Abstract
Purpose
Dark-adapted visual fields were obtained from patients with inherited retinal degeneration (IRD) and controls to evaluate the effect that age, retinal region, and disease had on scotopic sensitivity. Intra- and intersession test–retest repeatabilities for patients and controls were measured to establish significant change for longitudinal studies.
Methods
A total of 41 patients with IRD and 30 controls had one eye dilated and dark-adapted for 40 minutes. Scotopic sensitivity was measured with a Medmont dark-adapted chromatic (DAC) perimeter (size V stimulus, 200-ms duration, background luminance < 0.0001 cd/m2, dynamic range 0–75 decibel [dB]). Mixed effects analysis was performed to analyze age, retinal eccentricity, and sensitivity. The intra-/intersession coefficients of repeatability (CR) were calculated for controls and patients with IRD.
Results
Each additional year was associated with lower sensitivity (−0.22 dB) per year in normal controls over age 50 compared to younger controls (12–49 years). The superior field had lower sensitivity than the inferior, but the nasal field was not different compared to the temporal field in normal controls. The CR for intra- and intersession testing on mean sensitivity (MS)/pointwise sensitivity (PWS) were ±1.5/±8.5 and ±3.3/±9.8 dB, respectively, for patients with IRD. Control MS/PWS CR were ±1.5/±6.1 dB for intrasession and ±1.7/±6.8 dB for intersession DAC perimetry.
Conclusions
The DAC perimeter is an important asset because it tests a wide field of scotopic vision. The CR are comparable to those of other perimetry devices. Effects of age and retinal region should be considered when assessing scotopic sensitivity measured with the DAC perimeter.
Keywords: inherited retinal degeneration, scotopic sensitivity, static perimetry
Advances in genomics have linked approximately 250 genes to inherited retinal degenerations (IRD).1 Coupled with these advances, the first gene therapy targeted toward RPE65-associated IRD was approved for use in the United States in 2017 and by the European Commission as of November 2018. Clinical trials involving gene-specific IRDs have been increasing, which emphasizes a need for appropriate and informative outcome measures.
IRDs often begin with night blindness followed by defects and scotomas (blind spots) that form in the visual field due to dysfunction or death of the photoreceptors. Over time scotomas grow, constricting areas of vision and in some cases, continuing until the patient has no light perception. A perimeter maps the patients' visual field to monitor changes in patients with IRD. Common perimeters (Humphrey Field Analyzer [HFA], Zeiss, Dublin, CA, USA; Octopus, Haag-Streit, Koeniz, Switzerland) measure static sensitivity over the full field under light-adapted conditions, resulting in a cone-mediated visual field. Other perimeters are fundus guided, meaning that they test the central visual field while tracking eye movements by referencing a predetermined area of high contrast. Fundus-guided perimeters, the Macular Integrity Assessment (MAIA; CenterVue, Padova, Italy) and the MP1 (Nidek, Inc., Fremont, CA, USA), measure static sensitivity of the macula under photopic or mesopic conditions in which the cone photoreceptors are involved in stimulus detection.
Commercial perimeters were modified for scotopic testing through the addition of filters to change the light wavelengths and/or by blocking background beams and stray lights in the perimeter.2–5 Subsequently, fundus-guided perimeters were modified by inserting chromatic filters into the stimulus path and eliminating the mesopic background.2,3 CenterVue and Nidek now produce fundus-guided perimeters modified to accommodate scotopic testing. However, microperimeters test only the central field and the scotopic (S) perimeters; the S-MAIA and the MP1-S have a dynamic range of only 2 to 3 log units of sensitivity, which often results in floor and/or ceiling effects, not depicting the true depth of scotopic vision in patients with IRD and normal-sighted controls.4 Thus previous attempts at measuring scotopic sensitivity are problematic because they depended on customized devices to test the full visual field, had a limited dynamic range of stimulus intensities, or only tested the central retina.3,5–8 A commercial device to measure scotopic sensitivity throughout the visual field is desirable for multicenter trials following disease progression and evaluating potential treatments in patients with IRD.
We recently reported initial findings using a new two-color dark-adapted chromatic (DAC, Medmont International Pty. Ltd., Nunawading, VIC, Australia) perimeter that measures scotopic retinal sensitivity across the full visual field.9 The DAC perimeter differentiates rod and cone function by exploiting the differential spectral properties of photoreceptors. The chromatic light-emitting diode (LED) stimuli on the DAC perimeter are cyan and red. Cyan has a wavelength (505 nm) that is near the peak of the theoretical sensitivity function.10–12 Because rods are >2 log units more sensitive than cones to cyan, we quantified rod-mediated vision through two methods. First, the maximum sensitivity of cones to the cyan stimulus was determined in normal controls (+2 SD) and served as the reference cone threshold.9 The maximum sensitivity of cones was determined with the DAC perimeter by exposing normal controls to rod-desensitizing light (1.85 cd/m2; Goldmann-Weekers dark adaptometer, Haag-Streit) for 5 minutes prior to every 3 minutes of DAC testing. This method ensures testing is performed before the rod–cone break (maximum cone sensitivity). The upper limit of cone sensitivity (mean + 2 SD) was used to categorize detection as being rod mediated. Sensitivity greater than the cone threshold would therefore be rod mediated because cones cannot detect intensities dimmer than their threshold. The second method9,13 was through comparison of sensitivity to a red (625-nm) stimulus measured at the same loci, which indicates whether sensitivity was derived from rods, cones, or a mix of both receptors.9,13–15 We showed that rod function could be quantified with the DAC perimeter in the far-peripheral retina in some patients with advanced IRD who did not have detectable scotopic full-field electroretinography responses.9 Tan et al.16 used the DAC perimeter to determine the central field intra-/intersession pointwise coefficients of repeatability (CR), which were 8.4/8.2 decibel (dB) for control subjects and 9.1/11.7 dB for patients with age-related macular degeneration (AMD). Intra-/intersession repeatability testing for scotopic sensitivity in the far-peripheral field has yet to be determined. Intra-/intersession CR on the Medmont for patients with IRD also remains to be demonstrated.
Regional differences in retinal sensitivity have been reported using light-adapted perimetry.17–21 The superior retina is more sensitive than the inferior is, and the central retina is more sensitive than the periphery.17–19 It has been established that as we age, we lose sensitivity, and spatial differences in retinal sensitivity are exacerbated.17–20,22,23 These effects have also been evaluated to some extent using dark-adapted perimetry.24–28 For example, with the HFA perimeter, Jackson et al.26 measured lower sensitivity among individuals ∼70 years of age at four points (4°, 7°, 32°, and 38°) compared with younger individuals who were ∼27 years of age. However, spatial differences and age effects on scotopic sensitivity throughout the full field are unknown.
Here we measured the effects of age and test location on scotopic sensitivity, including the far-peripheral retina. Additionally, the intra-/intersession repeatabilities of the measurements derived from the DAC perimeter in normal controls and patients with IRD were determined. Establishing the effects on retinal sensitivity and the reliability of the DAC perimeter will be useful for interpreting functional changes in patients with IRD, in monitoring disease progression, and as an outcome measure in clinical trials.
Methods
Participants
A total of 41 patients with IRD and 30 normal controls participated in this research. Patients were diagnosed with IRD by a retinal specialist and referred to the Retina Foundation of the Southwest (Dallas, TX, USA) for further evaluation and genetic testing. All patients had experience with visual field testing. To minimize testing time, most patients had the eye with the lower best-corrected visual acuity (BCVA) dilated and patched for dark adaptation while the better-seeing eye performed other tasks. Patients were excluded if their BCVA was worse than 20/500 (1.4 logMAR). Other exclusion criteria were inability to complete the perimetry exams or any additional ocular abnormalities such as cataract, glaucoma, or nystagmus that would hinder stimulus detection. If there was no acuity difference between eyes, the right eye was tested. Left eye exams were transformed to right eye equivalent fields. Table 1 gives the patients' diagnosis, associated mutated gene, sex, age, refractive error, and BCVA, and indicates whether patients performed intrasession (*) testing, intersession (†) testing, or both (*†).
Table 1.
X-Linked Retinitis Pigmentosa (XLRP), Cone–Rod Dystrophy (CRD), RP Isolate (RPiso); Mainzer-Saldino Syndrome (MSS), Autosomal Recessive RP (arRP), Autosomal Dominant RP (adRP), Autosomal Dominant Pattern Dystrophy (adPD), Autosomal Dominant Macular Dystrophy (adMD), and Sphere (sph)
|
Subject # |
Age, Years |
Mutated Gene |
Diagnosis |
BCVA OD/OS, logMAR |
Refraction OD |
Refraction OS |
Exams |
| 1301 | 38 | RPGR | XLRP | 0.2/0.2 | −12.00 + 1.25 × 118 | −13.50 + 2.00 × 060 | † |
| 4338 | 69 | RPGR | XLRP carrier | 0.1/0.0 | +0.75 + 2.00 × 028 | Plano +2.00 × 036 | * |
| 4339 | 32 | RPGR | XLRP | 1.4/1.3 | −4.00 sph | −4.00 sph | *‡ |
| 4880 | 70 | RHO | adRP | 0.5/0.2 | +1.50 + 1.50 × 010 | −0.50 + 0.50 × 180 | *† |
| 5730 | 25 | CRB1 | CRD | 0.7/0.6 | +1.00 + 2.50 × 120 | +0.50 + 2.50 × 071 | * |
| 5931 | 64 | PRPH2 | adPD | 0.1/0.1 | +0.50 + 0.75 × 174 | −0.75 + 1.00 × 170 | *‡ |
| 6472 | 38 | RPGR | XLRP | 0.4/0.6 | −4.50 − 0.75 × 080 | −5.00 − 0.75 × 110 | * |
| 7566 | 25 | RPGR | XLRP | 0.5/0.5 | −9.50 + 2.00 × 100 | −9.50 + 2.25 × 085 | * |
| 7685 | 44 | RPGR | XLRP | 0.9/1.2 | −3.25 + 1.75 × 125 | −2.75 + 1.75 × 065 | † |
| 7773 | 37 | USH2A | arRP | 0.3/0.4 | −0.25 + 0.50 × 090 | −5.00 + 0.50 × 080 | †‡ |
| 7807 | 54 | Unknown | RPiso | −0.1/0.1 | −1.25 + 1.00 × 152 | −2.25 + 0.75 × 043 | * |
| 8126 | 20 | IMPDH1 | adRP | 0.0/0.1 | −4.25 + 2.50 × 90 | −4.75 + 2.75 × 082 | *‡ |
| 8538 | 42 | PRPH2 | adRP | 0.2/0.1 | +0.25 + 0.75 × 60 | −0.50 sph | *†‡ |
| 8866 | 59 | PRPH2 | adMD | 0.3/0.6 | +0.75 + 1.00 × 152 | +0.75 + 1.00 × 179 | * |
| 9457 | 75 | Unknown | RPiso | 0.3/0.5 | +0.75 + 0.50 × 160 | −1.00 + 1.25 × 178 | * |
| 9503 | 54 | Unknown | RPiso | 0.0/0.1 | +2.25 sph | +1.00 sph | * |
| 9795 | 43 | RPGR | XLRP carrier | 0.6/0.6 | Plano −2.00 × 075 | −1.00 − 0.75 × 175 | * |
| 9852 | 18 | RPGR | XLRP | 0.4/0.4 | Plano | Plano | * |
| 10313 | 42 | RHO | adRP | 0.3/1.0 | Plano +1.50 × 095 | Plano + 1.50 × 085 | † |
| 10669 | 17 | RP2 | XLRP | 0.5/0.9 | −2.25 + 2.00 × 104 | −1.50 + 2.50 × 085 | * |
| 10924 | 33 | USH2A | RPiso | 0.2/0.2 | −4.50 sph | −1.50 sph | *†‡ |
| 11179 | 45 | RPGR | CRD | 0.7/0.7 | −2.00 + 1.25 × 143 | −0.75 sph | * |
| 11313 | 31 | PRPH2 | adMD | 0.0/0.5 | −3.00 − 2.75 × 0 | −2.00 − 1.75 × 166 | *†‡ |
| 11314 | 16 | RHO | adRP | 0.3/0.2 | −5.25 + 0.75 × 119 | −5.50 + 1.50 × 036 | *†‡ |
| 11344 | 33 | USH2A | RPiso | 0.3/0.5 | Plano | Plano | * |
| 11685 | 10 | RPGR | XLRP | 0.2/0.2 | −3.75 + 2.75 × 104 | −4.75 + 2.00 × 072 | *†‡ |
| 11687 | 40 | RPGR | XLRP carrier | 0.5/0.1 | Plano | Plano | *†‡ |
| 11728 | 59 | PRPH2 | adPD | 0.3/0.4 | Plano | Plano | *†‡ |
| 91839 | 73 | PRPF31 | adRP | 0.0/−0.1 | Plano +1.25 × 170 | +0.25 + 1.00 × 011 | * |
| 92366 | 36 | USH2A | Usher II | 0.2/0.2 | P3.00 + 0.75 × 107 | −3.75 + 1.25 × 067 | * |
| 92388 | 52 | Unknown | Rpiso | 0.5/0.3 | +0.25 + 2.25 × 130 | +0.75 + 1.25 × 070 | * |
| 92394 | 58 | Unknown | adRP | 0.3/0.5 | −1.00 + 1.00 × 153 | −0.25 + 0.50 × 160 | * |
| 92416 | 15 | IFT140 | MSS | 0.3/0.2 | Plano | Plano | * |
| 92436 | 23 | RPGR | XLRP | 0.1/0.1 | −1.25 + 1.25 × 164 | −0.75 + 0.75 × 006 | * |
| 92437 | 44 | USH2A | Usher II | 0.2/0.1 | −1.50 − 0.75 × 110 | −2.00 − 0.25 × 090 | *†‡ |
| 94280 | 49 | USH2A | arRP | 0.2/0.1 | −1.00 + 1.00 × 153 | −1.00 + 0.75 × 065 | *† |
| 94308 | 19 | RP1 VUS | RPiso | 0.2/0.2 | −4.00 + 2.75 × 083 | −3.25 + 3.50 × 078 | * |
| 94388 | 33 | CNGA3 | CRD | 0.3/−0.1 | Plano | Plano | * |
| 94395 | 32 | Unknown | RPiso | 0.4/0.3 | −7.75 + 2.25 × 100 | −7.00 + 2.75 × 090 | * |
Intrasession repeat testing.
Intersession repeat testing.
Fixation testing.
Controls were employees (or their family and friends) that were age matched to the patients with IRD and were recruited from the Retina Foundation of the Southwest or Texas Retina Associates. None had evidence of age-related maculopathy (ARM) as reported by recent eye exams or by examination at the time of testing. Normal controls who had high refractive error (±6.00-diopter [D] sphere) were excluded. Controls were tested in their right eyes. The University of Texas Southwestern Institutional Review Board approved this study. Study procedures were explained and all participants signed an informed consent before testing. This research was conducted in accordance with institutional guidelines and the Declaration of Helsinki.
DAC Perimetry
One eye was fully dilated with tropicamide 1% and phenylephrine 2.5%. A black eye patch was secured over the test eye so that no light could be detected. Following 40 minutes of dark adaptation, the patch was moved to the fellow eye in a fully darkened room. A 505-nm (cyan or short wavelength) stimulus was used to test 103 points 144° horizontally and 72° vertically (Fig. 1A). The maximum luminance of the cyan stimulus was 12.58 cd/m2 and the dynamic range was approximately 75 dB. The spot size diameter was 1.72°, which is equivalent to the Goldman size V. The stimulus duration was 200 ms and the response time was 400 ms. The interval between stimuli was 1.1 seconds. The DAC perimeter has a black bowl and a background luminance of < 0.0001 cd/m2. Retinal sensitivities were determined using a four-down, two-up staircase threshold strategy. If the patient could not see the red fixation target, the intensity was gradually increased until the target was seen before beginning the exam. Infrared viewing of the test eye throughout examinations allowed monitoring of the correct alignment of the patient's pupil. The quality of each exam was assessed by a reliability factor, which was defined as the percentage of total catch trials where a false positive result was given. Exams with a reliability factor of >15% for normal controls or >25% for patients with IRD were excluded. Based on this criterion, three normal control and two IRD tests were excluded from intrasession analysis. For all participants in this study, the time for test 1 averaged 21.2 minutes ± 5.9 SD (range, 10.7–34.4 minutes), test 2 averaged 13.6 minutes ± 3.8 SD (range, 6.9–31.7 minutes), and test 3 averaged 13.7 minutes ± 2.0 SD (range, 9.8–18.8 minutes). These times include subject-controlled and requested breaks while the test was in progress.
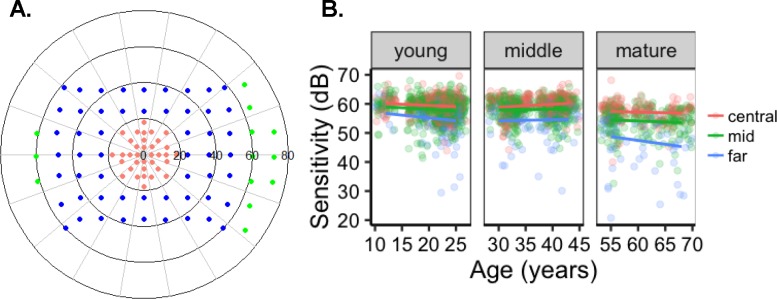
Figure 1.
Scotopic pointwise sensitivity. (A) Test locations for central (salmon), mid (blue), and far (green) periphery. (B) Normal control PWS was similar between the young and middle-age groups, but the mature group had a decrease in sensitivity in the central, mid-, and far-peripheral fields.
Points at the blind spot were removed as was a location with an artifact due to nasal shadowing (−48°, −36°).9 To determine scotopic sensitivity among differently aged normal controls and between retinal areas, we defined age groups by the two arbitrary gaps in age among the controls. These groups were labeled as young (≤29 years; n = 13, range, 12–25 years; mean age 21.3 years), middle-aged (30–49 years; n = 9, range, 30–43 years; mean age 37.1 years), and mature (≥50 years; n = 6; range, 55–72 years; mean = 62.8 years). Retinal areas were defined by eccentricity in degrees or by radial distance from foveal fixation (0°). Central points were located at ≤±18° (Fig. 1A, salmon). Midperipheral points (blue) were between ±19° and ±59°, and the far-peripheral points (green) were those positioned ≥±60°. The first intrasession DAC perimetry exam from normal controls was used for evaluating the effects of age and location on scotopic sensitivity. Hemifield (superior/inferior or nasal/temporal) differences were calculated by averaging the sensitivity of the points included in the specified region at all eccentricities (degree) within the specified region. Points did not overlap between the inferior/superior or nasal/temporal fields.
Fixation stability in patients (Table 1, ‡) was determined on a fundus perimeter (MP1-S; Nidek Technologies, Padova, Italy). Fixation was quantified with the bivariate contour ellipse area (BCEA)29 from 13 patients who performed fundus perimetry (MP1-S) on the same day as the DAC testing. The BCEA is an ellipse (in degrees) surrounding 1, 2, or 3 standard deviations (SD) of all fixation points recorded throughout the perimetry exam.30 Qualitative assessment by the Fujii method categorized the fixation data as “stable,” “relatively unstable,” or “unstable.”31
Statistics
Mixed effects analyses were performed in R 3.4.032 using the package lme433 to analyze the effect of age and retinal eccentricity on sensitivity. The random effects were the intercepts for subjects as well as by-subject random slopes. Visual inspection of residual plots did not reveal any obvious deviations from homoscedasticity or normality. Restricted maximum likelihood estimation was used to determine significant random effects.34,35 The maximum likelihood estimation was used to compare models by a leave-one-out method for modeling the data.34,36 Analysis of variance (ANOVA) was used to compare models.37 P values for individual effects within the model were determined by Satterthwaite's method.38 Analysis of deviance was calculated with Type II Wald χ2 tests with grouped data. Effects of groups on sensitivity are presented as mean ± standard error (SE). Welch's 2-sided t-test was used to determine differences for within-group means. Continuous data were analyzed with linear regression.
The CR is the range within which 95% of all retest values are expected to fall. The CR was calculated as 1.96 times the standard deviation (SD) of the difference between the two measurements.39 The upper and lower limits of agreement (ULoA and LLoA) are defined as the mean difference between sensitivity measured between tests ± CR. The magnitude of the repeatability was determined as suggested by Bland and Altman39 as the confidence intervals (CI95) around the ±ULoA and ±LLoA.39 The CR and the CI95 of the LoA are provided in Table 2.
Table 2.
Eccentricity (ecc), Mean Sensitivity, Pointwise Sensitivity, Inherited Retinal Degeneration (IRD), Coefficient of Repeatability (CR), Upper Limits of Agreement (ULoA), Lower LoA (LLoA), Upper (+) and Lower (−) Bounds for 90% Confidence Intervals
|
Ecc |
CR |
ULoA+ |
ULoA− |
LL0A+ |
LL0A− |
| Control | |||||
| Intrasession | |||||
| MS | 1.5 | 2.0 | 0.9 | −0.9 | −2.0 |
| Central MS | 1.5 | 1.9 | 0.9 | −1.0 | −2.0 |
| Mid MS | 1.6 | 2.2 | 1.0 | −1.0 | −2.1 |
| Far MS | 3.4 | 4.5 | 2.0 | −2.2 | −4.7 |
| PWS | 6.1 | 6.4 | 5.9 | −5.7 | −6.2 |
| Central PWS | 5.4 | 5.7 | 5.0 | −4.7 | −5.4 |
| Mid PWS | 6.2 | 6.6 | 5.9 | −5.7 | −6.4 |
| Far PWS | 7.2 | 7.9 | 6.5 | −6.5 | −8.0 |
| Intersession | |||||
| MS | 1.7 | 2.5 | 0.8 | −0.2 | −1.9 |
| Central MS | 2.3 | 3.5 | 1.0 | −0.3 | −2.8 |
| Mid MS | 2.2 | 3.4 | 0.9 | −0.4 | −2.9 |
| Far MS | 3.9 | 6.4 | 1.4 | −1.4 | −6.5 |
| PWS | 6.8 | 7.1 | 6.4 | −5.8 | −6.6 |
| Central PWS | 6.0 | 6.5 | 5.4 | −4.9 | −5.9 |
| Mid PWS | 6.4 | 6.8 | 5.9 | −5.2 | −6.1 |
| Far PWS | 9.3 | 10.6 | 8.0 | −8.0 | −10.6 |
| IRD | |||||
| Intrasession | |||||
| MS | 1.5 | 2.0 | 0.9 | −0.9 | −2.0 |
| Central MS | 1.4 | 1.9 | 0.9 | −1.0 | −2.0 |
| Mid MS | 1.6 | 2.2 | 1.0 | −1.0 | −2.1 |
| Far MS | 5.2 | 7.3 | 3.1 | −4.1 | −8.3 |
| PWS | 8.5 | 8.7 | 8.2 | −8.2 | −8.7 |
| Central PWS | 8.0 | 8.4 | 7.6 | −7.3 | −8.1 |
| Mid PWS | 8.6 | 9.0 | 8.3 | −8.7 | −9.4 |
| Far PWS | 8.8 | 9.5 | 8.2 | −7.4 | −8.7 |
| Intersession | |||||
| MS | 3.3 | 5.5 | 1.1 | −0.8 | −5.2 |
| Central MS | 3.2 | 5.7 | 0.7 | −1.4 | −6.3 |
| Mid MS | 3.4 | 5.7 | 1.1 | −0.8 | −5.4 |
| Far MS | 6.8 | 10.8 | 2.8 | −0.7 | −8.8 |
| PWS | 9.8 | 10.3 | 9.2 | −9.0 | −10.1 |
| Central PWS | 8.0 | 8.9 | 7.2 | −7.9 | −9.6 |
| Mid PWS | 10.0 | 10.8 | 9.2 | −8.9 | −10.5 |
| Far PWS | 12.4 | 14.2 | 10.6 | −8.5 | −12.1 |
Results
Effects of Age and Retinal Eccentricity
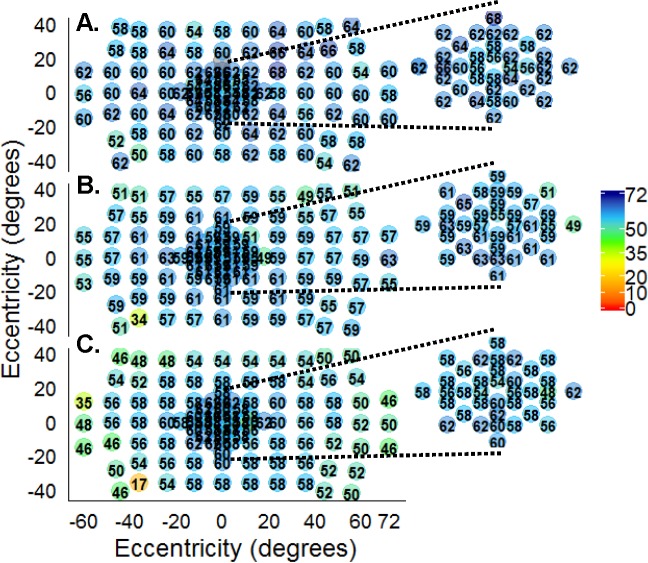
Pointwise sensitivities (PWS) were measured from 30 normal controls. To determine whether age or eccentricity of test point affected sensitivity, we evaluated the central, mid-, and far-peripheral points by age group (Table 3). The mean central PWS for the young (59.3 dB ± 3.2 SD), middle (59.9 dB ± 2.8 SD), and mature (55.7 dB ± 4.7 SD) groups was higher than the midperiphery (57.6, 57.9, and 52.1, respectively). Mean sensitivity (MS) was the lowest in the far periphery for the young, middle, and mature groups (blue; 54.3, 54.7, and 45.7, respectively; Fig. 1B; Table 3). The mature group had a ∼9-dB decrease in sensitivity in the far periphery (45.7 dB ± 10.5 SD) compared to PWS measured in their central field (55.7 dB ± 4.7 SD; Table 3; Fig. 1B). Inspection of PWS showed that the mature group had progressively lower sensitivity from the central to the mid- and to the far-peripheral fields when compared to the young and middle-aged groups (Fig. 1; X2 = 409.3 (6); P < 0.0001). Linear mixed effects with interaction between age and eccentricity (by radial distance from fixation) revealed that age significantly affected sensitivity by −1.2 dB per decade of associated control age (P < 0.0001). Eccentricity affected sensitivity by −1.1 dB per every 10° away from fixation (P < 0.0001). Examples of normal control sensitivity illustrates uniformity ranging from 50 to 68 dB in scotopic sensitivity from a 12-year-old control (Fig. 2A). Conversely, a 68-year-old control's field (Fig. 2C) had lower sensitivity (∼46–56 dB) at most points throughout the field but the peripheral field had the lowest sensitivity (∼35–48 dB) compared with the peripheral points from the middle (Fig 2B; ∼55–57 dB) and younger age (∼56–62 dB) groups.
Table 3.
Midperiphery (Mid), Far Periphery (Far)
|
Age Group |
Central, dB |
Mid, dB |
Far, dB |
Superior, dB |
Inferior, dB |
Temporal, dB |
Nasal, dB |
| Young | 59.3 ± 3.2 | 57.6 ± 4.7 | 54.3 ± 6.9 | 57.3 ± 4.2 | 58.7 ± 3.6 | 58.1 ± 4.0 | 57.7 ± 4.2 |
| Middle | 59.9 ± 2.8 | 57.9 ± 4.7 | 54.7 ± 5.2 | 57.8 ± 4.4 | 58.5 ± 3.5 | 58.2 ± 3.7 | 57.6 ± 4.4 |
| Mature | 55.7 ± 4.7 | 52.1 ± 8.3 | 45.7 ± 10.5 | 52.3 ± 6.9 | 55.7 ± 4.6 | 53.5 ± 6.4 | 54.3 ± 5.5 |
Figure 2.
Normal controls, pointwise sensitivities. (A) Sensitivity was consistent (50–64 dB) throughout the field for a 12-year-old. (B) There were slight decreases in sensitivity in the superior field for a 40-year-old control. (C) A 68-year-old control had lower sensitivity at most points throughout the field, with the lowest in the periphery compared to the younger and middle control groups. Macular sensitivities (insets).
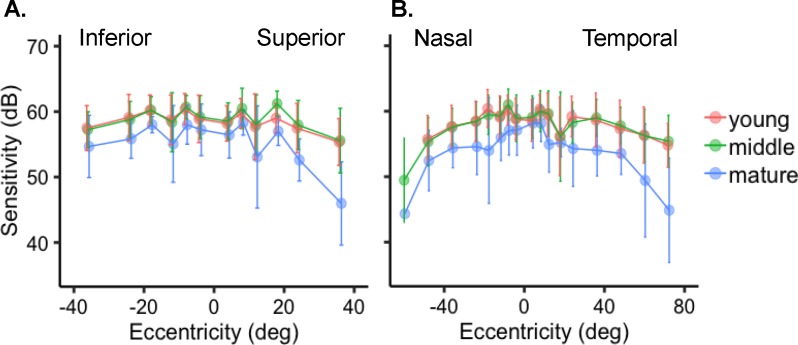
Previous studies have reported hemifield sensitivity differences.8 Hemifield differences were observed when the mean PWS for normal controls was plotted by degrees, with fixation set at 0° for superior/inferior (Fig. 3A) or nasal/temporal hemifields (Fig. 3B). Mean scotopic sensitivity measured in the superior (56.6 dB ± 5.2 SD) field was lower than in the inferior field (58.1 dB ± 4.0 SD; P < 0.0001; Fig. 3A). When sensitivity was compared between the nasal and temporal hemifields for normal controls, we found that the nasal field (57.1 dB ± 4.7 SD) had similar sensitivity to the points in the temporal (57.3 dB ± 4.8 SD) field (P = 0.4516).
Figure 3.
Hemifield sensitivity in normal controls. (A) Control mean sensitivity of points ± SD by eccentricity demonstrated greater sensitivity in the inferior compared to the superior field, but (B) sensitivity in the temporal was not different than in the nasal field. The mature (blue) group had lower sensitivity in all areas.
To determine the magnitude of age effects on sensitivity, we analyzed each age group separately. All three groups showed that MS was greatest in the central field and decreased by eccentricity, with the far periphery exhibiting the lowest sensivity (Table 3). Each additional year of control age was associated with −0.10 dB lower sensitivity for those in the young group, −0.04 dB per year for controls in the middle group, and −0.22 dB per year in normal controls over the age of 55. For the young participants, sensitivities in the superior (57.3 dB ± 4.2 SD) field were lower than in the inferior (58.7 dB ± 3.6 SD; P < 0.0001) field whereas the nasal (57.7 dB ± 4.2 SD) and temporal (58.1 dB ± 4.0 SD; P = 0.1302) fields were not different (Figs. 3A, 3B; Table 3). The middle age group had lower sensitivity in the superior (57.8 dB ± 4.4 SD) field compared to the inferior (58.5 dB ± 3.5 SD; P < 0.0001) field, but no difference was found between the nasal (57.6 dB ± 4.4 SD) and temporal (58.2 dB ± 3.7 SD) fields (P = 0.0773). The mature group had lower MS in the superior (52.3 dB ± 6.9 SD) than in the inferior (55.7 dB ± 4.6 SD) field (P < 0.0001; Fig. 3A; Table 3). The sensitivity in the nasal (54.3 dB ± 5.5 SD) field was not different than that in the temporal (53.5 dB ± 6.4 SD) field (p 0.1699; Fig. 3B; Table 3).
Intrasession Repeatability
Intrasession repeatability was analyzed using two exams that were obtained during the same visit for 24 normal controls (35 years ± 15 SD) and 34 patients with IRD (40 years ± 18 SD). Participants had a 5-minute break between tests 1 and 2 for the intrasession DAC perimetry exams. Grouped by individual, the average MS of tests 1 and 2 for normal controls was 57.4 dB ± 2.2 SD and 57.3 dB ± 2.1 SD (P = 0.962), respectively. The MS of tests 1 and 2 for patients with IRD was 25.9 dB ± 15.3 SD and 25.7 dB ± 15.5 SD (P = 0.259), respectively. The CR for MS in normal controls and patients with IRD was ±1.5 dB (Table 2). Controls and patients with IRD showed an increase in the CR with field eccentricity (Table 2), suggesting that measures were less reliable in the far periphery than in the central field.
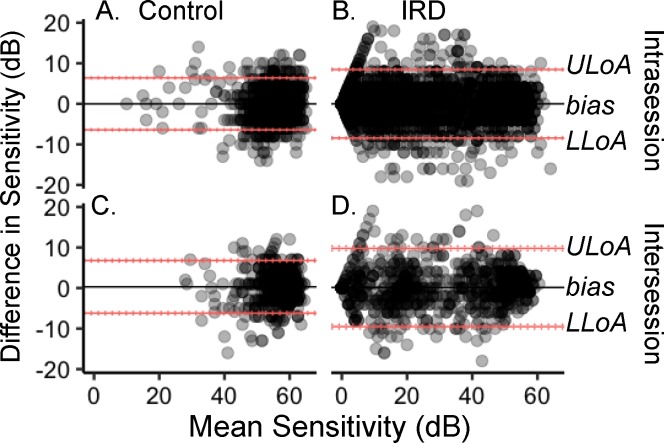
For PWS evaluated in normal controls and patients with IRD, the within-group, between-test means were not different (controls 58.2 dB ± 3.1 SD and 58.7 dB ± 4.1 SD, P = 0.5395; IRD 25.7 dB ± 4.6 SD and 25.7 dB ± 4.8 SD, P = 0.9888). Figure 4 is a Bland-Altman39 analysis with the pointwise MS on the x-axis and the pointwise-level differences for each participant on the y-axis. The average pointwise difference, or bias (solid lines), for intrasession (Figs. 4A, 4B) repeat testing was close to zero, indicating minimal systematic errors for controls or patients with IRD.
Figure 4.
Bland-Altman plots of (A) control intrasession pointwise sensitivity, (B) IRD intrasession PWS, (C) control intersession PWS, and (D) IRD intersession PWS. Sensitivity difference between tests is the bias (black solid lines). The upper and lower limits of agreement (ULoA and LLoA, respectively) are the red solid lines with the 95% CI shown as the dotted red lines around each LoA.
Intersession Repeatability
Intersession repeat testing PWS differences between tests revealed minimal systematic errors for controls (Fig. 4C) or patients with IRD (Fig. 4D) as indicated by the near-zero bias lines (black lines). The horizontal red lines indicate the ULoA and LLoA and the vertical red lines are the 95% CIs around each LoA.
Eight of the normal controls who performed intrasession testing plus two additional controls (10 total, 33 years ± 15 SD) returned to repeat testing with an average of 6.8 weeks ± 3.4 SD between visits. Fourteen patients with IRD (40 years ± 15 SD) returned within 8 weeks ± 6.5 SD for DAC intersession repeat testing. The MS for tests 1 and 3 were not different for controls (57 dB ± 2.0 SD and 57.2 dB ± 1.2 SD, P = 0.875) or for patients with IRD (27.1 dB ± 17.6 and 26.9 dB ± 17.6 SD; P = 0.983). Normal control CR for MS was ± 1.7 dB. Patients with IRD had CR for MS that was ±3.3 dB (Table 2). The CR for MS increased with eccentricity for both the control and the patient groups (Table 2).
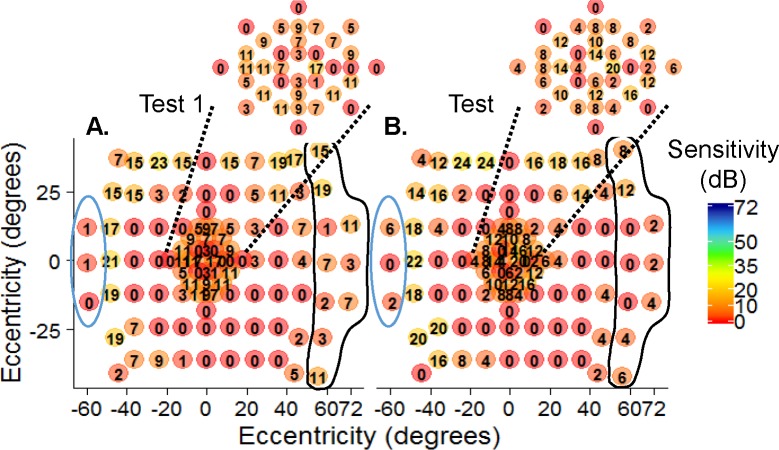
Intersession PWS for tests 1 and 3 were similar (57.2 dB ± 3.2 SD and 57.8 dB ± 3.1 SD, respectively; P = 0.3319) among normal controls. Likewise, patients with IRD showed no difference in the PWS measured at different visits (28.0 dB ± 4.1 SD and 27.8 dB ± 4.1 SD; P = 0.8885). The magnitude of the PWS differences between test 1 and 3 is shown in Figure 4 for controls (Fig. 4C) and patients with IRD (Fig. 4D). The intersession CR for PWS was ±6.8 dB (Table 2) for controls and ±9.8 dB for patients with IRD. Both groups had CR for PWS that was lower in the central field compared to the mid- and far-peripheral fields (Table 2). A representative example in a patient (subject #7773; autosomal recessive retinitis pigmentosa; arRP) shows variations in sensitivity (circled) between test 1 (Fig. 5A) and test 3 (Fig. 5B) but a similar midperipheral absolute (0 dB) ring scotoma at both visits.
Figure 5.
Intersession repeat testing. Subject #7773 (recessive retinitis pigmentosa) had comparable sensitivity between test 1 (A) and test 3 (B). This subject had a ring scotoma in the midperiphery (0 dB) measured with 44 days between visits. The far periphery (circled areas) had noticeable pointwise variability. Macular sensitivities (insets).
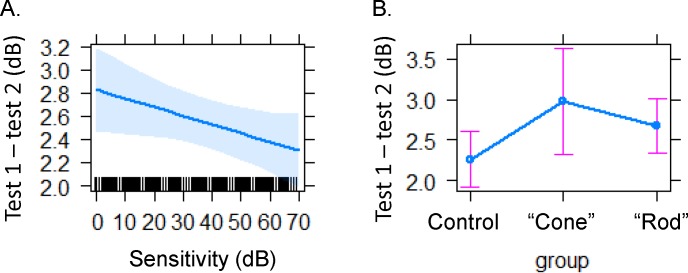
Patients with IRD consistently had higher CR than controls (Table 2). This prompted us to evaluate the effects of sensitivity on pointwise variability between tests. All participants with intrasession exams were included in the analysis. Indeed, the magnitude of PWS influenced the absolute variability between tests 1 and 2. Lower sensitivities had higher PWS absolute variability (P < 0.0012; Fig. 6A). However, variability could be the result of fixation instability, such as has been shown in patients with AMD.30 Here we used the absolute differences between tests to determine whether maculopathy patients had less reliable responses. Patients with IRD were separated into two groups. One was named the “cone” group; the primary disease was toward the cones or the macula, and the group included patients diagnosed with cone–rod dystrophy or autosomal dominant macular or pattern dystrophy. The second group, named the “rod” group, included diseases that initially affect the rods; these were patients diagnosed with Usher's syndrome II or recessive, dominant, X-linked, and isolate retinitis pigmentosa. The absolute variability between tests trended higher for the cone group and lowest for normal controls but was not statistically significant (Fig. 6B).
Figure 6.
Variability is lower with higher sensitivity (A) The absolute variability in pointwise sensitivity decreased with higher sensitivities for controls and patients with IRD. (B) Absolute PWS difference between tests was lower for controls and highest for patients in the cone group (autosomal dominant macular, pattern, or cone–rod dystrophy). The rod group (X-linked, X-linked carrier, recessive, isolate, or autosomal dominant retinitis pigmentosa and Usher's syndrome II).
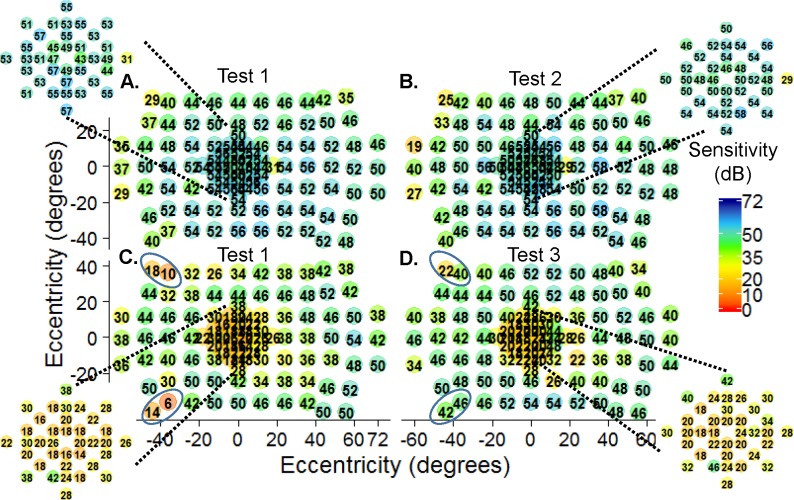
Fixation was recorded on the MP1-S to determine whether patients had unstable fixation. By the Fujii categories,31 only 3 out of 13 subjects (#8126, #11728, and #4339) had unstable fixation, that is, <75% of all fixation points within the 4° circle. Interestingly, all three subjects (#8126, #11728, and #4339) had good reliability (range, 0–4% false positives) for each of their DAC exams despite the unstable fixation measured by fundus perimetry. Figures 7A and 7B are the first and second intrasession tests, respectively, from subject #11728, who was diagnosed with autosomal dominant pattern dystrophy (adPD) due to a mutation (c. 629C>G, pPro210Arg) in the gene Peripherin-2 (previously known as Rod Degeneration Slow, PRPH2/RDS). Subject #11728 had BCVA of 20/50 (0.4 logMAR) in the tested eye and was the only cone group patient with “unstable” fixation (1 SD BCEA of 20.2 deg2). Interestingly, there was no evidence of fixation instability on the DAC fields as the two tests had similar PWS. Subject #11313 had the same PRPH2 mutation (c. 629C>G, pPro210Arg) as subject #11728 but was diagnosed with autosomal dominant macular degeneration (adMD). Test 1 (Fig. 7C) was similar to test 3 (Fig. 7D) except in the circled regions where nose and eyebrow artifacts were evident. This subject is a 36-year-old with a BCVA of 20/63 (0.5 logMAR). Despite the association of fixation instability and macular dystrophy, subject #11313 had “stable” fixation (1 SD BCEA 3.9 deg2). The two subjects, #11728 and #11313, had similar reliability for each DAC perimetry exam. Subject #11728 had 0%, 4%, and 0% and subject #11313 had 3%, 2%, and 4% false positives for tests 1, 2, and 3, respectively.
Figure 7.
Intra- (A, B) and intersession (C, D) repeat testing. Intrasession DAC perimetry for subject #11728 (adPD, BCVA: 20/50 or 0.4 logMAR) showed similar sensitivities between the (A) first and (B) second exams despite “unstable” fixation and 1 SD BCEA 20.2 deg2. (C) Test 1 was similar to (D) test 3 for subject #11313 (adMD, BCVA: 20/63 or 0.5 logMAR) and showed stable fixation (1 SD BCEA 3.9 deg2). Sensitivity due to nasal and eyebrow shadowing are circled for test 1 and test 3 for subject #11313. Macular sensitivities (insets).
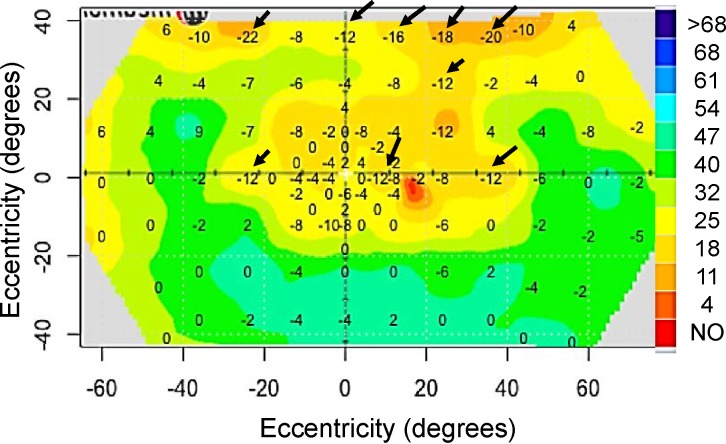
Greater variability in measured sensitivity may reflect measurements at transition zones, areas between healthy and degenerating retina, or areas without photoreceptors such as the optic nerve head.16,40,41 To validate this, we looked at five fields with the most difference in PWS between tests 1 and 2 and found that test points with highest variability were at an area of transition. Figure 8 highlights the greatest pointwise differences (arrows) at transition zones between tests for patient #4339. The controls also showed decreased sensitivity and higher variability at transition zones such as points of nasal and/or eyebrow shadowing points at or near the blind spot, and points tested in the far periphery in the mature group.
Figure 8.
Sensitivity difference between the first and second DAC exam. For patient #4339 there was more variability in pointwise sensitivity where the slope of the hill of vision is steep (arrows).
Discussion
Studies designed to test aging effects in normal controls have shown that scotopic retinal function starts decreasing in later years.24–28,42 Sturr et al.25 determined that scotopic sensitivity decreases at a mean rate of 0.4 dB per decade of life before age 53.4 years and then by 1.0 dB per decade thereafter. Here we found an overall 1.2-dB decrease in sensitivity per decade. For the mature group, each decade was associated with lower sensitivity by −2.0 dB whereas for the middle and young ages this was 1.0 and 0.4 dB, respectively. The difference in these studies was that we tested many points throughout the visual field whereas the previous study tested only one point at 10° nasal on the horizontal midline. In another study on aging effects, Jackson et al.26 reported that individuals ∼70 years of age lost 0.5 log unit of scotopic sensitivity compared to younger subjects at ∼27 years of age.26 They factored in lens density and ARM and found no difference in the older controls with and without ARM. Although we did not control for lens density, we are confident that ARM did not affect our mature group. The previous study used the MS of four points tested at 4°, 7°, 32°, and 38° along the horizontal midline.26 If we compare our young group to the mature group and exclude the far-peripheral points, we find that sensitivity in the mature group was affected by −4.7 dB ± 1.0 SE compared to the young group, which is similar to the previous study. True aging affects would be better established with a larger cohort of older controls that were tested multiple times over several years. Nevertheless, our results suggest that beginning at ∼55 years, scotopic sensitivity decreases and differences between the central and the far-peripheral retina are exacerbated, similarly to reports on aging effects on sensitivity determined with photopic perimetry.17,18,20
Differences were found between the superior and inferior fields for normal controls under dark-adapted conditions. This hemifield difference was magnified in the normal controls >55 years of age. Previous studies have shown that retinal sensitivity is lower in the superior field compared to the inferior field under both photopic20–22,43–45 and scotopic conditions.8 One proposed explanation is that the superior retina (inferior field) is exposed to less light compared to the inferior retina (superior field).17,46 Another possibility is that the topography of the human retina has a higher density of ganglion cells in the superior retina, which may account for the hemifield sensitivity differences.47 Also, eyelids can cast shadows and cause lower sensitivity in the superior field.21,48 Indeed, when we went back and looked for possible eyelid artifacts, we found that both the nasal and temporal fields had points with lower sensitivity and more variability between tests at the eyelid and nasal points (see Figs. 2B, 2C, 6D) compared to other nearby points. Additionally, locations corresponding to nose artifacts were excluded, thereby removing lower sensitivities from the mean, which may have led to an overestimation of the regional differences reported here.
We have recently shown two methods by which rod mediation can be detected in scotopic perimetry. The first compares cyan sensitivity to cone sensitivity at the same points. Any sensitivity above that of cone threshold would have to be mediated by rods, which are >2 log units more sensitive to short wavelengths than are cones. The second method isolates rod function by determining PWS differences between responses to cyan and red stimuli. Spectral properties of receptors dictate that more than a 20-dB difference in sensitivity to cyan than to red means that rods are detecting both stimuli at a given point. However, differences with sensitivity to red greater than to cyan would indicate that cone receptors were mediating detection of the stimuli. No attempt was made to determine whether the scotopic sensitivities in patients were being mediated by rods or cones, but we recognize that it would have been desirable to have used two-color perimetry to determine the locations where scotopic sensitivity was mediated by rods. In fact, the additional fields required for this assessment would have placed excessive demands on the patients, who already had to take three fields over a short period of time. The CR values reported here reflect the inherent variability in scotopic testing. For normal controls, this is the variability in rod-mediated sensitivity. However, for some patients/locations scotopic sensitivity may be rod or cone mediated, and therefore future work should include repeat measures after determining which receptor mediated detection of the stimuli.
As with any commercialized perimeter, variability in repeat measures needs to be established so that a significant change in function can be recognized. Patients with X-linked retinoschisis, a juvenile macular degeneration, had a similar CR for PWS for better/worse eye, which was 6.8 ± 3.6/5.4 ± 1.6 dB when tested under mesopic conditions with the MP1.49 Similar results were reported in patients with AMD (PWS CR ± 4.1 dB) who were tested on a MAIA microperimeter.41 This group discovered a learning effect between the first and second tests but not subsequent testing.41 We did not find significant learning effects on sensitivity in this study, but it is important to note that none of the participants in this study were naïve to perimetry. Another possibility is that learning effects were masked by the higher number of points tested in this study because learning effects would be more evident when a small number of points are tested.
The CR has been evaluated under dark-adapted conditions as well. The CR for MS/PWS in patients with maculopathy was ±2.2/±5.6 dB when tested with the S-MAIA.50 Similar results were found in another study using the DAC perimeter to determine the reliability for scotopic testing in the central field. To offset the low number of subjects tested and normalize distribution, an application of bootstrapping metric (random sampling with replacement), was used by Tan et al.16 to find the CR for MS/PWS for patients with AMD (±1.8/±4.2 dB). Additionally, they found that approximately 80% of all test points had a PWS difference less than 5 dB between tests for intra-/intersession measurements. That study reported that intrasession PWS CR for controls and patients with AMD was ±8.4 and ±9.5 dB, respectively.16 They also found worse repeatability for points tested at greater eccentricities (4°–24°).16 The CR for PWS reported in our study were lower than theirs (before bootstrapping) for controls (central ±5.4 Db and midperiphery ±6.2 dB) and for patients with IRD (central ±8.5 dB and midperiphery ±8.6 dB; Table 2). Intersession PWS CR was also different between ours (control ±6.8 dB/IRD ±9.8 dB) and the previous study (control: +8.2 dB/IRD ±11.7 dB). Differences may relate to the fact that we had more patients and controls and that we tested younger patients with IRD and age-matched controls. Our patients ranged in age from 10 years to 75, with 46 participants under the age of 50. Because the previous study was on patients with AMD, their patients and controls were older (range, 58–81 years).16 They argued that fixation should be steady in their patients with AMD because they were assessed early in disease when they had good acuities.16 In contrast, we included younger patients with acuities as low as 20/500 (1.4 logMAR; Table 1) but we found lower CR. We found no differences in the absolute variability between tests for the rod and cone groups (Fig. 5), which suggests that overall, patients in this study had stable fixation or that fixation instability does not contribute to variability in sensitivity measured with the DAC perimeter. The only patients in our study with “unstable” fixation also had a good reliability factor (few false positives). These results support the conclusion that older controls have lower scotopic sensitivity and more variability in sensitivity measured between tests. Recently, Cideciyan et al.51 reported ultrawide dark-adapted CR (9.6 dB) for PWS from patients with X-linked RP, which was comparable to the PWS CR we found in patients with IRD (8.5 dB).51 The CR was slightly higher in their study, but they also tested more points at greater eccentricity, and we showed here that the CR increases with eccentricity.
Similar to others,16,40,41 we found that the variability of responses in psychophysical tests was greater in patients than in normal subjects. The high CR is greater in patients with retinal disease (Table 2) and may reflect points tested at the transition zone, which is the area between healthy and degenerate retina.52,53 This area has a steep slope in the hill of vision, meaning that a slight adjustment in patient position may cause a point undetected in a deep scotoma to move to an area of retina where the point would be detected (Fig. 7). Comparison of PWS measured over time would likely be more variable for points tested at transition zones. A quantitative solution could be analyzing the volume of the hill of vision.54 This would specify the depth of scotopic vision whereby gross changes in scotomas or visual area would be noticed by visual inspection of the topographic rendering of a DAC perimetry exam.
The main drawback to the DAC perimeter, unlike fundus perimeters, is that it does not include a retina tracking feature for precision testing. Even so, the DAC is an important asset that tests the full field of scotopic vision in IRD. It provides a topographic map that makes it easy to know where healthy and degenerative changes occur. This information will be important for targeted therapy designed to prevent change or to rescue the diseased retina.
Acknowledgments
Supported by the Foundation Fighting Blindness (DGB), as well as the National Eye Institute of the National Institutes of Health under Award Numbers K99EY027460 (LDB) and EY09076 (DGB). The authors alone are responsible for the content and writing of the paper.
Disclosure: L.D. Bennett, None; G. Metz, None; M. Klein, None; K.G. Locke, None; A. Khwaja, None; D.G. Birch, None
References
- 1.Hafler BP. Clinical progress in inherited retinal degenerations: gene therapy clinical trials and advances in genetic sequencing. Retina. 2017;37:417–423. doi: 10.1097/IAE.0000000000001341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birch DG, Wen Y, Locke K, Hood DC. Rod sensitivity, cone sensitivity, and photoreceptor layer thickness in retinal degenerative diseases. Invest Ophthalmol Vis Sci. 2011;52:7141–7147. doi: 10.1167/iovs.11-7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crossland MD, Luong VA, Rubin GS, Fitzke FW. Retinal specific measurement of dark-adapted visual function: validation of a modified microperimeter. BMC Ophthalmol. 2011;11:5. doi: 10.1186/1471-2415-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu H, Bittencourt MG, Wang J, et al. Assessment of central retinal sensitivity employing two types of microperimetry devices. Trans Vis Sci Tech. 2014;3(5):3. doi: 10.1167/tvst.3.5.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roman AJ, Schwartz SB, Aleman TS, et al. Quantifying rod photoreceptor-mediated vision in retinal degenerations: dark-adapted thresholds as outcome measures. Exp Eye Res. 2005;80:259–272. doi: 10.1016/j.exer.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Kiser AK, Mladenovich D, Eshraghi F, Bourdeau D, Dagnelie G. Reliability and consistency of dark-adapted psychophysical measures in advanced eye disease. Invest Ophthalmol Vis Sci. 2006;47:444–452. doi: 10.1167/iovs.04-1146. [DOI] [PubMed] [Google Scholar]
- 7.Birch DG, Locke KG, Felius J, et al. Rates of decline in regions of the visual field defined by frequency-domain optical coherence tomography in patients with RPGR-mediated X-linked retinitis pigmentosa. Ophthalmology. 2015;122:833–839. doi: 10.1016/j.ophtha.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birch DG, Herman WK, deFaller JM, Disbrow DT, Birch EE. The relationship between rod perimetric thresholds and full-field rod ERGs in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1987;28:954–965. [PubMed] [Google Scholar]
- 9.Bennett LD, Klein M, Locke KG, Kiser K, Birch DG. Dark-adapted chromatic perimetry for measuring rod visual fields in patients with retinitis pigmentosa. Tran Vis Sci Tech. 2017;6(4):15. doi: 10.1167/tvst.6.4.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massof RW, Finkelstein D. Rod sensitivity relative to cone sensitivity in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1979;18:263–272. [PubMed] [Google Scholar]
- 11.Wald G. On rhodopsin in solution. J Gen Physiol. 1938;21:795–832. doi: 10.1085/jgp.21.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wald G. Human vision and the spectrum. Science. 1945;101:653–658. doi: 10.1126/science.101.2635.653. [DOI] [PubMed] [Google Scholar]
- 13.Cideciyan AV, Hood DC, Huang Y, et al. Disease sequence from mutant rhodopsin allele to rod and cone photoreceptor degeneration in man. Proc Natl Acad Sci U S A. 1998;95:7103–7108. doi: 10.1073/pnas.95.12.7103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobson SG, Voigt WJ, Parel JM, et al. Automated light- and dark-adapted perimetry for evaluating retinitis pigmentosa. Ophthalmology. 1986;93:1604–1611. doi: 10.1016/s0161-6420(86)33522-x. [DOI] [PubMed] [Google Scholar]
- 15.Birch DG, Anderson JL. Rod visual fields in cone-rod degeneration. Comparisons to retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1990;31:2288–2299. [PubMed] [Google Scholar]
- 16.Tan RS, Guymer RH, Luu CD. Repeatability of retinal sensitivity measurements using a Medmont dark-adapted chromatic perimeter in healthy and age-related macular degeneration cases. Trans Vis Sci Tech. 2018;7(3):3. doi: 10.1167/tvst.7.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hermann A, Paetzold J, Vonthein R, Krapp E, Rauscher S, Schiefer U. Age-dependent normative values for differential luminance sensitivity in automated static perimetry using the Octopus 101. Acta Ophthalmol. 2008;86:446–455. doi: 10.1111/j.1600-0420.2007.01055.x. [DOI] [PubMed] [Google Scholar]
- 18.Spry PG, Johnson CA. Senescent changes of the normal visual field: an age-old problem. Optom Vis Sci. 2001;78:436–441. doi: 10.1097/00006324-200106000-00017. [DOI] [PubMed] [Google Scholar]
- 19.Drance SM, Berry V, Hughes A. Studies on the effects of age on the central and peripheral isopters of the visual field in normal subjects. Am J Ophthalmol. 1967;63:1667–1672. doi: 10.1016/0002-9394(67)93644-6. [DOI] [PubMed] [Google Scholar]
- 20.Brenton RS, Phelps CD. The normal visual field on the Humphrey field analyzer. Ophthalmologica. 1986;193:56–74. doi: 10.1159/000309679. [DOI] [PubMed] [Google Scholar]
- 21.Katz J, Sommer A. Asymmetry and variation in the normal hill of vision. Arch Ophthalmol. 1986;104:65–68. doi: 10.1001/archopht.1986.01050130075023. [DOI] [PubMed] [Google Scholar]
- 22.Lorch L, Dietrich TJ, Schwabe R, Schiefer U. Comparison of local differential luminance sensitivity (dls) between Oculus Twinfield Perimeter and Humphrey Field Analyzer 630 (HFA I) in normal volunteers of varying ages [in German] Klin Monbl Augenheilkd. 2001;218:782–794. doi: 10.1055/s-2001-19689. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs NA, Patterson IH. Variability of the hill of vision and its significance in automated perimetry. Br J Ophthalmol. 1985;69:824–826. doi: 10.1136/bjo.69.11.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Werner JS. Night vision in the elderly: consequences for seeing through a “blue filtering” intraocular lens. Br J Ophthalmol. 2005;89:1518–1521. doi: 10.1136/bjo.2005.073734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sturr JF, Zhang L, Taub HA, Hannon DJ, Jackowski MM. Psychophysical evidence for losses in rod sensitivity in the aging visual system. Vision Res. 1997;37:475–481. doi: 10.1016/s0042-6989(96)00196-4. [DOI] [PubMed] [Google Scholar]
- 26.Jackson GR, Owsley C, Cordle EP, Finley CD. Aging and scotopic sensitivity. Vision Res. 1998;38:3655–3662. doi: 10.1016/s0042-6989(98)00044-3. [DOI] [PubMed] [Google Scholar]
- 27.Jackson GR, Owsley C. Scotopic sensitivity during adulthood. Vision Res. 2000;40:2467–2473. doi: 10.1016/s0042-6989(00)00108-5. [DOI] [PubMed] [Google Scholar]
- 28.Hammond BR, Jr, Wenzel AJ, Luther MS, Rivera RO, King SJ, Choate ML. Scotopic sensitivity: relation to age, dietary patterns, and smoking status. Optom Vis Sci. 1998;75:867–872. doi: 10.1097/00006324-199812000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Steinman RM. Effect of target size, luminance, and color on monocular fixation. J Opt Soc Am. 1965;55:1158–1164. [Google Scholar]
- 30.Bellmann C, Feely M, Crossland MD, Kabanarou SA, Rubin GS. Fixation stability using central and pericentral fixation targets in patients with age-related macular degeneration. Ophthalmology. 2004;111:2265–2270. doi: 10.1016/j.ophtha.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 31.Fujii GY, De Juan E, Jr, Humayun MS, Sunness JS, Chang TS, Rossi JV. Characteristics of visual loss by scanning laser ophthalmoscope microperimetry in eyes with subfoveal choroidal neovascularization secondary to age-related macular degeneration. Am J Ophthalmol. 2003;136:1067–1078. doi: 10.1016/s0002-9394(03)00663-9. [DOI] [PubMed] [Google Scholar]
- 32.Team RC. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 33.Bates D, Mächler M, Bolker B, Walker S. lme4: linear mixed-effects models using Eigen and S4. R package version 1.1-8. Available at: https://www.researchgate.net/publication/279236477_Package_Lme4_Linear_Mixed-Effects_Models_Using_Eigen_and_S4 Accessed March 14, 2019.
- 34.Millar RB. Maximum Likelihood Estimation and Inference. Indianapolis, IN: John Wiley & Sons; 2011. Latent variable models. [Google Scholar]
- 35.Ickstadt K, Wolpert RL. Spatial regression for marked point processes. In: Bernardo JM, Berger JO, Dawid AP, et al., editors. Bayesian Statistics 6. Oxford: Clarendon Press; 1999. pp. 323–341. [Google Scholar]
- 36.Robinson GK. That BLUP is a good thing: the estimation of random effects. Stat Sci. 1991;6:15–32. [Google Scholar]
- 37.Chambers JM, Hastie T. Statistical Models in S. Wadsworth & Brooks/Cole Advanced Books & Software; 1992. [Google Scholar]
- 38.Luke SG. Evaluating significance in linear mixed-effects models in R. Behav Res Methods. 2017;49:1494–1502. doi: 10.3758/s13428-016-0809-y. [DOI] [PubMed] [Google Scholar]
- 39.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 40.Palkovits S, Hirnschall N, Georgiev S, Leisser C, Findl O. Test-retest reproducibility of the microperimeter MP3 with fundus image tracking in healthy subjects and patients with macular disease. Tran Vis Sci Tech. 2018;7(1):17. doi: 10.1167/tvst.7.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu Z, Ayton LN, Guymer RH, Luu CD. Intrasession test-retest variability of microperimetry in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013;54:7378–7385. doi: 10.1167/iovs.13-12617. [DOI] [PubMed] [Google Scholar]
- 42.Birch DG, Anderson JL. Standardized full-field electroretinography. Normal values and their variation with age. Arch Ophthalmol. 1992;110:1571–1576. doi: 10.1001/archopht.1992.01080230071024. [DOI] [PubMed] [Google Scholar]
- 43.Heijl A, Lindgren G, Olsson J. Normal variability of static perimetric threshold values across the central visual field. Arch Ophthalmol. 1987;105:1544–1549. doi: 10.1001/archopht.1987.01060110090039. [DOI] [PubMed] [Google Scholar]
- 44.Casson EJ, Johnson CA, Nelson-Quigg JM. Temporal modulation perimetry: the effects of aging and eccentricity on sensitivity in normals. Invest Ophthalmol Vis Sci. 1993;34:3096–3102. [PubMed] [Google Scholar]
- 45.Zulauf M. Normal visual fields measured with Octopus Program G1. I. Differential light sensitivity at individual test locations. Graefes Arch Clin Exp Ophthalmol. 1994;232:509–515. doi: 10.1007/BF00181992. [DOI] [PubMed] [Google Scholar]
- 46.Curcio CA, Allen KA. Topography of ganglion cells in human retina. J Comp Neurol. 1990;300:5–25. doi: 10.1002/cne.903000103. [DOI] [PubMed] [Google Scholar]
- 47.Curcio CA. Photoreceptor topography in ageing and age-related maculopathy. Eye. 2001;15:376–383. doi: 10.1038/eye.2001.140. [DOI] [PubMed] [Google Scholar]
- 48.Dietrich TJ, Ata N, Sanger A, Selig B, Schiefer U, Benda N. Perimetry Update 1998/1999, Proceedings of the XIIIth International Perimetric Society Meeting. The Hague, The Netherlands: Kugler Publications; 1999. Age influences asymmetry in differential luminance sensitivity; pp. 223–227. [Google Scholar]
- 49.Jeffrey BG, Cukras CA, Vitale S, Turriff A, Bowles K, Sieving PA. Test-retest intervisit variability of functional and structural parameters in X-linked retinoschisis. Tran Vis Sci Tech. 2014;3(5):5. doi: 10.1167/tvst.3.5.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pfau M, Lindner M, Muller PL, et al. Effective dynamic range and retest reliability of dark-adapted two-color fundus-controlled perimetry in patients with macular diseases. Invest Ophthalmol Vis Sci. 2017;58:BIO158–BIO167. doi: 10.1167/iovs.17-21454. [DOI] [PubMed] [Google Scholar]
- 51.Cideciyan AV, Charng J, Roman AJ, et al. Progression in X-linked retinitis pigmentosa due to ORF15-RPGR mutations: assessment of localized vision changes over 2 years. Invest Ophthalmol Vis Sci. 2018;59:4558–4566. doi: 10.1167/iovs.18-24931. [DOI] [PubMed] [Google Scholar]
- 52.Chen FK, Patel PJ, Xing W, et al. Test-retest variability of microperimetry using the Nidek MP1 in patients with macular disease. Invest Ophthalmol Vis Sci. 2009;50:3464–3472. doi: 10.1167/iovs.08-2926. [DOI] [PubMed] [Google Scholar]
- 53.Anastasakis A, McAnany JJ, Fishman GA, Seiple WH. Clinical value, normative retinal sensitivity values, and intrasession repeatability using a combined spectral domain optical coherence tomography/scanning laser ophthalmoscope microperimeter. Eye. 2011;25:245–251. doi: 10.1038/eye.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weleber RG, Smith TB, Peters D, et al. VFMA: topographic analysis of sensitivity data from full-field static perimetry. Tran Vis Sci Tech. 2015;4(2):14. doi: 10.1167/tvst.4.2.14. [DOI] [PMC free article] [PubMed] [Google Scholar]