Abstract
Group V Phospholipase A2 (Pla2g5) is a member of the PLA2 family of lipid-generating enzymes. It is expressed in immune and non-immune cell types and is inducible during several pathologic conditions serving context-specific functions. In this review, we recapitulate the protective and detrimental functions of Pla2g5 investigated through preclinical and translational approaches.
1. Identification and characterization
Group V Phospholipases A2 (Pla2g5), identified by Chen and colleagues [1, 2] belongs to the group of secretory Ca2+-dependent Phospholipases A2 (PLA2). Its gene is located on mouse chromosome 4 (Pla2g5) and human chromosome 1 (PLA2G5) [3] in a cluster of genes containing 6 PLA2s, Pla2g2a, Pla2g2c, Pla2g5, Pla2g2d, Pla2g2e and Pla2g2f [4-7]. PLA2s hydrolyze membrane phospholipids to generate Lysophospholipids (LysoPL) and free fatty acids (FFAs). The FFA arachidonic acid (AA) can be further metabolized to generate eicosanoids, including prostaglandins and leukotrienes, or FFAs may signal through cognate receptors. To access membrane phospholipids, PLA2s need to bind membrane components [8]. Pla2g5, Pla2g2a and Pla2g2d bind to heparan sulfate proteoglycans through a cluster of negatively charged residues [9, 10]. Pla2g5 and Pla2g10 bind to membrane phosphatidylcholine (PC) with high affinity [8]; a tryptophan residue in position 31 of the human PLA2G5 was critical for binding PC-rich plasma membranes [8, 11, 12]. Pla2g5 is the only enzyme that uses both mechanisms. To some extent, PLA2s have substrate preferences [13]. While Pla2g5 and Pla2g10 may similarly bind PC, they have a preference for mono (or low) saturated FFAs or poly saturated FFAs (PUFA), respectively.
PLA2s have tissue- and cell-specific expression [14]. Pla2g5 was found in immune cells including macrophage cell lines P338D1 [15, 16], bone-marrow derived mast cells [17, 18], T-cells [19], and human neutrophils [20, 21]. It was also expressed in human cardiomyocytes during infarction [22], human lung epithelial cells [23, 24] in addition to the renal tubular epithelium, gastric fibroblasts and male reproductive organs [14, 25].
2. Defining Pla2g5 ability to generate AA and eicosanoids
Pivotal studies elucidated the role of Pla2g5 in AA release and eicosanoid generation. Addition of exogenous PLA2G5 to human neutrophils induced AA release and leukotriene B4 (LTB4) generation by activating cytosolic PLA2 (cPLA2)α [21, 26], a PLA2 absolutely required for AA release [27, 28]. The binding to membrane PC was necessary for AA release at the plasma membrane and internalization of PLA2G5 to which followed degradation in human neutrophils [21] or cysteinyl-leukotriene (CysLT) synthesis at the perinuclear membrane in HEK293 cells [29]. In human eosinophils internalization of PLA2G5, generated by epithelial cells [24], also led to CysLT release with a mechanism independent of cPLA2α [30]. As PLA2G5, PLA2G10 is expressed and secreted by epithelial cells [14, 31, 32]. Similarly to PLA2G5, PLA2G10 induced CysLT synthesis in eosinophils thorough transactivation but with a mechanism dependent on Lysophosphatidylcholine (LysoPC) generation, MAPK and cPLA2α activation [31].
Pla2g5 and Pla2g10 hydrolyze PC, the major phospholipid in the lung surfactant [33]. Interestingly, transgenic (Tg) overexpression of Pla2g5 or Pla2g10 had unanticipated consequences [34]. Pla2g5-Tg mice died within 8 hours from birth due to increased surfactant hydrolysis and lung damage, while CysLT levels in Pla2g5-Tg were similar to those of Wt mice. Pla2g10-Tg survived and had normal lungs, likely because Pla2g10 in the normal lung remained as an inactive proenzyme, whose activation may require proteolytic processing during inflammation. These reports established that although Pla2g5 may share cell expression and enzymatic properties with other conventional secreted PLA2s, the identification of each function underscores the specific roles of Pla2g5.
The first report of Pla2g5-null mice more than a decade ago focused on the contribution of endogenous Pla2g5 to eicosanoid generation in vitro and in vivo [35]. Pla2g5-null macrophages isolated from the peritoneal cavity and cultured for three hours had reduced prostaglandin E2 (PGE2) and CysLT generation following phagocytosis of zymosan, a yeast-derived particle [35]. Furthermore, Pla2g5-null mice had reduced vascular permeability and CysLT generation in vivo during zymosan-induced peritonitis. Phagocytosis, perhaps the primary function of macrophages, is a protective mechanism used by macrophages to fight infections and clear debris. Phagocytosis requires several steps: binding, uptake, and fusion. Pla2g5-null peritoneal macrophages cultured for two days had a marked reduction in phagocytosis of zymosan particles, complement- and IgG-opsonized sheep red blood cells [36]. Binding of zymosan was intact in resting Pla2g5-null peritoneal macrophages [37]. However, intracellular staining showed that Pla2g5 translocated to the phagosome and that the Pla2g5-null macrophages had a delay in the fusion of phagosomes containing zymosan with lysosomes. These data suggested that functions requiring intracellular fusion events, including the killing of pathogens and antigen presentation, could be regulated by Pla2g5 (see paragraphs on infectious diseases and asthma). In these latest reports [36, 37], CysLT generation in Pla2g5-null peritoneal macrophages following zymosan ingestion was comparable to Wild-type (Wt) peritoneal macrophages, likely due to differences in culture conditions or to Pla2g5-null mice being fully backcrossed to the C57BL/6J strain (N10-N11). Furthermore, these data suggested that the function of Pla2g5 in macrophages during phagocytosis of zymosan could be independent of its enzymatic activity or mediated by lipids other than CysLTs. Perhaps, Pla2g5 induces CysLT production by other cells including mast cells, at least in certain conditions [38–40].
3. An integrated view of Pla2g5 functions through preclinical studies: more than eicosanoid generation
An additional level of complexity is dictated by the changes happening in the cells after activation in the context of diseases. Ex vivo and in vivo studies have been instrumental in elucidating the inducible nature of Pla2g5 and its apparent opposite functions due to its expression in several cell types including macrophages [41, 42], epithelial cells [43], and adipocytes [44]. The information obtained through preclinical studies and human studies have elucidated the functions of Pla2g5 in peculiar cell populations that develop and are activated during the specific immune response associated with each disease (Table 1). The data discussed here demonstrate the need for establishing cell- and context-specific strategies for targeting PLA2G5 expression and function in complex diseases including asthma, obesity, arthritis, cardiovascular and infectious diseases.
Table 1.
Role of Pla2g5 determined by cell expression and its specific disease function
| Pathogenic /detrimental functions | ||||||
|---|---|---|---|---|---|---|
| Mouse cells | Function | Ref | Human cells | Function | Ref | |
| Asthma | BM-Dendritic cells | Antigen presentation | 18,19 | |||
| -Lung Macrophages -BM-M2 macrophages (GM-CSF, IL-4, IL-33) | M2 activation CCL22 generation T-cell recruitment | 10 | Monocyte-derived macrophages (IL-4) | M2 activation PGE2 generation TGM2 activation | 10, 22 | |
| -Lung Macrophages -BM-M2 macrophages (GM-CSF, IL-4, IL-33) | IL-33 generation LA and OA generation ILC2 activation | 11 | ||||
| Cardiovascular diseases | Cardiomyocytes | Myocardial injury | 41, 42 | Alpha smooth muscles Valve Macrophages | Aortic valve calcification | 47 |
| Atherosclerotic plaque | Hydrolysis of LDL Macrophage foam cell | 32, 33, 34 | Atherosclerotic plaque | Hydrolysis of LDL | 32 | |
| Resolving/protective functions | ||||||
| Infectious diseases | Peritoneal Macrophages | Phagocytosis of zymosan Phagocytosis and killing of Candida albicans | 8,9 | Monocyte-derived macrophages (IL-4) | M2 activation LysoPE generation Phagocytosis of zymosan | 49 |
| Structural cells and hematopoietic-derived cells, neutrophils | Neutrophil recruitment Clearance of Gram negative bacteria | 52, 53, 54 | Neutrophils | Bactericidal function | 50, 51 | |
| Metabolic syndrome | Adipocytes Adipose macrophages | LDL hydrolysis OA and LA generation M2 activation, GSIS | 13 | White adipose tissue | LDL Hydrolysis | 13 |
| Pancreatic beta-cells | GSIS in vivo | 57 | ||||
| Arthritis | Peritoneal macrophages | Phagocytosis of immunocomplexes | 55 | CD14+ synovial and blood cells | Exogenous Pla2g5 IC Phagocytosis | 55 |
3.1. Asthma
Asthma is a complex disease driven by inflammation and characterized by permanent modifications of the lung, reduced compliance, and exacerbations. Although there are two major asthma phenotypes, one characterized by increased numbers of eosinophils and the other by the prevalence of neutrophils, it’s clear that different mechanisms or endotypes may be involved [45]. Mouse models of allergic pulmonary inflammation mimic many features of human asthma and have helped to explore the contribution of PLA2s to asthma pathogenesis. Particularly, eicosanoids generated by the cPLA2α [28] had been implicated in the pathogenesis of asthma [46]. Using a model of Ovalbumin (OVA)-induced airway inflammation, which consists of a sensitization phase by intraperitoneal injection of OVA plus alum and a 3-day challenge phase with nebulized OVA, Munoz et al. reported that Pla2g5 was markedly induced in epithelial cells, airway smooth muscle cells and inflammatory cells infiltrating the lung [43]. Interestingly, administration of a blocking antibody against Pla2g5 at sensitization reduced airway narrowing and cell recruitment. When administered after challenge, the antibody decreased only airway resistance. However, a marked reduction in eosinophil numbers and airway resistance was reported in Pla2g5-null mice exposed to OVA compared to equally treated Wt mice [43], suggesting that Pla2g5 may act through multiple mechanisms. The effect of exogenous Pla2g5 on airway narrowing was independent of cPLA2α activation [43], while a previous report showed that PLA2G5 released by epithelial cells increased CysLT production by human eosinophils and was associated with increased cell adhesion induced by LysoPC [47].
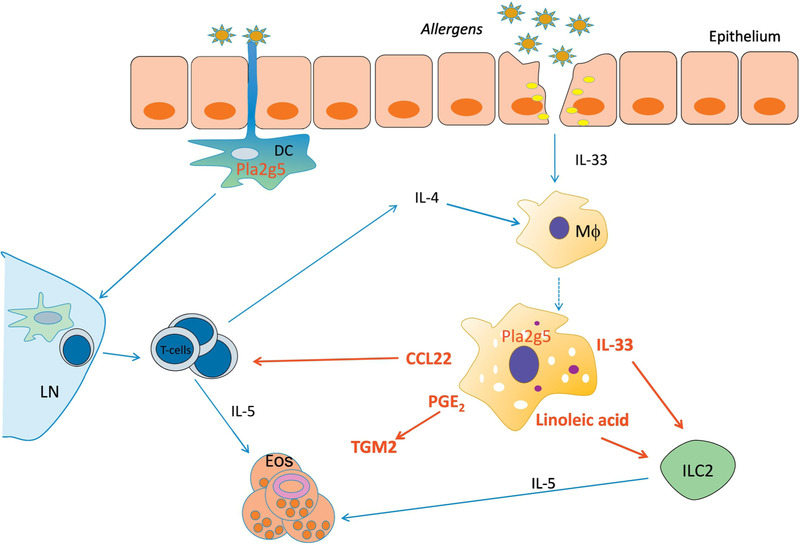
Using a model of pulmonary inflammation induced by the house dust mite (HDM) Dermatophagoides farinae and based on the notion that Pla2g5 regulates intracellular trafficking events in macrophages [37], in a subsequent study we proved that intracellular Pla2g5 in dendritic cells was required for antigen uptake, processing and T-cell activation, critical features of allergic sensitization [48] (Figure 1). The adaptive immune response to HDM was also impaired in the absence of Pla2g5, as restimulation with HDM of lymphocytes obtained from parabronchial lymph nodes resulted in significantly reduced production of IL-4, IL-5, and IL-13. Serum IgE were almost absent in HDM exposed Pla2g5-null mice [48]. Similar findings were obtained using an OVA model where the authors also showed that the function of Pla2g5 in inducing airway remodeling extended to a chronic model [49]. Mouse models based on clinical relevant allergens have significantly improved our understanding of the innate immune response to allergens involved in the pathogenesis of asthma. Transfers of Wt dendritic cells into HDM-exposed Pla2g5-null mice partially restored Th2 cytokine production by restimulated lymph node cells. However, they did not restore HDM-induced pulmonary inflammation (cell infiltration and goblet cell metaplasia) [48], suggesting that at least another lung resident cell expressing Pla2g5 was necessary for the development of pulmonary inflammation. Pla2g5 is expressed by lung epithelial cells and macrophages [34, 41, 43], among other cells. Bone marrow transplant experiment suggested that Pla2g5 was required in hematopoietic-derived cells to develop HDM-induced pulmonary inflammation [41]. Macrophages developing in Type 2 immune responses, including asthma, polarize toward a group of macrophages broadly referred as M2 macrophages [50, 51]. Hence, Wt lung macrophages isolated from mice exposed to HDM were activated toward an M2 phenotype characterized by the expression of the Resistin-like molecule-α (Relm-α), Arginase-1 (Arg-1) and YM1, all of which were reduced in lung macrophages isolated from equally treated Pla2g5-null mice. Lung macrophages required Pla2g5 for M2 activation, and to produce CCL22, a chemokine that recruits T-cells into the lung [41]. Furthermore, Pla2g5 was induced by IL-4, the prototypical Type 2 cytokine, in mouse bone marrow (BM)-macrophages and human macrophages derived from blood monocytes [41]. IL-13 also induced Pla2g5 expression but at lower levels compared to IL-4 in both mouse BM-macrophages [41, 44] and human monocyte-derived macrophages [41]. However, activation of BM-macrophages in vitro by IL-4 was not sufficient to elicit critical features of macrophages developing in the lungs of mice exposed to HDM. Indeed, only when BM-macrophages were activated in vitro with a cocktail of type 2 cytokines (GM-CSF, IL-33, and IL-4), they produced CCL22, which was significantly reduced in the absence of Pla2g5 [41] (Table 1). These data emphasize how the activation state of the macrophages can influence the role of Pla2g5 and vice versa.
Figure 1.
Illustration of Pla2g5 expression and function during allergen-induced pulmonary inflammation.
Dendritic cells (DCs) expressing Pla2g5 uptake and process allergens for presentation to T-cells in lymph nodes (LN). Activated T-cells are recruited to the lung by Pla2g5-expressing macrophages producing CCL22.
Epithelial cells release IL-33, which activates ILC2s and macrophages. Activated macrophages expressing Pla2g5 produce IL-33 and the FFAs, LA and OA, which further activate ILC2s to produce IL-5 for eosinophil recruitment and activation.
Human monocyte-derived macrophages cultured in M-CSF and polarized by IL-4 for 24h produced low amounts of CCL22 and Transglutaminase 2 (TGM2), a marker of human M2 macrophages developing in asthmatic patients [51]. However, culture of monocyte-derived macrophages in GM-CSF for 13 days and polarization with IL-4 for 24h sharply increased CCL22, TGM2, and PLA2G5 expression [52]. Compared to monocyte-derived macrophages treated with vector-siRNA, monocyte-derived macrophages treated with PLA2G5-siRNA had reduced PGE2 production and reduced transglutaminase activity, which was up-regulated by exogenous PLA2G5 or PGE2 [52]. Furthermore, PLA2G5 and TGM2 translocated during IL-4 activation and colocalized on the plasma membrane, suggesting possible crosstalk in human macrophages. In specimens obtained from patients with eosinophilic sinusitis and nasal polyposis, a chronic Type 2 disease which is frequently associated with asthma, PLA2G5 was highly expressed in macrophages co-expressing TGM2. These data suggest that the function of PLA2G5 in M2 macrophages could extend to human Type 2 diseases [52].
Alternaria alternata (Alternaria) is a major fungal allergen. Exposure to Alternaria spores induces development of asthma and asthma exacerbations [53, 54]. In mouse models, Alternaria triggers IL-33 release from epithelial cells and activation of Group 2 innate lymphoid cells (ILC2s), a type of innate cells responsive to IL-33 and central to the development of asthma [55, 56]. Pla2g5-null mice exposed to Alternaria had a significantly reduced number of activated lung ILC2s, expressing ST2, CD25, IL-5 and IL-13 and eosinophils [42]. Furthermore, intranasal administration of Alternaria induced IL-33 protein and mRNA expression in Wt lung macrophages which were both reduced in Pla2g5-null lung macrophages. In BM-macrophages IL-4 again was not sufficient to induce IL-33 in vitro, but a cocktail of Th2 cytokines including IL-4, GM-CSF and IL-33 [41] induced IL-33 protein and mRNA expression in Wt BM-macrophages, which were significantly reduced in equally activated Pla2g5-null BM-macrophages. Furthermore, mass spectrometry analysis revealed that Pla2g5-null BM-macrophages and macrophages isolated from the lungs of Alternaria-exposed Pla2g5-null mice had reduced production of oleic acid (OA), linoleic acid (LA), and arachidonic acid (AA) (OA> LA> AA). Transfers of Wt BM-Macrophages into Pla2g5-null mice significantly increased eosinophil numbers and ILC2 numbers, confirming that Pla2g5 in macrophages was required for ILC2 activation. However, when administered intranasally, LA and OA in the presence of IL-33 but not alone significantly potentiated ILC2 activation and increased lung eosinophils numbers, while in Pla2g5-null mice LA was ineffective [42]. LA binds the free fatty acid receptor (FFAR)-1 and FFAR4 [42]; in this study we reported for the first time that FFAR1 is expressed in Wt ILC2s and its expression is significantly reduced on Pla2g5-null ILC2s, which likely accounts for the reduced activation of Pla2g5-null ILC2s by LA in vivo and ex vivo. These findings highlight for the first time a critical innate pathway involving Pla2g5, macrophages, IL-33, FFA production and ILC2 activation (Figure 1).
In summary, endogenous Pla2g5 contributes to allergic asthma through at least 3 mechanisms: antigen presentation by dendritic cells [48, 49]; macrophage activation and production of the chemokine CCL22 for T cell recruitment into the lung [41], and macrophage production of IL-33 and FFAs for ILC2 activation [42] (Figure 1). The generation of mice lacking Pla2g5 in selected cell types will further elucidate the functions of Pla2g5-expressing cells in asthma and other diseases.
Transfers of Wt BM-macrophages into Pla2g5-null mice exposed to Alternaria increased eosinophil numbers and ILC2 numbers; however, these values did not reach the extent of Wt mice receiving Alternaria alone or Alternaria and Wt BM-Macrophages [42]. One possible explanation was that Pla2g5-null macrophages still present in Pla2g5-null mice prevented the full development of the allergic inflammatory response. Indeed, transfers of Pla2g5-null macrophages in Wt mice exposed to Alternaria significantly reduced the numbers of lung eosinophils and ILC2s [42]. These data suggest that cell therapy using Pla2g5-null macrophages represent potentially a new therapeutic approach at least in selected patients with asthma.
Pla2g10 also has a prominent role in asthma [57-59]. It was found in the bronchoalveolar lavage (BAL), in the airway epithelial cells and alveolar macrophages obtained from asthmatic patients [32, 58]. In addition, Pla2g10 levels were significantly elevated upon allergen inhalation in both murine and human airways [32, 59]. Pla2g10-null mice challenged with OVA had reduced lung inflammation and reduced eicosanoids release in BAL [59]. PLA2G10 highly expressed by lung epithelial cells, was released during inflammation acting on eosinophils to generate LysoPC and induce CysLTs generation in a cPLA2α dependent fashion [31]. Furthermore, Pla2g10 induced AA release by epithelial cells but not PGE2.
The role of Pla2g10 in asthma has been recently further investigated using the HDM murine model of airway hyperresponsiveness (AHR) [60]. Pla2g10 deletion protected mice from HDM-elicited AHR and resulted in reduced eosinophil and T cell infiltration in the lungs. While only IL-13 was reduced in in vitro restimulated Pla2g10-null lymphocytes, Pla2g10-null mice also showed reduced mucus formation and reduced M2 macrophage recruitment. Compared to HDM-exposed Wt mice, in Pla2g10-null mice exposed to HDM IL-33 prenzymatic properties with other conventional secreted PLA[isp]2s, theotein released in the BAL but not IL-33 mRNA in lung tissue was reduced and was associated with reduced ILC2 activation and mast cell production of IL-13 [60]. Furthermore, compared to HDM-exposed Wt mice, HDM-exposed Pla2g10-null mice had reduced CysLT in BAL as reported in the murine OVA model of AHR [32, 59, 60].
All together in vivo studies on the roles of Pla2g5 and Pla2g10 in asthma suggest that these enzymes synergistically contribute to asthma pathogenesis; however, their relative functions could be prevalent in selected cells and therefore in certain patients or asthma endotypes.
3.2. Cardiovascular diseases
Hydrolysis of phospholipids in low-density lipoproteins (LDL) and macrophage foam cell formation are some of the early events in the development of the atherosclerotic plaque. Pla2g5 promoted atherogenic functions in vitro [61, 62] and could be detected in mouse and human atherosclerotic lesions [62]. In LDL-receptor (LDLR)-null mice, overexpression of Pla2g5 resulted in an increased area of the atherosclerotic lesion, while Pla2g5 deficiency in bone marrow reduced the atherosclerotic lesion area in the aortic arch and thoracic aorta [63]. In vitro studies showed that these modifications were likely due to the capacity of Pla2g5 to modify LDL and induce foam cell formation [61] through Syndecan 4 (a proteoglycan receptor) uptake [64]. Additionally, hydrolysis of LDL and HDL by PLA2G5 could generate pro-atherogenic products acting on smooth muscle cells [65]. Accordingly, a recent report showed that hydrolysis of phospholipids in LDL is accomplished by several PLA2s, PLA2G10, PLA2G3, PLA2G2F, PLA2G2A, and PLA2G5. PLA2G5 targeted PC species containing 18:1 (OA) and 18:2 (LA), PLA2G10 preferred 20:4 (AA), and PLA2G3 was active on most PC species [66]. However, Pla2g5 was dispensable in developing atherosclerotic lesion in Apolipoprotein E (ApoE)-null mice, likely due to the different nature of circulating LDL [67]. In absence of Pla2g5, ApoE-null mice showed reduced aortal aneurysm but increased perivascular fibrosis and macrophage accumulation [68]. It is possible that this dual effect of Pla2g5 on LDL or perivascular fibrosis reflects the expression of Pla2g5 in different cell types or different activation of cells involved in the pathogenesis or resolution phase of cardiovascular diseases.
The elevated expression of PLA2G5 in the heart suggests that it may have a function in cardiac homeostasis [14]. Following different types of cardiac stress, including myocardial infarction and hypertension, the heterogeneous populations of cardiac cells including cardiomyocytes, fibroblasts, smooth muscle cells, endothelial cells, and immune cells, contribute to remodeling processes. In an ischemia/reperfusion model, the absence of PLA2G5 resulted in reduced size of the myocardial injury [22]. The effects of Pla2g5 in cardiomyocytes involved activation of cPLA2α through p38, a mitogen-activated protein kinase (MAPK) [69] as similarly reported in mast cells [39], a macrophage-like cell line [70], mesangial cells [71] and neutrophils [26]. PLA2G5 was also found in human aortic valves localized in cells expressing CD68 and alpha-smooth muscles [72]. By immunofluorescence, its expression and that of PLA2G2E, correlated with the expression of the pro-osteogenic molecules, bone morphogenetic protein (BMP)-2, Osteopontin and Alkaline Phosphatase (ALP) [72]. The role of PLA2G5 in valve calcifications was confirmed by knocking down PLA2G5 or PLA2G2E in valve interstitial cells which significantly reduced the expression of pro-osteogenic molecules. Furthermore, a study in humans showed that at least one PLA2G5 polymorphism, likely involving PLA2G5 expression, was associated with premature coronary artery disease [73].
Although these reports do not definitively support the use of PLA2G5 inhibitors as therapeutics for cardiovascular diseases, they warrant further investigation both through mouse models, ideally using a Pla2g5 conditional knock-out, and human studies.
3.3. Infectious diseases
The defect in phagocytosis of the yeast-derived particles zymosan by Pla2g5-null macrophages [36] prompted us to investigate the function of Pla2g5 in infectious diseases in vivo. Mice lacking Pla2g5 were injected intravenously with the fungus Candida albicans. Pla2g5-null mice showed increased fungal burden and mortality compared to Wt mice [37]. In vitro phagolysosome fusion, necessary for pathogen killing, was reduced in peritoneal macrophages lacking Pla2g5, due to a defect in intracellular trafficking of phagosomes containing Candida albicans. Pla2g5-null peritoneal macrophages also had a delay in the killing of Candida albicans likely responsible for the increased fungal burden in vivo. Therefore, Pla2g5 protected from Candida albicans infections in vivo and regulated several steps of the phagocytic cascade in vitro: uptake, fusion, and killing of Candida albicans [36, 37] (Table 1). Interestingly, some of these findings were reproduced in human monocyte-derived macrophages treated with PLA2G5-siRNA, which showed reduced phagocytosis of zymosan particles due to reduced Lysophosphathidylethanolamine (LysoPE) generation [74].
Among hematopoietic cells, PLA2G5 is expressed by human neutrophils, stored in azurophil granules as PLA2G10 [20]. However, following activation with formyl-Met–Leu–Phe (fMLP) or priming with pro-inflammatory cytokines [20, 75], only PLA2G5 is released extracellularly where it likely performs bactericidal functions. Pla2g5 has been involved in the inflammatory response to Gram-negative bacteria in vivo. In an LPS-induced acute lung injury model, Pla2g5 was expressed in epithelial cells and lung parenchyma 24h after LPS intratracheal administration. The absence of Pla2g5 reduced neutrophil recruitment into the lung [76]. Furthermore, in a model of LPS-induced cell migration, Pla2g5 was required for early leukocyte recruitment into the mouse air pouch, through ICAM and VCAM upregulation [77]. Consistently, infection of lungs with E. coli resulted in reduced leukocyte recruitment in BAL and reduced ICAM-1 and PECAM-1 expression in Pla2g5-null mice. Pla2g5 was required on structural cells and hematopoietic-derived cells to clear E. coli infection respectively from BAL and lung parenchyma. Eicosanoids levels were similar in BAL of E. coli-treated Pla2g5-null mice compared to equally treated Wt mice, while in lung parenchyma PGD2 and PGF2α were reduced. These data suggest that in the absence of Pla2g5 the decreased inflammatory response to bacteria was responsible for the reduced bacterial clearance and therefore worsening infection [78]. These studies point to the possible use of PLA2G5 as a therapeutic agent against infectious diseases, although its role in the development of type 2 inflammatory responses should be considered.
3.4. Arthritis
Another example of a worsening condition in the absence of Pla2g5 was shown by the K/bxN arthritis model [79]. PLA2G5, and to a greater extent PLA2G2A, were highly expressed in the joints of patients with rheumatoid arthritis [79]. Balb/cJ-Pla2g5-null mice [36], generated from embryonic stem cells from 129 strain which also lack Pla2g2a due to a frameshift mutation [80], had increased clinical features of serum-induced arthritis. Mechanistically, reduced phagocytosis of immune complexes (ICs) by Pla2g5-null macrophages caused increased IC deposition in the joints and worsening arthritis. Addition of exogenous Pla2g5 increased IC phagocytosis by mouse peritoneal macrophages and CD14+ human synovial macrophages in vitro. Furthermore, because CysLTs ameliorated disease in vivo and IC phagocytosis in vitro, the effect Pla2g5 in improving arthritis was attributable to increased CysLTs production and phagocytosis. This study suggested that in circumstances where phagocytosis by macrophages resolves detrimental conditions as in arthritis (Table 1), PLA2G5 could be used as therapeutic. Compared to Wt mice Pla2g2a-null mice had instead reduced clinical signs of arthritis using the same arthritis model [79], highlighting the opposite functions of Pla2g5 and Pla2g2a in this model.
Another study investigated the role of PLA2G5 in synovial fibroblasts obtained from a small group of patients with rheumatoid arthritis. In this report, PLA2G5 increased cartilage degradation and proinflammatory effects of endothelial protein C receptor (EPCR) activation [81].
Altogether these reports point once again to the cell-specific effect of Pla2g5 during pathological condition and the need of dissecting its cell-specific function with more sophisticated tools.
3.5. Metabolic syndrome
When fed a high fat diet (HFD) rich in OA, Pla2g5-null mice had increased body weight associated with increased plasma phospholipids and insulin resistance [44]. Pla2g5 was markedly induced in mouse white adipocytes following HFD feeding and human mesenteric white adipose tissue [44]. Low-density lipoproteins (LDL) from Pla2g5-null mice feeding HFD had significantly higher cholesterol and phospholipids (mainly PC containing OA and LA) compared to LDL from equally fed Wt mice [44]. Remarkably, the expression of PLA2G5 in human white adipose tissue inversely correlated with levels of plasma LDL. WAT macrophages from HFD-fed Pla2g5-null mice had reduced expression of CD206, one of the molecules expressed by mouse M2 macrophages, and increased ration M1/M2 macrophages. The addition of exogenous Pla2g5 to BM-derived macrophages polarized them toward a protective Type 2 phenotype [44]. However, the M2 polarization of BM-macrophages by IL-4 or IL-13, as monitored by Arg-1 expression, was not affected by Pla2g5 deficiency, suggesting that macrophage-derived Pla2g5 was insufficient to drive M2 polarization in this ex vivo experimental setting. These data are in line with other reports showing that IL-4 alone is not sufficient to elicit the role of endogenous Pla2g5 in M2 polarization of BM-macrophages [41, 42]. Interestingly in the model of HFD-induced obesity, transfers of Wt BM or Pla2g5-null BM into Pla2g5-null irradiated mice had similar HFD-induced obesity, insulin resistance, and hyperlipidemia, suggesting that the protective role of Pla2g5 in obesity was due to its expression in structural cells, namely adipocytes. Mechanistically, OA and to a lesser extent LA released from hyperlipidemic LDL by adipocyte-derived Pla2g5, attenuated (or counteracted) palmitate-induced M1 macrophage polarization in adipose tissue, resulting in protective effects in obesity and associated inflammation. Consistently, increased glucose-stimulated insulin secretion (GSIS) was reported in non-obese Pla2g5-null mice [82]. Interestingly, Pla2g2e-null mice had reduced HFD-induced obesity, and reduced phospholipids and cholesterol in plasma lipoproteins [44]. These findings highlight once more the opposite roles of Pla2g5 vs. other conventional PLA2s, Pla2g2e in the pathogenesis of obesity [44] and Pla2g2a in arthritis [79].
4. Conclusions
Pla2g5 is an enzyme that can generate an array of lipids including FFAs, particularly OA>LA>AA, and LysoPL. These lipids can act at cognate receptors or be further metabolized as AA in leukotrienes and prostaglandins, therefore potentially targeting multiple cells and multiple pathways within each cell. However, the ability of each cell type to express Pla2g5, produce lipid or respond to them depends also on the activation state of the cell, as proven for macrophages activating ILC2s during allergic pulmonary inflammation. Systemic administration of PLA2G5 inhibitors could ameliorate certain diseases including asthma but would exacerbate obesity, infections, and arthritis. However, in asthmatic patients, local administration of cells lacking PLA2G5 could prevent further activation of proinflammatory macrophages and release of dangerous lipid mediators. Personalized cell therapy could be a valuable option at least for selected patients with asthma or other diseases in which modulation of PLA2G5 expression and function must be locally targeted to particular cell types.
Acknowledgments
Funding
This work was supported by the National Institutes of Health grant number HL113071
Abbreviations:
- Pla2g5
Group V Phospholipase A2
- PGE2
prostaglandin E2
- CysLTs
cysteinyl-leukotriene
- Wt
Wild-type
- OVA
Ovalbumin
- HDM
house dust mite/ Dermatophagoides farinae
- Relm-α
Resistin-like molecule-α
- Arg-1
Arginase-1
- Alternaria
Alternaria alternata
- AHR
airway hyperresponsiveness
- PC
phosphatidylcholine
- LDL
low density lipoproteins
- BMP
bone morphogenetic protein
- ALP
Alkaline Phosphatase
- LysoPC
Lysophosphathidylcholine
- AA
arachidonic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest.
References
- [1].Chen J, Engle SJ, Seilhamer JJ, Tischfield JA, Cloning and recombinant expression of a novel human low molecular weight Ca(2+)-dependent phospholipase A2, J Biol Chem, 269 (1994) 2365–2368. [PubMed] [Google Scholar]
- [2].Chen J, Engle SJ, Seilhamer JJ, Tischfield JA, Cloning, expression and partial characterization of a novel rat phospholipase A2, Biochim Biophys Acta, 1215 (1994) 115–120. [DOI] [PubMed] [Google Scholar]
- [3].Tischfield JA, Xia YR, Shih DM, Klisak I, Chen J, Engle SJ, Siakotos AN, Winstead MV, Seilhamer JJ, Allamand V, Gyapay G, Lusis AJ, Low-molecular-weight, calcium-dependent phospholipase A2genes are linked and map to homologous chromosome regions in mouse and human, Genomics, 32 (1996) 328–333. [DOI] [PubMed] [Google Scholar]
- [4].Valentin E, Ghomashchi F, Gelb MH, Lazdunski M, Lambeau G, On the diversity of secreted phospholipases A(2). Cloning, tissue distribution, and functional expression of two novel mouse group II enzymes, J Biol Chem, 274 (1999) 31195–31202. [DOI] [PubMed] [Google Scholar]
- [5].Valentin E, Koduri RS, Scimeca JC, Carle G, Gelb MH, Lazdunski M, Lambeau G, Cloning and recombinant expression of a novel mouse-secreted phospholipase A2, J Biol Chem, 274 (1999) 19152–19160. [DOI] [PubMed] [Google Scholar]
- [6].Ishizaki J, Suzuki N, Higashino K, Yokota Y, Ono T, Kawamoto K, Fujii N, Arita H, Hanasaki K, Cloning and characterization of novel mouse and human secretory phospholipase A(2)s, J Biol Chem, 274 (1999) 24973–24979. [DOI] [PubMed] [Google Scholar]
- [7].Valentin E, Singer AG, Ghomashchi F, Lazdunski M, Gelb MH, Lambeau G, Cloning and recombinant expression of human group IIF-secreted phospholipase A(2), Biochem Biophys Res Commun, 279 (2000) 223–228. [DOI] [PubMed] [Google Scholar]
- [8].Singer AG, Ghomashchi F, Le Calvez C, Bollinger J, Bezzine S, Rouault M, Sadilek M, Nguyen E, Lazdunski M, Lambeau G, Gelb MH, Interfacial kinetic and binding properties of the complete set of human and mouse groups I, II, V, X, and XII secreted phospholipases A2, J Biol Chem, 277 (2002) 48535–48549. [DOI] [PubMed] [Google Scholar]
- [9].Murakami M, Nakatani Y, Kudo I, Type II secretory phospholipase A2 associated with cell surfaces via C-terminal heparin-binding lysine residues augments stimulus-initiated delayed prostaglandin generation, J Biol Chem, 271 (1996) 30041–30051. [DOI] [PubMed] [Google Scholar]
- [10].Murakami M, Shimbara S, Kambe T, Kuwata H, Winstead MV, Tischfield JA, Kudo I, The functions of five distinct mammalian phospholipase A2S in regulating arachidonic acid release. Type IIa and type V secretory phospholipase A2S are functionally redundant and act in concert with cytosolic phospholipase A2, J Biol Chem, 273 (1998) 14411–14423. [DOI] [PubMed] [Google Scholar]
- [11].Han SK, Kim KP, Koduri R, Bittova L, Munoz NM, Leff AR, Wilton DC, Gelb MH, Cho W, Roles of Trp31 in high membrane binding and proinflammatory activity of human group V phospholipase A2, J Biol Chem, 274 (1999) 11881–11888. [DOI] [PubMed] [Google Scholar]
- [12].Beers SA, Buckland AG, Giles N, Gelb MH, Wilton DC, Effect of tryptophan insertions on the properties of the human group IIA phospholipase A2: mutagenesis produces an enzyme with characteristics similar to those of the human group V phospholipase A2, Biochemistry, 42 (2003) 7326–7338. [DOI] [PubMed] [Google Scholar]
- [13].Murakami M, Koduri RS, Enomoto A, Shimbara S, Seki M, Yoshihara K, Singer A, Valentin E, Ghomashchi F, Lambeau G, Gelb MH, Kudo I, Distinct arachidonate-releasing functions of mammalian secreted phospholipase A2s in human embryonic kidney 293 and rat mastocytoma RBL-2H3 cells through heparan sulfate shuttling and external plasma membrane mechanisms, J Biol Chem, 276 (2001) 10083–10096. [DOI] [PubMed] [Google Scholar]
- [14].Masuda S, Murakami M, Ishikawa Y, Ishii T, Kudo I, Diverse cellular localizations of secretory phospholipase A2 enzymes in several human tissues, Biochim Biophys Acta, 1736 (2005) 200–210. [DOI] [PubMed] [Google Scholar]
- [15].Balboa MA, Balsinde J, Winstead MV, Tischfield JA, Dennis EA, Novel group V phospholipase A2 involved in arachidonic acid mobilization in murine P388D1 macrophages, J Biol Chem, 271 (1996) 32381–32384. [DOI] [PubMed] [Google Scholar]
- [16].Kessen UA, Schaloske RH, Stephens DL, Killermann Lucas K, Dennis EA, PGE2release is independent of upregulation of Group V phospholipase A2 during long-term stimulation of P388D1 cells with LPS, J Lipid Res, 46 (2005) 2488–2496. [DOI] [PubMed] [Google Scholar]
- [17].Reddy ST, Winstead MV, Tischfield JA, Herschman HR, Analysis of the secretory phospholipase A2that mediates prostaglandin production in mast cells, J Biol Chem, 272 (1997) 13591–13596. [DOI] [PubMed] [Google Scholar]
- [18].Bingham CO 3rd, Fijneman RJ, Friend DS, Goddeau RP, Rogers RA, Austen KF, Arm JP, Low molecular weight group IIA and group V phospholipase A(2) enzymes have different intracellular locations in mouse bone marrow-derived mast cells, J Biol Chem, 274 (1999) 31476–31484. [DOI] [PubMed] [Google Scholar]
- [19].Ho IC, Arm JP, Bingham CO 3rd, Choi A, Austen KF, Glimcher LH, A novel group of phospholipase A2s preferentially expressed in type 2 helper T cells, J Biol Chem, 276 (2001) 18321–18326. [DOI] [PubMed] [Google Scholar]
- [20].Degousee N, Ghomashchi F, Stefanski E, Singer A, Smart BP, Borregaard N, Reithmeier R, Lindsay TF, Lichtenberger C, Reinisch W, Lambeau G, Arm J, Tischfield J, Gelb MH, Rubin BB, Groups IV V, and X phospholipases A2s in human neutrophils: role in eicosanoid production and gram-negative bacterial phospholipid hydrolysis, J Biol Chem, 277 (2002) 5061–5073. [DOI] [PubMed] [Google Scholar]
- [21].Kim KP, Rafter JD, Bittova L, Han SK, Snitko Y, Munoz NM, Leff AR, Cho W, Mechanism of human group V phospholipase A2 (PLA2)-induced leukotriene biosynthesis in human neutrophils. A potential role of heparan sulfate binding in PLA2 internalization and degradation, J Biol Chem, 276 (2001) 11126–11134. [DOI] [PubMed] [Google Scholar]
- [22].Ishikawa Y, Komiyama K, Masuda S, Murakami M, Akasaka Y, Ito K, Akishima-Fukasawa Y, Kimura M, Fujimoto A, Kudo I, Ishii T, Expression of type V secretory phospholipase A2 in myocardial remodelling after infarction, Histopathology, 47 (2005) 257–267. [DOI] [PubMed] [Google Scholar]
- [23].Masuda S, Murakami M, Mitsuishi M, Komiyama K, Ishikawa Y, Ishii T, Kudo I, Expression of secretory phospholipase A2 enzymes in lungs of humans with pneumonia and their potential prostaglandin-synthetic function in human lung-derived cells, Biochem J, 387 (2005) 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wijewickrama GT, Kim JH, Kim YJ, Abraham A, Oh Y, Ananthanarayanan B, Kwatia M, Ackerman SJ, Cho W, Systematic evaluation of transcellular activities of secretory phospholipases A2. High activity of group V phospholipases A2 to induce eicosanoid biosynthesis in neighboring inflammatory cells, J Biol Chem, 281 (2006) 10935–10944. [DOI] [PubMed] [Google Scholar]
- [25].Masuda S, Murakami M, Matsumoto S, Eguchi N, Urade Y, Lambeau G, Gelb MH, Ishikawa Y, Ishii T, Kudo I, Localization of various secretory phospholipase A2 enzymes in male reproductive organs, Biochim Biophys Acta, 1686 (2004) 61–76. [DOI] [PubMed] [Google Scholar]
- [26].Kim YJ, Kim KP, Han SK, Munoz NM, Zhu X, Sano H, Leff AR, Cho W, Group V phospholipase A2 induces leukotriene biosynthesis in human neutrophils through the activation of group IVA phospholipase A2, J Biol Chem, 277 (2002) 36479–36488. [DOI] [PubMed] [Google Scholar]
- [27].Bonventre JV, Huang Z, Taheri MR, O'Leary E, Li E, Moskowitz MA, Sapirstein A, Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2, Nature, 390 (1997) 622–625. [DOI] [PubMed] [Google Scholar]
- [28].Uozumi N, Kume K, Nagase T, Nakatani N, Ishii S, Tashiro F, Komagata Y, Maki K, Ikuta K, Ouchi Y, Miyazaki J, Shimizu T, Role of cytosolic phospholipase A2 in allergic response and parturition, Nature, 390 (1997) 618–622. [DOI] [PubMed] [Google Scholar]
- [29].Kim YJ, Kim KP, Rhee HJ, Das S, Rafter JD, Oh YS, Cho W, Internalized group V secretory phospholipase A2 acts on the perinuclear membranes, J Biol Chem, 277 (2002) 9358–9365. [DOI] [PubMed] [Google Scholar]
- [30].Munoz NM, Kim YJ, Meliton AY, Kim KP, Han SK, Boetticher E, O'Leary E, Myou S, Zhu X, Bonventre JV, Leff AR, Cho W, Human group V phospholipase A2 induces group IVA phospholipase A2-independent cysteinyl leukotriene synthesis in human eosinophils, J Biol Chem, 278 (2003) 38813–38820. [DOI] [PubMed] [Google Scholar]
- [31].Lai Y, Oslund RC, Bollinger JG, Henderson WR Jr., Santana LF, Altemeier WA, Gelb MH, Hallstrand TS, Eosinophil cysteinyl leukotriene synthesis mediated by exogenous secreted phospholipase A2 group X, J Biol Chem, 285 (2010) 41491–41500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hallstrand TS, Lai Y, Altemeier WA, Appel CL, Johnson B, Frevert CW, Hudkins KL, Bollinger JG, Woodruff PG, Hyde DM, Henderson WR Jr., Gelb MH, Regulation and function of epithelial secreted phospholipase A2 group X in asthma, Am J Respir Crit Care Med, 188 (2013) 42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hite RD, Seeds MC, Safta AM, Jacinto RB, Gyves JI, Bass DA, Waite BM, Lysophospholipid generation and phosphatidylglycerol depletion in phospholipase A(2)-mediated surfactant dysfunction, Am J Physiol Lung Cell Mol Physiol, 288 (2005) L618–624. [DOI] [PubMed] [Google Scholar]
- [34].Ohtsuki M, Taketomi Y, Arata S, Masuda S, Ishikawa Y, Ishii T, Takanezawa Y, Aoki J, Arai H, Yamamoto K, Kudo I, Murakami M, Transgenic expression of group V, but not group X, secreted phospholipase A2 in mice leads to neonatal lethality because of lung dysfunction, J Biol Chem, 281 (2006) 36420–36433. [DOI] [PubMed] [Google Scholar]
- [35].Satake Y, Diaz BL, Balestrieri B, Lam BK, Kanaoka Y, Grusby MJ, Arm JP, Role of group V phospholipase A2 in zymosan-induced eicosanoid generation and vascular permeability revealed by targeted gene disruption, J Biol Chem, 279 (2004) 16488–16494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Balestrieri B, Hsu VW, Gilbert H, Leslie CC, Han WK, Bonventre JV, Arm JP, Group V secretory phospholipase A2 translocates to the phagosome after zymosan stimulation of mouse peritoneal macrophages and regulates phagocytosis, J Biol Chem, 281 (2006) 6691–6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Balestrieri B, Maekawa A, Xing W, Gelb MH, Katz HR, Arm JP, Group V secretory phospholipase A2 modulates phagosome maturation and regulates the innate immune response against Candida albicans, J Immunol, 182 (2009) 4891–4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Diaz BL, Satake Y, Kikawada E, Balestrieri B, Arm JP, Group V secretory phospholipase A2 amplifies the induction of cyclooxygenase 2 and delayed prostaglandin D2 generation in mouse bone marrow culture-derived mast cells in a strain-dependent manner, Biochim Biophys Acta, 1761 (2006) 1489–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kikawada E, Bonventre JV, Arm JP, Group V secretory PLA2 regulates TLR2-dependent eicosanoid generation in mouse mast cells through amplification of ERK and cPLA2alpha activation, Blood, 110 (2007) 561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Taketomi Y, Ueno N, Kojima T, Sato H, Murase R, Yamamoto K, Tanaka S, Sakanaka M, Nakamura M, Nishito Y, Kawana M, Kambe N, Ikeda K, Taguchi R, Nakamizo S, Kabashima K, Gelb MH, Arita M, Yokomizo T, Nakamura M, Watanabe K, Hirai H, Nakamura M, Okayama Y, Ra C, Aritake K, Urade Y, Morimoto K, Sugimoto Y, Shimizu T, Narumiya S, Hara S, Murakami M, Mast cell maturation is driven via a group III phospholipase A2-prostaglandin D2-DP1 receptor paracrine axis, Nat Immunol, 14 (2013) 554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ohta S, Imamura M, Xing W, Boyce JA, Balestrieri B, Group V secretory phospholipase A2 is involved in macrophage activation and is sufficient for macrophage effector functions in allergic pulmonary inflammation, J Immunol, 190 (2013) 5927–5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Yamaguchi M, Samuchiwal SK, Quehenberger O, Boyce JA, Balestrieri B, Macrophages regulate lung ILC2 activation via Pla2g5-dependent mechanisms, Mucosal Immunol, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Munoz NM, Meliton AY, Arm JP, Bonventre JV, Cho W, Leff AR, Deletion of secretory group V phospholipase A2 attenuates cell migration and airway hyperresponsiveness in immunosensitized mice, J Immunol, 179 (2007) 4800–4807. [DOI] [PubMed] [Google Scholar]
- [44].Sato H, Taketomi Y, Ushida A, Isogai Y, Kojima T, Hirabayashi T, Miki Y, Yamamoto K, Nishito Y, Kobayashi T, Ikeda K, Taguchi R, Hara S, Ida S, Miyamoto Y, Watanabe M, Baba H, Miyata K, Oike Y, Gelb MH, Murakami M, The adipocyte-inducible secreted phospholipases PLA2G5 and PLA2G2E play distinct roles in obesity, Cell Metab, 20 (2014) 119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Svenningsen S, Nair P, Asthma Endotypes and an Overview of Targeted Therapy for Asthma, Front Med (Lausanne), 4 (2017) 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Samuchiwal SK, Boyce JA, Role of lipid mediators and control of lymphocyte responses in type 2 immunopathology, J Allergy Clin Immunol, 141 (2018) 1182–1190. [DOI] [PubMed] [Google Scholar]
- [47].Munoz NM, Meliton AY, Lambertino A, Boetticher E, Learoyd J, Sultan F, Zhu X, Cho W, Leff AR, Transcellular secretion of group V phospholipase A2 from epithelium induces beta 2-integrin-mediated adhesion and synthesis of leukotriene C4 in eosinophils, J Immunol, 177 (2006) 574–582. [DOI] [PubMed] [Google Scholar]
- [48].Giannattasio G, Fujioka D, Xing W, Katz HR, Boyce JA, Balestrieri B, Group V secretory phospholipase A2 reveals its role in house dust mite-induced allergic pulmonary inflammation by regulation of dendritic cell function, J Immunol, 185 (2010) 4430–4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Henderson WR Jr., Ye X, Lai Y, Ni Z, Bollinger JG, Tien YT, Chi EY, Gelb MH, Key role of group v secreted phospholipase A2 in Th2 cytokine and dendritic cell-driven airway hyperresponsiveness and remodeling, PLoS One, 8 (2013) e56172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Martinez FO, Gordon S, The M1 and M2 paradigm of macrophage activation: time for reassessment, F1000Prime Rep, 6 (2014) 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Martinez FO, Helming L, Milde R, Varin A, Melgert BN, Draijer C, Thomas B, Fabbri M, Crawshaw A, Ho LP, Ten Hacken NH, Cobos Jimenez V, Kootstra NA, Hamann J, Greaves DR, Locati M, Mantovani A, Gordon S, Genetic programs expressed in resting and IL-4 alternatively activated mouse and human macrophages: similarities and differences, Blood, 121 (2013) e57–69. [DOI] [PubMed] [Google Scholar]
- [52].Yamaguchi M, Zacharia J, Laidlaw TM, Balestrieri B, PLA2G5 regulates transglutaminase activity of human IL-4-activated M2 macrophages through PGE2 generation, J Leukoc Biol, 100 (2016) 131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Downs SH, Mitakakis TZ, Marks GB, Car NG, Belousova EG, Leuppi JD, Xuan W, Downie SR, Tobias A, Peat JK, Clinical importance of Alternaria exposure in children, Am J Respir Crit Care Med, 164 (2001) 455–459. [DOI] [PubMed] [Google Scholar]
- [54].Salo PM, Arbes SJ Jr., Sever M, Jaramillo R, Cohn RD, London SJ, Zeldin DC, Exposure to Alternaria alternata in US homes is associated with asthma symptoms, J Allergy Clin Immunol, 118 (2006) 892–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Halim TY, Steer CA, Matha L, Gold MJ, Martinez-Gonzalez I, McNagny KM, McKenzie AN, Takei F, Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation, Immunity, 40 (2014) 425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Doherty TA, Khorram N, Lund S, Mehta AK, Croft M, Broide DH, Lung type 2 innate lymphoid cells express cysteinyl leukotriene receptor 1, which regulates TH2 cytokine production, J Allergy Clin Immunol, 132 (2013) 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hallstrand TS, Chi EY, Singer AG, Gelb MH, Henderson WR Jr., Secreted phospholipase A2 group X overexpression in asthma and bronchial hyperresponsiveness, Am J Respir Crit Care Med, 176 (2007) 1072–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Hallstrand TS, Lai Y, Ni Z, Oslund RC, Henderson WR Jr., Gelb MH, Wenzel SE, Relationship between levels of secreted phospholipase A(2) groups IIA and X in the airways and asthma severity, Clin Exp Allergy, 41 (2011) 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Henderson WR Jr., Chi EY, Bollinger JG, Tien YT, Ye X, Castelli L, Rubtsov YP, Singer AG, Chiang GK, Nevalainen T, Rudensky AY, Gelb MH, Importance of group X-secreted phospholipase A2 in allergen-induced airway inflammation and remodeling in a mouse asthma model, J Exp Med, 204 (2007) 865–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Nolin JD, Lai Y, Ogden HL, Manicone AM, Murphy RC, An D, Frevert CW, Ghomashchi F, Naika GS, Gelb MH, Gauvreau GM, Piliponsky AM, Altemeier WA, Hallstrand TS, Secreted PLA2 group X orchestrates innate and adaptive immune responses to inhaled allergen, JCI Insight, 2 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Boyanovsky BB, van der Westhuyzen DR, Webb NR, Group V secretory phospholipase A2-modified low density lipoprotein promotes foam cell formation by a SR-A- and CD36-independent process that involves cellular proteoglycans, J Biol Chem, 280 (2005) 32746–32752. [DOI] [PubMed] [Google Scholar]
- [62].Wooton-Kee CR, Boyanovsky BB, Nasser MS, de Villiers WJ, Webb NR, Group V sPLA2 hydrolysis of low-density lipoprotein results in spontaneous particle aggregation and promotes macrophage foam cell formation, Arterioscler Thromb Vasc Biol, 24 (2004) 762–767. [DOI] [PubMed] [Google Scholar]
- [63].Bostrom MA, Boyanovsky BB, Jordan CT, Wadsworth MP, Taatjes DJ, de Beer FC, Webb NR, Group v secretory phospholipase A2 promotes atherosclerosis: evidence from genetically altered mice, Arterioscler Thromb Vasc Biol, 27 (2007) 600–606. [DOI] [PubMed] [Google Scholar]
- [64].Boyanovsky BB, Shridas P, Simons M, van der Westhuyzen DR, Webb NR, Syndecan-4 mediates macrophage uptake of group V secretory phospholipase A2-modified LDL, J Lipid Res, 50 (2009) 641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Pruzanski W, Kopilov J, Kuksis A, Hydrolysis of lipoproteins by sPLA2's enhances mitogenesis and eicosanoid release from vascular smooth muscle cells: Diverse activity of sPLA2's IIA, V and X, Prostaglandins Other Lipid Mediat, 122 (2016) 64–68. [DOI] [PubMed] [Google Scholar]
- [66].Sato H, Kato R, Isogai Y, Saka G, Ohtsuki M, Taketomi Y, Yamamoto K, Tsutsumi K, Yamada J, Masuda S, Ishikawa Y, Ishii T, Kobayashi T, Ikeda K, Taguchi R, Hatakeyama S, Hara S, Kudo I, Itabe H, Murakami M, Analyses of group III secreted phospholipase A2 transgenic mice reveal potential participation of this enzyme in plasma lipoprotein modification, macrophage foam cell formation, and atherosclerosis, J Biol Chem, 283 (2008) 33483–33497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Boyanovsky B, Zack M, Forrest K, Webb NR, The capacity of group V sPLA2 to increase atherogenicity of ApoE-/- and LDLR-/- mouse LDL in vitro predicts its atherogenic role in vivo, Arterioscler Thromb Vasc Biol, 29 (2009) 532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Boyanovsky BB, Bailey W, Dixon L, Shridas P, Webb NR, Group V secretory phospholipase A2 enhances the progression of angiotensin II-induced abdominal aortic aneurysms but confers protection against angiotensin II-induced cardiac fibrosis in apoE-deficient mice, Am J Pathol, 181 (2012) 1088–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Yano T, Fujioka D, Saito Y, Kobayashi T, Nakamura T, Obata JE, Kawabata K, Watanabe K, Watanabe Y, Mishina H, Tamaru S, Kugiyama K, Group V secretory phospholipase A2 plays a pathogenic role in myocardial ischaemia-reperfusion injury, Cardiovasc Res, 90 (2011) 335–343. [DOI] [PubMed] [Google Scholar]
- [70].Ruiperez V, Astudillo AM, Balboa MA, Balsinde J, Coordinate regulation of TLR-mediated arachidonic acid mobilization in macrophages by group IVA and group V phospholipase A2s, J Immunol, 182 (2009) 3877–3883. [DOI] [PubMed] [Google Scholar]
- [71].Han WK, Sapirstein A, Hung CC, Alessandrini A, Bonventre JV, Cross-talk between cytosolic phospholipase A2 alpha (cPLA2 alpha) and secretory phospholipase A2 (sPLA2) in hydrogen peroxide-induced arachidonic acid release in murine mesangial cells: sPLA2 regulates cPLA2 alpha activity that is responsible for arachidonic acid release, J Biol Chem, 278 (2003) 24153–24163. [DOI] [PubMed] [Google Scholar]
- [72].Suzuki K, Takahashi S, Watanabe K, Fujioka D, Nakamura T, Obata JE, Kawabata K, Katoh R, Matsumoto M, Kugiyama K, The expression of groups IIE and V phospholipase A2 is associated with an increased expression of osteogenic molecules in human calcified aortic valves, J Atheroscler Thromb, 21 (2014) 1308–1325. [DOI] [PubMed] [Google Scholar]
- [73].Vargas-Alarcon G, Posadas-Romero C, Villarreal-Molina T, Alvarez-Leon E, Angeles-Martinez J, Soto ME, Monroy-Munoz I, Juarez JG, Sanchez-Ramirez CJ, Ramirez-Bello J, Ramirez-Fuentes S, Fragoso JM, Rodriguez-Perez JM, The (G>A) rs11573191 polymorphism of PLA2G5 gene is associated with premature coronary artery disease in the Mexican Mestizo population: the genetics of atherosclerotic disease Mexican study, Biomed Res Int, 2014 (2014) 931361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Rubio JM, Rodriguez JP, Gil-de-Gomez L, Guijas C, Balboa MA, Balsinde J, Group V secreted phospholipase A2 is upregulated by IL-4 in human macrophages and mediates phagocytosis via hydrolysis of ethanolamine phospholipids, J Immunol, 194 (2015) 3327–3339. [DOI] [PubMed] [Google Scholar]
- [75].Solodkin-Szaingurten I, Levy R, Hadad N, Differential behavior of sPLA2-V and sPLA2-X in human neutrophils, Biochim Biophys Acta, 1771 (2007) 155–163. [DOI] [PubMed] [Google Scholar]
- [76].Munoz NM, Meliton AY, Meliton LN, Dudek SM, Leff AR, Secretory group V phospholipase A2 regulates acute lung injury and neutrophilic inflammation caused by LPS in mice, Am J Physiol Lung Cell Mol Physiol, 296 (2009) L879–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Lapointe S, Brkovic A, Cloutier I, Tanguay JF, Arm JP, Sirois MG, Group V secreted phospholipase A2 contributes to LPS-induced leukocyte recruitment, J Cell Physiol, 224 (2010) 127–134. [DOI] [PubMed] [Google Scholar]
- [78].Degousee N, Kelvin DJ, Geisslinger G, Hwang DM, Stefanski E, Wang XH, Danesh A, Angioni C, Schmidt H, Lindsay TF, Gelb MH, Bollinger J, Payre C, Lambeau G, Arm JP, Keating A, Rubin BB, Group V phospholipase A2 in bone marrow-derived myeloid cells and bronchial epithelial cells promotes bacterial clearance after Escherichia coli pneumonia, J Biol Chem, 286 (2011) 35650–35662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Boilard E, Lai Y, Larabee K, Balestrieri B, Ghomashchi F, Fujioka D, Gobezie R, Coblyn JS, Weinblatt ME, Massarotti EM, Thornhill TS, Divangahi M, Remold H, Lambeau G, Gelb MH, Arm JP, Lee DM, A novel anti-inflammatory role for secretory phospholipase A2 in immune complex-mediated arthritis, EMBO Mol Med, 2 (2010) 172–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Kennedy BP, Payette P, Mudgett J, Vadas P, Pruzanski W, Kwan M, Tang C, Rancourt DE, Cromlish WA, A natural disruption of the secretory group II phospholipase A2 gene in inbred mouse strains, J Biol Chem, 270 (1995) 22378–22385. [DOI] [PubMed] [Google Scholar]
- [81].Xue M, Shen K, McKelvey K, Li J, Chan YK, Hatzis V, March L, Little CB, Tonkin M, Jackson CJ, Endothelial protein C receptor-associated invasiveness of rheumatoid synovial fibroblasts is likely driven by group V secretory phospholipase A2, Arthritis Res Ther, 16 (2014) R44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Shridas P, Noffsinger VP, Trumbauer AC, Webb NR, The dual role of group V secretory phospholipase A2 in pancreatic beta-cells, Endocrine, 58 (2017) 47–58 [DOI] [PMC free article] [PubMed] [Google Scholar]