Abstract
Stroke is a major social and health problem posing heavy burden on national economies. We provided detailed financial data on the direct in-hospital cost of acute stroke care in Lebanon and evaluated its drivers. This was an observational, quantitative, prospective, multicenter, incidence-based, bottom-up cost-of-illness study. Medical and billing records of stroke patients admitted to 8 hospitals in Beirut over 1 year were analyzed. Direct medical costs were calculated, and cost drivers were assessed using a multivariable linear regression analysis. In total, 203 stroke patients were included (male: 58%; mean age: 68.8 ± 12.9 years). The direct in-hospital cost for all cases was US$1 413 069 for 2626 days (US$538 per in-hospital day). The average in-hospital cost per stroke patient was US$6961 ± 15 663. Hemorrhagic strokes were the most costly, transient ischemic attack being the least costly. Cost drivers were hospital length of stay, intensive care unit length of stay, type of stroke, stroke severity, modified Rankin Scale, third party payer, surgery, and infectious complications. Direct medical cost of acute stroke care represents high financial burden to Lebanese health system. Development of targeted public health policies and primary prevention activities need to take priority to minimize stroke admission in future and to contain this cost.
Keywords: cost of illness, hospital costs, stroke, Lebanon, prospective studies, health policy, incidence, regression analysis, humans
What do we already know about this topic?
Information about cost of stroke care is not well known in Arab counties, and to our knowledge, no published literature on cost of stroke care in Lebanon exists to date.
How does your research contribute to the field?
In this article, we provide detailed financial data on the direct in-hospital cost of acute stroke care in Lebanon and evaluate its drivers.
What are your research’s implications toward theory, practice, or policy?
Stroke creates considerable social and economic burden to individuals and society and resources tend to be gradually limited; therefore, we found very interesting results, indicating the need to reduce this cost by development and management of new public health policies and medical insurance action plans for stroke.
Introduction
Stroke is the second most frequent cause of death1,2 and the major cause of disability2,3 worldwide. Being a disease with long-term consequences, stroke creates considerable social and economic burden to individuals and society,3 resulting from its high prevalence, hospitalization rates, morbidity, and mortality.4 Worldwide, stroke consumes about 2% to 4% of total health care costs.2 In the United States, total annual costs of stroke are expected to increase by 129%, reaching US$240.67 billion by 2030.5 Taken the scarcity of health care resources, cost-of-illness (COI) studies in stroke care are needed to provide insights into the distribution of the cost and its impact on the national health care expenditure.6
Because investigations into economic impact of stroke are lacking in Lebanon, this study aimed to estimate cost of medical care during hospital admission and to identify important variables that influence the cost in Beirut hospitals.
Methods
This study received ethical approval from the institutional review board of each participating hospital. Signed informed consent was obtained from each patient or his caregiver after explaining the purpose and methods of the study.
Study Design
This is an observational, prospective, incidence-based, multicenter, COI study. Adult patients (⩾18 years) diagnosed with acute stroke or transient ischemic attack (TIA) (primary or recurrent) supported by computed tomography scan and/or magnetic resonance imaging were included in this study between August 2015 and August 2016 from 8 hospitals in Beirut: 6 private university hospitals, 1 private community hospital, and 1 public university hospital.
Stroke was defined according to the International Classification of Diseases, Tenth Revision, including subarachnoid hemorrhage (SAH), primary intracerebral hemorrhage (PICH), and cerebral infarction. TIA was defined as a brief episode of neurologic dysfunction resulting from focal temporary cerebral ischemia and not associated with cerebral infarction.7
Patients admitted after 7 days of symptoms onset or those who have difficulty accepting follow-up visits were excluded. Patients were also excluded if they were already dependent regarding activities of daily living (Barthel Index [BI] score ⩽85); suffering from severe pathologies with unfavorable 1-year prognosis; disabling and progressive neurological diseases; cognitive decline (score >1 on Heteroanamnesis list Cognition)8 before their stroke.
Data Collection
Patients demographic (sex, age), socioeconomic profile (housing situation, socioeconomic status, employment status, third party payer [TPP], education level), risk factors, medical history including medical treatments, laboratory and imaging data, complications, and rehabilitation therapy (physiotherapy and speech therapy) were collected at baseline and/or during hospitalization period. Current smokers were defined as persons who reported smoking at least 100 cigarettes during their lifetime and who, at the time they participated in the study, reported smoking every day or some days. Former smokers were defined as those who have smoked at least 100 cigarettes in their lifetime but who have quit smoking since a minimum of 28 days. A researcher pharmacist did the data collection.
Billing data were collected using a bottom-up approach. Costs of hospitalization of patients admitted to another hospital before being transferred to a participating hospital were also included. Costs were calculated according to the quantity of resources consumed by each patient from admission till discharge from hospital. The total direct medical cost per patient for each resource item was calculated as follows: total direct cost = ∑unit cost × resource use. The bills for each patient were provided by the hospitals’ administration including information related to cost of hospitalization, laboratory, radiology and cardiology-related investigations, medication, nursing charges, physicians fees, and rehabilitation services. Costs calculated in Lebanese Pound (LBP) were converted to US$ (exchange rate: US$1 = LBP 1508).9
Study Tools
Prestroke functional disability was defined according to BI at admission, while functional disability at discharge was assessed using modified Rankin Scale (mRS) and BI. Patients were divided into 3 groups according to their mRS score—independence (mRS = 0-2), dependence (mRS = 3-5), and death (mRS = 6)—and into 4 groups according to their BI—independence (96-100), mild dependence (75-95), moderate dependence (46-74), and severe dependence (0-45).3,4,10
National Institution of Health Stroke Scale (NIHSS) score was used to classified stroke severity at admission into 5 categories (0 = no stroke symptoms, 1-4 = minor stroke, 5-14 = moderate stroke, 15-20 = moderate/severe, and 21-42 = severe stroke).11
Patients were classified into 5 etiologic/pathophysiological categories according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST system)12 and into 4 different stroke locations (lacunar stroke syndrome [LACS], partial anterior circulation stroke [PACS], posterior circulation stroke [POCS], and total anterior circulation stroke [TACS]) according to Bamford Scale (BS).13 Patients’ assessment for the mRS, BI, NIHSS, and stroke diagnosis; classification; and locations were performed by neurologists or neurologist resident.
Statistical Analysis
Data were entered and analyzed using Statistical Package for the Social Sciences (SPSS), version 20.0 (IBM Corporation, Armonk, New York). Cost data entry was doubled checked. Two researchers audited 5% randomly selected questionnaires. Data entry showed high reliability (error rate <1%). Data were presented as means ± SDs, except financial data presented also as medians and ranges. In bivariate analyses, Pearson correlation coefficients (or Spearman) were used for 2 continuous quantitative variables, Student test (or Mann-Whitney) for means comparison between 2 groups (for quantitative variables), and chi-square test (or Fisher exact test) for comparing percentages (for nominal, ordinal, and categorical variables) were used. ANOVA (analysis of variance) test (or Kruskal-Wallis) was used to compare between-group differences, followed by Bonferroni post hoc test when a significant difference was obtained. P ⩽ .05 indicated statistical significance. Bivariate analysis was done for the following dependent variables: length of stay (LOS), intensive care unit (ICU) LOS, and cost.
Predictors of total hospital cost (all stroke type and ischemic stroke [IS] only) and LOS were determined through multivariable stepwise linear regressions controlling for potential confounders, after ensuring sample and conditions adequacy. Logistic transformation ln(cost of stroke) and ln(LOS) were performed as their distributions were skewed. Transformed data were normally distributed and were entered in each model as dependent variable. Independent variables with P < 0.2 in the bivariate analysis were entered into the models. Regression was checked for collinearity (variance inflation factors [VIF] < 10 indicated noncollinearity). Confounders (age and sex) were entered to the model as independent variables.
Results
Demographic and Clinical Characteristics
In this study, 203 patients were enrolled (mean age: 69 ± 13 years, men: 58%) (Table 1). Approximately 5% of eligible patients did not give their written consent and were therefore excluded from the study. The mean LOS was 13 ± 18 days. More than 50% were admitted to an ICU with a mean LOS of 6 ± 13 days (Table 2).
Table 1.
Demographic Characteristics.
| All (n = 203; 100%) |
IS (n = 161; 79.3%) |
TIA (n = 12; 5.9%) |
PICH (n = 14; 6.9%) |
SAH (n = 16; 7.9%) |
P value | |
|---|---|---|---|---|---|---|
| Age, y, mean ± SD | 68.8 ± 12.9 | 70.3 ± 12.3 | 62.3 ± 16.0 | 72.6 ± 9.4 | 55.0 ± 9.9 | <.001a |
| Gender: Male, n (%) | 117 (57.6%) | 96 (59.6%) | 8 (66.7%) | 9 (64.3%) | 4 (25.0%) | .048 |
| TPP | NS | |||||
| Public | 166 (81.8%) | 129 (80.1%) | 11 (91.7%) | 11 (78.6%) | 15 (93.8%) | |
| Private | 37 (18.2%) | 32 (19.9%) | 1 (8.3%) | 3 (21.4%) | 1 (6.3%) | |
| Marital status | NSb | |||||
| Single/divorced | 19 (9.4%) | 13 (8.1%) | 1 (8.3%) | 3 (21.4%) | 2 (12.5%) | |
| Widowed | 62 (30.5%) | 54 (33.5%) | 2 (16.7%) | 2 (14.3%) | 4 (25.0%) | |
| Married | 122 (60.1%) | 94 (58.4%) | 9 (75.0%) | 9 (64.3%) | 10 (62.5%) | |
| Education | NSb | |||||
| Illiterate | 37 (18.2%) | 34 (21.1%) | 1 (8.3%) | 1 (7.1%) | 1 (6.3%) | |
| Elementary | 86 (42.4%) | 67 (41.6%) | 4 (33.3%) | 7 (50.0%) | 8 (50.0%) | |
| Secondary | 35 (17.2%) | 27 (16.8%) | 4 (33.3%) | 2 (14.3%) | 2 (12.5%) | |
| ⩾High school | 45 (22.2%) | 33 (20.5%) | 3 (25.0%) | 4 (28.5%) | 5 (31.3%) | |
| Professional condition | NSb | |||||
| Employed | 61 (30.0%) | 46 (28.6%) | 5 (41.7%) | 6 (42.9%) | 4 (25.0%) | |
| Housewife | 82 (40.4%) | 63 (39.1%) | 4 (33.3%) | 4 (28.6%) | 11 (68.8%) | |
| Retired | 21 (10.3%) | 16 (9.9%) | 1 (8.3%) | 4 (28.6%) | 0 (0%) | |
| Unemployed | 39 (19.2%) | 36 (22.4%) | 2 (16.7%) | 0 (0%) | 1 (6.3%) | |
| Monthly home income (US$) | NSb | |||||
| <500 | 60 (29.6%) | 52 (32.3%) | 2 (16.7%) | 2 (14.3%) | 4 (25.0%) | |
| [500-1000] | 62 (30.5%) | 48 (29.8%) | 5 (41.7%) | 5 (35.7%) | 4 (25.0%) | |
| [1000-1500] | 37 (18.2%) | 26 (16.1%) | 2 (16.7%) | 3 (21.4%) | 6 (37.5%) | |
| >1500 | 44 (21.7%) | 35 (21.7%) | 3 (25.0%) | 4 (28.6%) | 2 (12.5%) |
Note. IS = ischemic stroke; TIA = transit ischemic attack; PICH = primary intracerebral hemorrhage; SAH = subarachnoid hemorrhage; TPP = third party payer.
SAH vs PICH and SAH vs IS.
Nonparametric test.
Table 2.
Clinical Characteristics.
| All (n = 203; 100%) |
IS (n = 161; 79.3%) |
TIA (n = 12; 5.9%) |
PICH (n = 14; 6.9%) |
SAH (n = 16; 7.9%) |
P value | |
|---|---|---|---|---|---|---|
| Risk factors | ||||||
| Hypertension | 153 (75.7%) | 126 (78.3%) | 10 (83.3%) | 10 (76.9%) | 7 (43.8%) | .020 |
| Dyslipidemia | 76 (37.6%) | 60 (37.3%) | 9 (75.0%) | 4 (30.8%) | 3 (18.8%) | .020 |
| Diabetes mellitus | 83 (41.1%) | 70 (43.5%) | 4 (33.3%) | 7 (58.3%) | 2 (12.5%) | NS |
| Atrial fibrillation | 26 (12.9%) | 22 (13.7%) | 3 (25.0%) | 1 (7.7%) | 0 (0%) | NS |
| Smoker | NSa | |||||
| Former smoker | 31 (15.3%) | 24 (14.9%) | 3 (25.0%) | 4 (28.6%) | 0 (0%) | |
| Current smoker | 102 (50.5%) | 82 (50.9%) | 5 (41.7%) | 4 (28.6%) | 11 (68.8%) | |
| First ever stroke/TIA | 171 (84.2%) | 132 (82.0%) | 11 (91.7%) | 12 (85.7%) | 0 (0%) | NS |
| Prestroke BI (mean ± SD) | 98.7 ± 3.0 | 98.8 ± 3.0 | 98.3 ± 3.3 | 97.9 ± 3.8 | 99.4 ± 1.7 | NS |
| NIHSS on admission (mean ± SD) | 10.8 ± 9.9 | 10.0 ± 8.6 | 0.7 ± 1.0 | 19.4 ± 12.3 | 19.7 ± 12.7 | <.001ab |
| LOS (mean ± SD) | 12.9 ± 18.5 | 9.9 ± 8.8 | 3.4 ± 1.6 | 37.4 ± 46.9 | 30.1 ± 28.3 | <.001ac |
| ICU admission | 107 (52.7%) | 78 (48.4%) | 1 (8.3%) | 12 (85.7%) | 16 (100%) | <.001 |
| ICU LOS (mean ± SD) | 5.9 ± 13.2 | 3.6 ± 6.8 | 0.2 ± 0.6 | 20.2 ± 32.2 | 20.8 ± 20.9 | <.001ad |
| mRS at discharge (mean ± SD) | 3.5 ± 2.0 | 3.5 ± 1.9 | 0.6 ± 0.9 | 4.5 ± 1.8 | 4.6 ± 1.1 | <.001ad |
| BI at discharge (mean ± SD) | 58.6 ± 38.8 | 58.5 ± 38.1 | 97.9 ± 4.5 | 38.0 ± 43.8 | 38.5 ± 35.1 | <.001ad |
| TOAST classification | .014a | |||||
| LA | 37 (21.4%) | 36 (22.4%) | 1 (8.3%) | |||
| CE | 55 (31.8%) | 53 (32.9%) | 2 (16.7%) | |||
| SV | 27 (15.6%) | 27 (16.8%) | 0 (0%) | |||
| UC | 54 (31.2%) | 45 (27.9%) | 9 (75.0%) | |||
| Discharge destination | <.001a | |||||
| Home | 72 (35.5%) | 56 (34.8%) | 12 (100%) | 3 (21.4%) | 1 (6.3%) | |
| Home with help | 82 (40.4%) | 69 (42.9%) | 0 (0%) | 5 (35.7%) | 8 (50.0%) | |
| Rehabilitation center/nursing home | 22 (10.8%) | 16 (9.9%) | 0 (0%) | 2 (14.3%) | 4 (25.0%) | |
| Death | 27 (13.3%) | 20 (12.4%) | 0 (0%) | 4 (28.6%) | 3 (18.8%) | |
| Cost (US$) | <.001ae | |||||
| (mean ± SD)/ | 6961 ± 15663 | 4248 ± 4352 | 1277 ± 492 | 26 698 ± 50 400 | 21 257 ± 14 625 | |
| Median (25th-75th) | 2751 (1484-6396) | 2578 (1492-5436) | 1234 (947-1436) | 8028 (2382-28462) | 14 746 (12066-23957) | |
Note. IS = ischemic stroke; TIA = transit ischemic attack; PICH = primary intracerebral hemorrhage; SAH = subarachnoid hemorrhage; BI = Barthel Index; NIHSS= National Institution of Health Stroke Scale; LOS = length of stay; ICU = intensive care unit; mRS = modified Rankin Scale; TOAST = Trial of Org 10172 in Acute Stroke Treatment; LA = large-artery atherosclerosis; CE = cardioembolism; SV = small-vessel occlusion; UC = unclassified.
Nonparametric test.
Except SAH vs PICH.
Except SAH vs PICH and TIA vs IS.
TIA vs IS; PICH and SAH.
Except SAH vs PICH.
The mean NIHSS at admission was 11 ± 10 and 30% of the patients had an NIHSS ⩾ 15. Around 79% had an IS (22% due to large-artery atherosclerosis [LA], 33% cardioembolism [CE], 17% small-vessel occlusion [SV], and 28% unclassified [UC]), 6.9% had a PICH, 7.9% had a SAH, and 5.9% had a TIA. According to Bamford classification, the major affected territory was PACS (60%) (Table 2).
The mean mRS and BI scores at discharge were 3.5 ± 2.0 and 58.6 ± 38.8, respectively, and 30.0% of patients were independent at discharge (Table 2).
Patients with hemorrhage had more severe neurological deficits on admission, stayed longer in-hospital, required more ICU admissions, and had higher mortality rate; the survivors had worse functional outcome at discharge (Table 2).
Direct Cost of Stroke
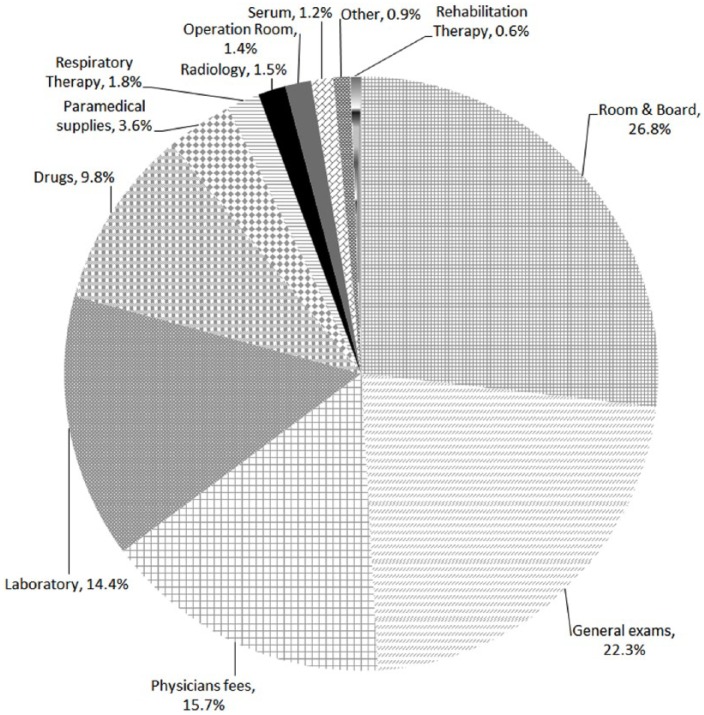
The direct in-hospital cost for all cases was US$1 413 069 for a total stay of 2626 days (US$538 per in-hospital day). The average cost per stroke patient was US$6961 ± 15 663. Of the total cost, 26.8% was attributed to the cost of room and board, 22.3% to general exams (including stroke and vascular imaging and cardiology-related investigations), 15.7% to physicians’ fees, 14.4% to laboratory tests, 14.6% to pharmacy, and 6.2% to other expenses (Figure 1).
Figure 1.
In-hospital cost distribution.
Predictors of Cost
Regarding stroke types, PICH were the most expensive (US$26 698 ± 50 400), followed by SAH (US$21 257 ± 14 625), which were significantly more expensive than IS (US$4248 ± 4352) and TIA (US$1277 ± 492) (Table 3).
Table 3.
Bivariable Analysis for Hospital LOS, ICU LOS and Cost of Stroke.
| N | LOS | ICU LOS | Cost (US$) | P valuea | |
|---|---|---|---|---|---|
| Total | 203 | 12.9 ± 18.5 | 5.9 ± 13.2 | 6961 ± 15663 | — |
| Type of stroke | <.001bc | ||||
| IS | 161 | 9.8 ± 8.8 | 3.6 ± 6.8 | 4248 ± 4352 | |
| TIA | 12 | 3.4 ± 1.6 | 0.2 ± 0.6 | 1277 ± 492 | |
| PICH | 14 | 37.3 ± 46.9 | 20.2 ± 32.2 | 26 698 ± 50 400 | |
| SAH | 16 | 30.1 ± 28.3 | 20.8 ± 20.9 | 21 257 ± 14 625 | |
| NIHSS | <.001bd | ||||
| No stroke symptoms | 13 | 5.1 ± 4.7 | 2.5 ± 4.7 | 3049 ± 3764 | |
| Minor stroke | 63 | 4.8 ± 3.0 | 0.7 ± 1.7 | 2372 ± 2214 | |
| Moderate stroke | 67 | 11.1 ± 16.0 | 2.8 ± 3.8 | 4451 ± 5129 | |
| Moderate/severe stroke | 18 | 14.7 ± 10.9 | 7.8 ± 10.3 | 7049 ± 5764 | |
| Severe stroke | 42 | 29.7 ± 28.0 | 18.7 ± 23.4 | 19 021 ± 30 734 | |
| mRS | <.001b | ||||
| Independent | 61 | 4.4 ± 2.8 | 0.8 ± 2.5 | 1971 ± 1741 | |
| Dependent | 115 | 14.9 ± 18.4 | 5.6 ± 9.1 | 7195 ± 9647 | |
| Dead | 27 | 23.7 ± 29.0 | 18.6±27.2 | 17 237 ± 36 370 | |
| BI | <.001e | ||||
| Independence | 49 | 4.3 ± 2.5 | 0.6 ± 1.9 | 1853 ± 1263 | |
| Mild dependence | 32 | 5.2 ± 3.3 | 1.4 ± 3.2 | 2405 ± 2070 | |
| Moderate dependence | 30 | 8.3 ± 5.0 | 3.3 ± 4.2 | 4779 ± 4762 | |
| Severe dependence | 65 | 20.9 ± 22.4 | 7.9 ± 11.2 | 9793 ± 11 753 | |
| BS | <.001fg | ||||
| LACS | 27 | 5.0 ± 2.8 | 0.4 ± 0.9 | 1827 ± 1092 | |
| POCS | 31 | 10.5 ± 8.6 | 5.6 ± 8.7 | 4365 ± 4188 | |
| TACS | 5 | 13.6 ± 6.6 | 7.4 ± 3.5 | 5732 ± 1819 | |
| PACS | 96 | 10.9 ± 9.8 | 3.6 ± 6.9 | 4896 ± 4854 | |
| POCS+PACS | 2 | 4.0 ± 1.4 | 0 ± 0 | 1546 ± 107 | |
| First ever vs recurrent stroke | NS | ||||
| First ever | 171 | 13.8 ± 19.9 | 6.5 ± 14.2 | 7495 ± 16 975 | |
| Recurrent | 32 | 8.6 ± 5.2 | 2.7 ± 4.1 | 4107 ± 2914 | |
| Infection status | <.001 | ||||
| Infection(–) | 141 | 6.6 ± 4.9 | 2.1 ± 3.9 | 3192 ± 3459 | |
| Infection(+) | 62 | 27.2 ± 27.8 | 14.5 ± 20.8 | 15 532 ± 26 028 | |
| Surgery | <.001 | ||||
| Surgery(–) | 175 | 9.8 ± 13.2 | 3.4 ± 6.8 | 4335 ± 6421 | |
| Surgery(+) | 28 | 32.3 ± 31.4 | 21.3 ± 26.7 | 23 374 ± 35 295 | |
| Gender | .015h | ||||
| Male | 117 | 12.6 ± 20.8 | 5.6 ± 14.5 | 6624 ± 19 009 | |
| Female | 86 | 13.3 ± 14.8 | 6.2 ± 11.2 | 7419 ± 9462 | |
| TPP | .039h | ||||
| Private | 37 | 13.7 ± 19.6 | 5.9 ± 13.4 | 8583 ± 13 061 | |
| Public | 166 | 12.8 ± 18.3 | 5.6 ± 12.1 | 6599 ± 16 199 | |
| TOAST | <.001fi | ||||
| LA | 37 | 10.2 ± 8.9 | 2.6 ± 3.7 | 4166 ± 2721 | |
| CE | 55 | 12.7 ± 10.2 | 6.0 ± 8.4 | 6064 ± 5865 | |
| SV | 27 | 5.0 ± 2.8 | 0.4 ± 1.0 | 1827 ± 1092 | |
| UC | 54 | 7.6 ± 7.5 | 2.5 ± 7.0 | 3003 ± 3251 | |
| Discharge destination | <.001bj | ||||
| Home | 72 | 4.5 ± 2.7 | 0.7 ± 2.3 | 1950 ± 1607 | |
| Home with help | 82 | 12.5 ± 12.9 | 5.6 ± 9.5 | 6555 ± 8864 | |
| Rehabilitation center/nursing home | 22 | 23.7 ± 29.0 | 7.8 ± 9.5 | 12 258 ± 12 591 | |
| Death | 27 | 28.9 ± 30.3 | 18.6 ± 27.2 | 17 237 ± 36 370 |
Note. Data are presented as mean ± SD. LOS = length of stay; ICU = intensive care unit; IS = ischemic stroke; TIA = transit ischemic attack; PICH = primary Intracerebral hemorrhage; SAH = subarachnoid hemorrhage; NIHSS = National Institution of Health Stroke Scale; mRS = modified Rankin Scale; BI = Barthel Index; BS = Bamford Scale; LACS = lacunar stroke syndrome; POCS = posterior circulation stroke; TACS = total anterior circulation stroke; PACS = partial anterior circulation stroke; TPP = third party payer; TOAST = Trial of Org 10172 in Acute Stroke Treatment; LA = large-artery atherosclerosis; CE = cardioembolism; SV = small-vessel occlusion; UC = unclassified.
For ICU LOS, nonparametric tests were used as the distribution could not be normal. LOS and cost were treated as ln(LOS) and ln(cost), parametric tests were used unless noted.
Nonparametric test used (due to nonhomogeneity of variances).
NS for SAH vs PICH.
LOS: NS for no stroke symptoms vs minor stroke and moderate stroke vs moderate/severe stroke / ICU LOS: only for severe stroke vs everything else / Cost: NS for no stroke symptoms vs minor stroke and moderate stroke, moderate stroke vs moderate/severe stroke.
LOS and cost: NS for independence vs mild dependence / ICU LOS: NS for independence vs mild and moderate dependence and mild vs moderate dependence.
Nonparametric test used, except for LOS (due to its homogeneity of variances).
Only for LACS vs POCS, PACS and TACS.
Only for cost, NS for LOS and ICU LOS.
LOS: NS for SV vs UC, CE vs LA, and LA vs UC / ICU LOS: NS for LA vs SV, LA vs UC, SV vs UC / Cost: NS for LA vs UC and SV vs UC.
LOS and cost: NS for rehabilitation center/nursing home vs death / ICU LOS: NS for home with help vs rehabilitation center/nursing home.
Among IS subtypes, the mean total cost was significantly higher for CE (US$6064 ± 5865) compared with SV and UC (US$1827 ± 1092, P < .001; US$3003 ± 3251, P = .003), respectively. According to Bamford classification, LACS had a significantly lower cost than POCS, TACS, PACS (P = .008, .008, <.001, respectively) (Table 3).
Patients with infectious complications (ie, pneumonia, urinary tract infection), or who underwent surgical intervention (ie, coiling, shunt, craniotomy, endarterectomy, gastrostomy, tracheotomy) had a higher cost (P < .001 for both) (Table 3).
LOS and total cost positively correlated with stroke severity. Patients who survived a severe stroke stayed in-hospital longer and had higher costs compared with those with less severe strokes (P < .001 in both comparisons). The higher cost of severe strokes was also associated with greater ICU use. Deceased patients used significantly more resources than survivors (US$17 237 ± 36 370 vs US$9166 ± 11 388; P < .001) (Table 3).
Total hospital costs strongly correlated with LOS (r = .835, P < .001), and ICU LOS (r = .794, P < .001), and moderately with admission NIHSS, mRS, and BI discharge scores (r = .657, r = .657, r = −.634, respectively, P < .001). Total hospital costs did not significantly correlate with age (r = .052, P = .459), unless when SAH patients were excluded (r = .227, P = .002) (Table 3).
Total cost varied by discharge destination; those discharged to rehabilitation centers or nursing homes had a considerably higher cost than home and home with help (P < .001) (Table 3).
Hospital LOS, ICU LOS, private TPP, hemorrhagic stroke, increased stroke severity on admission, having a surgery, infectious complication occurrence, and high mRS score at discharge were independent predictors of increased total cost after accounting for confounding factors. ICU LOS accounted for 57% of the variance in total cost. Hospital LOS, ICU LOS, and private TPP independently correlated with higher cost in ISs. In addition, LA and CE strokes, compared with SV and UC, and low BI at discharge were predictors of increased total cost (Table 4).
Table 4.
Multivariable Linear Regression Analysis of Overall Hospital Length of Stay and Cost for All Type of Strokes and for Ischemic Strokes.
| All type of strokesa |
Ischemic strokesb |
Hospital LOSc |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables explained |
77.8% of the variance of total cost (ln(cost)) |
71.3% of the variance of total cost (ln(cost)) |
73.4% of the variance of total LOS (ln(LOS)) |
||||||||||||
| ANOVA |
<0.001 |
<0.001 |
<0.001 |
||||||||||||
| Independent variables | Unstandardized coefficients |
Standardized coefficients | P value | VIF | Unstandardized coefficients |
Standardized coefficients | P value | VIF | Unstandardized coefficients |
Standardized coefficients | P value | VIF | |||
| B | SD | B | SD | B | SD | ||||||||||
| (Constant) | 6.240 | 7.281 | 0.799 | ||||||||||||
| LOS | 0.013 | 0.003 | 0.218 | <.001 | 2.6 | 0.038 | 0.006 | 0.400 | <.001 | 1.9 | |||||
| ICU LOS | 0.026 | 0.008 | 0.213 | .001 | 3.2 | 0.061 | 0.010 | 0.351 | <.001 | 1.6 | 0.033 | 0.006 | 0.295 | <.001 | 2.1 |
| TPP | <.001 | 1.0 | <.001 | 1.0 | |||||||||||
| Public | − | − | − | − | − | − | |||||||||
| Private | 0.444 | 0.089 | 0.181 | 0.529 | 0.086 | 0.284 | |||||||||
| Surgery | 0.609 | 0.135 | 0.208 | <.001 | 1.7 | 0.416 | 0.129 | 0.155 | .001 | 1.5 | |||||
| Type of stroke IS+TIA PICH+SAH |
− 0.320 |
− 0.135 |
− 0.114 |
.019 | 1.8 | ||||||||||
| NIHSS at admission | 0.100 | 0.046 | 0.117 | .032 | 2.3 | 0.116 | 0.046 | 0.148 | .013 | 2.3 | |||||
| mRS at discharge | 0.109 | 0.026 | 0.207 | <.001 | 1.9 | 0.145 | 0.027 | 0.300 | <.001 | 2.1 | |||||
| Infectious complication | 0.264 | 0.105 | 0.116 | .013 | 1.7 | 0.376 | 0.104 | 0.180 | <.001 | 1.6 | |||||
| BI at discharge | −0.003 | 0.001 | −0.164 | .005 | 1.6 | ||||||||||
| TOAST classification LA/CE UC/SV |
− –0.207 |
− 0.073 |
− –0.142 |
.005 | 1.2 | ||||||||||
| Discharge destination Home (with or without help) Rehabilitation center/Nursing home/Death |
− 0.419 |
− 0.116 |
− 0.159 |
<.001 | 1.3 | ||||||||||
| Sex (male reference) | −0.160 | 0.074 | −0.090 | .033 | 1.2 | ||||||||||
Note. ANOVA = analysis of variance; VIF = variance inflation factors; LOS = length of stay; ICU = intensive care unit; TPP = third party payer; IS = ischemic stroke; TIA = Transit Ischemic Attack; PICH = primary Intracerebral hemorrhage; SAH = subarachnoid hemorrhage; NIHSS = National Institution of Health Stroke Scale; mRS = modified Rankin Scale; BI = Barthel Index; TOAST = Trial of Org 10172 in Acute Stroke Treatment; LA = large-artery atherosclerosis; CE = cardioembolism; SV = small-vessel occlusion; UC = unclassified; LACS = lacunar stroke syndrome; PACS = partial anterior circulation stroke; POCS = posterior circulation stroke; TACS = total anterior circulation stroke.
Dependent variable: ln(cost). Independent variables: LOS, ICU LOS, TPP, surgery, type of stroke, NIHSS at admission, mRS at discharge, infectious complication, BI at discharge, discharge destination, sex, age.
Dependent variable: ln(cost). Independent variables: LOS, ICU LOS, TPP, surgery, NIHSS at admission, mRS at discharge, infectious complication, BI at discharge, TOAST classification, discharge destination, sex, age, Bamford classification (LACS vs other).
Dependent variable: ln(LOS). Independent variables: ICU LOS, TPP, surgery, type of stroke, NIHSS at admission, mRS at discharge, infectious complication, BI at discharge, discharge destination, sex, age.
Predictors of LOS
Predictors of higher LOS were high NIHSS at admission, high mRS score at discharge, ICU LOS, having a surgery, infectious complication, discharge destination to a rehabilitation center or nursing home or death, and female gender (Table 4).
Discussion
To the best of our knowledge, this is the first COI study analyzing the direct cost of in-patient medical care due to stroke in Lebanon and evaluating its drivers. The average in-hospital cost per stroke patient was US$6961 ± 15 663. Cost drivers were LOS, stroke types, severity, etiology, complications, dependency level, and TPP.
Although a direct comparison is not possible, mean hospital cost per patient (US$6961 ± 15 663) was close to that reported from high-income countries (Greece: US$7130)14 or lower (USA: US$9688),15 yet it was higher than figures reported from middle and low-income countries (Turkey: US$1917,4 Pakistan: US$1578,16 Brazil: US$4687 for PICH and US$2174 for IS,17 Argentina: US$14 904 for PICH and US$4717 for IS18) (all costs were adjusted to 2015 US$ by purchasing power parities and consumer prices index).
The mean LOS for patients in this study was close to similar studies done in Turkey, Greece, and Sweden,4,14,19 but considerably shorter than that reported in several high-income countries,20,21 though Spanish and US centers have reported shorter LOS.22-24
As in other studies,16,22,25 hospital LOS accounted for a large proportion of the variance of total cost than other variables entered to the regression model. The costs for bed and staff accounted for more than a quarter of total cost. Thus, as it was expected, LOS was highly correlated, in a direct and linear relationship, with total cost. Our study confirms that cost of in-patient care is largely driven by LOS14,21; decreasing the LOS might reduce in-hospital costs.26 Investigating interventions aiming at decreasing LOS from the societal perspective on the long run are necessary to ensure that they do not simply result in shifting of costs to follow-up care, resulting from poor quality of care, more complications, or more frequent readmissions.
Of interest, cost for beds and staffs was lower than in high- and middle-income countries.4,17,20,22 This might be partly due to the considerably shorter hospitalization in our study. In contrast, the cost for imaging and laboratory was similar or higher than in high- and middle-income countries.4,20,22
In this study, 53% of the patients were initially admitted to ICU with a mean LOS of 6 days. These figures are close to those from Japan20 and a bit lower than Argentina and Brazil.17,18 Admission criteria to ICU were not clearly predefined and depended on physicians in charge and hospitals policy; patients with severely reduced level of consciousness, those who required continuous cardiac monitoring, and those with massive infarction were usually admitted. Further studies are needed to elucidate the role of the stoke unit in acute stroke as a cost-effective model of care among stroke patients in Lebanon and advocate its implementation if found to be cost-effective.27 In fact, stroke service may result in reduced LOS and thus drive costs down.
We showed marked differences in in-patient costs, mortality, and LOS according to different stroke types. Patients with PICH incurred the greatest cost, averaging US$26 700; the median cost of PICH was 3 times higher than that of IS. Patients with a TIA were the least costly, averaging US$1300. Furthermore, mortality and LOS were significantly higher in patients with PICH and SAH than those with IS and TIA. As found in other high- and middle-income countries,18,19,23,28-30 patients with PICH or SAH bore higher costs, mortality, and LOS. Mean cost per discharge for PICH was higher than that in high-income countries23,28,30; however, costs of patients with SAH, TIA, and IS were lower than those in high-income countries.22,23,28,30 In opposition to US studies,23,28 mean cost of PICH was higher than SAH; however, the median is in line with their results. This could be due to 2 outlier patients in PICH group who spent 131 and 143 days in hospital. When these patients were removed from the analysis, mean cost of SAH exceeded that of PICH.
CE and LA strokes compared with SV and UC were predictors of increased total cost. As in previous studies, patients with CE stroke had more severe neurological deficits and poorer outcomes, resulting in greater resource utilization, relatively longer hospitalization and ICU LOS, and higher medical costs,20,21 as opposed to SV stroke.
As shown elsewhere,10,14,22 we found that cost of acute care rose with stroke severity; this was mostly driven by increased LOS. However, when these same factors were examined in multivariable analysis, stroke severity emerged as an independent predictor of cost after accounting for LOS effects.
Cost and LOS are dependent of functional outcome at discharge. Similarly to other studies, they increased with higher mRS scores10,14 and lower BI scores.3,10 Similarly to high-income countries,10,31 most patients (76%) were discharged home; however, more than half needed help. Patients were discharged from hospital mostly when their medical investigations were completed and their general medical condition was stable to continue domiciliary rehabilitation treatment.
Similarly to other middle-income studies’ findings,17,18 the cost of patients who underwent a surgery or developed infectious complication was significantly higher, due to the added cost of operating room and surgeons’ fees, extended hospital LOS, and antibiotic treatments. Katzan et al reported extended care and an incremental cost of US$20 413 (2015 US$) in stroke patients with pneumonia compared with infection-free patients.15
Patients who died in hospital had higher cost compared with survivors, as found elsewhere14; however, mortality rate in this study was considerably lower than other middle-income countries,4 but higher than some high-income countries20,22 yet very close to Greece.14 However, these former20,22 did not include hemorrhagic stroke patients, which show higher mortality rates than IS.32 Other possible reasons are the higher number of stroke severity in this study, the lack of stroke unit, and the underuse of thrombolysis in Lebanese hospitals.
Lebanon has a highly fragmented health care system and pluralistic.33 Many differences in health care system quality remain between rural and urban areas as well as between public and private health care with different types of managed health care plans. In Lebanon, 46.8% of the population reported having some form of insurance (either social or private).33 If one excludes the non-Lebanese population that is estimated at 7.6%, the government is responsible for the remaining 45.6% of the population.33 The total contribution of the public TPPs represented approximately 45% (US$634 626) of the total cost. TPP type significantly influenced total cost. In fact, in Lebanon, the cost of each resource varies based on TPP coverage tariffs. Public payers have lower tariffs for the same resource use compared with private.
Strengths and Limitations of This Study
The first strength of this study was related to the prospective data collection using validated tools used in previous similar research for data collection. It pioneered in assessing cost of stroke predictors through multivariable analysis. In addition, we estimated costs, including physicians’ fees, based on actual bills vs using proxy methods, rather than predetermined charges. We conducted a multicenter incidence-base study including a diversified population, thus increasing the generalizability of our results.
This study does, however, have limitations. Even though patients came from all governorates, hospitals were limited to Beirut region. In this study, we could not exclude some unintentional bias in patient care, due to its observational nature. Also, we did not have strict guidelines for the clinical management of patients, which depended primarily on the physician in charge.
Conclusion
This study is an important first step in evaluating the economic impact of hospitalization due to stroke in Lebanon. Cost of care is significantly influenced by level of stroke severity and LOS. This information may help policy makers to develop health care plans to minimize economic burden on health system. Future studies should focus on modifiable, often unmeasured parameters, related not only to stroke characteristics but also to hospital operational policies, potentially influencing LOS. Because stroke often results in permanent dependence, cost analysis of long-term care from a societal perspective should be established.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Rachel R. Abdo  https://orcid.org/0000-0001-8131-1676
https://orcid.org/0000-0001-8131-1676
Rana G. Rizk  https://orcid.org/0000-0002-8850-6502
https://orcid.org/0000-0002-8850-6502
References
- 1. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095-2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Donnan GA, Fisher M, Macleod M, Davis SM. Stroke. Lancet. 2008;371(9624):1612-1623. [DOI] [PubMed] [Google Scholar]
- 3. Fattore G, Torbica A, Susi A, et al. The social and economic burden of stroke survivors in Italy: a prospective, incidence-based, multi-centre cost of illness study. BMC Neurol. 2012;12:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Asil T, Celik Y, Sut N, et al. Cost of acute ischemic and hemorrhagic stroke in Turkey. Clin Neurol Neurosurg. 2011;113(2):111-114. [DOI] [PubMed] [Google Scholar]
- 5. Ovbiagele B, Goldstein LB, Higashida RT, et al. American Heart Association Advocacy Coordinating Committee and Stroke Council. Forecasting the future of stroke in the United States: a policy statement from the American Heart Association and American Stroke Association. Stroke. 2013;44(8):2361-2375. [DOI] [PubMed] [Google Scholar]
- 6. Lubitz J, Prihoda R. The use and costs of Medicare services in the last 2 years of life. Health Care Financ Rev. 1984;5:117-131. [PMC free article] [PubMed] [Google Scholar]
- 7. Easton JD, Saver JL, Albers GW, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009;40:2276-2293 [DOI] [PubMed] [Google Scholar]
- 8. Meijer R, van Limbeek J, de Haan R. Development of the stroke-unit discharge guideline: choice of assessment instruments for prediction in the subacute phase post-stroke. Int J Rehabil Res. 2006;29(1):1-8. [DOI] [PubMed] [Google Scholar]
- 9. Bank of Beirut. Date unknown. www.bdl.gov.lb/statistics/table.php?name¼t5282usd. Accessed March 11, 2016.
- 10. Alvarez- Sabín J, Quintana M, Masjuan J, et al. Economic impact of patients admitted to stroke units in Spain. Eur J Health Econ. 2017;18:449-458. doi: 10.1007/s10198-016-0799-9. [DOI] [PubMed] [Google Scholar]
- 11. The NINDS t-PA Stroke Study Group. Generalized efficacy of t-PA for acute stroke: subgroup analysis of the NINDS t-PA Stroke Trial. Stroke. 1998;28:2119-2125. [DOI] [PubMed] [Google Scholar]
- 12. Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35-41. [DOI] [PubMed] [Google Scholar]
- 13. Oxford Stroke Classification. http://www.medquarterly.com/mq88/MQPDF/MM/OxfordStrokeClassification.pdf. Accessed November 11, 2015.
- 14. Gioldasis G, Talelli P, Chroni E, Daouli J, Papapetropoulos T, Ellul J. In-hospital direct cost of acute ischemic and hemorrhagic stroke in Greece. Acta Neurol Scand. 2008;118:268-274. [DOI] [PubMed] [Google Scholar]
- 15. Katzan IL, Dawson NV, Thomas CL, Votruba ME, Cebul RD. The cost of pneumonia after acute stroke. Neurology. 2007;68:1938-1943. [DOI] [PubMed] [Google Scholar]
- 16. Khealani BA, Javed ZF, Syed NA, Shafqat S, Wasay M. Cost of acute stroke care at a tertiary care hospital in Karachi, Pakistan. J Pak Med Assoc. 2003;53(11):552-555. [PubMed] [Google Scholar]
- 17. Christensen MC, Valiente R, Silva Sampaio G. Acute treatment costs of stroke in Brazil. Neuroepidemiology. 2009;32:142-149. [DOI] [PubMed] [Google Scholar]
- 18. Christensen MC, Previgliano I, Capparelli FJ. Acute treatment costs of intracerebral hemorrhage and ischemic stroke in Argentina. Acta Neurol Scand. 2009;119:246-253. [DOI] [PubMed] [Google Scholar]
- 19. Claesson L, Gosman-Hedstrom G, Johannesson M, Fagerberg B, Blomstrand C. Resource utilization and costs of stroke unit care integrated in a care continuum: a 1-year controlled, prospective, randomized study in elderly patients: the Göteborg 70+ Stroke Study. Stroke. 2000;31:2569-2577. [DOI] [PubMed] [Google Scholar]
- 20. Yoneda Y, Uehara T, Yamasaki H, Kita Y, Tabuchi M, Mori E. Hospital-based study of the care and cost of acute ischemic stroke in Japan. Stroke. 2003;34:718-724. [DOI] [PubMed] [Google Scholar]
- 21. Caro JJ, Huybrechts KF, Duchesne I. Management patterns and costs of acute ischemic stroke: an international study. For the Stroke Economic Analysis Group. Stroke. 2000;31(3):582-590. [DOI] [PubMed] [Google Scholar]
- 22. Diringer MN, Edwards DF, Mattson DT, et al. Predictors of acute hospital costs for treatment of ischemic stroke in an academic center. Stroke. 1999;30:724-728. [DOI] [PubMed] [Google Scholar]
- 23. Reed SD, Blough DK, Meyer K, Jarvik JG. Inpatient costs, length of stay, and mortality for cerebrovascular events in community hospitals. Neurology. 2001;57(2):305-314. [DOI] [PubMed] [Google Scholar]
- 24. Grieve R, Hutton J, Bhalla A, et al. A comparison of the costs and survival of hospital-admitted stroke patients across Europe. Stroke. 2001;32:1684-1691. [DOI] [PubMed] [Google Scholar]
- 25. Mamoli A, Censori B, Casto L, Sileo C, Cesana B, Camerlingo M. An analysis of the costs of ischemic stroke in an Italian stroke unit. Neurology. 1999;53(1):112-116. [DOI] [PubMed] [Google Scholar]
- 26. Wentworth DA, Atkinson RP. Implementation of an acute stroke program decreases hospitalization costs and length of stay. Stroke. 1996;27:1040-1043. [DOI] [PubMed] [Google Scholar]
- 27. Briggs DE, Felberg RA, Malkoff MD, Bratina P, Grotta JC. Should mild or moderate stroke patients be admitted to an intensive care unit? Stroke. 2001;32(4):871-876. [DOI] [PubMed] [Google Scholar]
- 28. Holloway RG, Witter DM, Jr, Lawton KB, Lipscomb J, Samsa G. Inpatient costs of specific cerebrovascular events at five academic medical centers. Neurology. 1996;46:854-860. [PubMed] [Google Scholar]
- 29. Yoneda Y, Okuda S, Hamada R, et al. Hospital cost of ischemic stroke and intracerebral hemorrhage in Japanese stroke centers. Health Policy. 2005;73:202-211. [DOI] [PubMed] [Google Scholar]
- 30. Dodel RC, Haacke C, Zamzow K, et al. Resource utilization and costs of stroke unit care in Germany. Value Health. 2004;7:144-152. [DOI] [PubMed] [Google Scholar]
- 31. van Eeden M, van Heugten C, van Mastrigt GAPG, van Mierlo M, Visser-Meily JMA, Evers SMAA. The burden of stroke in the Netherlands: estimating quality of life and costs for 1 year poststroke. BMJ Open. 2015;5:e008220. doi: 10.1136/bmjopen-2015-008220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Broderick J, Connolly S, Feldmann E, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Circulation. 2007;116(16):391-413. [DOI] [PubMed] [Google Scholar]
- 33. Ammar W, Fakha H, Azzam O, et al. Lebanon National Health Accounts. World Bank; 2010. Date unknown. http://siteresources.worldbank.org/INTHSD/Resources/376278-1261143298590/6660179-1280173228245/LebanonNHA.pdf. Accessed May 24, 2018. [Google Scholar]