SUMMARY.
To investigate the effect of hepatitis B virus (HBV) infection on the development of diabetes mellitus (DM), we compared DM incidence and characteristics of Alaska Native persons with and without HBV infection. From 1990 to 2010, there were 52 incident DM cases among 1309 persons with infection vs 4557 DM cases among 85 698 persons without infection (log-rank test, P = 0.20). Compared to infected persons without DM, those with DM were significantly older (57.0 vs 47.4 years, P < 0.001) and had higher body mass index (34.5 vs 28.4 kg/m2, P < 0.001). Genotype, immune active disease and the presence of cirrhosis were not associated with DM. In this population-based cohort with over 20 years of follow-up, there was no effect of HBV infection on DM development.
Keywords: diabetes, hepatitis B, incidence, liver disease
INTRODUCTION
While a relationship between chronic hepatitis C virus (HCV) infection and type 2 diabetes mellitus (DM) has been established, the effect of chronic hepatitis B virus (HBV) infection on the development of insulin resistance and DM is less clear [1–3]. Chronic HBV infection has been a significant health disparity for Alaska Native persons, approximately 1600 (3%) of whom were found to be chronically infected when state-wide hepatitis B screening programmes were conducted in the 1980s [4]. Although there is no systematic screening for elevated glucose levels among Alaska Native persons, an active diabetes registry has been operative in Alaska for more than 25 years, and persons who fit the American Diabetic Association criteria for DM have been entered into this registry and actively monitored. The coexistence of the DM and HBV registries for the Alaska Native population provides a unique opportunity to study the relationship between DM and chronic HBV infection in a population-based setting. To determine whether the presence of chronic HBV infection was associated with the subsequent development of DM, we compared the incidence of DM over a 20-year period among Alaska Native persons with chronic HBV infection with that of the overall Alaska Native population, as well as demographic, clinical and virologic characteristics of HBV-infected Alaska Native persons with and without DM.
METHODS
Alaska Native persons with chronic HBV infection (positive hepatitis B surface antigen >6 months) receive care in the Alaska Native Tribal Health Consortium (ANTHC) Liver Disease and Viral Hepatitis Program. Liver function tests and HBV DNA levels are performed twice annually on all chronic hepatitis B patients. New cases are ascertained for the diabetes registry by searching patient visits in the ANT-HC electronic medical record for International Classification of Disease, Ninth revision codes for DM (types 1 and 2), gestational DM and any abnormal glucose. Cases and dates of diagnosis are verified with laboratory evidence. To identify persons in both cohorts, ANTHC staff conducted an electronic match of persons aged ≥ 18 years listed both in the DM and the chronic hepatitis B registries. Persons with HCV or HIV infection were excluded from the analyses. A ‘match’ was an adult who was listed in both registries for any length of time during 1990 through 2010.
To examine trends in DM incidence among Alaska Native persons with HBV infection vs Alaska Native persons in general, we used the 1990 populations of each group and compared the number of new DM cases diagnosed in each of 1990 cohorts over the ensuing 21 calendar years (i.e. 1990–2010). Demographic, clinical and virologic data were compared among persons who were a match (persons with chronic HBV and DM) and those in the chronic HBV registry who were not a match (persons with chronic HBV infection only), for the years 1990– 2010. Persons in the HBV infection cohort who developed type 1 DM, gestational DM or prediabetes during 1990–2010 were excluded from the analysis.
All analyses were conducted using SAS (Enterprise Guide 4.2, SAS Institute Inc. Cary, NC). The LIFETEST procedure was applied to produce Nelson–Aalen cumulative hazard in DM for the groups with and without HBV infection and the log-rank test was used to compare hazards. The t-test was used for comparison of means, and the Pearson’s chi-square or Fisher’s exact tests were used for the comparison of proportions. The study was approved by the CDC institutional review board and the Alaska Area Institutional Review Board. It received tribal approval through ANTHC and the Southcentral Foundation.
RESULTS
In 1990, there were 1309 Alaska Native persons with chronic HBV infection and no previous DM diagnosis. The Alaska Native population in 1990 of 85 698 persons constituted the cohort for assessment of DM incidence in the overall population during the ensuing 21 years. From 1990 to 2010, there were 52 patients in the matched DM-HBV infection data set and 1167 patients in the chronic hepatitis registry without DM (25 599 patient years of chronic hepatitis B follow-up).
The cumulative hazard of DM among Alaska Native persons with and without chronic HBV infection from 1990 to 2010 is shown in Fig. 1. There were 52 incident DM cases among 1309 persons with HBV infection and 4557 incident DM cases among 85 698 persons without infection. Although the cumulative hazard (DM incidence) was consistently less among Alaska Native persons with HBV infection compared with Alaska Native persons in general, the difference was not significant (log-rank test, P = 0.20).Compared with HBV-infected persons without DM, those with DM were significantly older (mean age 57.0 years vs 47.4 years, P < 0.001) and had higher BMI (34.5 vs 28.4 kg/m2, P < 0.001). Otherwise, there were no significant differences between those with or without DM with regard to gender, highest ALT level, phase of disease (immune active, immune tolerant, or inactive), the presence of cirrhosis or hepatocellular carcinoma (HCC) and HBV genotype (Table 1). In addition, the proportion of deceased persons (from any cause and from liver disease) was not significantly different between chronically infected persons with or without DM. In multivariable analysis, the only characteristics independently associated with DM were higher age group (adjusted odds ratio [aOR] 4.4 for age group 45–64 years and aOR 6.1 for age group ≥ 65 years, relative to age <45 years) and higher BMI (aOR 2.9 for BMI 25–30 kg/m2 and aOR 8.5 for BMI >30 kg/m2, relative to BMI <25 kg/m2). Neither gender, phase of disease or the presence of cirrhosis was independently associated with DM.
Fig. 1.
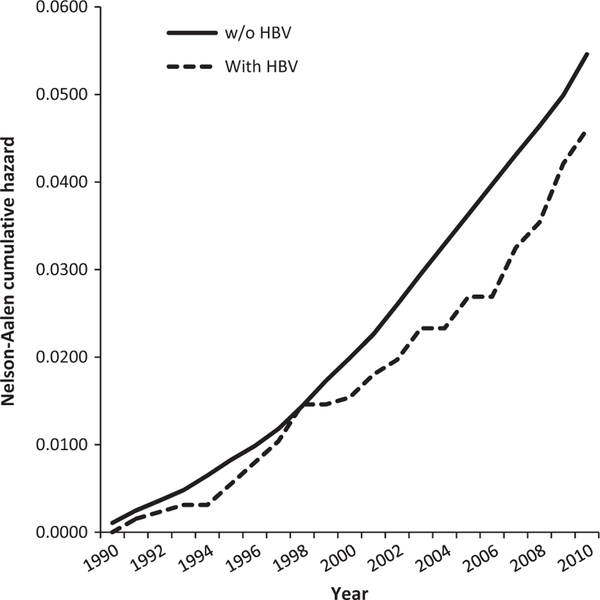
Nelson–Aalen cumulative hazard for type 2 diabetes among Alaska Native persons with and without hepatitis B virus infection, 1990–2010 (log-rank test, P = 0.20). From 1990 through 2010, there were 52 incident diabetes cases among 1309 persons with hepatitis B virus infection and 4557 incident diabetes cases among 85 698 persons without infection.
Table 1.
Demographic and clinical characteristics of Alaska Native persons with chronic hepatitis B with (n = 52) and without (n = 1167) type 2 diabetes mellitus, 1990–2010
| Diabetes (n = 52) | No diabetes (n = 1167) | P-value* | |
|---|---|---|---|
| Age, mean (years) | 57.0 | 47.4 | <0.001 |
| Gender | |||
| Male | 34 (65.4%) | 694 (59.5%) | 0.471 |
| Female | 18 (34.6%) | 473 (40.5%) | 0.471 |
| Body mass index, mean (kg/m2) | 34.5 | 28.4 | <0.001 |
| Clinical features | |||
| Highest alanine aminotransferase, mean | 198 | 138 | 0.276 |
| Inactive carrier† | 6 (11.5%) | 185 (15.9%) | 0.558 |
| Immune active carrier‡ | 12 (23.1%) | 225 (19.3%) | 0.477 |
| Alanine aminotransferase >40 and HBV DNA ≤ 2000 | 29 (55.8%) | 470 (40.3%) | 0.03 |
| Cirrhosis§ | 3 (5.8%) | 41 (3.5%) | 0.43 |
| Hepatocellular carcinoma | 2 (3.8%) | 28 (2.4%) | 0.369 |
| Genotype | |||
| A | 5 (9.6%) | 113 (9.7%) | 1.0 |
| B | 3 (5.8%) | 35 (3.0%) | 0.218 |
| C | 1 (1.9%) | 59 (5.1%) | 0.511 |
| D | 23 (44.3%) | 498 (42.7%) | 0.886 |
| F | 7 (13.4%) | 179 (15.3%) | 0.845 |
| Missing | 13 (25.0%) | 283 (24.2%) | — |
| Deceased | |||
| Any cause | 9 (17.3%) | 248 (21.2%) | 0.603 |
| Liver disease | 1 (11.0%) | 12 (4.8%) | 0.407 |
P-value: comparison of two means using t-test; comparison of two proportions using Pearson’s chi-square test. With cell counts <5, Fisher’s exact test was applied.
Alanine aminotransferase ≤ 40, HBV DNA ≤ 2000.
Alanine aminotransferase >40, HBV DNA >2000.
Determined by clinical signs.
There were an insufficient number of available liver biopsy results among chronically infected persons with DM to permit comparisons with biopsy results of those without DM (6 DM vs 105 non-DM biopsies). In univariate analysis, there was a significantly higher proportion of chronically infected persons with DM with maximum ALT >40 and maximum HBV DNA ≤ 2000 compared to those without DM (55.8 vs 40.3%, P = 0.03). The determination of whether this difference was attributable to nonalcoholic fatty liver disease (NAFLD) or to some other cause (e.g. alcohol intake) was not feasible, and most patients did not have sufficient information in their records to make the diagnosis of the metabolic syndrome.
DISCUSSION
In this population-based study with over 20 years of follow-up, we found no significant difference in the incidence of DM among Alaska Native persons with chronic HBV infection compared with the overall Alaska Native population. Among those with chronic HBV infection, DM was associated only with older age, higher BMI and elevated ALT in the presence of low viremia; there was no association with gender, immune active hepatitis, the presence of cirrhosis or HCC or HBV genotype. While some studies have reported a relationship between HBV infection and the development of insulin resistance [3,5,6], our data suggested neither a direct effect of chronic HBV infection on the subsequent development of DM, nor an association of DM with any particular manifestation or progression of HBV-related chronic liver disease. With respect to the association between DM and the presence of abnormal ALT levels in the absence of significant viremia (i.e. maximum ALT >40 and HBV DNA ≤ 2000), we could not confirm that these ALT elevations were the product of NAFLD or some other cause, such as alcoholic liver disease, because we did not have sufficient biopsy data, and we did not have information on alcohol intake.
Our study was retrospective, and we did not have complete laboratory, clinical and biopsy data for all persons in the HBV cohort. Thus, a precise determination of the extent of NAFLD and the degree to which DM-afflicted persons had evidence of fibrosis was not feasible. Alaskan Native persons with undiagnosed DM would not be included in the registry, and characteristics of the Alaska Native population could exist that would render our findings less applicable to other populations. Finally, our results are limited by sample size, given the relatively small number of patients with HBV infection and DM despite our large sample size of HBV-infected persons without DM.
Nonetheless, in this population-based cohort with two decades of follow-up, Alaska Native persons with chronic HBV infection were no more likely to develop DM than Alaska Native persons in general. Continued follow-up of this cohort and longitudinal studies of other cohorts HBV-infected persons, with the inclusion of complete biopsy, laboratory and clinical data, may provide greater insight on the relationship between DM, metabolic syndrome and the manifestations of liver disease among persons with HBV infection. If such a relationship exists, studies will be warranted to determine whether treatment of metabolic syndrome and DM, in addition to treatment of HBV infection, may ameliorate the progression of liver disease.
Acknowledgments
FUNDING
United States Centers for Disease Control and Prevention.
Abbreviations:
- ANTHC
Alaska Native Tribal Health Consortium
- aOR
adjusted odds ratio
- CDC
Centers for Disease Control and Prevention
- DM
diabetes mellitus
- HBsAg
hepatitis B surface antigen
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- NAFLD
nonalcoholic fatty liver disease
Footnotes
CONFLICT OF INTEREST
None.
REFERENCES
- 1.Huang ZS, Huang TS, Wu TH, Chen MF, Hsu CS, Kao JH. Asymptomatic chronic hepatitis B virus infection does not increase the risk of diabetes mellitus: a ten-year observation. J Gatroenterol Hepatol 2010; 25: 1420–1425. [DOI] [PubMed] [Google Scholar]
- 2.Li-Ng M, Tropp S, Danoff A, Bini EJ. Association between chronic hepatitis B virus infection and diabetes among Asian Americans and Pacific Islanders. Dig Liver Dis 2007; 39: 549–556. [DOI] [PubMed] [Google Scholar]
- 3.Lao TT, Tse KY, Chan LY, Tam KF, Ho LF. HBsAg carrier status and association between gestational diabetes with increased serum ferritin concentration in Chinese women. Diabetes Care 2007; 26: 3011–3016. [DOI] [PubMed] [Google Scholar]
- 4.McMahon BJ, Schoenberg S, Bulkow L et al. Seroprevalence of hepatitis B viral markers in 52,000 Alaska Natives. Am J Epidemiol 1993; 138: 544–549. [DOI] [PubMed] [Google Scholar]
- 5.Luo B, Wang Y, Wang K. Association of metabolic syndrome and hepatitis B infection in a Chinese population. Clin Chim Acta 2007; 380: 238–240. [DOI] [PubMed] [Google Scholar]
- 6.Alizadeh AHM, Fallahian F, Alavian SM et al. Insulin resistance in chronic hepatitis B and C. Indian J Gastroenterol 2006; 25: 286–289. [PubMed] [Google Scholar]


