Abstract
D-Arabinofuranose is a major glycosyl constituent of mycobacteria found in two essential cell envelope heteropolysaccharides, arabinogalactan and lipoarabinomannan. Seven different arabinosyltransferases at least are required to synthesize the arabinan domain of these two major glycans. Because of their interest from the perspective of drug development, these enzymes have been the object of intense investigations. In this chapter, we describe the protocols used to perform nonradioactive arabinosyltransferase assays with synthetic acceptor and donor substrates and characterize the enzymatic products of the reactions by mass spectrometry.
Keywords: arabinosyltransferase, D-arabinose, Mycobacteria, synthetic arabinoside acceptor, lipid donor
1. Introduction:
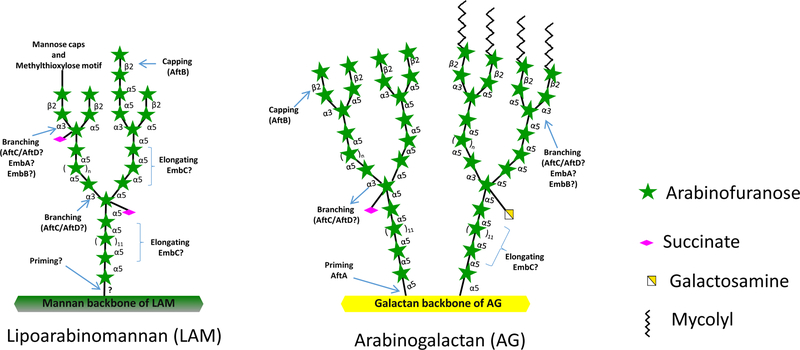
Mycobacterium tuberculosis causes tuberculosis (TB) in humans and kills about 1.7 million people annually. The emergence of multi-drug resistant M. tuberculosis strains threatens TB control (1). There is an unmet need for novel drugs to treat M. tuberculosis infections. Mycobacteria are known to produce a complex cell envelope which plays various important roles during infection and further forms an impermeable barrier to anti-TB drug treatment (2, 3). The unique cell envelope of mycobacteria is also the site of action of many anti-TB drugs and drug candidates (4). Two major D-arabinofuranose-containing heteropolysaccharides, arabinogalactan (AG) and lipoarabinomannan (LAM), found in the cell envelope of all mycobacteria are essential to the structural integrity of the bacilli (5). The structural organization of the arabinan domains of AG and LAM is highly conserved, consisting of three different glycosidic bonds viz α-(1,5), α-(1,3) and β-(1,2) (5) whose introduction in the course of the elongation of the two glycans is coordinated by a series of linkage-specific arabinosyltransferases (Fig. 1). The arabinosyltransferases AftA, AftB, AftC, AftD, EmbA and EmbB are involved in AG biosynthesis (6–11) whereas AftB, AftC, AftD and EmbC participate in the biosynthesis of the arabinan domain of LAM (10–13) (Fig. 1). Although EmbA, EmbB and EmbC are known targets of the front-line anti-TB drug, ethambutol, the enzymatic activities of these proteins have not yet been directly demonstrated in vitro. All mycobacterial arabinosyltransferases identified to date utilize decaprenylphosphoryl-β-D-arabinose (DPA) (Fig. 2a) as sole arabinose donor (14). The catalytic steps leading to DPA synthesis have been elucidated and shown to be targeted by the TB drug candidates benzothiazinones and dinitrobenzamides (15–16). Thus, both the DPA biosynthetic enzymes and arabinosyltransferases involved in AG and LAM biosynthesis are validated targets for the development of new anti-TB drugs. Up til now, the in vitro characterization of mycobacterial arabinosyltransferases has primarily relied on the use of rather cumbersome radioactive assays using mycobacterial lysates as enzyme source to monitor the transfer of 14C-labeled arabinose from DPA onto synthetic neoglycolipid acceptors (17). 14C-labeled DPA is generated in situ from 5-phosphoribosyl pyrophosphate p[14C]Rpp (18) and product formation is monitored by thin-layer chromatography and autoradiography upon partial purification (17). The synthetic neoglycolipid acceptors mimic the native structural organization of the arabinan domains of AG and LAM with their aglycon chain decreasing their polarity to allow their partitioning in organic solvents. While relatively straightforward, the radioactive nature of the enzymatic products generated by these assays limits their further structural characterization. We describe here a non-radioactive method to measure arabinosyltransferase activity that makes use of the same synthetic arabinoside acceptors and non-radiolabeled arabinose donor substrate(s) (Fig. 2). The enzymatic reaction products formed are extracted with ethanol and separated from interfering compounds using a strong anion exchange column. The partially purified enzymatic products are then permethylated and analyzed using liquid chromatography (LC) - mass spectrometry (MS)-based methods. Compared to radioactive assays, the nonradioactive LC-MS-based assays described herein not only offer the advantage of not generating any radioactive waste but also allow for the separation of the synthetic arabinose acceptors from their enzymatic products. The purified enzymatic products may be eluted at a preparative scale and their structure subsequently characterized by mass fragmentation (LC-MS/MS). Alternatively, the permethylated eluted products may be derivatized into permethylated alditol acetates to confirm the linkage of the enzymatically-transferred arabinosyl residue (21). Importantly, multiple glycosyltransferase activities on the same acceptor substrate may be analyzed in a single LC-MS run.
Figure 1.
Arabinosyltransferases involved in the biosynthesis of the arabinan domains of lipoarabinomannan (left panel) and arabinogalactan (right panel) in M. tuberculosis.
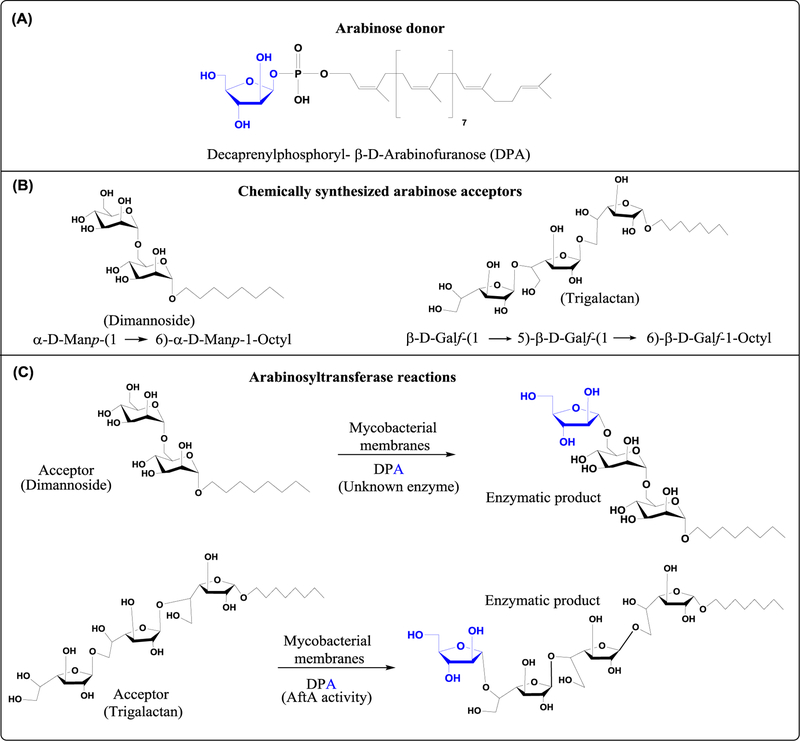
Figure 2. Structures of the synthetic acceptor and donor substrates used in the arabinosyltransferase reactions described in this chapter.
(A) Chemical structure of decaprenylphosphoryl-β-D-arabinose (DPA), the only known arabinose donor in mycobacteria.
(B) Structures of synthetic dimannoside (α-D-Manp-(1→6)- α-D-Manp-O(CH2)7CH3) and trigalactan (β-D-Galf-(1→5)-β-D-Galf-(1→6)-β-D-Galf-O(CH2)7CH3) acceptor substrates.
(C) Schematic representation of arabinosyltransferase reactions using M. smegmatis membranes as enzyme source, DPA as the arabinose donor and the synthetic dimannoside and trigalactan acceptors shown in (B).
2. Materials
2.1. Bacterial growth conditions
Frozen stock of M. smegmatis mc2155 (American Type culture collection). (Note 1)
Luria Bertani (LB) agar medium (Difco).
Luria Bertani (LB) broth medium (Difco).
Phosphate buffer saline pH 7.0.
37°C incubator with shaking.
250 mL harvesting bottles.
Bench top centrifuge.
2.2. Preparation of enzymatically active membrane fractions
Endotoxin free water.
Buffer A: 50 mM MOPS (pH 8.0 with KOH), 5 mM 2-mercaptoethanol and 10 mM MgCl2 (Buffer A should be kept at 4°C at all times).
Probe tip sonicator (Soniprep 150; MSE Ltd., Crawley Sussex, UK; 1-cm probe).
Oak Ridge round-bottom FEP tubes (30 ml).
Bench top centrifuge.
Ultra-centrifuge.
1 ml and 7 ml Dounce glass homogenizers.
Bicinchoninic acid (BCA) protein estimation kit (Pierce).
Spectrophotometer.
2.3. Arabinosyltransferase assay
Buffer B: 50 mM MOPS (adjust pH 8.0 with KOH), 5 mM 2-mercaptoethanol, 10 mM MgCl2 and 1 mM ATP.
Chemically synthesized α-D-Manp-(1→6)-α-D-Manp-1-Octyl (dimannoside) as arabinose acceptor (20). (Note 2)
Chemically synthesized β-D-Galf-(1→5)-β-D-Galf-(1→6)-β-D-Galf-1-Octyl (trigalactan) as arabinose acceptor (7). (Note 2)
Chemically synthesized decaprenylphosphoryl-β-D-arabinose (DPA) as arabinose donor (19). (Note 3)
Anhydrous ethyl alcohol, absolute.
37 °C water bath.
Bench top centrifuge.
HyperSep strong anion exchange (SAX) columns (60108–521 Thermo Scientific).
Vacuum concentrator, speed - vac (Savant).
Teflon-lined screw capped 13 × 100 mm glass tubes (Hot air oven dried).
2.4. Permethylation of enzymatic products
Vacuum concentrator, speed - vac (Savant).
Dried sample.
Anhydrous DMSO (MX 1457–7, Millipore Sigma).
NaOH pellets (306576–25G, Sigma).
Iodomethane (Sigma).
Water bath sonicator.
Endotoxin free water.
Mortar and Pestle (Hot air oven dried).
Glass syringe with needle.
Ice cold deionized water.
Teflon-lined screw capped 100 × 13 mm glass tubes (Hot air oven dried).
2.5. LC-MS Analysis
Water
Methanol
Formic acid
Mass spectrometer: Agilent 6220A time-of-flight (TOF) mass spectrometer equipped with an electrospray ionization/atmospheric pressure chemical ionization (ESI/APCI) multimode source.
Liquid chromatography: Agilent 1200 with binary pump and autosampler (Agilent technologies; Palo Alto, CA).
HPLC Column: 2.1 mm inner diameter (ID) X 150 mm, 3.5 µm X-Bridge reverse phase C18 column (Waters Corp.; Milford, MA).
Solvent A: LCMS-grade water with 0.1 % formic acid.
Solvent B: LC-MS-grade methanol with 0.1 % formic acid.
2 mL autosampler vials.
250 µL glass inserts with springs.
3. Methods
3.1. Bacterial growth conditions
Grow a M. smegmatis preculture from frozen stock by spreading on LB agar plate and incubating at 37 °C until a lawn has formed.
Aseptically transfer the entire plate of M. smegmatis into 2 L of LB broth and incubate at 37 °C with shaking for 48–72 h to obtain optimal biomass (Note 4).
Harvest cells by centrifugation at 3,500 rpm for 15 min at 4°C in a 250 mL conical tube.
Resuspend the harvested cell pellet with phosphate buffered saline (PBS) pH 7.0 and repeat centrifugation at 3,500 rpm for 15 min at 4°C.
Store PBS-washed cells at −80 °C.
3.2. Preparation of enzymatically active membrane fractions
All of the following steps are to be carried out on ice.
Thaw frozen M. smegmatis cells (10 g wet weight) on ice for 10 min and add pre-chilled 30 ml of buffer A to the thawed cell pellet. Vortex every 30 sec to create a homogeneous solution.
Transfer 30 mL of suspension to the sonication tube and break the cells using a 1-cm probe tip sonicator at 50% output for 10 cycles consisting of 60 sec pulses with 90 sec cooling intervals between pulses.
Transfer cell lysates into pre-chilled Oakridge round-bottom tubes on ice and remove the cell wall material and unbroken cells by centrifugation at 27,000 x g for 60 min at 4°C.
Carefully transfer the supernatant containing the membrane proteins to ultracentrifuge tubes and spin at 100,000 x g for 2 h at 4°C.
Discard the supernatant containing cytosolic proteins and resuspend the pellet containing the enzymatically active membrane fraction in 0.5 – 1.0 mL of Buffer A with gentle homogenization using a dounce homogenizer. Prepare aliquots of 50–100 µL of homogenized membranes and store until further use at −80 °C (Note 5).
Measure membrane proteins concentration using the Pierce BCA protein assay kit as described by the manufacturer (Note 6).
3.3. Arabinosyltransferase assay
The typical arabinosyltransferase reaction mixture contains the following in a total volume of 200 µL: buffer B, 200 µM acceptor substrate, 1 mM donor substrate, 0.5 mg of M. smegmatis membrane proteins (Note 7).
As a negative control, perform an enzymatic reaction lacking the acceptor substrate.
Incubate the enzymatic reaction mixtures at 37°C in a water bath for 2 h (Note 8).
After 2 h incubation, stop the enzymatic reaction by adding 200 µL of 100 % ethanol and vortex briefly.
Centrifuge the tube at 14,000 rpm for 10 min to clarify the mixture and transfer the supernatant containing the enzymatic product(s) into a clean Eppendorf tube.
Equilibrate a strong anion exchange (SAX) column with 5 mL of water, according to the manufacturer’s instructions.
Load the sample into the SAX column and elute with 5 mL 50 % ethanol.
Collect the flowthrough and the eluate in the same tube.
Transfer the eluate containing the enzymatic product(s) into clean 13 × 100 mm glass tube and dry in a savant.
3.4. Permethylation of the enzymatic reaction products for LC-MS analysis
The following permethylation procedure is to be performed inside a chemical hood.
Completely dry the extracted reaction products into a Teflon-lined screw capped glass tubes on the savant.
Add 100 µL of methanol and dry under nitrogen.
Add 100 µL DMSO, sonicate and allow to stand at room temperature for 10 min.
Mark a Pasteur pipet with 0.5 mL volume.
Place a small magnetic stir bar into each sample tubes.
NaOH/DMSO slurry: Place five NaOH pellets in a dry mortar and transfer 3 mL anhydrous DMSO into the mortar using a glass syringe. With a pestle, grind the NaOH pellets until a slurry is formed (Note 9).
Quickly add 0.5 mL of the NaOH/DMSO slurry directly on the sample without touching the side walls of the glass tube using a Pasteur pipet (0.5 ml marked), Cap the tubes and sonicate in water bath for 5 min.
Add 0.5 mL methyl iodide. Cap and allow to stir on a magnetic stir plate for 30 min at room temperature. The sample should look as a milky white slurry.
Add 200 uL of methyl iodide to the same tube and allow it to stir for another 30 min.
Quench the methylation reactions (exothermic) by dropwise addition of 1 mL ice cold water.
Add 2 mL of chloroform, vortex well and allow the mixture to settle into two layers.
Extract the permethylated products by spinning at 3,000 rpm for 5 min at room temperature.
Carefully remove the upper water layer and wash the bottom chloroform phase twice with 1ml water (Note 10).
Carefully transfer the lower chloroform layer containing the enzymatic product(s) into clean 13 × 100 mm glass tube and evaporate under a stream of nitrogen.
3.5. LC-MS analysis:
Resuspend the dried sample containing the enzymatic product(s) in 100 µL of methanol; briefly vortex and centrifuge at 3,000 rpm for 5 min at room temperature.
Using a Pasteur pipet, transfer the 100 µL methanol suspension into a autosampler vial with small volume insert.
Analyze the permethylated samples by LC-MS using an Agilent 6220A time-of-flight (TOF) mass spectrometer. Instrument parameters for the ion source: Gas temperature of 310°C, vaporizer drying gas flow of 10 L/min; nebulizer pressure of 45 psig, capillary voltage of 2,000 V. Acquire the scanning signals in positive ion mode. Select a mass range between m/z 115–2500 with a scanning rate of 1.32 spectra/sec.
Conditions for column chromatography: Set the column flow rate at 0.320 mL/min and column temperature to 45°C. Set initial first min at 90% solvent A and 10% solvent B. Then, ramp solvent B from 10 to 100% over 20 min and hold at 100% solvent B for 4 min. After 24 min, bring back to 10% solvent B in 0.10 min.
Set the post run to 10 min using 90% solvent A.
After all samples have been run, rinse the column prior to storage with 100% acetonitrile for 30 min at a flow rate of 0.5 mL/min.
Prior to running analytical samples, calibrate mass spectrometer in positive mode using the Agilent ESI-L tune mix.
Set the autosampler to inject 1 µL of permethylated reaction products into the LC-MS.
For LC-MS data analysis use the MassHunter Workstation Software Qualitative Analysis version B. 07.00.
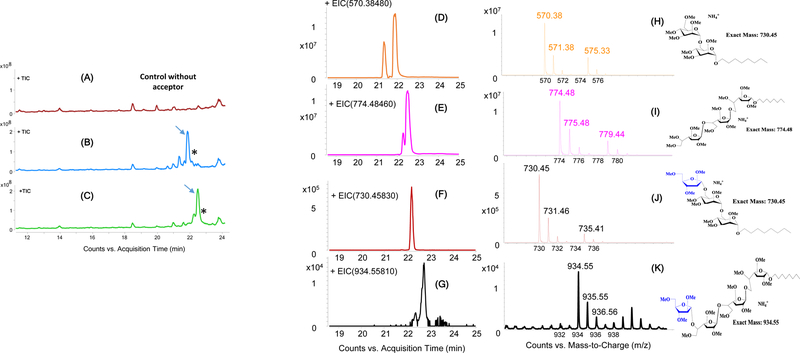
Figure 3. LC-MS analysis of permethylated reaction products from in vitro cell-free arabinosyltransferase reactions.
The arabinose acceptors shown in Fig. 2B and their enzymatic products are mostly detected as their ammonium adducts [M+NH4]+ in the positive ion mode. (A–C) Total ion chromatograms. The two major peaks indicated with an arrow correspond to the dimannoside (B) and trigalactan (C) acceptors. These peaks are lacking in the control reaction devoid of acceptor substrate (A). The asterisks indicate their corresponding enzymatic products.
Elution profiles (D–G) and extracted ion chromatograms (H–K). The elution profiles of the dimannoside acceptor (m/z 570.38) at 21.9 min (D) and trigalactan acceptor (m/z 770.48) at 22.4 min (E) are shown. Two new peaks with retention times 22.2 min (F) and 22.7 min (G) correspond to the addition of an arabinosyl residue to the dimannoside and trigalactan acceptors, respectively. The presence of ions with m/z 730.45 (J) and 934.55 (K) further confirms the enzymatic transfer of a single arabinosyl residue onto the synthetic dimannoside and trigalactan acceptors, respectively. The extracted ion chromatograms of the two acceptor substrates are shown in panels H and I. The chemical structures of the acceptors and their possible enzymatic products are shown alongside their corresponding extracted ion chromatograms.
Acknowledgements
Studies on mycobacterial arabinosyltransferases in the authors’ laboratory are supported by the National Institute of Allergy and Infectious Diseases (NIAID) / National Institutes of Health (NIH) grant AI064798. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Notes
Mycobacterium smegmatis is a non-pathogenic (BSL-2) fast-growing mycobacterial species. The structural organization and biosynthesis of the arabinan domains of AG and LAM is highly conserved across the Mycobacterium genus. For these reasons, M. smegmatis is often used as a surrogate model of M. tuberculosis to study arabinosyltransferase activities.
For the purpose of this chapter, we chose to present two examples of arabinosyl transfer reactions present in mycobacteria. One uses synthetic α-D-Manp-(1→6)-α-D-Manp-1-Octyl (dimannoside) (19) as the acceptor substrate and the other β-D-Galf-(1→5)-β-D-Galf-(1→6)-β-D-Galf-1-Octyl (trigalactan) (7) (Fig. 2).
Other functional arabinose donors harboring shorter acyl chains have also been reported (22).
Avoid adding Tween-80 to the LB broth as this detergent may interfere with LC-MS analysis.
Membranes may be stored at −80 °C. However, for best enzyme activity, it is recommended to use fresh membranes. Indeed, the freeze-thawing of membranes may lead to loss of enzymatic activity.
Usually 10 g of wet weight M. smegmatis cells yield nearly 15–20 mg membrane proteins.
DPA (stored in chloroform:methanol (2:1, by vol.)) is quickly evaporated to dryness under a stream of nitrogen in an Eppendorf tube. The dried DPA is then resuspended in 2% DMSO (final concentration) by gentle pipetting followed by a brief water bath sonication. Then add the remaining components of the arabinosyltransferase reaction mixture.
To increase product yield, it is recommended to perform overnight incubations by replenishing the reaction mixture with fresh membranes every 4 to 6 h.
Avoid weighing the NaOH pellets. They are very hygroscopic and presence of water negatively impacts the methylation reaction.
The water layer contains unreacted methyl iodide. It is a toxic chemical and it should be discarded as hazardous waste.
References:
- 1.World Health Organization. Global Tuberculosis Report 2016, WHO Press. [Google Scholar]
- 2.Queiroz A, and Riley LW (2017) Bacterial immunostat: Mycobacterium tuberculosis lipids and their role in the host immune response, Rev Soc Bras Med Trop 50, 9–18. [DOI] [PubMed] [Google Scholar]
- 3.Brennan PJ, and Nikaido H (1995) The envelope of mycobacteria, Annu Rev Biochem 64, 29–63. [DOI] [PubMed] [Google Scholar]
- 4.Lohrasbi V, Talebi M, et al. (2017) Trends in the discovery of new drugs for Mycobacterium tuberculosis therapy with a glance at resistance, Tuberculosis [DOI] [PubMed]
- 5.Angala SK, Belardinelli JM, et al. (2014) The cell envelope glycoconjugates of Mycobacterium tuberculosis, Crit Rev Biochem Mol Biol 49, 361–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alderwick LJ, Seidel M, et al. (2006) Identification of a Novel Arabinofuranosyltransferase (AftA) Involved in Cell Wall Arabinan Biosynthesis in Mycobacterium tuberculosis, J Biol Chem 281, 15653–15661. [DOI] [PubMed] [Google Scholar]
- 7.Shi L, Zhou R, Liu Z, et al. (2008) Transfer of the First Arabinofuranose Residue to Galactan Is Essential for Mycobacterium smegmatis Viability, J Bact 190, 5248–5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seidel M, Alderwick LJ, Birch, et al. (2007) Identification of a novel arabinofuranosyltransferase AftB involved in a terminal step of cell wall arabinan biosynthesis in Corynebacterianeae, such as Corynebacterium glutamicum and Mycobacterium tuberculosis, J Biol Chem 282, 14729–14740. [DOI] [PubMed] [Google Scholar]
- 9.Birch HL, Alderwick LJ, et al. (2008) Biosynthesis of mycobacterial arabinogalactan: identification of a novel α (1→ 3) arabinofuranosyltransferase, Mol Microbiol 69, 1191–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Škovierová H, Larrouy-Maumus G, et al. (2009) AftD, a novel essential arabinofuranosyltransferase from mycobacteria, Glycobiology 19, 1235–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Escuyer VE, Lety M-A, Torrelles JB, et al. (2001) The Role of the embA and embB Gene Products in the Biosynthesis of the Terminal Hexaarabinofuranosyl Motif of Mycobacterium smegmatis Arabinogalactan, J Biol Chem 276, 48854–48862. [DOI] [PubMed] [Google Scholar]
- 12.Jankute M, Alderwick LJ, et al. (2016) Disruption of mycobacterial AftB results in complete loss of terminal β (1→ 2) arabinofuranose residues of lipoarabinomannan, ACS chem Biol 12, 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alderwick LJ, Lloyd GS, et al. (2011) The C-terminal domain of the Arabinosyltransferase Mycobacterium tuberculosis EmbC is a lectin-like carbohydrate binding module, PLoS Pathog 7, e1001299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolucka BA, McNeil MR, et al. (1994) Recognition of the lipid intermediate for arabinogalactan/arabinomannan biosynthesis and its relation to the mode of action of ethambutol on mycobacteria, J Biol Chem 269, 23328–23335. [PubMed] [Google Scholar]
- 15.Makarov V, Manina G, et al. (2009) Benzothiazinones kill Mycobacterium tuberculosis by blocking arabinan synthesis, Science 324, 801–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christophe T, Jackson M, Jeon HK, et al. (2009) High content screening identifies decaprenyl-phosphoribose 2′ epimerase as a target for intracellular antimycobacterial inhibitors, PLoS Pathog 5, e1000645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee R, Mikusova K, et al. (1995) Synthesis of the arabinose Donor. beta.-d-arabinofuranosyl-1-monophosphoryldecaprenol, development of a basic arabinosyl-transferase assay, and identification of ethambutol as an arabinosyl transferase inhibitor, J Am Chem Soc 117, 11829–11832. [Google Scholar]
- 18.Scherman MS, Kalbe-Bournonville L, et al. (1996) Polyprenylphosphate-pentoses in mycobacteria are synthesized from 5-phosphoribose pyrophosphate, J Biol Chem 271, 29652–29658. [DOI] [PubMed] [Google Scholar]
- 19.Angala SK, McNeil MR, et al. (2016) Identification of a Novel Mycobacterial Arabinosyltransferase Activity Which Adds an Arabinosyl Residue to alpha-D-Mannosyl Residues, ACS Chem Biol 11, 1518–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tam PH, Besra GS, and Lowary TL (2008) Exploring the Substrate Specificity of a Mycobacterial Polyprenol Monophosphomannose-Dependent α-(1→ 6)-Mannosyltransferase, ChemBioChem 9, 267–278. [DOI] [PubMed] [Google Scholar]
- 21.Liav A, Huang H, et al. (2006) Stereoselective synthesis of decaprenylphosphoryl β-D-arabinofuranose, Tetrahedron Lett 47, 545–547. [Google Scholar]
- 22.Zhang J, Angala SK, et al. (2011) Reconstitution of functional mycobacterial arabinosyltransferase AftC proteoliposome and assessment of decaprenylphosphorylarabinose analogues as arabinofuranosyl donors, ACS Chem Biol 6, 819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]