information) and ESI mass spectroscopy.
DPPIV-Mediated Hydrolysis of Prodrugs Ex Vivo
This assay was done as previously reported in our FAP proteolysis assay [26] with slight modifications Solutions of tested compounds (50 mM in DMSO) were added to FAP buffer (100 mM Tris, 100 mM NaCl, pH 7.8) containing DPPIV (final DPPIV concentration is 30 nM; activity ≥4,500 units/μg protein). As a separate experiment, the compounds were also added to RPMI media± 10% FBS. In all these assays, the final drug concentration is 500 μM, and the final DMSO composition is 1% (v/v). The mixtures were incubated at 37°C for 24 hr in a shaking incubator. To measure the extent of prodrug hydrolysis by FAP, at different time points, 50 μl was withdrawn from the mixture and 100 μl of cold acetonitrile was added. The mixture was placed on ice for 10 min and then centrifuged at 13,000 rpm for 6 min. The supernatant was analyzed by the HPLC (using method 1, supporting information) and mass spectroscopy.
FAP/DPPIV-Mediated Hydrolysis of Prodrugs Ex Vivo
This assay was done as previously reported [26] with slight modifications. Solutions of each compound tested (10 mM in DMSO) were added to FAP buffer (100 mM Tris, 100 mM NaCl, pH 7.8) containing FAP (final FAP concentration is 300 nM). As a separate experiment, the compounds were also added to RPMI media 10% FBS. In all these assays the final drug concentration is 250 μM and the final DMSO composition is 2.5% (v/v). The mixtures were incubated at 37°C for 24 hr in a shaking incubator. To measure the extent of prodrug hydrolysis by FAP, at different time points, 50 μl was withdrawn from the mixture and 100 μl of cold acetonitrile was added. The mixture was placed on ice for 10 min and then centrifuged at 13,000 rpm for 6 min. The supernatant was analyzed by the high-performance liquid chromatography (using method 1, supporting information) and mass spectroscopy.
Investigation of Stability of Prodrug 11 in Human Plasma
Human plasma was collected in EDTA anticoagulant. Prodrug 11 (10 mM in DMSO) was added to the plasma to obtain a final drug concentration of 400 μM. A control experiment was done in PBS at the same concentration. The two drug solutions were incubated at 37°C for 24 hr. To analyze the mixture at different time points, 100 μl was withdrawn and 200 μl of cold methanol was added. The mixture was placed on ice for 10 min and then centrifuged at 13,000 rpm for 6 min. The supernatant was analyzed by the high-performance liquid chromatography (using method 1, supporting information) and mass spectroscopy.
In Vitro Efficacy (Cytotoxicity) of Emetine Analogs and Prodrugs in Cancer Cell Lines
To determine the efficacy of emetine and its prodrug analogs in cancer cell lines, LNCaP was plated at a density of 5 × 103 in 100 μl of medium per well. PC3, MCF-7, and MDA-MB-231 were plated at a density of 5 × 103 in 100 μl of medium per well in 100 μl medium per well in 96 well-plates. These cells were incubated for 24 hr to allow attachment to the tissue culture plates. Serial dilutions (10 μM, 50 μM, 100 μM, 1,000 μM, 10,0000 μM) of each drug (emetine and its analogs) were made in sterile DMSO and these were further diluted 500× (20 nM, 100 nM, 200 nM, 2,000 nM, 20,000 nM) using respective medium. One hundred microliter of each drug concentration was added in eight replicates to cells in the 96 well-plate to give final drug concentrations of 10 nM, 50 nM, 100 nM, 1,000 nM, and 10,000 nM of the drug and 0.1% v/v uniform DMSO concentration in all drug treated cells. A vehicle control of 0.2% v/v and blank of 0.0% v/v DMSO in the growth medium were made. One hundred microliter of these were also added in eight replicates to the cells plated in 100 μl of medium in the 96 well-plate to make vehicle of 0.1% DMSO and blank control of 0% DMSO. Each experiment was repeated at least twice. Population of viable cells was determined on day 0 and each of the 3rd and 5th day of incubating the cells with the drugs using the 3-[4,5-dimethylthiazol-2-yl]-2,5- diphenyltetrazolium bromide (MTT) cell proliferation assay.
The Cell Titer 96® Non-Radioactive Cell Proliferation Assay manufactured by Promega Corporation was employed for this study. This is based on cellular conversion of a tetrazolium salt into a formazan product that can be measured easily using a 96 well-plate reader. The proliferation assays were performed according to the manufacturers instructions. Absorbance was recorded at 570 nm with a correction wavelength of 650 nm on a SpectraMax Plus384 absorbance micro-plate reader (Molecular Devices, Sunnyvale, CA). In both the vehicle and blank control, the cell growth was in the logarithmic phase from day 0 to the 5th day.
In-Vitro Evaluation of the Cytotoxicity of Precursor 10 and Prodrug 11 in Normal Prostatic Epithelial Cell Lines From Human Prostate Tissue
This basal epithelial cell lines from prostate tissue were grown in keratinocyte serum free media (Invitrogen) according to manufacturer’s instruction. Cells were seeded at a density of 5 × 103 cells per well in 96 well-plates and allowed to attach overnight. Cells were then treated with different concentrations of emetine, DPPIV activatable precursor 10 and the FAP activatable prodrug 11 (with and without FAP and DPPIV). To treat the cells with prodrug 11 together with FAP and DPPIV addition, after preparing the media with appropriate drug concentration, the enzymes were added to the media at concentrations of 200 nM (for FAP) and 50 nM (for DPPIV). This incubation was left for 3 days and effect of the drug on the cell proliferation was determined using the The Cell Titer 96® Non-Radioactive Cell Proliferation Assay.
RESULTS AND DISCUSSION
Investigation of FAP Activation/Proteolysis of Prodrug to Emetine Without Amino Acid at P1′ Position
FAP is a type II integral membrane serine protease of the dipeptidyl peptidase IV family, which is characterized by post-prolyl cleavage specificity [24–25]. However, FAP is unique in this family of enzymes because, in addition to its dipeptidase (i.e., exopeptidase) activity, it also possesses a collagen type I-restricted gelatinase (i.e., endopeptidase) activity [25]. Previously, we generated a map of FAP cleavage sites within denatured collagen I (i.e., gelatin). In these studies, we demonstrated that FAP prefers the amino acids Gly-Pro in the P2-P1 positions [25]. We also documented that there is FAP activity present within the serum from a variety of species [26]. FAP-activity appeared most pronounced in fetal bovine serum used in tissue culture media and was lowest in human sera.
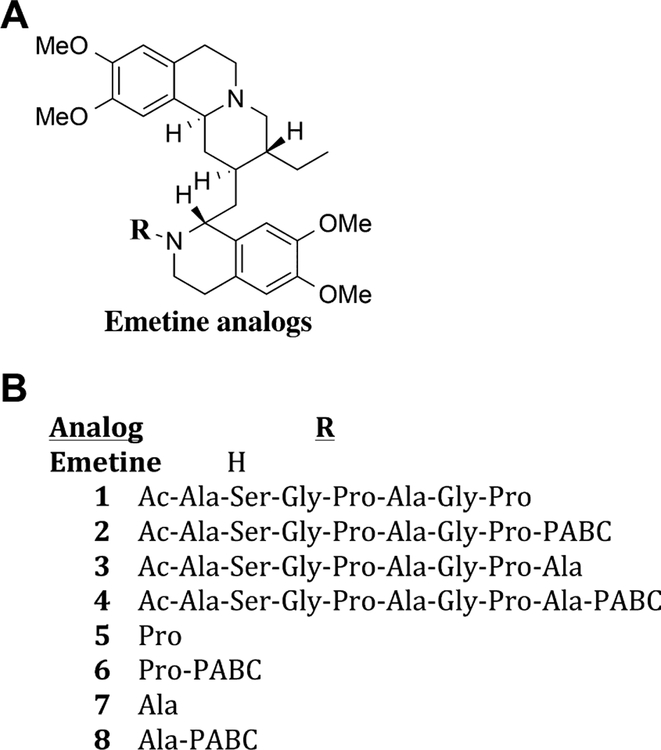
Based on these earlier studies, we selected the substrate Ac-Ala-Ser-Gly-Pro-Ala-Gly-Pro (ASGPAGP) as the peptide for the emetine prodrug. This peptide was synthesized using automated solid phase peptide synthesis and its N-terminal was capped with acetyl group. It was then directly coupled to emetine to produce 1 (Fig. 2A and B). Incubation of 1 with FAP in FAP buffer (10% FBS) at 37°C over a period of 24 hr did not release detectable amounts of emetine. LC-MS analysis of the assay product showed that the FAP peptide was cleaved after the first proline to release Ac-Ala-Ser-Gly-Pro and Ala-Gly-Pro-Emetine as the only products. Compound 1 was also not cytotoxic to human prostate cancer cells PC3 or LNCaP (Table I). Based on the potential for lack of cleavage due to steric hindrance at the cleavage site, a self-cleaving linker was next introduced between the peptide and emetine. We chose para-aminobenzyloxycarbonyl (PABC) as the self-cleaving linker, which undergoes 1,6-benzyl elimination upon cleavage of the amide bond resulting in spontaneous release of the free drug [32]. PABC has been utilized for spacing bulky drug from peptide substrate, thereby preventing steric hindrance [33,34]. Thus we synthesized compound 2 containing PABC between the proline and emetine (Scheme 2). Compound 2 was also not cytotoxic and just like 1, it was not cleaved by FAP containing media to release emetine (Table I).
Fig. 2.
(A) General structure of the N-2′ derived emetine analogs and prodrugs. (B) Description of N-2′ substituents on the emetine analogs and prodrugs.
Table I.
In Vitro Cytotoxicity and FAP Activation of the N-2′ Derived Peptidyl Emetine Analogs and Prodrugs
| Compounds | PC3 [IC50 (nM)] |
LNCaP [IC50 (nM)] |
% hydrolysis to emetine |
|---|---|---|---|
| Emetine | 29.43± 3.2 | 31.6± 2.4 | NA |
| 1 | 9820±87 | 7653± 81 | 0.0 |
| 2 | 8192±19 | 6271± 12 | 0.0 |
| 3 | >10,000 | >10,000 | 0.0 |
| 4 | 4712±12 | 3201± 10 | 0.0 |
| 5 | 8230± 51 | 6281± 98 | NA |
| 6 | >10,000 | >10,000 | NA |
| 7 | 9120± 91.8 | 7340± 88 | 0.0 |
| 8 | 8815± 161 | 9811± 98 | 0.0 |
Evaluating the Effect of Alanine at P1′ Position With and Without PABC
Previously, we demonstrated that preferred substrates for FAP were those containing either alanine or proline in the P1′ position [25]. Cleavage of FAP activatable emetine prodrugs containing an amino acid in the P1′ position would yield an amino acid containing emetine analogs in which the N-2′ position would be blocked. These analogs would be expected to be non-cytotoxic unless the amino acid was subsequently removed by aminopeptidases or other proteases in the tumor microenvironment. To explore whether addition of an amino acid in the P1′ position resulted in enhanced FAP cleavage we synthesized compounds 3 and 4 which incorporated alanine or alanine-PABC as the linker between the peptide and emetine (Fig. 2A and B). Incubation of 3 for 24 hr with FAP showed 100% cleavage to yield Ala-Gly-Pro-Ala-Emetine. In contrast, following 24 hr incubation of 4 with FAP, Ala-PABC-Emetine was observed as the major cleavage product (85%) with Ala-Gly-Pro-Ala-PABC-Emetine as a minor cleavage product (15%). Despite this cleavage, no further conversion to free emetine was observed for either 3 or 4 (Table I). Consistent with lack of release of free emetine, the IC50 of 3 and 4 against PC3 and LNCaP in FAP-containing FBS was more than 150-fold higher than that of emetine (Table I). Although alanine is placed at the P1′ position in 3 and 4, cleavage of 3 in the presence of FAP to only Ala-Gly-Pro-Ala-Emetine further confirms that there is steric hindrance at the cleavage site in 3 and a spacer/linker such as the PABC is needed.
Rationale for Using DPPIV for the Release of Free Emetine After Cleavage by FAP
To determine if the single amino acid containing emetine analogs could be further hydrolyzed by ubiquitous aminopeptidases present in the media or expressed by prostate cancer cells, we synthesized a series of amino acid-emetine analogs in which the amino acid proline 5, 6 or alanine 7, 8 was linked either directly or via the self-cleaving PABC linker to emetine in the N-2′ position. These analogs were screened for cytotoxicity over a 5-day incubation period against PC3 and LNCaP cells and were also found to be ~150–200-fold less cytotoxic than emetine (Table I). This indicates that these amino acid conjugated emetine analogs could not be further hydro-lyzed to emetine by ubiquitous aminopeptidases even in the presence of PABC as a spacer.
These results further emphasize the importance of the N-2′ position of emetine for cytotoxicity. They also highlight the need for a mechanism to release free emetine after cleavage by FAP. On this basis, we opted to take advantage of data from previous studies documenting the ubiquitous expression of dipeptidylpeptidase IV (DPPIV) within sites of most cancers, including prostate cancer [35–37]. DPPIV is a prolyl peptidase that cleaves dipeptides with the sequence X-Pro where X is preferably alanine. Modification of the N-terminus of the dipeptide blocks DPPIV hydrolysis [38]. Like FAP, DPPIV activity is also present in serum including the 10% FBS containing culture media [39]. The exopeptidase activity of DPPIV in the X-Pro (dipeptide) coupled small molecules have been previously reported [40–41]. Thus, as the next step in the development of an emetine prodrug, we synthesized compound 9 consisting of the dipeptide Ala-Pro coupled directly to emetine at the N-2′ position (Table II). Contrary to what we hypothesized, 9 was not converted to emetine in the presence of either purified DPPIV or 10% FBS, even at a very high concentration and with incubation lasting up to 48 hr at 37°C (Table II). We further tested the cytotoxicity potency of compound 9 in LNCaP, PC3, and the human breast cancer cell line MCF-7 and found that it was inactive compared to emetine (Table II). These in vitro cytotoxicity studies suggested that 9 is not activated to emetine. This was confirmed by LC-MS analysis demonstrating no production of emetine on incubation of 9 with DPPIV or in 10% FBS containing media (Table II).
Table II.
Structure, In Vitro Cytotoxicity and Hydrolysis of DPPIV Cleavable Emetine Analogs
| Compounds | R | IC50 (nM) | % emetine released | |||
|---|---|---|---|---|---|---|
| PC3 | LNCaP | MCF-7 | 10% FBS | DPPIV | ||
| Emetine | H | 29.43± 3.2 | 31.6± 2.4 | 35.8±1.6 | NA | NA |
| 9 | Ala-Pro | >10,000 | 9,520± 82 | >10,000 | 0 | 0 |
| 10 | Ala-Pro-PABC | 37.2± 1.6 | 38.2±1.0 | 38.4±1.2 | 78% | 98% |
Evaluation of the Release of Emetine From Ala-Pro-PABC-Emetine by DPPIV Activity
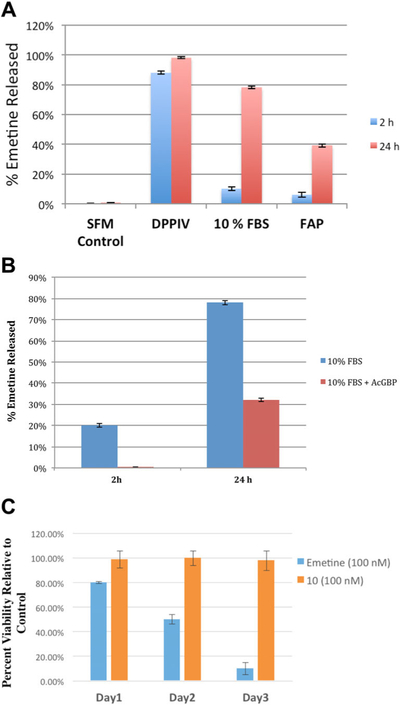
Given that steric hindrance could prevent DPPIV cleavage of Ala-Pro in compound 9, we next synthesized compound 10 in which the self-cleaving PABC linker is incorporated between emetine and the dipep-tide Ala-Pro to generate Ala-Pro-PABC-Emetine 10 (Fig. 2A, Table II). Compound 10 is completely stable in serum free media at 37°C over 72 hr incubation period (Fig. 3A). Both purified DPPIV and 10% FBS media converted 10 to emetine (Fig. 3A). Kinetic analysis of DPPIV mediated conversion of 10 (500 μM) to emetine showed that 88% conversion was accomplished within 2 hr of incubation with purified DPPIV and 98% conversion was measured after 24 hr of incubation. In 10% FBS, the activation occurs at a slower rate, producing 10% conversion in 2 hr and 78% after 24 hr. FAP was also able to convert 10 although much less efficiently than DPPIV with only 36% conversion after 24 hr (Fig. 3A). To further demonstrate that FAP/DPPIV activity is responsible for the activation of 10 in 10% FBS, we incubated 10 in the presence of AcGBP (Ac-Gly-boroPro), an inhibitor of FAP that is also a weak inhibitor of DPPIV [42]. The addition of this compound to 10% FBS containing media completely inhibited the release of emetine over 2 hr and decreased release by approximately 45% after 24 hr (Fig. 3B).
Fig. 3.
In vitro characterization of Ala-Pro-PABC-Emetine analog 10. (A) Conversion of compound 10 (200 μM) to emetine in the presence of serum free media (SFM), purified DPPIV (30 nM; activity ≥4,500units/μg protein), 10% fetal bovine serum (FBS), purified recombinant FAP (300 nM). (B) Inhibition of hydrolysis to free emetine by FAP inhibitor Ac-Gly-boroPro (200 μM) in 10% FBS. (C) Inhibition of growth of PrEC cell growing in FAP/DPPIV negative serum free media over 1–3 days exposure to emetine or 10 at 100 nM.
After observing that compound 10 is a prodrug precursor that would be activated to emetine in the presence of purified DPPIV and the DPPIV like activity of FBS, we evaluated its cytotoxicity compared to emetine. In a 3-day exposure, we tested compound 10 in prostate cancer cell lines (LNCaP and PC3) and breast cancer cell line (MCF-7) along with emetine (Table II). All the cells were grown in media containing 10% FBS. In this assay, 10 showed cytotoxicity comparable to that of emetine in all three cell-lines (Table II).
Compound 10 was highly active in 10% FBS containing media known to have DPPIV and FAP activity. However, to assay non-specific cytotoxicity in the absence of these enzymes, we utilized PrEC cells, a normal immortalized human prostatic epithelial cell line derived from non-malignant prostate tissue that grows in serum free media that lacks both FAP and DPPIV [43]. While emetine (100 nM) shows appreciable time dependent cytotoxicity as measured by the percentage of viable cells relative to control (day 1, 80%; day 2, 50%; and day 3, 10%), compound 10 has no observable cytotoxicity against these normal prostatic epithelial cell line growing in serum free media (Fig. 3C). The cells treated with 10 showed the same normal growth pattern observed in the untreated control. This further confirms both the stability of 10 to non-specific activation in the absence of serum and the need for DPPIV or DPPIV-like activity for mediating the conversion of 10 to emetine.
Evaluation of Tandem Activation of FAP-Activated Prodrug Obtained From Compound 10
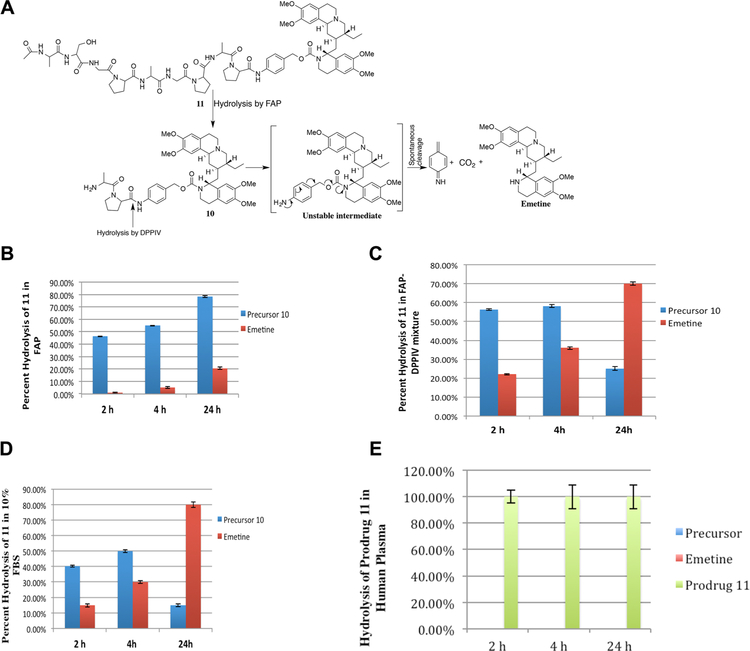
Having demonstrated that 10 could be cleaved to release free emetine by ubiquitous DPPIV produced within the cancer microenvironment, the final step in the generation of a FAP-activated prodrug was to couple 10 to the peptide (19) Ac-Ala-Ser-Gly-Pro-Ala-Gly-Pro to generate the prodrug 11, Ac-Ala-Ser-Gly-Pro-Ala-Gly-Pro-Ala-Pro-PABC-Emetine (Fig. 4A). This prodrug should only become activated when the peptide is released from 11 by FAP proteolysis to give 10 followed by cleavage by DPPIV to produce PABC-Emetine which spontaneously hydrolyzes through 1, 6-benzyl elimination to liberate the active emetine (Fig. 4A). Coupling the FAP peptide substrate to 10 was expected to be suitable for cleavage by FAP because the Ala of 10 becomes the P1′ of the FAP substrate.
Fig. 4.
In vitro characterization of FAP/DPPIV activated emetine prodrug 11. (A) Schematic depicting tandem activation by FAP and DPPIV and spontaneous elimination of the self-cleaving linker PABC to release free emetine. Production of Ala-Pro-PABC-emetine (precursor 10) and free emetine following incubation with (B) purified FAP (300 nM); (C) mixture of purified FAP and DPPIV (FAP, 300 nM; DPPIV, 30 nM); (D) 10% FBS; (E) human plasma.
In the presence of recombinant purified FAP, the prodrug 11 was progressively cleaved to the intermediate (precursor) 10, (Fig. 4B). Further, because of its dipeptidase activity, FAP was also able to further convert some of 10 to emetine (Fig. 4B). We further incubated 11 in an equimolar mixture of recombinant FAP and DPPIV to investigate the tandem conversion from 11 to emetine. A complimentary activity of both FAP and DPPIV was observed resulting in an enhanced conversion rate (2 hr: precursor 10, 56%; emetine, 22%) (Fig. 4C). Thus, as 11 is converted to 10 by FAP, DPPIV mediates the release of emetine from 10. Consequently, after 4 hr incubation, the conversion of 11 in the presence of both enzymes gave 58% of precursor 10 and 36 % of emetine. After 24 hr, only 25% of the reaction incubated prodrug solution is precursor 10 and 70% is emetine (Fig. 4C). Since in vitro cell based cytotoxicity assay will be done in the growth media supplemented with 10% FBS, we also investigated the activation of prodrug 11 in 10% FBS. Incubation of prodrug 11 in 10% FBS in growth media over a 24 hr period gave results that are similar to the purified FAP-DPPIV mixture (Fig. 4D). Since the prodrug will be administered systemically via the blood, we next assayed pro-drug 11 for stability following 24 hr incubatinon in human plasma. No detectable amount of either the precursor 10 or emetine was detected (Fig. 4E), further indicating that prodrug 11 is stable to non-specific proteolytic activation.
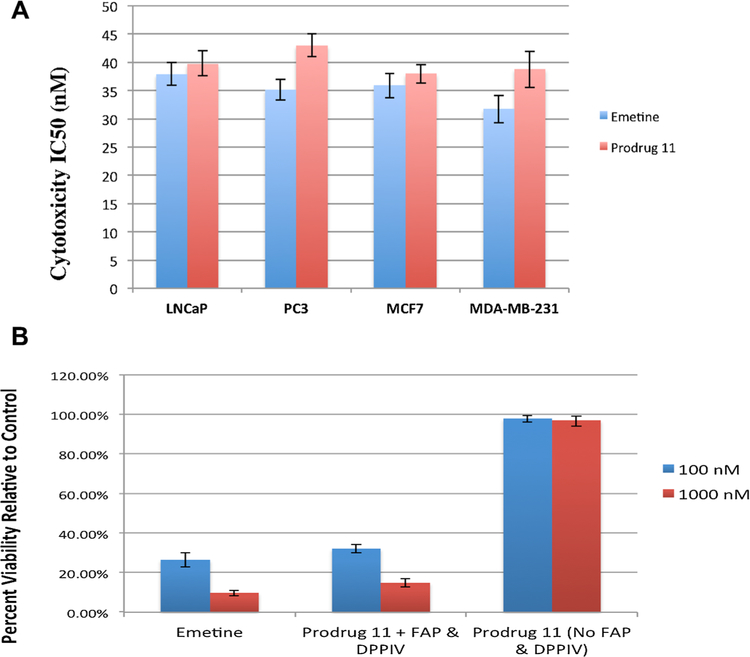
Having established the release of emetine from prodrug 11 in growth media containing 10% FBS, we next compared the cytotoxicity of a 3-day exposure prodrug 11 versus emetine across four human cancer cell lines (LNCaP, PC3, MCF-7, and MDA-MB-231) growing in 10% FBS supplemented media (Fig. 5A). Across these four lines, the IC50 for prodrug 11 was identical to that for emetine, consistent with almost complete activation of prodrug 11 to emetine in FAP/DPPIV positive 10% FBS supplemented growth media (Fig. 4D). To determine the degree of nonspecific in vitro hydrolysis of prodrug 11 in the absence of FAP, we utilized the PrEC normal prostatic basal epithelial cell line growing in serum free media (keratinocyte serum free media) [43]. These cells were treated with both emetine and prodrug 11 under the same conditions in a 3-day exposure in 100 nM and 1,000 nM of emetine and prodrug 11 (Fig. 5B). Under these conditions, prodrug 11 is not cytotoxic to the PrEC cells at 100 nM and 1,000 nM. In contrast, emetine is highly cytotoxic to these cells (Fig. 5B). Following exposure to an expanded dose range, the calculated IC50 of emetine in the PrEC cell lines is 35.7 nM while that of prodrug 11 is 7,730 nM indicating that the prodrug is more than 200 less cytotoxic than emetine in the absence of FAP and DPPIV.
Fig. 5.
Cytoxicity of emetine prodrug 11 against human prostate and breast cancer cells. (A) IC50 values for emetine and prodrug 11 against human prostate cancer cells LNCaP and PC3 and human breast cancer cells MCF-7 and MDA-MB-231 following 3-day incubation. (B) Effect of emetine or prodrug 11 on growth of PrEC cells following 3-day exposure to 100 and 1,000 nM of emetine in serum free media or prodrug 11 in serum free media (no FAP or DPPIV) or following addition of purified FAP and DPPIV to the media (FAP, 300 nM; DPPIV, 30 nM).
CONCLUSION
In line with our earlier finding that the secondary amine of the N-2′ position of emetine plays a crucial role in its cytotoxic activity [15–16], this present study shows that emetine could be converted to a peptidyl prodrug through its N-2′ amine. Such prodrug could be systemically delivered for activation by cancer selective enzymes within the cancer microenvironment, such as FAP that is overexpressed by the carcinoma-associated fibroblasts. The two-step enzymatic activation of prodrug 11, first by FAP specific activation to give the precursor 10, and then subsequently by ubiquitous DPPIV to yield emetine, is a strategic design for overcoming steric hindrance and also creates the right cleavage site for FAP. Thus, this strategy enables efficient controlled release of emetine in the tumor microenvironment.
In normal prostatic basal epithelial cells grown in serum free media lacking both FAP and DPPIV, prodrug 11 is more than 200× less cytotoxic than emetine, supporting the conclusion that this prodrug will potentially be minimally toxic to normal cells relative to emetine. Thus, this study provides evidence that prodrug 11 could potentially be safely delivered systemically. Also, restricted expression of FAP on the surface of cancer associated fibroblasts provides a mechanism for release of the cytotoxic free emetine payload into the extracellular fluid within the tumor microenvironment resulting in the potential for killing FAP-expressing cells as well as FAP-negative tumor cells via a bystander effect. This is a potential therapeutic strategy for multiple solid tumor types and not just for prostate and breast cancer alone. Future studies will be focused on in vivo safety, efficacy, and pharmacokinetic studies in appropriate tumor xenografts.
Supplementary Material
Grant sponsor:
Mass Spectrometry Core at Johns Hopkins; Grant number: P30 CA006973.
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of this article at the publisher’s web-site.
REFERENCES
- 1.Wiegrebe W, Kramer WJ, Shamma M. The emetine alkaloids. J Nat Prod 1984;47:397–408. [Google Scholar]
- 2.Grollman AP. Structural basis for inhibition of protein synthesis by emetine and cycloheximide based on an analogy between ipecac alkaloids and glutarimide antibiotics. Proc Natl Acad Sci USA 1966;56(6):1867–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jimenez A, Carrasco L, Vazquez D. Enzymic and nonenzymic translocation by yeast polysomes. Site of action of a number of inhibitors. Biochemistry 1977;16(21):4727–4730. [DOI] [PubMed] [Google Scholar]
- 4.Akinboye ES, Bakare O. Biological activities of emetine. Open Nat Prod J 2011;4:8–15. [Google Scholar]
- 5.Lambert AC, Toronto CM. The treatment of amoebic dysentery with emetine and bismuth iodide. Br Med J 1981; 1(2978):116–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matthews H, Usman-Idris M, Khan F, Read M, Nirmalan N. Drug repositioning as a route to anti-malarial drug discovery: Preliminary investigation of the in vitro anti-malarial efficacy of emetine dihydrochloride hydrate. Malar J 2013;12:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jondorf WR, Abbott BJ, Greenberg NA, Mead JAR. Anti-leukemic effectiveness of (–)-emetine and some of its derivatives. Pharmacologist 1970;12:282. [Google Scholar]
- 8.Panettiere F, Coltman CA. Phase I experience with emetine hydrochloride (NSC 33669) as an antitumor agent. Cancer 1971;27(4):835–841. [DOI] [PubMed] [Google Scholar]
- 9.Mastrangelo MJ, Grage TB, Bellet RE, Weiss AJ. A phase I study of emetine hydrochloride (NSC 33669) in solid tumors. Cancer 1973;31(5):1170–1175. [DOI] [PubMed] [Google Scholar]
- 10.Siddiqui S, Firat D, Olshin S. Phase II study of emetine (NSC-33669) in the treatment of solid tumors. Cancer Chemother Rep 1973;57:423–428. [PubMed] [Google Scholar]
- 11.Moertel CG, Schutt AJ, Hahn RG, Reitemeier RJ. Treatment of advanced gastrointestinal cancer with emetine (NCS-33669). Cancer Chemother Rep 1974;58:229–232. [PubMed] [Google Scholar]
- 12.Kane RC, Cohen MH, Broder LE, Bull MI, Creaven PJ, Fossieck BE. Phase I-II evaluation of emetine (NSC-33669) in the treatment of epidermoid bronchogenic carcinoma. Cancer Chemother Rep 1975;59:1171–1172. [PubMed] [Google Scholar]
- 13.Troconis M, Ma W, Nichols DE, McLaughlin J. Molecular modeling study of tubulosine and other related ipecac alkaloids. J Comput-Aided Mol Des 1998;12(5):411–418. [DOI] [PubMed] [Google Scholar]
- 14.Wong W, Bai XC, Brown A, Fernandez IS, Hanssen E, Condron M, Tan YH, Baum J, Scheres SH. Cryo-EM structure of the plasmodium falciparum 80S ribosome bound to the anti-protozoan drug emetine. eLife 2014;3:e03080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akinboye ES, Rosen DM, Denmeade SR, Kwabi-Addo B, BakareO. Design, synthesis, and evaluation of ph-dependent hydrolyzable emetine analogs as treatment for prostate cancer. J Med Chem 2012;55(17):7450–7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akinboye ES, Bamji ZD, Kwabi-Addo B, Ejeh D, Copeland RL, Denmeade SR, Bakare O. Design, synthesis and cytotoxicity studies of dithiocarbamate ester derivatives of emetine in prostate cancer cell lines. Bioorg Med Chem 2015;23(17): 5839–5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edosada CY, Quan C, Tran T, Pham V, Wiesmann C, Fairbrother W, Wolf BB. Peptide substrate profiling defines fibroblast activation protein as an endopeptidase of strict Gly(2)-Pro(1)-cleaving specificity. FEBS Lett 2006;580(6):1581–1586. [DOI] [PubMed] [Google Scholar]
- 18.Brennen WN, Isaacs JT, Denmeade SR. Rationale behind targeting fibroblast activation protein-expressing carcinoma-associated fibroblasts as a novel chemotherapeutic strategy. Mol Cancer Ther 2012;11(2):257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rettig WJ, Garin-Chesa P, Healey JH, Su SL, Ozer HL, Schwab M, Albino AP, Old LJ. Regulation and heterodimeric structure of the fibroblast activation protein in normal and transformed cells of mesenchymal and neuroectoderma origin. Cancer Res 1993;53(14):3327–3335. [PubMed] [Google Scholar]
- 20.Rettig WJ, Garin-Chesa P, Beresford HR, Oettgen HF, Melamed MR, Old LJ. Cell-surface glycoproteins of human sarcomas: Differential expression in normal and malignant tissues and cultured cells. Proc Natl Acad Sci USA 1988;85(9):3110–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levy MT, McCaughan GW, Abbott CA, Park JE, Cunningham AM, Müller E; Rettig WJ, Gorrell MD. Fibroblast activation protein: A cell surface dipeptidyl peptidase and gelatinase expressed by stellate cells at the tissue remodeling interface in human cirrhosis. Hepatology 1999;29(6):1768–1778. [DOI] [PubMed] [Google Scholar]
- 22.Tuxhorn JA, Ayala GE, Smith MJ, Smith VC, Dang TD, Rowley DR. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin Cancer Res 2002;8(9):2912–2923. [PubMed] [Google Scholar]
- 23.Yu DMT, Yao TW, Chowdhury S, Nadvi NA, Osborne B, Church WB, McCaughan GW, Gorrel MD. The dipeptidyl peptidase IV family in cancer and cell biology. FEBS J 2010;277(5):1126–1144. [DOI] [PubMed] [Google Scholar]
- 24.Park JE, Lenter MC, Zimmermann RN, Garin-Chesa P, Old LJ, Rettig WJ. Fibroblast activation protein, a dual specificity serine protease expressed in reactive human tumor stromal fibro-blasts. J Biol Chem 1999;274(51):36505–36512. [DOI] [PubMed] [Google Scholar]
- 25.Aggarwal S, Brennen WN, Kole TP, Schneider E, Topaloglu O, Yates M, Cotter RJ, Denmeade SR. Fibroblast activation protein peptide substrates identified from human collagen I derived gelatin cleavage sites. Biochemistry 2008;47(3):1076–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brennen WN, Rosen DM, Wang H, Isaacs JT, Denmeade SR. Targeting carcinoma-associated fibroblasts within the tumor stroma with a fibroblast activation protein-activated prodrug. J Natl Cancer Inst 2012;104(17):1320–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brennen WN, Rosen DM, Chaux A, Netto GJ, Isaacs JT, Denmeade SR. Pharmacokinetics and toxicology of a fibro-blast activation protein (FAP)-activated prodrug in murine xenograft models of human cancer. Prostate 2014;74(13): 1308–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.LeBeau AM, Brennen WN, Aggarwal S, Denmeade SR. Targeting the cancer stroma with a fibroblast activation protein-activated promelittin protoxin. Mol Cancer Ther 2009;8(5):1378–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Denmeade SR, Jakobsen C, Janssen S, Khan SR, Lilja H, Christensen SB, Isaacs JT. Prostate-specific antigen (psa) activated thapsigargin prodrug as targeted therapy for prostate cancer. J Natl Cancer Inst 2003;95(13):990–1000. [DOI] [PubMed] [Google Scholar]
- 30.Denmeade SR, Mhaka AM, Rosen DM, Brennen WN, Dalrymple S, Dach I, Olesen C, Gurel B, Demarzo AM, Wilding G, Carducci MA, Dionne CA, Møller JV, Nissen P, Christensen SB, Isaacs JT. Engineering a prostate-specific membrane antigen-activated tumor endothelial cell prodrug for cancer therapy. Sci Transl Med 2012;4(140):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan SR, Denmeade SR. In vivo activity of a psa-activated doxorubicin prodrug against psa-producing human prostate cancer xenografts. Prostate 2000;45(1):80–83. [DOI] [PubMed] [Google Scholar]
- 32.Carl PL, Chakravarty PK, Katzenellenbogen JA. A novel connector linkage applicable in prodrug design. J Med Chem 1981;24(5):479–480. [DOI] [PubMed] [Google Scholar]
- 33.Dubowchik GM, Firestone RA. Cathepsin B-sensitive dipeptide prodrugs. 1. A model study of structural requirements for efficient release of doxorubicin. Bioorg Med Chem Lett 1998; 8(23):3341–3346. [DOI] [PubMed] [Google Scholar]
- 34.Elsadek B, Graeser R, Warnecke A, Unger C, Saleem T, El-Melegy N, Madkor H, Kratz F. Optimization of an albumin-binding prodrug of doxorubicin that is cleaved by prostate-specific antigen. ACS Med Chem Lett 2010; 1(5):234–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bogenrieder T, Finstad CL, Freeman RH, Papandreou CN, Scher HI, Albino AP, Reuter VE, Nanus DM. Expression and localization of aminopeptidase A, aminopeptidase N, and dipeptidyl peptidase IV in benign and malignant human prostate tissue. Prostate 1997;33(4):225–232. [DOI] [PubMed] [Google Scholar]
- 36.Vanhoof G, De Meester I, van Sande M, Scharpé S, Yaron A. Distribution of proline-specific aminopeptidases in human tissues and body fluids. Eur J Clin Chem Clin Biochem 1992; 30(6):333–338. [DOI] [PubMed] [Google Scholar]
- 37.Wilson MJ, Ruhland AR, Quast BJ, Reddy PK, Ewing SL, Sinha AA. Dipeptidylpeptidase IV activities are elevated in prostate cancers and adjacent benign hyperplastic glands. J Androl 2000;21(2):220–226. [PubMed] [Google Scholar]
- 38.Yaron A, Naider F. Proline-dependent structural and biological properties of peptides and proteins. Crit Rev Biochem Mol Biol 1993;28(1):31–81. [DOI] [PubMed] [Google Scholar]
- 39.Buckley SJ, Collins PJ, O’Connor BF. The purification and characterization of novel dipeptidyl peptidase IV-like activity from bovine serum. Int J Biochem Cell Biol 2004;36(7): 1281–1296. [DOI] [PubMed] [Google Scholar]
- 40.Garcia-Aparicio C, Bonache M, Meeseter I, San-Felix A, Balzarini J, Camarasa M, Velazquez S. Design and discovery of a novel dipeptidyl-peptidase IV (CD26)-based prodrug approach. J Med Chem 2006;49(17):5339–5351. [DOI] [PubMed] [Google Scholar]
- 41.Diez-Torrubia A, Cabrera S, Castro S, Garcia-Aparicio C, Mulder G, De Meester I, Camarasa M, Balzarini J. Novel water-soluble prodrugs of acyclovir cleavable by dipeptidyl peptidase IV (DPPIV/CD26). Eur J Med Chem 2013;70:456–468. [DOI] [PubMed] [Google Scholar]
- 42.Edosada CY, Quan C, Wiesmann C, Tran T, Sutherlin D, Reynolds M, Elliott JM, Raab H, Fairbrother W, Wolf BB. Selective inhibition of fibroblast activation protein protease based on dipeptide substrate specificity. J Biol Chem 2006; 281(11):7437–7444. [DOI] [PubMed] [Google Scholar]
- 43.Litvinov IV, Griend DJV, Xu Y, Antony L, Dalrymple SL, Isaacs JT. Low-calcium serum-free defined medium selects for growth of normal prostatic epithelial stem cells. Cancer Res 2006; 66(17):8598–8607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.