Abstract
Context: Cancer-related fatigue (CRF) is one of the most burdensome symptoms in breast cancer survivors (BCSs), accompanied by reduced health-related quality of life (HRQOL). Objectives: This study investigated the influence of a multimodal therapy (MT; psychoeducation, eurythmy therapy, painting therapy, and sleep education/restriction), or a combination therapy (CT; MT plus aerobic training [AT]) on HRQOL in BCS with chronic CRF in comparison with AT alone. Methods: One hundred and twenty-six BCSs with CRF were included in a pragmatic comprehensive cohort study and allocated either per randomization or by preference to MT, CT, or AT. The EORTC QLQ-C30 core questionnaire was used to measure HRQOL. All analyses on HRQOL parameters were done in an explorative intention. Results: Patients were assigned to MT (n = 44), CT (n = 54), or AT (n = 28). CT was significantly superior to AT after 10 weeks of intervention (T1) in improving physical function. MT was found to have significant superiority over AT at T1 and T2 for physical functioning, emotional functioning, insomnia, and financial problems as well as role functioning, cognitive, social functioning, and fatigue 6 months later (T2). Conclusion: A multimodal approach appears to be a suitable concept for BCS with chronic CRF. A confirmatory study with larger samples should demonstrate the superiority of MT and adapted CT in HRQOL compared with the current treatment AT found in these explorative analyses.
Keywords: aerobic training, anthroposophic medicine, breast cancer, cancer-related fatigue, health-related quality of life, multimodal therapy
Introduction
Cancer-related fatigue (CRF) is one of the most common and burdensome consequences of cancer and its treatments.1 The National Comprehensive Cancer network defined CRF as “a distressing, persistent, subjective sense of physical, emotional and/or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning.”2 Factors describing the diversity of dimensions of CRF include energy deficiency, lack of concentration, sleep disturbances, emotional stress, and pain.2,3 CRF can occur during therapy in more than 70% of breast cancer patients, and after therapy in 34% of survivors, for more than 5 to 10 years after the first diagnosis.4-7 Previous studies have shown that CRF reduces health-related quality of life (HRQOL) in breast cancer patients by 20% to 30% during 3 to 48 months after completion of primary therapy compared with women without CRF.8 Research suggests that breast cancer survivors who consider their fatigue to be more of a disaster also have worse HRQOL than those who do not.8,9 The current therapy with the best available evidence for CRF is aerobic training (AT; small to medium effects).3,10-13 According to the National Comprehensive Cancer Network guidelines, AT is a category 1 recommendation,14 and according to the National Cancer Institute it is the only level 1 recommendation for CRF.15 Interventions such as sleep education/restriction and psychoeducational approaches capable of reducing CRF were also found.13,16-19 Nevertheless, there is insufficient evidence for the use of pharmacologic agents in CRF.11,20 Positive effects on HRQOL can also be achieved by painting therapy21 and eurythmy therapy.22 Due to the existing state of research, the manifold manifestations of CRF, and the insufficient clinical effects, the question arises whether multimodal therapy (MT) concepts could be more meaningful to improve CRF and HRQOL and to achieve larger effect sizes than monotherapies.23 The results of a pilot study investigating the effect of an MT approach (consisting of painting, eurythmy therapy, and psycho- and sleep-education) in breast cancer survivors showed positive effects on CRF, sleep quality, and autonomic and rest/activity regulation.24 In the next step, comparative effectiveness research appeared to be necessary. Therefore, a study was conducted comparing 10-week interventions of MT (psychoeducation, eurythmy therapy, painting therapy, and sleep-education) or a combination therapy (CT; MT with AT) with a control therapy of AT.25 Results on primary endpoint, safety outcome, and adherence were published elsewhere.25
This article reports the results of the exploratory analysis on whether an MT and CT concept for breast cancer survivors with CRF is superior to an AT on HRQOL after 10 weeks of intervention (T1) and 6 months later (T2). The results of HRQOL are of great importance, as this patient-reported outcome essentially complements the clinical relevance of the results of the primary endpoint, that is, the composite score of fatigue and disturbed sleep. In addition, we report the results of patients’ expectations.
Methods
The study was carried out between June 2011 and December 2013 in cooperation between the Research Institute Havelhöhe, Gemeinschaftskrankenhaus Havelhöhe, Gemeinschaftskrankenhaus Herdecke, the Hannover Medical School, the Institute of Integrative Medicine of Witten/Herdecke University, and the Gesellschaft für Klinische Forschung (Berlin, Germany).
The study design is a multicenter, prospective, parallel, 3-armed, openly randomized pragmatic trial in a comprehensive cohort design.
After obtaining informed consent, those patients who accepted randomization (balanced randomization list [1:1:1] with different permutation block sizes by a central randomization service for each center separately) were assigned either to MT, CT (CT = MT + AT), or the AT “control group.” If patients disagreed with randomization, they were allowed to choose an intervention according to their preference.
Interventions
The interventions were based on a manual defining the content, developed by a consensus of experts and tested in a pilot study.24,26 Before the start of the study, preparatory training and instruction for the therapists of all centers was carried out. Further details on the intervention are published in Kröz et al.25
Aerobic Training
The AT, conducted either as control therapy (AT) or in the CT arm, was conducted according to recommendations as home-based training (3-5 times per week/30-45 minutes) with 8 trainer-led sessions (45 minutes including 15 minutes’ rest). Participants were asked to document their practice at home in an exercise diary.
Multimodal Therapy
The MT arm uses 4 modules.
Sleep education
Basics of sleep and chronobiology were imparted in an information session. The aim of this module was to improve sleep quality by improving self-management in sleep disorders. The participants were asked to fill out a sleep diary, which was the basis for the recommendations at the end of sleep sessions and also served to record the individual adaption of sleep rhythm, sleep restriction and stimulus control, and adherence.
Psychoeducation
The session program began with information on the understanding of CRF to help improve coping and dealing with the disease and distressing feelings and thoughts. Body-orientated exercises to improve mindfulness, exercises to improve inner concentration, and stress management were also carried out by a psycho-oncologist.25
Eurythmy therapy
Eurythmy therapy, a mindfulness-orientated movement therapy in anthroposophic medicine, is characterized by (physical) “external movements,” which are transformed into (mental) “inner movements” in order to influence an awareness of one’s own body, mind, and soul.27-29 This module aims to improve the dysrhythmia associated with CRF through defined exercises, as described in detail by Kröz et al.25 Eurythmy therapy ended with a resting period of 15 minutes.
Painting therapy
Painting therapy is another therapeutic approach in anthroposophic medicine.30 Each therapy session began with the drawing of forms (dynamic closed forms) followed by watercolor painting. The method is described in detail in Kröz et al.25 Once a week, group sessions for MT and CT individuals were offered over a period of 10 weeks. Individuals in the MT and CT groups were required to attend group sessions for at least 7 days according to the protocol.
Participants
Most patients were recruited through local newspapers and physicians who informed their patients about the study; others spontaneously contacted the study centers.
Inclusion Criteria
The inclusion criteria included the following:
Women with breast cancer and CRF (18-75 years)
Time period after completion of chemotherapy and radiotherapy or surgery maximum 36 months
Fatigue Numerical Scale ≥4 and Cancer Fatigue Scale (CFS-D) ≥24
Duration of complaints at least 6 months
Time period after diagnosis maximum 45 months.
The exclusion criteria are as described in Kröz et al.25
Measures
Outcome parameters were assessed at baseline (T0), after 10 weeks of intervention (T1), and 6 months later (T2). Primary endpoint of the study was a composite score of the Pittsburgh Sleep Quality Index and the CFS-D.25 This article focuses on HRQOL measures as described below.
Health-Related Quality of Life (EORCT QLC-C30)
The cancer-specific, multidimensional 30-item EORTC QLQ-C30 core questionnaire was used to measure patients’ HRQOL. It is a widely used standard instrument that consists of functional scales, symptom scales, and a scale for global health status/quality of life (QoL).31,32 The 2 items forming the global health status/QoL scale are evaluated on a 7-point Likert-type scale, while all other items are evaluated on a 4-point Likert-type scale.31 The scales are converted from 0% to 100%.31,32 For the functional and global health status/QoL scale high scores correspond to good HRQOL. A high score in the symptom scales represents a high degree of symptomatology.33
Patients’ Expectations
The patients’ expectations were met with 5 items on physical changes (eg, “I expect a relief of my pain,” “I expect/hope for an improvement of my mobility,” and “I expect/hope for an improvement of my performance”), 4 items on psychological issues (eg, “I expect an increase of my self-confidence,” “I expect/hope for a psychological support,” and I expect/hope for a reduction in anxiety”), and 4 items on expectations in daily life, dealing with the disease, daily work, and making new acquaintances. Responses were rated on a 4-point Likert-type scale.34
Statistical Analysis
The sample size estimate of 114 patients focused on the primary parameters CFS-D and Pittsburgh Sleep Quality Index of the study; therefore, no performance calculations were performed on the HRQOL parameters.25 However, with this sample size a clinically relevant standardized difference of 0.5, corresponding to a 2-group difference of approximately 10% with a power of 75%, was detected.35 In order to control the possible bias between patients who prefer or reject randomization and between treatment arms of the preference group, 2 corresponding inclination scores were included in the model (to adjust to preference/randomization and to the choice of study arm in the nonrandomized group). No significant influence of these covariates could be found. Descriptive statistics for demographic and disease-specific variables were performed to show which relevant prognostic factors differ between the 3 treatment arms at the beginning. The van-Elteren and the Cochran-Mantel-Haenszel (CMH) tests (stratified for preference/randomization) were used to test for differences at a local significance level of 5%.
The treatment arms were tested for superiority of MT and CT over AT in relation to the dimension of EORTC, using a general linear model that included the parameters “preference/randomization” and “treatment” as fixed factors as well as the respective dimension baseline and the 2 propensity scores as covariates.
Model fit was assessed by graphical analysis of the quantile-quantile plot and by Akiake’s Information Criterion. According to the intention-to-treat principle, all patients were analyzed with valid baseline data (T0). Missing items were replaced according respective manuals of the questionnaire. The last-value-carried-forward method was used to calculate missing data for T1 in the main analysis. Statistical tests were performed bilaterally on a local α error level of α = 5%; corresponding 95% confidence intervals were calculated. No α error fitting was performed for multiple statistical testing as this exploratory analysis of the EORTC’s dimensions as secondary effectiveness parameters was intended to identify potentially clinically relevant treatment differences rather than to protect against a randomly occurring treatment difference. Sample size estimation, randomization lists, and analyses were performed using SAS for Windows 9.2 (SAS Institute, Cary, NC).
Results
Participants and Adherence to the Intervention
A total of 126 patients were assigned to 1 of the 3 treatment arms according to their preference (n = 61) or by randomization (n = 65). Before the intervention, 21 patients decided not to participate, so 105 patients started the intervention (AT = 20; CT = 51; and MT = 34). During the 10-week intervention period, 21 patients dropped out and later between the end of intervention and the 6-month follow-up of further patients (n = 25; the flowchart for recruitment and for drop-out reasons is described by Kröz et al25).
A total of 84 patients underwent the intervention (T1), and 81 patients underwent follow-up after 6 months (T2). The dropout rate for T1 was 25% in the AT group, 17% in the MT group, and 21.5% in the CT group. The CMH test, which tested whether there was a disposition difference between the 3 treatment arms, showed a statistically significant result (CMH P = .020).
Baseline Characteristics
At baseline, enrolled patients were on average 59 years old (SD = 10) in AT, 58 years old (SD = 11) in MT, and 56 years old (SD = 8) in CT. At baseline, no significant differences were observed between the 3 arms in terms of sociodemographic characteristics, tumor stage and biology, and treatment (Table 1).25 The baseline data related to the outcomes are presented in Table 2 without statistically significant differences for the EORTC QLQ-C30 dimensions.
Table 1.
| Treatment Group |
P | |||
|---|---|---|---|---|
| AT | MT | CT | ||
| Included | 28 | 44 | 54 | |
| Completed | 13 | 30 | 41 | |
| Marital status | ||||
| Single, n (%) | 1 (5.00) | 8 (23.53) | 8 (16.00) | |
| Married, n (%) | 14 (0.00) | 16 (47.06) | 27 (54.00) | |
| Divorced, n (%) | 3 (15.00) | 8 (23.53) | 13 (26.00) | |
| Widowed, n (%) | 2 (10.00) | 2 (5.88) | 2 (14.00) | |
| Children, n (%)/children at home, n (%) | 16 (84.21)/6 (31.58) | 23 (65.71)/8 (23.53) | 38 (74.51)/11 (24.44) | .2638/.6285 |
| Employment | .1842 | |||
| Employed, n (%) | 9 (47.37) | 11 (32.35) | 25 (52.08) | |
| Housewife, n (%) | 1 (5.26) | 3 (8.82) | 1 (2.08) | |
| Unemployed, n (%) | 0 (0.00) | 1 (2.94) | 6 (12.50) | |
| Pensioner, n (%) | 6 (31.58) | 13 (38.24) | 11 (22.92) | |
| Sickness certificate, n (%) | 3 (15.79) | 4 (11.76) | 4 (8.33) | |
| Other, n (%) | 0 (0.00) | 2 (5.88) | 1 (2.08) | |
| Vocational education | .2138 | |||
| Apprenticeship, n (%) | 9 (56.25) | 13 (46.43) | 20 (50.00) | |
| Technical college, n (%) | 4 (25.00) | 3 (10.71) | 3 (7.50) | |
| University of Applied Sciences, n (%) | 3 (18.75) | 2 (7.14) | 4 (10.00) | |
| University, n (%) | 0 (0.00) | 9 (32.14) | 12 (30.00) | |
| Other, n (%) | — | — | — | |
| No, n (%) | 0 (0.00) | 1 (3.57) | 1 (2.50) | |
| Age, mean (SD) | 58.6 (10.0) | 58 (10.6) | 56.4 (7.7) | .544 |
| Years since first diagnosis, mean (SD) | 2.0 (0.9) | 2.3 (0.8) | 1.8 (0.8) | .0861 |
| Surgery | ||||
| Yes, n/% | 28/100.0 | 44/100.0 | 54/100.0 | |
| Chemotherapy | ||||
| Yes, n (%) | 17 (60.71) | 26 (59.09) | 21 (38.89) | .0821 |
| Years since chemotherapy, mean (SD) | 1.9 (0.7) | 1.6 (0.7) | 1.8 (0.7) | .4462 |
| Radiotherapy | ||||
| Yes, n/% | 18/64.29 | 37/84.09 | 40/74.07 | .4024 |
| Antihormonal therapy | ||||
| Yes, n/% | 22/78.57 | 27/61.36 | 34/62.96 | .5815 |
| Mistletoe therapy | ||||
| Yes, n/% | 7/25.00 | 8/18.18 | 14/25.93 | .6038 |
| UICC stages | .1608 | |||
| CIS, n (%) | 1 (3.57) | 1 (2.27) | 0 (0.00) | |
| I, n (%) | 11 (39.29) | 28 (63.64) | 38 (70.37) | |
| II, n (%) | 6 (21.43) | 10 (22.73) | 10 (18.52) | |
| III, n (%) | 6 (21.43) | 5 (11.36) | 3 (5.56) | |
| Grading | .4551 | |||
| 1, n (%) | 4 (14.29) | 4 (9.09) | 12 (22.22) | |
| 2, n (%) | 12 (42.86) | 20 (45.45) | 19 (35.19) | |
| 3, n (%) | 6 (21.43) | 13 (29.55) | 14 (25.93) | |
| No data available | 6 (21.34) | 7 (15.91) | 9 (16.67) | |
Abbreviations: AT, aerobic training; MT, multimodal therapy; CT, combination therapy; UICC, Union for International Cancer Control; CIS, Cancer Information Service.
Test on difference: Cochran-Mantel-Haenszel (categorical data) or van-Elteren test (ordinal/interval data) stratified for preference/randomization.
Table 2.
Baseline Data for Outcomes of Study Population (Participants at Baseline).
| Outcomes | AT, Mean (SD) | MT, Mean (SD) | CT, Mean (SD) | Difference P |
|---|---|---|---|---|
| EORTC QLQ-C30 | ||||
| Physical Functioning | 66.7 (14.0) | 60.4 (19.1) | 63.6 (16.1) | .62 |
| Role Functioning | 55.8 (22.5) | 46.6 (27.1) | 50.3 (25.7) | .62 |
| Emotional Functioning | 47.5 (26.4) | 43.5 (21.6) | 41.7 (20.0) | .81 |
| Cognitive Functioning | 60.8 (28.7) | 53.9 (28.7) | 47.1 (23.0) | .24 |
| Social Functioning | 55.0 (32.0) | 56.9 (31.8) | 49.7 (32.2) | .65 |
| Global Health Status | 53.8 (17.6) | 53.5 (20.7) | 54.0 (18.1) | .92 |
| Fatigue | 66.1 (17.1) | 74.5 (17.2) | 68.2 (20.6) | .25 |
| Nausea/Vomiting | 2.5 (8.2) | 9.8 (14.3) | 7.2 (11.7) | .23 |
| Pain | 38.3 (30.2) | 48.0 (37.8) | 39.2 (34.9) | .51 |
| Dyspnea | 28.1 (33.8) | 42.2 (34.1) | 38.6 (29.4) | .24 |
| Insomnia | 65.0 (29.6) | 79.4 (27.2) | 66.7 (32.3) | .11 |
| Appetite Loss | 6.7 (17.4) | 20.6 (28.4) | 19.0 (26.9) | .10 |
| Constipation | 10.0 (19.0) | 19.6 (30.8) | 10.5 (22.6) | .42 |
| Diarrhea | 8.8 (21.8) | 12.7 (23.2) | 11.1 (23.7) | .57 |
| Financial Problems | 21.7 (32.9) | 37.3 (37.4) | 38.0 (38.7) | .42 |
Abbreviations: AT, aerobic training; MT, multimodal therapy; CT, combination therapy.
Comparison of Therapy Arms With Regard to Their Efficacy on HRQOL (EORTC QLQ-Q30)
In terms of functioning and global health status scales, increases in mean values for the CT group compared with the baseline, which represents a clinically relevant improvement in function (ie, an increase of about 10%), were observed in all dimensions for T1, and smaller, but partially clinically still relevant, increases (in terms of physical, role, and cognitive functioning) for T2. In the MT group, clinical improvements in functioning were equally observed in all dimensions at T1 and T2. For AT only for cognitive functioning (at T1) as well as for social functioning and global health status (at T1 and T2) an increase in mean values was shown, with clinically relevant improvement at T1. In addition, a clinical deterioration (ie, a decrease in mean values of 10% or more compared with baseline) was detected for emotional functioning at T2 (Table 3).
Table 3.
Differences From Baseline Values at T1 and T2a.
| AT | MT | P b | CT | P c | |
|---|---|---|---|---|---|
| EORTC Physical Functioning | |||||
| T1-T0 | −1.7 (16.1) | 11.9 (12.0) | .005 | 9.7 (14.9) | .007 |
| T2-T0 | −2.6 (14.5) | 11.6 (14.6) | .012 | 5.1 (6.8) | .186 |
| EORTC Role Functioning | |||||
| T1-T0 | 3.0 (20.9) | 19.5 (29.2) | .061 | 14.2 (27.8) | .178 |
| T2-T0 | −1.3 (17.3) | 20.4 (25.5) | .014 | 6.3 (31.0) | .185 |
| EORTC Emotional Functioning | |||||
| T1-T0 | −0.7 (16.8) | 14.9 (20.1) | .039 | 10.6 (24.4) | .193 |
| T2-T0 | −10.9 (22.4) | 13.5 (21.1) | .002 | 3.6 (25.2) | .072 |
| EORTC Cognitive Functioning | |||||
| T1-T0 | 13.9 (13.9) | 13.2 (22.4) | .513 | 11.8 (19.1) | .772 |
| T2-T0 | −2.6 (23.4) | 12.8 (25.1) | .034 | 10.4 (19.0) | .120 |
| EORTC Social Functioning | |||||
| T1-T0 | 8.4 (29.7) | 12.7 (25.8) | .239 | 14.2 (27.0) | .369 |
| T2-T0 | 1.3 (25.0) | 14.1 (26.5) | .045 | 3.1 (30.1) | .528 |
| EORTC Global Health Status | |||||
| T1-T0 | 13.9 (22.8) | 10.1 (18.8) | .934 | 9.4 (18.9) | .855 |
| T2-T0 | 5.1 (19.4) | 9.0 (20.1) | .421 | 3.2 (22.8) | .767 |
| EORTC Fatigue | |||||
| T1-T0 | −10.2 (18.6) | −20.3 (21.2) | .193 | −17.2 (21.1) | .268 |
| T2-T0 | −0.9 (23.8) | −20.6 (22.1) | .012 | −11.1 (26.1) | .149 |
| EORTC Nausea/Vomiting | |||||
| T1-T0 | 0.0 (7.1) | −1.2 (11.8) | .603 | −2.8 (12.9) | .709 |
| T2-T0 | 7.7 (14.6) | −0.6 (15.0) | .716 | 1.8 (22.1) | .872 |
| EORTC Pain | |||||
| T1-T0 | 2.8 (22.3) | −8.6 (17.0) | .119 | −1.2 (25.9) | .509 |
| T2-T0 | 5.1 (24.9) | −11.7 (33.0) | .119 | 3.1 (35.5) | .473 |
| EORTC Dyspnea | |||||
| T1-T0 | −3.0 (31.5) | −10.3 (26.9) | .949 | −9.8 (35.2) | .956 |
| T2-T0 | −2.8 (26.4) | −11.1 (29.3) | .432 | −16.7 (30.4) | .114 |
| EORTC Insomnia | |||||
| T1-T0 | −5.6 (27.8) | −32.2 (33.9) | .012 | −20.8 (28.9) | .075 |
| T2-T0 | −10.3 (21.1) | −33.3 (32.0) | .019 | −22.9 (34.1) | .061 |
| EORTC Appetite Loss | |||||
| T1-T0 | 2.8 (9.6) | −6.9 (22.5) | .345 | −4.1 (28.1) | .604 |
| T2-T0 | 10.3 (31.6) | −6.2 (26.2) | .561 | 3.6 (32.2) | .846 |
| EORTC Constipation | |||||
| T1-T0 | 0.0 (0.0) | −3.5 (24.1) | .538 | −0.8 (17.4) | .602 |
| T2-T0 | 5.1 (12.5) | 6.4 (23.1) | .334 | 6.3 (25.9) | .615 |
| EORTC Diarrhea | |||||
| T1-T0 | 6.1 (29.1) | −1.1 (33.9) | .818 | 1.7 (26.1) | .829 |
| T2-T0 | 13.9 (22.3) | −3.8 (28.8) | .133 | 4.5 (30.6) | .526 |
| EORTC Financial Problems | |||||
| T1-T0 | 8.3 (25.1) | −13.8 (30.2) | .047 | −3.3 (25.9) | .553 |
| T2-T0 | 12.8 (32.0) | −14.1 (28.6) | .028 | 0.9 (21.8) | .421 |
Abbreviations: AT, aerobic training; MT, multimodal therapy; CT, combination therapy; EORTC, European Organisation for Research and Treatment of Cancer.
Table shows the mean differences (± standard deviation) from T0 to T1 or T2 for the EORTC dimensions and the P values for the test for superiority of MT and CT over AT. All results are displayed for both T1 and T2 and are based on the imputed data set with missing value.
P values of the van-Elteren test stratified for preference/randomization for difference between MT versus AT.
P values of the van-Elteren test stratified for preference/randomization for difference between CT versus AT.
P values in boldface indicate statistically siginificance.
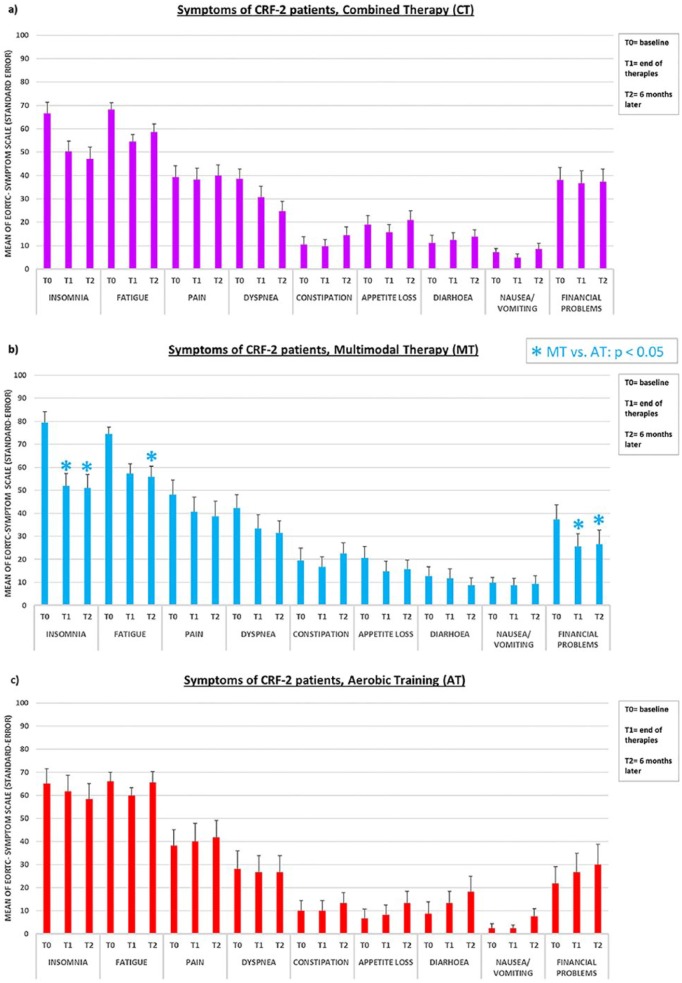
In the symptom scales, clinically relevant improvements over the baseline (ie, a decrease in mean values of 10% or more) in CT were shown only for fatigue, dyspnea, and insomnia at T1 and T2. Equivalent (and for insomnia even higher) clinical improvements of symptomatology were observed in the MT arm and additionally in pain and financial problems (also at T1 and T2; Table 3 and Figure 1b). A clinically relevant improvement in symptom scales in the AT group was observed only in fatigue at T1 and insomnia at T2, while a relevant deterioration (ie, an increase in symptom mean values of 10% or more) was detected at T2 for loss of appetite, diarrhea, and financial problems (Table 3 and Figure 1c).
Figure 1.
EORTC QLQ-C30: Mean values in the symptom scales in the CT, MT, and AT group at T0, T1, and T2, respectively.
Values are based on the ITT data set, with missing values imputed by LOCF. (a) CT: n = 51; (b) MT: n = 34; (c) AT: n = 20.
*Intention-to-treat analysis for MT versus AT, P < .05.
Combined Therapy (MT + AT) Versus AT
CT was significantly superior to AT compared only at T1 in improving physical functioning (P = .007, SES = 0.679). For none of the other dimensions the superiority test of CT over AT showed a significant result, neither for T1 nor for T2 (test for superiority of CT over AT; P values all >.05; Table 3).
Multimodal Therapy Versus AT
The results showed a significant superiority of MT over AT in improving the dimensions of physical functioning, emotional functioning, insomnia, and financial problems after 10 weeks of intervention. For all these areas, the superiority remained at T2, that is, physical functioning, emotional functioning, insomnia, and financial problems (all P < .05; details in Table 3). In addition, MT at T2 was also significantly better for the EORTC dimensions role functioning, cognitive functioning, social functioning, and fatigue than AT in T2 (all P < .05; details in Table 3).
Patients’ Expectations
With regard to physical changes, 63% in the AT group, 47% in the MT group, and 47% in the CT group expected a relief of their pain at baseline. A positive effect on their flexibility and discomforts was expected by more than 75% in all the 3 treatment arms. All participants of the 3 groups expected an increase in performance and most participants also expected physical (AT 89%; MT 91%; and CT 96%) and mental (AT 94%; MT 91%; and CT 86%) recovery. The majority of participants in each group expected to cope more effectively with everyday life (AT 94%; MT 97%; and CT 92%), work life (AT 83%; MT 58%; and CT 60%), illness (AT 75%; MT 82%; and CT 78%), anxiety reduction (AT 75%, MT 67%; and CT 60%), and an increase in self-confidence (AT 50%; MT 66%; and CT 68%). To make new acquaintances was expected before the intervention start by 60% in the AT group, 53% in the MT group, and 38% in the CT group. No significant difference between the 3 groups was found in any of the dimensions of patient expectation.
Discussion
The aim of this explorative study analysis was to investigate whether a multimodal and combined multimodal-aerobic therapy (CT) for breast cancer patients with a relevant CRF is superior to AT as monotherapy with regard to HRQOL. CT was found to be significantly superior to AT only in improving the physical functioning of the EORTC domain, while a significant superiority of the MT concept was found in the domains physical functioning, emotional functioning, insomnia, and financial problems compared with AT after 10 weeks of intervention, which was stable 6 months later, in addition to significantly better fatigue, cognitive functioning, and social functioning. This suggests a good sustainability of the MT concept for these HRQOL dimensions. Since the results are based on an exploratory analysis, the results are understood as indications with hypothesis-generating character. Nevertheless, they suggest that MT as monotherapy was more effective than AT in various dimensions. Previous studies have shown that a multimodal approach can be a valuable treatment to cope with the complexity of CRF in breast cancer patients.24 The strengths of the developed MT approach lies, among other things, in the demand-oriented, clinical, highly relevant improvement of symptom burden due to insomnia and fatigue of the target group. This may be related to the multimodal approach of various evidence-based interventions, including chronobiological adaptation through sleep intervention, psychoeducational, and possible mindfulness-oriented treatments3 that focus on the complex chronobiological, neuroendocrine-immune mechanisms of behavioral comorbidity in patients with CRF.36 In particular, the strong improvements in insomnia and fatigue of 30% and 20%, respectively, in the MT approached group are clinically highly significant and indicate a specific intervention effect. According to Osoba et al,35 mean changes of 5% to 10% in EORTC QLQ-C30 scores are already clinically relevant.
Therefore, the improvements in the CT also indicate a high clinical impact. In the context of a long-term follow-up, it should be investigated whether it is possible to sustainably reduce CRF and early retirement by participating in the developed MT or CT concept. The reason for the significant improvement in the dimension of the financial problems of EORTC in the MT group may be due to subjective changes in perception and improved well-being, which may make their financial situation less problematic. On the other hand, improved performance through reduced complaints can increase work capacity and thus reduce financial problems. One possible cause for the nonsuperiority of the more complex CT intervention compared with AT monotherapy could be a burden on patients in the CT arm, since greater effort is required to participate in several therapy sessions. It could be assumed that the number of treatment options implemented was so high that some individuals could not adapt mentally, emotionally, and physically, and therefore, positive effects were found only in the physical functioning domain. The relatively higher frequency of adverse events in the CT group compared with the MT arm indicates an excessive demand for the physical abilities of participants in this study arm.25
Further research is needed to clarify the appropriate “dose” and the most effective combination of modules for MT without exceeding the patients’ resources. Horneber et al3 summarized as central goals of CRF treatment the alleviation of factors that can negatively influence the symptoms of patients’ CRF by offering an individualized approach and activating individual resources. It will be challenging to develop customizable multimodal approaches and to demonstrate the best combination of treatment components for maximum effectiveness in patients’ CRF and HRQOL.37,38 The mean values for HRQOL (EORTC QLQ-C30) in all 3 arms after the intervention (T1, T2) differ clearly from the reference data of the general German population provided by Hinz et al.39 This is consistent with the results of studies that show that CRF affects the lives of breast cancer survivors even years after the first diagnosis.4,5,40 The results of this study underline the importance of understanding how sustained long-term therapeutic effects can be induced to improve HRQOL and to reduce early retirement rates in breast cancer survivors with chronic CRF.41
Limitations
Due to the chosen study design, the distinct contributions of the different treatment components remain unclear as they were applied as “packages.” Further studies are needed to investigate the contribution of each specific treatment.25 The analyses presented here were never intended as conclusive proof of efficacy, but as hypothesis-generating. In comparison to a conventional randomized study, participants were free to choose a treatment arm if they rejected randomization. These are 2 separate choices to consider, which introduce 2 types of selection bias in the distribution of patients across treatment arms and must be considered separately. This was done by including 2 respective propensity scores in the analysis with the aim of adjusting treatment effects to possible differences in prognostic and/or predictive markers. Even if the most important baseline values do not differ significantly, different means can be related by tendency to the preference group. This will be published in a separate article. In order to identify the relevance of an attrition bias, preference and randomized group were compared without finding relevant differences. In all statistical comparisons, propensity scores were included as compensating factors aiming to control possible bias.
In addition, the low number of participants in the 3 therapy arms and the unequal distribution of study participants (especially in the AT arm) are further limitations. The relatively high number of dropouts is not surprising for the relatively long intervention period, the known adherence problems in endurance training studies42 and the load on the intervention days in the CT and MT groups. The higher dropout rate in AT may be due to a lower improvement in insomnia and to the primary outcome of fatigue/sleep quality25 as well as the tendency to fatigue. To address this problem, a suitable method for calculating the missing values was chosen.
Conclusion
A combined therapy approach with a multimodal concept and aerobic training was superior compared with aerobic training only in improving physical functioning in breast cancer survivors with a chronic CRF, while for some relevant dimensions of patients’ HRQOL, especially insomnia and fatigue, a significant superiority of the multimodal approach over aerobic training alone was found. Future research is necessary to find the most effective combination of therapies for a multimodal therapy approach without exceeding the resources of the participants.
Acknowledgments
The authors thank the CRF-2 study group collaboration: Danilo Pranga, Fadime ten Brink, Bettina Berger, and Anette Zander for study group coordination and documentation; Annette Weninger for documentation; Nina Klara for recruitment; Augustine Glinz for recruitment and compiling graphics; Christian Bartsch for biochemical analysis; Benno Brinkhaus for scientific and methodological advice and support; Mahmud Naghavi, Peter Klug, Andrea Küssmann, and Petra Wundram for exercise training; Michaele Quetz, Sarah Zastrutzki, Dorothea Friemel, Anne Kristin Meyer, Judith Schulz, Martina Jackmuth, and Suzanne Mertens for psychoeducation; Sarah Zastrutzki and Christian Heckmann for sleep education; Barbara Trapp, Ursula Heusser, Frederika Rettigand, and Elisabeth Rieger for eurythmy therapy; and Astrid Diwidzius, Béatrice Gelin-Kröz, Karen Baumhöver-Wegener, Birgit Lindemann, and Sonja Steffens for painting therapy. We thank Ramona Beutke for the monitoring.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was funded by Mahle Stiftung, Stuttgart, Germany; Christophorus Stiftung, Stuttgart, Germany; Dr Hauschka Stiftung, Bad Holt/Eckwäldern, Germany; Gyllenberg Foundation, Helsinki, Finland; and Stiftung Helixor, Rosenfeld, Germany. The study sponsors had no involvement in the study design, collection, analysis, or interpretation of data. MK received financial support from Software AG Stiftung, Darmstadt, Germany. MK and RZ received financial support from Humanus Institute, Berlin, Germany.
References
- 1. Holz SACC, Smith SR. Cancer-related fatigue: what you need to know. Arch Phys Med Rehabil. 2017;98:1717-1718. [DOI] [PubMed] [Google Scholar]
- 2. National Comprehensive Cancer Network. Cancer-related fatigue. NCCN guidelines. https://www.nccn.org/professionals/physician_gls/PDF/fatigue.pdf. Accessed December 19, 2016.
- 3. Horneber M, Fischer I, Dimeo F, Ruffer JU, Weis J. Cancer-related fatigue: epidemiology, pathogenesis, diagnosis, and treatment. Dtsch Arztebl Int. 2012;109:161-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bower JE, Ganz PA, Desmond KA, et al. Fatigue in long-term breast carcinoma survivors: a longitudinal investigation. Cancer. 2006;106:751-758. [DOI] [PubMed] [Google Scholar]
- 5. Minton O, Stone P. How common is fatigue in disease-free breast cancer survivors? A systematic review of the literature. Breast Cancer Res Treat. 2008;112:5-13. [DOI] [PubMed] [Google Scholar]
- 6. Gutenbrunner C, Girke M, Dimeo F, et al. The cancer fatigue syndrome—an overview. Phys Med Rehabil Kurortmed. 2010;20:86-91. [Google Scholar]
- 7. Kröz M, Reif M, Bartsch C, et al. Impact of autonomic and self-regulation on cancer-related fatigue and distress in breast cancer patients—a prospective observational study. J Cancer Surviv. 2014;3:319-328. [DOI] [PubMed] [Google Scholar]
- 8. Alexander S, Minton O, Andrews P, Stone P. A comparison of the characteristics of disease-free breast cancer survivors with or without cancer-related fatigue syndrome. Eur J Cancer. 2009;45:384-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jacobsen PB, Andrykowski MA, Thors CL. Relationship of catastrophizing to fatigue among women receiving treatment for breast cancer. J Consult Clin Psychol. 2004;72:355-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cramp F, Daniel JB. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev. 2012;(2):CD006145. doi: 10.1002/14651858.CD006145.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Minton O, Berger A, Barsevick A, et al. Cancer-related fatigue and its impact on functioning. Cancer. 2013;119(suppl 11):2124-2130. [DOI] [PubMed] [Google Scholar]
- 12. Fong DYT, Ho JWC, Hui BPH, et al. Physical activity for cancer survivors: meta-analysis of randomised controlled trials. BMJ. 2012;344:e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Duijts SFA, Faber MM, Oldenburg HSA, van Beurden M, Aaronson NK. Effectiveness of behavioral techniques and physical exercise on psychosocial functioning and health-related quality of life in breast cancer patients and survivors—a meta-analysis. Psychooncology. 2011;20:115-126. [DOI] [PubMed] [Google Scholar]
- 14. Berger AM, Mooney K, Alvarez-Perez A, et al. ; National Comprehensive Cancer Network. Cancer-related fatigue, version 2.2015. J Natl Compr Canc Netw. 2015;13:1012-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Institute NC. Fatigue (PDQ)®–Health Professional Version. https://www.cancer.gov/about-cancer/treatment/side-effects/fatigue/fatigue-hp-pdq. Accessed September 29, 2017.
- 16. Ahlberg K, Ekman T, Gaston-Johansson F, Mock V. Assessment and management of cancer- related fatigue in adults. Lancet. 2003;362:640-650. [DOI] [PubMed] [Google Scholar]
- 17. Kangas M, Bovbjerg DH, Montgomery GH. Cancer-related fatigue: a systematic and meta-analytic review of non-pharmacological therapies for cancer patients. Psychol Bull. 2008;134:700-741. [DOI] [PubMed] [Google Scholar]
- 18. Berger AM, Kuhn BR, Farr LA, et al. Behavioral therapy intervention trial to improve sleep quality and cancer-related fatigue. Psychooncology. 2009;18:634-646. [DOI] [PubMed] [Google Scholar]
- 19. Aricò D, Raggi A, Ferri R. Cognitive behavioral therapy for insomnia in breast cancer survivors: a review of the literature. Front Psychol. 2016;7:1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Minton O, Richardson A, Sharpe M, Hotopf M, Stone P. Drug therapy for the management of cancer-related fatigue. Cochrane Database Syst Rev. 2010;(7):CD006704. doi: 10.1002/14651858.CD006704.pub3 [DOI] [PubMed] [Google Scholar]
- 21. Geue K, Goetze H, Buttstaedt M, Kleinert E, Richter D, Singer S. An overview of art therapy interventions for cancer patients and the results of research. Complement Ther Med. 2010;18:160-170. [DOI] [PubMed] [Google Scholar]
- 22. Lötzke D, Heusser P, Büssing A. A systematic literature review on the effectiveness of eurythmy therapy. J Integr Med. 2015;13:217-230. [DOI] [PubMed] [Google Scholar]
- 23. Escalante CP, Kallen MA, Valdres RU, Morrow PK, Manzullo EF. Outcomes of a cancer-related fatigue clinic in a comprehensive cancer center. J Pain Symptom Manage. 2010;39:691-701. [DOI] [PubMed] [Google Scholar]
- 24. Kröz M, Fink M, Reif M, et al. Multimodal therapy concept and aerobic training in breast cancer patients with chronic cancer-related fatigue. Integr Cancer Ther. 2013;12:301-311. [DOI] [PubMed] [Google Scholar]
- 25. Kröz M, Reif M, Glinz A, et al. ; CRF-2 Study Group. Impact of a combined multimodal-aerobic and multimodal intervention compared to standard aerobic treatment in breast cancer survivors with chronic cancer-related fatigue—results of a three-armed pragmatic trial in a comprehensive cohort design. BMC Cancer. 2017;17:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kröz M, Quetz M, Houben H, et al. Handbuch—zu einem multimodalen Interventionsprogramm bei Frauen mit Mammakarzinom mit einem Cancer Fatigue Syndrom. Berlin, Germany: Forschungsinstitut Havelhöhe; 2011. [Google Scholar]
- 27. von Laue HB, von Laue EE. Zur Physiologie der Heileurythmie: Lautgesetze und Therapieordnungen. Dornach, Switzerland: Verlag am Goetheanum; 2006. [Google Scholar]
- 28. Ritchie J, Wilkinson J, Gantley M, Feder G, Carter Y, Formby J. A model of integrated primary care: anthroposophic medicine. http://www.camphillwellbeing.org.uk/wp-content/uploads/2016/01/Integrated-Primary-Care-CWT-research.pdf. Published January 2001. Accessed December 6, 2018.
- 29. Kirchner-Bockholt M. Grundelemente der Heil-Eurythmie. Dornach, Switzerland: Verlag am Goetheanum; 2010. [Google Scholar]
- 30. Hauschka M, Fiechter C. Zur Künstlerischen Therapie, Band 2: Wesen und Aufgabe der Maltherapie. Gӧppingen, Germany: Schule für Künstlerische Therapie u. Massage; 1978. [Google Scholar]
- 31. Aaronson NK, Cull AM, Kaasa S, Sprangers M. The European Organization for Research and Treatment of Cancer (EORTC) Modular Approach to Quality of Life Assessment in Oncology: An Update. Quality of Life and Pharmacoeconomics in Clinical Trials. Philadelphia, PA: Lippincott-Raven; 1996:179-189. [Google Scholar]
- 32. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use. J Natl Cancer Inst. 1993;85:365-376. [DOI] [PubMed] [Google Scholar]
- 33. Scott NW, Fayers PM, Aaronson NK, et al. EORTC QLQ-C30 reference values. https://www.eortc.org/app/uploads/sites/2/2018/02/reference_values_manual2008.pdf. Published July 2008. Accessed December 6, 2018.
- 34. Gutenbrunner C, Hübner J, Hamann T, et al. Rehabilitationsbedarf bei Patientinnen mit Mammakarzinom. Phys Med Rehabil Kurortmed. 2007;17:A15. [Google Scholar]
- 35. Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139-144. [DOI] [PubMed] [Google Scholar]
- 36. Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. J Clin Oncol. 2008;26:971-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Koller M, Neugebauer EAM, Augustin M, et al. Assessment of quality of life in health services research - conceptual, methodological and structural prerequisites [in German]. Gesundheitswesen. 2009;71:864-872. [DOI] [PubMed] [Google Scholar]
- 38. Lenz G. Health care and rehabilitation. In: Kirch W, ed. Encyclopedia of Public Health. New York, NY: Springer; 2008:533-540. [Google Scholar]
- 39. Hinz A, Singer S, Brähler E. European reference values for the quality of life questionnaire EORTC QLQ-C30: results of a German investigation and a summarizing analysis of six European general population normative studies. Acta Oncol. 2014;53:958-965. [DOI] [PubMed] [Google Scholar]
- 40. Zerm R, Kröz M, Brauer D, et al. Cancer-related fatigue and disturbed rest–activity rhythm in recurrence-free breast cancer patients—results of a prospective study of CAM users. Eur J Integr Med. 2008;1:13-14. [Google Scholar]
- 41. Weis J, Bartsch HH. Fatigue bei Tumorpatienten: Prävalenz und Rehabilitationsbedarf. http://forschung.deutcshe-rentenversicherung.de/ForschPortalWeb/rehaDoc.pdf. Accessed December 25, 2016.
- 42. Kemmler W, Scharf M, Lell M, et al. High versus moderate intensity running exercise to impact cardiometabolic risk factors: the randomized controlled RUSH-study. BioMed Res Int. 2014;2014:e843095. [DOI] [PMC free article] [PubMed] [Google Scholar]