Abstract
Pulmonary arterial hypertension (PAH) can be found in patients suffering from a loss-of-function mutation of the gene encoding for the activin receptor-like kinase 1 (ALK-1), a bone morphogenetic protein (BMP) type 1 receptor. Interestingly, ALK-1 mutations also lead to hereditary hemorrhagic telangiectasia (HHT), an autosomal dominant disease characterized by arteriovenous malformations (AVMs) leading to potentially life-threatening bleeding complications such as epistaxis. Current therapeutic options for both diseases are limited and often only temporary or accompanied by severe side effects. Here, we report of a patient with a mutation of the ALK-1 gene suffering from both HHT and PAH. Recently, it was shown that tacrolimus increased ALK-1 signaling and had beneficial effects in selected end-stage PAH patients. We thus hypothesized that treatment with tacrolimus may prevent disease progression in this patient. Surprisingly, treatment with low-dose tacrolimus dramatically improved his HHT-associated epistaxis but did not attenuate progression of PAH.
Keywords: ALK-1 mutation, FK506, Morbus Osler, tacrolimus, VEGF
Introduction
In most patients with hereditary hemorrhagic telangiectasia (HHT) recurrent epistaxis occurs with increasing frequency during aging. Once nose-bleeding episodes started, reports of spontaneous regression can be scarcely found.1 Besides life-threatening complications of bleeding, the quality of life and the ability to work can be severely impaired in the face of irrepressible bleeding events. Increased production of the vascular endothelial growth factor (VEGF) by inhibition of BMP9-ALK-1 signaling has been attributed to the development of arteriovenous malformations (AVMs) in HHT patients.2 Although a combination of HHT and pulmonary arterial hypertension (PAH) is a rare condition, treatment is extremely difficult, as PAH (due to increased intravascular pressure) and PAH-targeted therapy may increase the bleeding in HHT.
Recently, it was reported that the immunosuppressant tacrolimus enhanced the ALK-1 signaling pathway in the endothelial cells of HHT patients, and inhibited increased VEGF signaling and hypervascularization in an HHT animal model.3 Moreover, tacrolimus was shown to activate the BMP type 2 receptor (BMPR-2) signaling pathway, which is suppressed in PAH, and reversed severe experimental pulmonary hypertension (PH) (4), as well as PAH in selected end-stage PAH patients (5).
Case description
A 51-year-old man with an ALK-1 mutation (exon 10, c.1451G > A) and a most likely non-pathogenetic BMPR-2 polymorphism (S775), suffering from both HHT and PAH, first presented to our hospital in 2001 (right heart catheterization [RHC] measurements: mean pulmonary arterial pressure [mPAP] = 50 mmHg, cardiac index = 2.99 L/min/m2, pulmonary vascular resistance [PVR] = 534 dyn; 6-min walk distance [6MWD] = 460 m). In recent years, the patient suffered from epistaxis several times a week, which necessitated interventional treatment with laser coagulation (Nd:YAG laser) at least twice yearly since October 2010. In February 2015, severe gastrointestinal bleeding required blood transfusion and endoscopic argon plasma laser coagulation. Furthermore, liver shunts were diagnosed. However, there was no evidence of intrapulmonary shunting from contrast-enhanced transthoracic echocardiographic examinations (performed in February 2016 and January 2017). PAH was treated with high dose calcium channel blockers (since 1995), sildenafil (since 2006), inhaled iloprost (since 2013), and macitentan (since 2014), resulting in improved pulmonary hemodynamics from 2011 to 2014. However, PVR and brain natriuretic peptide (BNP) levels increased between August 2014 and February 2016 (from 301 dyn to 379 dyn, and 42 pg/mL to 159 pg/mL, respectively), while 6MWD decreased (from 458 m to 390 m, see Supplementary Table 1). The concomitant increase of cardiac index was interpreted as an increase in systemic shunt. Due to the progress of PAH, we thus had to consider further treatment options. As systemic prostanoids can increase the susceptibility for bleeding, therapy escalation with systemic prostanoids seemed to imply a not tolerable high risk for life-threatening bleeding complications in this patient. Against the background of successful treatment of single PAH patients with tacrolimus,5 treatment with tacrolimus was thus started on a compassionate treatment basis in February 2016, with dosage aiming for a goal trough blood level of 1.5–2.5 ng/mL of this agent (immunosuppressive goal trough blood level = 6–8 ng/mL), in order to stabilize the patient’s PAH.
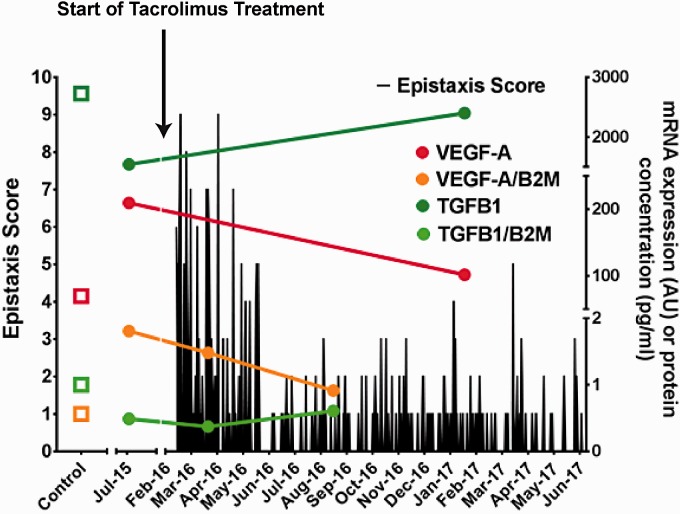
After initiation of treatment with tacrolimus, the frequency and severity of epistaxis decreased substantially determined by the nose-bleeding protocol of the patient (Fig. 1) and the epistaxis severity score (ESS) according Hoag et al.,6 which decreased from an ESS of 10.18 points before treatment to 3.06 points during treatment. During the observation period from February 2016 to August 2017, the patient required only one endonasal laser coagulation in May 2016 and there was no record of gastrointestinal bleeding. Side effects in terms of suppressed renal function or white blood cell counts were not observed. In contrast to HHT symptoms, PAH progressed from February 2016 to January 2017 (RHC in January 2017: PVR = 537 dyn, BNP = 391 pg/mL, 6MWD = 209 m), so that treatment with continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, was started in January 2017. In accordance with the course of HHT symptoms, the amount of mRNA in peripheral blood mononuclear cells (PBMCs), as well as circulating levels of VEGF-A, which were increased compared to healthy controls at baseline, normalized back to control levels 12 months after the initiation of tacrolimus treatment, as well as at baseline decreased TGFβ–1 levels (Fig. 1). In contrast, the mRNA level in PBMCs of biomarkers specifically related to canonical and non-canonical signaling of BMPR2 and thus to disease development of PAH (inhibitor of DNA binding [ID1] and LIM domain kinase 1 [LIMK1]/COFILIN1,7 respectively) did not return to control levels (Supplementary Table 1).
Fig. 1.
Nose-bleeding protocol from February 2016 to June 2017. For the assessment of severity of bleeding, an ESS taking into account duration and severity of bleed was used. Measurements of mRNA were performed in samples of peripheral blood mononuclear cells (PBMCs) from the patient and healthy controls (n = 3) using a quantitative polymerase chain reaction (PCR) assay with specific primers. Values are expressed as relative mRNA compared to control levels. Plasma levels of VEGF-A and TGFβ1 measured by ELISAs in blood samples obtained from the pulmonary artery during RHC of healthy controls (n = 3) and the patient. TGFβ, transforming growth factor β; VEGF-A, vascular endothelial growth factor A; B2M, β-2 microglobulin (as housekeeping gene).
Discussion
We here report for the first time that treatment with low-dose tacrolimus inhibited epistaxis in a patient with HHT due to an ALK-1 mutation. Current therapy, but also novel experimental treatment options such as thalidomide8 and the anti-VEGF therapy with bevacizumab,9 are limited by severe side effects.10 Thus, treatment with oral low-dose tacrolimus provides a novel therapeutic option for treatment of severe bleeding complications in HHT without recorded side effects. However, in contrast to previous reports5 progression of PAH could not be halted. One reason for the discrepant findings may be the fact that in the previous patients no mutations of BMP-ALK-1 signaling were detected while this patient suffered from an ALK-1 mutation which may affect tacrolimus signaling. As we detected signs of clinical worsening already before the initiation of the tacrolimus treatment, the progression of PAH was probably not directly related to the drug treatment, but rather a natural progression of the disease.
The opposing clinical effects of tacrolimus on HHT and PAH may be reflected by biomarkers determined in the PBMCs of the patient: while circulating and mRNA levels of VEGF-A were reversed during treatment, the mRNA levels of ID1, LIMK1, and COFILIN1, which are related to BMPR-2 signaling, did decrease during disease progression below the control level. However, the relevance of the changes of mRNA levels in PBMCs remains unclear. Of note, in accordance with previous investigations which, however, used tacrolimus in the immunosuppressive range,11 we found increased levels of circulating TGFβ, during treatment with tacrolimus which potentially could promote PAH.12 With regard to VEGF, its role in PAH is not completely resolved. While VEGF receptor inhibition may promote induction of development of PH in animal models, increased levels of VEGF have been found to promote established PH,13 and tacrolimus treatment reversed established PH in different animal models.4
In summary, this case report shows for the first time the striking beneficial effect of tacrolimus treatment in a HHT patient who suffered from severe epistaxis. Our findings support the study of tacrolimus for HHT treatment in clinical trials with careful attention to the potential effects on the pulmonary vasculature. How far VEGF, TGFβ ligands, and interaction partners of BMPR-2 may qualify as biomarkers for treatment efficiency has to be further investigated.
Supplementary Material
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.McDonald J, Wooderchak-Donahue W, VanSant Webb C, et al. Hereditary hemorrhagic telangiectasia: genetics and molecular diagnostics in a new era. Front Genet 2015; 6: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Botella LM, Albinana V, Ojeda-Fernandez L, et al. Research on potential biomarkers in hereditary hemorrhagic telangiectasia. Front Genet 2015; 6: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruiz S, Chandakkar P, Zhao H, et al. Tacrolimus rescues the signaling and gene expression signature of endothelial ALK1 loss-of-function and improves HHT vascular pathology. Hum Mol Genet 2017; 26: 4786–4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spiekerkoetter E, Tian X, Cai J, et al. FK506 activates BMPR2, rescues endothelial dysfunction, and reverses pulmonary hypertension. J Clin Invest 2013; 123(8): 3600–3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spiekerkoetter E, Sung YK, Sudheendra D, et al. Low-dose FK506 (Tacrolimus) in end-stage pulmonary arterial hypertension. Am J Respir Crit Care Med 2015; 192(2): 254–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoag JB, Terry P, Mitchell S, et al. An epistaxis severity score for hereditary hemorrhagic telangiectasia. Laryngoscope 2010; 120(4): 838–843. [DOI] [PubMed] [Google Scholar]
- 7.Foletta VC, Lim MA, Soosairajah J, et al. Direct signaling by the BMP type II receptor via the cytoskeletal regulator LIMK1. J Cell Biol 2003; 162(6): 1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lebrin F, Srun S, Raymond K, et al. Thalidomide stimulates vessel maturation and reduces epistaxis in individuals with hereditary hemorrhagic telangiectasia. Nat Med 2010; 16(4): 420–428. [DOI] [PubMed] [Google Scholar]
- 9.Thompson AB, Ross DA, Berard P, et al. Very low dose bevacizumab for the treatment of epistaxis in patients with hereditary hemorrhagic telangiectasia. Allergy Rhinol 2014; 5(2): 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geisthoff UW, Nguyen HL, Roth A, et al. How to manage patients with hereditary haemorrhagic telangiectasia. Br J Haematol 2015; 171(4): 443–452. [DOI] [PubMed] [Google Scholar]
- 11.Barbarino JM, Staatz CE, Venkataramanan R, et al. PharmGKB summary: cyclosporine and tacrolimus pathways. Pharmacogenet Genomics 2013; 23(10): 563–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Upton PD, Davies RJ, Tajsic T, et al. Transforming growth factor-beta(1) represses bone morphogenetic protein-mediated Smad signaling in pulmonary artery smooth muscle cells via Smad3. Am J Respir Cell Mol Biol 2013; 49(6): 1135–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tuder RM, Yun JH. Vascular endothelial growth factor of the lung: friend or foe. Curr Opin Pharmacol 2008; 8(3): 255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.