Abstract
Over the past 25 years, antibodies have emerged as extraordinarily promising vectors for the delivery of radionuclides to tumors for nuclear imaging. While radioimmunoconjugates often produce very high activity concentrations in target tissues, they also are frequently characterized by elevated activity concentrations in healthy organs as well. The root of this background uptake lies in the complex network of biological interactions between the radioimmunoconjugate and the subject. In this review, we seek to provide an overview of these interactions and thus paint a general picture of the in vivo fate of radioimmunoconjugates. In order to cover the entire story, we have divided our discussion into two parts. First, we will address the path of the entire radioimmunoconjugate as it travels through the body. And second, we will cover the fate of the radionuclide itself, as its course can diverge from the antibody under certain circumstances. Ultimately, our goal is to provide the nuclear imaging field with a resource covering these important — yet often underestimated — pathways.
Keywords: Monoclonal antibodies, antibodies, radioimmunoconjugates, pharmacokinetics, pharmacodynamics, FcRn, radioiodine, radiometals, copper-64, zirconium-89, indium-111, positron emission tomography, PET, immunoPET, single photon emission tomography, SPECT, immunoSPECT
INTRODUCTION
The advent of monoclonal antibodies as medical tools unquestionably represents one of the most important developments in healthcare over the last quarter century. Indeed, over this period, monoclonal antibodies (mAbs) have emerged as a leading class of biological drugs.1–2 If we include the 10 antibodies that received USFDA approval in 2017, a total of 63 monoclonal antibodies are now approved and marketed in the United States alone. Moreover, a growing pipeline of monoclonal antibodies in clinical trials suggests that several antibodies will garner regulatory approval per year in the near future.3 This growth has been fueled in part by several biotechnological advances, including improvements in (a) hybridoma and phage display technology, (b) recombinant DNA technology, (c) transgenic mice, and (d) industrial bioreactors.
Like many other fields, the nuclear imaging community — and specifically those interested in the imaging of cancer — has been quick to adopt mAbs. Indeed, the high affinity and specificity of mAbs for their in vivo targets have made antibodies extremely promising vectors for the delivery of radionuclides to tumor tissue for positron emission tomography (PET) and single photon emission computed tomography (SPECT). Over the past 25 years, a wide variety of radioimmunoconjugates labeled with nuclides ranging from 111In to 89Zr have been developed and translated to the clinic. Yet while radioimmunoconjugates often produce very high activity concentrations in target tissues, they also are frequently characterized by elevated activity concentrations in healthy organs such as the liver and spleen. The root of this background uptake lies in the complex network of interactions between the radioimmunoconjugate and the biochemical milieu of the subject.
In this review, we seek to provide an overview of these interactions and thus paint a general picture of the in vivo fate of radioimmunoconjugates. More specifically, we will seek to answer several questions about radioimmunoconjugates that are often asked by researchers new to the field, including “Why are their serum half-lives so long?”, “Why don’t they penetrate tumors particularly well?”, and “Why do radioimmunoconjugates produce so much background uptake in the liver (or the spleen)?” To provide answers to these queries, we have had to draw from a wide variety of fields, including literature on immunoPET, immunoSPECT, antibody pharmacokinetics, antibody-drug conjugates, immunotherapy, and immunology. In order to cover the entire story, we have divided our discussion into two parts. First — in The Fate of the Antibody — we will address the path of the entire radioimmunoconjugate as it travels through the body, covering topics such as absorption, distribution, elimination, and interaction with the immune system. And second — in The Fate of the Radionuclide — we will cover the journey of the radiolabel itself, as its course can diverge from the antibody under certain circumstances. Ultimately, our goal is to provide the nuclear imaging field with a resource covering these important yet often underestimated pathways and thereby facilitate the incorporation of these critical lessons into the creation of the next generation of radioimmunoconjugates.
THE FATE OF THE ANTIBODY
The Biochemical Processes Governing the In Vivo Behavior of Antibodies
Simply put, pharmacokinetics is the study of what the body does to a drug. When considering the pharmacokinetics of radioimmunoconjugates, it is absolutely critical to remember that the body has extensive machinery for the processing of its own endogenous antibodies that are created by B-cells and mass-produced by plasma cells in response to an infection or disease. As a result, any exogenous mAb — such as a radioimmunoconjugate — will be acted upon by the same cellular machinery that has been put in place for the catabolism of endogenous immunoglobulins (Ig).
Administration and Absorption:
The first step in the journey of any radioimmunoconjugate is entering the body. Most tumor-targeting mAbs are administered intravenously. This route provides the most direct access to the body’s vascular network for the systemic distribution of the immunoglobulin. In recent years, however, cost and logistical factors have led to the approval of the subcutaneous administration of a few antibody formulations.4–5 This approach relies on the diffusion of the mAb into the lymphatic system, which ultimately drains into the blood. While this method produces bioavailabilities between 50–80% over a period of 2–8 days4, it is relatively slow and subjects antibodies to pre-systemic elimination by soluble peptidases in the interstitial space of the extracellular matrix surrounding the site of injection. Collectively, these drawbacks as well as other practical concerns make subcutaneous administration unsuitable for radioimmunoconjugates.4, 6–7 As a result, radioimmunoconjugates are administered strictly intravenously.
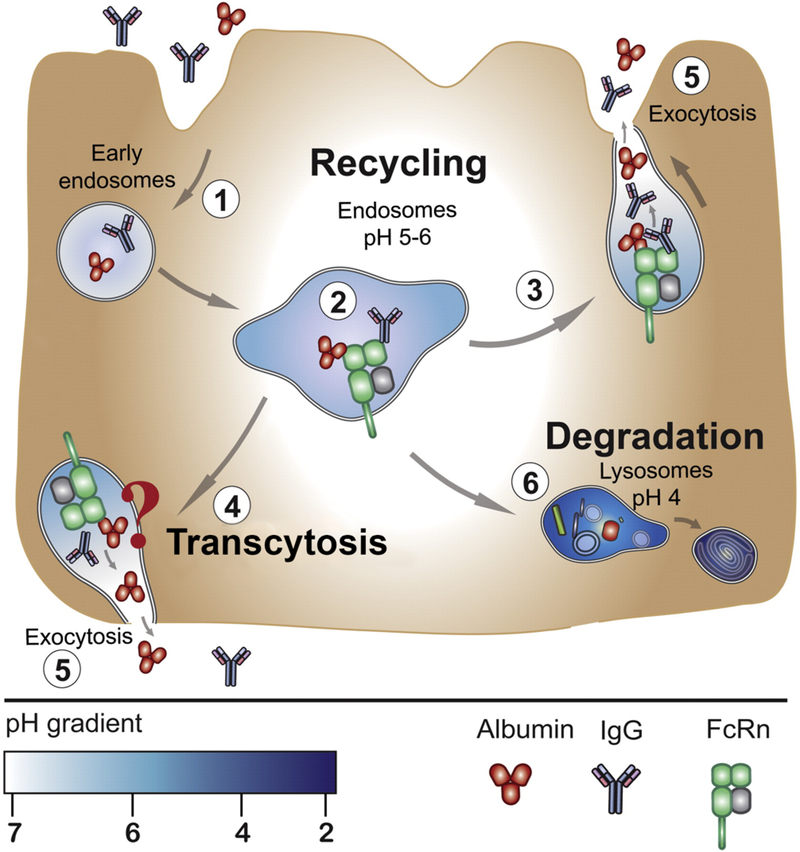
Regardless of the route of administration, circulating antibodies are immediately susceptible to cell uptake via fluid-phase pinocytosis or receptor-mediated endocytosis. Thankfully, antibodies can be rescued from intracellular catabolism through their interaction with the neonatal Fc-receptor (FcRn), which is expressed by a variety of cells in the body, including the endothelial cells lining blood vessels, hepatocytes, macrophages, monocytes, and dendritic cells (Figure 1).4, 8 Specifically, FcRn binds to the CH2-CH3 domains within the Fc portion of the antibody when the latter is engulfed within the acidified endosome (pH ~ 6.0–6.5) of the cell (Figure 2). The FcRn-bound antibody is protected from lysosomal degradation and is translocated to the apical or basolateral surface of the cell where it dissociates from the FcRn under conditions of neutral pH and is released back to the extracellular space or the blood. The FcRn-mediated recycling of antibodies is a key mechanism for maintaining the homeostasis of endogenous immunoglobulins and an important contributor to the bioavailability and long serum half-lives of exogenously introduced antibodies.9 To wit, mice lacking FcRn have demonstrated lower bioavailability of exogenous antibodies.10
Figure 1.
FcRn recycles monoclonal antibodies and contributes to their long serum half-life. (1) Antibodies and albumin in the blood undergo fluid-phase endocytosis and are captured within the early endosome of an endothelial cell lining the blood vessel. (2) FcRn binds to antibodies and albumin in the sorting vesicle and either (3) recycles them back to into the blood from the apical side of the cell or (4) transcytoses them through the basolateral side of the cell via exocytosis (5). In either case, the antibodies and serum albumin are protected from lysosomal degradation (6). Reproduced with permission from Bern et al.137
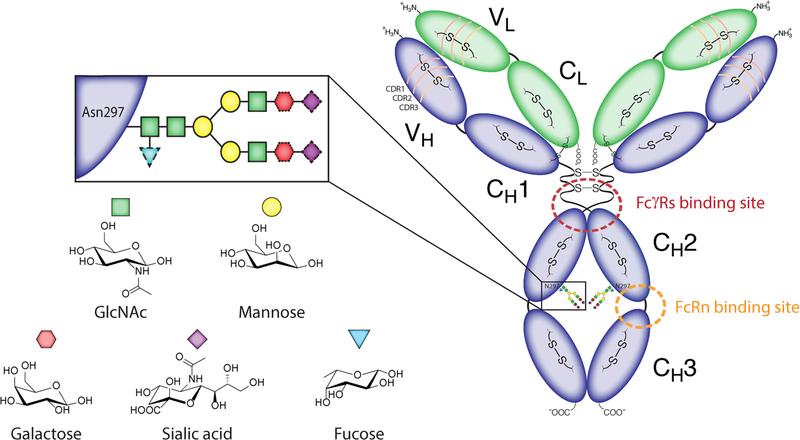
Figure 2.
The detailed structure of an IgG1 antibody, adapted from Adumeau et al.48
Distribution:
The next step along the antibody’s journey involves its transport from the blood vessels to the interstitial space of tissues where it can find tumor cells and bind to its intended target. Owing to their high molecular weight and polarity, antibodies diffuse across vascular endothelial cell membranes extremely slowly. As a result, antibodies rely on convective transport to facilitate their extravasation from blood vessels to the interstitial space of tissues.4 Convective transport, however, depends on the fluid flow rate from the blood to the tissue as well as the ‘sieving effect’ exercised by the paracellular pores of the vascular endothelium. The latter often results in higher concentrations of monoclonal antibodies being driven into tissues — such as the spleen, bone marrow, and liver — which have naturally fenestrated capillaries with large paracellular pores and sinusoids with ~100 nm clefts.4, 11 On the other hand, the presence of tight junctions in the vascular endothelium of the brain prevents the convective transport of antibodies across the blood brain barrier.4
The distribution of antibodies within tumor tissue occurs via diffusion and convection. Yet again, however, the macromolecular size of an antibody becomes a limiting factor, and both these locomotion processes proceed extremely slowly. The diffusion coefficient for a 150 kDa monoclonal antibody within solid tumors has been reported to be ~1.3 × 10−8 cm2/s, whereas that of a macromolecular protein such as bovine serum albumin is ~1.2 × 10−7 cm2/s.12–13 The intratumoral movement of an antibody is further hindered by interstitial fluid pressure (IFP) and binding site barriers, both of which act to further complicate the efficient transport of antibodies in solid tumors.13–16
Interstitial fluid pressure presents strong a physical resistance to the movement of antibodies within tumors. The binding site barrier, however, represents a biochemical resistance to the movement of the antibody that stems from its high affinity for its intended target. The latter phenomenon limits the antibody to the tumor cells that are situated in close proximity to the point of extravasation. This localization of antibodies within the perivascular space along the tumor periphery and gives rise to a heterogeneous pattern of uptake in solid tumors. One can imagine that antibodies that bind to tumor-associated shed antigens face a greater challenge from the binding site barrier due to the densely packed shed antigen present within the extracellular spaces of the tumor interstitium.17 Preclinical studies by Rudnick et al. and Glatt et al. have shown that antibodies with moderate binding affinity for the tumor target were able to achieve higher tumor uptake than counterparts with high affinity for the same target.18–20 However, since lowering the binding affinity of the antibody can compromise its specificity for binding to the target, it has been proposed that the binding site barrier may also be overcome by increasing the dose of unlabeled antibody. Doing so would facilitate the saturation of the target in the perivascular space of the tumor while allowing the radiolabeled antibody to extravasate further and achieve better tumor penetration.21–22
Biotransformation and Elimination:
Of the three modes of drug elimination — excretion, secretion, and biotransformation (via metabolism or catabolism) — the third is known to be the major contributor to the systemic elimination of monoclonal antibodies. Like endogenous immunoglobulins, exogenous antibodies are broken down into peptides within the reticuloendothelial system. The uptake of antibodies for intracellular catabolism occurs via 3 pathways: (i) non-specific fluid phase endocytosis (pinocytosis), which occurs throughout the body in endothelial cells lining the blood vessels; (ii) receptor-mediated endocytosis, which is carried out by Fc-receptors expressed by immune cells; and (iii) target-mediated disposition (TMD), which occurs when the Fab portions of the antibody bind to targets such as antigens expressed on tumor cells.
Once it has distributed inside a tumor, an antibody is predominantly eliminated by intracellular catabolism via target-mediated disposition (TMD). As the name implies, a prerequisite for TMD is the binding of the monoclonal antibody’s Fab domains to the target antigen. The finite expression of the target on a tumor makes this a ‘saturable’ process. The rate of this elimination pathway is governed by five factors: (i) the level of target expression; (ii) the dose of the antibody administered; (iii) the rate of target turnover; (iv) the kinetics of receptor internalization; and (v) the kinetics of intracellular catabolism. The strong dose dependence of this process causes it to follow non-linear kinetics, whereby the clearance of the antibody decreases as a function of dose.4 Moving beyond tumor tissue, pharmacokinetic models that account for the FcRn-mediated recycling of antibodies have shown that the liver acts as the principal site of antibody catabolism. However, when antibodies do not undergo receptor-mediated endocytosis and target-mediated disposition, organs such as the skin, muscle, and gastrointestinal tract contribute significantly to the in vivo catabolism of antibodies as well.6, 8, 10, 23 Notably, the size of full-length antibodies limits their glomerular filtration and renal excretion. However, full-length antibodies can be excreted via the kidneys in humans with pathologies such as multiple myeloma, which is frequently accompanied by renal dysfunction.24 Furthermore, antibody fragments including F(ab’)2 and Fab’ are excreted through the kidneys in both mice and humans.24–25
At this point, a brief shift in focus to the Fc region of the antibody can help us understand the role that this domain plays in elimination. Residues close to the hinge region within the distal portion of the CH2 domain interact with Fc-gamma receptors (FcγRs) expressed by several immune effector cells. This interaction can trigger the phagocytosis and catabolism of antibodies. However, at present, there is no clear consensus on the ability of FcγRs to influence the in vivo biodistribution and elimination of antibodies. Arguably, the relatively large pool of endogenous immunoglobulins should outcompete any exogenously injected antibodies for binding to FcγRs. Clinical investigations such as the one performed by Cartron et al. have demonstrated the role of FcγRs in the response to therapy with monoclonal antibodies. This work focused on a cohort of non-Hodgkin’s lymphoma patients bearing a single nucleotide polymorphism (SNP) in the gene for FcγRIIIa that gives the receptor higher affinity for IgG. It was found that patients with this SNP exhibited improved survival following treatment with rituximab compared to a cohort of patients who were given the same dose of the antibody but had a low affinity polymorph in their genotype.26 Despite these intriguing results, subsequent investigations found no statistically significant differences between the in vivo pharmacokinetics of rituximab in patients with high, medium, and low affinity FcγRIIIa polymorphs.21, 27 Similarly, preclinical studies using various FcγR knockout mice revealed no change in the disposition of targeted versus non-specific antibodies.21, 28–29 Taken together, these results support the role of the IgG-FcγR interaction in mediating immune effector cell functions and suggest that this interaction does not necessarily have any influence on the in vivo pharmacokinetics of antibodies. However, the in vivo pharmacokinetics could change if the exogenous antibody forms large immunocomplexes in vivo or has been specifically engineered to bind FcγR with high affinity.21
Finally, as discussed earlier, the expression of FcRn on a variety of cells plays a key role in both the homeostasis of endogenous immunoglobulin levels as well as the long serum half-lives of exogenously administered antibodies. Under physiologic conditions, endogenous immunoglobulins are present at concentrations between 65–130 μM (50–100 g in 5 L of blood in an average healthy adult human) and comprise ~ 20% of the total plasma protein by weight. While FcRn-mediated recycling is efficient, the limited expression of the receptor on cells limits the capacity of the process. This is an important consideration for the administration of exogenous antibodies. Notably, exogenous antibodies that are administered at ≤10 mg/kg do not perturb the in vivo homeostasis or efficiency of the FcRn recycling system. However, there are two scenarios in which the FcRn system can be saturated, leading to a dramatic systemic elimination of antibodies: (1) multiple myeloma patients have excessively high plasma IgG concentrations (100 mg/mL) and (2) extremely high concentrations (2 g/kg) of intravenous immunoglobulin (IVIG) are often used to treat patients suffering from autoimmune diseases.4, 30 Given that radioimmunoconjugates are typically administered in doses <100 mg, there is very little likelihood that immunoPET or immunoSPECT could interfere with the FcRn-mediated recycling system.
IgG Subclass and In Vivo Behavior
Perhaps not surprisingly, the in vivo fate of IgGs also depends upon their subtype. There are four subclasses of IgG antibodies — IgG1, IgG2, IgG3, and IgG4 — and each has a distinct yet critical role to play in the immune response. IgG1 antibodies are the most prevalent form and are induced primarily by both soluble protein antigens and membrane proteins. IgG2 antibodies, in contrast, are almost solely responsible for the response to bacterial capsular polysaccharide antigens. IgG3 antibodies are often the first to respond to viral infections and are particularly potent inductors of effector functions. And finally, IgG4 antibodies are often formed in response to long-term exposure to allergens.
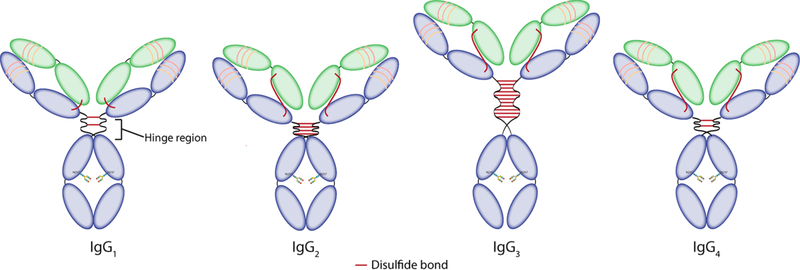
While the 4 subclasses share 90% of their amino acid sequence, small differences in their structures can translate into dramatic changes in their in vivo behavior. For example, the length of the hinge region — the flexible linker between the Fab arms and the Fc region — varies from 12 amino acids for the IgG2 and IgG4 subtypes to 15 amino acids for the IgG1 subtype and 62 amino acids for the IgG3 subtype (Figure 3).31 The length of this linker influences the affinity of each immunoglobulin for FcγRs receptors. More specifically, because the binding site is located in the lower hinge region, it can be partially or totally shielded by the Fab arms (Figure 2). As a result, a longer and more flexible hinge region facilitates stronger interactions between the immunoglobulin and FcγRs receptors, making IgG1 and IgG3 antibodies more potent inductors of effector mechanisms than IgG2 and IgG4 antibodies. Variations in the amino acid composition of the hinge regions, specifically the number of cysteine residues, can also result in different numbers of disulfide bonds between the heavy chains, ranging from 2 for the IgG1 and IgG4 subclasses to 11 for the IgG3 subtype (Figure 3).
Figure 3:
Structures of the different IgG subclasses.
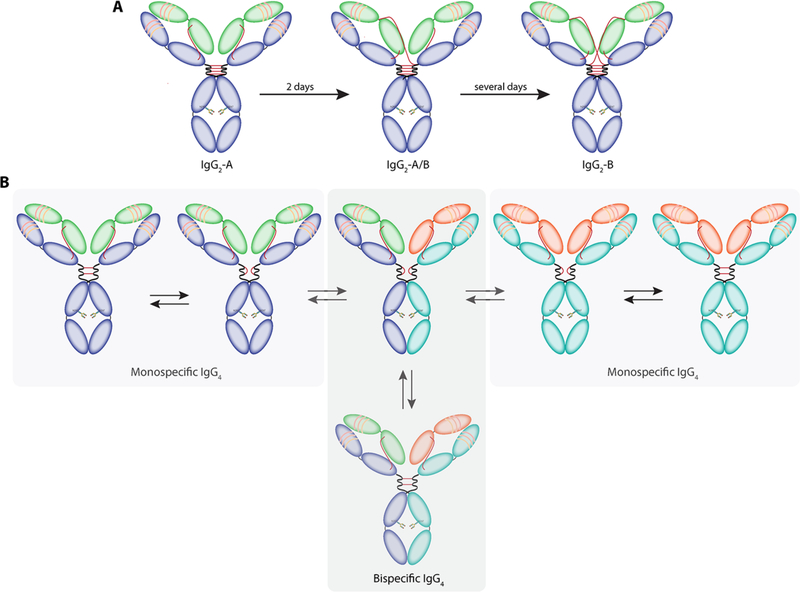
As we have noted, members of the IgG2 and IgG4 subclasses have lower affinity for FcγRs than their IgG1 and IgG3 cousins. As a result, the former have often been selected as platforms for the development of therapeutic antibodies in cases in which the recruitment of effector function may not be required. However, both of these subclasses seem to be sensitive to the redox environment in serum, leading to undesirable re-arrangements of their disulfide bonds.32 In the case of IgG2 antibodies, fully functional covalent dimers can be formed, presumably to improve the avidity of these antibodies to their target.33 Another phenomenon observed with IgG2 antibodies is the shuffling of disulfide bonds to generate three different isoforms: IgG2-A, IgG2-B, and IgG2-A/B (Figure 4A). Although the serum half-lives of the isoforms remain identical, the conversion of IgG2-A to IgG2-B induces a loss of activity in some cases.34 IgG4 antibodies present another unique feature: they can exchange Fab arms in vivo(!)35–36 More specifically, two different IgG4s can recombine to form a pair of monovalent-bispecific antibodies (Figure 4B).37
Figure 4:
Re-arrangement of disulfide bonds. (A) Different isoforms of IgG2 antibodies. (B) Fab arm exchange between IgG4 antibodies.
The structural differences between the IgG subclasses can also affect their pharmacokinetics. For instance, as we have explained earlier, the half-life of IgG antibodies is related to the interaction between the FcRn receptor and the CH2-CH3 interface of the immunoglobulin. In the case of IgG3 antibodies, a crucial histidine at position 435 is replaced with arginine. This lowers the affinity of IgG3 antibodies for the FcRn receptor and results in a dramatically shorter serum half-life: 7 days compared to 21 days for IgG1, IgG2 and IgG4 antibodies.31
Modulating the In Vivo Behavior of Antibodies
In the preceding pages, we have addressed the principal biochemical processes that govern the in vivo fate and pharmacokinetics of antibodies. In this section, we will ‘zoom in’ and cover several properties of immunoglobulins that have been artificially modulated — with varying degrees of success — to ‘improve’ the in vivo behavior of antibodies. Again, as above, much of this information has been gleaned from the antibody and antibody-drug conjugate literature. However, the overarching lessons apply to radioimmunoconjugates as well.
Fc-Mediated Interactions:
In an effort to produce therapeutic antibodies with longer-lived serum half-lives, several laboratories have synthesized Fc-mutated variants of antibodies that have stronger binding to FcRn at pH 6 but little to no binding to the receptor at pH 7.4. For example, the M252Y/S254T/T256E — nicknamed YTE — mutant of an antibody targeting respiratory syncytial virus (RSV) has shown an in vivo half-life of more than 60 days, 3 times longer than that of its parent antibody.5, 38 Conversely, antibodies with shorter in vivo half-lives can be created as well. One way to achieve this is by mutating key amino acid residues such as I253, H310, and H435 within the Fc portion of the antibody to lower the affinity of the antibody for FcRn and thus prevent FcRn-mediated recycling.39 Furthermore, the oxidation of the methionine residue at position 252 within the CH2-CH3 domain during the production of an antibody can decrease its binding affinity for FcRn. This has also been shown to dramatically reduce an antibody’s in vivo serum half-life.5, 40
A different approach to shortening the in vivo half-life of antibodies is predicated on the highly pH-driven nature of the Fc-FcRn interaction. It has been reported that antibodies having identical Fc regions but different antigen binding Fab domains can exhibit different binding affinities for FcRn, ultimately resulting in dramatically different in vivo serum half-lives. Interestingly, in this work, the serum half-lives of the antibodies correlated strongly with their kinetics of dissociation from FcRn at pH 7.4. In other words, antibodies with slower FcRn dissociation kinetics at pH 7.4 had shorter serum half-lives.5, 41 A similar observation was reported for a pair of interleukin-targeting antibodies with different Fab arms but almost identical Fc domains, identical binding affinities to FcRn at pH 6.0, and similar isoelectric points (pI). In this case, the antibody that had a patch of positively charged amino acid residues in the variable (Fv) domains of its Fab region displayed stronger binding to FcRn and only dissociated at pH >7.4. Predictably, the variant bearing a patch of positively charged amino acid residues in its Fab region had a significantly shorter in vivo half-life (~9 days) compared to its counterpart (~ 22 days).5, 42
Glycosylation:
Given the dominant role of FcRn as a mediator of the in vivo fate of antibodies, it is noteworthy that the FcRn-mediated recycling of antibodies is independent of their glycosylation status. Both genetically aglycosylated and chemoenzymatically deglycosylated variants have been reported to have serum half-lives similar to those of their glycosylated counterparts.5, 43 Furthermore, while the deglycosylation of antibodies can abrogate Fc-FcγR interactions, it is generally thought that this phenomenon will not influence the in vivo pharmacokinetics due to the occupancy of FcγR sites by endogenous Ig(s). On the other hand, the rapid clearance of glycosylated antibodies with high mannose glycans has been attributed to the processing of these antibodies by specific receptors, namely the mannose receptor (ManR) and the asialoglycoprotein receptor (ASGPR). Sinusoidal endothelial cells and hepatic parenchymal cells such as hepatocytes express ManR(s), whereas non-parenchymal cells of the liver such as the Kupffer cells are known to express ASGPR(s).5
Charge:
Because antibodies are glycoproteins with varying compositions of amino acids and sugars, they can have different isoelectric points. In general, antibodies are slightly more basic (pI > 6) than most serum proteins (pI < 5.5).44 This facilitates the purification of antibodies and allows them to interact with anionic glycan chains on cell surfaces as well as heparin sulfate proteoglycans present in the extracellular matrix. Antibodies can be ‘cationized’ to confer a more basic pI or ‘anionized’ to provide a more acidic pI. Importantly, ‘cationized’ antibodies are characterized by rapid clearance from circulation, short serum half-lives, and increased binding to tissues. In some cases, this augmented binding can be non-specific due to the electrostatic interactions between the ‘cationized’ antibody and negatively charged cell surfaces in vivo. Li et al. have recently shown that an antibody with a higher (more basic) pI produces more accumulation in the liver and spleen than an antibody with a lower (more acidic) pI.5, 44–45 On the other hand, antibodies modified to have more acidic pIs tend to repel negatively charged cell surfaces, thereby minimizing non-specific binding in both target and non-target tissues while concomitantly increasing serum half-lives. However, work from the groups of Khawli and Igawa has demonstrated that shifts in pI of at least 1–2 units are needed in order to produce meaningful alterations in the in vivo behavior of charge-modified antibodies.5, 46–47
In light of this article’s focus on radioimmunoconjugates, it is important to note that the attachment of bifunctional chelators to antibodies can influence the charge and biodistribution of antibodies as well. Despite a growing appreciation for the importance of site-specific modification, the vast majority of radioimmunoconjugates are synthesized via the indiscriminate coupling of amine-reactive bifunctional chelators to lysine resides on the surface of the immunoglobulin.48–49 Not surprisingly, this approach can alter the pI of immunoconjugates relative to their parent antibodies.50–51 This, in turn, can impact both the physicochemical properties and in vivo behavior of the immunoconjugate if the change in pI is >1–2 units. In addition, the conjugation of bifunctional chelators and prosthetic groups to IgGs can also dramatically affect the in vivo biodistribution of the resultant radioimmunoconjugate if the cargoes are inadvertently attached within the antibody’s antigen-binding domains, thus impairing the immunoreactivity of the construct. Ultimately, all alterations to the surface of an antibody should be performed cautiously, as these efforts can inadvertently (a) alter the tertiary structure of the antibody, (b) introduce new antigen determinant sites on the antibody, and (c) result in the loss of the antibody’s affinity for its antigen and/or FcRn.
Aggregation:
While the inter-chain disulfide bonds of the hinge region are important for the stability of an antibody, much of its macromolecular structure is held together by intra- and inter-domain hydrophobic interactions, hydrogen bonds, and van der Waals forces. These non-covalent interactions are critical for maintaining the tertiary structure of the immunoglobulin. However, non-covalent interactions can also be detrimental to the in vivo performance of antibodies. For example, hydrophobic patches of residues in the complementarity determining regions (CDR) and the Fc domain have been implicated in the aggregation of antibodies, though this behavior varies on an antibody-to-antibody basis. The process of modifying antibodies to form radioimmunoconjugates and antibody drug conjugates (ADC) makes them particularly susceptible to aggregation via the unfolding of subdomains and the exposure of residues and regions that are hotspots for aggregation.52 This phenomenon has been reported more frequently in the ADC world, as ADCs are often found to be more hydrophobic and prone to aggregation than their parent antibodies, due in part to the hydrophobicity of their cytotoxic payloads.52–54 As expected, the in vivo pharmacokinetic profiles of ADCs with higher drug-to-antibody ratios (DAR) differ significantly from those of their counterparts with lower DARs. While the former are more cytotoxic in vitro, they are cleared much more rapidly than the latter and are less effective in vivo.55–56
Unsurprisingly, the same principles extend to radioimmunoconjugates. The conjugation of hydrophobic chelators to antibodies can also perturb the tertiary structure of the immunoglobulins and increase their propensity for aggregation. Lub-de-Hooge et al., for example, have reported this behavior in their investigations using 111In-labeled trastuzumab.57 Likewise, desferrioxamine — the current ‘gold standard’ chelator for 89Zr — is a rather hydrophobic molecule and has been known to create immunoconjugates that are prone to aggregation.58–59 Price at al. have shown that the over-conjugation of DFO to trastuzumab yields radioimmunoconjugates with high chelator-to-antibody ratios and high specific activities but poor in vivo biodistribution profiles due to their increased propensity for aggregation.60 Specifically, this study demonstrates that aggregation prompts an increase in the uptake of radioimmunoconjugates in the liver of tumor-bearing mice. Intriguingly, it remains unclear whether hepatocytes in the parenchyma of the liver or non-parenchymal cells such as the Kupffer cells and endothelial cells of the liver are responsible for catabolizing aggregated proteins.61–65 It is also important to emphasize that aggregation is not the only cause of elevated uptake in the liver. Indeed, the in vivo formation of soluble immunocomplexes between radioimmunoconjugates and shed antigen can boost liver uptake as well.66–69 This phenomenon is distinct from the hepatic accumulation of protein aggregates, though, as the accumulation of immunocomplexes in the liver occurs soon after the administration of the radioimmunoconjugate and can be alleviated by the co-injection of unlabeled target-specific antibody.
Immunogenicity:
Like most xenobiotic drugs, monoclonal antibodies are capable of eliciting anti-drug antibody (ADA) responses.4–5 This can impact both the in vivo fate of the monoclonal antibody and — far more importantly — the subject to whom the antibody was administered. While many of the problems with ADA responses have been overcome due the field’s shift to humanized and fully human antibodies, some of these agents may nonetheless elicit ADA responses (from <1% up to 10%).8, 70 An infamous case is that of Humira (adalimumab), a TNF alpha-targeting fully human antibody that provokes an ADA response in 28% of patients.5, 71 Recent reports have shown that the co-administration of methotrexate as an immunosuppressant with adalimumab might help overcome the ADA-driven clearance and immunogenicity of the antibody.8, 72
THE FATE OF THE RADIONUCLIDE
Up until this point, we have been drawing upon data from biochemistry, cell biology, physiology, pharmacology, and biophysics to describe the factors that govern the behavior and pharmacokinetics of antibodies. Yet the focus of this review is not antibodies but rather radioimmunoconjugates. This is an important distinction. Of course, many of the important lessons that we have learned regarding the in vivo fate of antibodies also apply to radioimmunoconjugates. However, our discussion cannot stop there. The very entity that makes radioimmunoconjugates useful for immunoPET and immunoSPECT — the radionuclide — demands further attention. Most of the time, the radionuclide behaves exactly as we would hope: as a simple passenger along for the ride. However, in some circumstances — such as following the catabolism of the immunoglobulin — the paths of the antibody and the radionuclide can diverge. In these cases, it is critical to understand the fate of the radionuclide itself, as it can produce signal (and radiation dose) regardless of whether or not it remains attached to the antibody.
In the second half of this review, we will turn our attention to these radionuclides. We will provide an overview of the different radionuclides that have been used in immunoPET and immunoSPECT probes and discuss what is known regarding the in vivo fate of these nuclides when they are part of radioimmunoconjugates. In addition, we will address several other important lessons on the in vivo fate of antibodies that have been gleaned from the preclinical and clinical study of radioimmunoconjugates. The following section will be organized by radionuclide, as there are several considerations that are specific to individual isotopes.
Before we begin, a brief introduction to the structure of a radioimmunoconjugate might be helpful. General speaking, a radioimmunoconjugate has three parts: (a) an antibody, (b) a radionuclide, and (c) a linker between the radionuclide and the antibody. Radioimmunoconjugates labeled with metallic radionuclides have a fourth component: a chelator whose purpose is the stable sequestration of the radiometal during the radioimmunoconjugate’s in vivo journey. The identity of the antibody itself is, of course, determined by its molecular target. The radionuclide is chosen primarily on the grounds of its physical properties. Specifically, the radioactive emission must be appropriate for the desired imaging modality (either photons for SPECT or positrons for PET), and the radionuclidic half-life must be compatible with the multi-day serum half-life of the antibody. The choice of the linker between the moieties is governed by several factors, including the desired regioselectivity and site-specificity of the bioconjugation reaction. And finally, the chelator is selected in response to the radiometal, as different radiometals can have dramatically different coordination chemistries. With this quick anatomy lesson complete, it is now time to move on to our first radionuclide.
The Isotopes of Iodine
From a nuclear medicine perspective, iodine is a remarkably versatile element. Indeed, there are four different radioisotopes of iodine that can be used for nuclear imaging and therapy. Specifically, 123I and 125I are gamma emitters suitable for SPECT imaging, 124I is a positron-emitting isotope used for PET imaging, and 131I emits both β- and γ- rays and can thus be harnessed for both SPECT and radioimmunotherapy (RIT). Traditionally, the radioiodination of antibodies has been performed via the direct electrophilic radioiodination of tyrosine residues within the antibody to create radioiodotyrosines. Technically speaking, such directly labeled mAbs are not “radioimmunoconjugates” per se; however, several approaches for the synthesis of radiolabeled immunoconjugates based on radioiodinated prosthetic groups have gained popularity in recent years (vide infra). Interestingly, several factors — including cost, availability, and ease of radiochemistry — led to the widespread study of 131I-labeled constructs for RIT prior to the advent of therapeutic radioimmunoconjugates labeled with β-emitting radiometals such as 90Y, 177Lu, and 67Cu. As a result, many of the lessons learned about the in vivo behavior of radioiodine-bearing antibodies stem from preclinical and clinical investigations of 131I-labeled radioimmunoconjugates.
The chief concern surrounding the in vivo fate of radioiodinated antibodies is the ease with which the radionuclide can be separated from the antibody itself. Along these lines, several in vitro studies have demonstrated that the cellular internalization of radioiodinated antibodies leads to their rapid degradation within lysosomes and the extracellular release of monoiodotyrosine (Figure 5).73–75 This metabolite is further broken down by deoidination enzymes, releasing free radioiodide into the bloodstream. This release of radioiodide into the blood stream creates two interrelated problems:
First, the radioiodine will be taken up by any tissues that express the sodium-iodide (Na+/I−) symporter, an integral membrane protein present in the thyroid gland and the stomach among other tissues. This phenomenon has been reported in several preclinical in vivo studies.76–78 Moving to the clinic, the uptake of radioiodine in the thyroid was also observed by De Nardo et al. in 131I-Lym1-treated patients with non-Hodgkin’s lymphoma and chronic lymphocytic leukemia despite attempts to block this phenomenon via the infusion of Lugol’s solution or a saturated solution of potassium iodide.79–80 Interestingly, however, no hypothyroidism was detected during follow-up in these patients over several years.
Second, the lysosomal degradation of radioiodinated antibodies results in the rapid in vivo clearance of radioiodine from all tissues except those that metabolize or process iodine. Theoretically, this could be beneficial for nuclear imaging and therapy, as it leads to lower retention of radioactivity in non-target organs. However, this phenomenon translates into far lower activity concentrations in tumor tissue compared to those created by radioimmunoconjugates labeled with residualizing radionuclides such as 90Y, 177Lu, 64/67Cu or 89Zr. A study by De Nardo et al. comparing 67Cu- and 131I-labeled Lym-1 antibodies in patients with non-Hodgkin’s lymphoma demonstrated that the mean biological tumor half-life of 67Cu in the tumor was 8.8 days compared to only 2.3 days for 131I.80
Figure 5.
The importance of residualizing radiolabels. (A) After internalization, radioiodinated antibodies are routed to the lysosomes, where most of the catabolism occurs. This results in the extracellular release of monoiodotyrosine. (B) In contrast, radiometal-based catabolites are trapped in the lysosomes and thus remain in the cell.
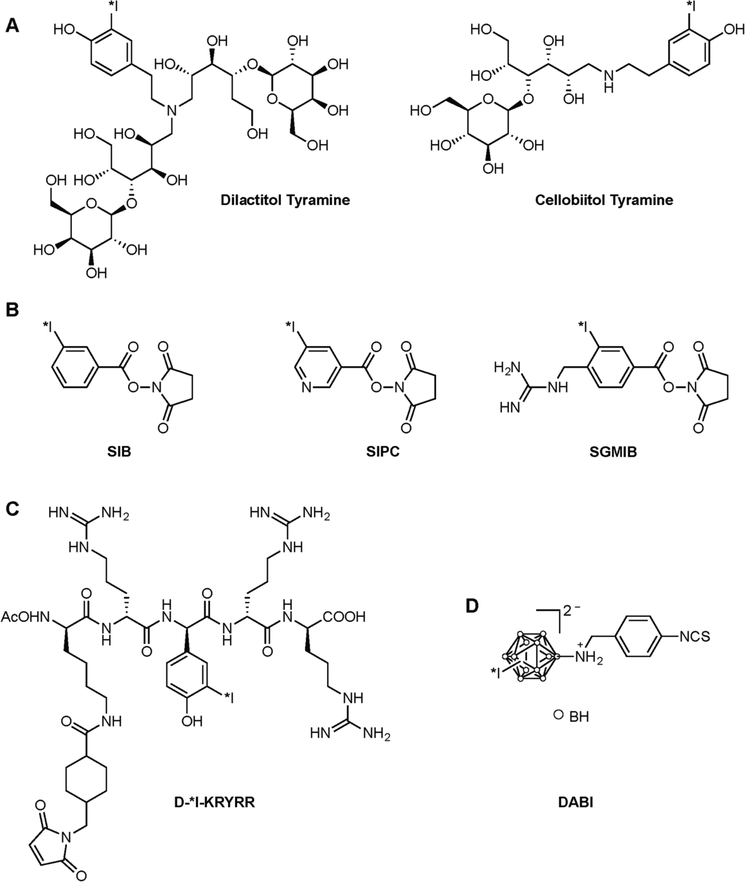
In order to circumvent this limitation, several residualizing prosthetic groups have been developed to help trap the nuclide within the lysosomes of target cells. These include non-metabolizable carbohydrates,81–85 positively- or negatively-charged aromatic compounds,86–89 bulky ionic polyhedral boron clusters,76 and short D-amino acid peptides that are resistant to proteolytic degradation (Figure 6).90–93 Diethylenetriaminepentaacetic acid (DTPA) has also been added to some short D-amino acid peptides owing to its own residualizing properties.94–95 In murine models of cancer, these strategies have been shown to increase the retention of radioiodine in tumor tissue as well as reduce the uptake of radioiodine in the thyroid.81–92, 94–96 Unfortunately, however, radiolabeling antibodies using these prosthetic groups is often less efficient than direct radioiodination. In addition, these residualizing prosthetic groups can also slow the clearance of radioactivity from non-target tissues. For example, radioimmunoconjugates bearing carbohydrate-based prosthetic groups have displayed higher uptake in the liver, whereas antibodies conjugated to positively charged residualizing agents have produced higher activity concentrations in the kidneys.85,88 To the best of our knowledge, no radioimmunoconjugates bearing any of these residualizing agents have reached clinical trials, leaving room for further improvement prior to clinical implementation.
Figure 6.
Examples of prosthetic groups designed to increase the residualization of radioiodine. (A) Nonmetabolizable carbohydrate-tyramine adducts. In these reagents, the cellobiitol carbohydrate is indigestible by lysosomal glycosidase, whereas the lactitol becomes non-degradable after attachment to a protein; (B) Aromatic acylation agents. SIB (N-succinimidyl 3-iodobenzoate), SIPC (N-succinimidyl 5-iodo-3-pyridine carboxylate) and SGIMB (N-succinimidyl 4-guanidinomethyl-3-iodobenzoate) produce positively charged metabolites at lysosomal pH that can escape cellular excretion; (C) Example of a short peptide containing D-amino acids that are — unlike L-amino acids — resistant to proteolytic degradation; (D) Bulky ionic polyhedral boron cluster DABI ([(4-isothiocyanatobenzylammonio)undecahydro-closododecaborate (1-)])
Radiometals
Over the past two decades, researchers have increasingly turned to the array of metallic radionuclides for immunoPET and immunoSPECT. Radiometals not only have promising physical properties but also can help eschew some of the issues surrounding the non-residualizing isotopes of radioiodine. As we mentioned in our introduction to this section, radiometals — unlike radiohalogens — require a chelator for their stable sequestration in vivo. Not surprisingly, different radiometals are compatible with different chelators. As a result, a wide range of bifunctional chelators have been developed and evaluated in both the laboratory and the clinic.97
The Isotopes of Copper:
Since the late 1990s, several research teams have focused their attention on the application of copper to nuclear medicine. While the metal has several radioisotopes suitable for medical applications, two are particularly compatible with antibodies: 67Cu and 64Cu. 67Cu is a β-emitter with a half-life of 2.6 days. Like 131I, its mean β energy of 141 keV (mean range of 0.2 mm) is suitable for the treatment of small tumors. 67Cu also emits γ-rays but does so in lower abundance and lower energy than 131I, reducing the radiation burden to non-target organs. In addition, its physical half-life of 2.6 days dovetails well with the biological half-life of antibodies. Nevertheless, its high cost and the lack of a reliable supply have limited its widespread use. In contrast, 64Cu is a positron-emitting radiometal that can be produced readily and relatively inexpensively in high specific activities. Unfortunately, its physical half-life of 12.7 hours is somewhat incompatible with the pharmacokinetics of full-length antibodies. That said, this has not stopped several laboratories from bringing 64Cu-labeled immunoPET agents into the clinic.98–100
The in vivo metabolism of both 64Cu- and 67Cu-labeled antibodies has been studied in several preclinical mouse models.101–103 Yet regardless of the antibody or tumor model, the catabolism of the antibody in the tumor was found to be fast and led to the formation of a final product consisting of the radiocopper-chelator complex attached to a short protein trapped within the lysosome. For example, in a study focusing on the in vitro fate of a 67Cu-CTPA-labeled variant of the chCE7 antibody in neuroblastoma cells, Novak-Hofer et al. found that the radionuclide was trapped in the lysosomes of tumor cells due to the accumulation of low molecular weight metabolites corresponding to 67Cu-CTPA with and without a lysine residue.104 Attempts to increase the lysosomal pH up to 6.0 did not result in the release of 67Cu from the cells, suggesting that the positive charge of the chelator was not the only thing responsible for the retention of the radionuclide in this subcellular compartment. These data, of course, stand in stark contrast to that obtained with radioiodine-labeled antibodies. Indeed, when compared to analogous antibodies labeled with isotopes of iodine, copper-based radioimmunoconjugates produce better tumor uptake across the board.
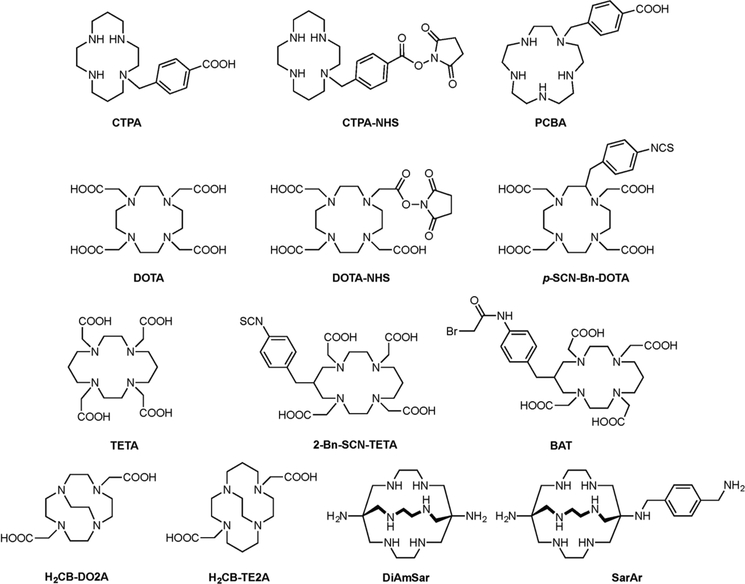
The one caveat of Cu-based radioimmunoconjugates is the uptake of the radionuclide in the liver. Despite the development of several highly stable macrocyclic chelators for 64Cu and 67Cu such as TETA, BAT, DOTA, and CTPA (Figure 7), 64Cu and 67Cu remain susceptible to copper-binding proteins that are abundant in the liver. Indeed, Rodgers et al. observed the formation of a 35 kDa metabolite of the 67Cu-CTPA-1A3 antibody that is believed to correspond to a transchelation product in which 67Cu is bound to superoxide dismutase (SOD), an endogenous antioxidant enzyme.105 Li et al. found a similar metabolite using a 64Cu-DOTA-labeled variant of cetuximab as well as a second metabolite with a molecular weight of 11 kDa, consistent with the mass of metallothionein.103 In humans, Mirick et al. demonstrated that the copper from 67Cu-2IT-BAT-Lym1 was not transferred to SOD but rather to ceruloplasmin, the major copper-carrying protein in the blood.106
Figure 7.
Selected chelators and bifunctional chelators for Cu(II).
To overcome this issue, several transchelation-resistant cross-bridged chelators have been created.107 Unfortunately, the harsh conditions required for the metalation of some of these chelators are incompatible with sensitive biomolecules such as immunoglobulins. More recently, a family of sarcophagine-based chelators has been developed by Sagerson and coworkers.108 Voss et al. used a member of this family to radiolabel the anti-GD2 monoclonal antibody 14.G2a with 64Cu and subsequently observed significantly reduced uptake and retention in the liver, a result which suggests that the chelator-radiometal complex is stable against in vivo transchelation.109
Due to their smaller size, the metabolism and excretion of antibody fragments differs from that of full-length antibodies and thus lie outside of the scope of this review. A few words, however, might be appropriate given that 64Cu is commonly used as a radiolabel for antibody fragments. Generally speaking, preclinical imaging and biodistribution studies reveal high non-target accumulation of radiolabeled antibody fragments in the kidneys regardless of the radionuclide employed, as the renal system is the typical route of elimination and clearance of these constructs. In the case of 64Cu-labeled antibody fragments, the formation of a small molecular weight metabolite — most likely a radiocopper complex attached to a lysine residue — that is retained in the cells of the proximal tubules of the kidney further exacerbates this issue.101 Rogers et al. demonstrated that the anomalous non-tumor-specific concentration of radioactivity in the kidneys can be circumvented by modulating the properties of the chelator.105 In this work, a comparative analysis of four different copper chelators — BAT, TETA, CTPA, and PCBA — revealed that more lipophilic and positively charged metabolites were more likely to accumulate in the kidneys. Zimmerman et al. have developed an alternative strategy by introducing a cleavable triglycine linker between the antibody fragment and the chelator in 67Cu-chCE7 F(ab’)2 to prevent the formation of metabolites bearing positively charged lysine residues. Interestingly, the efficacy of this approach proved to be dependent on the identity of the chelator. A construct bearing this linker and the cationic CTPA chelator produced significantly lower kidney retention than an analogous ‘traditional’ conjugate. However, a 67Cu-labeled fragment combining this linker with the anionic DO3A chelator yielded slower clearance from the blood and increased activity concentration in the tumor.110
Indium-111:
Unlike copper or iodine — each of which have several different medically useful radioisotopes — indium has only one radioisotope that can be used in nuclear medicine: 111In. 111In is a gamma-emitting radiometal with a half-life of 2.8 days and thus is very well suited for immunoSPECT imaging. In vitro studies have clearly illustrated that 111In possesses residualizing properties that can contribute to the improved uptake and retention of 111In-labeled radioimmunoconjugates in tumors compared to radioiodinated antibodies.74–75 Unfortunately, however, the residualizing nature of 111In also increases its retention in non-target organs. Moreover, the release of 111In from its chelator can create other issues, as it has been shown that the radionuclide can transchelate to iron carrying proteins such as transferrin and ferritin in the blood.111
In light of these concerns, the metabolism of 111In-labeled radioimmunoconjugates has been studied to better understand their uptake and retention in non-target tissues. Indeed, several articles explore the in vivo fate of 111In-labeled antibodies in healthy mice112 and rats.113–114 In these studies, it has been found that the intact 111In-labeled antibody is the only radioactive species present in the blood. However, up to four 111In-containing species can be — but are not always — detected in the liver and kidneys: the intact radioimmunoconjugate itself, a low molecular weight metabolite (likely the 111In-chelator attached to a small peptide), an 111In-labeled fragment of the original antibody, and an 111In-transferrin moiety. Rogers et al.114 have also shown that the low molecular weight metabolite — in their case positively identified as 111In-DTPA-ε-lysine — was also present alongside 111In-DTPA in the feces and urine as well.
Several of these observations were reinforced by the work of Motta-Hennessy et al. who studied the catabolism of a CEA-targeting 111In-SCN-Bz-DTPA-NP-4 antibody in athymic nude mice bearing GW-32 xenografts.115 Radioactivity from the tumor was recovered in 3 fractions corresponding to radioimmunoconjugate-antigen complexes, the native radioimmunoconjugate, and a low molecular weight fraction (LMWF). Further analyses of the LMWF via SDS-PAGE and instant thin-layer chromatography (iTLC) identified 111In-SCN-Bz-DTPA and 111In-SCN-Bz-DTPA-peptide fragments as the prime metabolites. In the same study, the authors confirmed the presence of the intact antibody and/or the low molecular weight fraction in other organs and biological fluids. Those findings were consistent with the metabolites found in healthy mice and rats.112,113–114 Furthermore, DeNardo et al. reported the presence of radiometabolites in the plasma of non-Hodgkin’s lymphoma patients injected with the 111In-labeled mouse IgG2a 111In-2IT-BAD-Lym that targets the (HLA)-DR10 antigen on the surface of malignant B lymphocytes.116 Interestingly, radiometabolites have not been described in the plasma of humans administered with 131I- or 67Cu-labeled variants of the same antibody.80 This example clearly underscores the influence of the nuclide itself on the in vivo processing of radiolabeled antibodies.
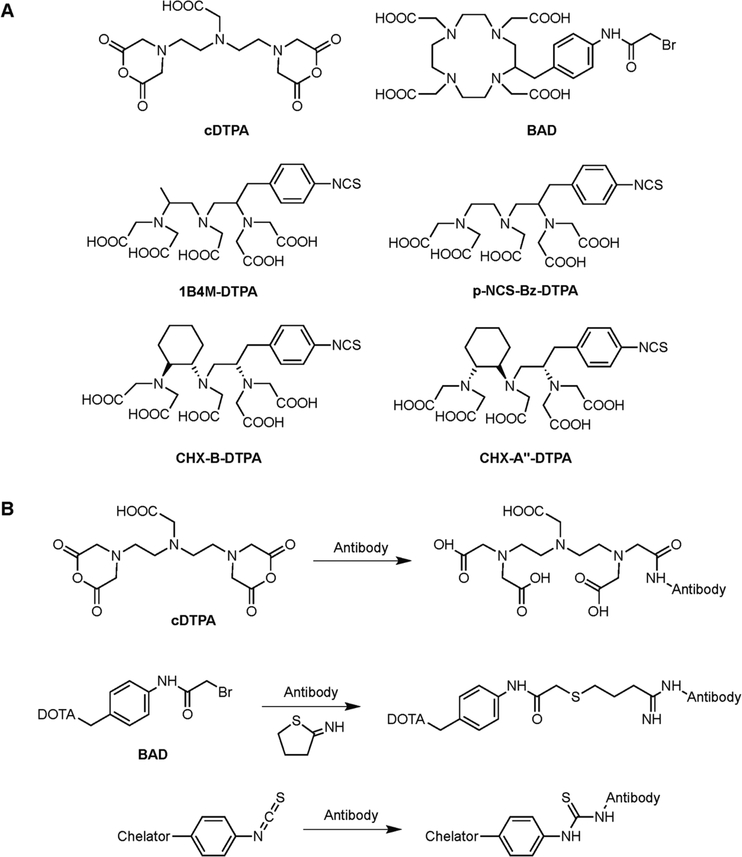
Interestingly, the identity of the chelator can also influence the number of metabolites. This was demonstrated effectively by Kinuya et al., who studied the effect of chelator structure on the metabolic fate of 111In-labeled radioimmunoconjugates of the monoclonal antibody T101 in normal mice. To this end, the antibody was conjugated with three different chelators — B4M-DTPA, CHX-B-DTPA and cDTPA (Figure 8) — and then radiolabeled with 111In. The authors found that the three radioimminoconjugates cleared from the whole body with similar rates. However, the two constructs bearing thiourea-linked variants of DTPA (1B4M-DTPA and CHX-B-DTPA) more quickly from the liver but more slowly from the blood than the cDTPA-bearing conjugate. This phenomenon was attributed to the greater stability of these two conjugates as well as the higher lipophilicity of the 1B4M-DTPA and CHX-B-DTPA chelators. In addition, differences were observed in the metabolites present. Two species were found in the liver after the administration of all three constructs: the intact radioimmunoconjugate itself and an 111In-labeled small molecular weight metabolite. However, another, minor metabolite was observed in the case of 111In-cDTPA-T101: 111In-labeled transferrin. The latter was also exclusively found in the serum of mice injected with the 111In-cDTPA-T101, although the rate of transchelation to transferrin was low (2% in serum after 5 days). These results suggest that all 5 carboxymethyl arms of the 1B4M-DTPA and CHX-B-DTPA chelators (compared to the 4 available for conjugated cDTPA) are necessary to stably coordinate 111In. Interestingly, however, a clinical study using the anti-CEA C110 antibody radiolabeled with 111In via a benzylisothiocyanate derivative of DTPA (p-SCN-Bz-DTPA) showed that the transchelation of 111In to circulating transferrin can occurs at a modest level in patients.117
Figure 8.
(A) Selected bifunctional chelators for 111In; (B) conjugation strategies associated with the various bifunctional chelators
Several strategies have been employed to reduce the uptake of radioactivity in the liver following the administration of 111In-labeled radioimmunoconjugates. One of the most common preclinical approaches is predicated on pre-dosing animals with unlabeled antibody prior to the administration of the radioimmunoconjugate in order to reduce the formation of radiolabeled immunocomplexes.118 An attempt at translating this strategy to the clinic seems to have had only limited success in decreasing the levels of shed antigen in the blood and thus signal in the liver.119 However, the pre- or co-injection of unlabeled antibodies alongside 111In-labeled mAbs has proven beneficial in other cases in which the target antigen is not shed.120–123 In a different approach, Studer et al. introduced a tetrapeptide linker (Ala-Leu-Ala-Leu) between the antibody and the chelator to promote the specific cleavage of the 111In-chelator complex in the liver.124 However, this approach led to very limited — if any — improvements in the activity concentrations retained in the liver of mice. The authors suggest that the 111In-chelate complexes were successfully cleaved from the antibody but were themselves subsequently (and unfortunately) trapped in the liver.
Zirconium-89:
Over the last decade, 89Zr has garnered a great deal of attention as an emergent radiometal for immunoPET imaging, primarily due to its emission of low energy positrons (~395.5 keV) and the advantageous match between its physical half-life (t1/2 ~ 3.3 d) and the in vivo pharmacokinetic profiles of antibodies. A wealth of 89Zr-labeled antibodies have been developed, evaluated in murine models of disease, and, in some cases, translated to the clinic over the past few years.58, 125 Both preclinical and clinical studies with 89Zr-labeled radioimmunoconjugates have shown the liver to be the non-target organ with the highest uptake and retention of radioactivity.126 This is most likely due to a number of factors, including the liver’s role as the primary site of the catabolism of antibodies as well as the residualizing nature of the radionuclide itself. Unfortunately, to the best of our knowledge, no extensive metabolic studies have yet been conducted using 89Zr-labeled antibodies.
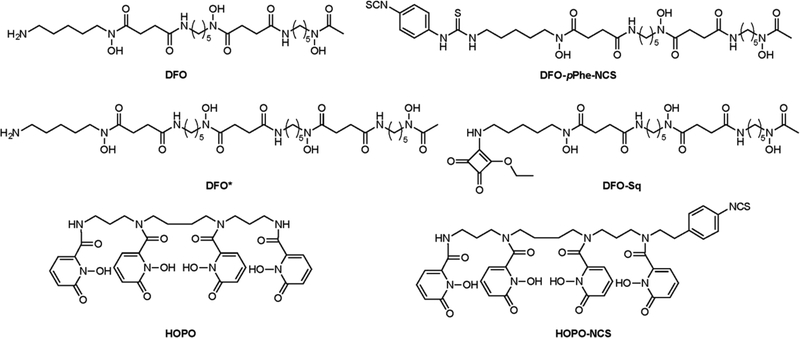
While 89Zr’s multiday half-life allows for the collection of imaging data as late as ten days after the administration of the radioimmunoconjugate, preclinical images taken at later time points often reveal relatively high activity concentrations in the bones of mice. It is well-known that Zr4+ is an osteophilic (bone-seeking) cation, as illustrated in several prior reports that employed 93Zr and 95Zr.127–128 This accumulation in bone suggests that desferrioxamine (DFO; Figure 9) — the most widely used chelator for 89Zr — may allow for the release of the radiometal into the bloodstream or its transchelation to plasma proteins. Abou et al. studied the biological fate of 89Zr by directly injecting the radiometal chelated by oxalate, citrate, and DFO.129 In this work, the authors confirmed that weakly-chelated 89Zr (i.e. 89Zr bound by bidentate oxalate or tridentate citrate ligands) was readily taken up by the bone. More specifically, 89Zr seems to accumulate in the non-soft tissue, mineralized constituents of the bone: most of the radioactivity was found in the epiphysis, whereas the cells of the marrow did not accumulate significant radioactivity. As a result, the authors suggest that 89Zr is most likely chelated by hydroxyapatite. In contrast, the administration of DFO-bound 89Zr produced no uptake in the bone. These data prompted the authors to suggest that in the case of 89Zr-DFO-labeled antibodies, the elevated activity concentrations in the bone arise not from the cleavage of 89Zr-DFO or the release of free 89Zr4+ but rather the rapid metabolism of the antibody and the 89Zr-DFO complex. Several groups have tried to develop more suitable chelators for 89Zr, specifically through the use of octadenate coordination environments. Readers interested in these efforts are directed to a comprehensive review by Heskamp et al.130
Figure 9.
Selected chelators and bifunctional chelators for 89Zr.
Though little clinical data is available on the metabolism of 89Zr-labeled antibodies, it is interesting to note that the bone uptake patterns observed in mice are typically not seen in humans, leading many to believe that DFO is indeed a suitable chelator for 89Zr in the clinic.96, 131–135 Studies using 89Zr-DFO-labeled trastuzumab in HER2-positive esophagogastric adenocarcinoma135 and 89Zr-labeled cmAb-U36 antibody in head and neck cancer131 reported relatively low activity accumulation and retention in the liver and spleen. In contrast, higher activity concentrations were observed in both organs during trials with 89Zr-huJ591,96 89Zr-cetuximab,132 or 89Zr-rituximab.133 These data suggest that the identity of the antibody may be the driving force behind the ultimate distribution of 89Zr in healthy tissues. That said, these differences may also be due to differences in the mass of antibody tracer that was used in each case and might therefore be resolved by the pre-injection of unlabeled antibody.
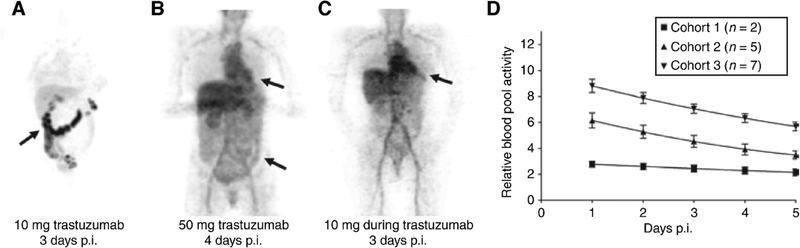
Notably, the growing popularity and clinical utility of 89Zr-labeled antibodies as immunoPET imaging agents for cancer has benefited greatly from our better understanding of the in vivo fate of antibodies. In a seminal feasibility study in patients with Her2-positive metastatic breast cancer, Dijkers et al. demonstrated the dramatic influence that the dose of antibody can have on the in vivo biodistribution of 89Zr-DFO-trastuzumab in patients who had been treated with therapeutic doses of trastuzumab versus those who had not.136 Their findings suggested that the optimal delineation of tumor lesions in treatment-naïve patients was achieved by co-dosing 1.5 mg of the 89Zr-DFO-trastuzumab admixed with 48.5 mg of un-labeled trastuzumab (Figure 10). Furthermore, the addition of significantly higher amounts of unlabeled trastuzumab — i.e. 48.5 mg unlabeled antibody in a 50 mg total dose vs. 8.5 mg unlabeled antibody in a 10 mg total dose — yielded a better in vivo biodistribution profile of the radiotracer. This has been attributed to the ability of a higher dose of unlabeled antibody to prevent the elimination of the tracer and its in vivo clearance due to binding the shed ectodomain of Her2 in treatment-naïve patients.
Figure 10.
The influence of antibody dose on the in vivo biodistribution of 89Zr-DFO-trastuzumab in patients with Her2-positive metastatic breast cancer. (A) PET image of a trastuzumab treatment-naïve patient (cohort 1) co-injected with 1.5 mg 89Zr-DFO-trastuzumab and 8.5 mg of unlabeled trastuzumab, showing non-tumor-specific physiologic uptake of the tracer in the gut; (B) PET image of a trastuzumab treatment-naïve patient (cohort 2) co-injected with 48.5 mg unlabeled trastuzumab and 1.5 mg 89Zr-DFO-trastuzumab, showing significantly reduced non-tumor-specific physiologic uptake in the gut and more widespread distribution of the tracer with the pre-indications of lesions that can be visualized in the liver and thoracic regions; (C) PET image of a patient previously treated with therapeutic doses of trastuzumab (cohort 3) co-injected with 1.5 mg 89Zr-DFO-trastuzumab and 8.5 mg of unlabeled trastuzumab, showing higher signal in the heart (indicative of radioactivity restricted to the blood pool) as well as the visualization of hotspots indicative of tumor lesions in the liver and abdominal region; (D) Relative blood pool activity in the patients of the 3 cohorts over a period of 5 days after the injection of the aforementioned doses of radioimmunoconjugate. Adapted and reproduced with permission from Dijkers et al.136
CONCLUDING REMARKS
In the preceding pages, we have tried to paint as clear a picture as possible of the in vivo journey of both the antibody and radionuclide components of radioimmunoconjugates. It is our intention that this work will be useful to both experienced researchers and those new to the field. For the latter, we hope this review provides a compelling introduction to the in vivo interactions that dictate the in vivo behavior of radioimmunoconjugates. For the more veteran researcher, we hope that this work reinforces the key features that govern the pharmacokinetics of antibodies and revisits how these might be leveraged to improve the performance of radioimmunoconjugates.
In the end, one thing became abundantly clear to us as we wrote this review: we need more data. The solution — more studies — is simple, but things may not be as straightforward as they seem. We work in a difficult funding climate, and metabolic studies aren’t exactly the ‘sexiest’ of investigations. Thus, we will end with the earnest hope that both funding agencies and grant reviewers remain mindful of the paramount importance of these fundamental investigations. They may not necessarily end up in the highest tier of journals, but they are nonetheless absolutely critical to the future success of the field.
Acknowledgments
Financial Support:The authors gratefully acknowledge Hunter College and the Research Foundation of the City University of New York. BZ thanks the NIH (4R00CA178205 and 1R01CA204167) for their generous funding. SKS acknowledges support from a Tow Postdoctoral Fellowship at Memorial Sloan Kettering Cancer Center.
REFERENCES
- 1.Strebhardt K; Ullrich A, Paul Ehrlich’s magic bullet concept: 100 years of progress. Nature Reviews Cancer 2008, 8 (6), 473–80. [DOI] [PubMed] [Google Scholar]
- 2.Ecker DM; Jones SD; Levine HL, The therapeutic monoclonal antibody market. MAbs 2015, 7 (1), 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reichert JM, Antibodies to watch in 2017. MAbs 2017, 9 (2), 167–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang W; Wang EQ; Balthasar JP, Monoclonal antibody pharmacokinetics and pharmacodynamics. Clinical Pharmacology and Therapeutics 2008, 84 (5), 548–58. [DOI] [PubMed] [Google Scholar]
- 5.Liu L, Pharmacokinetics of monoclonal antibodies and Fc-fusion proteins. Protein Cell 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryman JT; Meibohm B, Pharmacokinetics of monoclonal antibodies. CPT Pharmacometrics Syst Pharmacol 2017, 6 (9), 576–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao L; Ji P; Li Z; Roy P; Sahajwalla CG, The antibody drug absorption following subcutaneous or intramuscular administration and its mathematical description by coupling physiologically based absorption process with the conventional compartment pharmacokinetic model. Journal of Clinical Pharmacology 2013, 53 (3), 314–25. [DOI] [PubMed] [Google Scholar]
- 8.Ferri N; Bellosta S; Baldessin L; Boccia D; Racagni G; Corsini A, Pharmacokinetics interactions of monoclonal antibodies. Pharmacology Research 2016, 111, 592–599. [DOI] [PubMed] [Google Scholar]
- 9.Roopenian DC; Akilesh S, FcRn: the neonatal Fc receptor comes of age. Nature Reviews Immunology 2007, 7 (9), 715–25. [DOI] [PubMed] [Google Scholar]
- 10.Garg A; Balthasar JP, Physiologically-based pharmacokinetic (PBPK) model to predict IgG tissue kinetics in wild-type and FcRn-knockout mice. Journal of Pharmacokinetics and Pharmacodynamics 2007, 34 (5), 687–709. [DOI] [PubMed] [Google Scholar]
- 11.Tabrizi M; Bornstein GG; Suria H, Biodistribution mechanisms of therapeutic monoclonal antibodies in health and disease. American Association of Pharmaceutical Scientists Journal 2010, 12 (1), 33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown EB; Boucher Y; Nasser S; Jain RK, Measurement of macromolecular diffusion coefficients in human tumors. Microvascular Research 2004, 67 (3), 231–6. [DOI] [PubMed] [Google Scholar]
- 13.Jain RK; Baxter LT, Mechanisms of heterogeneous distribution of monoclonal antibodies and other macromolecules in tumors: significance of elevated interstitial pressure. Cancer Research 1988, 48 (24 Pt 1), 7022–32. [PubMed] [Google Scholar]
- 14.Jain RK, Physiological barriers to delivery of monoclonal antibodies and other macromolecules in tumors. Cancer Research 1990, 50 (3 Suppl), 814s–819s. [PubMed] [Google Scholar]
- 15.Fujimori K; Covell DG; Fletcher JE; Weinstein JN, A modeling analysis of monoclonal antibody percolation through tumors: a binding-site barrier. Journal of Nuclear Medicine 1990, 31 (7), 1191–8. [PubMed] [Google Scholar]
- 16.Juweid M; Neumann R; Paik C; Perez-Bacete MJ; Sato J; van Osdol W; Weinstein JN, Micropharmacology of monoclonal antibodies in solid tumors: direct experimental evidence for a binding site barrier. Cancer Research 1992, 52 (19), 5144–53. [PubMed] [Google Scholar]
- 17.Zhang Y; Pastan I, High shed antigen levels within tumors: an additional barrier to immunoconjugate therapy. Clinical Cancer Research 2008, 14 (24), 7981–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rudnick SI; Adams GP, Affinity and avidity in antibody-based tumor targeting. Cancer Biotherapy and Radiopharmaceuticals 2009, 24 (2), 155–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rudnick SI; Lou J; Shaller CC; Tang Y; Klein-Szanto AJ; Weiner LM; Marks JD; Adams GP, Influence of affinity and antigen internalization on the uptake and penetration of Anti-HER2 antibodies in solid tumors. Cancer Research 2011, 71 (6), 2250–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glatt DM; Beckford Vera DR; Parrott MC; Luft JC; Benhabbour SR; Mumper RJ, The interplay of antigen affinity, internalization, and pharmacokinetics on CD44-positive tumor targeting of monoclonal antibodies. Molecular Pharmaceutics 2016, 13 (6), 1894–903. [DOI] [PubMed] [Google Scholar]
- 21.Glassman PM; Abuqayyas L; Balthasar JP, Assessments of antibody biodistribution. Journal of Clinical Pharmacology 2015, 55 Suppl 3, S29–38. [DOI] [PubMed] [Google Scholar]
- 22.Blumenthal RD; Fand I; Sharkey RM; Boerman OC; Kashi R; Goldenberg DM, The effect of antibody protein dose on the uniformity of tumor distribution of radioantibodies: an autoradiographic study. Cancer Immunology, Immunotherapy 1991, 33 (6), 351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wright A; Sato Y; Okada T; Chang K; Endo T; Morrison S, In vivo trafficking and catabolism of IgG1 antibodies with Fc associated carbohydrates of differing structure. Glycobiology 2000, 10 (12), 1347–55. [DOI] [PubMed] [Google Scholar]
- 24.Kraj M; Kruk B; Lech-Maranda E; Warzocha K; Prochorec-Sobieszek M, High incidence of intact or fragmented immunoglobulin in urine of patients with multiple myeloma. Leuk Lymphoma 2015, 56 (12), 3348–56. [DOI] [PubMed] [Google Scholar]
- 25.Covell DG; Barbet J; Holton OD; Black CD; Parker RJ; Weinstein JN, Pharmacokinetics of monoclonal immunoglobulin G1, F(ab’)2, and Fab’ in mice. Cancer Research 1986, 46 (8), 3969–78. [PubMed] [Google Scholar]
- 26.Cartron G; Dacheux L; Salles G; Solal-Celigny P; Bardos P; Colombat P; Watier H, Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood 2002, 99 (3), 754–8. [DOI] [PubMed] [Google Scholar]
- 27.Dornan D; Spleiss O; Yeh RF; Duchateau-Nguyen G; Dufour A; Zhi J; Robak T; Moiseev SI; Dmoszynska A; Solal-Celigny P; Warzocha K; Loscertales J; Catalano J; Afanasiev BV; Larratt L; Rossiev VA; Bence-Bruckler I; Geisler CH; Montillo M; Wenger MK; Weisser M, Effect of FCGR2A and FCGR3A variants on CLL outcome. Blood 2010, 116 (20), 4212–22. [DOI] [PubMed] [Google Scholar]
- 28.Abuqayyas L; Zhang X; Balthasar JP, Application of knockout mouse models to investigate the influence of FcgammaR on the pharmacokinetics and anti-platelet effects of MWReg30, a monoclonal anti-GPIIb antibody. International Journal of Pharmacology 2013, 444 (1–2), 185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abuqayyas L; Balthasar JP, Application of knockout mouse models to investigate the influence of FcgammaR on the tissue distribution and elimination of 8C2, a murine IgG1 monoclonal antibody. International Journal of Pharmacology 2012, 439 (1–2), 8–16. [DOI] [PubMed] [Google Scholar]
- 30.Jin F; Balthasar JP, Mechanisms of intravenous immunoglobulin action in immune thrombocytopenic purpura. Human Immunology 2005, 66 (4), 403–10. [DOI] [PubMed] [Google Scholar]
- 31.Vidarsson G; Dekkers G; Rispens T, IgG subclasses and allotypes: From structure to effector functions. Frontiers in Immunology 2014, 5, 520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Correia IR, Stability of IgG isotypes in serum. mAbs 2010, 2 (3), 221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoo EM; Wims LA; Chan LA; Morrison SL, Human IgG2 can form covalent dimers. The Journal of Immunology 2003, 170 (6), 3134–3138. [DOI] [PubMed] [Google Scholar]
- 34.Dillon TM; Ricci MS; Vezina C; Flynn GC; Liu YD; Rehder DS; Plant M; Henkle B; Li Y; Deechongkit S; Varnum B; Wypych J; Balland A; Bondarenko PV, Structural and functional characterization of disulfide isoforms of the human IgG2 subclass. The Journal of biological chemistry 2008, 283 (23), 16206–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Neut Kolfschoten M; Schuurman J; Losen M; Bleeker WK; Martínez-Martínez P; Vermeulen E; den Bleker TH; Wiegman L; Vink T; Aarden LA; De Baets MH; van de Winkel JGJ; Aalberse RC; Parren PWHI, Anti-Inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science 2007, 317 (5844), 1554–1557. [DOI] [PubMed] [Google Scholar]
- 36.Zheng K; Teng F; Li X-M, Immunoglobulin G4-related kidney disease: Pathogenesis, diagnosis, and treatment. Chronic Diseases and Translational Medicine 2017, 3 (3), 138–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schuurman J; Perdok GJ; Gorter AD; Aalberse RC, The inter-heavy chain disulfide bonds of IgG4 are in equilibrium with intra-chain disulfide bonds. Molecular immunology 2001, 38 (1), 1–8. [DOI] [PubMed] [Google Scholar]
- 38.Robbie GJ; Criste R; Dall’acqua WF; Jensen K; Patel NK; Losonsky GA; Griffin MP, A novel investigational Fc-modified humanized monoclonal antibody, motavizumab-YTE, has an extended half-life in healthy adults. Antimicrobial Agents and Chemotherapy 2013, 57 (12), 6147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olafsen T, Fc engineering: serum half-life modulation through FcRn binding. Methods in Molecular Biology 2012, 907, 537–56. [DOI] [PubMed] [Google Scholar]
- 40.Gao X; Ji JA; Veeravalli K; Wang YJ; Zhang T; McGreevy W; Zheng K; Kelley RF; Laird MW; Liu J; Cromwell M, Effect of individual Fc methionine oxidation on FcRn binding: Met252 oxidation impairs FcRn binding more profoundly than Met428 oxidation. Journal of Pharmaceutical Sciences 2015, 104 (2), 368–77. [DOI] [PubMed] [Google Scholar]
- 41.Wang W; Lu P; Fang Y; Hamuro L; Pittman T; Carr B; Hochman J; Prueksaritanont T, Monoclonal antibodies with identical Fc sequences can bind to FcRn differentially with pharmacokinetic consequences. Drug Metabolism and Disposition 2011, 39 (9), 1469–77. [DOI] [PubMed] [Google Scholar]
- 42.Schoch A; Kettenberger H; Mundigl O; Winter G; Engert J; Heinrich J; Emrich T, Charge-mediated influence of the antibody variable domain on FcRn-dependent pharmacokinetics. Proceedings of the National Academy of Sciences of the United States of America 2015, 112 (19), 5997–6002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ju MS; Jung ST, Aglycosylated full-length IgG antibodies: steps toward next-generation immunotherapeutics. Current Opinions in Biotechnology 2014, 30, 128–39. [DOI] [PubMed] [Google Scholar]
- 44.Boswell CA; Tesar DB; Mukhyala K; Theil FP; Fielder PJ; Khawli LA, Effects of charge on antibody tissue distribution and pharmacokinetics. BIoconjugate Chemistry 2010, 21 (12), 2153–63. [DOI] [PubMed] [Google Scholar]
- 45.Li B; Tesar D; Boswell CA; Cahaya HS; Wong A; Zhang J; Meng YG; Eigenbrot C; Pantua H; Diao J; Kapadia SB; Deng R; Kelley RF, Framework selection can influence pharmacokinetics of a humanized therapeutic antibody through differences in molecule charge. MAbs 2014, 6 (5), 1255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khawli LA; Goswami S; Hutchinson R; Kwong ZW; Yang J; Wang X; Yao Z; Sreedhara A; Cano T; Tesar D; Nijem I; Allison DE; Wong PY; Kao YH; Quan C; Joshi A; Harris RJ; Motchnik P, Charge variants in IgG1: Isolation, characterization, in vitro binding properties and pharmacokinetics in rats. MAbs 2010, 2 (6), 613–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Igawa T; Tsunoda H; Tachibana T; Maeda A; Mimoto F; Moriyama C; Nanami M; Sekimori Y; Nabuchi Y; Aso Y; Hattori K, Reduced elimination of IgG antibodies by engineering the variable region. Protein Engineering and Design Science 2010, 23 (5), 385–92. [DOI] [PubMed] [Google Scholar]
- 48.Adumeau P; Sharma SK; Brent C; Zeglis BM, Site-specifically labeled immunoconjugates for molecular imaging—Part 1: Cysteine residues and glycans. Molecular Imaging and Biology 2016, 18 (1), 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adumeau P; Sharma SK; Brent C; Zeglis BM, Site-specifically labeled immunoconjugates for molecular imaging—Part 2: Peptide tags and unnatural amino acids. Molecular Imaging and Biology 2016, 18 (1), 153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boylan NJ; Zhou W; Proos RJ; Tolbert TJ; Wolfe JL; Laurence JS, Conjugation site heterogeneity causes variable electrostatic properties in Fc conjugates. Bioconjugate Chemistry 2013, 24 (6), 1008–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCombs JR; Owen SC, Antibody drug conjugates: design and selection of linker, payload and conjugation chemistry. AAPS J 2015, 17 (2), 339–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li W; Prabakaran P; Chen W; Zhu Z; Feng Y; Dimitrov DS, Antibody aggregation: Insights from sequence and structure. Antibodies 2016, 5 (19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wakankar AA; Feeney MB; Rivera J; Chen Y; Kim M; Sharma VK; Wang YJ, Physicochemical stability of the antibody-drug conjugate trastuzumab-DM1: changes due to modification and conjugation processes. Bioconjugate Chemistry 2010, 21 (9), 1588–95. [DOI] [PubMed] [Google Scholar]
- 54.Acchione M; Kwon H; Jochheim CM; Atkins WM, Impact of linker and conjugation chemistry on antigen binding, Fc receptor binding and thermal stability of model antibody-drug conjugates. MAbs 2012, 4 (3), 362–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lyon RP; Bovee TD; Doronina SO; Burke PJ; Hunter JH; Neff-LaFord HD; Jonas M; Anderson ME; Setter JR; Senter PD, Reducing hydrophobicity of homogeneous antibody-drug conjugates improves pharmacokinetics and therapeutic index. Nature Biotechnology 2015, 33 (7), 733–5. [DOI] [PubMed] [Google Scholar]
- 56.Sun X; Ponte JF; Yoder NC; Laleau R; Coccia J; Lanieri L; Qiu Q; Wu R; Hong E; Bogalhas M; Wang L; Dong L; Setiady Y; Maloney EK; Ab O; Zhang X; Pinkas J; Keating TA; Chari R; Erickson HK; Lambert JM, Effects of drug-antibody ratio on pharmacokinetics, biodistribution, efficacy, and tolerability of antibody-maytansinoid conjugates. Bioconjugate Chemistry 2017, 28 (5), 1371–1381. [DOI] [PubMed] [Google Scholar]
- 57.Lub-de Hooge MN; Kosterink JG; Perik PJ; Nijnuis H; Tran L; Bart J; Suurmeijer AJ; de Jong S; Jager PL; de Vries EG, Preclinical characterisation of 111In-DTPA-trastuzumab. British Journal of Pharmacology 2004, 143 (1), 99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Deri MA; Zeglis BM; Francesconi LC; Lewis JS, PET imaging with 89Zr: From radiochemistry to the clinic. Nuclear medicine and biology 2013, 40 (1), 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rudd SE; Roselt P; Cullinane C; Hicks RJ; Donnelly PS, A desferrioxamine B squaramide ester for the incorporation of zirconium-89 into antibodies. Chem Commun (Camb) 2016, 52 (80), 11889–11892. [DOI] [PubMed] [Google Scholar]
- 60.Price EW; Glaser JM; Edwards KJ; Lewis JS, Optimized antibody modification and radiolabeling conditions for zirconium-89 immuno-PET. Cancer Research 2016, 76. [Google Scholar]
- 61.Sancho J; Gonzalez E; Escanero JF; Egido J, Binding kinetics of monomeric and aggregated IgG to Kupffer cells and hepatocytes of mice. Immunology 1984, 53 (2), 283–9. [PMC free article] [PubMed] [Google Scholar]
- 62.Sands H; Jones PL, Methods for the study of the metabolism of radiolabeled monoclonal antibodies by liver and tumor. Journal of Nuclear Medicine 1987, 28 (3), 390–8. [PubMed] [Google Scholar]
- 63.Beatty JD; Beatty BG; O’Conner-Tressel M; Do T; Paxton RJ, Mechanisms of tissue uptake and metabolism of radiolabeled antibody--role of antigen: antibody complex formation. Cancer Research 1990, 50 (3 Suppl), 840s–845s. [PubMed] [Google Scholar]
- 64.Bogers WM; Stad RK; Janssen DJ; van Rooijen N; van Es LA; Daha MR, Kupffer cell depletion in vivo results in preferential elimination of IgG aggregates and immune complexes via specific Fc receptors on rat liver endothelial cells. Clinical and Experimental Immunology 1991, 86 (2), 328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Johansson AG; Lovdal T; Magnusson KE; Berg T; Skogh T, Liver cell uptake and degradation of soluble immunoglobulin G immune complexes in vivo and in vitro in rats. Hepatology 1996, 24 (1), 169–75. [DOI] [PubMed] [Google Scholar]
- 66.McQuarrie SA; Baum RP; Niesen A; Madiyalakan R; Korz W; Sykes TR; Sykes CJ; Hor G; McEwan AJ; Noujaim AA, Pharmacokinetics and radiation dosimetry of 99mTc-labelled monoclonal antibody B43.13 in ovarian cancer patients. Nuclear Medicine Communications 1997, 18 (9), 878–86. [DOI] [PubMed] [Google Scholar]
- 67.McQuarrie SA; Riauka T; Baum RP; Sykes TR; Noujaim AA; Boniface G; MacLean GD; McEwan AJ, The effects of circulating antigen on the pharmacokinetics and radioimmunoscintigraphic properties of 99m Tc labelled monoclonal antibodies in cancer patients. Journal of Pharmacy and Pharmaceutical Sciences 1998, 1 (3), 115–25. [PubMed] [Google Scholar]
- 68.Beatty BG; Beatty JD; Williams LE; Paxton RJ; Shively JE; O’Connor-Tressel M, Effect of specific antibody pretreatment on liver uptake of 111In-labeled anticarcinoembryonic antigen monoclonal antibody in nude mice bearing human colon cancer xenografts. Cancer Research 1989, 49 (6), 1587–1594. [PubMed] [Google Scholar]
- 69.Houghton JL; Abdel-Atti D; Scholz WW; Lewis JS, Preloading with unlabeled CA19.9 targeted human monoclonal antibody leads to improved PET imaging with (89)Zr-5B1. Molecular Pharmaceutics 2017, 14 (3), 908–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hwang WY; Foote J, Immunogenicity of engineered antibodies. Methods 2005, 36 (1), 3–10. [DOI] [PubMed] [Google Scholar]
- 71.Bartelds GM; Krieckaert CL; Nurmohamed MT; van Schouwenburg PA; Lems WF; Twisk JW; Dijkmans BA; Aarden L; Wolbink GJ, Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. Journal of the American Medical Association 2011, 305 (14), 1460–8. [DOI] [PubMed] [Google Scholar]
- 72.Seitz K; Zhou H, Pharmacokinetic drug-drug interaction potentials for therapeutic monoclonal antibodies: reality check. Journal of Clinical Pharmacology 2007, 47 (9), 1104–18. [DOI] [PubMed] [Google Scholar]
- 73.Geissler F; Anderson SK; Venkatesan P; Press O, Intracellular catabolism of radiolabeled anti-μ antibodies by malignant B-cells. Cancer Research 1992, 52 (10), 2907–2915. [PubMed] [Google Scholar]
- 74.Jules Mattes M; Griffiths GL; Diril H; Goldenberg DM; Ong GL; Shih LB, Processing of antibody-radioisotope conjugates after binding to the surface of tumor cells. Cancer 1994, 73 (S3), 787–793. [DOI] [PubMed] [Google Scholar]
- 75.Press OW; Shan D; Howell-Clark J; Eary J; Appelbaum FR; Matthews D; King DJ; Haines AMR; Hamann P; Hinman L; Shochat D; Bernstein ID, Comparative metabolism and retention of Iidine-125, yttrium-90, and indium-111 radioimmunoconjugates by cancer cells. Cancer Research 1996, 56 (9), 2123–2129. [PubMed] [Google Scholar]
- 76.Nestor M; Persson M; Cheng J; Tolmachev V; van Dongen G; Anniko M; Kairemo K, Biodistribution of the Chimeric Monoclonal Antibody U36 Radioiodinated with a closo-Dodecaborate-Containing Linker. Comparison with Other Radioiodination Methods. Bioconjugate Chemistry 2003, 14 (4), 805–810. [DOI] [PubMed] [Google Scholar]
- 77.Zalutsky MR; Colcher D; Kaplan WD; Kufe DW, Radioiodinated B6.2 monoclonal antibody: Further characterization of a potential radiopharmaceutical for the identification of breast tumors. International Journal of Nuclear Medicine and Biology 1985, 12 (3), 227–233. [DOI] [PubMed] [Google Scholar]
- 78.Zalutsky MR; Xu FJ; Yu Y; Foulon CF; Zhao XG; Slade SK; Affleck DJ; Bast RC, Radioiodinated antibody targeting of the HER-2/neu oncoprotein: Effects of labeling method on cellular processing and tissue distribution. Nuclear medicine and biology 1999, 26 (7), 781–790. [DOI] [PubMed] [Google Scholar]
- 79.DeNardo SJ; DeNardo GL; Kukis DL; Shen S; Kroger LA; DeNardo DA; Goldstein DS; Mirick GR; Salako Q; Mausner LF; Srivastava SC; Meares CF, 67Cu-2IT-BAT-Lym-1 pharmacokinetics, radiation dosimetry, toxicity and tumor regression in patients with lymphoma. Journal of Nuclear Medicine 1999, 40 (2), 302–10. [PubMed] [Google Scholar]
- 80.DeNardo GL; Kukis DL; Shen S; DeNardo DA; Meares CF; DeNardo SJ, 67Cu-versus 131I-labeled Lym-1 antibody: Comparative pharmacokinetics and dosimetry in patients with non-Hodgkin’s lymphoma. Clinical Cancer Research 1999, 5 (3), 533–541. [PubMed] [Google Scholar]
- 81.Stein R; Goldenberg DM; Thorpe SR; Basu A; Mattes MJ, Effects of radiolabeling monoclonal antibodies with a residualizing iodine radiolabel on the accretion of radioisotope in tumors. Cancer Research 1995, 55 (14), 3132–3139. [PubMed] [Google Scholar]
- 82.Thorpe SR; Baynes JW; Chroneos ZC, The design and application of residualizing labels for studies of protein catabolism. FASEB Journal 1993, 7 (5), 399–405. [DOI] [PubMed] [Google Scholar]
- 83.Ali SA; Warren SD; Richter KY; Badger CC; Eary JF; Press OW; Krohn KA; Bernstein ID; Nelp WB, Improving the tumor retention of radioiodinated antibody: Aryl carbohydrate adducts. Cancer Research 1990, 50 (3 Supplement), 783s–788s. [PubMed] [Google Scholar]
- 84.Maxwell JL; Baynes JW; Thorpe SR, Inulin-125I-tyramine, an improved residualizing label for studies on sites of catabolism of circulating proteins. The Journal of biological chemistry 1988, 263 (28), 14122–14127. [PubMed] [Google Scholar]
- 85.Pittman RC; Carew TE; Glass CK; Green SR; Taylor CA Jr.; Attie AD, A radioiodinated, intracellularly trapped ligand for determining the sites of plasma protein degradation in vivo. The Biochemical Journal 1983, 212 (3), 791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reist CJ; Garg PK; Alston KL; Bigner DD; Zalutsky MR, Radioiodination of internalizing monoclonal antibodies using N-succinimidyl 5-iodo-3-pyridinecarboxylate. Cancer Research 1996, 56 (21), 4970–4977. [PubMed] [Google Scholar]
- 87.Vaidyanathan G; Affleck DJ; Bigner DD; Zalutsky MR, Improved xenograft targeting of tumor-specific anti-epidermal growth factor receptor variant III antibody labeled using N-succinimidyl 4-guanidinomethyl-3-iodobenzoate. Nuclear medicine and biology 2002, 29 (1), 1–11. [DOI] [PubMed] [Google Scholar]
- 88.Shankar S; Vaidyanathan G; Affleck DJ; Peixoto K; Bigner DD; Zalutsky MR, Evaluation of an internalizing monoclonal antibody labeled using N-succinimidyl 3-[131I]iodo-4-phosphonomethylbenzoate ([131I]SIPMB), a negatively charged substituent bearing acylation agent. Nuclear medicine and biology 2004, 31 (7), 909–19. [DOI] [PubMed] [Google Scholar]
- 89.Choi J; Vaidyanathan G; Koumarianou E; McDougald D; Pruszynski M; Osada T; Lahoutte T; Lyerly HK; Zalutsky MR, N-Succinimidyl guanidinomethyl iodobenzoate protein radiohalogenation agents: Influence of isomeric substitution on radiolabeling and target cell residualization. Nuclear medicine and biology 2014, 41 (10), 802–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Foulon CF; Reist CJ; Bigner DD; Zalutsky MR, Radioiodination via D-amino acid peptide enhances cellular retention and tumor xenograft targeting of an internalizing enti-epidermal growth factor receptor variant III monoclonal antibody. Cancer Research 2000, 60 (16), 4453–4460. [PubMed] [Google Scholar]
- 91.Vaidyanathan G; Alston KL; Bigner DD; Zalutsky MR, Nε-(3-[*I]Iodobenzoyl)-Lys5-Nα-maleimido-Gly1-GEEEK ([*I]IB-Mal-d-GEEEK): A radioiodinated prosthetic group containing negatively charged d-glutamates for labeling internalizing monoclonal antibodies. Bioconjugate Chemistry 2006, 17 (4), 1085–1092. [DOI] [PubMed] [Google Scholar]
- 92.Pruszynski M; Koumarianou E; Vaidyanathan G; Chitneni S; Zalutsky MR, D-Amino acid peptide residualizing agents bearing N-hydroxysuccinimido- and maleimido-functional groups and their application for trastuzumab radioiodination. Nuclear medicine and biology 2015, 42 (1), 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee FT; Burvenich IJG; Guo N; Kocovski P; Tochon-Danguy H; Ackermann U; O’Keefe GJ; Gong S; Rigopoulos A; Liu ZQ; Gan HK; Scott AM, L-Tyrosine confers residualizing properties to a D-amino acid-rich residualizing peptide for radioiodination of internalizing antibodies. Mol. Imaging 2016, 15, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Govindan SV; Mattes MJ; Stein R; McBride BJ; Karacay H; Goldenberg DM; Hansen HJ; Griffiths GL, Labeling of monoclonal antibodies with diethylenetriaminepentaacetic acid-appended radioiodinated peptides containing D-amino acids. Bioconjugate Chemistry 1999, 10 (2), 231–240. [DOI] [PubMed] [Google Scholar]
- 95.Stein R; Govindan SV; Mattes MJ; Chen S; Reed L; Newsome G; McBride BJ; Griffiths GL; Hansen HJ; Goldenberg DM, Improved iodine radiolabels for monoclonal antibody therapy. Cancer Research 2003, 63 (1), 111–118. [PubMed] [Google Scholar]
- 96.Pandit-Taskar N; O’Donoghue JA; Beylergil V; Lyashchenko S; Ruan S; Solomon SB; Durack JC; Carrasquillo JA; Lefkowitz RA; Gonen M; Lewis JS; Holland JP; Cheal SM; Reuter VE; Osborne JR; Loda MF; Smith-Jones PM; Weber WA; Bander NH; Scher HI; Morris MJ; Larson SM, (89)Zr-huJ591 immuno-PET imaging in patients with advanced metastatic prostate cancer. European journal of nuclear medicine and molecular imaging 2014, 41 (11), 2093–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Price EW; Orvig C, Matching chelators to radiometals for radiopharmaceuticals. Chemical Society Reviews 2014, 43 (1), 260–290. [DOI] [PubMed] [Google Scholar]
- 98.Lockhart AC; Liu Y; Dehdashti F; Laforest R; Picus J; Frye J; Trull L; Belanger S; Desai M; Mahmood S; Mendell J; Welch MJ; Siegel BA, Phase 1 evaluation of [64Cu]DOTA-patritumab to assess dosimetry, apparent receptor occupancy, and safety in subjects with advanced solid tumors. Molecular Imaging and Biology 2016, 18 (3), 446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sasada S; Kurihara H; Kinoshita T; Yoshida M; Honda N; Shimoi T; Shimomura A; Yunokawa M; Yonemori K; Shimizu C; Hamada A; Kanayama Y; Watanabe Y; Fujiwara Y; Tamura K, 64Cu-DOTA-trastuzumab PET imaging for HER2-specific primary lesions of breast cancer. Annals of Oncology 2017, 28 (8), 2028–2029. [DOI] [PubMed] [Google Scholar]
- 100.Mortimer JE; Bading JR; Park JM; Frankel PH; Carroll MI; Tran TT; Poku EK; Rockne RC; Raubitschek AA; Shively JE; Colcher DM, Tumor Uptake of 64Cu-DOTA-Trastuzumab in Patients with Metastatic Breast Cancer. Journal of Nuclear Medicine 2018, 59 (1), 38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Novak-Hofer I; Zimmermann K; Maecke HR; Amstutz HP; Carrel F; Schubiger PA, Tumor uptake and metabolism of copper-67-labeled monoclonal antibody chCE7 in nude mice bearing neuroblastoma xenografts. Journal of Nuclear Medicine 1997, 38 (4), 536–544. [PubMed] [Google Scholar]
- 102.Novak-Hofer I; Schubiger PA, Copper-67 as a therapeutic nuclide for radioimmunotherapy. European journal of nuclear medicine and molecular imaging 2002, 29 (6), 821–30. [DOI] [PubMed] [Google Scholar]
- 103.Ping Li W; Meyer LA; Capretto DA; Sherman CD; Anderson CJ, Receptor-binding, biodistribution, and metabolism studies of 64Cu-DOTA-cetuximab, a PET-imaging agent for epidermal growth-factor receptor-positive tumors. Cancer Biotherapy and Radiopharmaceuticals 2008, 23 (2), 158–71. [DOI] [PubMed] [Google Scholar]
- 104.Novak-Hofer I; Amstutz HP; Mäcke HR; Schwarzbach R; Zimmermann K; Morgenthaler J-J; Schubiger PA, Cellular processing of copper-67-labeled monoclonal antibody chCE7 by human neuroblastoma cells. Cancer Research 1995, 55 (1), 46–50. [PubMed] [Google Scholar]
- 105.Rogers BE; Anderson CJ; Connett JM; Guo LW; Edwards WB; Sherman ELC; Zinn KR; Welch MJ, Comparison of four bifunctional chelates for radiolabeling monoclonal antibodies with copper radioisotopes: Biodistribution and metabolism. Bioconjugate Chemistry 1996, 7 (4), 511–522. [DOI] [PubMed] [Google Scholar]
- 106.Mirick GR; O’Donnell RT; DeNardo SJ; Shen S; Meares CF; DeNardo GL, Transfer of copper from a chelated 67Cu-antibody conjugate to ceruloplasmin in lymphoma patients 1. Nuclear medicine and biology 1999, 26 (7), 841–845. [DOI] [PubMed] [Google Scholar]
- 107.Boswell CA; Sun X; Niu W; Weisman GR; Wong EH; Rheingold AL; Anderson CJ, Comparative in vivo stability of copper-64-labeled cross-bridged and conventional tetraazamacrocyclic complexes. Journal of Medicinal Chemistry 2004, 47 (6), 1465–1474. [DOI] [PubMed] [Google Scholar]
- 108.Di Bartolo N; Sargeson AM; Smith SV, New 64Cu PET imaging agents for personalised medicine and drug development using the hexa-aza cage, SarAr. Organic & Biomolecular Chemistry 2006, 4 (17), 3350–3357. [DOI] [PubMed] [Google Scholar]
- 109.Voss SD; Smith SV; DiBartolo N; McIntosh LJ; Cyr EM; Bonab AA; Dearling JLJ; Carter EA; Fischman AJ; Treves ST; Gillies SD; Sargeson AM; Huston JS; Packard AB, Positron emission tomography (PET) imaging of neuroblastoma and melanoma with (64)Cu-SarAr immunoconjugates. Proceedings of the National Academy of Sciences of the United States of America 2007, 104 (44), 17489–17493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zimmermann K; Gianollini S; Schubiger PA; Novak-Hofer I, A triglycine linker improves tumor uptake and biodistributions of 67-Cu-Labeled anti-neuroblastoma MAb chCE7 F(ab′)2 fragments. Nuclear medicine and biology 1999, 26 (8), 943–950. [DOI] [PubMed] [Google Scholar]
- 111.Harris WR, Binding and transport of nonferrous metals by serum transferrin In Less Common Metals in Proteins and Nucleic Acid Probes, Springer Berlin Heidelberg: Berlin, Heidelberg, 1998; pp 121–162. [Google Scholar]
- 112.Himmelsbach M; Wahl RL, Studies on the metabolic fate of 111In-labeled antibodies. Int J Rad Appl Instrum B 1989, 16 (8), 839–45. [DOI] [PubMed] [Google Scholar]
- 113.Jones PL; Brown BA; Sands H, Uptake and metabolism of 111In-labeled monoclonal antibody B6.2 by the rat liver. Cancer Research 1990, 50 (3 Supplement), 852s–856s. [PubMed] [Google Scholar]
- 114.Rogers BE; Franano FN; Duncan JR; Edwards WB; Anderson CJ; Connett JM; Welch MJ, Identification of metabolites of 111In-diethylenetriaminepentaacetic acid-monoclonal antibodies and antibody fragments in vivo. Cancer Research 1995, 55 (23 Supplement), 5714s–5720s. [PubMed] [Google Scholar]
- 115.Motta-Hennessy C; Sharkey RM; Goldenberg DM, Metabolism of indium-111-labeled murine monoclonal antibody in tumor and normal tissue of the athymic mouse. Journal of Nuclear Medicine 1990, 31 (9), 1510–9. [PubMed] [Google Scholar]
- 116.DeNardo GL; DeNardo SJ; Kukis DL; O’Donnell RT; Shen S; Mirick GR; Meares CF, Metabolite production in patients with lymphoma after radiometal-labeled antibody administration. Journal of Nuclear Medicine 2001, 42 (9), 1324–33. [PubMed] [Google Scholar]
- 117.Hnatowich DJ; Rusckowski M; Brill AB; Siebecker DA; Misra H; Mardirossian G; Bushe H; Rescigno A; Stevens S; Johnson DK; et al. , Pharmacokinetics in patients of an anti-carcinoembryonic antigen antibody radiolabeled with indium-111 using a novel diethylenetriamine pentaacetic acid chelator. Cancer Research 1990, 50 (22), 7272–8. [PubMed] [Google Scholar]
- 118.Beatty BG; O’Conner-Tressel M; Do T; Paxton RJ; Beatty JD, Mechanism of decreasing liver uptake of 111In-labeled anti-carcinoembryonic antigen monoclonal antibody by specific antibody pretreatment in tumor bearing mice. Cancer Research 1990, 50 (3 Suppl), 846s–851s. [PubMed] [Google Scholar]
- 119.Davidson BR; Babich J; Young H; Waddington W; Clarke G; Short M; Boulos P; Styles J; Dean C, The effect of circulating antigen and radiolabel stability on the biodistribution of an indium labelled antibody. Br J Cancer 1991, 64 (5), 850–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Murray JL; Lamki LM; Shanken LJ; Blake ME; Plager CE; Benjamin RS; Schweighardt S; Unger MW; Rosenblum MG, Immunospecific saturable clearance mechanisms for indium-111-labeled anti-melanoma monoclonal antibody 96.5 in humans. Cancer Res 1988, 48 (15), 4417–22. [PubMed] [Google Scholar]
- 121.Patt YZ; Lamki LM; Haynie TP; Unger MW; Rosenblum MG; Shirkhoda A; Murray JL, Improved tumor localization with increasing dose of indium-111-labeled anti-carcinoembryonic antigen monoclonal antibody ZCE-025 in metastatic colorectal cancer. J Clin Oncol 1988, 6 (8), 1220–30. [DOI] [PubMed] [Google Scholar]
- 122.Taylor A Jr.; Milton W; Eyre HP; Christian P; Wu F; Hagan P; Alazraki N; Datz FL; Unger M, Radioimmunodetection of human melanoma with indium-111-labeled monoclonal antibody. J Nucl Med 1988, 29 (3), 329–37. [PubMed] [Google Scholar]
- 123.Patt YZ; Lamki LM; Shanken J; Jessup JM; Charnsangavej C; Ajani JA; Levin B; Merchant B; Halverson C; Murray JL, Imaging with indium111-labeled anticarcinoembryonic antigen monoclonal antibody ZCE-025 of recurrent colorectal or carcinoembryonic antigen-producing cancer in patients with rising serum carcinoembryonic antigen levels and occult metastases. J Clin Oncol 1990, 8 (7), 1246–54. [DOI] [PubMed] [Google Scholar]
- 124.Studer M; Kroger LA; DeNardo SJ; Kukis DL; Meares CF, Influence of a peptide linker on biodistribution and metabolism of antibody-conjugated benzyl-EDTA. Comparison of enzymatic digestion in vitro and in vivo. Bioconjug Chemistry 1992, 3 (5), 424–9. [DOI] [PubMed] [Google Scholar]
- 125.van de Watering FCJ; Rijpkema M; Perk L; Brinkmann U; Oyen WJG; Boerman OC, Zirconium-89 labeled antibodies: A new tool for molecular imaging in cancer patients. BioMed Research International 2014, 2014, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Meijs WE; Haisma HJ; Klok RP; van Gog FB; Kievit E; Pinedo HM; Herscheid JD, Zirconium-labeled monoclonal antibodies and their distribution in tumor-bearing nude mice. Journal of Nuclear Medicine 1997, 38 (1), 112–8. [PubMed] [Google Scholar]
- 127.Rama Sastry BV; Owens LK; Ball COT, Differences in the distribution of zirconium-95 and niobium-95 in the rat. Nature 1964, 201 (4917), 410–411. [DOI] [PubMed] [Google Scholar]
- 128.Bäckström J; Hammarström L; Nelson A, Distribution of zirconium and niobium in mice: Autoradiographic study. Acta Radiologica: Diagnosis 1967, 6 (2), 122–128. [DOI] [PubMed] [Google Scholar]
- 129.Abou DS; Ku T; Smith-Jones PM, In vivo biodistribution and accumulation of 89Zr in mice. Nuclear medicine and biology 2011, 38 (5), 675–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Heskamp S; Raavé R; Boerman OC; Rijpkema M; Goncalves V; Denat F, 89Zr-immunoPET in oncology: state of the art 89Zr-radiochemistry. Bioconjugate Chemistry 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Börjesson PKE; Jauw YWS; de Bree R; Roos JC; Castelijns JA; Leemans CR; van Dongen GAMS; Boellaard R, Radiation dosimetry of 89Zr-labeled chimeric monoclonal antibody U36 as used for immuno-PET in head and neck cancer patients. Journal of Nuclear Medicine 2009, 50 (11), 1828–1836. [DOI] [PubMed] [Google Scholar]
- 132.Makris NE; Boellaard R; van Lingen A; Lammertsma AA; van Dongen GA; Verheul HM; Menke CW; Huisman MC, PET/CT-derived whole-body and bone marrow dosimetry of 89Zr-cetuximab. Journal of Nuclear Medicine 2015, 56 (2), 249–54. [DOI] [PubMed] [Google Scholar]
- 133.Muylle K; Flamen P; Vugts DJ; Guiot T; Ghanem G; Meuleman N; Bourgeois P; Vanderlinden B; van Dongen GA; Everaert H; Vaes M; Bron D, Tumour targeting and radiation dose of radioimmunotherapy with (90)Y-rituximab in CD20+ B-cell lymphoma as predicted by (89)Zr-rituximab immuno-PET: impact of preloading with unlabelled rituximab. European journal of nuclear medicine and molecular imaging 2015, 42 (8), 1304–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Laforest R; Lapi SE; Oyama R; Bose R; Tabchy A; Marquez-Nostra BV; Burkemper J; Wright BD; Frye J; Frye S; Siegel BA; Dehdashti F, [89Zr]Trastuzumab: Evaluation of Radiation Dosimetry, Safety, and Optimal Imaging Parameters in Women with HER2-Positive Breast Cancer. Molecular Imaging and Biology 2016, 18 (6), 952–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.O’Donoghue JA; Lewis JS; Pandit-Taskar N; Fleming SE; Schoder H; Larson SM; Beylergil V; Ruan S; Lyashchenko S; Zanzonico PB; Weber WA; Carrasquillo JA; Janjigian YY, Pharmacokinetics, biodistribution, and radiation dosimetry for 89Zr-trastuzumab in patients with esophagogastric cancer. Journal of Nuclear Medicine 2017, Online Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dijkers EC; Oude Munnink TH; Kosterink JG; Brouwers AH; Jager PL; de Jong JR; van Dongen GA; Schroder CP; Lub-de Hooge MN; de Vries EG, Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clinical Pharmacology and Therapeutics 2010, 87 (5), 586–92. [DOI] [PubMed] [Google Scholar]
- 137.Bern M; Sand KM; Nilsen J; Sandlie I; Andersen JT, The role of albumin receptors in regulation of albumin homeostasis: Implications for drug delivery. Journal of Controlled Release 2015, 211, 144–62. [DOI] [PubMed] [Google Scholar]