Abstract
Background: Surgical resection followed by adjuvant chemotherapy is the only therapeutic option in pancreatic cancer. However, there is limited research evaluating methods of improving adherence to adjuvant treatment after curative resection. Methods: From January 1995 to December 2014, 323 patients with pancreatic cancer who underwent pancreatectomy at the Severance Hospital were enrolled in this study. We retrospectively analyzed clinicopathologic factors with propensity score matching method. Results: The final study population was 217, after excluding patients undergoing neoadjuvant treatment or palliative resection, those who died within 30 days after operation, and those lost to follow-up after discharge. Among them, 161 received adjuvant treatment after curative resection. Cox’s proportional hazard models revealed that nodal metastasis, perioperative transfusion, and completion of adjuvant treatment were significantly correlated with cancer recurrence and cancer-related death (P < .05). Phellinus linteus (PL) medication was the only significant predictor for completion of adjuvant treatment after curative resection in logistic regression analysis (P = .039). Disease-free and overall survival of the PL medication group were significantly higher than the no PL medication group (P < .05). Conclusions: PL medication potentially contributed to long-term oncologic outcomes by increasing patients’ adherence to postoperative adjuvant chemotherapy, which resulted from PL medication associated with low toxicity of chemotherapy.
Keywords: pancreatic cancer, adjuvant treatment, Phellinus linteus, chemotherapy, survival
Introduction
Pancreatic cancer is one of the most lethal malignancies among major solid tumors.1 There has been little improvement of oncologic outcomes even after implementation of various therapeutic approaches.2-4 Surgical resection with curative intent, followed by adjuvant chemotherapy, is the only therapeutic option that has demonstrated long-term survival without tumor recurrence in pancreatic cancer.5,6 Current evidence indicates that adherence and completion of adjuvant treatment after radical pancreatectomy are crucial factors for improved oncologic outcomes.7 However, there is limited research evaluating potential methods for improving adherence to adjuvant treatment after curative resection of pancreatic cancer. We previously published our interim results for potential clinical application of Phellinus linteus (PL) in treatment of pancreatic cancer.8 In this study, based on accumulating clinical data, we attempted to perform further analysis to identify the potential role of PL medication in patients who underwent radical pancreatectomy for pancreatic ductal adenocarcinoma.
Methods
Study Design
This study is a retrospective cohort clinical study based on a single institutional database. From January 1995 to December 2014, 323 patients with pancreatic cancer who underwent pancreatectomy at the Severance Hospital were enrolled in this study. We retrospectively collected and analyzed data regarding clinicopathologic factors, including demographics, perioperative management, short-term outcomes, and administration of PL medication of the patients from our institutional pancreatic cancer cohort. Oncologic outcomes, including recurrences and cancer-related death, were obtained from medical records. This study was approved by the institutional review board of the Severance Hospital, Seoul, Korea (2017-1566-001).
Phellinus linteus Extract Medication
PL is a medical mushroom that has been used in East Asia for centuries to manage several diseases; its extract, which contains various bioactive substances, including proteoglycans, polysaccharides, cyclophellitol, hispidin, furan derivatives, and hispolon,9 was commercialized as an oral medication in Korea, and it has been administered to pancreatic cancer patients who underwent curative intent pancreatectomy since 2005 to enhance postoperative recovery. PL Mycelium Extract (Aclang, Kwangdong Pharmaceutical Co, Ltd, Korea), which was approved by the Korean Food & Drug Administration (KFDA), was administered at a dose of 1100 mg 3 times per day orally, after acquiring the informed consent of enrolled patients.
Clinical Setting for PL Medication After Curative Resection of Pancreatic Ductal Adenocarcinoma
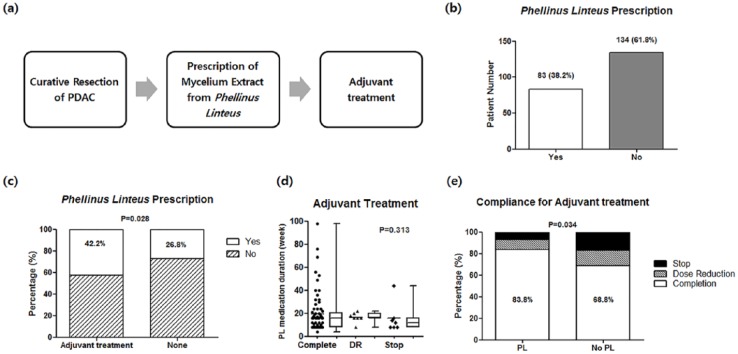
PL medication has been prescribed as a supplementary drug to promote general health for postoperative patients who underwent radical pancreatectomy by a surgical oncologist (Figure 3). This drug was administered only after the patient was provided detailed information about the drug and consented to its use. The administration of medication continued for the duration the patient agreed to continue adjuvant treatment for pancreatic cancer.
Figure 3.
Prescription details of Phellinus linteus (a, b, c), and effects of adjuvant treatment after curative resection of pancreatic ductal adenocarcinoma (PDAC) (d, e).
Propensity Score Matching
To reduce statistical selection bias in this retrospective study, propensity score matching was used for the comparisons in both the adjuvant treatment group, and no adjuvant treatment group. Major confounding factors for administering PL medication in each group were selected and propensity scores were measured by logistic regression. Proper matching procedures were performed in each group with nearest selection methods (1:1 matching in adjuvant group and 1:2 matching in no adjuvant group).
Statistics
Comparisons between the 2 separate groups were conducted by statistical tests using Student’s t test or Wilcoxon rank sum test for continuous variables, and chi-square test or Fisher’s exact test for categorical variables. Survival analysis was performed using the Kaplan-Meier method, and log-rank test was used for comparisons of survival differences between the 2 groups. Cox’s proportional hazard model was used to evaluate prognostic factors for recurrence and cancer-related death, and a logistic regression model was applied to estimate predictive variables for the completion of adjuvant treatment after curative resection of pancreatic cancer. A P value less than .05 was considered statistically significant.
Results
Enrolled Patients
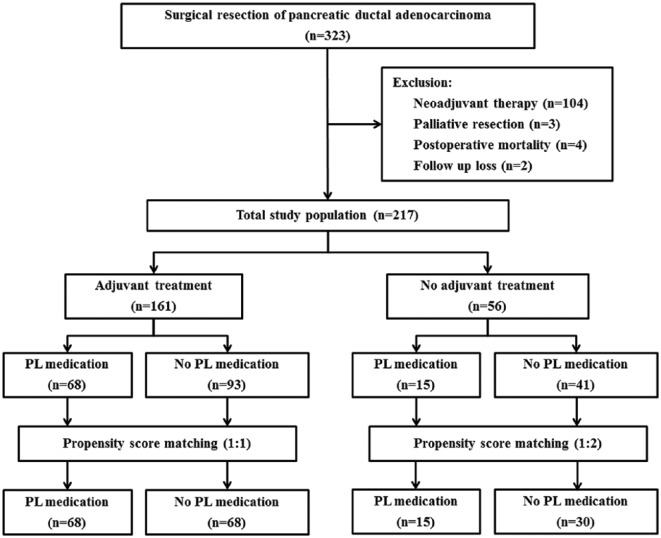
The total study population was 217, after excluding patients who underwent neoadjuvant treatment or palliative resection, those who died within 30 days of resection, and those lost to follow-up after discharge (Figure 1). Of the total study population, 161 patients received adjuvant treatment after curative resection, and 56 patients did not receive adjuvant treatment due to various reasons, including patient preference and an early stage cancer diagnosis, which insurance did not cover. The number of patients with PL medication was 68 in the group of adjuvant treatment and 15 in the no adjuvant treatment group. There was no adverse event related to PL medication during follow-up. Propensity score matching was performed for the groups with or without adjuvant treatment (1:1 matching in group with adjuvant treatment, 1:2 matching in group without adjuvant treatment).
Figure 1.
Flow diagram of the study cohort of pancreatic ductal adenocarcinoma patients.
Prognostic Factors for Recurrence and Cancer-Related Death
Cox’s proportional hazard models were analyzed to evaluate major prognostic factors for recurrence and cancer-related death (Table 1). Univariate and multivariate analysis revealed that tumor size, nodal metastasis, perioperative transfusion, and completion of adjuvant treatment were significantly correlated with cancer recurrence and cancer-related death (P < .05).
Table 1.
Univariate and Multivariate Regression Model for Recurrence and Cancer-Related Death by Using Cox’s Proportional Hazard Model in Total Study Population (n = 217).
| Variables | Recurrence | Cancer-Related Death | ||||||
|---|---|---|---|---|---|---|---|---|
| Crude HR (95% CI) | P | Adjusted HR (95% CI) | P | Crude HR (95% CI) | P | Adjusted HR (95% CI) | P | |
| Patient characteristics | ||||||||
| Age (years) | 0.996 (0.978-1.014) | .666 | 1.003 (0.983-1.023) | .763 | ||||
| Sex (male/female) | 0.934 (0.671-1.299) | .683 | 1.144 (0.799-1.637) | .462 | ||||
| BMI (kg/m2) | 1.003 (0.988-1.019) | .664 | 0.963 (0.911-1.018) | .186 | ||||
| ASA score (1-2/3-4) | 1.278 (0.844-1.934) | .247 | 1.029 (0.477-1.563) | .894 | ||||
| Comorbidity (yes/no) | 0.983 (0.673-1.435) | .928 | 1.183 (0.786-1.779) | .421 | ||||
| Pathologic factors | ||||||||
| Tumor location (Head-Neck/Body-Tail) | 1.143 (0.699-1.868) | .594 | 1.033 (0.931-1.149) | .542 | ||||
| Tumor size (cm) | 1.197 (1.072-1.338) | .001 | 1.211 (1.074-1.365) | .002 | 1.123 (0.992-1.272) | .066 | ||
| T stage (1-2/3-4) | 2.483 (0.997-5.357) | .087 | 1.105 (0.699-1.745) | .671 | ||||
| Nodal metastasis (yes/no) | 1.931 (1.361-2.738) | .001 | 1.866 (1.288-2.704) | .001 | 1.872 (1.286-2.719) | .001 | 2.122 (1.292-3.486) | .003 |
| Cell differentiation (well and moderate/poor and undifferentiated) | 1.787 (0.907-3.520) | .093 | 1.282 (0.931-1.766) | .128 | ||||
| Perineural invasion (yes/no) | 0.967 (0.684-1.367) | .851 | 0.927 (0.645-1.333) | .684 | ||||
| Lymphovascular invasion (yes/no) | 1.207 (0.859-1.697) | .278 | 1.165 (0.811-1.674) | .408 | ||||
| R status (R0/R1-2) | 1.201 (0.755-1.909) | .439 | 1.280 (0.776-2.114) | .334 | ||||
| Perioperative factors | ||||||||
| Operation method (open/MIS) | 1.354 (0.891-1.984) | .243 | 1.054 (0.978-1.024) | .458 | ||||
| Intraoperative bleeding (mL) | 1.001 (0.999-1.011) | .122 | 1.125 (0.997 - 1.548) | .265 | ||||
| Perioperative transfusion (yes/no) | 1.469 (0.999-2.160) | .051 | 1.464 (0.992-2.160) | .055 | 1.781 (1.191 - 2.661) | .005 | 1.791 (1.112-2.881) | .017 |
| Postoperative major complication (yes/no) | 0.836 (0.517-1.353) | .466 | 0.803 (0.492-1.311) | .381 | ||||
| Postoperative factors | ||||||||
| Adjuvant treatment (yes/no) | 0.829 (0.572-0.998) | 0.048 | 0.673 (0.456-0.995) | .047 | 0.775 (0.524-0.985) | .042 | 0.487 (0.342-0.876) | .001 |
| Completion without dose reduction (yes/no) | 0.863 (0.547-1.012) | .107 | 0.841 (0.642-0.947) | .048 | 0.251 (0.251-0.925) | .038 | ||
| Phellinus linteus medication (yes/no) | 1.024 (0.734-1.429) | .889 | 0.772 (0.534-1.116) | .168 | ||||
| Phellinus linteus medication duration (weeks) | 1.003 (0.999-1.015) | .695 | 0.993 (0.974-1.012) | .449 | ||||
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index; CI, confidence interval; HR, hazard ratio; MIS, minimally invasive surgery.
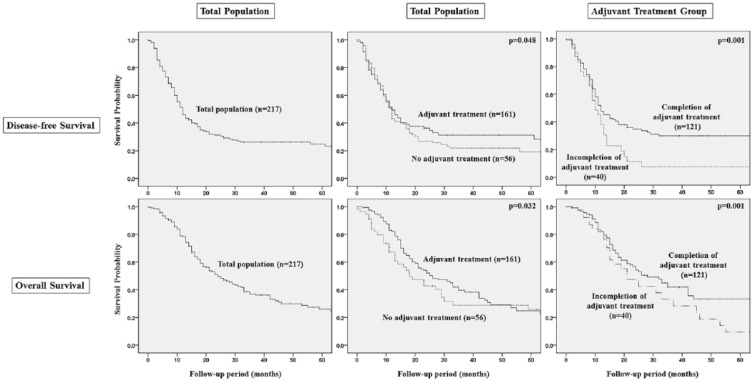
Survival Analysis According to Adjuvant Treatment
The provision of adjuvant treatment was a major prognostic factor for disease-free and cancer-specific overall survival (Figure 2). The group with adjuvant treatment showed significantly better survival outcomes than the group with no adjuvant treatment (P < .05). Completion of adjuvant treatment was an especially important predictor for survival outcomes in the group with adjuvant treatment (P = .001).
Figure 2.
Survival analysis of the total population (n=217) and adjuvant treatment group (n = 161) after curative resection of pancreatic ductal adenocarcinoma.
Predictive Factors for Completion of Adjuvant Treatment
Logistic regression analysis was performed to evaluate the predictors for completion of adjuvant treatment after radical pancreatectomy (Table 2). PL medication and several perioperative factors, including operation time, intraoperative bleeding, and postoperative major complications more than grade IV according to the Clavien-Dindo classification system, were revealed as significant factors in univariate analysis (P < .05). However, PL medication was the only significant predictor for completion of adjuvant treatment after curative resection in multivariate analysis (adjusted odds ratio [OR] 2.141, 95% CI 1.039-4.411, P = .039).
Table 2.
Predictors for Completion of Adjuvant Treatment After Curative Resection of PDAC Using Logistic Regression Analysis in Adjuvant Treatment Group (n=161).
| Covariates | Crude OR | 95% CI | P | Adjusted OR | 95% CI | P |
|---|---|---|---|---|---|---|
| Patient characteristics | ||||||
| Age (years) | 1.004 | 0.972-1.038 | 0.797 | |||
| Sex (male/female) | 1.001 | 0.536-1.869 | 0.998 | |||
| BMI (kg/m2) | 0.985 | 0.956-1.015 | 0.334 | |||
| ASA score (1-2/3-4) | 0.957 | 0.355-2.580 | 0.931 | |||
| Comorbidity (yes/no) | 1.382 | 0.696-2.742 | 0.355 | |||
| Pathologic factors | ||||||
| Tumor size (cm) | 1.112 | 0.679-21487 | 0.687 | |||
| T stage (1-2/3-4) | 1.148 | 0.293-4.497 | 0.843 | |||
| Nodal metastasis (yes/no) | 1.109 | 0.597-2.058 | 0.744 | |||
| Perioperative factors | ||||||
| Operation type (PD/DP) | 0.838 | 0.439-1.601 | 0.593 | |||
| Operation method (open/MIS) | ||||||
| Operation time (minutes) | 1.003 | 1.000-1.005 | 0.031 | 0.999 | 0.995-1.002 | 0.532 |
| Intraoperative bleeding (mL) | 1.001 | 1.000-1.002 | 0.054 | 1.001 | 0.999-1.002 | 0.292 |
| Perioperative transfusion (yes/no) | 1.164 | 0.570-2.375 | 0.677 | |||
| Postoperative major complication (yes/no) | 0.229 | 0.051-1.021 | 0.053 | 0.299 | 0.065-1.372 | 0.121 |
| Postoperative factors | ||||||
| Phellinus linteus medication (yes/no) | 2.273 | 1.151-4.492 | 0.018 | 2.141 | 1.039-4.411 | 0.039 |
| Phellinus linteus medication duration (weeks) | 1.014 | 0.971-1.058 | 0.532 | |||
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index; CI, confidence interval; DP, distal pancreatectomy; MIS, minimally invasive surgery; OR, odds ratio; PD, pancreaticoduodenectomy; PDAC, pancreatic ductal adenocarcinoma.
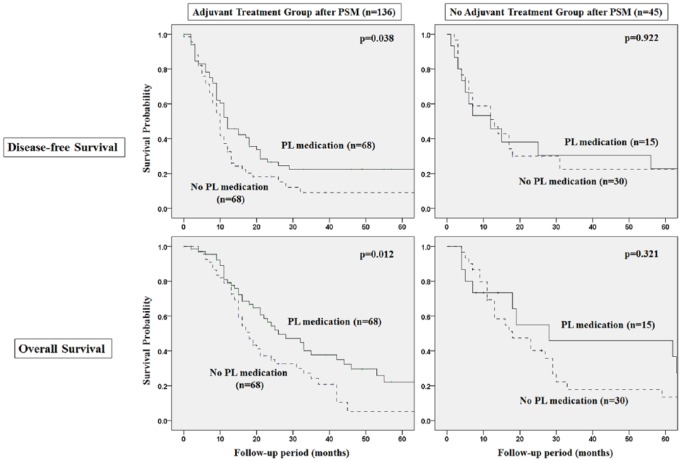
Association Between PL Medication and Adherence to Adjuvant Treatment
In subgroup analysis, PL medication contributed to improved adherence to adjuvant chemotherapy regarding reduction of chemotherapy toxicity less than grade 3 complications (PL vs no PL medication: 14.7% vs 33.8%, P = .001), completion of chemotherapy without dose reduction, or incomplete stop (PL vs no PL medication: 83.8% vs 63.2%, P = .005), even after propensity score matching (Figure 3, Table 3). Disease-free survival (DFS) and overall survival (OS) of the PL medication group were significantly higher than the no PL medication group in patients with adjuvant treatment after curative resection (DFS; PL vs no PL medication 33 vs 12 months, P = .038, OS: PL vs no PL medication 39 vs 18 months, P = .012) (Figure 4).
Table 3.
Cliniopathologic Factors and Detailed Outcomes of Adjuvant Treatment in PL and No PL Medication Group in Adjuvant Treatment Group With Propensity Score Matching.
| Variables | Adjuvant Treatment Group (n = 161) | Propensity Score–Matched Group (n = 136) | ||||
|---|---|---|---|---|---|---|
| PL Medication (n = 68) | No PL Medication (n = 93) | P | PL Medication (n = 68) | No PL Medication (n = 68) | P | |
| Patient characteristics | ||||||
| Age (years) | 62.9 ± 8.9 | 61.9 ± 9.3 | .469 | 62.9 ± 8.9 | 61.7 ± 9.7 | .434 |
| Sex (male, %) | 47.1% | 68.8% | .006 | 47.1% | 61.1% | .055 |
| BMI (kg/m2) | 25.1 ± 17.4 | 22.5 ± 3.3 | .185 | 25.1 ± 17.4 | 22.6 ± 3.5 | .268 |
| ASA score (1/2/3/4, %) | 24.2/50.0/22.7/1.5 | 20.4/63.4/16.1/0 | .271 | 24.2/50.0/22.7/1.5 | 19.1/64.7/16.2/0 | .346 |
| Comorbidity (%) | 82.4% | 79.6% | .691 | 82.4% | 80.9% | .925 |
| Perioperative factors | ||||||
| Pancreatioduodenectomy/distal pancreatectomy | 36/32 | 49/44 | .609 | 36/32 | 35/33 | .958 |
| Operation time (minutes) | 390.1 ± 117.1 | 350.7 ± 147.9 | .073 | 390.1 ± 117.1 | 364.1 ± 132.7 | .136 |
| Combined resection (%) | 30.9% | 23.7% | .368 | 30.9% | 25.0% | .567 |
| R status (R0/R1/R2, %) | 80.9/14.7/4.4 | 82.8/16.1/1.1 | .401 | 80.9/14.7/4.4 | 79.4/19.1/1.5 | .496 |
| Bleeding (cm3) | 591.3 ± 384.1 | 571.1 ± 474.3 | .778 | 591.3 ± 384.1 | 574.4 ± 504.1 | .832 |
| Transfusion (%) | 20.9% | 22.0% | .515 | 20.9% | 21.8% | .544 |
| Pathologic factors | ||||||
| Tumor size (cm) | 2.7 ± 1.0 | 3.1 ± 1.3 | .048 | 2.7 ± 1.0 | 3.0 ± 1.2 | .104 |
| Pathologic T stage (T1/T2/T3/T4, %) | 1.5/1.5/92.6/4.4 | 0/2.2/97.8/0 | .589 | 1.5/1.5/92.6/4.4 | 0/1.5/98.5/0 | .741 |
| Pathologic N stage (N0/N1, %) | 72.1% | 60.2% | .134 | 72.1% | 62.5% | .284 |
| Cell differentiation (%) | ||||||
| Well/moderate/poor/undifferentiated | 11.9/82.1/4.5/1.5 | 17.6/70.3/11.0/1.1 | .314 | 11.9/82.1/4.5/1.5 | 16.2/73.5/8.8/1.5 | .636 |
| Retrieved lymph node | 19.7 ± 11.5 | 18.8 ± 12.9 | .634 | 19.7 ± 11.5 | 19.2 ± 11.7 | .791 |
| Positive lymph node | 2.0 ± 2.1 | 1.9 ± 2.8 | .791 | 2.0 ± 2.1 | 2.6 ± 3.0 | .213 |
| Perineural invasion | 73.5% | 66.3% | .387 | 73.5% | 66.2% | .445 |
| Lymphovascular invasion | 41.2% | 35.9% | .514 | 41.2% | 39.7% | .721 |
| Adjust organ invasion | 16.2% | 21.5% | .426 | 16.2% | 23.5% | .391 |
| Postoperative major complicationa | 16.7% | 13.4% | .621 | 16.7% | 10.0% | .395 |
| Hospital stay (days) | 18.0 ± 9.4 | 19.9 ± 36.3 | .643 | 18.0 ± 9.4 | 19.8 ± 13.2 | .845 |
| Detailed outcomes of adjuvant treatment | ||||||
| Time interval from resection to adjuvant treatment (days) | 60.8 ± 34.2 | 46.9 ± 24.7 | .004 | 60.8 ± 34.2 | 46.3 ± 27.1 | .009 |
| Adjuvant treatment regimen | ||||||
| Chemoradiation | 18 (26.5%) | 21 (22.6%) | .457 | 18 (26.5%) | 17 (25%) | .898 |
| Chemotherapy only | 50 (73.5%) | 72 (77.4%) | 50 (73.5%) | 51 (75%) | ||
| Radiation dose (range, Gy) | 40.5 ± 15.5 | 41.3 ± 12.2 | .885 | 40.5 ± 15.5 | 40.8 ± 13.8 | .628 |
| Chemotherapy regimen | ||||||
| Gemcitabine base | 47 (69.1%) | 61 (65.5%) | .728 | 47 (69.1%) | 42 (61.8%) | .345 |
| 5-FU base | 21 (30.9%) | 32 (34.5%) | 21 (30.9%) | 26 (38.2%) | ||
| Chemotherapy toxicity (%) | ||||||
| ≥Grade 3 toxicity | 10 (14.7%) | 24 (25.8%) | .026 | 10 (14.7%) | 23 (33.8%) | .001 |
| Result of chemotherapy (%) | ||||||
| Completion | 57 (83.8%) | 64 (68.8%) | .027 | 57 (83.8%) | 43 (63.2%) | .005 |
| Dose reduction | 6 (8.8%) | 13 (14.0%) | 6 (8.8%) | 10 (14.7%) | ||
| Stop | 5 (7.4%) | 16 (17.2%) | 5 (7.4%) | 15 (22.1%) | ||
Abbreviations: 5-FU, fluorouracil; ASA, American Society of Anesthesiologists; BMI, body mass index; PL, Phellinus linteus.
Postoperative major complication includes more than Clavien-Dindo classification grade III.
Figure 4.
Survival analysis of propensity matched population on Phellinus linteus (PL) medication, with or without adjuvant treatment, after curative resection.
Discussion
Current evidence indicates that adherence and completion of adjuvant treatment for the duration of planned schedules, rather than for a short interval between surgery and adjuvant chemotherapy, is crucial for improving survival outcomes in patients who underwent radical pancreatectomy with curative intent.7,10 Identifying specific clinical factors affecting completion of chemotherapy, therefore, is mandatory for improving survival outcomes in patients who underwent radical pancreatectomy for pancreatic cancer. However, there seems to be few studies to investigate this issue.
Postoperative complications after radical pancreatectomy frequently develop11-13 and are a major cause of delay or failure of adjuvant treatment, due to the poor general condition of patients.13-15 Major complications, including especially postoperative pancreatic fistula and postpancreatectomy hemorrhage, induce life-threatening conditions and hinder postoperative recovery after curative resection of pancreatic cancer.16-19 Our data also showed that perioperative bleeding and postoperative complications are correlated with completion of adjuvant treatment after curative resection for pancreatectomy. Interestingly, PL medication after curative resection for pancreatic cancer was a significant prognostic factor for completion of adjuvant chemotherapy.
There is substantial evidence demonstrating the antitumor effects of PL based on in vitro and in vivo experiments.20,21 Despite the potential of PL medication as an anticancer treatment, few clinical studies have been published regarding the medication.8,22 There have been no prior publications regarding well-designed clinical studies analyzing the anticancer effects of PL medication in pancreatic cancer. This study revealed that PL medication promotes adherence to adjuvant treatment by reducing the rate at which patients reduce their dose or cease adjuvant chemotherapy. The exact underlying mechanism needs to be further investigated; however, this protective effect of PL medication in the setting of adjuvant therapy might be due to its action in reducing clinically relevant chemotherapy-related toxicities in the postoperative period after radical pancreatectomy.
Although this study used propensity score–matched comparisons, a limitation of this study is the retrospective and single institutional study. External validation in large cohorts would be necessary to verify the results of this study regarding oncologic effects of PL medication. In addition, a well-designed randomized control trial study is necessary for confirming antitumor, immune promoting or toxicity reduction effects of PL medication in the postoperative period following radical pancreatectomy.
In conclusion, PL medication contributed to improved survival by increasing adherence to chemotherapy in the group with adjuvant treatment. Oncologic effects of PL medication appear to be due to its action in lowering the toxicity of chemotherapy, according to our retrospective cohort analysis. Considering the limited literature on methods to improve adherence to postoperative chemotherapy in patients with radical pancreatectomy for pancreatic cancer, the findings of this study suggest the value in conducting randomized control studies to further evaluate and confirm this hypothesis.
Footnotes
Authors’ Note: Parts of this study were presented in oral presentation session of Joint congress of the 6th biennial congress of the Asian-Pacific Hepato-Pancreato-Biliary Association, June 7-10, 2017, at PACIFICO Yokohama, Yokohama, Japan.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through High Value-added Food Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (31605503).
ORCID iD: Sung Hwan Lee  https://orcid.org/0000-0003-3365-0096
https://orcid.org/0000-0003-3365-0096
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30. [DOI] [PubMed] [Google Scholar]
- 2. Tempero MA, Malafa MP, Behrman SW, et al. Pancreatic adenocarcinoma, version 2.2014: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2014;12:1083-1093. [DOI] [PubMed] [Google Scholar]
- 3. Khorana AA, Mangu PB, Berlin J, et al. Potentially curable pancreatic cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34:2541-2556. doi: 10.1200/JCO.2016.67.5553 [DOI] [PubMed] [Google Scholar]
- 4. Sohal DP, Mangu PB, Khorana AA, et al. Metastatic pancreatic cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2016;34:2784-2796. doi: 10.1200/JCO.2016.67.1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Neoptolemos JP, Stocken DD, Friess H, et al. ; European Study Group for Pancreatic Cancer. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200-1210. [DOI] [PubMed] [Google Scholar]
- 6. Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267-277. [DOI] [PubMed] [Google Scholar]
- 7. Valle JW, Palmer D, Jackson R, et al. Optimal duration and timing of adjuvant chemotherapy after definitive surgery for ductal adenocarcinoma of the pancreas: ongoing lessons from the ESPAC-3 study. J Clin Oncol. 2014;32:504-512. doi: 10.1200/JCO.2013.50.7657 [DOI] [PubMed] [Google Scholar]
- 8. Kang CM, Han DH, Hwang HK, Choi SH, Lee WJ. Anticancer effect of Phellinus linteus; potential clinical application in treating pancreatic ductal adenocarcinoma. J Carcinog Mutagen. 2013;(suppl 9):001. doi: 10.4172/2157-2518.S9-001 [DOI] [Google Scholar]
- 9. Zhu T, Kim SH, Chen CY. A medicinal mushroom: Phellinus linteus. Curr Med Chem. 2008;15:1330-1335. [DOI] [PubMed] [Google Scholar]
- 10. Saeed H, Hnoosh D, Huang B, et al. Defining the optimal timing of adjuvant therapy for resected pancreatic adenocarcinoma: a statewide cancer registry analysis. J Surg Oncol. 2016;114:451-455. [DOI] [PubMed] [Google Scholar]
- 11. Trede M, Schwall G. The complications of pancreatectomy. Ann Surg. 1988;207:39-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pugalenthi A, Protic M, Gonen M, et al. Postoperative complications and overall survival after pancreaticoduodenectomy for pancreatic ductal adenocarcinoma. J Surg Oncol. 2016;113:188-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Beger HG, Rau B, Gansauge F, Poch B, Link KH. Treatment of pancreatic cancer: challenge of the facts. World J Surg. 2003;27:1075-1084. [DOI] [PubMed] [Google Scholar]
- 15. Aahlin EK, Olsen F, Uleberg B, Jacobsen BK, Lassen K. Major postoperative complications are associated with impaired long-term survival after gastro-esophageal and pancreatic cancer surgery: a complete national cohort study. BMC Surg. 2016;16:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bassi C, Marchegiani G, Dervenis C, et al. ; International Study Group on Pancreatic Surgery (ISGPS). The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 2017;161:584-591. [DOI] [PubMed] [Google Scholar]
- 17. Serrano PE, Kim D, Kim PT, et al. Effect of pancreatic fistula on recurrence and long-term prognosis of periampullary adenocarcinomas after pancreaticoduodenectomy. Am Surg. 2016;82:1187-1195. [PubMed] [Google Scholar]
- 18. Wente MN, Veit JA, Bassi C, et al. Postpancreatectomy hemorrhage (PPH)—an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;142:20-25. [DOI] [PubMed] [Google Scholar]
- 19. Roulin D, Cerantola Y, Demartines N, Schäfer M. Systematic review of delayed postoperative hemorrhage after pancreatic resection. J Gastrointest Surg. 2011;15:1055-1062. [DOI] [PubMed] [Google Scholar]
- 20. Han SB, Lee CW, Jeon YJ, et al. The inhibitory effect of polysaccharides isolated from Phellinus linteus on tumor growth and metastasis. Immunopharmacology. 1999;41:157-164. [DOI] [PubMed] [Google Scholar]
- 21. Li G, Kim DH, Kim TD, et al. Protein-bound polysaccharide from Phellinus linteus induces G2/M phase arrest and apoptosis in SW480 human colon cancer cells. Cancer Lett. 2004;216:175-181. [DOI] [PubMed] [Google Scholar]
- 22. Cho SH, Kim JH, Park BK, et al. The effects of Mesima-Ex, the immunomodulator in curatively resected gastric cancer. J Korean Cancer Assoc. 1997;29:800-806. [Google Scholar]