Abstract
Background.
Data describing influenza– or respiratory syncytial virus (RSV)–associated hospitalized illness in children aged <5 years in Africa are limited.
Methods.
During 2011–2016, we conducted surveillance for severe respiratory illness (SRI) in children aged <5 years in 3 South African hospitals. Nasopharyngeal aspirates were tested for influenza and RSV using real-time reverse transcription polymerase chain reaction. We estimated rates of influenza- and RSV-associated hospitalized SRI by human immunodeficiency virus (HIV) status and compared children who tested positive for influenza vs RSV using multivariable penalized logistic regression.
Results.
Among 3650 hospitalized children, 203 (5.6%) tested positive for influenza viruses, 874 (23.9%) for RSV, and 19 (0.5%) for both. The median age of children hospitalized with influenza was 13.9 months vs 4.4 months for RSV (P < .01). Annual influenza-associated hospitalization rates per 100 000 were highest among infants aged 6–11 months (545; 95% confidence interval [CI], 409–703), while RSV-associated hospitalization rates were highest in infants aged 0–2 months (6593; 95% CI, 5947–7217). HIV exposure was associated with increased incidence of influenza- and RSV-associated hospitalization in infants aged 0–5 months, with relative risk (RR) 2.2 (95% CI, 1.4–3.4) and 1.4 (95% CI, 1.3–1.6), respectively. HIV infection was associated with increased incidence of influenza- and RSV-associated hospitalization in all age groups; RR 2.7 (95% CI, 2.0–3.5) and 3.8 (95% CI, 3.1–4.8), respectively.
Conclusions.
Influenza- and RSV-associated hospitalizations are common among South African infants. HIV infection and HIV exposure in infants increase risk of influenza- and RSV-associated hospitalization.
Keywords: influenza, respiratory syncytial virus, human immunodeficiency virus, incidence, South Africa
Influenza and respiratory syncytial virus (RSV) are leading causes of hospitalization and death among children globally [1–4]. However, data describing the spectrum of influenza- or RSV-associated hospitalized illness in infants and young children in low- and middle-income countries (LMIC) are limited because children hospitalized in these settings are not routinely tested for influenza or RSV infection [5]. These data are essential for informing decisions about vaccine introduction. Recent investments in strengthening respiratory disease surveillance in LMIC have demonstrated a sizeable burden of influenza- and RSV-associated hospitalizations and deaths. It is estimated that 99% of influenza- and RSV-associated deaths in children aged <5 years occur in developing countries [2, 3].
Published rates of influenza-associated lower respiratory tract infection (LRTI) hospitalizations in South African children aged 0–4 years are from the pandemic period (2009–2011) when surveillance at a single hospital estimated a rate of 766 (95% confidence interval [CI], 553–1021) per 100 000 human immunodeficiency virus (HIV)–infected children and 314 (95% CI, 284–349) per 100 000 HIV-uninfected children [6]. Likewise, rates of RSV-associated LRTI hospitalizations in children aged 0–4 years from the same hospital were estimated to be 10.1 (95% CI, 9.6–11.7) per 1000 children in 2010 and 11.2 (95% CI, 10.6–11.8) per 1000 children in 2011 [7]. Surveillance from a private hospital network in South Africa during 2007–2012 estimated mean annual influenza- and RSV-associated hospitalization rates in children aged <1 year of 255 (95% CI, 143–358) and 7601 (95% CI, 4312–10 817) per 100 000 infants, respectively [8]. However, estimates from the pandemic period may not reflect the burden of seasonal influenza, and existing estimates of RSV-associated hospitalization rates are highly variable.
Few prior studies of influenza and RSV in children have assessed the impact of antenatal HIV exposure on hospitalization risk independently from HIV infection. In an analysis of data from 2010–2011, we found that HIV exposure was associated with increased risk of RSV-associated hospitalization (incidence rate ratio [IRR], 1.4; 95% CI, 1.3–1.6) and death (odds ratio [OR], 2.1; 95% CI, 1.1–3.8) in infants aged 0–5 months [9]. The same trend was noted for influenza-associated hospitalization, but the association was not statistically significant (IRR, 1.2; 95% CI, 0.8–1.8). HIV exposure has also been associated with increased risk of invasive pneumococcal disease (IPD) (IRR, 2.7; 95% CI, 2.0–3.7) post-vaccine introduction and IPD-associated mortality (adjusted relative risk ratio [aRR], 1.76; 95% CI, 1.09–2.85) in infants aged 0–5 months [10]. In both studies [9, 10], HIV exposure was not associated with increased risk of hospitalization or death in older infants or children; therefore, we limited our assessment on the impact of HIV exposure to infants aged 0–11 months.
Given the limitations of available data, we aimed to describe the epidemiology of hospitalized influenza and RSV in South African children aged 0–59 months from 3 surveillance hospitals during 2011–2016.
METHODS
Severe Respiratory Illness Surveillance
During January 2011–December 2016, prospective surveillance for severe respiratory illness (SRI) at 3 hospitals (Edendale Hospital in Pietermaritzburg, KwaZulu-Natal Province, and Klerksdorp and Tshepong Hospitals in Klerksdorp, North West Province) was conducted among children aged 0–59 months. SRI case definitions were age specific. In infants aged 2 days to <3 months, SRI was defined as a hospitalized infant with a diagnosis of suspected sepsis or physician-diagnosed acute LRTI. In children aged 3–59 months, SRI was defined as a hospitalized child with physician-diagnosed LRTI, including bronchitis, bronchiolitis, pneumonia, or pleural effusion.
Specimen Collection and Laboratory Procedures
Surveillance procedures have been previously described [11]. Briefly, surveillance officers completed case report forms for all consenting SRI cases. Nasopharyngeal aspirates were collected from enrolled children, placed in universal transport medium, and transported at 4°C–8°C to the National Institute for Communicable Diseases within 72 hours of specimen collection. For 2011–2014, a multiplex real-time reverse transcription polymerase chain reaction (rRT-PCR) assay was used to test for influenza A and B viruses; RSV; parainfluenza virus types 1, 2, and 3; adenoviruses; rhinoviruses; human metapneumovirus; and enteroviruses [12]. For 2015–2016, a commercial multiplex rRT-PCR (FTD Flu/RSV assay, FastTrack Diagnostics, Sliema, Malta) was used to test for influenza A and B viruses and RSV. For infants aged 0–11 months, HIV exposure status was determined by medical record review, maternal or infant rapid HIV testing, or maternal or infant enzyme-linked immunosorbent assay (ELISA). HIV infection status in children aged ≥18 months was determined by rapid HIV testing or ELISA of a dried blood spot. HIV infection status in children aged <18 months with positive ELISA or unknown serostatus was determined by HIV PCR of an anonymized linked dried blood spot. Blood samples were collected for real-time PCR testing for Streptococcus pneumoniae targeting the lytA gene [13].
Rates of Influenza- and RSV-associated Hospitalization
Average annual influenza- and RSV-associated hospitalization rates per 100 000 population were calculated using the number of SRI hospitalizations (from all sites) multiplied by the age-specific proportion testing positive for influenza or RSV and dividing by the sum of the mid-year population estimate for the combined catchment area. Rates were adjusted for non-enrollment (refusals, weekend admissions), healthcare-seeking behavior, and the attributable fraction of infection to illness [14]. Where published population estimates were unavailable (infants aged 0–2 months, 3–5 months, and 6–11 months), we estimated from the relative proportion of infants aged 0–11 months assuming the birth rate was constant throughout the year and adjusting for neonatal and infant mortality [15]. We stratified hospitalization rates by HIV status for children aged 0–59 months and HIV exposure status for infants only. Log binomial regression was used to estimate age-specific and overall age-adjusted relative risk (aRR) for influenza-associated SRI among HIV-infected children aged 0–59 months and HIV-exposed uninfected (HEU) infants compared to HIV-unexposed uninfected (HUU) infants.
Comparison of Influenza- and RSV-associated Hospitalizations
We compared children who tested positive for influenza viruses to those who tested positive for RSV, excluding children who tested positive for both pathogens. We also described HIV status of influenza- and RSV-associated in-hospital deaths. Pearson χ2 test and Wald χ2 test using logistic regression were used to assess differences in categorical variables, and Wilcoxon rank-sum test was used to test for differences in median age. We also performed unconditional multivariable penalized logistic regression for factors associated with influenza vs RSV infection in hospitalized children. Variables with P values < .2 in the univariate analysis were assessed in multivariable analysis. Backward elimination was used to determine the final model. Statistical significance was assessed at P < .05 for all analysis using Stata 14.2 (StataCorp, College Station, TX).
Ethical Approval
The University of the Witwatersrand Human Research Ethics Committee and the University of KwaZulu-Natal Human Biomedical Research Ethics Committee approved SRI surveillance (protocols M140824 and BF157/08; updated protocol BE496/14). The US Centers for Disease Control and Prevention (CDC) determined that this surveillance did not constitute human subjects research (NRD ID2012–6197 and NRD CGH2015–210).
RESULTS
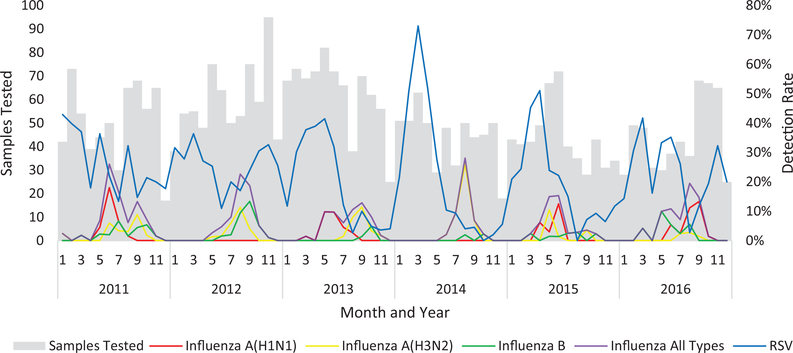
Influenza viruses were primarily detected in the winter months (May–September), while RSV demonstrated variable seasonality (Figure 1). In 2013–2015, RSV peaked in the first half of the year and was rarely detected after August; however, in 2011, 2012, and 2016, RSV was detected throughout the year. Among 3650 children aged 0–59 months, 203 (5.6%) tested positive for influenza viruses, 874 (23.9%) for RSV, and 19 (0.5%) for both. The highest detection for influenza occurred among children aged 24–59 months (8.6%), and the highest detection for RSV occurred among infants aged 0–2 months (38.8%). The median age of children hospitalized with influenza was 13.9 (interquartile range [IQR], 7.5–27.4) months, while the median age of children hospitalized with RSV infection was 4.4 months (IQR, 2.0–10.5; P < .01; Table 1). HIV status was available for 975 (90.5%) children who tested positive for influenza or RSV, and 11.1% and 5.8% were HIV infected, respectively (P = .01). HIV exposure status was available for 666 (84.5%) of the 777 infants who tested positive for influenza or RSV. Among the 83 infants with HIV exposure data who tested positive for influenza, 9 (10.8%) were HIV infected, 27 (32.5%) were HEU, and 47 (56.6%) were HUU. Among the 583 infants with HIV exposure data who tested positive for RSV, 19 (3.3%) were HIV infected, 243 (41.7%) were HEU, and 321 (55.1%) were HUU.
Figure 1.
Total samples tested and detection rate for influenza and respiratory syncytial virus in children aged 0–59 months enrolled in severe respiratory illness surveillance, Klerksdorp and Pietermaritzburg, South Africa, 2011–2016. Abbreviation: RSV, respiratory syncytial virus.
Table 1.
Clinical and Epidemiologic Characteristics of Children Aged 0–59 Months Hospitalized With Influenza Compared to Those With Respiratory Syncytial Virus Infection, Klerksdorp and Pietermaritzburg, South Africa, 2011–2016
| Total Samples Tested (N = 3631) | Total Positive for Influenza or RSV (N = 1077) | Influenza Positive (n = 203) | RSV Positive (n = 874) | Univariate OR (95% CI) | Multivariable OR (95% CI) | |
|---|---|---|---|---|---|---|
| Detection rate (%) | … | 29.7 | 5.6 | 24.1 | … | … |
| Sex (n, %) | ||||||
| Male | 2051 (56.6) | 619 (57.5) | 121 (59.6) | 498 (57.0) | 0.9 (0.7–1.2) | … |
| Age Group (months) (n, %) | ||||||
| 0–2 | 840 (23.1) | 338 (31.4) | 12 (5.9) | 326 (37.3) | Ref | Ref |
| 3–5 | 647 (17.8) | 226 (21.0) | 26 (12.8) | 200 (22.9) | 3.5 (1.7–7.2) | 3.4 (1.7–7.0) |
| 6–11 | 753 (20.7) | 211 (19.6) | 57 (28.1) | 154 (17.6) | 10.1 (5.2–19.3) | 9.3 (4.9–17.9) |
| 12–23 | 729 (20.1) | 168 (15.6) | 51 (25.1) | 117 (13.4) | 11.8 (6.1–23.0) | 10.0 (5.1–19.4) |
| 24–59 | 662 (18.2) | 134 (12.4) | 57 (28.1) | 77 (8.8) | 20.1 (10.3–39.3) | 15.8 (8.0–30.9) |
| Median age (IQR)a | 8.2 (3.3–18.5) | 5.5 (2.4–13.5) | 13.9 (7.5–27.4) | 4.4 (2.0–10.5) | … | … |
| Underlying medical conditions (n, %) | ||||||
| HIV infection | 385/3302 (11.7) | 66/973 (6.8) | 20/180 (11.1) | 46/795 (5.8) | 2.0 (1.2–3.5) | … |
| Non-HIV chronic illnessb | 526/3623 (14.5) | 241/1010 (23.9) | 62/183 (33.9) | 121/769 (15.7) | 1.9 (1.3–2.6) | … |
| Weight for age <–2 z scores | 656/2794 (23.5) | 149/808 (18.4) | 41/158 (26.0) | 108/650 (16.6) | 1.8 (1.2–2.7) | … |
| Height for age <–2 z scores | 3/2681 (0.1) | 2/783 (2.3) | 1/153 (4.8) | 1/630 (1.5) | 4.1 (0.3–66.5) | … |
| Coinfection and vaccination status (n, %) | ||||||
| Tuberculosis | 67/1067(6.3) | 17/300 (5.7) | 3/70 (4.3) | 14/230 (6.1) | 0.7 (0.2–2.5) | … |
| Pneumococcus | 263/2696 (9.8) | 74/792 (9.3) | 19/153 (12.4) | 55/639 (8.6) | 1.5 (0.9–2.6) | … |
| Other viral coinfectionc | 1953/3367 (58.0) | 378/1007 (37.5) | 47/188 (25.0) | 331/819 (40.4) | 0.5 (0.3–0.7) | … |
| Received ≥2 doses PCVd | 1726/2310 (74.7) | 453/622 (72.8) | 110/147 (74.8) | 343/475 (72.2) | 1.1 (0.7–1.7) | … |
| Clinical characteristics and course (n, %) | ||||||
| Fever | 2267/3607 (62.9) | 708/1073 (66.0) | 159/203 (78.3) | 549/870 (63.1) | 2.1 (1.5–3.0) | … |
| Cough | 3207/3583 (89.5) | 1033/1068 (96.7) | 187/200 (93.5) | 846/868 (97.5) | 0.4 (0.2–0.8) | … |
| Oxygen saturation <90% | 303/2701 (11.2) | 108/777 (13.9) | 15/154 (9.7) | 93/623 (14.9) | 0.6 (0.3–1.1) | … |
| Tachypneae | 2059/3591 (57.3) | 631/1069 (59.0) | 98/201 (48.8) | 533/868 (61.4) | 0.6 (0.4–0.8) | … |
| Given supplemental oxygen | 2133/3600 (59.3) | 713/1071 (66.6) | 95/200 (47.5) | 618/871 (71.0) | 0.4 (0.3–0.5) | 0.6 (0.4–0.8) |
| Mechanical ventilation | 93/3593 (2.6) | 24/1068 (2.2) | 7/200 (3.5) | 17/868 (2.0) | 1.8 (0.7–4.4) | 3.0 (1.1–7.8) |
| Antibiotics on admission | 3454/3561 (97.0) | 1048/1068 (98.1) | 195/200 (97.5) | 853/868 (98.3) | 0.7 (0.2–1.9) | … |
| Nonrespiratory admission diagnosis (eg, sepsis, diarrhea, seizure) | 425/3583 (11.9) | 66/1066 (6.2) | 24/200 (12.0) | 42/866 (4.8) | 2.7 (1.6–4.5) | 2.2 (1.2–4.1) |
| Symptom duration prior to admission (days) | ||||||
| 0–1 | 1255/3517 (35.7) | 262/1041 (25.2) | 50/194 (25.8) | 212/847 (25.0) | Ref | … |
| 2–4 | 1580/3517 (44.9) | 554/1041 (53.2) | 83/194 (42.8) | 471/847 (55.6) | 0.7 (0.5–1.1) | … |
| 5 or more | 682/3517 (19.4) | 225/1041 (21.6) | 61/194 (31.4) | 164/847 (19.4) | 1.6 (1.0–2.4) | … |
| Duration of hospitalization (days) | ||||||
| 0–2 | 980/3515 (27.9) | 244/1046 (23.3) | 54/196 (27.6) | 190/850 (22.4) | Ref | … |
| 3–6 | 1605/3515 (45.7) | 552/1046 (52.8) | 92/196 (46.9) | 460/850 (54.1) | 0.7 (0.5–1.0) | … |
| 7 or more | 930/3515 (26.5) | 250/1046 (23.9) | 50/196 (25.5) | 200/850 (23.5) | 0.9 (0.6–1.4) | … |
| –Median length of hospitalization in days (IQR)a | 4 (2–7) | 4 (3–6) | 4 (2–7) | 4 (3–6) | P = 0.6 | … |
| –Intensive care (n, %) | 158/3595 (4.4) | 35/1069 (3.3) | 8/200 (4.0) | 27/869 (3.1) | 1.3 (0.6–2.9) | … |
| –Deaths, case fatality ratio (%) | 51/3574 (1.4) | 9/1067 (0.8) | 2/201 (1.0) | 7/866 (0.8) | 1.2 (0.3–6.0) |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; IQR, interquartile range; OR, odds ratio; PCV, pneumococcal conjugate vaccine; RSV, respiratory syncytial virus.
Wilcoxon rank sum for difference of median age among influenza-positive and RSV-positive children, P < .01.
Non-HIV chronic illnesses among influenza- or RSV-positive children include malnutrition (129), prematurity (98), asthma (15), seizure disorder (7), and other illnesses (5).
Other viruses tested include adenovirus, enterovirus, parainfluenza viruses 1,2,3, human metapneumovirus, and rhinovirus. Tested 2012–2014, therefore, not included in multivariable model.
Includes children aged 3–59 months.
Tachypneic defined as a child with respiratory rate >60 breaths/minute in children aged <2 months, >50 breaths/minute in children aged 2–12 months of age, and >40 breaths/minute in children aged ≥1 year at the time of hospital admission.
Point estimates of the rates of influenza-associated hospitalization were highest among infants aged 6–11 months (545/100 000) and decreased in the second year of life (Table 2). RSV-associated hospitalization rates were highest among infants aged 0–2 months (6593/100 000) and rapidly declined during the first year of life. HIV infection was associated with increased risk of influenza- and RSV-associated hospitalization in nearly all age groups, with an overall RR of 2.7 (95% CI, 2.0–3.5) and 3.8 (95% CI, 3.1–4.8) for children aged 0–59 months for influenza and RSV, respectively. Risk of influenza-associated hospitalization was higher in HIV-infected compared to HIV-uninfected (HEU and HUU combined) infants aged 6–11 months (RR, 2.7; 95% CI, 1.7–4.3), children aged 12–23 months (RR, 2.3; 95% CI, 1.4–4.0), and children aged 24–59 months (RR, 3.5; (95% CI, 2.1–5.9). Risk of RSV-associated hospitalization was higher in HIV-infected compared to HIV-uninfected (HEU and HUU combined) infants aged 6–11 months (RR, 3.6; 95% CI, 2.2–5.9), children aged 12–23 months (RR, 5.9; 95% CI, 4.0–8.7), and children aged 24–59 months (RR, 6.7; 95% CI, 4.3–10.6; Table 2). When comparing HIV-infected to HIV-uninfected (HEU and HUU combined) infants aged 0–2 and 3–5 months, there were no statistically significant differences in risk of influenza-associated hospitalization and only modest increased risk of RSV-associated hospitalization (Table 2). However, once stratified by HIV exposure status, there were statistically significant differences between HUU and both HEU and HIV-infected infants. Compared to HUU infants, HEU infants had increased risk of influenza-associated hospitalization among those aged 0–2 (RR, 3.0; 95% CI, 1.4–6.6) and 3–5 months (RR, 1.9; 95% CI, 1.1–3.2) (Table 3). Likewise, HIV exposure was also associated with increased risk of RSV-associated hospitalization among infants aged 0–2 (RR, 1.4; 95% CI, 1.2–1.6) and 3–5 months (RR, 1.4; 95% CI, 1.2–1.7). Compared to HUU infants, HIV-infected infants also had increased risk of influenza- and RSV-associated hospitalization, with RR 3.1 (95% CI, 2.0–4.6) and 2.8 (95% CI, 2.0–3.9), respectively (Table 3).
Table 2.
Estimated Mean Annual Rates of and Relative Risk Associated With Human Immunodeficiency Virus Infection for Influenza- or Respiratory Syncytial Virus–associated Severe Respiratory Illness Hospitalization in Children Aged 0–59 Months, Klerksdorp and Pietermaritzburg, South Africa, 2011–2016
| Severe Respiratory Illness Hospitalization Ratea,b (95% CI) |
||||
|---|---|---|---|---|
| Age Group (in months) | All | HIV Infected | HIV Uninfected | Relative Risk HIV Infected vs HIV Uninfected (95% Confidence Interval) |
| Influenza associated | ||||
| 0–2 | 235 (108–381) | 431 (27–1413) | 248 (84–424) | 1.7 (0.5–5.9) |
| 3–5 | 434 (281–630) | 591 (20–1964) | 282 (171–416) | 2.1 (0.6–6.9) |
| 0–5 c | 334 (194–505) | 499 (23–1645) | 268 (136–419) | 1.9 (0.8–4.5) |
| 6–11 | 545 (409–703) | 1305 (428–2449) | 486 (352–655) | 2.7 (1.7–4.3) |
| 0–11d | 439 (301–603) | 967 (249–2112) | 364 (231–524) | 2.5 (1.6–3.7) |
| 12–23 | 216 (157–280) | 439 (87–898) | 189 (129–256) | 2.3 (1.4–4.0) |
| 24–59 | 79 (59–101) | 222 (72–417) | 63 (43–85) | 3.5 (2.1–5.9) |
| 0–59e | 178 (127–237) | 422 (110–868) | 154 (101–215) | 2.7 (2.0–3.5) |
| Respiratory syncytial virus associated | ||||
| 0–2 | 6593 (5947–7217) | 13 973 (27–37 328) | 6529 (5842–7201) | 2.1 (1.0–4.4) |
| 3–5 | 3411 (2973–3865) | 7067 (20–16 145) | 3491 (3017–3980) | 2.0 (1.1–3.7) |
| 0–5c | 5010 (4468–5550) | 8760 (0–21 339) | 5055 (4472–5638) | 2.1 (1.3–3.3) |
| 6–11 | 1494 (1271–1739) | 5595 (1194–11 201) | 1545 (1308–1801) | 3.6 (2.2–5.9) |
| 0–11d | 3262 (2878–3655) | 6864 (715–15 266) | 3331 (2918–3754) | 2.6 (1.9–3.6) |
| 12–23 | 409 (330–491) | 2278 (953–3917) | 384 (301–472) | 5.9 (4.0–8.7) |
| 24–59 | 89 (69–113) | 553 (215–972) | 82 (61–106) | 6.7 (4.3–10.6) |
| 0–59e | 787 (682–896) | 1483 (426–2860) | 804 (690–922) | 3.8 (3.1–4.8) |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus.
Rates expressed per 100 000 population.
Estimated rates adjusted for the attributable fraction of influenza/respiratory syncytial virus, the proportion positive among those enrolled, and healthcare-seeking practices.
Relative risk adjusted by age within the following categories: 0–2 months and 3–5 months.
Relative risk adjusted by age within the following categories: 0–2 months, 3–5 months, and 6–11 months.
Relative risk adjusted by age within the following categories: 0–2 months, 3–5 months, 6–11 months, 12–23 months, and 24–59 months.
Table 3.
Estimated Mean Annual Rates of and Relative Risk Associated With Human Immunodeficiency Virus (HIV) Infection and HIV Exposure for Influenza- or Respiratory Syncytial Virus–associated Severe Respiratory Illness Hospitalization in Infants Aged 0–11 Months, Klerksdorp and Pietermaritzburg, South Africa, 2011–2016
| Severe Respiratory Illness Hospitalization Ratea,b (95% CI) |
||||||
|---|---|---|---|---|---|---|
| Age Group (in Months) | All | HIV Infected | HIV Exposed Uninfected | HIV Unexposed Uninfected | RR HIV Infected vs HIV Unexposed Uninfected (95% CI) | RR HIV Exposed Uninfected vs HIV Unexposed Uninfected (95% CI) |
| Influenza associated | ||||||
| 0–2 | 235 (108–381) | 431 (27–1413) | 258 (51–516) | 85 (9–214) | 5.1 (1.4–18.8) | 3.0 (1.4–6.6) |
| 3–5 | 434 (281–630) | 591 (20–1964) | 312 (105–577) | 165 (84–258) | 3.6 (1.1–11.9) | 1.9 (1.1–3.2) |
| 0–5c | 334 (194–505) | 499 (23–1645) | 268 (70–492) | 125 (53–216) | 4.2 (1.7–10.0) | 2.2 (1.4–3.4) |
| 6–11 | 545 (409–703) | 1305 (428–2449) | 543 (245–901) | 460 (308–630) | 2.8 (1.8–4.5) | 1.2 (0.8–1.7) |
| 0–11d | 439 (301–603) | 967 (249–2112) | 332 (119–595) | 240 (142–355) | 3.1 (2.0–4.6) | 1.5 (1.1–2.0) |
| Respiratory syncytial virus associated | ||||||
| 0–2 | 6593 (5947–7217) | 13 973 (27–37 328) | 9415 (7983–10 942) | 6664 (9–7516) | 2.1 (1.0–4.3) | 1.4 (1.2–1.6) |
| 3–5 | 3411 (2973–3865) | 7067 (20–16 145) | 4851 (3874–5956) | 3435 (2869–4077) | 2.1 (1.1–3.8) | 1.4 (1.2–1.7) |
| 0–5c | 5010 (4468–5550) | 8760 (0–21 339) | 6419 (5335–7604) | 4488 (3852–5148) | 2.1 (1.3–3.3) | 1.4 (1.3–1.6) |
| 6–11 | 1494 (1271–1739) | 5595 (1194–11 201) | 1331 (1049–1654) | 1194 (952–1472) | 4.7 (2.9–7.7) | 1.1 (0.9–1.3) |
| 0–11d | 3262 (2878–3655) | 6864 (715–15 266) | 2933 (2403–3521) | 2687 (2272–3129) | 2.8 (2.0–3.9) | 1.3 (1.2–1.5) |
Abbreviations: CI, confidence intervals; HIV, human immunodeficiency virus; RR, relative risk.
Rates expressed per 100 000 population.
Estimated rates adjusted for the attributable fraction of influenza/respiratory syncytial virus, the proportion positive among those enrolled, and healthcare-seeking practices.
RR adjusted by age within the following categories: 0–2 months and 3–5 months.
RR adjusted by age within the following categories: 0–2 months, 3–5 months, and 6–11 months.
In the multivariable analysis comparing influenza vs RSV-infected children, older age (multivariable OR increased from 3.4–15.8 vs 0–2 months), mechanical ventilation (OR, 3.0; 95% CI, 1.1–7.8), and nonrespiratory admission diagnosis (OR, 2.2; 95% CI, 1.2–4.1) were associated with influenza infection, while receipt of supplemental oxygen (OR, 0.6; 95% CI, 0.4–0.8) was associated with RSV infection (Table 1).
Among 1067 children hospitalized with SRI who tested positive for influenza or RSV with outcome data, there were 9 in-hospital deaths (case fatality rate [CFR], 0.8%): 2/201 (1.0%) influenza-positive and 7/866 (0.8%) RSV-positive children. Among these 9 deaths, HIV status was available for 8, and 7 were infants. Among the 7 infant deaths, 1 (14.3%) was HIV infected, 4 (57.1%) were HEU, 1 was HUU, and 1 had unknown HIV status. Of the 2 children aged 12–23 months who died, 1 was HIV infected and 1 was HEU. Of the 2 children who tested positive for influenza and died, 1 (50.0%) was HIV infected and 1 (50.0%) was HEU. Among the 7 children who tested positive for RSV and died, 1 (14.3%) was HIV infected, 4 (57.1%) were HEU, 1 was HUU, and 1 had unknown HIV status.
DISCUSSION
We identified differences in influenza and RSV seasonality, detection rate, and burden by age group, HIV exposure, and HIV status. This could have important implications for the targeting and timing of influenza and RSV vaccines. The burden of RSV-associated hospitalization in infants was approximately 8 times that of influenza-associated hospitalization. RSV-associated hospitalization was also more common than influenza-associated hospitalization in children aged 12–23 months; however, hospitalization rates in children aged 24–59 months were similar for influenza and RSV. HIV exposure in infants and HIV infection in all age groups were associated with increased rates of both influenza- and RSV-associated hospitalization. In the multivariable analysis comparing influenza vs RSV-infected hospitalized children, influenza was associated with increased mechanical ventilation and RSV with receipt of supplemental oxygen, both indicators of disease severity.
The burden of RSV-associated hospitalization was highest in infants, especially in those aged 0–2 months. Because hospitalization rates peak at such a young age, maternal immunization may be an effective strategy for reducing RSV-associated hospitalizations. Published estimates from Kenya show the burden of RSV-associated hospitalization to be 13.4 (95% CI, 7.5–23.8) per 1000 infants aged <6 months [16]. Prior estimates from South Africa showed RSV-associated hospitalizations at 24 (95% CI, 22–26) per 1000 HIV-uninfected infants (HEU and HUU combined) aged <6 months and 128 (95% CI, 104–157) per 1000 HIV-infected infants aged <6 months [7]. Our estimates of RSV-associated hospitalization in infants aged <6 months are nearly 4 times the Kenya estimates. Likewise, our estimates for HIV-uninfected infants (HEU and HUU combined) aged <6 months are more than twice the prior estimate from South Africa. However, for HIV-infected infants aged <6 months, our estimates are comparable to the earlier estimate. In our surveillance, we used a modified SRI case definition that includes suspected sepsis or diagnosis of lower respiratory tract infection in infants aged 0–2 months. This case definition may improve sensitivity for the detection of RSV and/or influenza virus infections in young infants because it does not require specific symptoms (ie, fever and cough) [17, 18] and may contribute to the observed differences with published rates.
In this analysis, RSV-associated disease burden remained high in infants aged 6–11 months and in children aged 12–23 months. Given that maternal RSV-specific antibodies wane in the first 6 months of life [19], alternative strategies such as infant RSV vaccination or extended half-life monoclonal antibodies [20] may be useful to prevent RSV-associated hospitalizations in these age groups.
The burden of influenza-associated hospitalization was highest in infants aged 6–11 months. This rate is comparable to published estimates of 4.7 (95% CI, 1.8–11.9) per 1000 Kenyan infants aged 6–11 months [16]. Likewise, our estimated rate of influenza-associated hospitalization in infants aged 0–11 months was comparable to a prior published estimate from Soweto, South Africa, of 300–469 per 100 000 [6]. Also, the relative burden of influenza-associated hospitalization in infants aged 0–2 months was lower than in infants aged 3–5 months. Given that duration of maternal influenza antibody protection may wane in the third and fourth months of life [21–23], the proportion of influenza-associated hospitalizations preventable through maternal influenza vaccination may be lower than our estimates using rates in infants aged 0–5 months. Maternal influenza vaccine coverage in South Africa remains below 15% [24] but has increased annually since 2011. Current influenza vaccines are approved for administration from 6 months of age; however, there are concerns about the effectiveness of influenza vaccines in children aged 6–23 months [25–28]. Given the issue of waning maternal immunity and the lower effectiveness of current influenza vaccines in young children, alternative strategies for preventing these influenza-associated hospitalizations are urgently needed.
The relative risk of influenza-associated hospitalization in HIV-infected vs HIV-uninfected (HEU and HUU combined) children was similar in all age groups; however, the relative risk of RSV-associated hospitalization in HIV-infected vs HIV-uninfected (HEU and HUU combined) children varied by age and was highest among children aged 24–59 months (Table 2). HIV exposure and HIV infection were independently associated with increased rates of influenza- and RSV-associated hospitalization in infants aged 0–5 months (Table 3). The effect of HIV exposure was not seen in infants aged 6–11 months. This is the first study to demonstrate an association between HIV exposure and influenza-associated hospitalization in infants aged 0–5 months that may be associated with decreased transplacental antibody transfer from HIV-infected mothers [22].
The overall in-hospital CFR (0.8%) among influenza- and RSV-infected children was low in our surveillance. Recent modeling of influenza- and RSV-associated deaths in South African children estimated that 57% (95% CI, 54–60) of all-cause influenza-associated and 26% (95% CI, 24–28) of all-cause RSV-associated deaths in children aged <5 years occurred out-of-hospital [29]. Global studies of influenza-associated LRTI estimate 1–2 million influenza-associated severe cases and 3.0% CFR in developing countries (including an estimate of 5.6% from Soweto, South Africa) [2]. Similar studies of RSV estimate 3.4 million RSV-associated severe cases and 2.1% CFR in developing countries (including an estimate of 1.3% from Soweto, South Africa) [3]. These earlier studies were conducted prior to 2004 when HIV antiretroviral therapy was introduced in the public sector for children. In addition, programs for the prevention of mother-to-child transmission have dramatically reduced transmission of HIV from more than 20% in 2004 to less than 2% in 2015 [30]. The overall reduction in HIV prevalence among children and the high proportion of out-of-hospital deaths may explain the much lower CFR noted in our surveillance.
There are several limitations to this analysis. In calculating rates of influenza- and RSV-associated hospitalization, we assumed that the proportion positive among children enrolled in surveillance was comparable to that of children with similar diagnosis codes who were not tested. There may be bias in enrollment practices (eg, not enrolling critically ill children or the youngest infants) that may impact that assumption. Also, these sites may not be representative of urban and rural South Africa. In our model, the attributable fraction of influenza and RSV were calculated for infants aged <1 year and for children aged 1–4 years. It is possible that the attributable fraction for infants aged 0–2 months might be different than that for older infants. Similarly, our health utilization survey was not powered to assess differences in hospitalization by refined age groups. Finally, we did not analyze the impact of the duration of maternal antiretroviral exposure on infant influenza- or RSV-associated hospitalization.
CONCLUSIONS
HIV exposure (in infants aged 0–5 months) and HIV infection are risk factors for influenza- and RSV-associated hospitalization. More than one-third of infants aged 0–2 months who met our SRI case definition tested positive for RSV. RSV-associated hospitalization rates were much higher than influenza-associated hospitalization rates in the first 2 years of life. Maternal immunization or passive immunization with monoclonal antibody may prevent influenza- or RSV-associated hospitalizations in young infants. Influenza vaccination of infants aged 6 months and older should be encouraged.
Acknowledgments.
The authors thank the surveillance officers and participants who made this work possible.
Financial support. This work was supported by the CDC through a cooperative agreement with the National Institute for Communicable Diseases, South Africa (5U01IP001048).
Potential conflicts of interest. C. C. reports grants from the CDC during the conduct of the study, support from Parexel, and grants from Sanofi Pasteur outside the submitted work. S. A. M. reports grants from the CDC, the Bill and Melinda Gates Foundation (BMGF), Pfizer, GlaxoSmithKline, and Novartis and personal fees from BMGF outside the submitted work. A. v. G. reports grants from the CDC during the conduct of the study and grants from Pfizer and Sanofi Pasteur outside the submitted work. All remaining authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Footnotes
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
References
- 1.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380:2095–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nair H, Brooks WA, Katz M, et al. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet 2011; 378:1917–30. [DOI] [PubMed] [Google Scholar]
- 3.Nair H, Nokes DJ, Gessner BD, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 2010; 375:1545–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lafond KE, Nair H, Rasooly MH, et al. ; Global Respiratory Hospitalizations—Influenza Proportion Positive Working Group. Global role and burden of influenza in pediatric respiratory hospitalizations, 1982–2012: a systematic analysis. PLoS Med 2016; 13:e1001977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nair H, Simões EA, Rudan I, et al. ; Severe Acute Lower Respiratory Infections Working Group. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. Lancet 2013; 381:1380–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen C, Moyes J, Tempia S, et al. Severe influenza-associated respiratory infection in high HIV prevalence setting, South Africa, 2009–2011. Emerg Infect Dis 2013; 19:1766–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moyes J, Cohen C, Pretorius M, et al. Epidemiology of respiratory syncytial virus-associated acute lower respiratory tract infection hospitalizations among HIV-infected and HIV-uninfected South African children, 2010–2011. J Infect Dis. 2013;208(Suppl 3):S217–26. [DOI] [PubMed] [Google Scholar]
- 8.Kyeyagalire R, Tempia S, Cohen AL, et al. Hospitalizations associated with influenza and respiratory syncytial virus among patients attending a network of private hospitals in South Africa, 2007–2012. BMC Infect Dis 2014; 14:694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen C, Moyes J, Tempia S, et al. Epidemiology of acute lower respiratory tract infection in HIV-exposed uninfected infants. Pediatrics. 2016; 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Mollendorf C, von Gottberg A, Tempia S, et al. ; Group for Enteric, Respiratory and Meningeal Disease Surveillance in South Africa. Increased risk for and mortality from invasive pneumococcal disease in HIV-exposed but uninfected infants aged <1 year in South Africa, 2009–2013. Clin Infect Dis 2015; 60:1346–56. [DOI] [PubMed] [Google Scholar]
- 11.Cohen C, Walaza S, Moyes J, et al. Epidemiology of viral-associated acute lower respiratory tract infection among children <5 years of age in a high HIV prevalence setting, South Africa, 2009–2012. Pediatr Infect Dis J 2015; 34:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pretorius MA, Madhi SA, Cohen C, et al. Respiratory viral coinfections identified by a 10-plex real-time reverse-transcription polymerase chain reaction assay in patients hospitalized with severe acute respiratory illness—South Africa, 2009–2010. J Infect Dis. 2012;206(Suppl 1):S159–65. [DOI] [PubMed] [Google Scholar]
- 13.Carvalho Mda G, Tondella ML, McCaustland K, et al. Evaluation and improvement of real-time PCR assays targeting lytA, ply, and psaA genes for detection of pneumococcal DNA. J Clin Microbiol 2007; 45:2460–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tempia S, Walaza S, Moyes J, et al. Attributable fraction of influenza virus detection to mild and severe respiratory illnesses in HIV-infected and HIV-uninfected patients, South Africa, 2012–2016. Emerg Infect Dis 2017; 23:1124–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Department of Health SSASS, South African Medical Research Council, and ICF. South Africa Demographic and Health Survey 2016: key indicators. Pretoria, South Africa and Rockville, Maryland, USA: NDoH, Stats SA, SAMRC and ICF, 2017. [Google Scholar]
- 16.Emukule GO, Khagayi S, McMorrow ML, et al. The burden of influenza and RSV among inpatients and outpatients in rural western Kenya, 2009–2012. PLoS One 2014; 9:e105543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall CB, Simőes EA, Anderson LJ. Clinical and epidemiologic features of respiratory syncytial virus. Curr Top Microbiol Immunol 2013; 372:39–57. [DOI] [PubMed] [Google Scholar]
- 18.Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med 2009; 360:588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ochola R, Sande C, Fegan G, et al. The level and duration of RSV-specific maternal IgG in infants in Kilifi Kenya. PLoS One 2009; 4:e8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu Q, McLellan JS, Kallewaard NL, et al. A highly potent extended half-life antibody as a potential RSV vaccine surrogate for all infants. Sci Transl Med 2017; 9. [DOI] [PubMed] [Google Scholar]
- 21.Steinhoff MC, Omer SB, Roy E, et al. Influenza immunization in pregnancy–antibody responses in mothers and infants. N Engl J Med 2010; 362:1644–6. [DOI] [PubMed] [Google Scholar]
- 22.Nunes MC, Cutland CL, Dighero B, et al. ; Matflu Team. Kinetics of hemagglutination-inhibiting antibodies following maternal influenza vaccination among mothers with and those without HIV infection and their infants. J Infect Dis 2015; 212:1976–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tapia MD, Sow SO, Tamboura B, et al. Maternal immunisation with trivalent inactivated influenza vaccine for prevention of influenza in infants in Mali: a prospective, active-controlled, observer-blind, randomised phase 4 trial. Lancet Infect Dis 2016; 16:1026–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramkrishna W Trends in influenza vaccine coverage of high risk groups in South Africa (2011–2015), prior to influenza policy implementation Options IX for the Control of Influenza; August 27, 2016. Chicago, Illinois: International Society for Influenza and other Respiratory Virus Diseases, 2016. [Google Scholar]
- 25.Hoberman A, Greenberg DP, Paradise JL, et al. Effectiveness of inactivated influenza vaccine in preventing acute otitis media in young children: a randomized controlled trial. JAMA 2003; 290:1608–16. [DOI] [PubMed] [Google Scholar]
- 26.Vesikari T, Knuf M, Wutzler P, et al. Oil-in-water emulsion adjuvant with influenza vaccine in young children. N Engl J Med 2011; 365:1406–16. [DOI] [PubMed] [Google Scholar]
- 27.Rolfes MA, Goswami D, Sharmeen AT, et al. Efficacy of trivalent influenza vaccine against laboratory-confirmed influenza among young children in a randomized trial in Bangladesh. Vaccine. 2017; 35:6967–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Claeys C, Zaman K, Dbaibo G et al. Prevention of vaccine-matched and mismatched influenza in children aged 6–35 months: a multinational randomised trial across five influenza seasons. Lancet Child Adolesc Health. 2018; 2:338–49. [DOI] [PubMed] [Google Scholar]
- 29.Cohen C, Walaza S, Treurnicht FK, et al. In- and out-of-hospital mortality associated with seasonal and pandemic influenza and respiratory syncytial virus in South Africa, 2009–2013. Clin Infect Dis; 2018; 66:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diseases NIfC. Prevention of HIV mother to child transmission: a South African success story. Communicable Diseases Communique 2015.