Abstract
Motor cortex stimulation (MCS) is an effective therapy for refractory neuropathic pain. MCS increases the nociceptive threshold in healthy rats via endogenous opioids, inhibiting thalamic nuclei and activating the periaqueductal gray. It remains unclear how the motor cortex induces top-down modulation of pain in the absence of persistent pain. Here, we investigated the main nuclei involved in the descending analgesic pathways and the spinal nociceptive neurons in rats that underwent one session of MCS and were evaluated with the paw pressure nociceptive test. The pattern of neuronal activation in the dorsal raphe nucleus (DRN), nucleus raphe magnus (NRM), locus coeruleus (LC), and dorsal horn of the spinal cord (DHSC) was assessed by immunoreactivity (IR) for Egr-1 (a marker of activated neuronal nuclei). IR for serotonin (5HT) in the DRN and NRM, tyrosine hydroxylase (TH) in the LC, and substance P (SP) and enkephalin (ENK) in the DHSC was also evaluated. MCS increased the nociceptive threshold of the animals; this increase was accompanied by activation of the NRM, while DRN activation was unchanged. However, cortical stimulation induced an increase in 5HT-IR in both serotonergic nuclei. MCS did not change the activation pattern or TH-IR in the LC, and it inhibited neuronal activation in the DHSC without altering SP or ENK-IR. Taken together, our results suggest that MCS induces the activation of serotonergic nuclei as well as the inhibition of spinal neurons, and such effects may contribute to the elevation of the nociceptive threshold in healthy rats. These results allow a better understanding of the circuitry involved in the antinociceptive top-down effect induced by MCS under basal conditions, reinforcing the role of primary motor cortex in pain control.
Electronic supplementary material
The online version of this article (10.1186/s12993-019-0156-0) contains supplementary material, which is available to authorized users.
Keywords: Motor cortex, Neurostimulation, Antinociception, Raphe nuclei, Spinal cord
Introduction
Epidural motor cortex stimulation (MCS) is an effective therapeutic option for refractory neuropathic pain of either peripheral or central origin [59, 60, 62, 74, 93, 97]. MCS provides pain relief in 55 to 64% of patients refractory to other treatments [13, 15, 42, 44, 88], but the neural mechanism underlying its analgesic effect remains poorly understood. In patients with neuropathic pain, MCS modulates nociceptive spinal reflexes [21] and induces analgesia by activating top-down controls originating from intracortical horizontal fibers or interneurons [39]. Electrophysiology and functional imaging studies have shown that MCS activates supraspinal areas involved in the perception and/or emotional appraisal of pain, including the lateral thalamus, anterior cingulate cortex (ACC), anterior insula and periaqueductal gray (PAG) [20, 21, 70, 71]. The analgesic effect of MCS is also correlated with the release of endogenous opioids in the ACC, insula and PAG [47, 48]. In healthy rats, with no neuropathic conditions, MCS reduces the responsiveness of spinal nociceptive neurons [16, 80], thereby raising the nociceptive threshold via endogenous opioids [12], this response is associated with inhibition of the thalamic nuclei and activation of the PAG [65]. In neuropathic rats, MCS reverses central and peripheral pain [8, 45, 64, 78, 96, 99, 100], activating the limbic system and PAG and inhibiting the thalamic nuclei and spinal nociceptive neurons [64]. It has been hypothesized that, in humans and animals, MCS induces analgesia by activating the descending analgesic pathways [12, 70, 71, 100]; however, it is not clear which midbrain nuclei are modulated after cortical stimulation or how they act on spinal nociceptive neurons to elevate the nociceptive threshold.
The serotonergic dorsal raphe nucleus (DRN) is an important brainstem nucleus involved in the pain modulation system, which target different brain areas because its widespread ascending and descending projections, including a few fibers to the spinal cord directly [101]. Other pivotal areas involved in the descending analgesic system include the opioidergic PAG, the noradrenergic locus coeruleus (LC), and the rostral ventromedial medulla (RVM), which consists of the serotonergic nucleus raphe magnus (NRM) and adjacent nucleus reticularis magnocellularis [4, 10, 55]. Activation of these descending pathways induces the release of serotonin (5HT) and noradrenaline (NA) in the dorsal horn of the spinal cord (DHSC); these neurotransmitters acting on 5HT1A and α2-adrenergic receptors directly or indirectly inhibit projection neurons, central terminals of primary afferent fibers and excitatory interneurons by decreasing the release of excitatory neurotransmitters, such as glutamate, substance P (SP) and calcitonin gene-related peptide (CGRP) [10, 106]. A dysregulation in descending pain modulation is observed in persistent pain condition, since that central sensitization phenomena changes the subtype of spinal 5HT/NA receptors, which can have inhibitory or facilitatory role on pain [43, 69, 85, 102]. Additionally, the descending analgesic system induces the activation of inhibitory interneurons in the DHSC; these neurons release GABA, glycine and enkephalin (ENK), which also contribute to the inhibition of ascending nociceptive transmission [4, 106].
Considering pain control induced by cortical stimulation, while descending noradrenergic pathway may not be critical for the MCS-induced analgesia in healthy or neuropathic rats [99], descending serotonergic and dopaminergic pathways contribute to spinal antinociception induced by MCS in neuropathic rats [98, 100]. However, its role in spinal modulation is not totally clear. Therefore, a more thorough investigation of the role of motor cortex in the nociceptive threshold in healthy conscious rats could facilitate the understanding of its function in pain control. Considering that the neurocircuitry involved in MCS-induced analgesia needs to be better clarified, we investigated the activation pattern of the serotonergic and noradrenergic nuclei involved in the descending analgesic system, as well as their effect on the spinal nociceptive neurons, in response to MCS in healthy rats.
Materials and methods
Experimental design
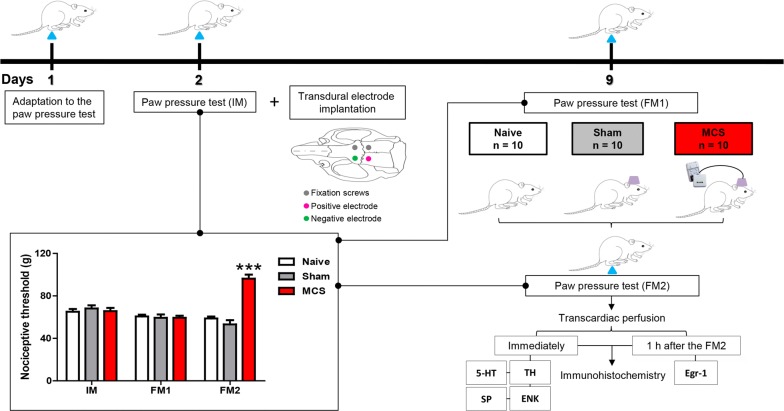
Adult rats were evaluated in a nociceptive test (described in Measuring nociceptive threshold below), and subsequently, under anesthesia, transdural electrodes were implanted over the functional area of the left primary motor cortex associated with the right hind limb [12]. After 1 week, the nociceptive test was performed again, and a group of rats undertook one session of MCS (15 min); at the end of this period, while still under stimulation, they were re-evaluated on the test. Rats that underwent surgical procedures but were not electrically stimulated (sham) and rats that did not receive any surgical procedures (naive) were also evaluated. Immediately after the last nociceptive evaluation, the animals (5 animals per group) were anesthetized and immediately perfused to have their brains and spinal cords processed to evaluate immunoreactivity for 5HT, tyrosine hydroxylase (TH) and SP/ENK in the DRN/NRM, LC and DHSC, respectively. Additionally, another group of animals (5 animals per group) was anesthetized 1 h after the last nociceptive test and then subjected to an Egr-1 immunohistochemistry assay to evaluate the neuronal activation pattern in the DRN, NRM, LC and DHSC (Fig. 1).
Fig. 1.
Experimental design of the study and effect of cortical stimulation on mechanical nociceptive threshold. Rats were habituated to the nociceptive test the day preceding the electrode implantation (day 1). The paw pressure test was conducted prior to the electrode implantation (initial measurement, IM; day 2) and 7 days later (day 9), as well as before (final measurement 1, FM1) and during MCS (final measurement 2, FM2). Naive and sham rats were also evaluated. Animals were divided into three groups: rats with no surgical procedure (Naive), rats with transdural electrodes and false stimulation (Sham), and stimulated rats (MCS). The transdural electrodes were implanted over the motor cortex of the left hemisphere, and the nociceptive threshold of the right hind paw was determined. Values represent the mean ± SEM of 10 animals from each group. Statistically significant differences from the naive group (***p < 0.0001, after Bonferroni’s post hoc test) are indicated. Half of the animals in each group were perfused immediately after the last nociceptive test and evaluated for 5HT-, TH-, SP- and ENK-IR; the other half was perfused 1 h after the last nociceptive test for evaluation of Egr-1-IR. 5HT serotonin, ENK enkephalin, IR immunoreactivity, MCS motor cortex stimulation, SP substance P, TH tyrosine hydroxylase
Animals
Thirty-six male Wistar rats (180–220 g) were housed in acrylic boxes (three rats per cage) for at least 5 days before the initiation of the experimental procedures. The boxes, containing wood shavings, were kept in a room with a stable, controlled ambient temperature (22 ± 2 °C) and a light/dark cycle of 12 h/12 h, and the animals had free access to water and rat chow pellets. All experimental procedures were in accordance with the guidelines for the ethical use of animals in research involving pain and nociception [108] and were reported in accordance with the ARRIVE guidelines (http://www.nc3rs.org.uk/arrive-guidelines). The study was approved by the Ethics Committees on the Use of Animals of both the Hospital Sírio Libanês (protocol number CEUA 2011/13) and the Institute of Biomedical Sciences of the University of São Paulo (no. 055, page 103, book 02, 2011).
Electrode implantation and electrical stimulation parameters
Rats were deeply anesthetized with ketamine/xylazine (0.5/2.3 mg/kg, i.m.) and received a local scalp injection of 2% lidocaine (100 µL/animal, s.c.). Whenever necessary, supplementary doses of ketamine were administered to the animals to ensure an anesthetized state. Then, under stereotaxic guidance using a functional map developed by our group [14], two transdural stainless steel electrodes (cylinders of 0.8 mm in diameter) were fixed over the left primary motor cortex in the functional area corresponding to the right hind limb (1.0 mm rostral and 1.5 mm caudal to the bregma, 1.5 mm lateral to the midline). Two fixation screws (implanted 4 to 6 mm away from the site of stimulation) and acrylic polymer were used to stabilize the implant and to ensure electrical isolation. The contacts of each electrode pole were inserted into a connector, which was also fixed to the whole ensemble. For 3 consecutive days, the animals received anti-inflammatory (Ketoprofen 5 mg/kg/day, via s.c.) to prevent pain from the surgery. All implanted rats were allowed to recover for 1 week before testing began. Electrical stimulation was applied according to an earlier study, which showed changes in mechanical nociceptive threshold without interfering with thermal nociceptive threshold and with general or motor activities [12]. One week after implantation of the electrodes, electrical stimulation was delivered in a single 15-min session (1.0 V, 60 Hz, and 210 µs; Medtronic electrical stimulator, Minneapolis, MN), and the final measurements during the nociceptive test were recorded while the rats were still under stimulation. The cathode was always chosen to be the posterior contact of the electrode because, according to the functional map, that site has greater surface area corresponding to the hind limb [14]. The sham group was subjected to the same conditions but did not receive stimulation. The rats were randomly divided into the sham and stimulated groups.
Measuring nociceptive threshold
On the day of the nociceptive tests, the animals were brought into a separate, quiet room 1 h before the tests to allow them to habituate to the environment. The mechanical nociceptive threshold was determined using a pressure apparatus (EEF-440, Insight, SP, Brazil), which has been previously described [73]. Briefly, a mass with increasing magnitude (16 g/s) was applied to the right hind paw. The mass (in grams) required to induce the withdrawal response represented the nociceptive threshold. Antinociception was defined as a significant increase in the pressure necessary to induce the withdrawal response in experimental animals compared either with initial measurement of the same animal and with the control animals (naive group). Aiming to reduce animal stress, the rats were handled by the experimenter and were habituated to the paw pressure test the day preceding the electrode implantation. Nociceptive tests were conducted prior to the electrode implantation (initial measurement) and 7 days later, as well as before (final measurement 1) and during MCS (final measurement 2). Naive and sham rats were also evaluated.
Immunohistochemistry
The animals were divided into three groups: rats that had not undergone surgery (naive, n = 10), rats with electrodes implants, but not electrically stimulated (sham, n = 13), and stimulated rats (MCS, n = 13). Randomly, half of the animals in each group were perfused immediately after the last nociceptive test to evaluate 5HT, TH, SP and ENK immunoreactivity (IR), and the other half was perfused 1 h after the last nociceptive test to evaluate Egr-1-IR. In this last group, the animals were perfused 1 h after the last nociceptive stimulus because the expression levels of inducible transcription factor proteins (including c-Fos, c-Jun and Egr-1) peak at approximately 1 h after the stimulus and fade by 3 to 4 h [29]. Rats were deeply anesthetized with ketamine and xylazine and then subjected to transcardiac perfusion with saline solution followed by 4% paraformaldehyde (PFA) dissolved in 0.1 M phosphate buffer (PB). The brain and lumbar spinal cord (L4–L6 segments) were collected and postfixed in PFA for 4 h, followed by incubation with 30% sucrose solution in PB for 48 h at 4 °C. Tissue sections (30 µm) were cut on a freezing microtome, washed in PB, and incubated for 12 to 16 h at 4 °C with the following primary antibodies: rabbit anti-Egr-1 (Early growth response protein 1, C-19; Santa Cruz Biotechnology, CA, USA), rabbit anti-5TH (NT-102, Protos Biotech, NY, USA), mouse anti-TH (MAB5280, Millipore, MA, USA), rabbit anti-SP (AB962, Millipore) or mouse anti-ENK (MAB350, Millipore) diluted 1:1000 in 0.3% of Triton X-100 containing 5% normal donkey serum (Jackson ImmunoResearch, ME, USA). After being washed (3 × 10 min) with PB, tissue sections were incubated for 2 h at room temperature with biotinylated secondary antibodies (1:200, Jackson ImmunoResearch). After additional washes, the sections were incubated for 2 h at room temperature with avidin-biotin complex (1:100; ABC Elite kit, VectorLabs, CA, USA), and visualized with 0.05% diaminobenzidine tetrahydrochloride (DAB, Sigma-Aldrich, MO, USA) and 0.03% (final concentration) hydrogen peroxide in PB. The sections were then washed and mounted on glass slides, and the staining was intensified with 0.05% osmium tetroxide in water. Afterwards, the sections were dehydrated through graded ethanol solutions, followed by xylene, and coverslipped with Permount (Fisher Scientific, PA, USA). The IR was captured by means of a light microscope (Eclipse E1000, Nikon, NY, USA) and was analyzed using ImageJ software (National Institute of Health, MD, USA; http://rsbweb.nih.gov/ij/). Figures were assembled using Adobe Photoshop (Adobe Systems, CA, USA); the images were optimized using contrast and brightness only. Quantitative analysis was performed to determine the density of nuclei showing positive IR for Egr-1 in the dorsomedial and ventromedial DRN, NRM, LC and DHSC (laminae I–IV) in animals perfused 1 h after the behavior analyses. Additionally, the densities of 5HT-IR in the DRN and NRM, TH-IR in the LC and SP and ENK-IR in the DHSC were analyzed in the animals perfused immediately after the nociceptive tests. The areas analyzed were defined for each structure by using a 10× objective for the DRN (B7; − 7.32 to − 7.92 mm from bregma), NRM (B3; − 11.04 to − 11.76 from bregma) and LC (A6; − 9.72 to − 9.96 mm from bregma) [9] and a 40× objective for the DHSC (laminae I–IV). For each assay, total number of positive profile (immunostained particles) was used to provide a mean immunolabel value (compared with pre-defined threshold) in five tissue sections per animal and five animals per group. The regions of interest were identified according to histological landmarks based on the adjoining Nissl-stained sections in the brain atlas [67] and spinal cord atlas [56].
Statistical analysis
Data are presented as the mean ± standard error of the mean (SEM). Statistical analyses were conducted with GraphPad Prism 5.0 software (GraphPad Software Inc; CA, USA). The results of the nociceptive tests were analyzed using two-way (2-w) repeated measures (rm) analysis of variance (ANOVA) followed by the Bonferroni’s post hoc test. Immunohistochemistry data were normalized (by defining the naive group as 100% for Egr-1 and 1.0 ratio for 5HT, TH, SP and ENK) and were analyzed using one-way (1-w) ANOVA followed by the Bonferroni’s post hoc test. In all cases, p < 0.05 was considered statistically significant.
Results
Twenty-six animals underwent electrode implantation, as stated above; however, six implanted rats were excluded from the study because they removed their subdural implants before the final nociceptive tests. Hence, the results concern naive animals n = 10, sham animals n = 10 and MCS animals n = 10.
MCS increased the mechanical nociceptive threshold in rats in the paw contralateral to the stimulation side compared with the thresholds of the control group, non-operated naive animals (Fig. 1, Additional file 1: Table S1). The nociceptive threshold increased in 62% when compared to the nociceptive threshold observed in the naive animals (2-w-rm-ANOVA, Treatment × Time, F2,42 = 24.91, p = 0.0001; followed by Bonferroni’s post hoc test, p < 0.001; Fig. 1).
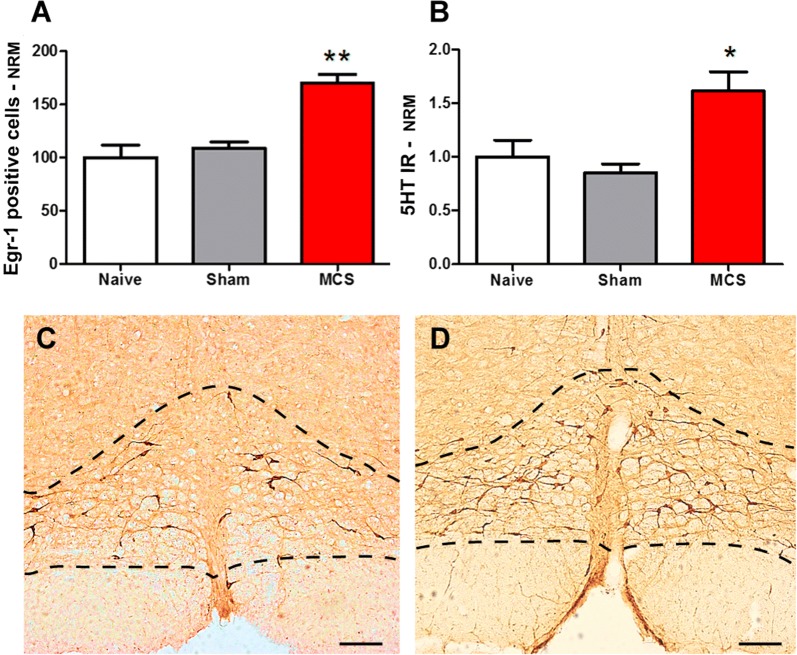
Regarding the NRM, we observed that naive and sham-stimulated animals presented the same pattern of neuronal activation and 5HT-IR in the NRM (Fig. 2A, B). MCS induced an increase of 70% in Egr-1-IR (1-w-ANOVA, F(2,12) = 20.06, p = 0.0003, followed by Bonferroni’s post hoc test, p < 0.01; Fig. 2A) and 62% in 5HT-IR (1-w-ANOVA, F(2,12) = 6.43, p = 0.0126, followed by Bonferroni’s post hoc test, p < 0.01; Fig. 2B–D) in the NRM over the levels in the naive group (Additional file 1: Table S1).
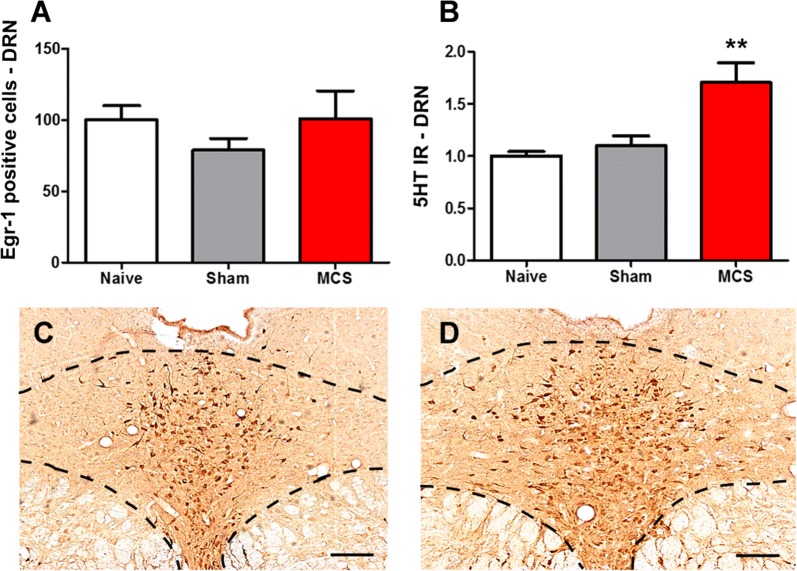
Fig. 2.
MCS, antinociception, and their correlation with NRM activation. Quantification of Egr-1 (A) and 5HT (B) IR in the NRM of naive (without surgical intervention), sham (with epidural electrodes but without stimulation) and stimulated (MCS, 1.0 V/60 Hz/210 µs, 15 min) rats. Values represent the mean ± SEM (n = 5 animals per group). **p < 0.01 compared to the naive group, after Bonferroni’s post hoc test. Photomicrographs illustrating the 5HT-IR in the NRM of sham (C) and stimulated (D) rats. 5HT serotonin, IR immunoreactivity, MCS motor cortex stimulation, NRM nucleus raphe magnus. Scale bars: 200 μm
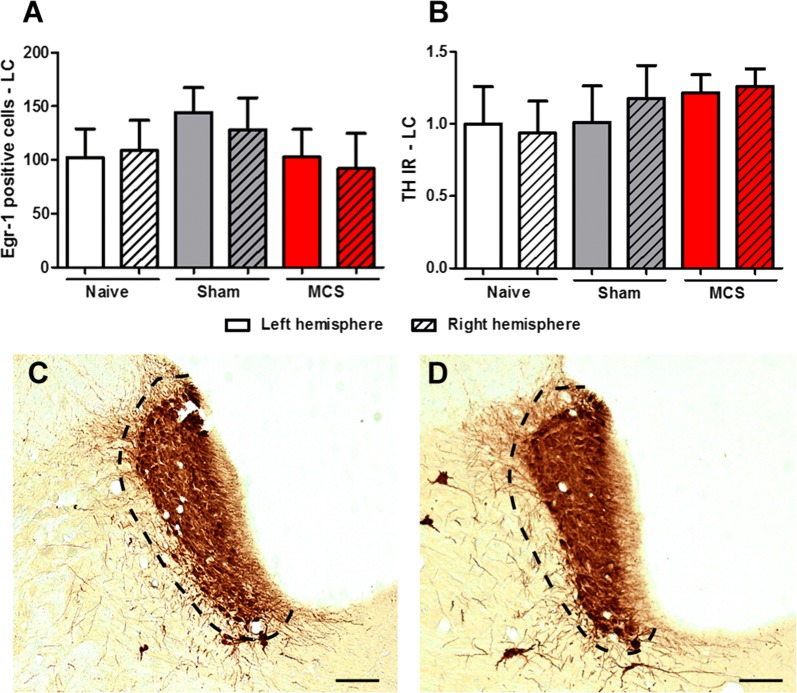
In the DRN, we observed the same staining intensity for Egr-1 (Fig. 3A) and for 5HT (Fig. 3B) between naive animals and sham-stimulated animals. MCS did not interfere with DRN activity (1-w-ANOVA, F(2,12) = 0.74, p = 0.4945; Fig. 3A); however, it induced an increase of 70% in the 5HT-IR of the DRN compared with that of the naive group (1-w-ANOVA, F(2,12) = 8.42, p = 0.0029, followed by Bonferroni’s post hoc test, p < 0.01; Fig. 3B–D; Additional file 1: Table S1). Within the LC, animals submitted to MCS presented similar staining of Egr-1-positive neurons to naive and sham-stimulated animals in both cerebral hemispheres (1-w-ANOVA, F(5,25) = 0.49, p = 0.7797; Fig. 4A) and TH-IR (1-w-ANOVA, F(5,25) = 0.42, p = 0.8285; Fig. 4B–D; Additional file 1: Table S1).
Fig. 3.
Participation of the DRN in MCS-induced antinociception. Quantification of Egr-1 (A) and 5HT (B) IR in the DRN of naive (without surgical intervention), sham (with epidural electrodes but without stimulation) and stimulated (MCS, 1.0 V/60 Hz/210 µs, 15 min) rats. Values represent the mean ± SEM (n = 5 animals per group). *p < 0.05 and **p < 0.01 in comparison to the naive group, after Bonferroni’s post hoc test. Photomicrographs illustrating the 5HT-IR in the DRN of sham (C) and stimulated (D) rats. 5HT serotonin, DRN dorsal raphe nucleus, IR immunoreactivity, MCS motor cortex stimulation. Scale bars: 200 µm
Fig. 4.
Involvement of LC in antinociception induced by MCS. Quantification of Egr-1 (A) and TH (B) IR in the LC of naive (without surgical intervention), sham (with epidural electrodes but without stimulation) and stimulated (MCS, 1.0 V/60 Hz/210 µs, 15 min) rats. Values represent the mean ± SEM (n = 5 animals per group). Photomicrographs illustrating the TH-IR in the LC of sham (C) and stimulated (D) rats. IR immunoreactivity, LC locus coeruleus, MCS motor cortex stimulation, TH tyrosine hydroxylase. Scale bars: 200 µm
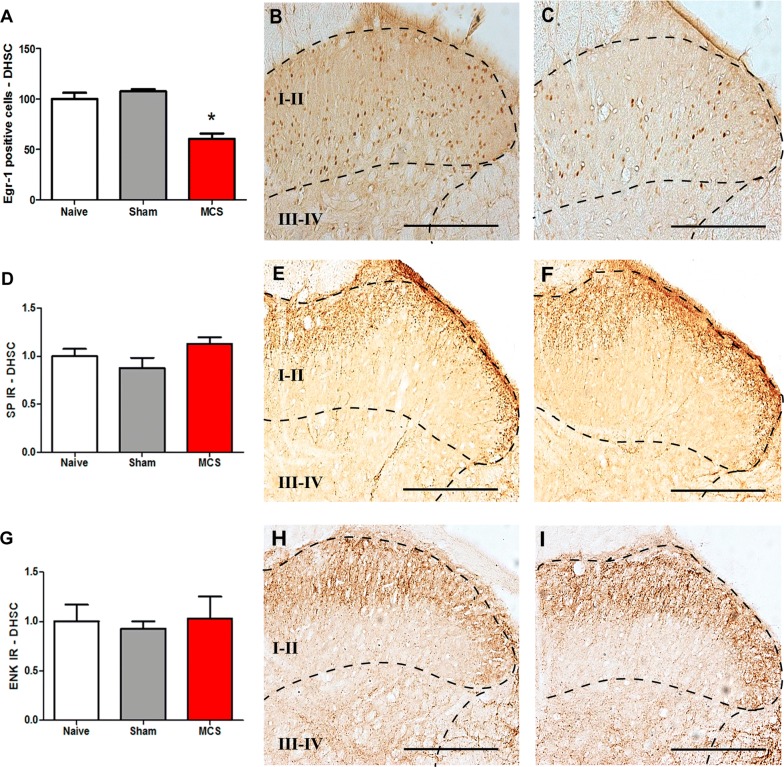
Concerning the spinal cord (laminae I–IV), animals subjected to MCS showed a decrease of 72% in the Egr-1-IR in the DHSC compared to the level in the naive group (1-w-ANOVA, F(2,12) = 26.90, p = 0.0002; followed by Bonferroni’s post hoc test, p < 0.05; Fig. 5A–C; Additional file 1: Table S1). Moreover, MCS did not change the pattern of SP-IR (1-w-ANOVA, F(2,12) = 2.40, p = 0.2423; Fig. 5D–F) and ENK-IR (1-w-ANOVA, F(2,12) = 0.0978, p = 0.9075; Fig. 5G–I) in the DHSC compared with the patterns in naive animals. Naive animals showed the same staining pattern for Egr-1, SP and ENK as sham-stimulated animals (Fig. 5A, D, G) (Additional file 1: Table S1).
Fig. 5.
Effect of cortical stimulation on DHSC activation. Quantification of Egr-1 (A), SP (D) and ENK (G) IR in the DHSC of naive (without surgical intervention), sham (with epidural electrodes but without stimulation) and stimulated (MCS, 1.0 V/60 Hz/210 µs, 15 min) rats. Values represent the mean ± SEM (n = 5 animals per group). *p < 0.05 compared to the naive group, after Bonferroni’s post hoc test. Photomicrographs illustrating Egr-1-IR (B, C), SP-IR (e, F) and ENK-IR (H, I) in sections of the DHSC (laminae I-IV, right side) from sham (B, E, H) and stimulated (C, F, I) rats. DHSC dorsal horn of the spinal cord, ENK enkephalin, IR immunoreactivity, MCS motor cortex stimulation, SP substance P. Scale bars: 200 µm
Discussion
Are serotoninergic nuclei involved in MCS-induced antinociception in the absence of neuropathic conditions?
MCS alleviates neuropathic pain in humans and animals; nevertheless, the subcortical relay mechanisms involved in this response are not yet fully understood. In human studies, it has been suggested that the neurocircuitry involved in the emotional component of pain and in the descending analgesic system mediate analgesia induced by MCS [21, 39, 70, 71]. In naive and neuropathic rats, MCS-induced antinociception is accompanied by PAG activation [64, 65], which plays a pivotal role in the activation of descending analgesic pathways [55]. The antinociceptive response observed in naive rats is specific to motor cortex, considering that stimulation of posterior parietal or somatosensory cortices did not elicit any changes in the general activity or nociceptive response [12]. In neuropathic rats, MCS-induced analgesia is modulated by the action of RVM on spinal 5HT1A receptors [100]; however, the role of motor cortex in the activation of the descending serotonergic nuclei, in healthy conscious rats, has not been evaluated yet.
Neuronal activity mapping with inducible transcription factors (ITFs) or immediate early genes, such as c-fos, c-jun and egr-1 (zif268, krox-24 or zenk), has been widely used to study the neurocircuitry underlying nociception [28–30, 35, 38]. Although this tool is widely used, it is limited in the interpretation of the cell type and mechanism of action; however, it makes it possible to determine which structures are more or less activated in relation to the applied intervention, allowing for a more detailed investigation in this altered area. ITFs showed to be overexpressed in neurons in response to extracellular stimuli including peripheral nociceptive stimulation in different areas of the brain and spinal cord [26, 28, 38, 83]. Supporting earlier findings [12, 16, 65, 78, 99], we showed here that MCS raised the mechanical nociceptive threshold of the hind paw contralateral to the stimulation side in healthy, conscious animals. To substantiate the hypothesis of the role of the descending serotoninergic system in MCS-induced antinociception, we investigated Egr-1-IR and 5HT-IR in the NRM, a critical relay for descending pain control [55]. The NRM contains serotonergic neurons, which exert facilitatory and inhibitory influence on spinal nociceptive transmission [11, 63, 79]. The NRM serotonergic neurons are considered essential for descending analgesic control, and although they constitute only approximately 20% of the total population of the NRM, they are one of the main sources of 5HT to the DHSC [9, 54]. Our results showed that, in healthy animals, MCS not only induced activation of the NRM, observed by Egr-1 labeling, but also increased the 5HT production within this nucleus. Considering that RVM blockade has spinal nociceptive effects in healthy control animals [22, 27], we can suggest that the NRM is activated after cortical stimulation and that this response may contribute to the elevation of the nociceptive threshold in healthy rats.
The DRN, the major source of serotonergic neurons in the brain [36, 92], is another important nucleus involved in pain control [18, 63, 101]. The DRN projections to the forebrain area contribute effectively to the affective-motivational component of pain [101], and since MCS-induced analgesia appears to specifically modulate the emotional appraisal of pain, rather that its intensity [70], we hypothesized that the DRN may also be involved in the effect of MCS. To test this hypothesis, we assessed Egr-1 and 5HT immunolabeling in the DRN after MCS-induced antinociception. We observed here that MCS increased the amount of 5HT staining in the DRN but did not interfere with the pattern of neuronal activation in this nucleus. The DRN has a high degree of neuronal heterogeneity; this nucleus comprises groups of neurons containing 5HT, glutamate, ENK, GABA and dopamine, which may be combined with different neuropeptides [19, 46, 84] and this neuronal diversity is not restricted to inhibition; some DRN neurons are involved in facilitation of nociceptive transmission [101]. The DRN synaptic circuits are finely regulated by glutamate and GABA arising from extra-raphe areas as well as from local sources, influencing the activity of 5HT cells [84]. Regarding nociceptive inhibition, serotonergic DRN neurons receive excitatory glutamatergic connections, leading to 5TH release directly to the DHSC or indirectly to the NRM, modulating the spinal nociceptive response [101]. The inhibition of nociceptive response depends on the activation of 5-HT receptor subtype and its anatomical location [34, 55]. In this sense, under healthy conditions, the activation of spinal 5HT1A receptor inhibits the nociceptive response in the DHSC [23]. Concerning nociceptive facilitation, serotonergic DRN neurons receive inhibitory connections from GABAergic neurons, which contribute to the inhibition of 5HT release within this nucleus [84, 86, 101]. As no changes in neuronal activity were found in the DRN following MCS, it is plausible that the sum of the inhibitory and excitatory signals within the DRN amounts to zero, reflecting balanced neuronal changes that modulate the activity of this nucleus. In this regard, our hypothesis is that the MCS inhibits the local GABAergic interneurons, leading to an increase in the activity of 5HT cells, resulting in a lack of change in the pattern of neuronal activation in the DRN. This hypothesis is supported by the fact that the MCS-induced antinociception was accompanied by considerable enhancement of 5HT in the DRN, converging with other studies that showed an increase in 5HT or its synthesizing enzyme tryptophan hydroxylase in analgesic conditions [66, 87, 101, 104]. Our data suggest for the first time that the DRN, an important nucleus involved in the emotional appraisal of pain, can play a significant role in the elevation of the nociceptive threshold after MCS in healthy rats.
Is the LC not really involved in descending analgesic control induced by MCS?
The brainstem nuclei A5, LC (A6) and A7 are the main sources of the noradrenergic nerve terminals in the spinal cord [37, 103], with the LC providing the predominant noradrenergic input to the DHSC [5, 33, 72]. The LC exerts a predominant inhibitory effect on spinal nociceptive transmission by descending noradrenergic fibers; however, it also exerts a facilitatory effect on the nociceptive response by ascending fibers [37, 43, 68, 69, 107]. Since the noradrenergic neurons of the LC are critical for descending analgesic control [37, 68], we evaluated the pattern of Egr-1-IR and TH-IR (for NA neurons) in this area. Our data showed that MCS did not change neuronal activation or TH staining in the LC compared with the values of the non-operated naive animals.
It was previously shown that MCS increased the discharge rates of LC neurons in neuropathic rats; however, it did not change the neuronal firing rates of LC cells in sham-operated animals [99]. Additionally, the same authors showed that local pharmacological inactivation of the LC and blockade of spinal α2-adrenergic receptors failed to reverse MCS-induced antinociception in the neuropathic and sham-operated rats, suggesting that the descending noradrenergic pathway originating in the LC may do not play a crucial role in spinal antinociception induced by cortical stimulation [99]. Under healthy conditions, the coeruleospinal noradrenergic system has only a slight influence on nociceptive response, whereas, with sustained pain and pathophysiological states, it plays a critical role in pain control [25, 58, 68, 94, 95]. On this topic, it was shown that blockade of α2-adrenergic receptors and lesion of the LC had no effect on spinal nociceptive neurons in healthy animals but modulated the magnitude and duration of the neuronal responses in animals with peripheral inflammation [25, 94]. Our results emphasize the idea that the coeruleospinal noradrenergic pathway might play a secondary role in the control of nociceptive response under basal conditions, particularly in MCS-induced antinociception.
How is the spinal circuitry affected in response to elevation of the nociceptive threshold after MCS?
The DHSC is the complex site where several ascending and descending sensory pathways modulate nociceptive information, acting on projection neurons, primary afferent neurons and excitatory and inhibitory interneurons to contribute to pain processing in both facilitatory and inhibitory systems [89, 90, 105]. Considering that the activation of descending serotonergic and noradrenergic analgesic pathways results in inhibition of the firing of spinal nociceptive-specific neurons in healthy conditions [10, 41, 106] and that the involvement of these pathways in the MCS-induced antinociception is very clear in the literature and corroborate with our findings, we investigated the spinal modulation after MCS. For that, we applied Egr-1-IR to evaluate the pattern of neuronal activation in the DHSC after cortical stimulation. We showed here that MCS decreased the Egr-1-positive neurons in the DHSC, corroborating the idea that there is a direct correlation between a decrease in ITF expression and antinociception in the DHSC [7, 24, 57]. In line with this idea, we showed previously in neuropathic rats that the MCS-induced analgesia is accompanied by complete reversion of spinal hyperactivity induced by peripheral neuropathy, which was manifested by a decrease in Egr-1-IR in the spinal cord [64]. Moreover, MCS in healthy rats attenuated the neuronal discharge rates [75, 80] and Egr-1-IR [16] in the DHSC in response to peripheral mechanical stimulation. Our results support the hypothesis that MCS inhibits the DHSC neurons directly through activation of corticospinal pathways and/or indirectly through activation of the descending analgesic pathways.
SP is a neuropeptide that binds to neurokinin 1 (NK1) receptors and plays an important role in the transmission of nociceptive signals from primary afferent neurons in the spinal cord, contributing to excitability of projection neurons and central sensitization [2, 3, 51, 82]. In the spinal cord, SP is present in interneurons, descending fibers, and central terminals of the primary afferent neurons located superficially in the DHSC [31, 77, 81]. Taking into account that SP is one of the main excitatory neurotransmitters released in the DHSC that mediate nociceptive transmission from the peripheral to the central nervous system, we evaluated the pattern of immunolabeling for SP in the DHSC in response to MCS. In the DHSC, 80% of SP is in primary afferent terminals and is co-localized with NK1 in projection neurons in lamina I of spinal cord [31, 52, 91]. Corroborating these findings, we also observed a high concentration of SP in superficial laminas of the DHSC; however, no difference was observed in the staining intensity of this neuropeptide among naive, sham-stimulated and stimulated animals.
The intensity and modality of a stimulus and the degree of inflammation it induces are critical issues that determine spinal SP release and the extent to which this neuropeptide contributes to the transmission of nociceptive signals [1, 40, 49]. Occupancy of NK1 leads to internalization of the receptor, which has been used as a quantitative indicator of SP release by sensory neurons in response to noxious stimuli [1, 49, 50, 53]. However, it was identified by tracking NK1 internalization in the DHSC neurons that SP is released only under conditions of intense pain [2]. In addition, the loss of NK1-expressing spinal neurons decreases the nociceptive hypersensitivity associated with chronic pain conditions; however, responses to nociceptive stimuli of lower intensity are unaffected by this loss [61], suggesting that the SP-NK1 system is not crucial to spinal nociceptive transmission under healthy conditions. Considering our findings that MCS inhibits the extent of spinal neuronal activation and does not alter SP immunoreactivity in the DHSC, we can hypothesize the following: (1) The modulation of SP action can occur postsynaptically, with direct inhibition of NK1 receptor internalization within projection neurons; (2) Under basal conditions, in the absence of intense painful stimuli, the inhibition of projection neurons appears not to be related to the inhibition of SP release from primary afferent fiber terminals, and the inhibition of other neurotransmitters such as glutamate or CGRP may be the main drivers of this antinociceptive effect; (3) The control of spinal nociceptive transmission in the absence of persistent pain can rely more on the activation of inhibitory systems in the spinal cord circuitry than on the inhibition of excitatory systems.
A brainstem-spinal cord inhibitory circuit controls the mechanical pain threshold through ENK- and GABA-mediated inhibition of spinal nociceptive neurons [17]. Taking into account the crucial role of ENK in the inhibition of the spinal projection neurons [6, 76] and the involvement of the opioid system in MCS-induced antinociception in healthy rats [12], we investigated ENK labeling in the spinal cord in response to cortical stimulation. Our results are consistent with a previous report demonstrating that in the spinal cord, the highest concentrations of ENK-positive fibers are observed in laminae I and II [32]; therefore, we did not detect any changes in ENK labeling between the different experimental groups. There are several polysynaptic inhibitory circuits in the DHSC that can be critical in controlling the spinal nociceptive neurons. Further investigation of the spinal neurocircuitry is required to understand how the motor cortex modulates the nociceptive threshold in the spinal cord in the absence of persistent pain.
Conclusion
Taken together, our results suggest that MCS induces the activation of serotonergic NRM and DRN as well as the inhibition of spinal neurons, and such effects may contribute to the elevation of the nociceptive threshold in healthy conscious rats. Moreover, MCS-induced antinociception, under baseline conditions, may not involve both the noradrenergic coeruleospinal pathway and changes in SP or ENK release in the spinal cord. A better understanding of how these areas respond to MCS under normal conditions will help clarify the role of motor cortex in pain control and how malfunctions of that region could drive responsiveness to cortical stimulation under neuropathic conditions. Our findings expand the scientific knowledge regarding the role of primary motor cortex in pain control, emphasizing that it may be one of the most rostral structures in the neuroaxis related to the pain modulatory system.
Additional file
Additional file 1: Table S1. Pain behavior and immunoreactivity data from naive, sham and stimulated rats.
Authors’ contributions
Study design and data analysis: PSSL, ACPC, ETF, and RLP. Data collection and composition of the paper: PSSL, ACPC, ETF, LRGB and RLP. All authors read and approved the final manuscript.
Acknowledgements
This research was supported by the Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP: 2009/50772-4 and 2010/13748-5) and Hospital Sírio Libanês.
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Funding
This research was supported by the Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP: 2009/50772-4 and 2010/13748-5) and Hospital Sírio Libanês.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- 5HT
serotonin
- ACC
anterior cingulate cortex
- DHSC
dorsal horn of the spinal cord
- DRN
dorsal raphe nucleus
- ENK
enkephalin
- IR
immunoreactivity
- LC
locus coeruleus
- MCS
motor cortex stimulation
- NA
noradrenaline
- NRM
nucleus raphe magnus
- PAG
periaqueductal gray
- RVM
rostral ventromedial medulla
- SP
substance P
- TH
tyrosine hydroxylase
References
- 1.Allen BJ, Rogers SD, Ghilardi JR, Menning PM, Kuskowski MA, Basbaum AI, Simone DA, Mantyh PW. Noxious cutaneous thermal stimuli induce a graded release of endogenous substance P in the spinal cord: imaging peptide action in vivo. J Neurosci. 1997;17:5921–5927. doi: 10.1523/JNEUROSCI.17-15-05921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basbaum AI. Spinal mechanisms of acute and persistent pain. Reg Anesth Pain Med. 1999;24:59–67. doi: 10.1016/s1098-7339(99)90167-0. [DOI] [PubMed] [Google Scholar]
- 3.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- 5.Bruinstroop E, Cano G, Vanderhorst VG, Cavalcante JC, Wirth J, Sena-Esteves M, Saper CB. Spinal projections of the A5, A6 (locus coeruleus), and A7 noradrenergic cell groups in rats. J Comp Neurol. 2012;520:1985–2001. doi: 10.1002/cne.23024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Budai D, Fields HL. Endogenous opioid peptides acting at mu-opioid receptors in the dorsal horn contribute to midbrain modulation of spinal nociceptive neurons. J Neurophysiol. 1998;79:677–687. doi: 10.1152/jn.1998.79.2.677. [DOI] [PubMed] [Google Scholar]
- 7.Buritova J, Honoré P, Besson JM. Indomethacin reduces both Krox-24 expression in the rat lumbar spinal cord and inflammatory signs following intraplantar carrageenan. Brain Res. 1995;674:211–220. doi: 10.1016/0006-8993(95)00009-f. [DOI] [PubMed] [Google Scholar]
- 8.Cha M, Ji Y, Masri R Motor cortex stimulation activates the incertothalamic pathway in an animal model of spinal cord injury. J Pain. 2013;14:260–269. doi: 10.1016/j.jpain.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dahlstrom A, Fuxe K. Evidence for the existence of monoamine-containing neurons in the central nervous system. 1. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol Scand Suppl. 1964;232:231–255. [PubMed] [Google Scholar]
- 10.Fields HL, Basbaum AI, Heinricher MM. Central nervous system mechanisms of pain modulation. In: McMahon SB, Koltzenburg M, editors. Wall and Melzack’s textbook of pain. 5. Edinburg: Elsevier/Churchill Livingstone; 2006. pp. 125–142. [Google Scholar]
- 11.Fields HL, Heinricher MM, Mason P. Neurotransmitters in nociceptive modulatory circuits. Annu Rev Neurosci. 1991;14:219–245. doi: 10.1146/annurev.ne.14.030191.001251. [DOI] [PubMed] [Google Scholar]
- 12.Fonoff ET, Dale CS, Pagano RL, Paccola CC, Ballester G, Teixeira MJ, Giorgi R. Antinociception induced by epidural motor cortex stimulation in naive conscious rats is mediated by the opioid system. Behav Brain Res. 2009;196:63–70. doi: 10.1016/j.bbr.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 13.Fonoff ET, Hamani C, Ciampi de Andrade D, Yeng LT, Marcolin MA, Jacobsen Teixeira M. Pain relief and functional recovery in patients with complex regional pain syndrome after motor cortex stimulation. Stereotact Funct Neurosurg. 2011;89:167–172. doi: 10.1159/000324895. [DOI] [PubMed] [Google Scholar]
- 14.Fonoff ET, Pereira JP, Jr, Camargo LV, Dale CS, Pagano RL, Ballester G, Teixeira MJ. Functional mapping of the motor cortex of rat using transdural electrical stimulation. Behav Brain Res. 2009;202:138–141. doi: 10.1016/j.bbr.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 15.Fontaine D, Hamani C, Lozano A. Efficacy and safety of motor cortex stimulation for chronic neuropathic pain: Critical review of the literature. J Neurosurg. 2009;110:251–256. doi: 10.3171/2008.6.17602. [DOI] [PubMed] [Google Scholar]
- 16.França NR, Toniolo EF, Franciosi AC, Alves AS, de Andrade DC, Fonoff ET, Britto LR, Dale CS. Antinociception induced by motor cortex stimulation: somatotopy of behavioral response and profile of neuronal activation. Behav Brain Res. 2013;250:211–221. doi: 10.1016/j.bbr.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 17.François A, Low SA, Sypek EI, Christensen AJ, Sotoudeh C, Beier KT, Ramakrishnan C, Ritola KD, Sharif-Naeini R, Deisseroth K, Delp SL, Malenka RC, Luo L, Hantman AW, Scherrer G. A Brainstem-spinal cord inhibitory circuit for mechanical pain modulation by GABA and enkephalins. Neuron. 2017;93:822–839. doi: 10.1016/j.neuron.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freitas RL, Bassi GS, de Oliveira AM, Coimbra NC. Serotonergic neurotransmission in the dorsal raphe nucleus recruits in situ 5-HT (2A/2C) receptors to modulate the post-ictal antinociception. Exp Neurol. 2008;213:410–418. doi: 10.1016/j.expneurol.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Fu W, Le Maître E, Fabre V, Bernard JF, David Xu ZQ, Hökfelt T. Chemical neuroanatomy of the dorsal raphe nucleus and adjacent structures of the mouse brain. J Comp Neurol. 2010;518:3464–3494. doi: 10.1002/cne.22407. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Larrea L, Peyron R. Motor cortex stimulation for neuropathic pain: from phenomenology to mechanisms. Neuroimage. 2007;37:S71–S79. doi: 10.1016/j.neuroimage.2007.05.062. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Larrea L, Peyron R, Mertens P, Gregoire MC, Lavenne F, Le Bars D, Convers P, Mauguière F, Sindou M, Laurent B. Electrical stimulation of motor cortex for pain control: a combined PET-scan and electrophysiological study. Pain. 1999;83:259–273. doi: 10.1016/s0304-3959(99)00114-1. [DOI] [PubMed] [Google Scholar]
- 22.Gilbert AK, Franklin KB. GABAergic modulation of descending inhibitory systems from the rostral ventromedial medulla (RVM). Dose–response analysis of nociception and neurological deficits. Pain. 2001;90:25–36. doi: 10.1016/s0304-3959(00)00383-3. [DOI] [PubMed] [Google Scholar]
- 23.Gjerstad J, Tjolsen A, Hole K. The effect of 5-HT1A receptor stimulation on nociceptive dorsal horn neurons in rats. Eur J Pharmacol. 1996;318:315–321. doi: 10.1016/s0014-2999(96)00819-9. [DOI] [PubMed] [Google Scholar]
- 24.Gogas KR, Presley RW, Levine JD, Basbaum AI. The antinociceptive action of supraspinal opioids results from an increase in descending inhibitory control: correlation of nociceptive behavior and c-fos expression. Neuroscience. 1991;42:617–628. doi: 10.1016/0306-4522(91)90031-i. [DOI] [PubMed] [Google Scholar]
- 25.Green GM, Lyons L, Dickenson AH. α2-Adrenoceptor antagonists enhance responses of dorsal horn neurones to formalin induced inflammation. Eur J Pharmacol. 1998;47:201–204. doi: 10.1016/s0014-2999(98)00217-9. [DOI] [PubMed] [Google Scholar]
- 26.Harris JA. Using c-fos as a neural marker of pain. Brain Res Bull. 1998;45:1–8. doi: 10.1016/s0361-9230(97)00277-3. [DOI] [PubMed] [Google Scholar]
- 27.Heinricher MM, Tortorici V. Interference with GABA transmission in the rostral ventromedial medulla: disinhibition of off-cells as a central mechanism in nociceptive modulation. Neuroscience. 1994;63:533–546. doi: 10.1016/0306-4522(94)90548-7. [DOI] [PubMed] [Google Scholar]
- 28.Herdegen T, Kovary K, Leah J, Bravo R. Specific temporal and spatial distribution of JUN, FOS, and KROX-24 proteins in spinal neurons following noxious transsynaptic stimulation. J Comp Neurol. 1991;313:178–191. doi: 10.1002/cne.903130113. [DOI] [PubMed] [Google Scholar]
- 29.Herdegen T, Leah JD. Inducible and constitutive transcription factors in the mammalian nervous system: control of gene expression by Jun, Fos and Krox, and CREB/ATF proteins. Brain Res Brain Res Rev. 1998;28:370–490. doi: 10.1016/s0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- 30.Herdegen T, Walker T, Leah JD, Bravo R, Zimmermann M. The KROX-24 protein, a new transcription regulating factor: expression in the rat central nervous system following afferent somatosensory stimulation. Neurosci Lett. 1990;120:21–24. doi: 10.1016/0304-3940(90)90158-6. [DOI] [PubMed] [Google Scholar]
- 31.Hökfelt T, Kellerth JO, Nilsson G, Pernow B. Substance p: localization in the central nervous system and in some primary sensory neurons. Science. 1975;190:889–890. doi: 10.1126/science.242075. [DOI] [PubMed] [Google Scholar]
- 32.Hökfelt T, Ljungdahl A, Terenius L, Elde R, Nilsson G. Immunohistochemical analysis of peptide pathways possibly related to pain and analgesia: enkephalin and substance P. Proc Natl Acad Sci U S A. 1977;74:3081–3085. doi: 10.1073/pnas.74.7.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howorth PW, Teschemacher AG, Pickering AE. Retrograde adenoviral vector targeting of nociresponsivepontospinal noradrenergic neurons in the rat in vivo. J Comp Neurol. 2009;512:141–157. doi: 10.1002/cne.21879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- 35.Hunt SP, Pini A, Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987;328:632–634. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- 36.Jacobs BL, Azmitia E. Structure and function of the brain serotonin system. Physiol Rev. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- 37.Jones SL. Descending noradrenergic influences on pain. Prog Brain Res. 1991;88:381–394. doi: 10.1016/s0079-6123(08)63824-8. [DOI] [PubMed] [Google Scholar]
- 38.Lanteri-Minet M, Isnardon P, de Pommery J, Menetrey D. Spinal and hindbrain structures involved in visceroception and visceronociception as revealed by the expression of Fos, Jun and Krox-24 proteins. Neuroscience. 1993;55:737–753. doi: 10.1016/0306-4522(93)90439-m. [DOI] [PubMed] [Google Scholar]
- 39.Lefaucheur JP, Holsheimer J, Goujon C, Keravel Y, Nguyen JP. Descending volleys generated by efficacious epidural motor cortex stimulation in patients with chronic neuropathic pain. Exp Neurol. 2010;223:609–614. doi: 10.1016/j.expneurol.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 40.Levine JD, Fields HL, Basbaum AI. Peptides and the primary afferent nociceptor. J Neurosci. 1993;13:2273–2286. doi: 10.1523/JNEUROSCI.13-06-02273.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li P, Zhuo M. Cholinergic, noradrenergic, and serotonergic inhibition of fast synaptic transmission in spinal lumbar dorsal horn of rat. Brain Res Bull. 2001;54:639–647. doi: 10.1016/s0361-9230(01)00470-1. [DOI] [PubMed] [Google Scholar]
- 42.Lima MC, Fregni F. Motor cortex stimulation for chronic pain: systematic review and meta-analysis of the literature. Neurology. 2008;70:2329–2337. doi: 10.1212/01.wnl.0000314649.38527.93. [DOI] [PubMed] [Google Scholar]
- 43.Llorca-Torralba M, Borges G, Neto F, Mico JA, Berrocoso E. Noradrenergic locus coeruleus pathways in pain modulation. Neuroscience. 2016;338:93–113. doi: 10.1016/j.neuroscience.2016.05.057. [DOI] [PubMed] [Google Scholar]
- 44.Lopez WO, Barbosa DC, Teixera MJ, Paiz M, Moura L, Monaco BA, Fonoff ET. Pain relief in CRPS-II after spinal cord and motor cortex simultaneous dual stimulation. Pain Physician. 2016;19:E631–E635. [PubMed] [Google Scholar]
- 45.Lucas JM, Ji Y, Masri R. Motor cortex stimulation reduces hyperalgesia in an animal model of central pain. Pain. 2011;152:1398–1407. doi: 10.1016/j.pain.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luo M, Zhou J, Liu Z. Reward processing by the dorsal raphe nucleus: 5-HT and beyond. Learn Mem. 2015;22:452–460. doi: 10.1101/lm.037317.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maarrawi J, Peyron R, Mertens P, Costes N, Magnin M, Sindou M, Laurent B, Garcia-Larrea L. Motor cortex stimulation for pain control induces changes in the endogenous opioid system. Neurology. 2007;69:827–834. doi: 10.1212/01.wnl.0000269783.86997.37. [DOI] [PubMed] [Google Scholar]
- 48.Maarrawi J, Peyron R, Mertens P, Costes N, Magnin M, Sindou M, Laurent B, Garcia-Larrea L. Brain opioid receptor density predicts motor cortex stimulation efficacy for chronic pain. Pain. 2013;154:2563–2568. doi: 10.1016/j.pain.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 49.Mantyh PW. Neurobiology of substance P and the NK1 receptor. J Clin Psychiatry. 2002;63:6–10. [PubMed] [Google Scholar]
- 50.Mantyh PW, Allen CJ, Ghilardi JR, Rogers SD, Mantyh CR, Liu H, Basbaum AI, Vigna SR, Maggio JE. Rapid endocytosis of a G protein-coupled receptor: substance P evoked internalization of its receptor in the rat striatum in vivo. Proc Natl Acad Sci U S A. 1995;92:2622–2626. doi: 10.1073/pnas.92.7.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mantyh PW, Rogers SD, Honore P, Allen BJ, Ghilardi JR, Li J, Daughters RS, Lappi DA, Wiley RG, Simone DA. Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science. 1997;278:275–279. doi: 10.1126/science.278.5336.275. [DOI] [PubMed] [Google Scholar]
- 52.Marshall GE, Shehab SA, Spike RC, Todd AJ. Neurokinin-1 receptors on lumbar spinothalamic neurons in the rat. Neuroscience. 1996;72:255–263. doi: 10.1016/0306-4522(95)00558-7. [DOI] [PubMed] [Google Scholar]
- 53.Marvizón JC, Wang X, Matsuka Y, Neubert JK, Spigelman I. Relationship between capsaicin-evoked substance P release and neurokinin 1 receptor internalization in the rat spinal cord. Neuroscience. 2003;118:535–545. doi: 10.1016/s0306-4522(02)00977-6. [DOI] [PubMed] [Google Scholar]
- 54.Mason P. Physiological identification of pontomedullary serotonergic neurons in the rat. J Neurophysiol. 1997;77:1087–1098. doi: 10.1152/jn.1997.77.3.1087. [DOI] [PubMed] [Google Scholar]
- 55.Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 56.Molander C, Xu Q, Grant G. The cytoarchitectonic organization of the spinal cord in the rat. I. The lower thoracic and lumbosacral cord. J Comp Neurol. 1984;230:133–141. doi: 10.1002/cne.902300112. [DOI] [PubMed] [Google Scholar]
- 57.Morgan JI, Curran T. Stimulus-transcription coupling in neurons: role of cellular immediate-early genes. Trends Neurosci. 1989;12:459–462. doi: 10.1016/0166-2236(89)90096-9. [DOI] [PubMed] [Google Scholar]
- 58.Muto Y, Sakai A, Sakamoto A, Suzuki H. Activation of NK1 receptors in the locus coeruleus induces analgesia through noradrenergic-mediated descending inhibition in a rat model of neuropathic pain. Br J Pharmacol. 2012;166:1047–1057. doi: 10.1111/j.1476-5381.2011.01820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nguyen JP, Lefaucheur JP, Decq P, Uchiyama T, Carpentier AC, Fontaine D, Brugières P, Pollin B, Fève A, Rostaing S, Cesaro P, Keravel Y. Chronic motor cortex stimulation in the treatment of central and neuropathic pain: correlations between clinical, electrophysiological and anatomical data. Pain. 1999;82:245–251. doi: 10.1016/S0304-3959(99)00062-7. [DOI] [PubMed] [Google Scholar]
- 60.Nguyen JP, Velasco F, Brugières P, Velasco M, Keravel Y, Boleaga B, Brito F, Lefaucheur JP. Treatment of chronic neuropathic pain by motor cortex stimulation: results of a bicentric controlled crossover trial. Brain Stim. 2008;1:89–96. doi: 10.1016/j.brs.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 61.Nichols ML, Allen BJ, Rogers SD, Ghilardi JR, Honore P, Luger NM, Finke MP, Li J, Lappi DA, Simone DA, Mantyh PW. Transmission of chronic nociception by spinal neurons expressing the substance P receptor. Science. 1999;286:1558–1561. doi: 10.1126/science.286.5444.1558. [DOI] [PubMed] [Google Scholar]
- 62.Nuti C, Peyron R, Garcia-Larrea L, Brunon J, Laurent B, Sindou M, Mertens P. Motor cortex stimulation for refractory neuropathic pain: four-year outcome and predictors of efficacy. Pain. 2005;118:43–52. doi: 10.1016/j.pain.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 63.Ossipov MH, Dussor GO, Porreca F. Central modulation of pain. J Clin Invest. 2010;120:3779–3787. doi: 10.1172/JCI43766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pagano RL, Assis DV, Clara JA, Alves AS, Dale CS, Teixeira MJ, Fonoff ET, Britto LR. Transdural motor cortex stimulation reverses neuropathic pain in rats: a profile of neuronal activation. Eur J Pain. 2011;15:268.e1–268.e14. doi: 10.1016/j.ejpain.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 65.Pagano RL, Fonoff ET, Dale CS, Ballester G, Teixeira MJ, Britto LR. Motor cortex stimulation inhibits thalamic sensory neurons and enhances activity of PAG neurons: possible pathways for antinociception. Pain. 2012;153:2359–2369. doi: 10.1016/j.pain.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 66.Palazzo E, de Novellis V, Petrosino S, Marabese I, Vita D, Giordano C, Di Marzo V, Mangoni GS, Rossi F, Maione S. Neuropathic pain and the endocannabinoid system in the dorsal raphe: pharmacological treatment and interactions with the serotonergic system. Eur J Neurosci. 2006;24:2011–2020. doi: 10.1111/j.1460-9568.2006.05086.x. [DOI] [PubMed] [Google Scholar]
- 67.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6. New York: Elsevier/Academic Press; 2007. [Google Scholar]
- 68.Pertovaara A. Noradrenergic pain modulation. Prog Neurobiol. 2006;80:53–83. doi: 10.1016/j.pneurobio.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 69.Pertovaara A. The noradrenergic pain regulation system: a potential target for pain therapy. Eur J Pharmacol. 2013;716:2–7. doi: 10.1016/j.ejphar.2013.01.067. [DOI] [PubMed] [Google Scholar]
- 70.Peyron R, Faillenot I, Mertens P, Laurent B, Garcia-Larrea L. Motor cortex stimulation in neuropathic pain. Correlations between analgesic effect and hemodynamic changes in the brain. A PET study. Neuroimage. 2007;34:310–321. doi: 10.1016/j.neuroimage.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 71.Peyron R, Garcia-Larrea L, Deiber MP, Cinotti L, Convers P, Sindou M, Mauguière F, Laurent B. Electrical stimulation of precentral cortical area in the treatment of central pain: electrophysiological and PET study. Pain. 1995;62:275–286. doi: 10.1016/0304-3959(94)00211-V. [DOI] [PubMed] [Google Scholar]
- 72.Proudfit HK, Clark FM. The projections of locus coeruleus neurons to the spinal cord. Prog Brain Res. 1991;88:123–141. doi: 10.1016/s0079-6123(08)63803-0. [DOI] [PubMed] [Google Scholar]
- 73.Randall LO, Selitto JJ. A method for measurement of analgesic activity of inflamed tissue. Arch Int Pharmacodyn Ther. 1957;111:409–419. [PubMed] [Google Scholar]
- 74.Rasche D, Ruppolt M, Stippich C, Unterberg A, Tronnier VM. Motor cortex stimulation for long-term relief of chronic neuropathic pain: a 10 year experience. Pain. 2006;121:43–52. doi: 10.1016/j.pain.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 75.Rojas-Piloni G, Martinez-Lorenzana G, Condes-Lara M, Rodriguez-Jimenez J. Direct sensorimotor corticospinal modulation of dorsal horn neuronal C-fiber responses in the rat. Brain Res. 2010;1351:104–114. doi: 10.1016/j.brainres.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 76.Ruda MA. Opiates and pain pathways: demonstration of enkephalin synapses on dorsal horn projection neurons. Science. 1982;215:1523–1525. doi: 10.1126/science.6121374. [DOI] [PubMed] [Google Scholar]
- 77.Ruda MA, Bennett GJ, Dubner R. Neurochemistry and neural circuitry in the dorsal horn. Prog Brain Res. 1986;66:219–268. doi: 10.1016/s0079-6123(08)64606-3. [DOI] [PubMed] [Google Scholar]
- 78.Rusina R, Vaculin S, Yamamotova A, Barek S, Dvorakova H, Rokyta R. The effect of motor cortex stimulation in deafferentated rats. Neuro Endocrinol Lett. 2005;26:283–288. [PubMed] [Google Scholar]
- 79.Salas R, Ramirez K, Vanegas H, Vazquez E. Activity correlations between on-like and off-like cells of the rostral ventromedial medulla and simultaneously recorded wide-dynamic-range neurons of the spinal dorsal horn in rats. Brain Res. 2016;1652:103–110. doi: 10.1016/j.brainres.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 80.Senapati AK, Huntington PJ, Peng YB. Spinal dorsal horn neuron response to mechanical stimuli is decreased by electrical stimulation of the primary motor cortex. Brain Res. 2005;1036:173–179. doi: 10.1016/j.brainres.2004.12.043. [DOI] [PubMed] [Google Scholar]
- 81.Seybold V, Elde R. Immunohistochemical studies of peptidergic neurons in the dorsal horn of the spinal cord. J Histochem Cytochem. 1980;28:367–370. doi: 10.1177/28.4.6154731. [DOI] [PubMed] [Google Scholar]
- 82.Seybold VS. The role of peptides in central sensitization. Handb Exp Pharmacol. 2009;194:451–491. doi: 10.1007/978-3-540-79090-7_13. [DOI] [PubMed] [Google Scholar]
- 83.Sheng M, Greenberg ME. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990;4:477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- 84.Soiza-Reilly M, Commons KG. Unraveling the architecture of the dorsal raphe synaptic neuropil using high-resolution neuroanatomy. Front Neural Circuits. 2014;8:105. doi: 10.3389/fncir.2014.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Suzuki R, Rygh LJ, Dickenson AH. Bad news from the brain: descending 5-HT pathways that control spinal pain processing. Trends Pharmacol Sci. 2004;25:613–617. doi: 10.1016/j.tips.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 86.Tao R, Auerbach SB. Influence of inhibitory and excitatory inputs on serotonin efflux differs in the dorsal and median raphe nuclei. Brain Res. 2003;961:109–120. doi: 10.1016/s0006-8993(02)03851-9. [DOI] [PubMed] [Google Scholar]
- 87.Tazawa T, Kamiya Y, Kobayashi A, Saeki K, Takiguchi M, Nakahashi Y, Shinbori H, Funakoshi K, Goto T. Spinal cord stimulation modulates supraspinal centers of the descending antinociceptive system in rats with unilateral spinal nerve injury. Mol Pain. 2015;11:36. doi: 10.1186/s12990-015-0039-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Teixeira MJ, de Andrade DC, Fonoff ET. Intra-operative transdural electric stimulation in awake patient: target refining for motor cortex stimulation. Acta Neurochir Suppl. 2013;117:73–78. doi: 10.1007/978-3-7091-1482-7_12. [DOI] [PubMed] [Google Scholar]
- 89.Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci. 2010;11:823–836. doi: 10.1038/nrn2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Todd AJ. Identifying functional populations among the interneurons in laminae I-III of the spinal dorsal horn. Mol Pain. 2017;13:1744806917693003. doi: 10.1177/1744806917693003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Todd AJ, Puskar Z, Spike RC, Hughes C, Watt C, Forrest L. Projection neurons in lamina I of rat spinal cord with the neurokinin 1 receptor are selectively innervated by substance p-containing afferents and respond to noxious stimulation. J Neurosci. 2002;22:4103–4113. doi: 10.1523/JNEUROSCI.22-10-04103.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tork I. Anatomy of the serotonergic system. Ann N Y Acad Sci. 1990;600:9–34. doi: 10.1111/j.1749-6632.1990.tb16870.x. [DOI] [PubMed] [Google Scholar]
- 93.Tsubokawa T, Katayama Y, Yamamoto T, Hirayama T, Koyama S. Chronic motor cortex stimulation for the treatment of central pain. Acta Neurochir Suppl (Wien) 1991;52:137–139. doi: 10.1007/978-3-7091-9160-6_37. [DOI] [PubMed] [Google Scholar]
- 94.Tsuruoka M, Matsutani K, Inoue T. Coeruleospinal inhibition of nociceptive processing in the dorsal horn during unilateral hindpaw inflammation in the rat. Pain. 2003;104:353–361. doi: 10.1016/s0304-3959(03)00042-3. [DOI] [PubMed] [Google Scholar]
- 95.Tsuruoka M, Tamaki J, Maeda M, Hayashi B, Inoue T. Biological implications of coeruleospinal inhibition of nociceptive processing in the spinal cord. Front Integr Neurosci. 2012;6:87. doi: 10.3389/fnint.2012.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vaculín S, Franek M, Yamamotová A, Rokyta R. Motor cortex stimulation in rats with chronic constriction injury. Exp Brain Res. 2008;185:331–335. doi: 10.1007/s00221-007-1158-y. [DOI] [PubMed] [Google Scholar]
- 97.Velasco F, Arguelles C, Carrillo-Ruiz JD, Castro G, Velasco AL, Jiménez F, Velasco M. Efficacy of motor cortex stimulation in the treatment of neuropathic pain: a randomised double-blind trial. J Neurosurg. 2008;108:698–706. doi: 10.3171/JNS/2008/108/4/0698. [DOI] [PubMed] [Google Scholar]
- 98.Viisanen H, Ansah OB, Pertovaara A. The role of the dopamine D2 receptor in descending control of pain induced by motor cortex stimulation in the neuropathic rat. Brain Res Bull. 2012;89:133–143. doi: 10.1016/j.brainresbull.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 99.Viisanen H, Pertovaara A. Antinociception by motor cortex stimulation in the neuropathic rat: does the locus coeruleus play a role? Exp Brain Res. 2010;201:283–296. doi: 10.1007/s00221-009-2038-4. [DOI] [PubMed] [Google Scholar]
- 100.Viisanen H, Pertovaara A. Roles of the rostroventromedial medulla and the spinal 5-HT(1A) receptor in descending antinociception induced by motor cortex stimulation in the neuropathic rat. Neurosci Lett. 2010;476:133–137. doi: 10.1016/j.neulet.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 101.Wang QP, Nakai Y. The dorsal raphe: an important nucleus in pain modulation. Brain Res Bull. 1994;34:575–585. doi: 10.1016/0361-9230(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 102.Wei F, Dubner R, Zou S, Ren K, Bai G, Wei D, Guo W. Molecular depletion of descending serotonin unmasks its novel facilitatory role in the development of persistent pain. J Neurosci. 2010;30(25):8624–8636. doi: 10.1523/JNEUROSCI.5389-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Westlund KN, Bowker RM, Ziegler MG, Coulter JD. Noradrenergic projections to the spinal cord of the rat. Brain Res. 1983;263:15–31. doi: 10.1016/0006-8993(83)91196-4. [DOI] [PubMed] [Google Scholar]
- 104.Wu YY, Jiang YL, He XF, Zhao XY, Shao XM, Sun J, Shen Z, Shou SY, Wei JJ, Ye JY, Yan SS, Fang JQ. 5-HT in the dorsal raphe nucleus is involved in the effects of 100-Hz electro-acupuncture on the pain-depression dyad in rats. Exp Ther Med. 2017;14:107–114. doi: 10.3892/etm.2017.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yasaka T, Tiong SY, Hughes DI, Riddell JS, Todd AJ. Populations of inhibitory and excitatory interneurons in lamina II of the adult rat spinal dorsal horn revealed by a combined electrophysiological and anatomical approach. Pain. 2010;151:475–488. doi: 10.1016/j.pain.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yoshimura M, Furue H. Mechanisms for the anti-nociceptive actions of the descending noradrenergic and serotonergic systems in the spinal cord. J Pharmacol Sci. 2006;101:107–117. doi: 10.1254/jphs.crj06008x. [DOI] [PubMed] [Google Scholar]
- 107.Zhang C, Yang SW, Guo YG, Qiao JT, Dafny N. Locus coeruleus stimulation modulates the nociceptive response in parafascicular neurons: an analysis of descending and ascending pathways. Brain Res Bull. 1997;42:273–278. doi: 10.1016/s0361-9230(96)00262-6. [DOI] [PubMed] [Google Scholar]
- 108.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Pain behavior and immunoreactivity data from naive, sham and stimulated rats.