Abstract
To maximize the physiological relevance of in vivo brain tumor mouse models designed to study the downstream effects of oncogenic mutations, it is important to express the mutated genes at appropriate levels, in relevant cell types, and in the proper developmental context. For recurrent mutations found in the heterozygous state in tumors, expression of the mutation from the endogenous locus is a more physiologically relevant recapitulation of the brain tumor genome. Here, we describe an approach to generate knock-in mice with an inducible mutation recombined into the endogenous locus. In these engineered mice, the mutated allele is designed for expression controlled by the endogenous promoter and regulatory elements after Cre recombinase-mediated deletion of a loxP-STOP-loxP cassette inserted upstream of the translational start site. To preserve the structure of the endogenous locus, mutations or additional elements may need to be inserted at a considerable distance from the loxP-STOP-loxP cassette. We used recombineering to build a construct with two selectable markers and multiple genetic alterations that can be introduced into the endogenous allele in cis with a single ES cell targeting.
Keywords: recombineering, knock-in, transgenic, mutation, oncogene, mouse, Cre recombinase, loxP, brain tumor, neuro-oncology
1. Introduction
Genetically engineered mouse models are extremely useful tools for the study of normal brain development and neuropathologies including brain tumors. Diverse approaches are available to generate transgenic mice expressing mutated oncogenes in brain, with varying levels of regulation. Mutated genes may be introduced into a small number of somatic cells through injection of virus, or in vivo delivery of DNA constructs [1, 2]. While expeditious, the oncogenes are not expressed under the physiological regulation conferred by the endogenous promoter and may not reflect a heterozygous state if desired. The small number of cells targeted with such approaches may limit brain tumor development, showing greatest success with very strong oncogenic drivers that may not be most representative of mutations in human tumors. Furthermore, injection-based delivery poses varying technical challenges in different anatomic locations and developmental stages. Germline mutations may highlight connections between normal development and functions in tumorigenic transformation. Many cancer-associated mutations cause embryonic lethality, precluding the use of germline mutations to study brain tumor development. Multiple approaches are used to successfully introduce mutations into the mouse germline such as random integration of transgenic constructs by pronuclear injection (i.e., transgenes) and genome editing using tools such as CRISPR/Cas9-mediated genome editing in zygotes or embryonic stem (ES) cells [3–6]. Genome editing is currently most successful for simple gene knockouts or constitutive mutations, but remains more challenging for complex designs in which multiple elements must be reliably introduced in cis within a single allele, particularly through the introduction of large segments of DNA [7, 8]. Currently, more precise control over induction of a particular mutation in specific cell types and at diverse developmental stages can be achieved by Cre recombinase-inducible germline alleles.
Recombineering uses homologous recombination through gap repair in bacteria to genetically introduce alterations in plasmids, and is a powerful method to construct complex vectors to create knock-in alleles with multiple genetic features [9]. As an example, we discuss the design to generate a Cre recombinase-inducible K27M mutation knocked-in to the endogenous H3f3a gene to model mutations found in human diffuse intrinsic pontine glioma. We detail the protocol to recover the genomic region of interest from a bacterial artificial chromosome (BAC), introduce a loxP-STOP-loxP cassette to render the gene Cre recombinase-inducible, and introduce additional mutations or genomic elements and selectable markers. The final knock-in targeting construct is electroporated into mouse ES cells. Designing the knock-in construct with two selectable markers allows simultaneous engineering of multiple alterations in a single ES targeting experiment. The targeted ES cells described in this protocol would be used to generate mice [10] which can be bred with mice expressing Cre recombinase in a tissue-specific and/or inducible manner. The resulting models provide spatial and temporal regulation of the engineered knock-in oncogenic mutation under control of the endogenous promoter and regulatory elements.
2. Materials
2.1. Recombineering
BAC containing the genomic locus of the gene of interest, ideally from the same mouse strain as the embryonic stem cells (ES cells) that you will use (see Note 1). For this example, mouse H3f3a BAC in chloramphenicol-resistant plasmid pBACe3.6 (Source BioScience, #bMQ164i21).
Recombineering bacterial strain SW102 (NCI, https://ncifrederick.cancer.gov/research/brb/reagents/recombineeringReagent.aspx). A modified DH10B strain derived from DY380 [9] with the galactokinase (galK) gene deleted from the galactose operon for galK selection. These cells express bacteriophage λ Red genes exo, bet and gam from a temperature-responsive promoter that facilitate gap repair homologous recombination. The gap repair genes are repressed at 30 °C and expressed at 42 °C. These cells are tetracycline resistant.
Recombineering bacterial strain SW105 (NCI, https://ncifrederick.cancer.gov/research/brb/reagents/recombineeringReagent.aspx). Similar to SW102 derived from EL250 [9] but contains an arabinose-inducible araC-PBAD promoter expressing enhanced Flippase (Flpe) and carries no antibiotic resistance.
Recombineering plasmid PL451 (NCI, https://ncifrederick.cancer.gov/research/brb/reagents/recombineeringReagent.aspx). Contains Neomycin (Neo) CDS expressed by eukaryotic phosphoglycerate kinase (PGK) and prokaryotic Em7 promoters [9] to confer G418 resistance in mammalian cells and kanamycin resistance in bacteria, respectively. This cassette is flanked by two Flp recombinase target (Frt) sites and one loxP site.
Recombineering plasmid PL451-no loxP derived from PL451. loxP site present in original PL451 has been removed to be compatible for use with loxP-STOP-loxP cassette.
Recombineering plasmid pFrtF3-Neo-FrtF3. Derived from PL451-no loxP (2.1–5 above). This cassette is flanked by two Flp recombinase target (Frt) sites containing F3 spacer mutation [11]. This design allows Flp to direct deletion between matched FrtF3 sites, but helps prevent cross-recombination between Frt and FrtF3 sites.
Ultrapure DNAse/RNAse-free water (Invitrogen).
10% L-(+)-Arabinose (SIGMA) stock solution: 100 mg/mL dissolved in Ultrapure DNAse/RNAse-free water and 0.22 μm sterile-filtered.
LoTE (3 mM Tris, 0.2 mM EDTA, 0.22 μm filter sterilized).
Dimethyl sulfoxide (DMSO).
2.2. Vector Cloning
pBluescript KS (+) plasmid for subcloning.
pBR322-DT targeting plasmid equipped to express negative selection diphtheria toxin (DT) in mammalian cells to eliminate cells that undergo incorrect homologous recombination. Backbone provides ampicillin resistance.
pLox-Stop-lox-TOPO plasmid (loxP-STOP-loxP) [12]. Contains a cassette flanked by loxP sites equipped with an adenovirus splice acceptor-tetrameric SV40 polyadenylation array designed to halt transcription. Adjacent to the splice acceptor in the opposite orientation is a PGK promoter-puromycin (Puro) gene-PGK polyadenylation cassette designed to express puromycin in mammalian cells.
Luria-Bertani (LB) media: 10 g/L Tryptone, 5 g/L Yeast extract-B, 10 g/L NaCl (MP Biomedicals, #3002-031). Autoclave 25 capsules/1 L purified water.
LB (Lennox L) agar: 9.14 g/L Enzymatic digest of casein, 4.57 g/L Yeast extract (low sodium), 4.57 g/L NaCl, 13.72 g/L Bacteriological agar (SIGMA, #L7025). Autoclave 1 tablet/50 mL purified water.
Tetracycline hydrochloride (SIGMA) stock solution: 15 mg/mL dissolved in methanol.
Chloramphenicol (SIGMA) stock solution: 30 mg/mL dissolved in ethanol.
Ampicillin sodium salt (SIGMA) stock solution: 100 mg/mL dissolved in Ultrapure DNAse/RNAse-free water.
Kanamycin (SIGMA) stock solution: 50 mg/mL dissolved in Ultrapure DNAse/RNAse-free water.
QIAquick Gel Extraction Kit (QIAGEN).
QIAEX II Gel Extraction Kit (QIAGEN).
QIAGEN Large-Construct Kit (QIAGEN).
QIAGEN EB buffer from QIAprep Spin Miniprep Kit (QIAGEN).
Various restriction enzymes (New England Biolabs, use high fidelity versions when possible).
DNA Polymerase I, Large (Klenow) Fragment (New England Biolabs).
Expand Hifi PCR System dNTP Pack (Roche).
pCR2.1-TOPO Cloning Kit w/ TOP10 chemically competent cells (Invitrogen).
pCR-BluntII-TOPO Cloning Kit w/ TOP10 chemically competent cells (Invitrogen).
QuickChange II SDM Kit (Agilent Technologies).
MegaX DH10B T1R Electrocompetent E.coli cells (Invitrogen).
One Shot Stbl3 Chemically Competent E.coli cells (Invitrogen).
UltraPure Phenol:Chloroform:Isoamyl Alcohol (P:C:I, 25:24:1) (Invitrogen).
Chloroform.
Isopropanol.
Ethanol.
Methanol.
3 M sodium acetate.
2.3. Electroporation
Gene Pulser Electroporation Cuvette with 0.1 cm gap (BIORAD).
Gene Pulser Electroporation Cuvette with 0.4 cm gap (BIORAD).
Gene Pulser Xcell Total System (BIORAD).
Gene Pulser II System with Capacitance Extender Plus (BIORAD).
S.O.C. Medium: 2% Tryptone, 5% Yeast Extract, 10 mM NaCl, 2.5 mM KCl, 10 mM MgCl2, 10 mM MgSO4, 20 mM glucose (Invitrogen).
2.4. ES Cell Targeting
Mouse 129 strain-derived ES.
Multi-drug resistant mouse embryonic fibroblasts (MEF DR4) (ATCC, #SCRC-1045).
G418 sulfate liquid (Geneticin, 50 mg/mL) (Gibco).
Puromycin dihydrochloride (SIGMA) stock solution: 2 mg/mL dissolved in Ultrapure DNAse/RNAse-free water.
3. Methods
3.1. Designing the Inducible Knock-In Targeting Construct
Accurately planning all steps to build the knock-in targeting vector before construction begins is critical. For highest efficiency, distinct antibiotic selection and introduction of new restriction enzyme sites need to be considered and designed for each step.
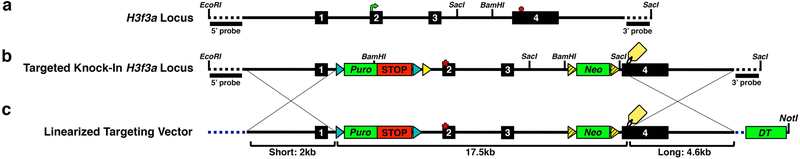
Generate a sequence map of the entire wild-type gene locus. Identify key features including endogenous translational start and stop sites, and the location to introduce the selective mutation (Fig. 1a). Plan the alterations that need to be introduced. Examples from the engineered H3f3a locus include a loxP-STOP-loxP cassette, point mutation, Frt-Neo-Frt cassette, and an epitope tag (Fig. 1b). Recombineering allows introduction of elements at any location. However, when possible, avoid repetitive genomic sequences for recombineering-mediated homologous recombination (see Note 2). Furthermore, ideally position targeting cassettes (e.g., loxP-STOP-loxP) at least 200 bp away from splice donors and splice acceptors. Map restriction enzyme sites to assist designing recombineering vectors that will be used for locus retrieval and incorporating engineered elements (Fig. 1a, b).
Plan the location for the loxP-STOP-loxP cassette. For an inducible knock-in, a transcription termination sequence (e.g., loxP-STOP-loxP) should ideally be placed in an intron or 5′ untranslated region upstream of the endogenous translation initiation codon. To avoid disruption of regulatory elements for your gene of interest or surrounding genes, check the ENCODE database to mark potential promoters and enhancers. For H3f3a where the canonical translation initiation start is located in exon 2, we designed the loxP-STOP-loxP cassette to be positioned in intron 1 (Fig. 1b).
Plan additional engineered features (e.g., point mutations, epitope tags, etc.). The likelihood of homologous recombination occurring in between the loxP-STOP-loxP cassette and an additional introduced mutation is directly proportional to genomic distance. For large distances, incorporation of a second selectable marker will greatly enhance the efficiency of identifying the correctly targeted locus in ES cells. In the H3f3a locus, we introduced a K27M point mutation in exon 2, approximately 900 bp from the loxP-STOP-loxP cassette, which provides puromycin positive selection. If the alterations to be introduced are close together as in this case, the targeting vector could be complete with this single selection. However, we also inserted an in-frame epitope tag to be positioned just upstream of the canonical stop codon in exon 4, approximately 8.7 kb from the K27M point mutation. To allow double selection to maximize identification of ES cells that incorporated all of these elements, we inserted a second Frt-Neo-Frt cassette. Insert the selection cassette within a nearby intron to avoid altering the desired protein sequence after induction (Fig. 1b). This approach allows a single targeting in ES cells for integration of all elements rather than sequential targeting.
Plan the homology arms to direct homologous recombination in ES cells. Regions of exact homology between the knock-in vector and the endogenous locus will direct homologous recombination in ES cells. To promote crossover events outside of the engineered elements, use homology arms approximately 4 kb on one side and 2 kb on the other side. For the H3f3a targeting vector, short and long homology arms (2 and 4.6 kb, respectively) facilitate homologous recombination of the final 17.5 kb engineered locus (Fig. 1b, c).
Plan the strategy to identify correctly targeted ES cells. Southern blot analysis remains the gold standard to assess accurate homologous recombination in ES cells.To discriminate between homologous recombination and random integration, a PCR approach must span from the endogenous locus outside of the knock-in targeting construct and connect with the alterations introduced into the locus. There is no positive control for this event until homologous recombination occurs, and spurious PCR results often give misleading false positives or false negatives. To plan the Southern blot approach, consider overall restriction enzyme digest map of the endogenous locus with additional sites added by engineered elements. Select restriction enzyme sites that reside within and outside the engineered locus and are efficient in cutting genomic DNA. Design probes 500–1000 bp residing outside the targeting construct to provide adequate resolution to discriminate bands from targeted and wild-type alleles (see Note 2). In general, a range of bands of 5–12 kb with at least a 20% difference between wild-type and targeted fragments is ideal. Test probes on wild-type DNA to verify clear results before proceeding. For the H3f3a design, we identified EcoRI/BamHI double digest suitable to assess DNA banding patterns with 5′ probe and SacI to assess DNA banding patterns with 3′ probe (Fig. 1a, b).
Plan a negative selection element in the knock-in construct. The final targeting vector also includes diphtheria toxin (DT) outside of the regions of homology to select against ES cells with random integration. The H3f3a final knock-in vector confers DT negative selection plus puro and neo double positive selection (Fig. 1c).
Identify a unique restriction site to linearize the knock-in construct. The final targeting vector should contain a unique restriction enzyme cut site in the vector backbone that can be used to linearize the construct without disrupting the targeted locus or any engineered elements. If such a site does not exist, you can engineer it into the vector before construction begins. We used NotI to linearize the final H3f3a knock-in vector (Fig. 1c).
Fig. 1. Designing An Inducible H3f3a Knock-In Vector.
(a) Schematic map of the H3f3a gene locus defining intron and exon configuration, endogenous translation initiation (green arrow, exon 2) and stop (red hexagon, exon 4) sites. (b) Final design of targeted knock-in H3f3a locus illustrating important engineering features including intron 1 placement ofloxP-STOP-loxP (STOP) transcription termination sequence that will also provide puromycin (Puro) positive selection in ES cells, exon 2 point mutation (red asterisk), exon 4 in-frame epitope tag (yellow tag) associated with intron 3 placement of Frt-Neo-Frt cassette to provide G418 positive selection in ES cells. loxP (cyan) Frt (yellow) and FrtF3 (yellow striped) sequences are marked with triangular arrowheads. Dotted line shows genomic locus not included in targeting construct. EcoRI/BamHI sites and SacI sites generate restriction fragments detected by 5′ and 3′ probes, respectively, to create distinguishable Southern blot DNA fragment sizes before and after targeting, with a SacI site designed into the Frt-Neo-Frt cassette cloning. Probes are designed to hybridize outside homologous recombination events for proper Southern blot verification. (c) Final design of H3f3a NotI-linearized targeting vector. Dotted blue line indicates targeting vector backbone. Vector backbone provides Diphtheria toxin (DT) negative selection in ES cells. Short (2 kb) and long (4.6 kb) homology arms flank engineered locus (17.5 kb) to guide homologous recombination outside of engineered elements. Not drawn to scale so that elements can be clearly marked.
3.2. Retrieve the Genomic Region of interest from a BAC Clone
This step uses recombineering to isolate the desired genomic region of your gene of interest from the much larger BAC clone insert into the retrieval vector.
3.2.1. Make Electrocompetent SW102 Bacteria
Begin starter culture of SW102 bacteria: 5 μL/5 mL LB with tetracycline (15 μg/mL). Incubate/shake at 30 °C overnight approximately 18 h.
Transfer 800 μL starter culture to 40 mL LB with tetracycline (15 μg/mL), save remaining culture as a reference for measuring O.D.600. Incubate/shake diluted culture at 30 °C until O.D.600 is 0.6.
Cool cells in ice water for 2 min. Transfer 12.5 mL of culture into individual ice cold 14 mL round-bottom tube in ice water.
Spin 5 min at ~2000 × g at 4 °C. Discard supernatant, add 1 mL ice cold nuclease-free water. Hand-shake in ice water slurry until pellet is resuspended. Add 9 mL ice cold nuclease-free water. Invert tubes two times.
Repeat step 4 above.
Spin 5 min at ~2000 × g at 4 °C. Decant supernatant, be careful not to lose pellet and resuspend by hand-shaking in residual approximately 200 μL nuclease-free water. These are electrocompetent cells, ready for electroporation.
If electrocompetent cells are to be saved for later use, replace ice cold water with ice cold 10% glycerol in 3–6 above and store final 200 μL of cells at −80 °C.
3.2.2. Electroporate BAC DNA into SW102 Bacteria
Transfer 50 μL electrocompetent SW102 cells into ice cold 0.1 cm gap Gene Pulser Electroporation Cuvette. Add 1–10 μL (approximately 1 μg) BAC DNA (124 kb) to cuvette cells. Include cuvettes for two additional conditions: a no DNA negative control, and a known circular plasmid with appropriate antibiotic selection positive control. Electroporate with the following parameters: 1750 V, 200 Ω, 25 μF. Monitor time constant and voltage output (usually approximately 5 and 1725, respectively).
Recover electroporated cells in 350 μL S.O.C. medium and incubate/shake at 30 °C for 1 h.
Plate recovered cells onto chloramphenicol (12.5 μg/mL) LB agar plates. Incubate overnight at 30 °C.
Select 5–10 chloramphenicol-resistant colonies, grow 5 mL cultures in LB-chloramphenicol (12.5 μg/mL) media overnight at 30 °C.
Make multiple 10% DMSO stocks from individual colonies by adding 100 μL of DMSO to 900 μL culture. Store at −80°C. These are SW102-BAC bacteria.
Isolate DNA from 5 mL cultures using a modified protocol from QIAGEN Large-Construct Kit (see Note 3).
3.2.3. Construct the Retrieval Vector
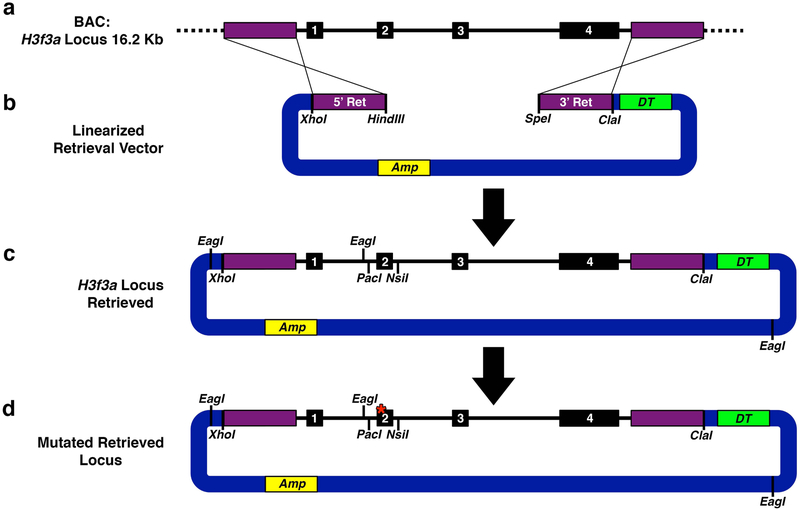
Design retrieval arms at the 5′ and 3′ ends of the genomic region that you would like to include in your knock-in construct (see Notes 4–6). For H3f3a, we retrieved 16.2 kb encompassing the entire H3f3a locus.
Using the BAC DNA as a template, PCR amplify 5′ retrieval arm (5′ Ret). Engineer complimentary restriction enzyme sites in your PCR primers for ligating into the main targeting vector, pBR322-DT (see Notes 7 and 8) and assembling with the 3′ retrieval arm. For H3f3a, we engineered the 5′ retrieval arm to contain flanking XhoI and HindIII sites (Fig. 2a, b). TA-subclone the PCR fragment into pCR2.1-TOPO, transform into TOP10 cells, and verify the sequence.
Using the BAC DNA as a template, PCR amplify 3′ retrieval arm (3′ Ret). Again, engineer complimentary restriction enzyme sites in your PCR primers for ligating into the main targeting vector, pBR322-DT, and assembling with the 5′ retrieval arm. For H3f3a, we used flanking HindIII/SpeI and ClaI sites (Fig. 2a, b). TA-subclone into pCR2.1-TOPO, transform into TOP10 cells and verify the sequence.
Subclone the 5′ and 3′ retrieval arms into pBR322-DT. Linearize pBR322-DT with XhoI and ClaI, isolate 5′ Ret with XhoI and HindIII, and 3′ Ret with HindIII and ClaI. Perform a triple ligation to clone 5′ and 3′ retrieval arms into pBR322-DT. Transform into TOP10 bacteria, extract DNA, and verify by restriction digest. This is the final retrieval vector (Fig. 2b).
Fig. 2. H3f3a Locus Retrieval.
(a) Schematic of desired 16.2 kb H3f3a locus including exons 1–4 within a 124 kb BAC clone. Dotted black line indicates genomic regions outside of region to be retrieved. (b) Retrieval vector with specific locus homology retrieval arms (5′ Ret and 3′ Ret), linearized with HindIII and SpeI to facilitate gap repair homologous recombination. pBR322-DT Vector (blue) is equipped to express Diphtheria toxin (DT) for negative selection in mammalian cells and ampicillin resistance (Amp) in bacterial cells. (c) Successfully retrieved locus. EagI sites mark diagnostic digest points to verify vector fidelity. PacI and NsiI sites flank exon 2 facilitating mutant exon 2 replacement. (d) Retrieved locus with successfully replaced mutated exon 2 (red asterisk). If mutation in the gene of interest is located distally to the loxP-STOP-loxP cassette, it would be introduced by recombineering as a distal element in Targeting 2 rather than at this early stage.
3.2.4. Retrieve the Genomic Region of Interest into pBR322-DT
Linearize retrieval vector by restriction enzyme digest to cut at the junction between the 5′ and 3′ retrieval arms (Fig. 2b). HindIII and SpeI were used for H3f3a retrieval. Purify by gel extraction (QIAquick Gel Extraction Kit).
For gap repair-mediated homologous recombination, begin starter culture of SW102-BAC bacteria prepared in Subheading 3.2.2, step 5: 5 μL/5 mL LB with chloramphenicol (25 μg/mL). Incubate/shake at 30 °C overnight approximately 18 h.
Transfer 800 μL starter culture to 40 mL LB with chloramphenicol (25 μg/mL), save remaining culture as a reference for measuring O.D.600. Incubate/shake diluted culture at 30 °C until O.D.600 is 0.6.
Separate 12.5 mL of culture into a 50 mL conical tube, keep remainder at 30 °C (to be used as a no heat-induced gap repair negative control that should result in no colonies after selection). Shake 50 mL conical tube in 42 °C water bath for 15 min to heat-shock the bacteria.
Shake heat-shocked bacteria in ice water slurry for 2 min. Do the same with 10 mL of negative control bacteria that were not heat shocked. Incubate each culture in ice water for 5 min. Transfer 10 mL of each culture into individual prechilled 14 mL round-bottom tubes in ice water slurry.
Spin 5 min at ~2000 × g at 4 °C. Discard supernatant, add 1 mL ice cold nuclease-free water. Hand-shake in ice water slurry until pellet is resuspended. Add 9 mL ice cold nuclease-free water. Invert tubes two times.
Repeat step 6 above.
Spin 5 min at ~2000 × g at 4 °C. Decant supernatant, be careful not to lose pellet and resuspend by hand-shaking in residual approximately 200 μL nuclease-free water.
For each culture, transfer 50 μL cells into ice cold 0.1 cm gap Gene Pulser Electroporation Cuvette. Add 1–10 μL (approximately 20 ng) linearized retrieval vector to each cuvette. Include two additional controls for each culture: a no DNA negative control, and a known circular plasmid with appropriate antibiotic selection positive control. Electroporate with the following parameters: 1750 V, 200 Ω, 25 μF. Monitor time constant and voltage output (usually approximately 5 and 1725, respectively).
Recover electroporated cells in 350 μL S.O.C. medium and incubate/shake at 30 °C for 1 h.
Plate recovered cells onto LB-ampicillin (100 μg/mL) agar plates. Incubate overnight at 30 °C.
Select 5–10 colonies, grow 5 mL cultures in LB-ampicillin (100 μg/mL) media overnight at 30 °C, isolate DNA from a portion of each culture (see Note 3). This is the retrieved locus vector (Fig. 2c).
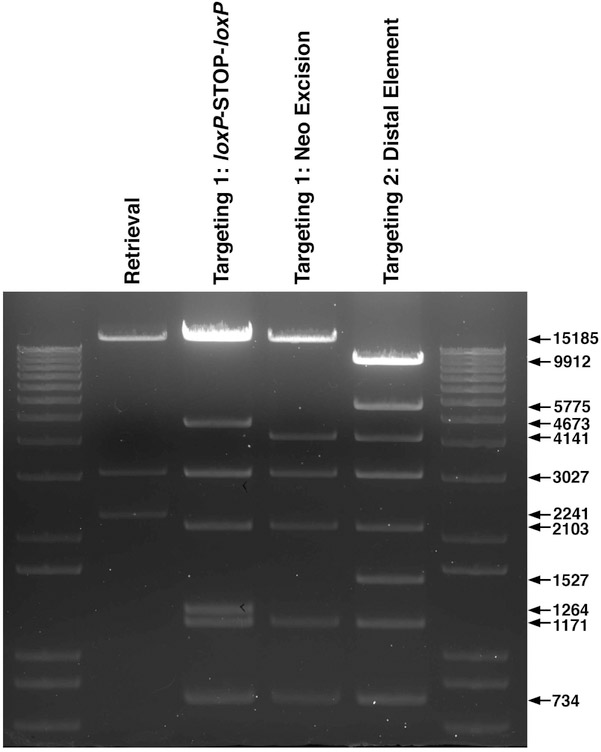
Perform diagnostic EagI restriction enzyme digest to verify correct banding pattern for the retrieved locus vector (Fig. 2c and 7). Make 10% DMSO stocks of validated clones, store at −80°C.
Fig. 7. Verifying H3f3a Knock-In Targeting Vector Construction.
EagI restriction enzyme digests to verify correct assembly for each step of H3f3a knock-in targeting vector construction.
3.3. Engineer Proximal Point Mutations
In this example, we introduced a K27M mutation into H3f3a exon 2.
Using the BAC DNA as a template, PCR amplify H3f3a exon 2 and flanking PacI and NsiI restriction enzyme sites (Fig. 2c). TA-subclone into pCR2.1-TOPO and verify sequence.
Design appropriate primers and use exon 2 pCR2.1-TOPO subclone as a template and perform site-directed mutagenesis PCR (QuickChange II SDM Kit) to incorporate desired mutation.
Verify sequence of mutated exon 2.
Linearize retrieved locus vector and isolate mutated exon 2 fragment with PacI/NsiI double digest. Since the retrieved locus vector is >10 kb, gel purify pieces with QIAEX II Gel Extraction Kit.
Ligate mutated exon 2 fragment into retrieved locus vector. Purify half of the ligations with QIAEX II gel extraction kit to maximize electroporation efficiency in next step.
Electroporate MegaX DH10B T1R Electrocompetent E.coli cells with the following parameters: 2000 V, 200 Ω, 25 μF.
Recover electroporated cells in 350 μL S.O.C. medium and incubate/shake at 30 °C for 1 h.
Plate recovered cells onto LB-ampicillin (100 μg/mL) agar plates. Incubate overnight at 30 °C.
Select 5–10 colonies, grow 5 mL cultures in LB-ampicillin (100 μg/mL) media overnight at 30 °C.
Isolate DNA from a portion of each culture (see Note 3).
Confirm EagI digest banding pattern (Fig. 2d and 7) and verify mutated exon 2 sequence (Fig. 2d) with primers flanking exon 2. Make 10% DMSO stocks of validated clones.
Electroporate 1–10 μL (approximately 20 ng) of this exon 2 mutated retrieved locus vector back into SW102 bacteria following steps in Subheading 3.2.1 to make them electrocompetent and steps Subheading 3.2.2, steps 1–3 for electroporation selecting with LB-ampicillin (100 μg/mL).
Select 5–10 colonies, grow 5 mL cultures in LB-ampicillin (100 μg/mL) media overnight at 30 °C.
Isolate DNA from a portion of each culture (see Note 3). This is the mutated retrieved locus vector (Fig. 2d).
Re-verify EagI digest banding pattern (Fig. 2d and 7). Make 10% DMSO stocks of validated clones and use these SW102 bacteria containing the mutated retrieved locus for subsequent engineering.
3.4. Targeting 1: inserting the loxP-STOP-loxP Cassette
To insert the loxP-STOP-loxP cassette, build a construct with 5′ targeting and 3′ targeting arms to direct integration by homologous recombination at the desired location in the mutated retrieved locus. Include a Frt-Neo-Frt cassette, which confers kanamycin resistance to bacteria for selecting desired clones.
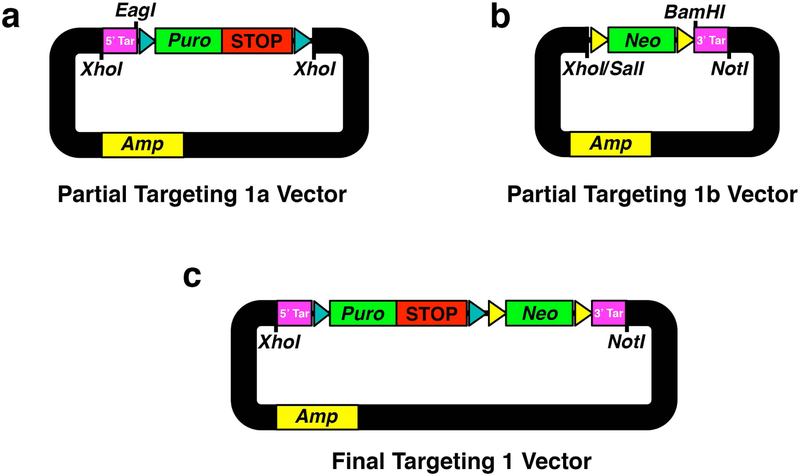
3.4.1. Constructing the loxP-STOP-loxP Targeting 1 Vector.
Using the BAC DNA as a template, PCR amplify 5′ targeting arm (see Notes 9 and 10) (5′ Tar, Fig. 3a) to contain flanking XhoI and EagI sites, and TA-subclone into pCR2.1-TOPO, transform into TOP10 cells and verify sequence.
Isolate 5′ Tar by double digest with XhoI/EagI, loxP-STOP-loxP cassette by NotI/XhoI from pLox-Stop-lox-TOPO, and linearize pBluescript KS(+) with XhoI. Gel purify these 3 fragments and perform a triple ligation to assemble a partial Targeting 1a vector backbone (Fig.3a). This ligation eradicates NotI site between 5′ Tar and loxP-STOP-loxP cassette, and leaves the EagI restriction enzyme site for diagnostic size verification of the assembled targeting vector at this stage (see Note 10).
Transform into chemically competent Stbl3 cells (see Note 11), incubate overnight at 30 °C and select with ampicillin (100 μg/mL). Select 5–10 clones and verify correct ligation using EagI restriction site banding pattern.
Using the BAC DNA as a template, PCR amplify 3′ targeting arm (see Note 9), (3′ Tar, Fig. 3b) to contain flanking BamHI and NotI sites, and TA-subclone into pCR2.1-TOPO, transform into TOP10 cells and verify sequence.
Isolate 3′ Tar fragment by double digest with BamHI/NotI, Frt-Neo-Frt cassette from PL451-no loxP plasmid by XhoI/BamHI and linearize pBluescript KS(+) backbone with XhoI/NotI. Gel purify these 3 fragments and perform a triple ligation to assemble a partial Targeting 1b vector backbone (Fig. 3b).
Transform into chemically competent Stbl3 cells (see Note 11). Incubate overnight at 30 °C and select with kanamycin (50 μg/mL). Select 5–10 clones and verify correct ligation using EagI or KpnI restriction site banding pattern.
Isolate partial Targeting 1a fragment by XhoI digest, partial Targeting 1b fragment by SalI/NotI double digest, and linearize pBluescript KS(+) backbone with SalI/NotI. Gel purify these 3 fragments and perform a triple ligation to assemble complete Targeting 1 vector (Fig. 3c). This ligation eradicates XhoI/SalI sites between partial Targeting 1a and Targeting 1b fragments.
Transform into chemically competent Stbl3 cells (see Note 11) and select with kanamycin (50 μg/mL). Select 5–10 clones and verify correct ligation using separate XhoI/NotI, BamHI or EcoRI restriction site banding pattern. This is the Targeting 1 vector to insert the loxP-STOP-loxP cassette (Fig. 3c).
Fig. 3. Assembling Targeting 1 Vector For loxP-STOP-loxP Insertion.
(a) Partial targeting 1a vector containing subcloned XhoI-flanked H3f3a intron 1 5′ homology targeting arm (5′ Tar) and loxP-STOP-loxP (STOP) cassette equipped to express puromycin (Puro) in mammalian cells. EagI is the designed diagnostic subcloning remnant restriction enzyme site. (b) Partial targeting 1b vector containing subcloned XhoI/SalI and NotI-flanked Frt-Neo-Frt (Neo) cassette equipped to express neomycin in bacterial and mammalian cells, and H3f3a intron 1 3′ targeting homology arm (3′ Tar). BamHI is a subcloning remnant restriction enzyme site. (c) Final targeting 1 vector. loxP sites (cyan arrowheads), Frt sites (yellow arrowheads), ampicillin resistance in bacterial cells (Amp).
3.4.2. Insert loxP-STOP-loxP/Neo Selection Cassette into Mutated Retrieved Locus Vector
Isolate targeting 1 cassette from targeting 1 vector with XhoI/NotI double digest (Fig. 4a). Purify by gel extraction using QIEX II Gel Extraction Kit.
Using SW102 bacteria containing mutated retrieved locus from Subheading 3.3, follow Subheading 3.2.4 steps 2–12 with LB-ampicillin (100 μg/mL) selection prior to electroporation and LB-kanamycin (50 μg/mL) selection after electroporating isolated targeting cassette 1.
Perform diagnostic EagI restriction enzyme digest to verify correct banding pattern for targeting 1 (Fig. 4b and 7).
Select one correct Targeting 1 clone DNA from Subheading 3.4.2, step 3 above, electroporate into MegaX DH10B T1R Electrocompetent E.coli cells following Subheading 3.3, steps 6–10 above selecting with LB-kanamycin (50 μg/mL). Grow cultures and isolate DNA from a portion of each culture (see Note 3).
Re-verify EagI digest banding pattern (Fig. 4b and 7). Make 10% DMSO stocks of validated clones. This is the intermediate step of targeting 1 (Fig. 4b).
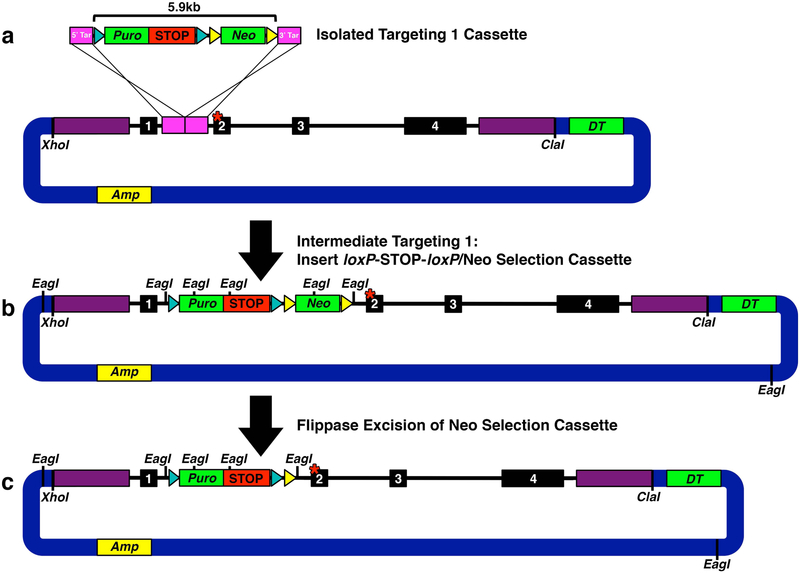
Fig. 4. Targeting 1: Inserting loxP-STOP-loxP.
(a) Isolated targeting 1 cassette excised with XhoI and NotI to facilitate gap repair homologous recombination. (b) Intermediate step of targeting 1: Successful insertion of targeting 1 loxP-STOP-loxP/Neo selection cassette (5.9 kb) into H3f3a intron 1. EagI sites mark diagnostic digest points to verify vector fidelity. (c) Successful Flippase excision of Neo selection cassette leaving behind a remnant Frt site and stable loxP-STOP-loxP. EagI sites mark diagnostic digest points to verify vector fidelity.
3.4.3. Flippase-Mediated in vitro Removal of Targeting 1 Neo Selection Cassette
Begin starter culture of SW105 bacteria 5 μL/5 mL LB with no antibiotic. Incubate/shake at 30 °C overnight approximately 15 h.
Transfer 800 μL starter culture to 40 mL LB with no antibiotic, make 1 duplicate dilution and save remaining culture as a reference for measuring O.D.600. Incubate/shake diluted cultures at 30 °C until O.D.600 is 0.4.
To 1 duplicate culture dilution, add 400 μL 10% L-(+)-Arabinose to induce expression of Flippase.
Incubate/shake both diluted cultures at 30 °C for 1 h.
Make cells electrocompetent using conditions in Subheading 3.2.1, steps 3–6.
For each culture, transfer 50 μL cells into 2 separate ice cold 0.1 cm gap Gene Pulser Electroporation Cuvette (sets 1a and 1b). Add 1–10 μL (approximately 20 ng) intermediate targeting 1 loxP-STOP-loxP + Neo vector DNA produced in Subheading 3.4.2 to each cuvette. For each culture, transfer another 50 μL cells into 2 separate ice cold 0.1 cm gap Gene Pulser Electroporation Cuvette to serve as no DNA controls (sets 2a and 2b). For each culture, also prepare 2 cuvettes to electroporate MegaX DH10B T1R Electrocompetent E.coli cells with a 1:100 dilution of Targeting 1 clone DNA (sets 3a and 3b) to serve as electroporation controls. Include one additional electroporation for each culture: a known circular plasmid with appropriate antibiotic selection positive control. Electroporate with the following parameters: 1750 V, 200 Ω, 25 μF. Monitor time constant and voltage output (usually approximately 5 and 1725, respectively).
Recover electroporated cells in 350 μL S.O.C. medium and incubate/shake at 30 °C for 1 h.
Plate half of recovered cells for each set onto LB-ampicillin (100 μg/mL) and the other half on kanamycin (50 μg/mL) agar plates. Incubate overnight at 30 °C. Colony growth on ampicillin plates and lack of growth on kanamycin plates after arabinose treatment indicates efficiency of Flippase-mediated excision of Neo cassette. Colony growth on kanamycin plates without arabinose treatment indicated the specificity of the arabinose-induced Flippase expression in the SW105 bacteria.
Select 5–10 colonies, grow 5 mL cultures in LB-ampicillin (100 μg/mL) media overnight at 30 °C, isolate DNA from a portion of each culture (see Note3).
Perform diagnostic EagI restriction enzyme digest to verify correct banding pattern (Figs. 4c and 7). Make 10% DMSO stocks of validated clones.
3.4.4. Transfer Mutant Locus + loxP-STOP-loxPVector into SW102 Cells.
Make new electrocompetent SW102 cells as in Subheading 3.2.1.
Transfer in duplicate 50 μL cells into ice cold 0.1 cm gap Gene Pulser Electroporation Cuvette. Add 1–10 μL (approximately 20 ng) mutant locus + targeting 1 vector (from Subheading 3.4.3) to cuvette cells. Include duplicate cuvettes for two additional conditions: a no DNA negative control, and a known circular plasmid with appropriate antibiotic selection positive control. Electroporate with the following parameters: 1750 V, 200 Ω, 25 μF. Monitor time constant and voltage output (usually approximately 5 and 1725, respectively).
Recover electroporated cells in 350 μL S.O.C. medium and incubate/shake at 30 °C for 1 h.
Plate recovered cells onto LB-ampicillin (100 μg/mL) or kanamycin (50 μg/mL) agar plates. Incubate overnight at 30 °C.
Colony growth on kanamycin plates indicates contamination of un-excised Neo cassette. Select 5–10 ampicillin-resistant colonies, grow 5 mL cultures in LB-ampicillin (100 μg/mL) media overnight at 30 °C, isolate DNA from a portion of each culture (see Note 3).
Reconfirm diagnostic EagI restriction enzyme digest banding pattern (Figs. 4c and 7). Make 10% DMSO stocks of validated clones. This completes targeting 1: loxP-STOP-loxP inserted into mutant retrieved locus (Fig. 4c). If there were no additional distal genetic elements to engineer, this would be the finished knock-in targeting construct. Use these SW102 bacteria containing the mutated retrieved locus + loxP-STOP-loxP vector for subsequent engineering.
3.5. Targeting 2: Distal Genetic Element and Neo Selection Cassette
To insert an additional genetic element, build a construct with 5′ targeting and 3′ targeting arms to direct integration by homologous recombination at the desired location. To increase the frequency of recovering ES clones that have incorporated all of the engineered events, include a FrtF3-Neo-FrtF3 cassette so that double selection can be used. The FrtF3 sites will not recombine with the residual Frt site adjacent to the loxP-STOP-loxP cassette (see Note 12).
3.5.1. Constructing Targeting 2 Distal Element Insertion Vector.
This vector is designed to incorporate an additional genetic element, which could include alterations to the coding sequence of the target gene, or insertions of epitope tags or GFP fusions. As before, targeting arms include approximately 300 bp of endogenous sequence flanking any engineered sequence. In the H3f3a example, we inserted an in frame epitope tag immediately upstream of the endogenous exon 4 translation STOP codon. Since this epitope will be approximately 8.5 kb away from the exon 2 mutation, a Frt-flanked Neo cassette was included upstream of exon 4 to facilitate double positive selection when isolating ES clones.
Using the BAC DNA as a template, PCR amplify 5′ targeting arm (5′ Tar) to contain flanking HindIII and EcoRI restriction enzyme sites (see Note 9). TA-subclone into pCR2.1-TOPO to assemble partial Targeting 2a vector (Fig. 5a). Verify sequence.
Using the BAC DNA as a template, PCR amplify 3′ targeting arm (3′ Tar), to contain flanking BglII/SacI and SacII restriction enzyme sites (see Note 9). For H3f3a, this arm includes the endogenous STOP codon, and the engineered SacI restriction enzyme site serves as a new DNA cut site for Southern blot verification (Fig. 5b). TA-subclone into pCR2.1-TOPO and verify sequence.
Add the epitope tag to the 3′ targeting arm using the 3′ Tar subclone as a template for splice overlap extension PCR [13]. The epitope was designed with a linker sequence containing an EagI restriction enzyme site for diagnostic size verification of the assembled targeting vector at this stage (Fig. 5b).
Isolate 3′ Tar + epitope insert by double digest with BglII/SacII, FrtF3-Neo-FrtF3 cassette from pFrtF3-Neo-FrtF3 plasmid by HindIII/BamHI and linearize pBluescript KS(+) backbone with HindIII/SacII. Gel purify these 3 fragments and perform a triple ligation to assemble a partial Targeting 2b vector (Fig. 5b). This ligation eradicates BamHI and BglII sites between 3′ Tar and Neo cassette.
Subclone 5′ Tar arm into the partial Targeting 2b vector by isolating 5′ Tar insert by HindIII/EcoRI double digest and linearize partial Targeting 2b vector backbone above by HindIII/EcoRI. Gel purify these 2 fragments and ligate to assemble final Targeting 2 vector (Fig. 5c).
Transform into chemically competent Stbl3 cells (see Note 11) and select with kanamycin (50 μg/mL). Select 5–10 clones and verify correct ligation using diagnostic restriction enzyme cutting patterns.
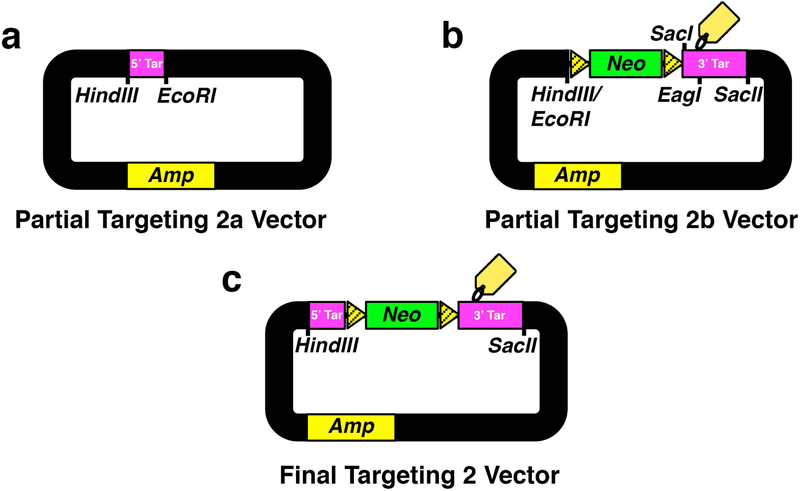
Fig. 5. Assembling Targeting 2 Vector For Distal Element Insertion.
(a) Partial targeting 2a vector containing subcloned HindIII and EcoRI-flanked H3f3a intron 3 5′ targeting homology arm (5′ Tar). (b) Partial targeting 2b vector containing subcloned HindIII/EcoRI and SacII-flanked Frt-Neo-Frt (Neo) cassette to express neomycin in bacterial and mammalian cells and H3f3a intron 3/exon 4 3′ targeting homology arm (3′ Tar). SacI is the designed subcloning remnant restriction enzyme site for Southern blot verification. (c) Final targeting 2 vector. Mutated Frt sites (striped yellow). 3′ Tar is engineered to contain the diagnostic linker EagI restriction enzyme site and epitope tag sequence.
3.5.2. Insert Distal Genetic Element and Neo Selection Cassette into Mutant Locus + loxP-STOP-loxP Targeting 1 Vector
Isolate Targeting 2 cassette from final Targeting 2 vector by HindIII/SacII double digest (Fig. 6a). Purify by gel extraction (QIAquick Gel Extraction Kit).
Using SW102 bacteria containing mutant locus + loxP-STOP-loxP vector (from Subheading 3.4.4), follow Subheading 3.2.4, steps 2–12 with LB-ampicillin (100 μg/mL) selection prior to electroporation and LB-kanamycin (50 μg/mL) selection after electroporating isolated Targeting cassette 2.
Perform diagnostic EagI restriction enzyme digest to verify correct banding pattern (Fig. 6b and 7). Make 10% DMSO stocks of validated clones.
Select one correct clone DNA from Subheading 3.5.2, step 3 above.
Electroporate MegaX DH10B T1R Electrocompetent E.coli cells with up to 100 ng DNA using the following parameters: 2000 V, 200 Ω, 25 μF. Also electroporate a no DNA negative control and a known circular plasmid with appropriate antibiotic selection positive control.
Recover electroporated cells in 350 μL S.O.C. medium and incubate/shake at 30 °C for 1 h.
Plate recovered cells onto LB-kanamycin (50 μg/mL) agar plates. Incubate overnight at 30 °C.
Select 2–3 colonies, grow 5 mL cultures in LB-kanamycin (50 μg/mL) media overnight at 30 °C, isolate DNA from a portion of each culture (see Note 3).
Re-verify EagI digest banding pattern (Fig. 6b and 7). Make 10% DMSO stocks of validated clone.
This is the final H3f3a knock-in targeting vector (Fig. 6b).
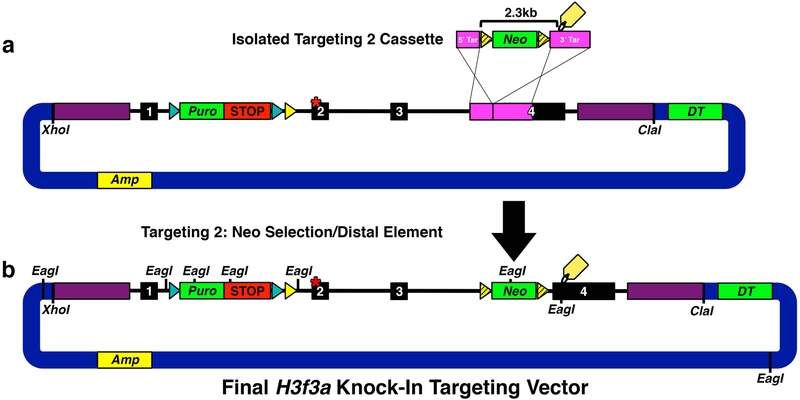
Fig. 6. Targeting 2: Inserting Distal Element.
(a) Targeting 2 cassette fragment isolated with HindIII and SacII to facilitate gap repair homologous recombination. (b) Final H3f3a knock-in targeting vector generated by successful insertion of targeting 2 Neo selection/distal element cassette (2.3 kb) into H3f3a intron 3/exon 4. EagI sites mark diagnostic digest points to verify final gene targeting vector fidelity.
3.6. Homologous Recombination into Mouse ES Cells
3.6.1. Prepare Linearized Construct for ES Cell Electroporation.
Linearize 40 μg of final knock-in targeting vector with unique restriction site that cuts in the vector backbone. NotI was used for this H3f3a example.
Bring reaction volume to 350 μL with nuclease-free water.
Extract with 350 μL P:C:I.
Spin 5 min at ~16,000 × g. Transfer aqueous (top) phase to a fresh tube (~350 μL).
Extract with equal volume chloroform (~350 μL).
Spin 5 min at ~16,000 × g. Transfer aqueous (top) phase to a fresh tube (~350 μL).
Add 1/10 volume 3 M sodium acetate (~30 μL), mix gently.
Add 660 μL ethanol, mix gently. DNA cloud becomes visible.
Spin at ~16,000 × g, 4 °C for 15 min.
Remove supernatant and wash pellet with 1 mL ice cold 70% ethanol.
Spin at ~16,000 × g, 4 °C for 10 min.
Repeat 70% ethanol wash and spin.
Remove supernatant and air dry pellet.
Resuspend in 10 μL LoTE.
3.6.2. Electroporate ES Cells with Linearized Knock-In Targeting Construct
For guidelines on propagating ES cells, including all media and solutions, conditions to generate irradiated MEF feeder cells, picking ES clones, and DNA extraction from clones, please refer to [10].
Grow ES cells on a monolayer of irradiated DR4 MEFs.
When ES cells reach 80% confluence, trypsinize in 2 mL, add 8 mL media.
Spin 5 min at ~180 × g.
Wash pellet gently with 10 mL PBS.
Spin 5 min at ~180 × g.
Resuspend pellet gently in 1.6 mL ice cold PBS.
Divide dilution into two 800 μL aliquots and add to ice cold 0.4 cm gap Gene Pulser Electroporation Cuvette.
To each cuvette, add 20 μg linearized targeting vector DNA from Subheading 3.6.1.
Electroporate with the following parameters: 250 V, 500 μF. Monitor time constant and voltage output (usually approximately 7 and 250, respectively). Add each electroporation to 30 mL pre-warmed media.
Split each electroporation onto three 10 cm2 plates seeded with irradiated MEFs.
Incubate cells overnight.
Replace media with double-positive selection media (2 μg/mL Puromycin, 100 μg/mL G418).
Replace double positive selection media every day for 4 days. Small colonies become visible.
Replace double positive selection media with G418 selection media (100 μg/mL G418) every day until colonies become large enough to pick (approximately 1 week after electroporation).
Pick clones, expand to create duplicate clone sets. Freeze viable cells for one set and use duplicate set for DNA extraction and Southern blot analysis to identify correctly targeted clones (see Note 13).
Correctly targeted ES clones are used for blastocyst injection and subsequent breeding to create knock-in mice, following procedures outlined in detail in [10] (see Notes 14 and 15).
4. NOTES
Obtain mouse BAC clones generated from the same mouse strain (e.g., 129) as the mouse ES cells to be used for targeting. This will aid in proper homologous recombination of the final targeting vector in ES cells.
The design for homologous recombination events and Southern blot probes should avoid sequences with repetitive elements when possible to help ensure specificity. RepeatMasker on UCSC genome browser (http://genome.ucsc.edu/), as well as BLAST searches can help identify repetitive elements.
Modified protocol for isolating large construct DNA. Pellet 4 mL of culture, resuspend in 250 μL chilled QIAGEN P1 with RNAse A. Add 250 μL QIAGEN P2, mix gently by inverting five times, incubate at room temperature 5 min. Add 350 μL chilled QIAGEN P3, mix gently by inverting five times, incubate on ice for 10 min. Spin at ~16,000 × g for 10 min at 4 °C. Gently transfer supernatant to a fresh tube. Add 750 μL isopropanol, mix gently by inverting five times, incubate at room temperature 10 min. Spin at ~16,000 × g at 4 °C, gently remove supernatant without disturbing DNA pellet. Wash pellet with 1 mL ice cold 70% ethanol. Spin at ~16,000 × g at 4 °C, gently remove supernatant without disturbing DNA pellet. Wash pellet with 1 mL ice cold 70% ethanol. Spin at ~16,000 × g at 4 °C, gently remove supernatant without disturbing DNA pellet. Air dry pellet. Resuspend in 10 μL LoTE.
The fragment size to be retrieved will vary depending on the locus and design. Retrieve the region that you need to engineer as well as 5′ and 3′ homology arms.
Retrieval arms should ideally be approximately 500 bp to help enrich for correctly targeted fragments [9].
Consider designing retrieval vector to linearize with two incompatible restriction enzymes to avoid self-ligation during retrieval.
We used pBR322-DT as the vector backbone for the knock-in targeting vector because it is more amenable to cloning and growth of large DNA inserts compared to newer generation cloning vectors.
Plan the full strategy before beginning. The order of introducing different elements may vary depending on the specific locus and design. For example, because Subheading 3.3 required digestion of a PacI site in the H3f3a locus, we eliminated the PacI site in the vector backbone of pBR322-DT by Klenow fill-in and blunt-end ligation before Subheading 3.2.
Targeting arms should be approximately 300 bp to help enrich for correctly targeted fragments [9].
Subcloning strategy to build the loxP-STOP-loxP targeting vector using restriction enzymes should be carefully considered to avoid PCR amplifying the loxP-STOP-loxP cassette sequence due to its tetrameric SV40 polyA tandem array (3.5 kb) [12]. This array can undergo spontaneous recombination resulting in loss of one or more of the SV40 polyA elements (860 bp each), therefore it is very important to monitor the proper size of this array with diagnostic restriction enzyme digest at each step of cloning.
Use chemically competent Stbl3 cells combined with selected growth at 30 °C when cloning unstable vectors containing repeat sequences such as the loxP-STOP-loxP and Frt-Neo-Frt cassettes.
For distal element insertion in Targeting 2 (Subheading 3.5), we used a Frt F3 spacer mutant that was shown to maintain self-recognition, but lack cross-recombination potential with unmutated Frt sites [11]. This prevents Flippase-mediated recombination between FrtF3 sites flanking the Neo selectable marker in the distal element and a residual Frt site that remains in intron 1 after Targeting 1.
Targeting efficiency will vary depending on locus targeted and specific knock-in targeting construct.
The positive selection Neo cassette can be removed by transient ES cell transfection or transduction with a vector expressing Flippase, followed by isolation and screening of individual clones. Alternatively, to avoid additional ES cell manipulation, the resulting mice can be bred with mice engineered to ubiquitously express Flippase (e.g., 129S4/SvJaeSor-Gt(ROSA)26Sortm1(FLP1)Dym/J, Jackson Labs #003946) to delete the Neo cassette in the germline of all progeny mice.
The combination of multiple mutated alleles is generally required for spontaneous mouse brain tumor formation [5, 14, 15]. Depending on the oncogene, knock-in oncogenic mutations would typically need to be combined with additional mutations through breeding with other engineered mice, or by layering on injection-mediated delivery of other mutations.
Acknowledgments
This work was supported in part by NIH grant CA096832 to SJB, and by ALSAC.
References
- 1.Wiesner SM, Decker SA, Larson JD, Ericson K, Forster C, Gallardo JL, Long C, Demorest ZL, Zamora EA, Low WC, SantaCruz K, Largaespada DA, Ohlfest JR (2009) De novo induction of genetically engineered brain tumors in mice using plasmid DNA. Cancer Research 69 (2):431–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hambardzumyan D, Amankulor NM, Helmy KY, Becher OJ, Holland EC (2009) Modeling Adult Gliomas Using RCAS/t-va Technology. Transl Oncol 2 (2):89–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang H, Wang H, Shivalila CS, Cheng AW, Shi L, Jaenisch R (2013) One-step generation of mice carrying reporter and conditional alleles by CRISPR/Cas-mediated genome engineering. Cell 154 (6):1370–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F, Jaenisch R (2013) One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 153 (4):910–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rankin SL, Zhu G, Baker SJ (2012) Review: insights gained from modelling high-grade glioma in the mouse. Neuropathol Appl Neurobiol 38 (3):254–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kersten K, de Visser KE, van Miltenburg MH, Jonkers J (2017) Genetically engineered mouse models in oncology research and cancer medicine. EMBO Molecular Medicine 9 (2):137–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanchez-Rivera FJ, Jacks T (2015) Applications of the CRISPR-Cas9 system in cancer biology. Nat Rev Cancer 15 (7):387–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huijbers IJ, Del Bravo J, Bin Ali R, Pritchard C, Braumuller TM, van Miltenburg MH, Henneman L, Michalak EM, Berns A, Jonkers J (2015) Using the GEMM-ESC strategy to study gene function in mouse models. Nat Protoc 10 (11):1755–1785 [DOI] [PubMed] [Google Scholar]
- 9.Liu P, Jenkins NA, Copeland NG (2003) A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Research 13 (3):476–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Behringer R, Gertsenstein M, Nagy K, Nagy A (2014) Manipulating the Mouse Embryo: A Laboratory Manual. Fourth edition. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York [Google Scholar]
- 11.Turan S, Kuehle J, Schambach A, Baum C, Bode J (2010) Multiplexing RMCE: versatile extensions of the Flp-recombinase-mediated cassette-exchange technology. Journal of Molecular Biology 402 (1):52–69 [DOI] [PubMed] [Google Scholar]
- 12.Jackson EL, Willis N, Mercer K, Bronson RT, Crowley D, Montoya R, Jacks T, Tuveson DA (2001) Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes & Development 15 (24):3243–3248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR (1989) Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77 (1):51–59 [DOI] [PubMed] [Google Scholar]
- 14.Zhu G, Rankin SL, Larson JD, Zhu X, Chow LM, Qu C, Zhang J, Ellison DW, Baker SJ (2017) PTEN Signaling in the Postnatal Perivascular Progenitor Niche Drives Medulloblastoma Formation. Cancer Research 77 (1):123–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chow LM, Endersby R, Zhu X, Rankin S, Qu C, Zhang J, Broniscer A, Ellison DW, Baker SJ (2011) Cooperativity within and among Pten, p53, and Rb pathways induces high-grade astrocytoma in adult brain. Cancer Cell 19 (3):305–316 [DOI] [PMC free article] [PubMed] [Google Scholar]