Abstract
Microbicidal nitric oxide (NO) production is reliant on inducible NO synthase (iNOS)- mediated L-arginine metabolism in macrophages (MΦs). L-arginine supply, however, can be restricted by arginase activity, resulting in inefficient NO and inhibition of anti-microbial MΦ function. MΦs circumvent this by converting L-citrulline to L-arginine, thereby resupplying substrate for NO production. Here we define the metabolic signature of mycobacteria-infected murine MΦs supplied L-arginine, L-citrulline, or both amino acids (AAs). Using liquid chromatography tandem mass spectrometry (LC-MS/MS), we determined L-arginine synthesized from L-citrulline was less effective as a substrate for arginase-mediated L-ornithine production as compared to L-arginine directly imported from the extracellular milieu. Following Mycobacterium bovis BCG infection and co-stimulation with interferon-γ (IFN-γ), we observed MΦ arginase activity did not inhibit production of NO derived from L-citrulline, contrary to NO inhibition witnessed when MΦs were cultured in L-arginine. Furthermore, we found arginase-expressing MΦs preferred L-citrulline, as compared to L-arginine, to promote anti-mycobacterial activity. We expect defining the consequences of L-citrulline metabolism in MΦs will provide novel approaches for enhancing immunity, especially in the context of mycobacterial disease.
Introduction
Leukocytes are readily equipped to defend against invading pathogens. One such defense mechanism, NO, is produced by MΦs in high concentrations in response to pathogen associated molecular patterns in combination with IFN-γ produced by innate and/or adaptive effector lymphocytes. This defensive mechanism is crucial in mice infected with mycobacterial species. A loss of function mutation in Nos2, which encodes iNOS, results in increased lethality to Mycobacterium tuberculosis (Mtb) and M. bovis (Mb) BCG (1, 2). The contribution of iNOS in human immunity remains less defined than in mice. For instance, many have shown human MΦs to make significantly less NO as mouse MΦs in similar in vitro conditions (3). However, in vivo evidence suggests some protective role for iNOS in human tuberculosis (TB). Genome- wide association studies link NOS2 with increased incidence of TB (4–8), although the expression and/or activity of iNOS resulting from these polymorphisms are unknown. Still, others have described that iNOS and NO production in TB patients correlates with less severe disease, and its production by human alveolar MΦs ex vivo inhibits mycobacterial growth (9–15).
iNOS catalyzes the generation of NO in MΦs by converting the AA L-arginine to L-citrulline. This process can be interrupted by arginase activity, which hydrolyzes L-arginine to L-ornithine and urea, thereby competing with iNOS for bioavailable L-arginine. Similar to iNOS, Argl (type 1 arginase) is induced in human MΦs within TB granulomas and in the lungs of mice infected with Mb BCG (16–19), suggesting substrate competition for L-arginine in vivo. Indeed, mice lacking Arg1 in MΦs make more NO and clear Mtb faster than control mice (20).
As L-arginine becomes limiting, MΦs replenish intracellular stores of L-arginine via L-citrulline metabolism (21). We have recently described that mice unable to convert L-citrulline to L-arginine in their hematopoietic compartment produce less NO and are more susceptible to Mtb and Mb BCG infection (22). These data led us to ask if synthesized L-arginine had similar metabolic consequences as imported L-arginine in mycobacteria-infected MΦs. Here, we describe that synthesized L-arginine from L-citrulline has increased availability for NO production versus conversion to L-ornithine, as compared to imported L-arginine. Moreover, we show that the combination of both L-arginine and L-citrulline provides MΦs with an elevated anti-mycobacterial potential than either AA alone.
Methods and Materials
Mice.
C57BL/6 and Aslflox/flox;Tie2-cre mice (and controls) were bred within the Division of Veterinary Services at CCHMC. Strains were obtained from The Jackson Laboratories (C57BL/6J, 000664; B6.129S7-Asltm1Brle/J, 018830; B6.Cg-Tg(Tek-cre)1Ywa/J, 008863). Procedures were approved by the IACUC at CCHMC.
Tissue Culture.
Complete Dulbecco’s Modified Eagles Media (C-DMEM, 400 μM L-arginine, 10–013-CV, Cellgro, Corning Life Sciences) was prepared by adding bovine calf serum (SH30073.03, Thermo Fisher Scientific) to 10%, and penicillin / streptomycin (15140–122, Gibco, Life Technologies) to 1% final concentration. L-arginine-free C-DMEM (R-free C- DMEM, A14431–01, Gibco, Life Technologies) was prepared by adding dialyzed fetal bovine serum (35–071-CV, Cellgro, Corning Life Sciences) to 10% final concentration. L-arginine and L-citrulline were prepared at a stock concentration of 100 mM in sterile water. L-arginine and L-citrulline stocks were added to R-free C-DMEM to concentrations noted in the text. Cells were cultured in a humidified atmosphere at 37°C plus 5% CO2.
Macrophages.
Inflammatory peritoneal-derived MΦs (PDMs): Mice were administered 1 ml sterile thioglycollate (R064710, Thermo Fischer Scientific) by intraperitoneal injection. After 4 days peritoneal cells were collected by lavage. Following red cell lysis, cells were plated on tissue culture plastic in C-DMEM. C-DMEM and non-adherent cells were aspirated after 4 hours, and fresh R-free C-DMEM was added with L-arginine and/or L-citrulline. Bone marrow- derived MΦs (BMDMs): Bone marrow was flushed from femurs and tibias and re-suspended in BM-C-DMEM (10% FBS, 1% P/S, 40 ng/ml human M-CSF (gift from P. Murray, St. Jude Children’s Research Hospital, Memphis, TN)). Bone marrow was plated on tissue culture plastic (2× 75 cm2 flasks per mouse, 15 ml per flask) for 5–7 days. 5 ml fresh BM-C-DMEM was added every 48 hours until harvest. BMDMs were collected by scraping and cells were plated on tissue culture plastic in BM-C-DMEM. The following morning, media was aspirated and fresh R-free C-DMEM was added with L-arginine and/or L-citrulline. In all experiments, initial culture in L-arginine containing C-DMEM did not result in appreciable amounts of extracellular or intracellular L-arginine or L-citrulline after reconstituting with R-free C-DMEM (data not shown), so this method was utilized to conform to previously published methods (19, 20, 22)
Mycobacterium bovis BCG.
M. bovis bacillus Calmette-Guérin Pasteur strain (gift from P. Murray, St. Jude Children’s Research Hospital, Memphis, TN) was cultured in 7H9 (M0178, Sigma-Aldrich) plus OADC (R450605, Thermo Fisher Scientific) containing 0.05% tween-80 (P4780, Sigma-Aldrich) at 37°C shaking ~50 r.p.m. Cultures were used for infection within 5–14 days following thaw. Bacilli were washed twice with sterile PBS and passed through a 40 μm strainer. A600 = 0.3 by spectrophotometric analysis consistently resulted in 1×107 CFUs per 100 μl volume. Multiplicity of infection (moi) ranged from 1 to 5 bacilli per MΦ.
Liquid chromatography tandem mass spectrometry (LC-MS/MS).
Intracellular: 5×106 to 6×106 cells were washed twice with PBS and then lysed in 1 mL ice-cold methanol. Cell debris was pelleted and methanol lysates were collected. Extracellular: Supernatants from above cells were collected and mixed (1:1, v/v) with ice-cold methanol. AAs were measured by LC-MS/MS with selected-reaction-monitoring (SRM) and with the use of stable isotopic-labeled AAs as internal standards. Samples were analyzed with the LC20AD HPLC system (Shimadzu) coupled to the TSQ Quantum Ultra™ Triple Quadrupole Mass Spectrometer (Thermo Scientific). Chromatographic separation of AAs was achieved on a 50 × 2.1mm Atlantis HILIC column (Waters). A gradient mobile phase was used with a binary solvent system, which changed from 90% mobile phase B (95% acetonitrile/5% water/0.1% formic acid/1.5 mM ammonium formate) to 28% mobile phase A (water/0.1% formic acid/1.5 mM ammonium formate) at a flow rate of 0.6 ml/minute. The total run time was 10 minutes and the injection volume was 10 μL. The optimal signal for the analytes was achieved in positive ion mode with the instrument settings: spray voltage: 4 kV; Sheath gas pressure: 35; Auxiliary gas flow: 10; and capillary temperature: 350°C. Argon was used as the collision gas. Data were acquired and processed with Xcalibur™ 2.2 (Thermo Scientific).
RNA analysis.
RNA was collected using Trizol (Invitrogen). cDNA was synthesized using SuperScript II reverse transcriptase (Invitrogen) and analyzed by SybrGreen (Applied Biosytems) qRT-PCR.
Protein analysis.
Protein RIPA lysates were separated by Tris-HCl buffered 4–15% gradient SDS-PAGE, followed by transfer to Protran membranes. Membranes were analyzed by Ponceau S staining and then blocked in 3% milk in Tris-buffered saline plus 0.05% Tween followed by immunoblot to detect Asl (PA5–22300, Thermo Scientific) and Grb2 (610112, BD Biosciences).
Griess Assay.
Equal volumes of cell culture supernatant and Griess reagent were mixed in a 96 well plate. Sodium nitrite (237213, Sigma-Aldrich) was used as a standard. Absorbance values were measured at A492 using a DTX-880 Mutltimode plate reader and detection software (Beckman Coulter).
Colony Forming Unit (CFU) analysis.
Media was removed, and cells were washed with sterile PBS followed by aspiration to remove extracellular Mb BCG. Following wash, MΦs were lysed in water containing 1% IGEPAL (18896, Sigma-Aldrich) for 10 minutes at 37°C. Serial dilutions were plated on 7H10 agar (262710, BD Biosciences) containing OADC. Agar plates were incubated at 37°C for 14–18 days prior to counting colonies.
Reagents.
Lipopolysaccharide (LPS) from Escherichia coli 0111 :B4 (L3012), L-arginine (A8094), and L-citrulline (C7629) were obtained from Sigma-Aldrich; IFN-γ (14–8311-63), IL-4 (14–8041-62), and IL-10 (14–8101-62) were obtained from eBioscience; BEC (S-(2- boronoethyl)-L-cysteine) was obtained from Cayman Chemical; 1,2,3,4,5-13C5 L-citrulline; 1,2,3,4,5-13C5 L-arginine, 13C5 L-ornithine, 13C6 15N4 L-arginine, ureido 13C L-citrulline, and 1,2- 13C2 L-ornithine were obtained from Cambridge Isotope Laboratories, Inc.
Primers.
Asl (forward: 5’ ACTCTTGGAGGTGCAGAAGC 3’, reverse: 5’ AGTAGCTCCCGGTCCACAC 3 ); Gapdh (forward: 5’ GGTGCTGAGTATGTCGTGGA 3’, reverse: 5’ CGGAGATGATGACCCTTTTG 3).
Statistics.
Error bars represent the standard deviation (SD) from the mean. Data were analyzed for statistical significance by Student’s t test and p values are represented as * p < 0.05, ** p < 0.01, and *** p < 0.001.
Results
Synthesized L-arginine is sequestered for iNOS-mediated metabolism
We first sought to determine if differences exist in metabolism of distinct intracellular Larginine sources in mycobacteria-infected MΦs. Thioglycollate-elicited peritoneal-derived MΦs (PDMs) were utilized to address this question due to their increased basal arginase activity as compared with other MO sources, and the subsequent implications this activity may have on anti-mycobacterial NO production. PDMs were cultured in media containing equimolar amounts of L-arginine, L-citrulline, or both AAs followed by Mb BCG infection. Analysis of extra-and intracellular AA contents revealed that MΦs cultured in L-citrulline lacked L-ornithine production to amounts observed from MΦs cultured in L-arginine (Fig. 1A, B). This effect was most pronounced when infected MOs were also stimulated with IFN-γ. Similar differences in L-ornithine synthesis were observed in PDMs and bone marrow-derived MΦs (BMDMs) co-stimulated with IFN-γ and the Toll-like receptor agonist, LPS (Supp. Fig. 1A, B). L-citrulline has been reported to inhibit arginase activity (23–25), yet we did not observe this effect as MΦs cultured in L-arginine alone, or in combination with L-citrulline, produced similar amounts of L-ornithine in all stimulation conditions (Supp. Fig. 1A, B). When AAs were lowered to 0.2 mM (within physiological ranges), L-arginine-cultured MΦs converted nearly all the L-arginine to L-ornithine or L-citrulline following infection (Fig. 1C, D). As with cells cultured in 1 mM AAs, L-citrulline resulted in less L-ornithine synthesis than L-arginine, suggesting imported L-arginine is the preferred substrate for L-ornithine synthesis as compared to L-arginine synthesized intracellularly from L-citrulline. This was further confirmed when infected MΦs were cultured in normal (12C5) L-arginine and heavy isotope (13C5) L-citrulline to detect the source of L-ornithine (Fig. 2A). Greater than 90% of all L-ornithine detected within or released from Mb BCG-infected MΦs with IFN-γ stimulation contained the 12C label whether cultured in 1 mM (Fig. 2B, C) or 0.2 mM (Fig. 2D, E) AAs.
Figure 1.
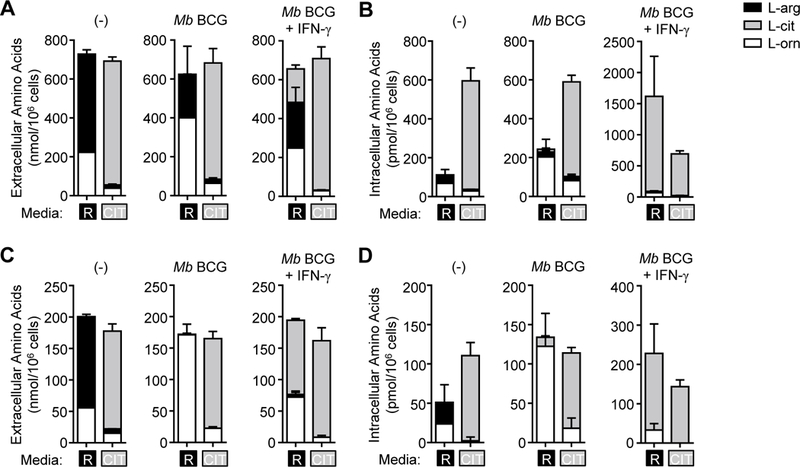
L-arginine and L-citrulline metabolism in mycobacteria-infected MΦs. (A-D) PDMs were cultured in R-free C-DMEM containing L-arginine [R] or L-citrulline [CIT] (1.0 mM (A, B) or 0.2 mM (C, D)), and were infected with Mb BCG (moi = 5) with and without IFN-γ stimulation for 48 hours. Uninfected PDMs (–) were also cultured for 48 hours. Supernatants (A, C)and cell lysates (B, D) were analyzed by LC-MS/MS to detect L-arginine, L-citrulline, and L- ornithine (n = 4). Data are combined from 2 experiments. Error bars, SD.
Figure 2.
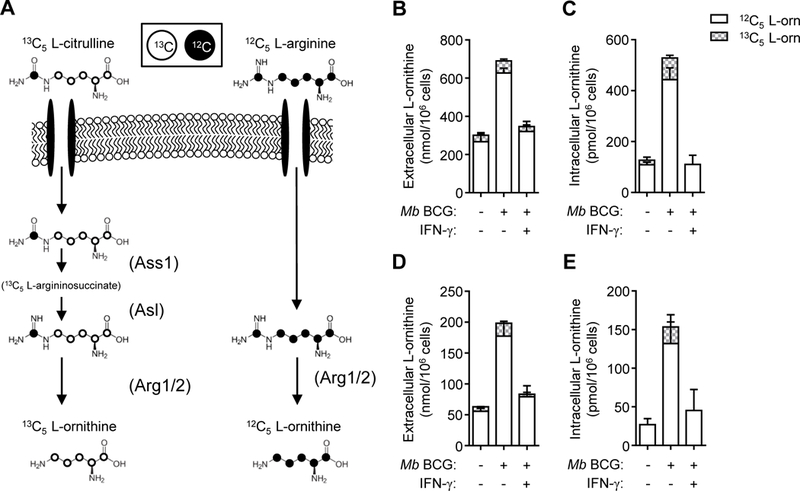
L-ornithine synthesis from L-arginine or 13C5 L-citrulline. (A) Schematic of normal (12C5) L-ornithine synthesis from L-arginine (right) and heavy (13C5) L-ornithine synthesis from L-citrulline (left). Enzymes are depicted in parentheses. (B-E) PDMs, cultured in media containing L-arginine and heavy 13C5 isotope L-citrulline (1.0 mM (B, C) or 0.2 mM (D, E)), were infected with Mb BCG (moi = 5) with and without IFN-y stimulation for 48 hours. Uninfected PDMs (–) were also cultured for 48 hours. Supernatants (B, D) and cell lysates (C, E) were analyzed by LC-MS/MS to detect native (12C5) and heavy (13C5) L-ornithine (n = 4). Data are combined from 2 experiments. Error bars, SD.
It was possible that the decrease in L-ornithine from synthesized L-arginine was entirely due to insufficient metabolism of L-citrulline to L-arginine, so we next analyzed NO production. We detected less NO from infected MΦs cultured in L-citrulline (Fig. 3A, B); however, the deficiency in total L-ornithine production was even larger (Fig. 3C, D). When comparing the NO:L-ornithine production ratio, we found MΦs cultured in L-citrulline were more likely to produce NO versus L-ornithine as compared to those cultured in L-arginine at both 1 mM and 0.2 mM conditions (Fig. 3E, F, Supp. Fig. 1C-F). Taken together, our data indicate a preferential use of L-arginine synthesized from L-citrulline by iNOS that may not be impacted by arginase- mediated inhibition of NO and NO-mediated anti-mycobacterial MΦ functions.
Figure 3.

Differential NO and L-ornithine production in mycobacteria-infected MΦs cultured in L-arginine or L-citrulline. (A-F) Mycobacteria-infected PDMs with and without IFN-γ stimulation were cultured in L-arginine, L-citrulline, or both AAs at 1.0 mM (A, C, E) or 0.2 mM (B, D, F). (A, B) NO production was determined by analyzing supernatant nitrite (NO2-) amounts by Griess assay (n = 8). (C, D) Total L-ornithine combining intracellular and extracellular amounts. (E, F) The ratio of NO2- to L-ornithine was determined by dividing the concentration of NO2- by L-ornithine (intracellular plus extracellular). Data are presented as fold change compared to MΦs cultured in L-arginine media (n = 4). Data are combined from 2 experiments. Error bars, SD. * p < 0.05, *** p < 0.001 by Student’s t test.
L-arginine synthesis is necessary for L-citrulline-mediated anti-mycobacterial MΦ function
Because MΦs cultured in L-citrulline displayed differential NO and L-ornithine production compared to those cultured in L-arginine, we next evaluated if metabolism of L-citrulline to L-arginine was necessary to elicit these effects. The sole known pathway of L-arginine synthesis from L-citrulline is mediated by the sequential action of argininosuccinate synthase 1 (Assl) and argininosuccinate lyase (Asl). Assl converts L-citrulline to L- argininosuccinate, and Asl mediates L-arginine synthesis from L-argininosuccinate. To determine if this metabolism was necessary for L-citrulline-mediated NO production and anti- mycobacterial MO function, mice were generated to facilitate conditional deletion of Asl in hematopoietic cells by crossing Aslflox/flox mice (26) with Tie2-cre mice (Aslflox/flox;Tie2-cre) (Fig. 4A). As compared to control mice, PDMs from Aslflox/flox;Tie2-cre mice displayed markedly diminished Asl mRNA and protein (Fig. 4B, C). PDMs were then harvested from Aslflox/flox;Tie2- cre and control mice to characterize the effect of Asl deletion on anti-mycobacterial functions. MΦs from both groups displayed similar NO production and reduction in live Mb BCG CFUs when cultured in L-arginine media (Fig. 4D, E). NO production and anti-mycobacterial function from control PDMs cultured in L-citrulline mirrored that found from MΦs cultured in L-arginine. However, Aslflox/flox;Tie2-cre PDMs cultured in L-citrulline did not produce NO or decrease Mb BCG burden, similar to our previous report analyzing MΦs lacking functional Assl (22). These data confirm that conversion of L-citrulline to L-arginine is required for L-citrulline-mediated anti-mycobacterial MΦ function.
Figure 4.

L-arginine synthesis is required for L-citrulline-mediated NO production and mycobacterial control. (A) Schematic of Asl gene disruption and location of qRT-PCR primers in Aslflox conditional knock out mice. The AslΔ allele forms following cre-mediated recombination. (B- C) qRT-PCR (B) and immunoblot (C) of Asl from unstimulated PDMs (n = 4) of indicated mice. Immunoblot of Grb2 and Ponceau S staining are shown as loading controls. (D-E) PDMs (n ≥ 8) cultured in R-free C-DMEM containing 1.0 mM L-arginine [R] or 1.0 mM L-citrulline [CIT] were infected with Mb BCG (moi = 1) with and without IFN-γ. At 72 hours post-infection/stimulation, NO was determined by Griess assay (D). Data are the mean NO2- amounts. Mb BCG colony forming units (CFU) were determined from lysed MOs at 72 hours post-infection/stimulation (E). Data are the individual CFU. Black line represents the mean. Data are representative of two (B, C) or combined from at least two of three experiments (D, E). Error bars, SD. ** p < 0.01, *** p < 0.001 by Student’s t test. ns, not statistically significant.
Synthesized L-arginine enhances mycobactericidal MΦ activity
Thus far, our data have shown a significantly larger NO:L-ornithine ratio from MΦs that use synthesized L-arginine from L-citrulline as compared to those utilizing imported L-arginine. This suggests that MΦ polarization prior to infection – altering the balance of iNOS versus arginase activity – may dictate the anti-mycobacterial potency of L-citrulline. Further, in IL-4 + IL-10 polarized MOs, the use of L-citrulline as a source of L-arginine may bypass arginase-mediated inhibition of iNOS function and elicit better anti-mycobacterial immunity. This method of polarization is likely relevant in vivo as Mtb-infected patients with elevated type 2 cytokines have increased prevalence of TB (27–29). To test this, PDMs were pre-stimulated with IL-4 + IL-10 for 24 hours prior to infection to further induce Argl activity. PDMs were washed following pre-stimulation to remove residual cytokines. Upon infection, PDMs were stimulated with IFN-γ with or without the arginase inhibitor BEC in culture containing 1 mM L-arginine, L-citrulline, or both AAs (Fig. 5A). We found that pre-treatment of PDMs with IL-4 + IL-10 did not have a dramatic effect on NO production or recovered Mb BCG. Furthermore, PDMs treated with BEC did not drastically increase NO production, or result in any significant change in Mb BCG CFUs (Fig. 5B, C). We expected this phenomenon might be due to supraphysiological concentrations of L-arginine and L-citrulline, so we modified subsequent experiments to contain 0.2 mM L-arginine and L-citrulline. In contrast to PDMs cultured in supraphysiological Larginine and L-citrulline, those cultured at physiological concentrations exhibited more NO production and greater mycobacterial killing when cultured with L-arginine than solely L- citrulline (Fig. 6). Notably, this effect was reversed when MΦs were prestimulated with IL-4 and IL-10. In prestimulated PDMs, L-citrulline provided better NO production and mycobacterial control than L-arginine alone, and the combination of the two provided the best anti-mycobacterial effects (Fig. 6B, C). Interestingly, this difference was reversed in MΦs treated with BEC. Together, these data suggest MΦ polarization prior to infection dictates the requirement for L-citrulline-mediated anti-mycobacterial function, but only when these AAs approach physiological concentrations.
Figure 5.
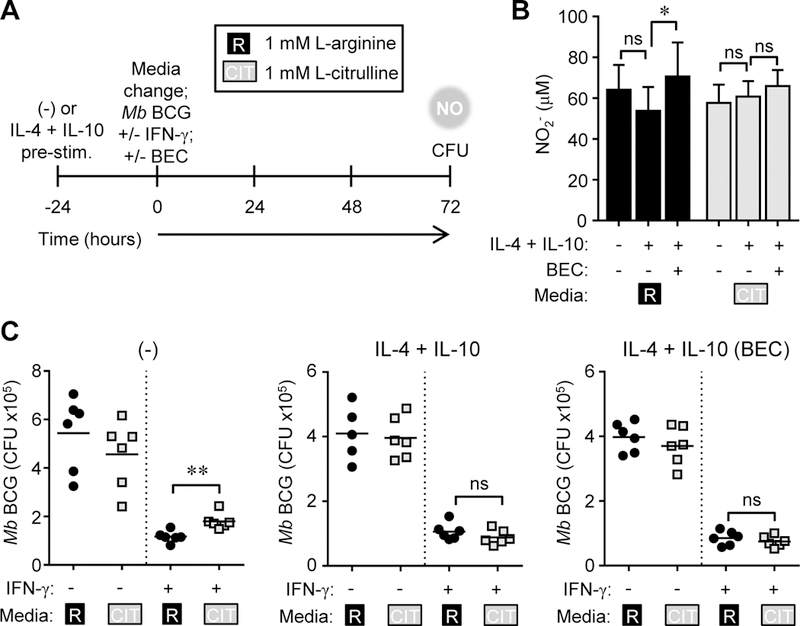
NO production and mycobacterial control in MΦs cultured in supraphysiological L-arginine and L-citrulline. (A-C) PDMs (n ≥ 5) were left alone or pre-stimulated with IL-4 + IL- 10 for 24 hours. Following PBS wash, PDMs were cultured in R-free C-DMEM containing 1.0 mM L-arginine [R] or 1.0 mM L-citrulline [CIT] and infected with Mb BCG plus IFN-γ, with and without BEC for 72 hours. At 72 hours post-infection, NO was determined by Griess assay.(B).Data are the mean NO2- amounts. Mb BCG CFU were determined from lysed MΦs (C). Data are the individual CFU. Black lines represent the mean. Data are combined from 2 experiments. Error bars, SD. * p < 0.05, ** p < 0.01 by Student’s t test. ns, not statistically significant.
Figure 6.
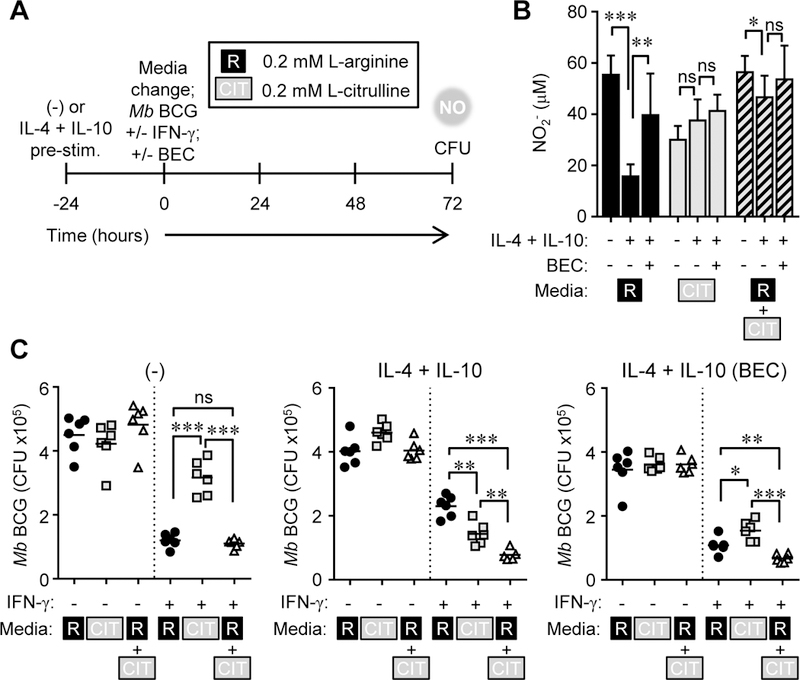
MΦ polarization dictates the requirement for L-citrulline-mediated mycobacterial control. (A-C) Experimental design. PDMs (n ≥ 5) were left alone or pre-stimulated with IL-4 + IL-10 for 24 hours. Following PBS wash, PDMs were cultured in R-free C-DMEM containing 0.2 mM L-arginine [R], 0.2 mM L-citrulline [CIT], or both and infected with Mb BCG plus IFN-γ, with and without BEC for 72 hours. At 72 hours post-infection, NO was determined by Griess assay (B). Data are the mean NO2- amounts. Mb BCG CFU were determined from lysed MΦs.(C).Data are the individual CFU. Black lines represent the mean. Data are combined from 2 experiments. Error bars, SD. * p < 0.05, ** p < 0.01, *** p < 0.001 by Student’s t test. ns, not statistically significant.
As an additional method to alter iNOS and arginase-mediated metabolism, we modified the timing of IFN-γ stimulation, resulting in a delay in iNOS expression, and thereby NO production. We expect a scenario of delayed IFN-γ stimulation may occur early during primary infection, prior to amplified IFN-γ responses delivered by effector T cells. We infected PDMs cultured in L-arginine, L-citrulline, or both for 24 hours prior to IFN-γ addition, and determined anti-mycobacterial activity after an additional 72 hours (Fig. 7A). Similar to the data in Fig. 5, the combination of AAs at supraphysiological concentrations did not enhance anti-mycobacterial MΦ activity compared to those cultured in L-arginine alone (Fig. 7B, C). Even when AA concentrations were decreased, the delay in IFN-γ stimulation resulted in no statistical differences in NO or the amount of recovered Mb BCG from MΦs cultured in L-arginine as compared to L-citrulline. However, the combination of L-arginine and L-citrulline provided MΦs the ability to increase NO production, and control Mb BCG better than MΦs cultured in either AA alone (Fig. 7D, E).
Figure 7.

Delay in IFN-γ stimulation increases the anti-mycobacterial benefit of L-citrulline metabolism in infected MΦs. (A-E) PDMs (n ≥ 8) cultured in R-free C-DMEM containing L-arginine [R], L-citrulline [CIT], or both were infected with Mb BCG (moi = 1). Following 24 hours of infection, IFN-γ was added to appropriate wells. (B, D) 72 hours post-IFN-γ stimulation, NO was determined by Griess assay. Data are the mean NO2- amounts. (C, E) Mb BCG CFU were determined from lysed MΦs. Data are the individual CFU. Black lines represent the mean. Data are combined from 2 experiments. Error bars, SD. ** p < 0.01, *** p < 0.001 by Student’s t test. ns, not statistically significant.
Discussion
These data reveal that L-citrulline and L-arginine are essential for anti-mycobacterial defense. Depending on the activation state of MΦs, one or the other of these AAs may be the dominant contributor to mycobactericidal effectors brought about by differential metabolism of synthesized versus imported L-arginine (Fig. 8). Importantly, the enhancement of anti- mycobacterial activity in MΦs cultured in L-citrulline or the combination of L-citrulline and L-arginine as compared to L-arginine alone, only came to light under physiological culture conditions, and was not observed when using supraphysiological concentrations of L-arginine and L-citrulline. This observation should be stressed for designing cell culture experiments, considering common cell culture media, DMEM and RPMI, contain 2 to 10 times the concentration of L-arginine found in plasma. Furthermore, these media do not contain L-citrulline, concealing the impact this AA may have on cellular biology. Current investigations are aimed at determining how differential usage of L-arginine occurs dependent on its source. One mechanism of interest that has been described in other models (26, 30) is the possibility of a protein complex containing Asl, Ass1, and iNOS that aids in compartmentalizing synthesized Larginine, so that it is not available for arginase-mediated metabolism.
Figure 8.

Working model: Utilization of L-citrulline and L-arginine for NO production in mycobacteria-infected MΦs. (A) Schematic showing a common pool of L-arginine (either imported or synthesized), available for arginase or iNOS-mediated metabolism. (B) In contrast, this schematic shows imported L-arginine (Ri, black arrows) is available for arginase-mediated inhibition of NO production, whereas synthesized L-arginine (Rs, dashed blue arrows) derived from L-citrulline is sequestered for iNOS utilization.
One might expect the metabolic consequences for L-arginine and L-citrulline we observe in MΦs might extend to NO synthases in other tissues. Two additional NOS isoforms exist in mammals – neuronal NOS (nNOS, encoded by Nosi) and endothelial NOS (eNOS, encoded by Nos3) – which may benefit from L-citrulline metabolism. Arginase activity regulates NO output in endothelial cells during hypertension and in aged rodents (31–33). Furthermore, it has been suggested that eNOS relies on L-arginine synthesis from L-citrulline to mediate optimal NO production (34, 35). Arginase 1 is expressed in the mouse brain and upregulated under inflammatory conditions in the central nervous system where nNOS-derived NO serves as a neurotransmitter (36–38). And while evidence of nNOS and Arg1 in neuronal cells is limited, Arg1 is capable of inhibiting NO production in Arg1/nNOS co-transfected cells (39), suggesting L-citrulline metabolism could rescue nNOS function if such an environment exists in vivo. Moreover, astrocytes utilize both nNOS and iNOS for NO production, upregulate Ass1 upon stimulation, and produce NO in L-citrulline-only conditions (40–43), supporting the notion that L-citrulline metabolism provides metabolic advantages to NOS isoforms in multiple cell types.
How do our results relate to AA metabolism and NO production in human MΦs? As mentioned in the introduction, in vitro human mononcyte-derived MΦs produce very little NO. Yet, evidence that human tissue MΦs make iNOS and NO – both in vivo and ex vivo – suggests in vitro conditions are missing an essential factor(s) that would enable NO production (3). With the recent work by Mattilla et al., demonstrating iNOS and arginase protein expression within human TB granuloma MΦs (17), we expect competition for L-arginine – that we have attempted to model in this work – applies to in vivo pathologies. Broad implications of these findings include the possibility of enhancing immune function with AAs in vivo. L-arginine supplementation, aimed at enhancing NO-dependent immunity against TB, has been attempted in Mtb-infected patients in two independent studies. Schön and colleagues treated TB patients with oral L-arginine and observed subtle benefits, including increased sputum conversion, reduced cough and chest pain in HIV negative patients (44). Yet a more recent study has found no clinical benefit in supplementing L-arginine to Mtb-infected patients (45). Metabolic consequences of oral L-arginine supplementation may be responsible for these results. Following ingestion, the bulk of L-arginine is metabolized within intestinal enterocytes or imported into liver hepatocytes, reducing the concentration available for immune utilization (46). L-citrulline, on the other hand, bypasses enterocyte metabolism and liver import (46, 47). Taken together with our present data that show that L-citrulline-derived L-arginine bypasses arginase-mediated suppression of mycobactericidal MΦ activity, future studies investigating the benefits of supplementing L-citrulline on mycobacterial host defenses are warranted.
Supplementary Material
Acknowledgements
We thank Drs. S. S. Way, D. Haslam, and members of their laboratories for discussions in preparing this manuscript.
This work was supported by Cincinnati Children’s Hospital Medical Center Trustee Grant Award (JEQ), American Heart Association Scientist Development Grant 15SDG21550007 (JEQ) and the Division of Infectious Diseases at Cincinnati Children’s Hospital Medical Center.
References
- 1.Garcia I, Guler R, Vesin D, Olleros ML, Vassalli P, Chvatchko Y, Jacobs M, and Ryffel B. 2000. Lethal Mycobacterium Bovis Bacillus Calmette Guerin Infection in Nitric Oxide Synthase 2-Deficient Mice: Cell-Mediated Immunity Requires Nitric Oxide Synthase 2. Lab Invest 80: 1385–1397. [DOI] [PubMed] [Google Scholar]
- 2.MacMicking JD, North RJ, LaCourse R, Mudgett JS, Shah SK, and Nathan CF. 1997. Identification of Nitric Oxide Synthase as a Protective Locus against Tuberculosis. Proc Natl Acad Sci U S A 94: 5243–5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas AC, and Mattila JT. 2014. “Of Mice and Men”: Arginine Metabolism in Macrophages. Front Immunol 5: 479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azad AK, Sadee W, and Schlesinger LS. 2012. Innate Immune Gene Polymorphisms in Tuberculosis. Infect Immun 80: 3343–3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomez LM, Anaya JM, Vilchez JR, Cadena J, Hinojosa R, Velez L, Lopez- Nevot MA, and Martin J. 2007. A Polymorphism in the Inducible Nitric Oxide Synthase Gene Is Associated with Tuberculosis. Tuberculosis (Edinb) 87: 288–294. [DOI] [PubMed] [Google Scholar]
- 6.Jamieson SE, Miller EN, Black GF, Peacock CS, Cordell HJ, Howson JM, Shaw MA, Burgner D, Xu W, Lins-Lainson Z, Shaw JJ, Ramos F, Silveira F, and Blackwell JM. 2004. Evidence for a Cluster of Genes on Chromosome 17q11-Q21 Controlling Susceptibility to Tuberculosis and Leprosy in Brazilians. Genes Immun 5: 46–57. [DOI] [PubMed] [Google Scholar]
- 7.Moller M, Nebel A, Valentonyte R, van Helden PD, Schreiber S, and Hoal EG. 2009. Investigation of Chromosome 17 Candidate Genes in Susceptibility to Tb in a South African Population. Tuberculosis (Edinb) 89: 189–194. [DOI] [PubMed] [Google Scholar]
- 8.Velez DR, Hulme WF, Myers JL, Weinberg JB, Levesque MC, Stryjewski ME, Abbate E, Estevan R, Patillo SG, Gilbert JR, Hamilton CD, and Scott WK. 2009. Nos2a, Tlr4, and Ifngrl Interactions Influence Pulmonary Tuberculosis Susceptibility in African-Americans. Hum Genet 126: 643–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ralph AP, Yeo TW, Salome CM, Waramori G, Pontororing GJ, Kenangalem E, Sandjaja E Tjitra R. Lumb GP. Maguire RN. Price MD. Chatfield P. Kelly M, and Anstey NM. 2013. Impaired Pulmonary Nitric Oxide Bioavailability in Pulmonary Tuberculosis: Association with Disease Severity and Delayed Mycobacterial Clearance with Treatment. J Infect Dis 208: 616–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicholson S, Bonecini-Almeida Mda G. Lapa e, Silva JR, Nathan C, Xie QW, Mumford R, Weidner JR, Calaycay J, Geng J, Boechat N, Linhares C, Rom W, and Ho JL. 1996. Inducible Nitric Oxide Synthase in Pulmonary Alveolar Macrophages from Patients with Tuberculosis. J Exp Med 183: 2293–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rich EA, Torres M, Sada E, Finegan CK, Hamilton BD, and Toossi Z. 1997. Mycobacterium Tuberculosis (Mtb)-Stimulated Production of Nitric Oxide by Human Alveolar Macrophages and Relationship of Nitric Oxide Production to Growth Inhibition of Mtb. Tuber Lung Dis 78: 247–255. [DOI] [PubMed] [Google Scholar]
- 12.Wang CH, Liu CY, Lin HC, Yu CT, Chung KF, and Kuo HP. 1998. Increased Exhaled Nitric Oxide in Active Pulmonary Tuberculosis Due to Inducible No Synthase Upregulation in Alveolar Macrophages. Eur Respir J 11: 809–815. [DOI] [PubMed] [Google Scholar]
- 13.Jagannath C, Actor JK, and Hunter RL Jr. 1998. Induction of Nitric Oxide in Human Monocytes and Monocyte Cell Lines by Mycobacterium Tuberculosis. Nitric Oxide 2: 174–186. [DOI] [PubMed] [Google Scholar]
- 14.Choi HS, Rai PR, Chu HW, Cool C, and Chan ED. 2002. Analysis of Nitric Oxide Synthase and Nitrotyrosine Expression in Human Pulmonary Tuberculosis. Am J Respir Crit Care Med 166: 178–186. [DOI] [PubMed] [Google Scholar]
- 15.Nozaki Y, Hasegawa Y, Ichiyama S, Nakashima I, and Shimokata K. 1997. Mechanism of Nitric Oxide-Dependent Killing of Mycobacterium Bovis Bcg in Human Alveolar Macrophages. Infect Immun 65: 3644–3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pessanha AP, Martins RA, Mattos-Guaraldi AL, Vianna A, and Moreira LO. 2012. Arginase-1 Expression in Granulomas of Tuberculosis Patients. FEMS Immunol Med Microbiol 66: 265–268. [DOI] [PubMed] [Google Scholar]
- 17.Mattila JT, Ojo OO, Kepka-Lenhart D, Marino S, Kim JH, Eum SY, Via LE, Barry CE 3rd, Klein E, Kirschner DE, Morris SM Jr., Lin PL, and Flynn JL. 2013. Microenvironments in Tuberculous Granulomas Are Delineated by Distinct Populations of Macrophage Subsets and Expression of Nitric Oxide Synthase and Arginase Isoforms. J Immunol 191: 773–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duque-Correa MA, Kuhl AA, Rodriguez PC, Zedler U, Schommer-Leitner S, Rao M, Weiner J 3rd, Hurwitz R, Qualls JE, Kosmiadi GA, Murray PJ, Kaufmann SH, and Reece ST. 2014. Macrophage Arginase-1 Controls Bacterial Growth and Pathology in Hypoxic Tuberculosis Granulomas. Proc Natl Acad Sci U S A 111: E4024–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qualls JE, Neale G, Smith AM, Koo MS, DeFreitas AA, Zhang H, Kaplan G, Watowich SS, and Murray PJ. 2010. Arginine Usage in Mycobacteria-Infected Macrophages Depends on Autocrine-Paracrine Cytokine Signaling. Sci Signal 3: ra 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El Kasmi K. C., Qualls JE, Pesce JT, Smith AM, Thompson RW, Henao-Tamayo M, Basaraba RJ, Konig T, Schleicher U, Koo MS, Kaplan G, Fitzgerald KA, Tuomanen EI, Orme IM, Kanneganti TD, Bogdan C, Wynn TA, and Murray PJ. 2008. Toll-Like Receptor-Induced Arginase 1 in Macrophages Thwarts Effective Immunity against Intracellular Pathogens. Nat Immunol 9: 1399–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu GY, and Brosnan JT. 1992. Macrophages Can Convert Citrulline into Arginine. Biochem J 281 ( Pt 1): 45–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qualls JE, Subramanian C, Rafi W, Smith AM, Balouzian L, DeFreitas AA, Shirey KA, Reutterer B, Kernbauer E, Stockinger S, Decker T, Miyairi I, Vogel SN, Salgame P, Rock CO, and Murray PJ. 2012. Sustained Generation of Nitric Oxide and Control of Mycobacterial Infection Requires Argininosuccinate Synthase 1. Cell Host Microbe 12: 313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Bassossy HM, El-Fawal R, Fahmy A, and Watson ML. 2013. Arginase Inhibition Alleviates Hypertension in the Metabolic Syndrome. Br J Pharmacol 169: 693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shearer JD, Richards JR, Mills CD, and Caldwell MD. 1997. Differential Regulation of Macrophage Arginine Metabolism: A Proposed Role in Wound Healing. Am J Physiol 272: E181–190. [DOI] [PubMed] [Google Scholar]
- 25.Romero MJ, Platt DH, Caldwell RB, and Caldwell RW. 2006. Therapeutic Use of Citrulline in Cardiovascular Disease. Cardiovasc Drug Rev 24: 275–290. [DOI] [PubMed] [Google Scholar]
- 26.Erez A, Nagamani SC, Shchelochkov OA, Premkumar MH, Campeau PM, Chen Y, Garg HK, Li L, Mian A, Bertin TK, Black JO, Zeng H, Tang Y, Reddy AK, Summar M, O’Brien WE, Harrison DG, Mitch WE, Marini JC, Aschner JL, Bryan NS, and Lee B. 2011. Requirement of Argininosuccinate Lyase for Systemic Nitric Oxide Production. Nat Med 17: 1619–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashenafi S, Aderaye G, Bekele A, Zewdie M, Aseffa G, Hoang AT, Carow B, Habtamu M, Wijkander M, Rottenberg M, Aseffa A, Andersson J, Svensson M, and Brighenti S. 2014. Progression of Clinical Tuberculosis Is Associated with a Th2 Immune Response Signature in Combination with Elevated Levels of Socs3. Clin Immunol 151: 84–99. [DOI] [PubMed] [Google Scholar]
- 28.Heitmann L, Abad Dar M., Schreiber T, Erdmann H, Behrends J, McKenzie AN, Brombacher F, Ehlers S, and Holscher C. 2014. The Il-13/Il-4ralpha Axis Is Involved in Tuberculosis-Associated Pathology. J Pathol 234: 338–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rook GA, Hernandez-Pando R, Dheda K, and Teng Seah G. 2004. Il-4 in Tuberculosis: Implications for Vaccine Design. Trends Immunol 25: 483–488. [DOI] [PubMed] [Google Scholar]
- 30.Flam BR, Hartmann PJ, Harrell-Booth M, Solomonson LP, and Eichler DC. 2001. Caveolar Localization of Arginine Regeneration Enzymes, Argininosuccinate Synthase, and Lyase, with Endothelial Nitric Oxide Synthase. Nitric Oxide 5: 187–197. [DOI] [PubMed] [Google Scholar]
- 31.Xu W, Kaneko FT, Zheng S, Comhair SA, Janocha AJ, Goggans T, Thunnissen FB, Farver C, Hazen SL, Jennings C, Dweik RA, Arroliga AC, and Erzurum SC. 2004. Increased Arginase Ii and Decreased No Synthesis in Endothelial Cells of Patients with Pulmonary Arterial Hypertension. FASEB J 18: 1746–1748. [DOI] [PubMed] [Google Scholar]
- 32.Zhang C, Hein TW, Wang W, Miller MW, Fossum TW, McDonald MM, Humphrey JD, and Kuo L. 2004. Upregulation of Vascular Arginase in Hypertension Decreases Nitric Oxide-Mediated Dilation of Coronary Arterioles. Hypertension 44: 935–943. [DOI] [PubMed] [Google Scholar]
- 33.White AR, Ryoo S, Li D, Champion HC, Steppan J, Wang D, Nyhan D, Shoukas AA, Hare JM, and Berkowitz DE. 2006. Knockdown of Arginase I Restores No Signaling in the Vasculature of Old Rats. Hypertension 47: 245–251. [DOI] [PubMed] [Google Scholar]
- 34.Flam BR, Eichler DC, and Solomonson LP. 2007. Endothelial Nitric Oxide Production Is Tightly Coupled to the Citrulline-No Cycle. Nitric Oxide 17: 115–121. [DOI] [PubMed] [Google Scholar]
- 35.Mun GI, Kim IS, Lee BH, and Boo YC. 2011. Endothelial Argininosuccinate Synthetase 1 Regulates Nitric Oxide Production and Monocyte Adhesion under Static and Laminar Shear Stress Conditions. JBiol Chem 286: 2536–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu H, Iyer RK, Kern RM, Rodriguez WI, Grody WW, and Cederbaum SD. 2001. Expression of Arginase Isozymes in Mouse Brain. JNeurosci Res 66: 406–422. [DOI] [PubMed] [Google Scholar]
- 37.Xu L, Hilliard B, Carmody RJ, Tsabary G, Shin H, Christianson DW, and Chen YH. 2003. Arginase and Autoimmune Inflammation in the Central Nervous System. Immunology 110: 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knott AB, and Bossy-Wetzel E. 2009. Nitric Oxide in Health and Disease of the Nervous System. AntioxidRedox Signal 11: 541–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Que LG, George SE, Gotoh T, Mori M, and Huang YC. 2002. Effects of Arginase Isoforms on No Production by Nnos. Nitric Oxide 6: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simic G, Lucassen PJ, Krsnik Z, Kruslin B, Kostovic I, Winblad B, and Bogdanovi. 2000. Nnos Expression in Reactive Astrocytes Correlates with Increased Cell Death Related DNA Damage in the Hippocampus and Entorhinal Cortex in Alzheimer’s Disease. Exp Neurol 165: 12–26. [DOI] [PubMed] [Google Scholar]
- 41.Caggiano AO, and Kraig RP. 1998. Neuronal Nitric Oxide Synthase Expression Is Induced in Neocortical Astrocytes after Spreading Depression. J Cereb Blood Flow Metab 18: 75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmidlin A, and Wiesinger H. 1998. Argininosuccinate Synthetase: Localization in Astrocytes and Role in the Production of Glial Nitric Oxide. Glia 24: 428–436. [PubMed] [Google Scholar]
- 43.Wiesinger H 2001. Arginine Metabolism and the Synthesis of Nitric Oxide in the Nervous System. ProgNeurobiol 64: 365–391. [DOI] [PubMed] [Google Scholar]
- 44.Schon T, Elias D, Moges F, Melese E, Tessema T, Stendahl O, Britton S, and Sundqvist T. 2003. Arginine as an Adjuvant to Chemotherapy Improves Clinical Outcome in Active Tuberculosis. Eur Respir J 21: 483–488. [DOI] [PubMed] [Google Scholar]
- 45.Ralph AP, Waramori G, Pontororing GJ, Kenangalem E, Wiguna A, Tjitra E, Sandjaja DB Lolong TW. Yeo MD. Chatfield RK. Soemanto I. Bastian R Lumb GP. Maguire J Eisman RN Price PS Morris P. Kelly M, and Anstey NM. 2013. L-Arginine and Vitamin D Adjunctive Therapies in Pulmonary Tuberculosis: A Randomised, Double-Blind, Placebo-Controlled Trial. PLoS One 8: e70032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Curis E, Nicolis I, Moinard C, Osowska S, Zerrouk N, Benazeth S, and Cynober L. 2005. Almost All About Citrulline in Mammals. Amino Acids 29: 177–205. [DOI] [PubMed] [Google Scholar]
- 47.Bahri S, Zerrouk N, Aussel C, Moinard C, Crenn P, Curis E, Chaumeil JC, Cynober L, and Sfar S. 2013. Citrulline: From Metabolism to Therapeutic Use. Nutrition 29: 479–484. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


