ABSTRACT
Eukaryotic cells respond to an overload of unfolded proteins in the endoplasmic reticulum (ER) by activating signaling pathways that are referred to as the unfolded protein response (UPR). Much UPR research has been conducted in cultured cells that exhibit no baseline UPR activity until they are challenged by ER stress initiated by chemicals or mutant proteins. At the same time, many genes that mediate UPR signaling are essential for the development of organisms ranging from Drosophila and fish to mice and humans, indicating that there is physiological ER stress that requires UPR in normally developing animal tissues. Recent studies have elucidated the tissue-specific roles of all three branches of UPR in distinct developing tissues of Drosophila, fish and mammals. As discussed in this Review, these studies not only reveal the physiological functions of the UPR pathways but also highlight a surprising degree of specificity associated with each UPR branch in development.
KEY WORDS: Unfolded protein response, Endoplasmic reticulum, IRE1, XBP1, PERK, eIF2α, ATF4, ATF6, Pancreas, Eye
Summary: We review evidence that normally developing tissues have physiological ER stress, and the three branches of the unfolded protein response (UPR) exhibit a high level of specificity in promoting their development.
Introduction
In eukaryotic cells, the endoplasmic reticulum (ER) serves as the primary site of folding and maturation of secretory and transmembrane proteins. Nascent polypeptides undergo a complex, multistep chaperone-assisted process of maturation to attain their native structure (Braakman and Hebert, 2013). Failure of proteins to undergo proper protein folding and maturation in this organelle imposes stress to the ER, eventually activating an array of cellular responses that are widely referred to as the unfolded protein response of the ER (UPRER; henceforth abbreviated as UPR). These signaling pathways reduce global protein synthesis and enhance the cellular protein folding capacity, which, in concert, help to restore the viability of the organism in the face of altered proteostasis (Han and Kaufman, 2017; Hetz and Papa, 2018; Walter and Ron, 2011).
In metazoans ranging from Drosophila, Caernorhabditis elegans and fish to mammals, the UPR is mediated by the three well-known ER stress sensor molecules: inositol requiring 1 (IRE1), pancreatic ER eIF2α kinase (PERK), and activating transcription factor 6 (ATF6). Upon sensing the presence of unfolded proteins inside the ER, these sensors activate downstream transcription factors (XBP1, ATF4 and ATF6, respectively) to induce stress response gene expression (Han and Kaufman, 2017; Hetz and Papa, 2018; Walter and Ron, 2011).
A large body of work has characterized in detail the molecular mechanisms of UPR regulation, and has been reviewed extensively elsewhere (Han and Kaufman, 2017; Hetz and Papa, 2018). A majority of the studies are based on cultured cells that show no baseline UPR activity until they are challenged by ER stress-causing chemicals or mutant proteins. However, the idea that healthy cells have no baseline UPR activity is inconsistent with the observation that UPR-mediating genes are essential for development and survival of a number of species ranging from Drosophila and fish to mice and humans (see below for details). The requirement for UPR-inducing genes indicates that certain cell types require UPR activity, not only to respond to mutant proteins and to stress imposed by external sources, but also to respond to physiological conditions associated with normal development. However, compared to what has been elucidated in cultured cells exposed to ER stress-causing agents, our understanding of the physiological roles of UPR in metazoan tissues remains poor. Here, we review the recent advances with regard to the in vivo role of UPR during metazoan development.
The role of the IRE1–XBP1 branch of the UPR in development and differentiation
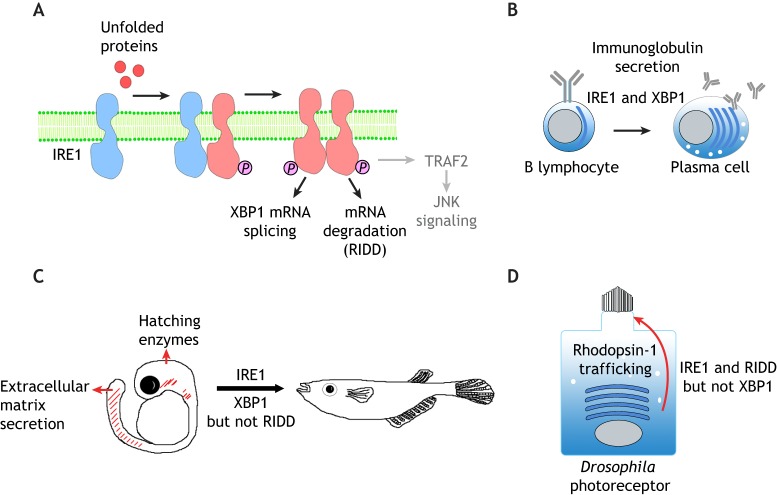
IRE1 is an ER-resident transmembrane protein with both kinase and endoribonuclease activities (Cox et al., 1993; Cox and Walter, 1996; Mori et al., 1993). Accumulation of unfolded proteins inside the ER lumen drives the oligomerization and trans-autophosphorylation of the cytosolic domain of IRE1 (Shamu and Walter, 1996). There are two distinct homologs of IRE1 in mammals, IRE1α and IRE1β (also known as ERN1 and ERN2, respectively) (Tirasophon et al., 1998; Wang et al., 1998). IRE1α is the primary UPR mediator in mammals as it is ubiquitously expressed, whereas the expression of IRE1β is limited to mucin-producing goblet cells in the digestive tract (Bertolotti et al., 2001). Medaka fish also have two IRE1 genes, encoding IRE1α and IRE1β, but unlike in mice, both genes are expressed ubiquitously (Ishikawa et al., 2011). In response to ER stress, IRE1 gains activity to splice the mRNA of X-box-binding protein 1 (XBP1) to induce the expression of stress response genes, analogous to what occurs with Hac1 mRNA in yeast (Cox and Walter, 1996; Calfon et al., 2002; Shen et al., 2001; Yoshida et al., 2001) (Fig. 1A).
Fig. 1.
The IRE1 branch of the UPR in development and differentiation. (A) A schematic diagram of IRE1 signaling. IRE1 is a transmembrane protein of the ER. Misfolded and/or unfolded proteins in the ER (red circles) promote the oligomerization and trans-autophosphorylation of IRE1, which activate its cytoplasmic RNase function. Active IRE1 splices the mRNA of XBP1 in the cytoplasm to induce stress-responsive gene transcription. In addition, active IRE1 cleaves and degrades a number of other mRNAs through a process that is referred to as RIDD. IRE1 also binds to TRAF2 to activate JNK signaling (gray), but whether this axis plays an active role in animal development remains unclear. (B) IRE1–XBP1 signaling promotes the differentiation of B lymphocytes into plasma cells, which involves expansion of the ER network to allow efficient secretion of immunoglobulins. This differentiation process requires both IRE1 and spliced XBP1. (C) Medaka fish development requires IRE1 and splicing of XBP1 mRNA, but not RIDD. Loss of IRE1 or XBP1 impairs the function of secretory tissues that include the embryonic tail, hatching gland and liver. Spliced XBP1 can rescue the IRE1 mutant phenotype, indicating that the sole function of IRE1 in these tissues is to splice XBP1 mRNA. (D) The requirement for IRE1 in the Drosophila photoreceptor differentiation. Rhodopsin-1 is synthesized in the ER (blue curved bars) and is trafficked to the rhabdomeres, a specialized microvilli-derived structure at the apical membrane (top of the cell). In the absence of IRE1, Rhodopsin-1 fails to traffic properly through the secretory pathway, resulting in photoreceptor differentiation and rhabdomere morphogenesis. This process requires IRE1-mediated RIDD, but not XBP1.
The identification of XBP1 as a UPR mediator has begun to shed light on the developmental role of UPR in mammals, as this transcription factor had been previously recognized for its essential requirement in the developing liver and in the B cell lineage (Reimold et al., 2000, 2001). Within the B cell lineage, pre-B cells respond to antigens by differentiating into antibody-secreting plasma cells. This differentiation process is accompanied by the expression of a large amount of immunoglobulins into the secretory pathway and a dramatic expansion of the ER network (Fig. 1B). Such differentiation is also associated with the appearance of IRE1-mediated XBP1 mRNA splicing. Indeed, overexpression of a spliced XBP1 isoform is sufficient to drive pre-B cells to gain plasma-cell-like properties (Iwakoshi et al., 2003; van Anken, et al., 2003). Together, these results indicate that during the humoral immune response, pre-B cells activate the UPR to expand their ER capacity to secrete large amounts of immunoglobulins.
The physiological importance of the IRE1–XBP1 axis has been further validated in Drosophila, frogs and fish. In Drosophila, Ire1 and Xbp1 mutants fail to survive beyond early larval stages, and the lethality could be rescued by re-introducing wild-type transgenes (Huang et al., 2017). In medaka fish, IRE1α or IRE1β single mutants develop normally, but the double mutants fail to survive beyond two weeks of fertilization (Ishikawa et al., 2017). Similarly, XBP1-knockout medaka fish show defects during early development (Ishikawa et al., 2017). The tissues that require IRE1 and XBP1 in fish and Drosophila are those with high protein secretory loads. For example, Drosophila requires XBP1 in the digestive tract, perhaps because many of the cells are specialized in secreting digestive enzymes (Fig. 1C) (Huang et al., 2017). In fish, XBP1 is also highly expressed in organs with highly secretory cell types, such as the hatching gland, notochord and tail (Bennett et al., 2007; Ishikawa et al., 2017). These organs derive from mesoderm and endoderm cells that express Nodal, a member of the TGFβ family member growth factor family, and zebrafish studies have shown that XBP1 is among the Nodal-induced genes (Bennett et al., 2007). Inhibition of XBP1 through morpholino antisense oligonucleotide injection in zebrafish interfered with Nodal-induced differentiation of the hatching gland, which normally secretes large amounts of chorion-digesting proteases (Bennett, et al., 2007). Similarly, IRE1α and IRE1β double-knockout or XBP1-knockout medaka fish show defects in hatching gland development (Ishikawa et al., 2017). In Xenopus, XBP1 has been noted as a gene that is highly expressed in the developing notochord, an organ that provides structural support to the embryo by secreting large amounts of proteins to form a stiff sheath (Zhao et al., 2003; Tanegashima et al., 2009). Knockdown of XBP1 using morpholinos impaired proper notochord development in Xenopus, establishing a functional significance for the expression pattern of XBP1 in that organ (Tanegashima et al., 2009).
Recent studies have further examined in detail the roles of individual domains within IRE1 and XBP1. In Drosophila, a mutant Ire1 transgene with impaired ability to sense misfolded peptides could not rescue the Drosophila Ire1 mutant phenotype, supporting the idea that it is the misfolded peptides within the normally developing cells that drive IRE1 activation. The XBP1 mutant phenotypes in fish and flies could be further rescued by expressing the spliced XBP1 isoform, but not by a transgene with silent mutations that make it resistant to splicing by IRE1 (Huang et al., 2017; Ishikawa et al., 2017). These results indicate that IRE1-mediated XBP1 mRNA splicing is essential for development.
If a major role of IRE1 during development is to activate XBP1 splicing in response to physiological ER stress, one would expect that the IRE1 loss-of-function phenotype would be similar to that of loss of XBP1. As described above, this has been established in a number of tissues in fish and Drosophila (Ishikawa et al., 2017; Huang et al., 2017). However, in mice, there are similarities, as well as stark differences, in the reported IRE1α- and XBP-knockout phenotypes. Xbp1−/− mice die during embryogenesis due to liver dysfunction (Reimold et al., 2000), and when this function was restored with a liver-specific rescue of Xbp1, later requirements for Xbp1 for the proper secretion of digestive enzymes from pancreatic acinar cells and salivary glands were revealed (Lee et al., 2005). Similarly, defects in liver hepatocytes, pancreas and salivary glands have been reported in conditional IRE1α-knockout mice (Zhang et al., 2011; Iwawaki et al., 2010). However, other tissues show clear differences. It has been reported that a major developmental phenotype associated with IRE1α loss of function occurs in extraembryonic tissues such as the placenta (Iwawaki et al., 2009). Indeed, the embryonic lethality associated with IRE1α loss could be rescued by its expression in placenta. Intriguingly, there are no reported extraembryonic tissue phenotypes associated with the loss of Xbp1 in mice. Although it cannot be ruled out that differences in the genetic background between the IRE1α- and XBP1-knockout mice (Reimold et al., 2000; Iwawaki et al., 2009) are contributing factors to the different phenotypes, these observations point to the possibility that IRE1 and XBP1 may have developmental functions that are independent of each other.
There is now a significant amount of evidence supporting the idea that IRE1 has XBP1-independent functions. First, IRE1 in mammalian cells has been shown to also activate TRAF2-mediated Jun N-terminal kinase (JNK) signaling, independently of its RNase activity, to stimulate a proinflammatory response (Urano et al., 2000). Among others, this axis contributes to obesity-induced type 2 diabetes-like symptoms in mouse models, because JNKs hyperphosphorylate insulin receptor substrate-1 (IRS-1) in obese animals (Ozcan et al., 2004). Whether the IRE1–TRAF2–JNK axis is required to resolve physiological ER stress during normal development remains unclear.
As part of its XBP1-independent functions, IRE1 also targets a group of mRNAs on the surface of the ER for degradation, widely referred to as regulated IRE1-dependent decay of mRNAs (RIDD) (Hollien and Weissman, 2006) (Fig. 1A). As this degradation of mRNAs reduces the amount of new protein synthesis in the ER, this mechanism is thought to alleviate the burden on the ER protein-folding system. The functional significance of RIDD varies among species. At one end of the spectrum is Saccharomyces pombe, which has IRE1 that is solely devoted to RIDD, and there is no XBP1-like transcription factor whose mRNA undergoes splicing in this organism downstream of IRE1 (Kimmig et al., 2012). At the other extreme is medaka fish, which does not require any XBP1-independent IRE1 function during development, and expression of the spliced isoform of XBP1 fully rescues the developmental defects of the IRE1α and IRE1β double mutants (Ishikawa et al., 2017). In Drosophila, IRE1 has both XBP1-dependent and -independent roles during development. In the larva, the Ire1 mutant phenotype cannot be rescued solely with the expression of spliced XBP1, suggestive of a requirement for IRE1 that is independent of XBP1 (Huang et al., 2017). The role of the RIDD function of IRE1 has been characterized in more detail in the developing photoreceptors of Drosophila pupal eyes (Coelho et al., 2013). In this tissue, Xbp1 mRNA expression is largely undetectable during normal development (Ryoo et al., 2007, 2013), and consistent with this, there is no obvious phenotype associated with Xbp1 loss-of-function in the eye (Coelho et al., 2013). However, loss of Ire1 impairs proper Rhodopsin-1 trafficking and photoreceptor differentiation, indicative of an XBP1-independent role for IRE1 (Fig. 1D). In cells devoid of Ire1 in the eye, RIDD targets accumulate, including those that are involved in fatty acid transport, which contributes to the eye phenotype, establishing the in vivo significance of RIDD during normal metazoan development (Coelho et al., 2013).
The role of the PERK–ATF4 branch of the UPR during development
The second ER stress sensor, PERK (also known as EIF2AK3 in mammals), is an ER-resident transmembrane protein that has sequence homology with the luminal domain of IRE1 (Harding et al., 1999; Shi et al., 1998). Upon sensing the misfolded protein pool, PERK dissociates from the major ER chaperone BiP (also known as HSPA5) to activate its kinase function (Bertolotti et al., 2000; Ma et al., 2002). Once active, PERK phosphorylates Ser-51 of the α-subunit of eukaryotic translation initiation factor 2 (eIF2α), thereby reducing the overall rate of mRNA translation initiation (Harding et al., 1999; Shi et al., 1998) (Fig. 2A). Such translational attenuation is thought to help relieve the burden of the protein folding system in the ER, and, consistent with this idea, loss-of-function mutations of PERK cause dysfunction in a number of cell types. For example, a loss-of-function mutation of PERK underlies a rare autosomal recessive disease known as Walcott–Rallison syndrome, in which the afflicted individuals suffer from infantile-onset diabetes, exocrine pancreas atrophy and other problems in the bone, liver and kidney (Delépine et al., 2000; Durocher et al., 2006). The cell types that become dysfunctional are mostly those with high protein secretory load, including the β-islet cells that secrete insulin. Similarly, PERK-knockout mice show dysfunction of β-islet cells, pancreatic acinar cells, which secrete digestive enzymes, and cells within the skeletal system (Harding et al., 2001; Iida et al., 2007; Zhang et al., 2002) (Fig. 2B). In PERK-mutant β-islet cells, where PERK function has been investigated in more detail, pro-insulin molecules have been found to fail to traffic out of the ER to the Golgi because of a reduction in the expression of ER chaperones in these cells (Gupta et al., 2010; Sowers et al., 2018). Mutating the downstream target of PERK also has similar effects. Knock-in mice with a Ser51Ala eIF2α mutation, which is resistant to PERK-mediated phosphorylation, also results in defects in proinsulin trafficking within β-islet cells (Back et al., 2009; Scheuner et al., 2001).
Fig. 2.
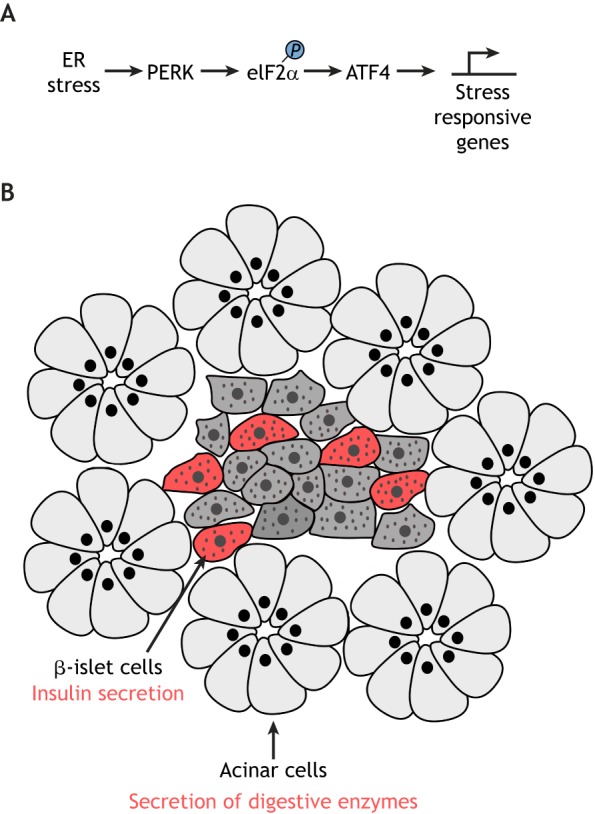
The PERK branch of the UPR. (A) A schematic diagram of PERK–ATF4 signaling. PERK phosphorylates the translational initiation factor eIF2α in response to ER stress, which prompts an attenuation in general mRNA translation. Paradoxically, these conditions stimulate the synthesis of ATF4, a transcription factor that induces stress-responsive mRNA transcription. (B) Impairment of the PERK–eIF2α–ATF4 pathway causes dysfunction of the pancreas, including secretion of insulin and digestive enzymes. Specifically, deletion of PERK or ATF4, or an introduction of eIF2α with a point mutation that means it resists phosphorylation by PERK, commonly result in abnormal pancreas function and development. The cell types affected in these mouse models are indicated with arrows. β-islet cells are marked in red, and other cell types are marked in gray.
Aside from PERK alleles that affect β-islet cell function, another set of human PERK variants was found through a genome-wide association study (GWAS) in patient samples of progressive supranuclear palsy (PSP), a tauopathy neurodegenerative disease associated with abnormal tau deposits (Höglinger et al., 2011). Whereas many of the PERK alleles that cause Wolcott–Rallison syndrome completely abrogate PERK expression or impair PERK kinase function, the tauopathy-associated PERK alleles are hypomorphs with reduced signaling activity. Induced pluripotent stem cells containing these tauopathy-associated PERK alleles are more vulnerable to chemicals that cause ER stress (Yuan et al., 2018). Although GWAS studies are correlative in nature, these observations suggest that PERK must respond to physiological stress in the nervous system, and a failure to do so increases the risk of neurodegeneration. Notably, our understanding of the role of PERK in tauopathy still remains rudimentary. For example, we do not know which cell types in the brain require PERK, and what type of physiological stress is associated with PERK activation in this organ. Furthermore, as tau is a cytoplasmic protein, how the aggregation of this protein is linked to ER stress also remains to be elucidated.
It is worth noting that physiological PERK activity is not always associated with high protein secretory activity. In the Drosophila intestinal epithelium, wounded and stressed cells send mitogenic signals to intestinal stem cells (ISCs) to promote tissue regeneration. It has been shown that eIF2α is phosphorylated in ISCs during this process, and that knockdown or knockout of PERK in ISCs reduces the rate of ISC proliferation (Wang et al., 2015). ISCs are not known for high protein secretory activity, and it is not yet known what causes PERK activation in ISCs, but these findings indicate that PERK has other roles beyond assisting the physiological function of secretory cells.
In addition to reducing general translational initiation, eIF2α phosphorylation by PERK paradoxically stimulates the translation of a few mRNAs that contain short upstream open-reading frames in their 5′-untranslated region. Recent studies have identified a handful of factors that are activated in this way (Andreev et al., 2015; Palam et al., 2011; Zhou et al., 2008), and of these, ATF4 is best characterized (Harding et al., 2000; Lu et al., 2004; Vattem and Wek, 2004) (Fig. 2A). Consistent with the essential role of ATF4 as a downstream factor of PERK, Atf4-knockout mice show developmental defects that include pancreatic hypertrophy (Iida et al., 2007). The Atf4 loss-of-function phenotype in the mouse pancreas is more severe than that of PERK mutants. Although the underlying reason for this severity has not been determined, it might be because ATF4 responds to other signals, including those mediated by three other eIF2α kinases: GCN2 (also known as EIF2AK4), which responds to amino acid deprivation, PKR (also known as EIF2AK2), which responds to double-stranded RNAs, and HRI (also known as EIF2AK1), which responds to heme deficiency (Hinnebusch et al., 2016). Recent studies indicate that ATF4 also responds to various types of stress imposed on the mitochondria (Quirós et al., 2017).
Atf4-knockout mice show a few additional tissue phenotypes, including defects in the lens, growth retardation and anemia (Tanaka et al., 1998; Hettmann et al., 2000; Masuoka and Townes, 2002; Zhao et al., 2015). How could ATF4 regulate general animal development? As a transcription factor, ATF4 induces the expression of chaperones, other ER-quality-control factors, amino-acid transporters and mRNA translational regulators that could help the cells return to their basal states (Harding et al., 2003; Malzer et al., 2018). Specific examples of ATF4 targets include growth arrest and DNA damage-inducible 34 (GADD34; also known as PPP1R15A) and constitutive repressor of eIF2α phosphorylation (CReP; also known as PPP1R15B) that help to de-phosphorylate phospho-eIF2α as part of a negative-feedback loop (Connor et al., 2001; Fawcett et al., 1999; Jousse et al., 2003; Novoa et al., 2003), as well as 4E-BP (EIF4EBP1 in mammals), which binds to eIF-4E to specifically inhibit cap-dependent translation (Kang et al., 2017; Vasudevan et al., 2017; Yamaguchi et al., 2008). A recent study has found that this ATF4–4E-BP axis plays an important role in the patterning of the Drosophila wing. Specifically, the authors found that BMP signaling, which is required for proper wing patterning in this organism, is altered in developing Drosophila with abnormal ATF4–4E-BP signaling (Malzer et al., 2018). This study provides an example of how UPR signaling could shape tissue patterning during animal development.
The role of ATF6 in animal development
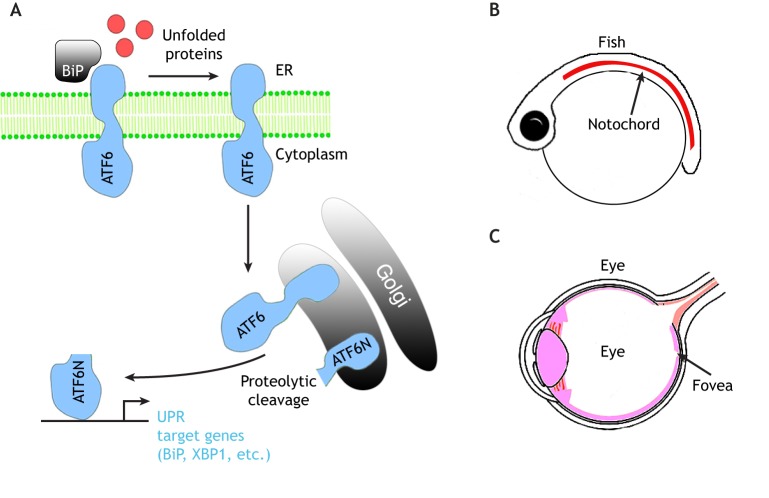
The third UPR stress sensor, ATF6, is a type II ER-transmembrane protein with a DNA-binding domain facing the cytoplasmic side. In the presence of misfolded and/or unfolded proteins, ATF6 is released from BiP and leaves the ER compartment. The destined trafficking of ATF6 to the Golgi (Schindler and Schekman, 2009) facilitates the protease-dependent (Site-1 and Site-2 protease) removal of the luminal domain and the transmembrane anchor, respectively (Haze et al., 1999; Ye et al., 2000). The released cytoplasmic domain of ATF6 (ATF6-N) then translocates to the nucleus and induces the expression of a broad range of UPR target genes that include the chaperone BiP and the UPR mediator XBP1 (Fig. 3A) (Yamamoto et al., 2007; Yoshida et al., 2000). Vertebrate species encode two isoforms of ATF6 (ATF6α, also called just ATF6 in mammals, and ATF6β, or ATF6B), which have partially redundant roles in UPR (Thuerauf et al., 2002, 2004). ATF6α or ATF6β single-knockout mice are viable and fertile, but the double-knockouts are lethal at the early embryonic stage, indicating that the two isoforms have essential but overlapping functions (Yamamoto et al., 2007). The early embryonic lethal phenotype of ATF6α and ATF6β double-knockout mice is more severe than that of other UPR pathway knockouts that delete IRE1α, XBP1, PERK or ATF4. It has been argued that this is because vertebrate ATF6 genes have evolved to play a broader role in UPR in mediating the induction of ER chaperones and UPR signaling mediators (Yamamoto et al., 2007; Yoshida et al., 2000, 2001). Such a role of ATF6 is somewhat different from the situation in S. cerevisiae, which does not encode ATF6 and where the IRE1 branch is responsible for the induction of ER chaperones in response to stress (Cox et al., 1993; Mori et al., 1993). Single ATF6 genes are encoded in the genomes of C. elegans, as well as Drosophila. In C. elegans, ATF6 does not have an apparent role in BiP (hsp-3) induction in response to ER, as mutating XBP1 together with PERK is sufficient to block its induction (Calfon et al., 2002; Shen et al., 2001). ATF6 in Drosophila has not yet been characterized in detail, but BiP induction is partially suppressed by the loss of IRE1 in this organism (Ryoo et al., 2007). Unlike the situation in invertebrates, medaka fish have an important early embryonic requirement for ATF6. Similar to what is found for mammals, there are two ATF6-encoding genes, ATF6α and ATF6β (Ishikawa et al., 2011). ATF6α mutant fish fail to induce BiP in response to ER stress. While single knockouts are viable, the ATF6α and ATF6β double knockouts are embryonic lethal, with impaired notochord development (Fig. 3B) (Ishikawa et al., 2013).
Fig. 3.
The ATF6 branch of the UPR. (A) A schematic diagram of ATF6 signaling. Upon sensing accumulation of misfolded and/or unfolded proteins in the ER lumen, the chaperone BiP is released from ATF6 (an ER-residing transmembrane protein, in blue) and ATF6 translocates to the Golgi compartment. There, ATF6 is proteolytically cleaved to produce cytosolic ATF6 (ATF6-N), which moves to the nucleus to induce the expression of UPR target genes, including those encoding BiP and XBP1. (B,C) Phenotypes associated with the loss of ATF6 in fish (B) and humans (C). (B) ATF6α and ATFβ double mutant medaka fish show abnormal notochord development due to the lack of extracellular matrix protein secretion. (C) ATF6 mutation in humans causes photoreceptor dysfunction and therefore disrupts the proper development of the fovea, where cone photoreceptors reside.
In humans, the two ATF6 genes are referred to as ATF6 (also known as ATF6α) and ATF6B (Haze et al., 2001). It has been recently found that hyper-activation of ATF6 in human embryonic stem cells (hESCs) not only promotes their expansion of the ER network, but also biases their differentiation into the mesodermal lineage (Kroeger et al., 2018). Conversely, loss-of-function mutations of ATF6 in induced pluripotent stem cells impede this mesodermal fate specification (Kroeger et al., 2018). Mesoderm differentiation during embryogenesis requires many secreted morphogens and their receptors, and, therefore, one possibility through which ATF6 could promote mesodermal fate during development is by aiding with the folding and trafficking of the factors that determine mesoderm cell fate. Human genetics studies have provided more direct in vivo evidence that human ATF6 is involved in cell fate specification. Specifically, loss-of-function mutations in ATF6 were identified from patients suffering from two diseases that impair vision. The first is achromatopsia (ACHM), an autosomal recessive disease characterized by a lack of cone photoreceptor function and color blindness (Kohl et al., 2015; Ansar et al., 2015; Xu et al., 2015). The other is cone-rod dystrophy (CRD), which is characterized by the progressive loss of cone function followed by the loss of rod photoreceptor function (Skorczyk-Werner et al., 2017). ACHM patients with ATF6 mutations show morphological defects in the fovea, an area within the eye that contains cone photoreceptors. Some of these patients entirely lack foveal pits, indicating that the phenotype is due to developmental defects of the eye (Fig. 3C) (Kohl et al., 2015; Ansar et al., 2015; Xu et al., 2015). The mutations that have been identified in ACHM impair many different aspects of ATF6 function, including its ability to detect misfolded peptides in the ER lumen as determined by studies on patient-derived fibroblast cells (Chiang et al., 2017; Tam et al., 2018), strongly supporting the idea that misfolded peptides in the ER drive ATF6 activation required for proper cell fate determination in different tissues, including eye and mesoderm. The ATF6 mutation that underlies CRD was recently identified based on two siblings in Poland. The encoded mutant protein changes Asp564 into Gly in the luminal domain, which senses unfolded proteins, and the mutant protein is impaired in its ability to respond to ER stress-causing chemicals. Both CRD patients exhibit missing foveal pit and foveal hypoplasia (Skorczyk-Werner et al., 2017), further corroborating the role of ATF6 in fovea development.
Specificity of the three UPR signaling branches during animal development
As all three branches of the UPR are activated by the overload of misfolded and/or unfolded proteins in the ER, all three of these should be activated in developing tissues with a protein secretory burden that exceeds their protein folding capacity. If all three UPR branches are commonly activated by a given physiological stress, and each pathway plays non-redundant roles, one would expect to see similar phenotypes for each of the UPR mediator mutants in those tissues. A mammalian tissue that fits this assumption is the pancreas. As discussed above, PERK, ATF4 and XBP1 single-knockout mice commonly suffer from pancreatic acinar and β cell dysfunction. Although IRE1α is not required in the pancreas for embryonic survival (Iwawaki et al., 2009), a closer inspection of IRE1α-knockout mice revealed that there are histological abnormalities in pancreatic acinar cells (Iwawaki et al., 2010), and defects in proinsulin folding in β-islet cells (Tsuchiya et al., 2018). Similarly, in Xenopus, IRE1α knockdown has an inhibitory effect on pancreatic development. Possible roles of mammalian ATF6 genes in acinar cells have not been characterized in detail, but it has been reported that siRNA-mediated knockdown of ATF6α triggers the death of insulinoma cells, a cell line derived from pancreatic β-islet cells (Teodoro et al., 2012). Taken together, these observations indicate that the three UPR pathways have non-redundant roles in this tissue, and each UPR branch probably regulates the expression of a distinct set of downstream target genes, all of which are required for proper pancreatic function.
At the other extreme to the above tissues, are examples of tissues that require only some of the UPR branches for their normal development and function. For example, loss of XBP1 impairs B cell differentiation into plasma cells, but deletion of PERK and/or ATF6 do not give rise to similar phenotypes (Gass et al., 2002), suggestive of a specialized role of the XBP1 branch of the UPR. The tissue-specific requirement for UPR is evident from studies of several other tissues, including a retarded liver growth phenotype found in both IRE1α- and XBP1-deficient mice (Reimold et al., 2000; Zhang et al., 2005) and abnormal thinning of bone in PERK-null mice (Delépine et al., 2000; Zhang et al., 2002). As discussed above, IRE1α-knockout mice have phenotypes in the placenta, which is not observed when other UPR-mediating genes are knocked out (Fig. 4A). With regard to the eye, ATF4-knockout mice suffer from microphthalmia due to excessive cell death of epithelial lens cells (Hettmann et al., 2000) (Fig. 4B). This lens phenotype has not been observed when the other UPR inducers are knocked out. As discussed above, ATF6 is required for fovea development in a different part of the eye (Kohl, et al., 2015; Ansar et al., 2015; Xu et al., 2015) (Fig. 4B), but similar phenotypes have not yet been reported in mice that lack the other UPR mediators. Among the ATF6 mutations that give rise to the fovea phenotype are those that impair its ability to respond to misfolded peptides in the ER, which suggests that the conditions that activate ATF6 during fovea development should also activate the other UPR branches. Although, it cannot be ruled that other UPR branches have as-yet-uncharacterized roles in fovea development, these observations raise the more likely possibility that ATF6-mediated UPR has a specialized role in promoting foveal development, whereas the ATF4 branch has a distinct role in the development of epithelial lens cells.
Fig. 4.
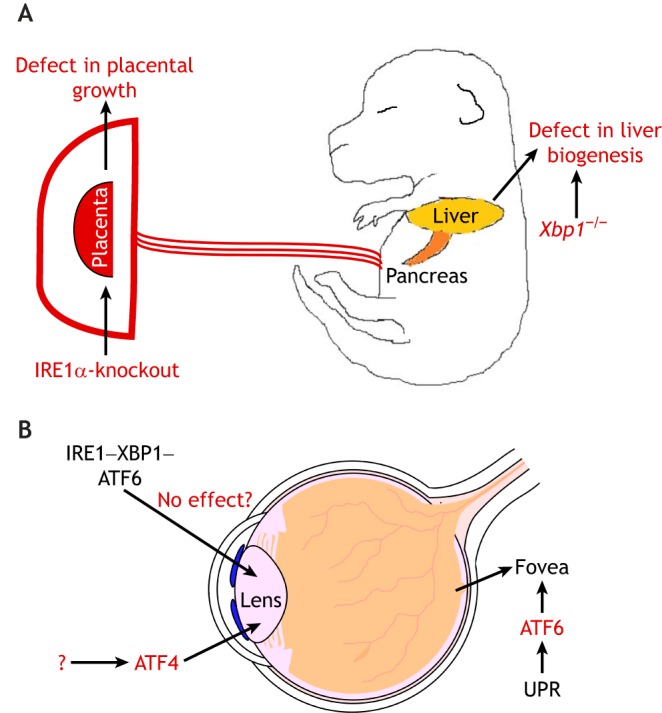
Selective roles of specific branches of the UPR in animal development. (A) IRE1α-knockout impairs placenta development in mice. However, this placenta phenotype has not been reported in mice lacking any of the other UPR mediators, including PERK, ATF6 or XBP1, suggesting a surprisingly selective role for IRE1 in placenta development for reasons that remain unclear. (B) Different parts of the mammalian eye require distinct branches of the UPR for development. In the posterior eye, the fovea is a region that contains cone cells involved in color vision. The development of the fovea is impaired in individuals with ATF6 mutations, resulting in color blindness or progressive loss of cone and rod cells. Knockout of ATF4, which mediates the PERK branch of the UPR, in mice does not give rise to the reported defects in fovea development, but instead impairs the differentiation of the lens epithelium. However, the upstream signals leading to ATF4 activation in this context are not clear. There are no reported defects in the lens epithelium that are associated with deletions of IRE1, XBP1 or ATF6, suggesting that there is a surprising degree of specificity in the roles of each UPR branch in animal tissues.
The reasons underlying such specificity are only subject to speculation at this point. For instance, it is possible that there is a specific demand for genes that are downstream targets of ATF6, but not of XBP1 and ATF4, during foveal development. Similarly, there may be a specific demand for ATF4 target genes in the lens epithelium, and not of those of the other UPR branches. It is also possible that certain tissues, such as lens cells, activate ATF4 through specific signals other than through misfolded peptides that commonly activate all three UPR pathways. It is noteworthy that ATF4 has been shown to be activated by other conditions, including amino acid deprivation and double-stranded RNAs, which do not activate the other UPR branches (Hinnebusch et al., 2016). Finally, it is possible that conditions that activate each branch of the UPR are more nuanced than the notion typically put forward by studies of cultured cells exposed to ER-stress-causing chemicals. According to this idea, specific conditions could activate one branch of the UPR, but not the others, in certain tissues. Perhaps those nuanced differences may lie in the unfolded peptide that is recognized by domains of PERK, IRE1 and ATF6. Alternatively, the differences in the membrane bilayer environment may allow for some specificity. Supporting the latter idea are recent studies that have examined in detail how lipids affect the activation of IRE1 and ATF6 branches. For example, IRE1 in yeast and cultured mammalian cells could be activated by lipid imbalances in cell membranes (Promlek et al., 2011; Volmer et al., 2013). Furthermore, biophysical analysis together with molecular dynamics simulations indicate that IRE1 activation could be affected by thickness or orderliness of the membrane lipid bilayer (Halbleib et al., 2017). Moreover, ATF6 activation could be affected by a very different type of lipids – specific types of sphingolipids. When these sphingolipids are added to cells, they activate the ATF6 arm of the UPR (Tam et al., 2018). Therefore, it is possible that some of the tissues have a specific membrane bilayer environment that readily activates one branch of the UPR over the other, and this idea that awaits further validation in vivo.
Concluding remarks
Recent studies on the developmental roles of UPR have shed light on the nature of the physiological ER stress in animal tissues, and the mechanisms required to cope with such stress. Yet, many outstanding questions remain unanswered. Perhaps one of the most fundamental questions is regarding the specific roles of the UPR signaling branches in development. For instance, why is only the IRE–XBP1 branch required during B-cell differentiation, but not the others? Why is ATF6, but not the other UPR mediators, required for fovea development? Why are the lens epithelial cells sensitive to the loss of ATF4, but not to loss of ATF6? Are certain branches activated during development by ligands other than misfolded peptides present in the ER lumen? Many new mechanistic discoveries are being made with cultured cells that have been exposed to chemicals, but the in vivo significance and the possible roles that they may play during animal development remain unclear. These questions may be clarified in future studies by examining the branch-specific UPR reporters in animal tissues in detail, and by introducing domain-specific mutations of major UPR mediators in animal models and determining their effects in normal development and disease.
Acknowledgements
We appreciate the suggestions of the reviewers that led us to expand the topics covered in this review.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
The work of our laboratory is supported by the National Institutes of Health (NIH) (grants R01 EY020866 and R01 GM125954) and also by the March of Dimes Foundation (1-FY18-335). Deposited in PMC for release after 12 months.
References
- Andreev D. E., O'Connor P. B. F., Fahey C., Kenny E. M., Terenin I. M., Dmitriev S. E., Cormican P., Morris D. W., Shatsky I. N. and Baranov P. V. (2015). Translation of 5′ leaders is pervasive in genes resistant to eIF2 repression. eLife 4, e03971 10.7554/eLife.03971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansar M., Santos-Cortez R. L. P., Saqib M. A. N., Zulfiqar F., Lee K., Ashraf N. M., Ullah E., Wang X., Sajid S., Khan F. S. et al. (2015). Mutation of ATF6 causes autosomal recessive achromatopsia. Hum. Genet. 134, 941-950. 10.1007/s00439-015-1571-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back S. H., Scheuner D., Han J., Song B., Ribick M., Wang J., Gildersleeve R. D., Pennathur S. and Kaufman R. J. (2009). Translation attenuation through eIF2alpha phosphorylation prevents oxidative stress and maintains the differentiated state in beta cells. Cell Metab. 10, 13-26. 10.1016/j.cmet.2009.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J. T., Joubin K., Cheng S., Aanstad P., Herwig R., Clark M., Lehrach H. and Schier A. F. (2007). Nodal signaling activates differentiation genes during zebrafish gastrulation. Dev. Biol. 304, 525-540. 10.1016/j.ydbio.2007.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolotti A., Zhang Y., Hendershot L. M., Harding H. P. and Ron D. (2000). Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2, 326-332. 10.1038/35014014 [DOI] [PubMed] [Google Scholar]
- Bertolotti A., Wang X. Z., Novoa I., Jungreis R., Schlessinger K., Cho J. H., West A. B. and Ron D. (2001). Increased sensitivity to dextran sodium sulfate colitis in IRE1beta-deficient mice. J. Clin. Invest. 107, 585-593. 10.1172/JCI11476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braakman I. and Hebert D. N. (2013). Protein folding in the endoplasmic reticulum. Cold Spring Harb. Perspect. Biol. 5, a013201 10.1101/cshperspect.a013201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calfon M., Zeng H., Urano F., Till J. H., Hubbard S. R., Harding H. P., Clark S. G. and Ron D. (2002). IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415, 92-96. 10.1038/415092a [DOI] [PubMed] [Google Scholar]
- Chiang W.-C., Chan P., Wissinger B., Vincent A., Skorczyk-Werner A., Krawczyński M. R., Kaufman R. J., Tsang S. H., Héon E., Kohl S. et al. (2017). Achromatopsia mutations target sequential steps of ATF6 activation. Proc. Natl. Acad. Sci. USA 114, 400-405. 10.1073/pnas.1606387114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho D. S., Cairrão F., Zeng X., Pires E., Coelho A. V., Ron D., Ryoo H. D. and Domingos P. M. (2013). Xbp1-independent Ire1 signaling is required for photoreceptor differentiation and rhabdomere morphogenesis in Drosophila. Cell Rep. 5, 791-801. 10.1016/j.celrep.2013.09.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. H., Weiser D. C., Li S., Hallenbeck J. M. and Shenolikar S. (2001). Growth arrest and DNA damage-inducible protein GADD34 assembles a novel signaling complex containing protein phosphatase 1 and inhibitor 1. Mol. Cell Biol. 21, 6841-6850. 10.1128/MCB.21.20.6841-6850.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J. S. and Walter P. (1996). A novel mechanism for regulating activity of a transcription factor that controls the unfolded protein response. Cell 87, 391-404. 10.1016/S0092-8674(00)81360-4 [DOI] [PubMed] [Google Scholar]
- Cox J. S., Shamu C. E. and Walter P. (1993). Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell 73, 1197-1206. 10.1016/0092-8674(93)90648-A [DOI] [PubMed] [Google Scholar]
- Delépine M., Nicolino M., Barrett T., Golamaully M., Mark Lathrop G. and Julier C. (2000). EIF2AK3, encoding translation initiation factor 2-alpha kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nat. Genet. 25, 406-409. 10.1038/78085 [DOI] [PubMed] [Google Scholar]
- Durocher F., Faure R., Labrie Y., Pelletier L., Bouchard I. and Laframboise R. (2006). A novel mutation in the EIF2AK3 gene with variable expressivity in two patients with Wolcott-Rallison syndrome. Clin. Genet 70, 34-38. 10.1111/j.1399-0004.2006.00632.x [DOI] [PubMed] [Google Scholar]
- Fawcett T. W., Martindale J. L., Guyton K. Z., Hai T. and Holbrook N. J. (1999). Complexes containing activating transcription factor (ATF)/cAMP-responsive-element-binding protein (CREB) interact with the CCAAT/enhancer-binding protein (C/EBP)-ATF composite site to regulate Gadd153 expression during the stress response. Biochem. J. 339, 135-141. [PMC free article] [PubMed] [Google Scholar]
- Gass J. N., Gifford N. M. and Brewer J. W. (2002). Activation of an unfolded protein response during differentiation of antibody-secreting B cells. J. Biol. Chem 277, 49047-49054. 10.1074/jbc.M205011200 [DOI] [PubMed] [Google Scholar]
- Gupta S., McGrath B. and Cavener D. R. (2010). PERK (EIF2AK3) regulates proinsulin trafficking and quality control in the secretory pathway. Diabetes 59, 1937-1947. 10.2337/db09-1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbleib K., Pesek K., Covino R., Hofbauer H. F., Wunnicke D., Hänelt I., Hummer G. and Ernst R. (2017). Activation of the unfolded protein response by lipid bilayer stress. Mol. Cell 67, 673-684.e8. 10.1016/j.molcel.2017.06.012 [DOI] [PubMed] [Google Scholar]
- Han J. and Kaufman R. J. (2017). Physiological/pathological ramifications of transcription factors in the unfolded protein response. Genes Dev. 31, 1417-1438. 10.1101/gad.297374.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding H. P., Zhang Y. and Ron D. (1999). Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397, 271-274. 10.1038/16729 [DOI] [PubMed] [Google Scholar]
- Harding H. P., Novoa I., Zhang Y., Zeng H., Wek R., Schapira M. and Ron D. (2000). Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell 6, 1099-1108. 10.1016/S1097-2765(00)00108-8 [DOI] [PubMed] [Google Scholar]
- Harding H. P., Zeng H., Zhang Y., Jungries R., Chung P., Plesken H., Sabatini D. D. and Ron D. (2001). Diabetes mellitus and exocrine pancreatic dysfunction in perk−/− mice reveals a role for translational control in secretory cell survival. Mol. Cell 7, 1153-1163. 10.1016/S1097-2765(01)00264-7 [DOI] [PubMed] [Google Scholar]
- Harding H. P., Zhang Y., Zeng H., Novoa I., Lu P. D., Calfon M., Sadri N., Yun C., Popko B., Paules R. et al. (2003). An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell 11, 619-633. 10.1016/S1097-2765(03)00105-9 [DOI] [PubMed] [Google Scholar]
- Haze K., Yoshida H., Yanagi H., Yura T. and Mori K. (1999). Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol. Cell 10, 3787-3799. 10.1091/mbc.10.11.3787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haze K., Okada T., Yoshida H., Yanagi H., Yura T., Negishi M. and Mori K. (2001). Identification of the G13 (cAMP-response-element-binding protein-related protein) gene product related to activating transcription factor 6 as a transcriptional activator of the mammalian unfolded protein response. Biochem. J. 355, 19-28. 10.1042/bj3550019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettmann T., Barton K. and Leiden J. M. (2000). Microphthalmia due to p53-mediated apoptosis of anterior lens epithelial cells in mice lacking the CREB-2 transcription factor. Dev. Biol. 222, 110-123. 10.1006/dbio.2000.9699 [DOI] [PubMed] [Google Scholar]
- Hetz C. and Papa F. R. (2018). The unfolded protein response and cell fate control. Mol. Cell 69, 169-181. 10.1016/j.molcel.2017.06.017 [DOI] [PubMed] [Google Scholar]
- Hinnebusch A. G., Ivanov I. P. and Sonenberg N. (2016). Translational control by 5'-untranslated regions of eukaryotic mRNAs. Science 352, 1413-1416. 10.1126/science.aad9868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höglinger G. U., Melhem N. M., Dickson D. W., Sleiman P. M. A., Wang L.-S., Klei L., Rademarkers R., de Silva R., Litvan I., Riley D. E. et al. (2011). Identification of common variants influencing risk of the tauopathy progressive supranuclear palsy. Nat. Genet. 43: 699-705. 10.1038/ng.859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollien J. and Weissman J. S. (2006). Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science 313, 104-107. 10.1126/science.1129631 [DOI] [PubMed] [Google Scholar]
- Huang H.-W., Zeng X., Rhim T., Ron D. and Ryoo H. D. (2017). The requirement of IRE1 and XBP1 in resolving phyiological stress during Drosophila development. J. Cell Sci. 130, 3040-3049. 10.1242/jcs.203612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida K., Li Y., McGrath B. C., Frank A. and Cavener D. R. (2007). PERK eIF2 alpha kinase is required to regulate the viability of the exocrine pancreas in mice. BMC Cell Biol. 8, 38 10.1186/1471-2121-8-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T., Taniguchi Y., Okada T., Takeda S. and Mori K. (2011). Vertebrate unfolded protein response: mammalian signaling pathways are conserved in Medaka fish. Cell Struct. Funct. 36, 247-259. 10.1247/csf.11036 [DOI] [PubMed] [Google Scholar]
- Ishikawa T., Okada T., Ishikawa-Fujiwara T., Todo T., Kamei Y., Shigenobu S., Tanaka M., Saito T. L., Yoshimura J., Morishita S. et al. (2013). ATF6a/b-mediated adjustment of ER chaperone levels is essential for development of the notochord in medaka fish. Mol. Biol. Cell 24, 1387-1395. 10.1091/mbc.e12-11-0830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T., Kashima M., Nagano A. J., Ishikawa-Fujiwara T., Kamei Y., Todo T. and Mori K. (2017). Unfolded protein response transducer IRE1-mediated signaling independent of XBP1 mRNA splicing is not required for growth and development of medaka fish. eLife 6, e26845 10.7554/eLife.26845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwakoshi N. N., Lee A.-H., Vallabhajosyula P., Otipoby K. L., Rajewsky K. and Glimcher L. H. (2003). Plasma cell differentiation and the unfolded protein response intersect at the transcription factor xbp-1. Nat. Immunol. 4, 321-329. 10.1038/ni907 [DOI] [PubMed] [Google Scholar]
- Iwawaki T., Akai R., Yamanaka S. and Kohno K. (2009). Function of IRE1 alpha in the placenta is essential for placental development and embryonic viability. Proc. Natl. Acad. Sci. USA 106, 16657-16662. 10.1073/pnas.0903775106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwawaki T., Akai R. and Kohno K. (2010). IRE1alpha disruption causes histological abnormality of exocrine tissues, increase of blood glucose level, and decrease of serum immunoglobulin level. PLoS ONE 5, e13052 10.1371/journal.pone.0013052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jousse C., Oyadomari S., Novoa I., Lu P., Zhang Y., Harding H. P. and Ron D. (2003). Inhibition of a constitutive translation initiation factor 2alpha phosphatase, CReP, promotes survival of stressed cells. J. Cell Biol. 163, 767-775. 10.1083/jcb.200308075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M.-J., Vasudevan D., Kang K., Kim K., Park J.-E., Zhang N., Zeng X., Neubert T. A., Marr M. T. II and Ryoo H. D. (2017). 4E-BP is a target of the GCN2-ATF4 pathway during Drosophila development and aging. J. Cell Biol. 216, 115-129. 10.1083/jcb.201511073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmig P., Diaz M., Zheng J., Williams C. C., Lang A., Aragón T., Li H. and Walter P. (2012). The unfolded protein response in fission yeast modulates stability of select mRNAs to maintain protein homeostasis. eLife 1, e00048 10.7554/eLife.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl S., Zobor D., Chiang W.-C., Weisschuh N., Staller J., Gonzalez Menendez I., Chang S., Beck S. C., Garcia Garrido M., Sothilingam V. et al. (2015). Mutations in the unfolded protein response regulator ATF6 cause the cone dysfunction disorder achromatopsia. Nat. Genet. 47, 757-765. 10.1038/ng.3319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeger H., Grimsey N., Paxman R., Chiang W. C., Plate L., Jones Y., Shaw P. X., Trejo J., Tsang S. H., Powers E. et al. (2018). The unfolded protein response regulator ATF6 promotes mesodermal differentiation. Sci. Signal. 11, eaan5785 10.1126/scisignal.aan5785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A.-H., Chu G. C., Iwakoshi N. N. and Glimcher L. H. (2005). XBP-1 is required for biogenesis of cellular secretory machinery of exocrine glands. EMBO J. 24, 4368-4380. 10.1038/sj.emboj.7600903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P. D., Harding H. P. and Ron D. (2004). Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J. Cell Biol. 167, 27-33. 10.1083/jcb.200408003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma K., Vattem K. M. and Wek R. C. (2002). Dimerization and release of molecular chaperone inhibition facilitate activation of eukaryotic initiation factor-2 kinase in response to endoplasmic reticulum stress. J. Biol. Chem. 277, 18728-18735. 10.1074/jbc.M200903200 [DOI] [PubMed] [Google Scholar]
- Malzer E., Dominicus C. S., Chambers J. E., Dickens J. A., Mookerjee S. and Marciniak S. J. (2018). The integrated stress response regulates BMP signalling through effects on translation. BMC Biol. 16, 34 10.1186/s12915-018-0503-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuoka H. C. and Townes T. M. (2002). Targeted disruption of the activating transcription factor 4 gene results in severe fetal anemia in mice. Blood 99, 736-745. 10.1182/blood.V99.3.736 [DOI] [PubMed] [Google Scholar]
- Mori K., Ma W., Gething M.-J. and Sambrook J. (1993). A transmembrane protein with a cdc2+/cdc28-related kinase activity is required for signaling from the ER to the nucleus. Cell 74, 743-756. 10.1016/0092-8674(93)90521-Q [DOI] [PubMed] [Google Scholar]
- Novoa I., Zhang Y., Zeng H., Jungreis R., Harding H. P. and Ron D. (2003). Stress-induced gene expression requires programmed recovery from translational repression. EMBO J. 22, 1180-1187. 10.1093/emboj/cdg112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan U., Cao Q., Yilmaz E., Lee A. H., Iwakoshi N. N., Ozdelen E., Tuncman G., Gorgun C., Glimcher L. H. and Hotamisligil G. S. (2004). Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306, 457-461. 10.1126/science.1103160 [DOI] [PubMed] [Google Scholar]
- Palam L. R., Baird T. D. and Wek R. C. (2011). Phosphorylation of eIF2 facilitates ribosomal bypass of an inhibitory upstream ORF to enhance CHOP translation. J. Biol. Chem. 286, 10939-10949. 10.1074/jbc.M110.216093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Promlek T., Ishiwata-Kimata Y., Shido M., Sakuramoto M., Kohno K. and Kimata Y. (2011). Membrane aberrancy and unfolded proteins activate the endoplasmic reticulum stress sensor Ire1 in different ways. Mol. Biol. Cell 22, 3520-3532. 10.1091/mbc.e11-04-0295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirós P. M., Prado M. A., Zamboni N., D'Amico D., Williams R. W., Finley D., Gygi S. P. and Auwerx J. (2017). Multi-omics analysis identifies ATF4 as a key regulator of the mitochondrial stress response in mammals. J. Cell Biol. 216, 2027-2045. 10.1083/jcb.201702058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimold A. M., Etkin A., Clauss I., Perkins A., Friend D. S., Zhang J., Horton H. F., Scott A., Orkin S. H., Byrne M. C. et al. (2000). An essential role in liver development for transcription factor XBP-1. Genes Dev. 14, 152-157. [PMC free article] [PubMed] [Google Scholar]
- Reimold A. M., Iwakoshi N. N., Manis J., Vallabhajosyula P., Szomolanyi-Tsuda E., Gravallese E. M., Friend D., Grusby M. J., Alt F. and Glimcher L. H. (2001). Plasma cell differentiation requires the transcription factor XBP-1. Nature 412, 300-307. 10.1038/35085509 [DOI] [PubMed] [Google Scholar]
- Ryoo H. D., Domingos P. M., Kang M.-J. and Steller H. (2007). Unfolded protein response in a Drosophila model for retinal degeneration. EMBO J. 26, 242-252. 10.1038/sj.emboj.7601477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryoo H. D., Li J. and Kang M.-J. (2013). Drosophila XBP1 expression reporter marks cells under endoplasmic reticulum stress and with high protein secretory load. PLoS ONE 8, e75774 10.1371/journal.pone.0075774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuner D., Song B., McEwen E., Liu C., Laybutt R., Gillespie P., Saunders T., Bonner-Weir S. and Kaufman R. J. (2001). Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol. Cell 7, 1165-1176. 10.1016/S1097-2765(01)00265-9 [DOI] [PubMed] [Google Scholar]
- Schindler A. J. and Schekman R. (2009). In vitro reconstitution of ER-stress induced ATF6 transport in COPII vesicles. Proc. Natl. Acad. Sci. USA 106, 17775-17780. 10.1073/pnas.0910342106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamu C. E. and Walter P. (1996). Oligomerization and phosphorylation of the Ire1p kinase during intracellular signaling from the endoplasmic reticulum to the nucleus. EMBO J. 15, 3028-3039. 10.1002/j.1460-2075.1996.tb00666.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X., Ellis R. E., Lee K., Liu C.-Y., Yang K., Solomon A., Yoshida H., Morimoto R., Kurnit D. M., Mori K. et al. (2001). Complementary signaling pathways regulate the unfolded protein response and are required for C. elegans development. Cell 107, 893-903. 10.1016/S0092-8674(01)00612-2 [DOI] [PubMed] [Google Scholar]
- Shi Y., Vattem K. M., Sood R., An J., Liang J., Stramm L. and Wek R. C. (1998). Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved in translational control. Mol. Cell Biol. 18, 7499-7509. 10.1128/MCB.18.12.7499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorczyk-Werner A., Chiang W.-C., Wawrocka A., Wicher K., Jarmuż-Szymczak M., Kostrzewska-Poczekaj M., Jamsheer A., Płoski R., Rydzanicz M., Pojda-Wilczek D. et al. (2017). Autosomal recessive cone-rod dystrophy can be caused by mutations in the ATF6 gene. Eur. J. Hum. Genet. 25, 1210-1216. 10.1038/ejhg.2017.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers C. R., Wang R., Bourne R. A., McGrath B. C., Hu J., Bevilacqua S. C., Paton J. C., Paton A. W., Collardeau-Frachon S., Nicolino M. et al. (2018). The protein kinase PERK/EIF2AK3 regulates proinsulin processing not via protein synthesis but by controlling endoplasmic reticulum chaperones. J. Biol. Chem. 293, 5134-5149. 10.1074/jbc.M117.813790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam A. B., Roberts L. S., Chandra V., Rivera I. G., Nomura D. K., Forbes D. J. and Niwa M. (2018). The UPR activator ATF6 responds to proteotoxic and lipotoxic stress by distinct mechanisms. Dev. Cell 46, 327-343.e7. 10.1016/j.devcel.2018.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Tsujimura T., Takeda K., Sugihara A., Maekawa A., Terada N., Yoshida N. and Akira S. (1998). Targeted disruption of ATF4 discloses its essential role in the formation of eye lens fibres. Genes Cells 3, 801-810. 10.1046/j.1365-2443.1998.00230.x [DOI] [PubMed] [Google Scholar]
- Tanegashima K., Zhao H., Rebbert M. L. and Dawid I. B. (2009). Coordinated activation of the secretory pathway during notochord formation in the Xenopus embryo. Development 136, 3543-3548. 10.1242/dev.036715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teodoro T., Odisho T., Sidorova E. and Volchuk A. (2012). Pancreatic β-cells depend on basal expression of active ATF6α-p50 for cell survival even under nonstress conditions. Am. J. Physiol. Cell Physiol. 302, C992-C1003. 10.1152/ajpcell.00160.2011 [DOI] [PubMed] [Google Scholar]
- Thuerauf D. J., Morrison L. E., Hoover H. and Glembotski C. C. (2002). Coordination of ATF6-mediated transcription and ATF6 degradation by a domain that is shared with the viral transcription factor, VP16. J. Biol. Chem. 277, 20734-20739. 10.1074/jbc.M201749200 [DOI] [PubMed] [Google Scholar]
- Thuerauf D. J., Morrison L. and Glembotski C. C. (2004). Opposing roles for ATF6alpha and ATF6beta in endoplasmic reticulum stress response gene induction. J. Biol. Chem. 279, 21078-21084. 10.1074/jbc.M400713200 [DOI] [PubMed] [Google Scholar]
- Tirasophon W., Welihinda A. A. and Kaufman R. J. (1998). A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (Ire1p) in mammalian cells. Genes Dev. 12, 1812-1824. 10.1101/gad.12.12.1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya Y., Saito M., Kadokura H., Miyazaki J.-I., Tashiro F., Imagawa Y., Iwawaki T. and Kohno K. (2018). IRE1-XBP1 pathway regulates oxidative proinsulin folding in pancreatic β cells. J. Cell Biol. 217, 1287-1301. 10.1083/jcb.201707143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urano F., Wang X., Bertolotti A., Zhang Y., Chung P., Harding H. P. and Ron D. (2000). Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287, 664-666. 10.1126/science.287.5453.664 [DOI] [PubMed] [Google Scholar]
- van Anken E., Romijn E. P., Maggioni C., Mezghrani A., Sitia R., Braakman I. and Heck A. J. R. (2003). Sequential waves of functionally related proteins are expressed when B cells prepare for antibody secretion. Immunity 18, 243-253. 10.1016/S1074-7613(03)00024-4 [DOI] [PubMed] [Google Scholar]
- Vasudevan D., Clark N. K., Sam J., Cotham V. C., Ueberheide B., Marr M. T. II and Ryoo H. D. (2017). The GCN2-ATF4 signaling pathway induces 4E-BP to bias translation and boost antimicrobial peptide synthesis in response to bacterial infection. Cell Rep. 21, 2039-2047. 10.1016/j.celrep.2017.10.096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vattem K. M. and Wek R. C. (2004). Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc. Natl. Acad. Sci. USA 101, 11269-11274. 10.1073/pnas.0400541101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volmer R., van der Ploeg K. and Ron D. (2013). Membrane lipid saturation activates endoplasmic reticulum unfolded protein response transducers through their transmembrane domains. Proc. Natl. Acad. Sci. USA 110, 4628-4633. 10.1073/pnas.1217611110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P. and Ron D. (2011). The unfolded protein response: from stress pathway to homeostatic regulation. Science 334, 1081-1086. 10.1126/science.1209038 [DOI] [PubMed] [Google Scholar]
- Wang X.-Z., Harding H. P., Zhang Y., Jolicoeur E. M., Kuroda M. and Ron D. (1998). Cloning of mammalian Ire1 reveals diversity in the ER stress responses. EMBO J. 17, 5708-5717. 10.1093/emboj/17.19.5708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Ryoo H. D., Qi Y. and Jasper H. (2015). PERK limits Drosophila lifespan by promoting intestinal stem cell proliferation in response to ER stress. PLoS Genet. 11, e1005220 10.1371/journal.pgen.1005220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M., Gelowani V., Eblimit A., Wang F., Young M. P., Sawyer B. L., Zhao L., Jenkins G., Creel D. J., Wang K. et al. (2015). ATF6 is mutated in early onset photoreceptor degeneration with macular involvement. Invest. Ophthalmol. Vis. Sci. 56, 3889-3895. 10.1167/iovs.15-16778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S., Ishihara H., Yamada T., Tamura A., Usui M., Tominaga R., Munakata Y., Satake C., Katagiri H., Tashiro F. et al. (2008). ATF4-mediated induction of 4E-BP1 contributes to pancreatic beta cell survival under endoplasmic reticulum stress. Cell Metab. 7, 269-276. 10.1016/j.cmet.2008.01.008 [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Sato T., Matsui T., Sato M., Okada T., Yoshida H., Harada A. and Mori K. (2007). Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and xbp1. Dev. Cell 13, 365-376. 10.1016/j.devcel.2007.07.018 [DOI] [PubMed] [Google Scholar]
- Ye J., Rawson R. B., Komuro R., Chen X., Davé U. P., Prywes R., Brown M. S. and Goldstein J. L. (2000). ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol. Cell 6, 1355-1364. 10.1016/S1097-2765(00)00133-7 [DOI] [PubMed] [Google Scholar]
- Yoshida H., Okada T., Haze K., Yanagi H., Yura T., Negishi M. and Mori K. (2000). ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol. Cell. Biol. 20, 6755-6767. 10.1128/MCB.20.18.6755-6767.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Okada T., Haze K., Yanagi H., Yura T., Negishi M. and Mori K. (2001). Endoplasmic reticulum stress-induced formation of transcription factor complex ERSF including NF-Y (CBF) and activating transcription factors 6alpha and 6beta that activates the mammalian unfolded protein response. Mol. Cell. Biol. 21, 1239-1248. 10.1128/MCB.21.4.1239-1248.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S. H., Hiramatsu N., Liu Q., Sun X. V., Lenh D., Chan P., Chiang K., Koo E. H., Kao A. W., Litvan I. et al. (2018). Tauopathy-associated PERK alleles are functional hypomorphs that increase neuronal vulnerability to ER stress. Hum. Mol. Genet. 27, 3951-3963. 10.1093/hmg/ddy297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., McGrath B., Li S., Frank A., Zambito F., Reinert J., Gannon M., Ma K., McNaughton K. and Cavener D. R. (2002). The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol. Cell Biol. 22, 3864-3874. 10.1128/MCB.22.11.3864-3874.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Wong H. N., Song B., Miller C. N., Scheuner D. and Kaufman R. J. (2005). The unfolded protein response sensor IRE1alpha is required at 2 distinct steps in B cell lymphopoiesis. J. Clin. Invest. 115, 268-281. 10.1172/JCI200521848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Wang S., Malhotra J., Hassler J. R., Back S. H., Wang G., Chang L., Xu W., Miao H., Leonardi R. et al. (2011). The unfolded protein response transducer IRE1α prevents ER stress-induced hepatic steatosis. EMBO J. 30, 1357-1375. 10.1038/emboj.2011.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Cao Y. and Grunz H. (2003). Xenopus X-box binding protein 1, a leucine zipper transcription factor, is involved in the BMP signaling pathway. Dev. Biol. 257, 278-291. 10.1016/S0012-1606(03)00069-1 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Zhou J., Liu D., Dong F., Cheng H., Wang W., Pang Y., Wang Y., Mu X., Ni Y. et al. (2015). ATF4 plays a pivotal role in the development of functional hematopoietic stem cells in mouse fetal liver. Blood 126, 2383-2391. 10.1182/blood-2015-03-633354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D., Palam L. R., Jiang L., Narasimhan J., Staschke K. A. and Wek R. C. (2008). Phosphorylation of eIF2 directs ATF5 translational control in response to diverse stress conditions. J. Biol. Chem. 283, 7064-7073. 10.1074/jbc.M708530200 [DOI] [PubMed] [Google Scholar]