Abstract
In the placenta, the breast cancer resistance protein (BCRP)/ABCG2 efflux transporter limits the maternal-to-fetal transfer of drugs and chemicals. Previous research has pointed to the estrogenic mycotoxin zearalenone as a potential substrate for BCRP. Here, we sought to assess the role of the BCRP transporter in the transplacental disposition of zearalenone during pregnancy. In vitro transwell transport assays employing BCRP/Bcrp-transfected Madine-Darby canine kidney cells and BeWo trophoblasts with reduced BCRP expression were used to characterize the impact of BCRP on the bidirectional transport of zearalenone. In both models, the presence of BCRP protein increased the basolateral-to-apical transport and reduced the apical-to-basolateral transport of zearalenone over a 2-h period. In vivo pharmacokinetic analyses were then performed using pregnant wild-type and Bcrp−/− mice after a single tail vein injection of zearalenone. Zearalenone and its metabolite α-zearalenol were detectable in serum, placentas, and fetuses from all animals, and β-zearalenol was detected in serum and fetuses, but not placentas. There were no significant differences in the maternal serum concentrations of any analytes between the two genotypes. In Bcrp−/− mice, the free fetal concentrations of zearalenone, α-zearalenol, and β-zearalenol were increased by 115%, 84%, and 150%, respectively, when compared with wild-type mice. Concentrations of free zearalenone and α-zearalenol were elevated 145% and 78% in Bcrp−/− placentas, respectively, when compared with wild-type placentas. Taken together, these data indicate that the placental BCRP transporter functions to reduce the fetal accumulation of zearalenone, which may impact susceptibility to developmental toxicities associated with in utero zearalenone exposure.
Keywords: BCRP, placenta, ABCG2, zearalenone, mycotoxin
During pregnancy, the placenta develops from the blastocyst and regulates the flow of nutrients, waste, and gases between the maternal and fetal circulations. Trophoblasts, the parenchymal cells of the placenta, fuse to form a syncytium that inhibits direct contact of the two blood supplies providing a physical and biochemical protective barrier for the developing fetus (Gupta et al., 2016). The breast cancer resistance protein (BCRP/ABCG2) is an efflux transporter enriched on the maternal-facing surface of syncytiotrophoblasts. On the apical membrane, BCRP prevents the transepithelial passage of xenobiotics. Substrates of BCRP include endogenous chemicals, such as certain steroids and bile acids, pharmaceuticals, such as the diabetes drug glyburide and the antibiotic nitrofurantoin, as well as dietary contaminants, such as the plasticizer bisphenol A and phytoestrogen genistein. Compromised BCRP function in the placenta may consequently increase the risk of the fetus to chemical exposures during pregnancy. It is therefore critical to characterize the interaction of environmental and dietary contaminants with placental BCRP.
Recently, in vitro screening performed by our laboratory identified zearalenone as a substrate of the human BCRP transporter (Xiao et al., 2015). Zearalenone is an estrogenic mycotoxin produced by Fusarium fungi that grows on cereal crops in moist climates. The European Union has established the acceptable maximum for zearalenone in food at 4 µg/kg (European Commission, 2006), but multiple studies worldwide have demonstrated that commonly consumed foods often exceed this level (Iqbal et al., 2014; Lahouar et al., 2018; Ok et al., 2014; Tralamazza et al., 2016). Furthermore, analysis of urine from a cohort of prepubescent girls in New Jersey demonstrated that free zearalenone was present in the range of 0.2–8.4 ng/ml and was correlated with delayed puberty onset (Bandera et al., 2011). It should also be noted that α-zearalanone, a zearalenone metabolite marketed under the tradename Ralgro, is commonly used to increase feed-to-weight ratios in cattle. Ralgro is currently used as a growth promoter in the United States but has been banned by the European Union. Exposure to xenoestrogens in utero is well-understood to induce adverse developmental effects (Hines, 2011). Specifically, in utero exposure to zearalenone causes precocious puberty and mammary proliferation in both female Wistar rats and C57BL/6 mice (Belli et al., 2010; Hilakivi-Clarke et al., 1998). Zearalenone exposure is potentially common during pregnancy, with one study reporting detectable levels of zearalenone in the urine of 11 out of 30 pregnant women tested (Fleck et al., 2016).
From prior in vivo rodent studies, zearalenone does cross the placenta into the fetal compartment, but the transplacental transfer of zearalenone appears to be limited (Appelgren et al., 1982; Bernhoft et al., 2001; Koraichi et al., 2012). Although multiple factors can regulate xenobiotic disposition, these data could point to placental efflux as a potential mechanism for regulating fetal exposure to zearalenone. To date, no studies have evaluated the ability of the BCRP transporter to regulate the fetoplacental disposition of zearalenone. Therefore, the purpose of this study was to comprehensively assess whether BCRP restricts the maternal-to-fetal transfer of zearalenone using in vitro and in vivo models of the human placental barrier.
MATERIALS AND METHODS
Chemicals
Unless stated otherwise, all chemicals were purchased from Sigma-Aldrich (St. Louis, Missouri).
Cell culture and lentiviral knockout of BCRP
All cell culture and transport experiments were performed in an incubator at 37°C with 5% CO2 in HEPA-filtered air. Marine-Darby canine kidney (MCDK) cells (passages 3–15), transfected with human (hBCRP) or mouse (mBcrp) BCRP constructs or empty vector, were provided by Dr Alfred Schinkel (Netherlands Cancer Institute) and maintained in DMEM (Life Technologies, Carlsbad, California) supplemented with 10% fetal bovine serum (Atlantic Biologicals, Frederick, Maryland) and 1% penicillin-streptomycin (Durmus et al., 2012). BeWo-b30 human choriocarcinoma cells (passages 25–45) were provided by Dr Nicholas Illsley (Hackensack University Medical Center) and maintained in DMEM: F12 (Life Technologies) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin (Vardhana and Illsley, 2002). Stable BeWo knockdown cells were generated using ABCG2 (sc-41151-V, Santa Cruz) or control (sc-108080) lentiviral shRNA particles. Cells were grown to 70% confluence on a 96-well plate before incubation for 24 h in DMEM:F12 containing 5 μg/ml polybrene (Santa Cruz) and 2 viral particles per cell (60 000 particles/well). Subsequently, cells were subcultured in a 24-well plate, and stable transfected clones were selected using 6.5 μg/ml puromycin over 48 h.
Animal treatment
Bcrp−/− mice were obtained from Taconic Biosciences (Taconic, New York) and backcrossed to the C57BL/6 background (027 strain, Charles River Laboratories, Wilmington, Massachusetts) until >99% congenic (Rutgers RUCDR Infinite Biologics, Piscataway, New Jersey). Adult female and male C57BL/6 wild-type and Bcrp−/− mice were mated overnight with the same genotype. The presence of the sperm plug denoted gestational day 0. Mice were provided phytoestrogen-free food and water ad libitum. At gestation day 14, mice were administered 10 mg/kg zearalenone dissolved in DMSO:PEG400:Saline (1:5:4 v/v) by tail vein injection (n = 4–5 dams per genotype). Two additional dams per genotype received vehicle to generate tissue matrices used for standard curves. At 1 h post injection, blood (cardiac puncture), placentas, and fetuses were collected. Blood samples were centrifuged for 15 min at 600 × g to isolate sera. All samples were stored at −80°C until analysis by LC-MS. Animal studies were approved by the Rutgers IACUC Committee.
Western blotting
MDCK and BeWo cell lysates for Western blot were collected and stored in buffer containing 20 mM Tris-HCl, 150 mM NaCl, 5 mM ethylenediamine tetraacetic acid, 1% Triton X-100 and a protease inhibitor cocktail (Sigma P8340, 1%, v/v). Mouse placenta homogenates were prepared in sucrose-Tris-HCl buffer (250 mM sucrose and 10 mM Tris-HCl, pH 7.5) supplemented with protease inhibitor cocktail (Sigma P8340, 1%, v/v) using a bead homogenizer (Tissue-Lyser LT, Qiagen) for 3 min at 40 Hz. Unless indicated otherwise, all Western blotting was performed with equipment from BioRad (Hercules, California) as described previously (Zheng et al., 2013). All samples were spun down at 1000 × g for 10 min. Thirty micrograms of protein homogenates were loaded onto 4%–12% Tris-HCl gels, electrophoretically separated, and transferred to nitrocellulose membranes. After blocking in 5% nonfat milk for 2 h, membranes were incubated overnight at 4°C in 2% nonfat milk containing primary antibodies used to detect BCRP (BXP-53, 1:5000; Enzo Life Sciences, Farmingdale, New York), β-actin (ab8227, 1:2000, Abcam, Cambridge, Massachusetts), or glyceraldehyde 3-phosphate dehydrogenase (GAPDH, 1:2000, ab9485, Abcam), followed by incubation with either HRP-linked rabbit or HRP-linked rat secondary antibodies (1:1000, 2 h, Cell Signaling Technologies, Danvers, Massachusetts). After incubating membranes briefly with Luminata Forte Western HRP substrate (Millipore, Billerica, Massachusetts), protein-antibody complexes were visualized using a Fluorchem Imager (ProteinSimple, Santa Clara, California).
Fluorescent substrate retention
For fluorescent substrate retention transport studies, BeWo or MDCK cells were added to 96-well round-bottom plates (100 000 cells/well). Uptake phase. Cells were incubated in 100 μl DMEM (MDCK) or DMEM: F12 (BeWo) containing 5 μM Hoechst 33342, a fluorescent BCRP substrate, or 10 μM Rhodamine 123, a fluorescent MDR1 substrate for 30 min. A parallel treatment group included cells also incubated with 1 μM Ko143, an established BCRP inhibitor (Bircsak et al., 2013), or 5 μM PSC833, an established MDR1 inhibitor (Wen et al., 2014). Efflux phase. The substrate-containing media was removed and replaced with substrate-free media. Cells were then incubated an additional 1 h. The cells were then washed and re-suspended in 50 μl ice-cold HBSS and set on ice for analysis. A Cellometer Vision automated cell counter (Nexcelom Bioscience, Lawrence, Massachusetts) fitted with a VB-450-302 filter (excitation/emission = 375/450 nm) or VC-535-403 (excitation/emission = 470/535 nm) was used to quantify intracellular fluorescence of Hoechst 33342 or Rhodamine 123, respectively. The total number of cells analyzed for each sample ranged from 500 to 2000, and fluorescence was normalized for cell size.
Transwell transport
MDCK or BeWo cells were seeded at a density of 100 000 and 200 000 cells per well, respectively, on a collagen-coated 24-well multiwall insert system (cat. no. 351181, 1.0 μM pore, high-density PET membrane, Corning, Tewksbury, Massachusetts). Transport assays were performed at 37°C without agitation on days 3–4 post seeding on those wells with a transepithelial electrical resistance (TEER) value greater than 250 Ω * cm2 for MDCK cells (Yang et al., 2016) and 80–160 Ω * cm2 for BeWo cells (Li et al., 2013a) as measured immediately before and after the experiment. Monolayer integrity was also assessed by measuring the rejection percentage of Lucifer Yellow (20 μM) (Hidalgo et al., 1989) using % Rejection = (1 – Cr/(Cr + Cd)) × 100, where Cr is the final concentration in the receiver compartment and Cd is the final concentration in the donor compartment (Nkabinde et al., 2012). On the day of experiments, media in both the apical and basolateral compartment were replaced with HBSS. The test compound (1 μM BODIPY-glyburide, 50 μM zearalenone, or 10 μM Rhodamine 123) was added to the donor compartment at time = 0 and a 100-μl aliquot was collected from the receiver compartment every 30 min. Fluorescence was measured using a SpectraMax M3 spectrophotometer (Molecular Devices, San Jose, California). BODIPY-glyburide and Lucifer Yellow were measured using Ex/Em: 428/540 nm, and Rhodamine was measured using Ex/Em: 507/529 nm. Zearalenone was quantified by HPLC-UV. Permeability coefficients were calculated using Papp (cm/s) = (Q/t)/(A * C0), where Q is glyburide (nmol) transported to the receiver compartment at time t (s), A is the cell surface area (cm2), and C0 is the initial concentration of test substrate (μM) (Li et al., 2013a). Flux ratios were calculated using the equation (PappB − A)/(PappA − B).
Messenger RNA quantification
Mouse placentas were homogenized using a bead homogenizer (Tissue-Lyser LT, Qiagen) for 4 min at 40 Hz in RNAzol RT. Total RNA was isolated from lysates according to the manufacturer’s protocol. RNA content and purity were determined by measuring absorbance at 260 nm using a NanoDrop (Fisher Scientific). cDNA was generated from total RNA (1000 ng) with the High-Capacity cDNA Reverse Transcription Kit (ThermoFisher) and a MultiGene OptiMax Thermal Cycler (Labnet International Inc., Edison, New Jersey) according to the manufacturer’s instructions. Quantitative PCR was performed with cDNA, Sybr Green dye (Life Technologies), forward and reverse primers (see Supplementary Table 1) (Integrated DNA Technologies, Inc., Coralville, Iowa), and a ViiA7 RT-PCR System (Life Technologies). Ct values were converted to ΔΔCt values in comparison with the housekeeping gene ribosomal protein 13A (Rpl13A).
Quantification of zearalenone and metabolites
Quantification of free zearalenone (parent compound only) in aliquots from transwell transport experiments was performed using an HPLC-UV system (Jasco, Easton, Maryland) equipped with a PU-4185 binary pump, UV 4075 detector (254 nm), AS-2055 autosampler, Zorbax Eclipse 3 mm × 15 cm C18 column (Agilent, Santa Clara, California) (adapted from De Baere et al., 2012). Peak areas were quantified using ChromNav V2 and compared with a standard curve. An isocratic mobile phase consisting of H2O:acetonitrile (3:2, formic acid added to pH = 3.0) was used.
To quantify free and total zearalenone and metabolites from in vivo experiments, analyte extracts were prepared from sera and tissue and quantified by LC-MS. Neither zearalenone nor its metabolites were detected in sera or tissues of vehicle-treated dams (data not shown). For sera, samples were added onto ChemElut solid-phase extraction columns (1 ml, unbuffered, 12198002, Agilent), and analytes were eluted using methyl tert-butyl ether (3 × 2 ml). Eluent samples were then dried under N2, re-dissolved in methanol, added onto preconditioned Discovery DSC-NH2 solid-phase extraction columns, and eluted with methanol (1 ml). Samples were dried again before being re-dissolved in LC-MS mobile phase (see below). Placentas and fetuses were first weighed and homogenized in sodium acetate buffer (0.2 M, pH 4.65) before incubating overnight in the presence or absence of β-glucuronidase (1000 U/sample) at 37°C with gentle shaking. Liquid-liquid extraction was then performed on the homogenates by adding methyl tert-butyl ether (3 × 2 ml), vortexed for 30 s, and centrifuged at 1000 × g for 10 min. The ether phase was removed and the extraction was repeated 2 more times. Extracts were dried under N2, reconstituted in n-hexane:dichloromethane (3:2, 1 ml), and added onto preconditioned silica Sep-Pak solid-phase extraction columns (500 mg/3 cc, 186004615, Waters, Milford, Massachusetts). Samples were washed with ethyl acetate: n-hexane (6:94, 2 ml), and then the analytes were eluted with ethyl acetate:n-hexane (25:75, 2 ml) then ethyl acetate (neat, 3 ml). Eluates were dried under N2 before being re-dissolved in LC-MS mobile phase (see below).
Quantification of zearalenone and its metabolites in extracts prepared from mouse tissues and serum was performed using an LC-MS system (Thermo Fisher) equipped with an Accela UPLC pump and autosampler (4°C), a 100 × 4.6-mm betasil phenyl hexyl column (35°C) (Phenomenex, Torrance, California), and a Thermo Scientific LTQ XL mass spectrometer (Waltham, Massachusetts) with an atmospheric pressure chemical ionization (APCI) source. The mobile-phase gradient used was started at water:methanol:(0.1% formic acid added):acetonitrile (2:1:1), then ramped to 5:47.5:47.5 over 5–11 min then back to initial after 15 min. Spiked sample matrices were used for quality control (>80% recovery) and run with each sample batch. The inter/intra-day variability (%RSD) was 4.5/4.0, 3.2/3.1, and 2.6/2.5 for zearalenone, α-zearalenol, and β-zearalenol, respectively. The detection limit for this method was 0.05 ng/ml, the lowest used in the standard curve (signal-to-noise ratio > 3). Peak areas were quantified using Xcalibur and normalized to milliliter (serum) or milligram (tissues).
Statistical analysis
Data are presented as mean ± SD and analyzed using GraphPad Prism 5.0 software (GraphPad Software Inc., La Jolla, California). Depending upon the number of comparisons, either 1-way or 2-way analysis of variance with Newman-Keuls or Bonferroni posttest, respectively, or an unpaired student’s t test was used to assess statistical significance (p < .05).
RESULTS
Transporter Expression and Activity in Transfected hBCRP and mBcrp MDCK Cells
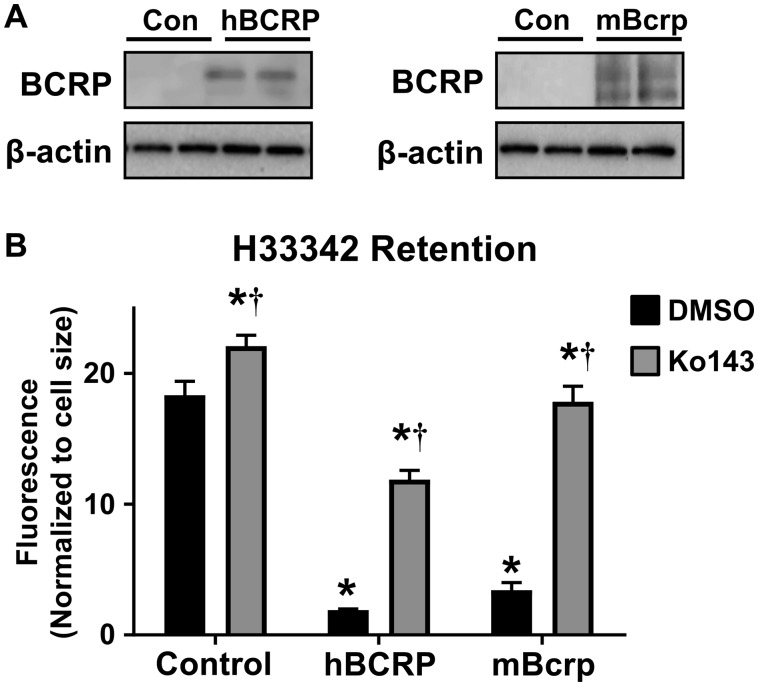
The ability of human BCRP and mouse Bcrp proteins to transport zearalenone was first assessed in MDCK cells transfected with full-length BCRP/Bcrp plasmids (Durmus et al., 2012). Western blot analysis of MDCK cells demonstrated that only the hBCRP and mBcrp transfected cell lines expressed BCRP/Bcrp protein, with no detectable bands in the empty vector control cells (Figure 1A). H33342, a fluorescent substrate of BCRP, was used to assess BCRP activity using a cell accumulation assay. The intracellular retention of H33342 was reduced 80%–90% in MDCK cells transfected with hBCRP and mBcrp (Figure 1B). Ko143, an inhibitor of BCRP, increased the cellular accumulation of H33342 by 20%, 1000%, and 425% in control, hBCRP, and mBcrp MDCK cells, respectively (Figure 1B).
Figure 1.
Characterization of BCRP expression and activity in hBCRP- and mBcrp-transfected MDCK cells. A, Western blot of transfected and control MDCK cells analyzing BCRP/Bcrp protein expression (72 kDa). β-actin (42 kDa) was used as a loading control. B, Retention of H33342 (10 μM) in empty vector control and transfected MDCK cells. Ko143 (1 μM) was used as a pharmacological inhibitor of BCRP/Bcrp (n = 6). Asterisks (*) represent statistically significant differences (p < .05) compared with control/vehicle. Daggers (†) represent statistically significant differences (p < .05) compared with vehicle within treatment group. Data represent the mean ± SD.
Transepithelial Transport of Zearalenone in Transfected hBCRP and mBcrp MDCK Cells
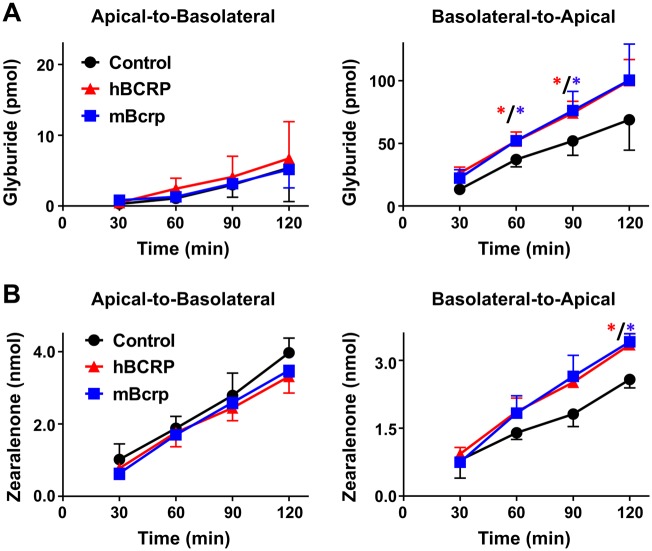
As an efflux transporter that localizes to the apical membrane of cells, BCRP/Bcrp enables the basolateral-to-apical transport of chemicals in polarized epithelium. In transwell cultures, BODIPY-glyburide was used as a probe BCRP/Bcrp substrate (Bircsak et al., 2016; Gedeon et al., 2008; Hemauer et al., 2010; Zhou et al., 2008) to confirm the polarization and transepithelial activity of BCRP/Bcrp. For these experiments, BODIPY-glyburide was added to the donor compartment and fluorescence quantified in the receiver compartment. The time-dependent increase of glyburide in the receiver compartment was linear (Figure 2, R2 = 0.95–0.99). When grown in transwell inserts, MDCK cells exhibited minimal apical-to-basolateral transport of glyburide that was unaffected by the expression of BCRP/Bcrp (Figure 2A). As expected, both hBCRP and mBcrp MDCK cells significantly increased the basolateral-to-apical transport of glyburide (Figure 2A).
Figure 2.
Transport of glyburide and zearalenone by MDCK cells in transwell cultures. MDCK cells grown on transwell inserts were assessed for translocation of (A) BODIPY-glyburide (1 μM) and (B) zearalenone (50 μM) across cell monolayers for 2 h as described in the Materials and Methods. Data represent the mean pmol detected in the receiver compartment ± SD (n = 3 independent experiments). Asterisks (*) represent statistically significant differences (p < .05) compared with control cells.
Similar to experiments with glyburide, the increase of zearalenone in the receiver compartment was linear and time dependent (Figure 2B, R2 = 0.98–0.99). Although zearalenone was transported in the apical-to-basolateral direction, this transfer was minimally affected by expression of BCRP/Bcrp (Figure 2B). By comparison, both hBCRP- and mBcrp-transfected MDCK cells exhibited significantly increased the basolateral-to-apical transport of zearalenone (Figure 2B).
BCRP shares an overlapping substrate profile with the multidrug resistance protein 1 (MDR1, P-glycoprotein). Notably, MDCK cells express endogenous canine MDR1 (Li et al., 2013b) (Supplementary Figure 1). We confirmed canine MDR1 activity using the MDR1 substrate, Rhodamine 123. In MDCK cells, the MDR1 inhibitor PSC833 significantly reduced the basolateral-to-apical transport of Rhodamine 123 at all time points (Figure 3A) but had no impact on the disposition of zearalenone (Figure 3B). Interestingly, transfection of hBCRP reduced expression of endogenous canine MDR1 protein mBcrp in MDCK cells (Supplementary Figure 1). Taken together, these data point to an ability for mouse and human BCRP, but not canine MDR1, to transport zearalenone in MDCK cells.
Figure 3.
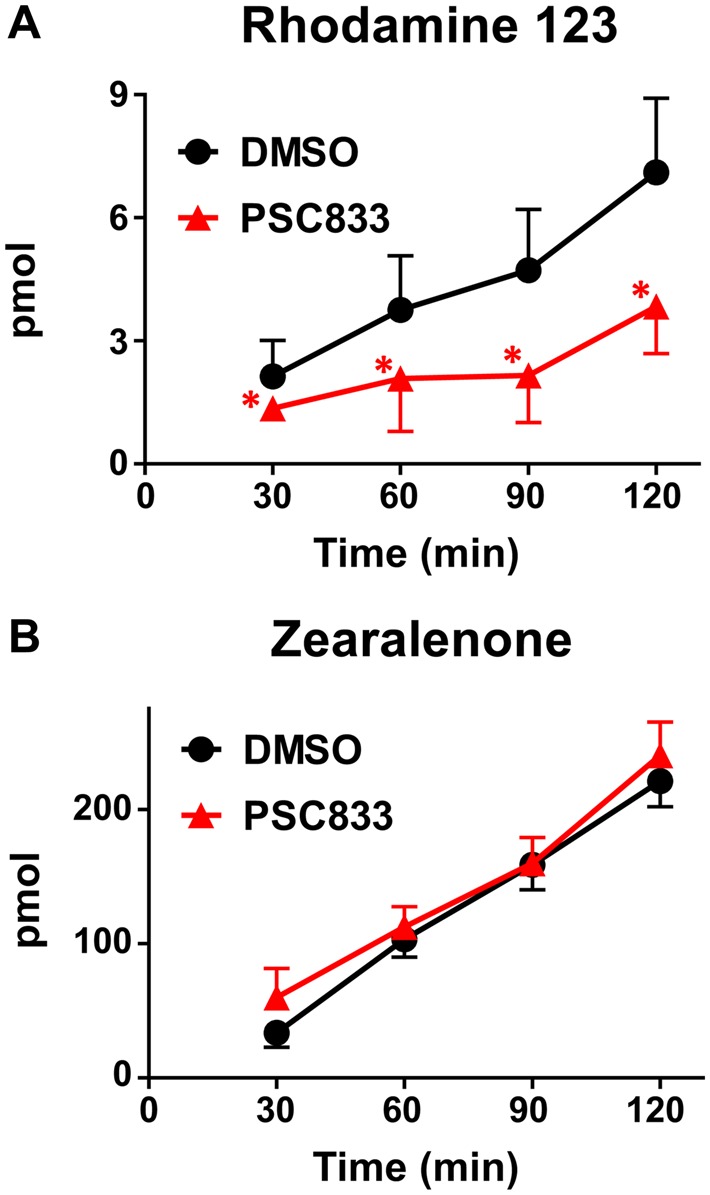
Basolateral-to-apical transport of zearalenone and Rhodamine 123 in MDCK cells in the presence of a MDR1 inhibitor. Control MDCK cells grown on transwell inserts were assessed for translocation of (A) the MDR1 substrate Rhodamine 123 (10 μM) and (B) zearalenone (50 μM) across cell monolayers for 2 h as described in the Materials and Methods. PSC833 (5 μM) was used as a pharmacological inhibitor of MDR1. Data represent the mean pmol detected in the receiver compartment ± SD (n = 3–4). Asterisks (*) represent statistically significant differences (p < .05) compared with control cells.
BCRP Expression and Activity in Human Placental Cells Following Lentiviral Knockdown
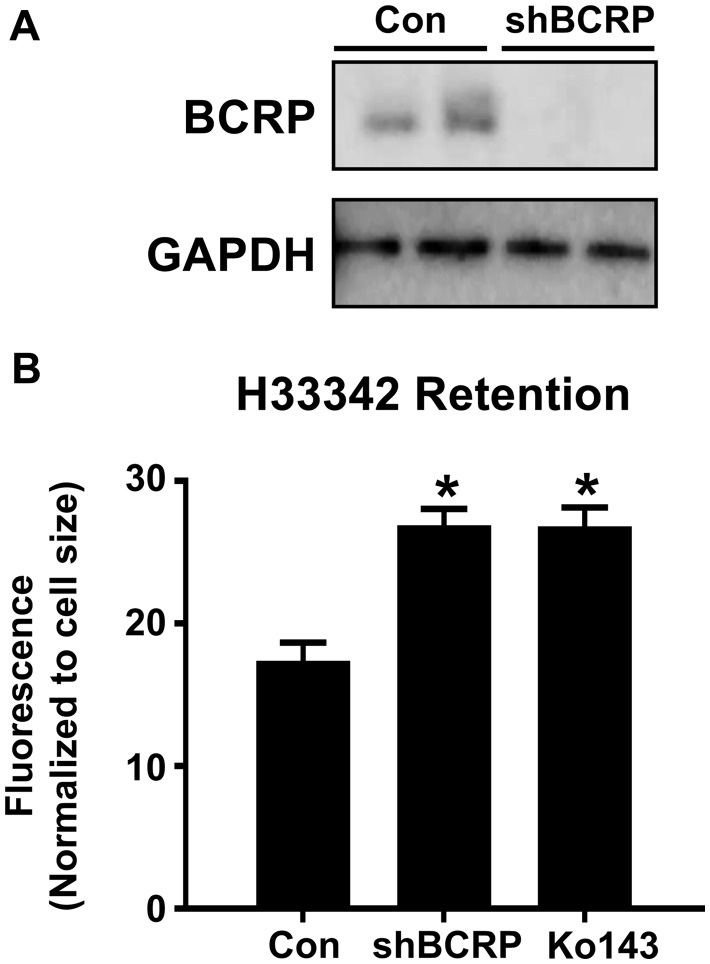
To recapitulate the human placental barrier, a second set of studies were performed using human BeWo b30 trophoblast cells that polarize when grown on transwell inserts. Consistent with prior reports (Ceckova et al., 2006; Mitra and Audus, 2010), BeWo cells highly express BCRP protein (Figure 4A). Lentiviral knockdown of BCRP in BeWo b30 cells using targeted shRNA particles reduced BCRP protein to levels below detection (Figure 4A). Similarly, compared with BeWo b30 cells infected with control shRNAs, BCRP mRNA expression was reduced 88% (data not shown). As a result of BCRP knockdown, H33342 retention was increased by 53% in BeWo b30 cells (Figure 4B). The enhanced accumulation of H33342 in BCRP knockdown cells was similar to the pharmacological inhibitor of BCRP, Ko143 (Figure 4B).
Figure 4.
Characterization of BCRP protein and activity in BeWo shBCRP cells. A, Western blot of BCRP protein (72 kDa) in lysates from BeWo cells after treatment with control or shBCRP lentiviral particles. GAPDH (37 kDa) was used as a loading control. B, Retention of H33342 (5 μM) in control and shBCRP BeWo cells. Ko143 (1 μM) was used as a pharmacological inhibitor of BCRP (n = 6). Asterisks (*) represent statistically significant differences (p < .05) compared with control. Data represent the mean ± SD.
Transepithelial Transport of Zearalenone in Human Placental Cells Following Lentiviral Knockdown of BCRP
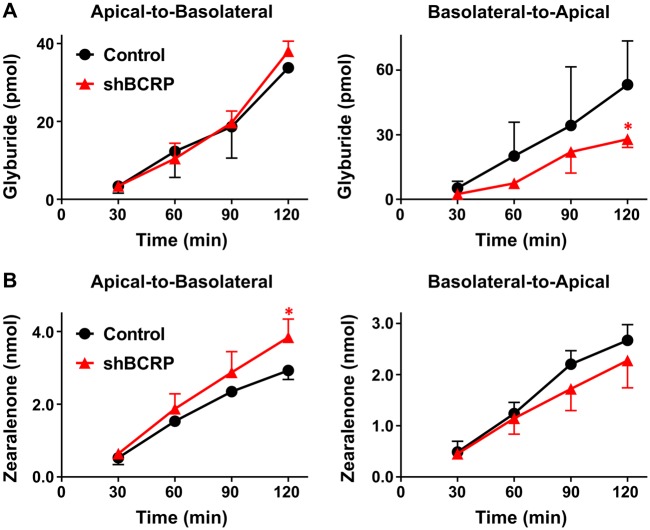
In the placenta, BCRP mediates the fetal (basolateral) to maternal (apical) translocation of xenobiotics. When grown in transwell inserts, BeWo b30 cells exhibited greater transfer of BODIPY-glyburide in the basolateral-to-apical direction compared with the apical-to-basolateral direction. Although there was no difference in the apical-to-basolateral transport of glyburide between control and shBCRP BeWo cells (Figure 5A), the basolateral-to-apical transport of glyburide was significantly decreased by 50% in shBCRP BeWo cells (Figure 5A). These data confirm that BCRP was properly polarized to the apical surface of BeWo b30 cells and functional in transferring the known substrate glyburide. By comparison, the apical-to-basolateral transport of zearalenone was significantly increased in shBCRP BeWo cells compared with control cells (Figure 5B), and this difference was observed when the starting concentration was as low as 5 μM (Supplementary Figure 2). The basolateral-to-apical transport of zearalenone also tended to decrease in shBCRP cells (Figure 5B).
Figure 5.
Transport of glyburide and zearalenone by BeWo cells in transwell cultures. BeWo cells grown on transwell inserts were assessed for translocation of (A) BODIPY-glyburide (1 μM) and (B) zearalenone (50 μM) across cell monolayers for 2 h as described in the Materials and Methods. Data represent the mean pmol detected in the receiver compartment ± SD (n = 3 independent experiments). Asterisks (*) represent statistically significant differences (p < .05) compared with control cells.
For all transwell experiments, the observed effects of BCRP on glyburide and zearalenone transport were reflected in the respective permeability coefficients and flux ratios, presented in Table 1. Lucifer yellow was used in transwell experiments to confirm monolayer integrity (determined by TEER values), and the percent rejection of Lucifer yellow was 98.6% ± 1.5% and 95.0% ± 0.6% in MDCK and BeWo cell transwell cultures, respectively. BCRP expression did not impact Lucifer yellow rejection in either MDCK or BeWo cells (data not shown).
Table 1.
Permeability Coefficients of Glyburide and Zearalenone in BeWo and MDCK Transwell Culture Experiments
| Glyburide |
Zearalenone |
|||||
|---|---|---|---|---|---|---|
| Papp A-B | Papp B-A | Flux Ratio | Papp A-B | Papp B-A | Flux Ratio | |
| b30 | 1.4E-03 | 2.2E-03 | 1.58 | 2.3E-06 | 2.3E-06 | 0.98 |
| b30 shBCRP | 1.6E-03 | 1.2E-03 | 0.73 | 3.2E-06 | 1.9E-06 | 0.60 |
| MDCK | 2.2E-04 | 2.9E-03 | 13.03 | 3.2E-05 | 2.2E-05 | 0.68 |
| MDCK hBCRP | 2.1E-04 | 4.3E-03 | 20.39 | 2.8E-05 | 2.8E-05 | 1.01 |
| MDCK mBcrp | 2.2E-04 | 4.2E-03 | 19.45 | 2.7E-05 | 2.9E-05 | 1.07 |
Papp expressed in cm/s.
Distribution of Zearalenone and Its Metabolites in Wild-Type and Bcrp−/− Mice
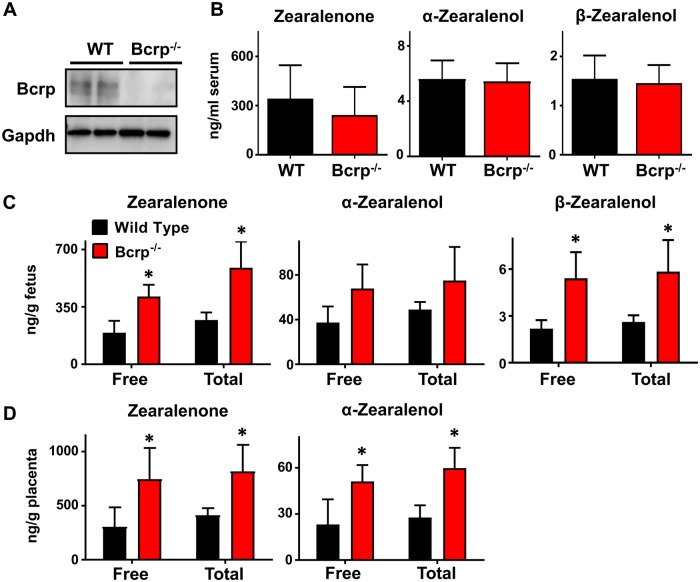
After confirming that both mouse and human BCRP proteins could transport zearalenone and that BCRP can influence the directional transfer of the mycoestrogen in placental cells, the transplacental transfer of zearalenone was assessed in pregnant wild-type and Bcrp−/− mice. In placentas from gestation day 14, Bcrp protein was detected in wild-type but not Bcrp−/− mice (Figure 6A). Placentas from Bcrp−/− mice also displayed a > 99% reduction in Bcrp mRNA (data not shown).
Figure 6.
Quantification of zearalenone and its metabolites in pregnant wild-type and Bcrp−/− mice. A, Western blot of placental homogenates from pregnant wild-type and Bcrp−/− mice analyzing BCRP protein expression (72 kDa) on gestation day 14. GAPDH (37 kDa) was used as a loading control. B–D, Concentration of zearalenone and its metabolites detected in serum (B), fetuses (C), and matched placentas (D) 1 h after tail vein injection as determined by LC-MS. Total concentration was determined after incubation of samples with β-glucuronidase overnight at 37°C. Data represent the mean ± SD (n = 4–5). Asterisks (*) represent statistically significant differences (p < .05) compared with wild-type mice.
After an IV injection of zearalenone to pregnant wild-type and Bcrp−/− mice, zearalenone and its metabolites were quantified in mouse serum, placenta, and fetuses by LC-MS. After 1 h, serum concentrations of free zearalenone, α-zearalenol, and β-zearalenol ranged from 108 to 588, 4 to 7, and 1 to 2 ng/ml, respectively (Figure 6B). There were no significant differences in the serum concentrations of any analyte between wild-type and Bcrp−/− mice (Figure 6B). For placental and fetal tissues, both free and total (free + deconjugated) zearalenone and its metabolites were quantified. In fetal tissues of Bcrp−/− mice, free/total zearalenone, α-zearalenol, and β-zearalenol concentrations increased by 115%/118%, 84%/53%, and 150%/100%, respectively, compared with wild-type mice (Figure 6C). In matched placentas of Bcrp−/− mice, free/total zearalenone and α-zearalenol concentrations were increased by 145%/99% and 122%/114%, respectively, compared with wild-type mice (Figure 6D). β-zearalenol was not detected in placentas from either genotype (data not shown). All samples were also assessed for the presence for additional metabolites including zearalanone, α-zearalanol, and β-zearalanol, but these analytes were below the detection limit (data not shown).
Relative Expression of Transporters and Drug-Metabolizing Enzymes in Wild-Type and Bcrp−/− Mouse Placentas
To rule out compensatory changes in placental gene expression that could impact the metabolism and disposition of zearalenone in vivo, the mRNA expression of transporters and drug-metabolizing enzymes were compared between wild-type and Bcrp−/− placentas (Table 2). The majority of transcripts analyzed demonstrated no statistically significant difference between genotypes. Oatp2b1, Oatp3a1, and Oatp5a1 mRNA levels were significantly but minimally altered in Bcrp−/− placentas (+31%, +29%, and −27% compared with wild-type placentas, respectively). Cyp1a1 and sulfatase 1 were also significantly altered in Bcrp−/− placentas (+37% and −24%, respectively). Transcripts for Cyp3a11, Oatp1a5, and Oatp2a1 were also analyzed but could not be detected.
Table 2.
Expression of Uptake and Efflux Transporters and Metabolism Genes in Bcrp−/− Mouse Placentas
| Gene | Fold Change | p < .05 |
|---|---|---|
| Slc Transporters | ||
| Oct1 | 1.12 | N |
| Oct2 | 0.87 | N |
| Oct3 | 0.88 | N |
| Oat1 | 0.95 | N |
| Oat2 | 0.92 | N |
| Oat3 | 1.13 | N |
| Octn1 | 0.97 | N |
| Octn2 | 0.97 | N |
| Octn3 | 0.96 | N |
| Ent1 | 1.07 | N |
| Mate1 | 1.07 | N |
| Mate2 | 0.96 | N |
| Oatp1a4 | 0.89 | N |
| Oatp2b1 | 1.31 | Y |
| Oatp3a1 | 1.29 | Y |
| Oatp4a1 | 1.05 | N |
| Oatp5a1 | 0.73 | Y |
| Abc Transporters | ||
| Abca1 | 1.19 | N |
| Bcrp | 2.70E-04 | Y |
| Mdr1a | 1.08 | N |
| Mdr1b | 1.13 | N |
| Mrp1 | 1.16 | N |
| Mrp2 | 0.79 | N |
| Mrp3 | 1.27 | N |
| Mrp4 | 1.23 | N |
| Mrp5 | 1.14 | N |
| Mrp6 | 1.19 | N |
| Mrp7 | 1.13 | N |
| Phase I and II Enzymes | ||
| Cyp1a1 | 1.37 | Y |
| Cyp1a2 | 1.31 | N |
| Cyp1b1 | 0.94 | N |
| Cyp27a1 | 1.01 | N |
| Cyp2b10 | 1.19 | Y |
| Cyp2e1 | 1.57 | N |
| Gusb | 1.02 | N |
| Sulf1 | 0.76 | Y |
| Sulf2 | 1.02 | N |
| Sult1a1 | 1.09 | N |
| Sult1e1 | 1.03 | N |
Data represent the mean fold-changes in Bcrp−/− placentas compared with wild-type placentas (n = 6). Y represents statistically significant differences (p < .05) compared with wild-type mice.
DISCUSSION
In the placenta, BCRP protects the fetus from toxicant exposure by preventing transport from the maternal to the fetal circulation. Therefore, we sought to determine whether BCRP can prevent the maternal-to-fetal transfer of the mycotoxin, zearalenone. Previous work from our laboratory demonstrated that zearalenone is a novel substrate of BCRP using basic screening techniques including BCRP substrate retention and ATPase activity (Xiao et al., 2015). Zearalenone inhibited BCRP activity in membrane vesicles (using ATPase activity with sulfasalazine and Lucifer yellow uptake) and in BeWo cells (using the H33342 retention assay) (Xiao et al., 2015). Furthermore, the presence of the BCRP inhibitor Ko143 increased zearalenone retention in BeWo cells (Xiao et al., 2015). Building on these initial data, the current study employed complementary in vitro and in vivo approaches to address whether BCRP could limit the transplacental transfer of zearalenone. We compared the transport of zearalenone by mouse Bcrp and human BCRP isoforms, utilized an in vitro model of the human placental barrier, and quantified placental and fetal zearalenone concentrations in wild-type and Bcrp−/− dams.
In order to determine whether BCRP prevents fetal exposure to zearalenone, this study utilized techniques designed to more accurately represent the human placental barrier than cell retention-based transport methods. MDCK cells transfected with the hBCRP and mBCRP genes were used to elucidate the specific effect of BCRP on zearalenone disposition and to account for differences between homologs (human and mouse). After establishing that BCRP was present and functional in transfected MDCK cells, glyburide, a previously published substrate of BCRP in BeWo cells (Bircsak et al., 2016), was used to probe the impact of BCRP on vectoral transport. In MDCK cells, BCRP greatly increased the basolateral-to-apical transport of glyburide. The flux ratios of glyburide in control, hBCRP, and mBcrp MDCK cells were 6.38, 14.87, and 17.70, respectively. Only minimal glyburide was transported from the apical to the basolateral compartment, which could account for a lack of difference between the transfected and nontransfected MDCK cells. MDCK cells also express the canine Mdr1 transporter, which can transport glyburide. In MDCK cells, the presence of BCRP increased the basolateral-to-apical transport and decreased the apical-to-basolateral transport of zearalenone. Importantly, the MDR1 inhibitor PSC833 had no impact on the basolateral-to-apical transport of zearalenone, indicating that our observations with MDCK cells were specific to BCRP/Bcrp.
B30 cells, a subclone of the BeWo cell line that can form a monolayer, were used to recapitulate syncytiotrophoblasts. It has been previously demonstrated that BeWo cells secrete hormones, including human chorionic gonadotropin, similar to syncytiotrophoblasts (Pattillo and Gey, 1968; Pattillo et al., 1968). After confirming that shBCRP knockdown successfully reduced BCRP protein expression and activity, we examined the impact of BCRP on the bidirectional transport of glyburide and zearalenone. Similar to our observations with MDCK cells, BCRP knockdown reduced the basolateral-to-apical transport of glyburide by BeWo cells but had no effect in the opposite direction. In BeWo cells, the genetic knockdown of BCRP decreased the basolateral-to-apical transport and increased the apical-to-basolateral transport of zearalenone pointing to BCRP as a determinant of the transplacental disposition of this mycoestrogen.
In considering the data presented here, it is also important to recognize the potential role of other uptake and efflux transporters present at both the apical and basolateral membranes of either cell line. BeWo b30 cells have been reported to express MDR1 protein (Albekairi et al., 2015), but, contrary to those studies, we did not detect MDR1 transport of rhodamine (data not shown). As aforementioned, our data demonstrate that MDR1 does not transport zearalenone (Figure 3). Instead, zearalenone has also been shown to interact with MRP1-3, OAT1-4, OCT1, and OCT2 in vitro (Tachampa et al., 2008; Videmann et al., 2009). Because some of these transporters are also expressed in MDCK and BeWo cells, they may impact the efflux ratios of probe substrates as well as zearalenone (Table 1). This study focused specifically on the role of BCRP, and further work is needed to fully characterize involvement by other transporters in the transplacental disposition of zearalenone.
Pharmacokinetics studies with nitrofurantoin and glyburide in Bcrp−/− mice demonstrate that BCRP/Bcrp activity in the placenta prevents the fetal accumulation of BCRP substrates (Zhang et al., 2007; Zhou et al., 2008). The findings from the in vivo experiments in this study reflect those observed from in vitro transwell assays. Bcrp−/− mice displayed greater placental retention and maternal-to-fetal transfer of zearalenone and its metabolites compared with control without any significant differences in sera concentrations, litter size, placental weights, or fetal weights. It is therefore evident that Bcrp plays a role in protecting the fetus from exposure to zearalenone present in the maternal circulation. In considering the impact of BCRP on zearalenone pharmacokinetics, it is also important to note any compensatory transcriptional changes in other transporters and enzymes that result from deleting the Bcrp gene. Bcrp−/− placentas displayed a similar transcriptional profile of transporters to those of wild-type mice, with a few exceptions. Oatp2b1 and 3a1 were both slightly up-regulated (29%–31%) and Oatp5a1 was slightly down-regulated (27%). Oatp2b1 is basolateral transporter, and, if zearalenone is an Oatp2b1 substrate, an increase in transcript levels would decrease the transplacental transfer of zearalenone which was not observed in this study. By comparison, the subcellular localization of Oatp3a1 and 5a1 in mouse syncytiotrophoblasts and their contribution to zearalenone disposition are not currently understood. It is also unclear in this study if the metabolites of zearalenone cross the placenta or are formed from fetal hepatic metabolism of the parent compound. By gestation day 17, fetal livers of C57BL/6 mice do express a number of Cyps, including 2d26, 2d10, 2c68, and 2c69, but these isoforms have not yet been shown to metabolize zearalenone (Peng et al., 2012). It is known that zearalenone is metabolized to α-zearalenol and β-zearalenol by both human and murine 3α- and 3β-hydroxysteroid dehydrogenase, and both the parent compound and its metabolites can be glucuronidated in humans by UGT 1A1, 1A3, 1A8, and 2B7. BCRP is known to transport glucuronidated phase II metabolites of other compounds (An and Morris, 2011; Imai et al., 2003; Kiessling and Pettersson, 1978; Wu et al., 2012; Zamek-Gliszczynski et al., 2011). Although the glucuronidated metabolites have not been shown to be estrogenic, human fetal livers do express glucuronidase enzymes that may deconjugate the glucuronide-conjugated zearalenone metabolites. Additional work is therefore needed to thoroughly characterize the transport of these metabolites individually.
Prior studies have demonstrated that zearalenone crosses the placenta and impacts reproductive development in both female Wister rats and C57BL/6 mice (Belli et al., 2010; Hilakivi-Clarke et al., 1998). Specifically, zearalenone (0.2–5000 µg/kg/day for Wistar rats on GD9-PND5 and 2 µg/day for C57BL/6 mice on GD15-20) induced precocious puberty and mammary proliferation in both studies, raising the concern that similar effects may be seen in humans. Conversely, however, urinary zearalenone concentration has been correlated to delayed puberty in a cohort of New Jersey girls (Bandera et al., 2011). It is possible, however, that urinary concentrations of zearalenone indicate an increase in excretion rather than an increase in consumption. Alternately, zearalenone may act as an antagonist to endogenous estrogens. Zearalenone interacts with all three isoforms of estrogen receptor (ERα, ERβ, and GPR30) but is not as potent as 17β-estradiol (Kuiper-Goodman et al., 1987; Takemura et al., 2007; Zinedine et al., 2007). The specific effects of zearalenone within the fetus and placenta, therefore, may depend on the presence of endogenous estrogens.
There exist genetic, pathological, and toxicological factors that decrease placental BCRP activity and may therefore increase fetal exposure to zearalenone. The 421C > A (Q141K) BCRP polymorphism, for instance, significantly decreases BCRP protein expression (40%–50%) but not transcription (Bircsak et al., 2018). The 421C > A allele varies between ethnic groups, but is relatively common among Asian (32%) and Hispanic (28%) populations (Bircsak et al., 2018). Previous in vitro experiments with Q141K BCRP expressing human embryonic kidney cells demonstrate increased retention of H33342, glyburide, and zearalenone compared with those expressing wild-type BCRP protein (Bircsak et al., 2016; Xiao et al., 2015). However, further studies are needed to determine the impact of the 421C > A (Q141K) polymorphism on fetal drug accumulation in vivo. BCRP activity can also be directly inhibited by the previously mentioned substrates glyburide, genistein, and bisphenol A, which can all be encountered during pregnancy (Bircsak et al., 2016; Dankers et al., 2013). Moreover, BCRP expression is reduced by diseases of pregnancy involving placental dysfunction. Placentas from pregnancies with preeclampsia and intrauterine growth restriction, for instance, have demonstrated a marked reduction in BCRP mRNA and protein expression (Evseenko et al., 2007; Jebbink et al., 2015). Therefore, a number of factors that reduce BCRP expression and/or function in the placenta may alter fetal exposure to zearalenone.
Using a complement of in vitro and in vivo approaches, we have demonstrated that zearalenone is a substrate of human BCRP and mouse Bcrp and that BCRP/Bcrp in the placenta reduces the maternal-to-fetal transfer of zearalenone. There are a number of conditions in which placental BCRP function can be compromised which could potentially increase the maternal-to-fetal transfer of zearalenone. Taken together, these data suggest that women with compromised BCRP efflux may be at risk for higher fetal zearalenone exposure during pregnancy. Therefore, further studies are needed to characterize the effects of real world in utero exposure to zearalenone.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
FUNDING
National Institutes of Environmental Health Sciences (ES029275, ES020522, ES005022, ES007148, ES029794), a component of the National Institutes of Health.
CONFLICT OF INTEREST
No conflicts to declare.
Supplementary Material
REFERENCES
- Albekairi N. A., Al-Enazy S., Ali S., Rytting E. (2015). Transport of digoxin-loaded polymeric nanoparticles across BeWo cells, an in vitro model of human placental trophoblast. Ther. Deliv. 6, 1325–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An G., Morris M. E. (2011). The sulfated conjugate of biochanin A is a substrate of breast cancer resistant protein (ABCG2). Biopharm. Drug Disp. 32, 446–457. [DOI] [PubMed] [Google Scholar]
- Appelgren L. E., Arora R. G., Larsson P. (1982). Autoradiographic studies of [3h]zearalenone in mice. Toxicology 25, 243–253. [DOI] [PubMed] [Google Scholar]
- Bandera E. V., Chandran U., Buckley B., Lin Y., Isukapalli S., Marshall I., King M., Zarbl H. (2011). Urinary mycoestrogens, body size and breast development in New Jersey girls. Sci. Total Environ. 409, 5221–5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belli P., Bellaton C., Durand J., Balleydier S., Milhau N., Mure M., Mornex J. F., Benahmed M., Le Jan C. (2010). Fetal and neonatal exposure to the mycotoxin zearalenone induces phenotypic alterations in adult rat mammary gland. Food Chem. Toxicol. 48, 2818–2826. [DOI] [PubMed] [Google Scholar]
- Bernhoft A., Behrens G. H., Ingebrigtsen K., Langseth W., Berndt S., Haugen T. B., Grotmol T. (2001). Placental transfer of the estrogenic mycotoxin zearalenone in rats. Reprod. Toxicol. 15, 545–550. [DOI] [PubMed] [Google Scholar]
- Bircsak K. M., Gibson C. J., Robey R. W., Aleksunes L. M. (2013). Assessment of drug transporter function using fluorescent cell imaging. Curr. Protoc. Toxicol. 57, Unit 23.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bircsak K. M., Gupta V., Yuen P. Y., Gorczyca L., Weinberger B. I., Vetrano A. M., Aleksunes L. M. (2016). Genetic and dietary regulation of glyburide efflux by the human placental breast cancer resistance protein transporter. J. Pharmacol. Exp. Ther. 357, 103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bircsak K. M., Moscovitz J. E., Wen X., Archer F., Yuen P. Y. S., Mohammed M., Memon N., Weinberger B. I., Saba L. M., Vetrano A. M., et al. (2018). Interindividual regulation of the breast cancer resistance protein/ABCG2 transporter in term human placentas. Drug Metab. Disp. 46, 619–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceckova M., Libra A., Pavek P., Nachtigal P., Brabec M., Fuchs R., Staud F. (2006). Expression and functional activity of breast cancer resistance protein (BCRP, ABCG2) transporter in the human choriocarcinoma cell line BeWo. Clin. Exp. Pharmacol. Physiol. 33, 58–65. [DOI] [PubMed] [Google Scholar]
- Dankers A. C., Roelofs M. J., Piersma A. H., Sweep F. C., Russel F. G., van den Berg M., van Duursen M. B., Masereeuw R. (2013). Endocrine disruptors differentially target ATP-binding cassette transporters in the blood-testis barrier and affect Leydig cell testosterone secretion in vitro. Toxicol. Sci. 136, 382–391. [DOI] [PubMed] [Google Scholar]
- De Baere S., Osselaere A., Devreese M., Vanhaecke L., De Backer P., Croubels S. (2012). Development of a liquid-chromatography tandem mass spectrometry and ultra-high-performance liquid chromatography high-resolution mass spectrometry method for the quantitative determination of zearalenone and its major metabolites in chicken and pig plasma. Anal. Chim. Acta 756, 37–48. [DOI] [PubMed] [Google Scholar]
- Durmus S., Sparidans R. W., Wagenaar E., Beijnen J. H., Schinkel A. H. (2012). Oral availability and brain penetration of the B-RAFV600E inhibitor vemurafenib can be enhanced by the P-GLYCOprotein (ABCB1) and breast cancer resistance protein (ABCG2) inhibitor elacridar. Mol. Pharm. 9, 3236–3245. [DOI] [PubMed] [Google Scholar]
- European Commision. (2006). Commission regulation (EC) no 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union L364, 5–24. [Google Scholar]
- Evseenko D. A., Murthi P., Paxton J. W., Reid G., Emerald B. S., Mohankumar K. M., Lobie P. E., Brennecke S. P., Kalionis B., Keelan J. A. (2007). The ABC transporter BCRP/ABCG2 is a placental survival factor, and its expression is reduced in idiopathic human fetal growth restriction. FASEB J. 21, 3592–3605. [DOI] [PubMed] [Google Scholar]
- Fleck S. C., Churchwell M. I., Doerge D. R., Teeguarden J. G. (2016). Urine and serum biomonitoring of exposure to environmental estrogens II: Soy isoflavones and zearalenone in pregnant women. Food Chem. Toxicol. 95, 19–27. [DOI] [PubMed] [Google Scholar]
- Gedeon C., Anger G., Piquette-Miller M., Koren G. (2008). Breast cancer resistance protein: Mediating the trans-placental transfer of glyburide across the human placenta. Placenta 29, 39–43. [DOI] [PubMed] [Google Scholar]
- Gupta S. K., Malhotra S. S., Malik A., Verma S., Chaudhary P. (2016). Cell signaling pathways involved during invasion and syncytialization of trophoblast cells. Am. J. Reprod. Immunol. 75, 361–371. [DOI] [PubMed] [Google Scholar]
- Hemauer S. J., Patrikeeva S. L., Nanovskaya T. N., Hankins G. D. V., Ahmed M. S. (2010). Role of human placental apical membrane transporters in the efflux of glyburide, rosiglitazone, and metformin. Am. J. Obstet. Gynecol. 202, 383.e381–383.e387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo I. J., Raub T. J., Borchardt R. T. (1989). Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 96, 736–749. [PubMed] [Google Scholar]
- Hilakivi-Clarke L., Cho E., Clarke R. (1998). Maternal genistein exposure mimics the effects of estrogen on mammary gland development in female mouse offspring. Oncol. Rep. 5, 609–616. [DOI] [PubMed] [Google Scholar]
- Hines M. (2011). Prenatal endocrine influences on sexual orientation and on sexually differentiated childhood behavior. Front. Neuroendocrinol. 32, 170–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y., Asada S., Tsukahara S., Ishikawa E., Tsuruo T., Sugimoto Y. (2003). Breast cancer resistance protein exports sulfated estrogens but not free estrogens. Mol. Pharmacol. 64, 610–618. [DOI] [PubMed] [Google Scholar]
- Iqbal S. Z., Asi M. R., Jinap S., Rashid U. (2014). Detection of aflatoxins and zearalenone contamination in wheat derived products. Food Control 35, 223–226. [Google Scholar]
- Jebbink J., Veenboer G., Boussata S., Keijser R., Kremer A. E., Elferink R. O., van der Post J., Afink G., Ris-Stalpers C. (2015). Total bile acids in the maternal and fetal compartment in relation to placental ABCG2 expression in preeclamptic pregnancies complicated by HELLP syndrome. Biochim. Biophys. Acta 1852, 131–136. [DOI] [PubMed] [Google Scholar]
- Kiessling K. H., Pettersson H. (1978). Metabolism of zearalenone in rat liver. Acta Pharmacol. Toxicol. 43, 285–290. [DOI] [PubMed] [Google Scholar]
- Koraichi F., Videmann B., Mazallon M., Benahmed M., Prouillac C., Lecoeur S. (2012). Zearalenone exposure modulates the expression of ABC transporters and nuclear receptors in pregnant rats and fetal liver. Toxicol. Lett. 211, 246–256. [DOI] [PubMed] [Google Scholar]
- Kuiper-Goodman T., Scott P. M., Watanabe H. (1987). Risk assessment of the mycotoxin zearalenone. Regul. Toxicol. Pharmacol. 7, 253–306. [DOI] [PubMed] [Google Scholar]
- Lahouar A., Jedidi I., Sanchis V., Said S. (2018). Aflatoxin B1, ochratoxin A and zearalenone in sorghum grains marketed in Tunisia. Food Addit. Contam. Part B Surveil. 11, 103–110 [DOI] [PubMed] [Google Scholar]
- Li H., van Ravenzwaay B., Rietjens I. M., Louisse J. (2013). Assessment of an in vitro transport model using BeWo B30 cells to predict placental transfer of compounds. Arch. Toxicol. 87, 1661–1669. [DOI] [PubMed] [Google Scholar]
- Li J., Wang Y., Hidalgo I. J. (2013). Kinetic analysis of human and canine P-glycoprotein-mediated drug transport in MDR1-MDCK cell model: Approaches to reduce false-negative substrate classification. J. Pharm. Sci. 102, 3436–3446. [DOI] [PubMed] [Google Scholar]
- Mitra P., Audus K. L. (2010). MRP isoforms and bcrp mediate sulfate conjugate efflux out of BeWo cells. Int. J. Pharm. 384, 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkabinde L. A., Shoba-Zikhali L. N., Semete-Makokotlela B., Kalombo L., Swai H. S., Hayeshi R., Naicker B., Hillie T. K., Hamman J. H. (2012). Permeation of PLGA nanoparticles across different in vitro models. Curr. Drug Deliv. 9, 617–627. [DOI] [PubMed] [Google Scholar]
- Ok H. E., Choi S. W., Kim M., Chun H. S. (2014). HPLC and UPLC methods for the determination of zearalenone in noodles, cereal snacks and infant formula. Food Chem. 163, 252–257. [DOI] [PubMed] [Google Scholar]
- Pattillo R. A., Gey G. O. (1968). The establishment of a cell line of human hormone-synthesizing trophoblastic cells in vitro. Cancer Res. 28, 1231–1236. [PubMed] [Google Scholar]
- Pattillo R. A., Gey G. O., Delfs E., Mattingly R. F. (1968). Human hormone production in vitro. Science 159, 1467–1469. [DOI] [PubMed] [Google Scholar]
- Peng L., Yoo B., Gunewardena S. S., Lu H., Klaassen C. D., Zhong X-B. (2012). RNA sequencing reveals dynamic changes of mRNA abundance of cytochromes p450 and their alternative transcripts during mouse liver development. Drug Metab. Disp. 40, 1198–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachampa K., Takeda M., Khamdang S., Noshiro-Kofuji R., Tsuda M., Jariyawat S., Fukutomi T., Sophasan S., Anzai N., Endou H. (2008). Interactions of organic anion transporters and organic cation transporters with mycotoxins. J. Pharmacol. Sci. 106, 435–443. [DOI] [PubMed] [Google Scholar]
- Takemura H., Shim J.-Y., Sayama K., Tsubura A., Ting Zhu B., Shimoi K.. 2007. Characterization of the estrogenic activities of zearalenone and zeranol in vivo and in vitro. J Steroid Biochem Mol Biol. 103, 170-177. [DOI] [PubMed]
- Tralamazza S. M., Bemvenuti R. H., Zorzete P., de Souza Garcia F., Correa B. (2016). Fungal diversity and natural occurrence of deoxynivalenol and zearalenone in freshly harvested wheat grains from Brazil. Food Chem. 196, 445–450. [DOI] [PubMed] [Google Scholar]
- Vardhana P. A., Illsley N. P. (2002). Transepithelial glucose transport and metabolism in bewo choriocarcinoma cells. Placenta 23, 653–660. [DOI] [PubMed] [Google Scholar]
- Videmann B., Mazallon M., Prouillac C., Delaforge M., Lecoeur S. (2009). ABCC1, ABCC2 and ABCC3 are implicated in the transepithelial transport of the myco-estrogen zearalenone and its major metabolites. Toxicol. Lett. 190, 215–223. [DOI] [PubMed] [Google Scholar]
- Wen X., Gibson C. J., Yang I., Buckley B., Goedken M. J., Richardson J. R., Aleksunes L. M. (2014). MDR1 transporter protects against paraquat-induced toxicity in human and mouse proximal tubule cells. Toxicol. Sci. 141, 475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B., Jiang W., Yin T., Gao S., Hu M. (2012). A new strategy to rapidly evaluate kinetics of glucuronide efflux by breast cancer resistance protein (BCRP/ABCG2). Pharm. Res. 29, 3199–3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J., Wang Q., Bircsak K. M., Wen X., Aleksunes L. M. (2015). Screening of environmental chemicals identifies zearalenone as a novel substrate of the placental BCRP/transporter. Toxicol. Res. 4, 695–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Ma Z., Zhou S., Weng Y., Lei H., Zeng S., Li L., Jiang H. (2016). Multiple drug transporters are involved in renal secretion of entecavir. Antimicrob. Agents Chemother. 60, 6260–6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamek-Gliszczynski M. J., Day J. S., Hillgren K. M., Phillips D. L. (2011). Efflux transport is an important determinant of ethinylestradiol glucuronide and ethinylestradiol sulfate pharmacokinetics. Drug Metab. Disp. 39, 1794–1800. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wang H., Unadkat J. D., Mao Q. (2007). Breast cancer resistance protein 1 limits fetal distribution of nitrofurantoin in the pregnant mouse. Drug Metab. Disp. 35, 2154–2158. [DOI] [PubMed] [Google Scholar]
- Zheng R., Po I., Mishin V., Black A. T., Heck D. E., Laskin D. L., Sinko P. J., Gerecke D. R., Gordon M. K., Laskin J. D. (2013). The generation of 4-hydroxynonenal, an electrophilic lipid peroxidation end product, in rabbit cornea organ cultures treated with UVB light and nitrogen mustard. Toxicol. Appl. Pharmacol. 272, 345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Naraharisetti S. B., Wang H., Unadkat J. D., Hebert M. F., Mao Q. (2008). The breast cancer resistance protein (BCRP1/ABCG2) limits fetal distribution of glyburide in the pregnant mouse: An Obstetric-Fetal Pharmacology Research Unit Network and University of Washington Specialized Center of Research Study. Mol. Pharmacol. 73, 949–959. [DOI] [PubMed] [Google Scholar]
- Zinedine A., Soriano J. M., Moltó J. C., Mañes J. (2007). Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 45, 1–18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.