Abstract
Epidemiological studies have reported positive associations between serum perfluorooctanoic acid (PFOA) and total and non-high-density lipoprotein cholesterol (non-HDL-C) although the magnitude of effect of PFOA on cholesterol lacks consistency. The objectives of this study were to evaluate the effect of PFOA on plasma cholesterol and triglyceride metabolism at various plasma PFOA concentrations relevant to humans, and to elucidate the mechanisms using APOE*3-Leiden.CETP mice, a model with a human-like lipoprotein metabolism. APOE*3-Leiden.CETP mice were fed a Western-type diet with PFOA (10, 300, 30 000 ng/g/d) for 4–6 weeks. PFOA exposure did not alter plasma lipids in the 10 and 300 ng/g/d dietary PFOA dose groups. At 30 000 ng/g/d, PFOA decreased plasma triglycerides (TG), total cholesterol (TC), and non-HDL-C, whereas HDL-C was increased. The plasma lipid alterations could be explained by decreased very low-density lipoprotein (VLDL) production and increased VLDL clearance by the liver through increased lipoprotein lipase activity. The concomitant increase in HDL-C was mediated by decreased cholesteryl ester transfer activity and changes in gene expression of proteins involved in HDL metabolism. Hepatic gene expression and pathway analysis confirmed the changes in lipoprotein metabolism that were mediated for a major part through activation of the peroxisome proliferator-activated receptor (PPAR)α. Our data confirmed the findings from a phase 1 clinical trial in humans that demonstrated high serum or plasma PFOA levels resulted in lower cholesterol levels. The study findings do not show an increase in cholesterol at environmental or occupational levels of PFOA exposure, thereby indicating these findings are associative rather than causal.
Keywords: perfluorooctanoic acid (PFOA), cholesterol, lipoproteins, non-HDL cholesterol, triglycerides, peroxisome proliferator-activated receptor (PPAR)α
Salts of perfluorooctanoic acid (PFOA) were widely used as an emulsifier in the manufacture of fluoropolymers. Because PFOA is extremely stable (due to strong carbon-fluorine bond strength) and nonflammable, it cannot be readily degraded by strong acids, alkalis, or oxidizing agents; and as a result, it persists in the environment and is detected ubiquitously in humans and wildlife (Lau et al., 2007).
In general populations with ambient exposures or a community exposed to environmental levels of PFOA, several cross-sectional epidemiological studies have reported associations of serum PFOA with increased serum concentrations of total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) in adults and children (Eriksen et al., 2013; Frisbee et al., 2010; Geiger et al., 2014; Jain and Ducatman, 2018; Liu et al., 2018; Nelson et al., 2010; Steenland et al., 2009). However, the magnitude of effect on cholesterol appears to be less in more highly exposed occupational workers (Costa et al., 2009; Olsen and Zobel 2007; Olsen et al., 2000, 2003, 2012; Sakr et al., 2007a, 2007b; Steenland et al., 2010). In addition, there has been no reported increased risk for coronary artery disease incidence related to PFOA exposure in an exposed community (Steenland et al., 2015) or in occupational cohorts when using internal referent comparisons (Raleigh et al., 2014; Steenland and Woskie, 2012).
Several studies have evaluated the effect of PFOA on plasma lipids, including mice (Loveless et al., 2006; Rebholz et al., 2016; Xie et al., 2003), rats (Haughom and Spydevold, 1992; Loveless et al., 2006), and monkeys (Butenhoff et al., 2002). In general, plasma lipids were either lowered or unchanged under these toxicological study conditions. These findings were consistent with one of the known toxicodynamic properties of PFOA in which it can activate nuclear receptor peroxisome proliferator-activated receptor alpha (PPARα) and subsequently leads to increased fatty acid (FA) oxidation and an overall lowering of serum/plasma lipid levels (Buhrke et al., 2015; Elcombe et al., 2010; Takacs and Abbott, 2007). The activation of PPARα is the operative mechanism of fibrate drugs that reduce serum/plasma lipids in both laboratory animals and humans (Bijland et al., 2010; Staels et al., 2008). In a phase 1 clinical trial study, PFOA was investigated as an antitumor agent due to its ability to inhibit PIM kinase activity (Convertino et al., 2018). These patients received weekly ammonium PFOA doses that ranged between 50 and 1200 mg for 6 weeks. A reduction in plasma TC levels (LDL, not HDL-C) occurred at plasma PFOA concentrations between 420 and 565 µM (175 000–230 000 ng/ml). Albeit the exact mechanism was not fully elucidated, the finding reported by Convertino et al. (2018) was consistent with the toxicodynamic property of PFOA described previously where hypolipidemic responses were observed in laboratory animals (Haughom and Spydevold, 1992; Loveless et al., 2006; Xie et al., 2003).
It is worth noting that, unlike humans, rodent lipoprotein metabolism is characterized by fast clearance of apoB-containing lipoproteins and the absence of cholesteryl ester transfer protein (CETP) resulting in a higher proportion of HDL-C relative to LDL-C (Princen et al., 2016). CETP is responsible for transferring cholesterol ester (CE) from HDL-C to the apoB-containing lipoproteins in exchange for triglycerides (TG). In contrast, human and nonhuman primates have a higher proportion of LDL-C relative to HDL-C due to the presence of CETP (Princen et al., 2016). Given the difference in lipid-handling that can hamper the extrapolation of rodent lipid data to human (Princen et al., 2016), we undertook this study to evaluate the effects of PFOA on lipid metabolism using the APOE*3-Leiden.CETP mouse model. This genetically engineered mouse model was designed to mimic the human lipoprotein metabolism with CETP expression and a delayed apoB clearance (Princen et al., 2016). It has been widely used to study the effect of drugs on atherosclerosis (Ason et al., 2014; Dewey et al., 2017; Kühnast et al., 2014, 2015), cardiovascular safety (de Vries-van der Weij et al., 2009; Pouwer et al., 2018), and lipid metabolism (de Haan et al., 2008; van der Hoogt et al., 2007; van der Hoorn et al., 2008). Therefore, the objectives of the study reported herein are to (1) evaluate the effect of PFOA on plasma cholesterol at different PFOA concentrations that had been reported in human observational and experimental studies; and (2) elucidate the mechanism for the hypolipidemic responses with PFOA exposures, including hepatic gene expression and pathway analysis.
MATERIALS AND METHODS
Animals
Sixty-four male APOE*3-Leiden.CETP transgenic mice of 7–12 weeks (experiment 1) and 6–10 weeks (experiment 2) of age at the start of the experiment were used in this study. Mice were housed under standard conditions with a 12-h light-dark cycle and had free access to food and water. When fasting was required, only food was removed for the length specified by the study protocol. Body weight, food intakes, and clinical signs of behavior were monitored regularly during the study. Animal experiments were approved by the Institutional Animal Care and Use Committee of The Netherlands Organization for Applied Research (TNO) under registration number 3682. All procedures involving animals were conformed to Guide for the Care and Use of Laboratory Animals (ILAR, 2011).
Human Plasma Samples
Plasma from healthy anonymized donors was obtained after their written informed consent from Sanquin blood bank (the Netherlands), in accordance with the Declaration of Helsinki.
Materials
PFOA ammonia salt (FC-143, lot 332) was provided by 3M Company (St Paul, Minnesota). It consisted of 77.6% linear and 22% branched (12.6% internal monomethyl (nonalpha), 9% isopropyl, 0.2% tert-butyl, 0.1% gem-dimethyl, and 0.1% alpha monomethyl). This test material, a white solid, was 97.99% pure and was stored at room temperature. All other chemicals were reagent-grade.
Study Design
In this study, mice were fed a semisynthetic Western-type diet for 4 weeks of a dietary run-in (acclimation) period prior to group allocation (Figure 1). The semisynthetic Western-type diet consisted of 0.25% cholesterol (wt/wt), 1% corn oil (wt/wt), and 14% bovine fat (wt/wt; Hope Farms, Woerden, The Netherlands).
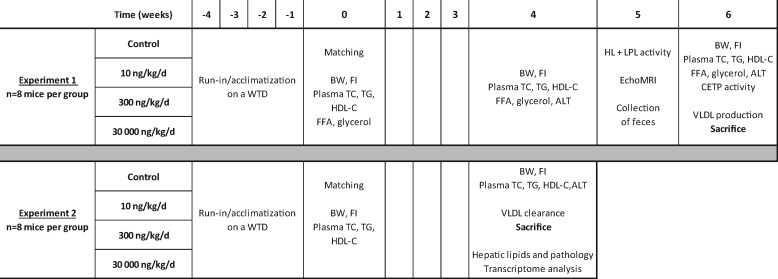
Figure 1.
Study set-up. At the end of the 4-week run-in period (t = week 0), mice were randomized into 4 groups based on age, body weight, and baseline plasma TC, TG, and HDL-C levels measured at the end of the run-in period. Upon randomization, mice were either fed with Western-type diet alone (control group) or Western-type diet containing ammonium PFOA at 10, 300, or 30 000 ng/g/d (n = 8 mice per dose group). Abbreviations: BW, body weight; CETP, cholesteryl ester transfer protein; FFA, free fatty acids; FI, food intake; HDL-C, high-density lipoprotein cholesterol; HL, hepatic lipase; LPL, lipoprotein lipase; MRI, magnetic resonance imaging; TC, total cholesterol; TG, triglycerides; VLDL, very-low-density lipoprotein; WTD, Western-type diet.
For test material administration, ammonium PFOA was incorporated into this Western-type diet at either 10, 300, or 30 000 ng/g/d (verification by liquid chromatography-tandem mass spectrometry [LC-MS/MS]). These dietary PFOA doses were chosen to achieve serum or plasma PFOA concentrations that had been reported in human observational or experimental studies. At the end of 4–6 weeks of dietary PFOA treatment at either 10, 300, or 30 000 ng/g/d, the mice were aimed to achieve comparable plasma PFOA levels found in the mid-Ohio river valley residents whose drinking water was contaminated with PFOA (ie, environmental exposure), fluorochemical production workers (ie, occupational exposure), or laboratory toxicological studies where reduced serum/plasma lipids were observed (ie, toxicological exposure), respectively.
Experiment 1 (6-week dietary treatment containing ammonium PFOA)
At the end of the 4-week run-in period (t = week 0), mice were randomized into four groups based on age, body weight, and baseline plasma TC, TG, and HDL-C levels measured at the end of run-in period (n = 8 mice per dose group) after a 4-h fast by tail vein bleeding. Upon randomization, mice were fed with the Western-type diet alone (control group) or Western-type diet containing ammonium PFOA at either 10, 300, or 30 000 ng/g/d for 6 weeks (see Table 1). Body weight and food intakes were monitored and recorded throughout the study for all mice. At the end of week 4, plasma TC, TG, free fatty acids (FFA), glycerol and alanine aminotransferase (ALT) were measured in blood samples from 4-h fasted mice collected by tail vein bleeding. At the end of week 5, hepatic lipase (HL) and lipoprotein lipase (LPL) activities were determined in plasma (after heparin injection). In addition, total fat mass was determined by EchoMRI image and 48–72 h feces were collected to measure excretion of bile acids and neutral sterols. At the end of week 6, in addition to plasma TC, TG, FFA, glycerol, and ALT determination, very low-density lipoprotein (VLDL) production was measured for all mice (Figure 1). Aliquots of plasma samples (approximately 20 μl) were collected from mice at week 0, 4, and 6 for PFOA concentration determination by LC-MS/MS. All mice were euthanized at the end of week 6, and perigonadal white adipose tissue (pWAT) and brown adipose tissue (BAT) were weighed.
Table 1.
Dietary PFOA Intake and Mean Plasma Concentrations of PFOA
| Control | 10 ng/g/d PFOA | 300 ng/g/d PFOA | 30 000 ng/g/d PFOA | |
|---|---|---|---|---|
| [PFOA] in diet (ng/g) | <0.5 | 9 ± 1 | 273 ± 12 | 26 380 ± 683 |
| Experiment 1 | ||||
| Dietary PFOA intake (ng/g bw/day) | 0.0 | 10 | 291 | 30 238 |
| Plasma [PFOA], t = 4 weeks (ng/ml) | <1.0 | 49 ± 4 | 1350 ± 88 | 90 663 ± 8867 |
| Plasma [PFOA], t = 6 weeks (ng/ml) | 5 ± 1 | 65 ± 7 | 1524 ± 54 | 144 000 ± 13 406 |
| Experiment 2 | ||||
| Dietary PFOA intake (ng/g bw/day) | 0.0 | 10 | 298 | 29 476 |
| Plasma [PFOA], t = 4 weeks (ng/ml) | <1.0 | 51 ± 5 | 1395 ± 100 | 93 713 ± 4827 |
Mice received a Western-type diet without or with 10, 300 or 30 000 ng/g/d PFOA, for 6 weeks (experiment 1) or 4 weeks (experiment 2). Dietary and plasma PFOA concentrations were measured by LC-MS/MS and dietary PFOA intake was calculated. Data are presented as mean ± SD. (n = 8 mice per group, n = 6 dietary samples). LC-MS/MS, liquid chromatography-tandem mass spectrometry.
Experiment 2 (4-week dietary treatment containing ammonium PFOA)
Similar to experiment 1, at the end of the 4-week run-in period (t = week 0), mice were randomized into 4 groups based on age, body weight, and baseline plasma TC, TG and HDL-C levels measured at the end of the run-in period (n = 8 mice per dose group) after a 4-hour fast by tail vein bleeding. Upon randomization, mice were either fed with Western-type diet alone (control group) or Western-type diet containing ammonium PFOA at 10, 300, or 30 000 ng/g/d similar to experiment 1 described above (see Table 1). Body weight and food intakes were monitored and recorded throughout the study for all mice. At the end of week 4, plasma TC, TG and ALT enzyme activity were measured for all mice in addition to VLDL-clearance evaluation. Aliquots of plasma samples (approximately 20 μl) were collected from mice at week 0 and 4 for PFOA concentration determination by LC-MS/MS. In experiment 2, all mice were euthanized on week 4 for liver-related analysis, including microscopic evaluation, detailed hepatic lipid profiling, and transcriptome analysis (Figure 1).
Determination of Serum and Dietary Concentrations of PFOA
Dietary PFOA concentrations were determined by LC-MS/MS. Briefly, one gram of each dietary sample (sampled from top, middle, and bottom of the bag) were pulverized and followed by addition of 50% methanol in water (9 ml). After overnight incubation at room temperature on a roller shaker, samples were then sonicated for 15 min followed by centrifugation (2500 × g, 5 min). One hundred microliters of the top layer was transferred to a new tube which contained a fixed amount of the stable isotope-labeled internal standard (13C-PFOA), followed by the addition of 1 N formic acid (1 ml) and 100 μl of saturated ammonium sulfate. The solution was mixed by vortexing and then subjected to solid phase extraction (SPE) with Phenomenex Strata-X 3-ml SPE columns and LC-MS/MS according to the method described in Ehresman et al. (2007). Serum PFOA concentrations were also determined by LC-MS/MS as described previously (Ehresman et al., 2007).
Plasma Biochemical Analysis
EDTA plasma samples were collected in week 0, 4, and 6 after a 4-h fast. Plasma TC, TG, FFA, and free glycerol were determined using enzymatic kits (TC: Roche/Hitachi, Mannheim, Germany, catalogue# 11491458216 TG: Roche/Hitachi, catalogue# 11730711216, FFA: Wako diagnostics, Richmond, USA, catalogue# 434-91795 and 436-91995, free glycerol: Sigma-Aldrich, St. Louis, USA, catalogue# F6428) according to the manufacturer’s protocols. HDL-C was measured after precipitation as described previously (Kühnast et al., 2015). Non-HDL-C levels were calculated by subtracting HDL-C from TC. The distribution of cholesterol over plasma lipoproteins was determined in group-wise pooled plasma by fast protein liquid chromatography (FPLC; Kooistra et al., 2006). ALT enzymatic activity was measured by reflectance photometry using a Reflotron® Plus analyzer (Hoffman-La Roche, Mannheim, Germany).
CETP Activity Assay
Differences in CETP activity between APOE*3-Leiden.CETP mice and humans may affect the magnitude of the PFOA-induced increase of plasma HDL-C. Therefore, endogenous CETP activity was measured in human and mouse plasma with a fluorescent assay using donor liposomes enriched with nitrobenzoxadiazole-labeled cholesteryl esters (RB-CETP, Roar Biomedical, New York, New York) as described previously (Gautier et al., 2007).
Hepatic VLDL-TG and VLDL-apoB Production
All mice were fasted for 4 h prior to the start of the experiment. During the experiment, mice were sedated with acepromazine-midazolam-fentanyl intraperitoneally [6.25 mg/kg acepromazine (Ceva Santé Animale B.V., Naaldwijk, The Netherlands), 6.25 mg/kg midazolam (Actavis, Baarn, The Netherlands), and 0.3125 mg/kg fentanyl (Bipharma B.V., Almere, The Netherlands)]. At t = 0 min, blood was taken via tail bleeding and mice were intravenously (IV) injected with 100 µl phosphate-buffered saline (PBS) containing 20 μCi Trans35S-labeled methionine/cysteine (ICN Biomedicals, Irvine, California) to measure de novo apoB synthesis. After 30 min, the mice received a Triton WR1339 IV injection (500 mg/kg body weight), which inhibits LPL-mediated lipolysis, thereby blocking VLDL clearance. Blood samples were drawn at 0, 15, 30, 60, and 90 min after Triton WR1339 injection and used for determination of the plasma TG concentration. After 90 min, the animals were sacrificed by cervical dislocation and blood was collected by heart puncture for subsequent isolation of VLDL by density-gradient ultracentrifugation. 35S-apoB was measured in the VLDL fraction after apoB-specific precipitation, and VLDL-apoB production rate was calculated as disintegration per minute (dpm)/h, as previously reported (Bijland et al., 2011; Geerling et al., 2014; van der Hoorn et al., 2008).
In Vivo Clearance of VLDL-Like Particles
All mice were fasted for 4 h and injected in the tail vein with VLDL-like particles (80 nm) containing 3H-labeled FA (as glycerol tri[3H]-oleate, [3H]-TO) and 14C-labeled cholesteryl oleate (as [14C]-cholesteryl oleate, [14C]-CO). At t = 2, 5, 10, and 15 min post-injection, blood was collected to determine the plasma decay of [3H]-TO and [14C]-CO. At 15 min, mice were euthanized by cervical dislocation and perfused with heparin 10 U/ml in ice-cold PBS for 5 min. Organs (ie, liver, subcutaneous WAT [sWAT], BAT, spleen, lung and skeletal femoral muscle) were harvested and saponified overnight in 500 μl Solvable (Perkin-Elmer, Wellesley, Massachusetts) to determine [3H]-TO and [14C]-CO uptake. Retention of radioactivity in the saponified tissues was measured as % of the injected dose and the half-life of VLDL-[3H]-TO and [14C]-CO was calculated from the slope after linear fitting of semilogarithmic decay curves as described previously (Bijland et al., 2011; Geerling et al., 2014; van der Hoorn et al., 2008).
HL and Lipoprotein Lipase Assay
Lipolytic activity of both LPL and HL was determined at t = 5 weeks. To liberate LPL from the endothelium, 4-h fasted mice were injected IV with heparin (0.1 U/g body weight; Leo Pharmaceutical Products BV, Weesp, The Netherlands) and blood was collected after 20 min. Postheparin plasma was incubated with 0.2 ml of TG substrate mixture containing triolein (4.6 mg/ml) and [3H]-TO (2.5 μCi/ml) for 30 min at 37°C in the presence or absence of 1 M NaCl, which completely inhibits LPL activity, to estimate both the HL and LPL activity. The LPL activity was calculated as the fraction of total triacylglycerol hydrolase activity that was inhibited by the presence of 1 M NaCl and is expressed as the amount of FFAs released per hour per ml of plasma (Bijland et al., 2011; Post et al., 2003).
Liver Histology
Liver samples (lobus sinister medialis hepatis) were collected, fixed in formalin and paraffin embedded, and sections (3 µm) were stained with hematoxylin and eosin (H&E). The level of macrovesicular and microvesicular steatosis and hypertrophy relative to the total liver area was determined by a board-certified pathologist and expressed as the percentage of total liver area. Inflammation was scored by counting the number of aggregates of inflammatory cells per mm2. Inflammatory aggregates, as marker of liver inflammation, are defined as a cluster, not a row, of ≥ 5 inflammatory cells (Liang et al., 2015).
Hepatic Lipid Analysis
Liver tissue samples of lobus sinister lateralis hepatis were homogenized in phosphate-buffered saline, and the protein content was measured using a Lowry protein assay. Lipids were extracted as described previously (Post et al., 1999), separated by high-performance thin-layer chromatography on silica gel plates, stained and analyzed with ChemiDoc Touch Imaging System (Bio-Rad Laboratories Inc, Hercules, USA). TG, CE, and free cholesterol (FC) content were quantified using Image-lab version 5.2.1 software (Bio-Rad Laboratories Inc, Hercules, USA) and expressed per milligram liver protein.
Excretion of Fecal Sterols and Bile Acids
Fecal excretion of neutral sterols and bile acids was determined in feces, collected during a 48- to 72-h time period at 2 consecutive time points at week 5, by gas chromatographic analysis as described previously (Post et al., 2003).
Hepatic Gene Expression and Pathway Analysis
Total RNA was extracted from the liver using the RNAbee isolation kit (Tel-Test Inc., Friendswood, USA, catalogue# CS-105B) and the Nucleospin RNA II mini spin kit (Macherey-Nagel GmbH & Co, Düren, Germany, catalogue# 740.955.50). Total RNA concentration was determined spectrophotometrically using Nanodrop 1000 (Isogen Life Science, De Meern, The Netherlands), and RNA quality was assessed using the 2100 Bioanalyzer (Agilent Technologies, Amstelveen, The Netherlands). The NEBNext Ultra Directional RNA Library Prep Kit for Illumina was used to process the samples according to the protocol “NEBNext Ultra Directional RNA Library Prep Kit for Illumina” (NEB #E7420S/L). Strand-specific messenger RNA sequencing libraries were generated and sequenced at GenomeScan (Leiden, The Netherlands). The libraries were multiplexed, clustered, and sequenced on an Illumina NextSeq 500 with a single-read 75-cycle sequencing protocol, 15 million reads per sample. The genome reference and annotation file Mus_Musculus.GRCm38 was used for analysis in FastA and GTF format. The reads were aligned to the reference sequence using Tophat 2.0.14 combined with Bowtie 2.1.0, and based on the mapped read locations and the gene annotation. HTSeq-count version 0.6.1p1 was used to count how often a read was mapped on the transcript region. Selected differentially expressed genes (DEGs) were used as an input for pathway and upstream regulator analysis through Ingenuity Pathway Analysis suite (www.ingenuity.com; Accessed November 2016). Calculated p-values <.01 were used as threshold for significance in all analysis except for those in the 30 000 ng/g/d dose group for which we used the adjusted p-value of <.05, the latter indicating a higher level of stringency. Gene set enrichment analysis was used to highlight the most important processes and pathways ranked based on their p-value of overlap (Bijland et al., 2011; Liang et al., 2015; Pouwer et al., 2018).
Statistical Analysis
Data are presented as means ± SD. A Kruskal–Wallis test was used to determine the significance of differences between the groups. Significance of differences of the individual groups with the control was calculated nonparametrically using a Mann–Whitney U-test and the rejection criteria were adjusted using a Bonferroni–Holm correction. IBM SPSS v24.0 was used for all analyses. p-Values ≤.05 were considered statistically significant.
RESULTS
Clinical Observations and Food Intakes
No clinical signs of abnormal behavior were noted in any treatment group during the study. There was no PFOA treatment-related mortality observed in the study and PFOA dietary treatment did not appear to affect food intakes in the mice. Four mice died in experiment 1 due to anesthetic complications (2 mice in 10 ng/g/d, 1 mouse in 300 ng/g/d, and 1 mouse in 30 000 ng/g/d) and this was not considered to be treatment related.
Dietary and Plasma Concentrations of PFOA
Dietary PFOA concentrations were determined for each dose group and based on 5–6 samples per dose group. The mean dietary PFOA concentrations were 8.5 ± 0.8, 273.0 ± 11.6, and 26 380 ± 683.3 ng/g for the 10, 300, and 30 000 ng/g/d dose groups, respectively, which represented 89.1 ± 8.6%, 95.0 ± 4.0%, and 91.8 ± 2.4% of the target doses (Table 1). Based on body weight and food intake, these dietary PFOA intakes are approximately 10, 300 and 30 000 ng/g/d, respectively (Table 1).
Mean plasma PFOA concentrations were measured in all animals from both experiments and are presented in Table 1. In both experiments, there were dose-dependent increases in plasma PFOA concentrations. After 4 weeks of dietary PFOA exposures, plasma PFOA concentrations in either experiment 1 or experiment 2 were very similar; they were at 49–51 ng/ml (from 10 ng/g dose group), 1350–1395 ng/ml (from 300 ng/g dose group), and 90 663–93 713 ng/ml (from 30 000 ng/g/d dose group). In experiment 1, plasma PFOA concentrations continued to increase through week 6 (Table 1).
Body Weight and Liver Weight
In experiment 1, there was a statistically significant decrease in body weight of the 30 000 ng/g/d dose group mice at t = 4 weeks (−10%, p < .01) and at t = 6 weeks (−18%, p < .001) when compared with control. The body weight reduction was not observed in week 4 in experiment 2 (Table 2). There was also a statistically significant increase in liver weight from the 30 000 ng/g/d group in experiment 1 (+150%, p < 0.001) and in experiment 2 (+190%, p < .001), but not with the 10 or 300 ng/g/d dietary dose (Table 2).
Table 2.
Body Weight, Food Intake, Liver Weight, and Plasma Parameters of Mice in Experiment 1 and Experiment 2
| Experiment 1 (t = 4 weeks) |
||||
|---|---|---|---|---|
| Control | 10 ng/g/d | 300 ng/g/d | 30 000 ng/g/d | |
| Body weight (gram) | 28.9 ± 1.9 | 28.5 ± 2.5 | 28.5 ± 2.4 | 25.9 ± 1.1** |
| Food intake (gram/day/mouse) | 2.8 ± 0.1 | 3.0 ± 0.4 | 2.8 ± 0.3 | 2.8 ± 0.3 |
| Liver weight (gram) | NA | NA | NA | NA |
| Liver weight (% of body weight) | NA | NA | NA | NA |
| ALT (U/l) | 145 ± 84 | 83 ± 27 | 217 ± 151 | 437 ± 112*** |
| TC (mmol/l) | 6.2 ± 0.9 | 6.8 ± 1.5 | 5.9 ± 1.1 | 4.3 ± 0.7** |
| HDL-C (mmol/l) | 1.8 ± 0.3 | 1.5 ± 0.3 | 1.7 ± 0.3 | 3.6 ± 0.5*** |
| non-HDL-C (mmol/l) | 4.4 ± 0.9 | 5.3 ± 1.4 | 4.2 ± 1.1 | 0.7 ± 0.2*** |
| TG (mmol/l) | 1.7 ± 0.4 | 1.6 ± 0.3 | 1.2 ± 0.2 | 0.3 ± 0.0*** |
|
Experiment 1 (t = 6 weeks) |
||||
| Control | 10 ng/g/d | 300 ng/g/d | 30 000 ng/g/d | |
| Body weight (gram) | 30.2 ± 1.9 | 28.9 ± 3.0 | 29.6 ± 2.4 | 25.5 ± 1.0*** |
| Food intake (gram/day/mouse) | 3.0 ± 0.1 | 2.9 ± 0.4 | 3.0 ± 0.4 | 2.6 ± 0.3 |
| Liver weight (gram) | 1.4 ± 0.1 | 1.4 ± 0.3 | 1.5 ± 0.3 | 3.5 ± 0.2*** |
| Liver weight (% of body weight) | 4.5 ± 0.3 | 4.8 ± 0.5 | 5.2 ± 0.6 | 13.6 ± 0.1*** |
| ALT (U/l) | 95 ± 27 | 118 ± 70 | 123 ± 90 | 740 ± 161** |
| TC (mmol/l) | 7.6 ± 1.3 | 6.9 ± 1.5 | 7.8 ± 2.7 | 5.1 ± 0.6*** |
| HDL-C (mmol/l) | 1.4 ± 0.3 | 1.5 ± 0.4 | 1.5 ± 0.2 | 3.2 ± 0.3*** |
| non-HDL-C (mmol/l) | 6.3 ± 1.4 | 5.4 ± 1.2 | 6.3 ± 2.7 | 2.0 ± 0.5*** |
| TG (mmol/l) | 1.8 ± 0.4 | 1.5 ± 0.4 | 1.5 ± 0.4 | 0.5 ± 0.0*** |
|
Experiment 2 (t = 4 weeks) |
||||
| Control | 10 ng/g/d | 300 ng/g/d | 30 000 ng/g/d | |
| Body weight (gram) | 27.6 ± 1.9 | 28.7 ± 2.5 | 28.3 ± 1.6 | 25.8 ± 1.9 |
| Food intake (gram/day/mouse) | 2.7 ± 0.2 | 3.0 ± 0.2 | 2.9 ± 0.1 | 2.6 ± 0.1 |
| Liver weight (gram) | 1.3 ± 0.1 | 1.4 ± 0.1 | 1.5 ± 0.1 | 3.8 ± 0.3*** |
| Liver weight (% of body weight) | 4.9 ± 0.4 | 4.8 ± 0.4 | 5.2 ± 0.2 | 14.8 ± 0.6*** |
| ALT (U/l) | 178 ± 52 | 211 ± 118 | 533 ± 950 | 553 ± 81** |
| TC (mmol/l) | 6.1 ± 0.7 | 6.4 ± 1.5 | 6.0 ± 1.1 | 4.9 ± 1.2 |
| HDL-C (mmol/l) | 2.4 ± 0.4 | 2.2 ± 0.3 | 2.0 ± 0.2 | 4.5 ± 0.9*** |
| non-HDL-C (mmol/l) | 3.7 ± 0.9 | 4.3 ± 1.5 | 4.0 ± 1.1 | 0.3 ± 0.2*** |
| TG (mmol/l) | 1.5 ± 0.5 | 1.6 ± 0.5 | 1.5 ± 0.2 | 0.4 ± 0.1*** |
Mice received a Western-type diet without or with 10, 300 or 30 000 ng/g/d PFOA for 6 weeks in experiment 1 or for 4 weeks in experiment 2. All parameters were measured individually, except for food intake which was measured per cage. Data are presented as mean ± SD (n = 8 per group, n = 5–7 per group for ALT, n = 3–4 cages per group). **p < .01, ***p < .001 as compared with the control group.
Abbreviations: ALT, alanine transaminase; HDL-C, high-density lipoprotein cholesterol; NA, not applicable; non-HDL-C, non-high-density lipoprotein cholesterol; TC, total cholesterol; TG, triglycerides.
The Effect of PFOA on Plasma Lipids
PFOA decreased plasma TC and TG and increased HDL-C
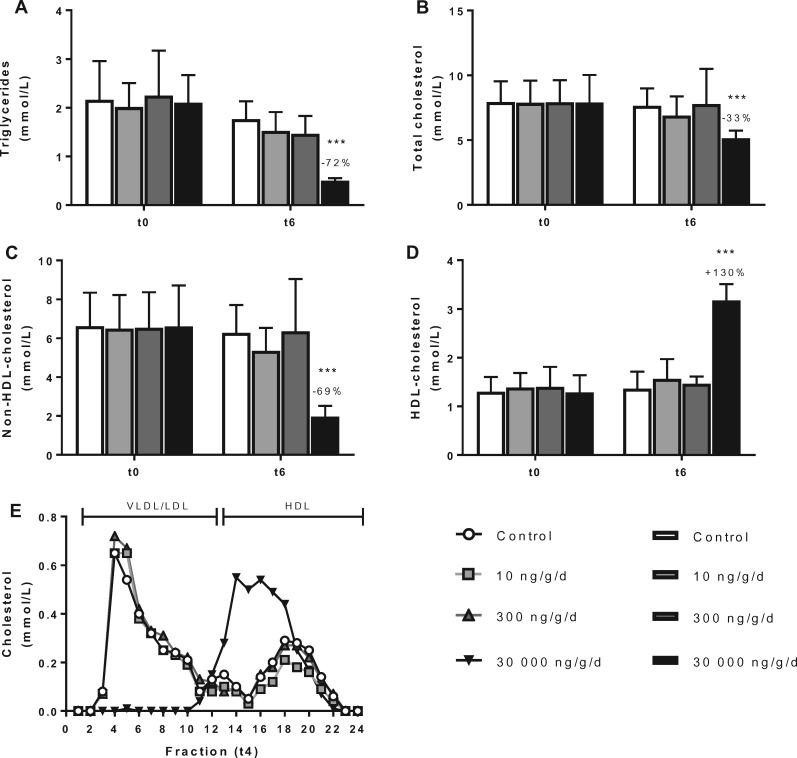
PFOA exposure did not alter plasma lipids at 10 and 300 ng/g/d dietary PFOA dose groups, respectively (Figs. 2A–D). In contrast, at 30 000 ng/g/d dietary dose group where plasma PFOA reached 144 000 ng/ml after 6 weeks of dietary PFOA exposure, it decreased plasma TG (−72%, p < .001; Figure 2A), TC (−33%, p < .001; Figure 2B), and non-HDL-C (−69%, p < .001; Figure 2C) relative to controls. There was a concomitant increase in HDL-C (+130%, p < .001; Figure 2D). The lipoprotein profile at this high PFOA exposure exhibited a distribution pattern where most cholesterol was confined to large cholesterol ester-rich HDL (Kooistra et al., 2006) and almost none in VLDL-LDL (non-HDL; Figure 2E).
Figure 2.
PFOA at low dose does not alter plasma lipids. Mice received a Western-type diet without or with 10, 300 or 30 000 ng/g/d PFOA. At baseline (t0) and after 6 weeks of exposure (t6), 4-h fasted blood was taken and plasma was assayed for TG (A), TC (B), non-HDL-C (C), and HDL-C (D). After 4 weeks of intervention, cholesterol distribution over lipoproteins was determined by FPLC in group-wise pooled plasma (E). Data are presented as means + SD (n = 8 per group). ***p < .001 as compared with the control group. Abbreviations: FPLC, fast protein liquid chromatography; HDL-C, high-density lipoprotein cholesterol; VLDL/LDL, (very) low-density lipoprotein.
PFOA decreased plasma FFA, plasma glycerol, pWAT, and BAT weight
After 6 weeks of PFOA exposure at the high dietary dose of 30 000 ng/g/d, total body fat mass (−66%, p < .01), pWAT (−61%, p < .01), and intrascapular BAT (−47%, p < .001) were decreased compared with control (Table 3). These observations were accompanied by decreased plasma FFAs (−48%, p < .001) and glycerol levels (−42%, p < .001; Table 3), both of which are primarily derived from TG lipolysis in adipose tissue.
Table 3.
PFOA Decreases Plasma FFAs, Plasma Glycerol, and Body Fat
| Control | 10 ng/g/d PFOA | 300 ng/g/d PFOA | 30 000 ng/g/d PFOA | |
|---|---|---|---|---|
| Total body fat (gram) | 3.5 ± 1.9 | 2.8 ± 0.9 | 3.3 ± 1.2 | 1.2 ± 0.3** |
| pWAT (gram) | 0.8 ± 0.3 | 0.7 ± 0.3 | 0.6 ± 0.2 | 0.3 ± 0.1** |
| BAT (gram) | 0.15 ± 0.03 | 0.13 ± 0.02 | 0.12 ± 0.02 | 0.08 ± 0.03** |
| FFA (mmol/l) | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.8 ± 0.2 | 0.5 ± 0.1*** |
| Free glycerol (mmol/l) | 0.4 ± 0.1 | 0.3 ± 0.0 | 0.3 ± 0.1 | 0.1 ± 0.0*** |
Mice received a Western-type diet without or with 10, 300 or 30 000 ng/g/d PFOA. In experiment 1, total body fat was measured by EchoMRI at week 5. At week 6, blood samples were taken after a 4-hour fast and plasma was assayed for FFAs and glycerol. At the end of experiment 1, BAT and pWAT were collected and weighted. Data are presented as mean ± SD (n = 6–8 per group). **p < .01, ***p < .001 as compared with the control group.
Abbreviations: BAT, brown adipose tissue; FFAs, free fatty acids; MRI, magnetic resonance imaging; pWAT, perigonadal white adipose tissue.
PFOA increased plasma HDL-C levels by reducing CETP activity
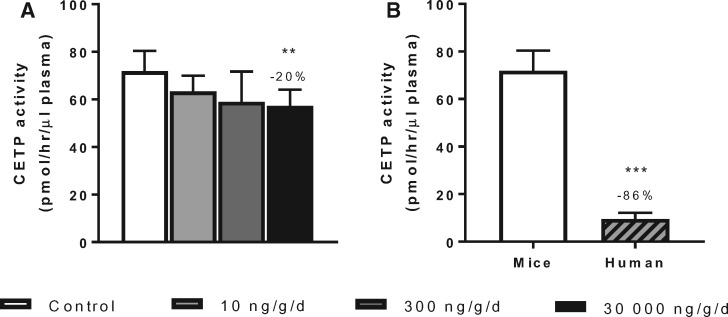
Because high dose of PFOA at 30 000 ng/g/d did result in increased plasma HDL-C levels and HDL size (Figs. 2D and E), we investigated whether this was caused by reduction of CETP activity. Indeed, PFOA significantly reduced CE transfer activity by 20% (p < .01) in plasma of APOE*3-Leiden.CETP mice (Figure 3A). When we compared the CETP activity in plasma of the control APOE*3-Leiden.CETP mice with the activity in human plasma, we found a 7.5-fold higher activity in mice (Figure 3B). Taken together, the HDL-raising effect observed in this mouse model with PFOA treatment appears to be a consequence of the reduced CETP activity. This effect might be less pronounced in humans due to substantially lower CETP activity as illustrated in Figure 3B.
Figure 3.
PFOA at high dose increases HDL-C by reducing CETP activity. Mice received a Western-type diet without or with PFOA in 3 different doses, 10, 300 and 30 000 ng/g/d. After 6 weeks of PFOA exposure in experiment 1, CETP activity was determined (A), and the activity in mice of the control group was compared with the activity in human plasma samples (B). Data are represented as mean + SD (n = 6–8 mice per group and n = 4 human plasma samples). **p < .01 as compared with the control group and ***p < .001 as compared with control APOE*3-Leiden.CETP mice. Abbreviations: CETP, cholesteryl ester transfer protein.
The Effect of PFOA on Hepatic Lipoprotein and Lipid Metabolism
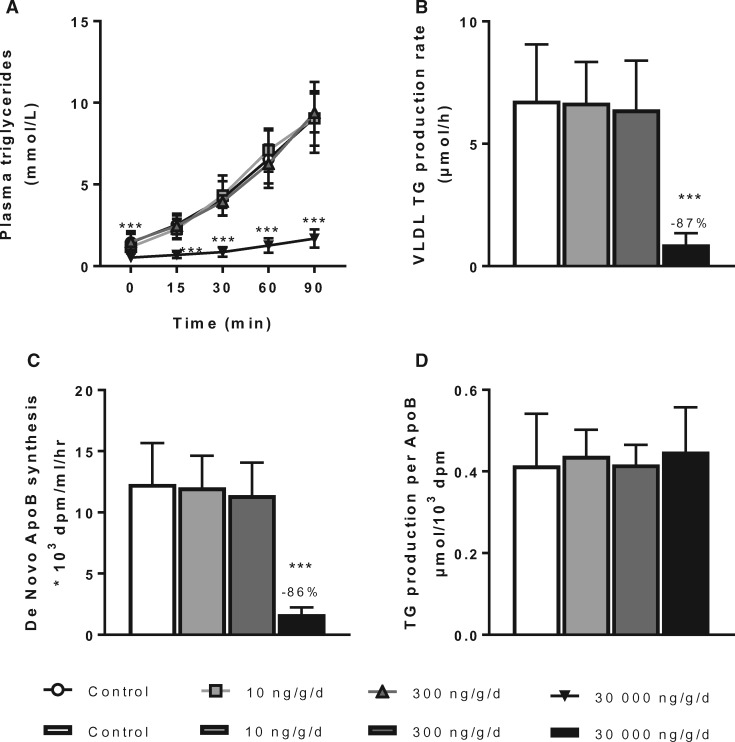
PFOA decreased hepatic VLDL-production rate
Because a decreased VLDL production may contribute to the overall TC- and TG-lowering effects, the VLDL production rate was determined in mice after 6 weeks of PFOA exposure. The VLDL-TG production rate was markedly decreased (−87%, p < .001) by high dose PFOA exposure (30 000 ng/g/d dietary dose group; Figs. 4A and B); and a similar decrease was observed in the VLDL-ApoB production rate (−86%, p < .001; Figure 4C). When normalized by ApoB, the overall TG production rate per ApoB was similar across all dose groups (Figure 4D), indicating that PFOA reduced the production rate of VLDL particles but did not alter the ratio between TG to ApoB. These findings also provide an explanation for the marked reduction in total fat and pWAT and intrascapular BAT mass, and the correspondingly decreased plasma FFAs and glycerol levels through a reduced supply of VLDL-TG-derived FFAs for storage in adipose tissue. In contrast, VLDL production was not affected by either 10 or 300 ng/g/d dietary PFOA dose groups.
Figure 4.
PFOA at high dose decreases VLDL-TG production and ApoB synthesis. Mice received a Western-type diet without or with 10, 300 or 30 000 ng/g/d PFOA. After 6 weeks, 4-h fasted mice of experiment 1 were injected with Tran35S-label and Triton after which blood samples were drawn up to 90 min. Plasma VLDL-TGs (A) were plotted and used to calculate the rate of TG production (B) from the slope of the individual curves. Ninety minutes after Triton injection plasma was used to isolate VLDL by ultracentrifugation, and the rate of de novo ApoB synthesis was determined (C). The TG production per ApoB was then calculated (D). Data are represented as mean ± SD (n = 6–8 per group). ***p < .001 as compared with the control group. Abbreviations: ApoB, apolipoprotein B; TG, triglycerides; VLDL, very low-density lipoproteins.
PFOA increased plasma VLDL clearance through enhanced LPL activity
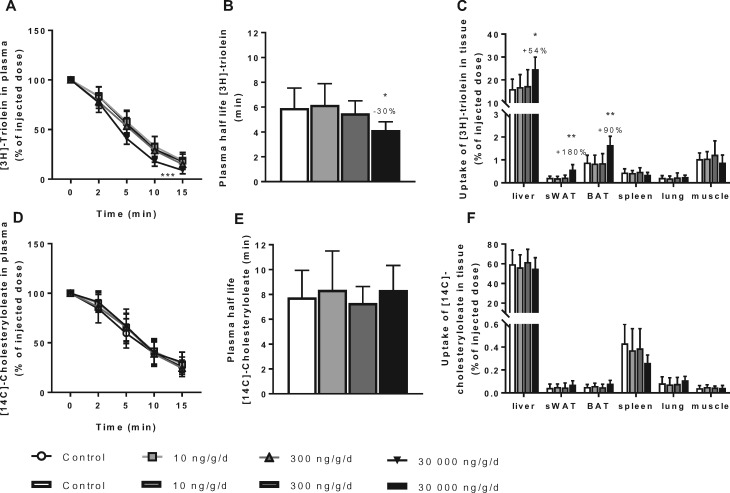
Plasma VLDL-TG levels are defined by the balance between VLDL-TG production and VLDL-TG clearance, hence this part of the experiment evaluated the plasma clearance and tissue uptakes of [3H]-TO- and [14C]-CO-labeled VLDL-like particles after 4 weeks of PFOA exposure. At 10 or 300 ng/g/d dietary PFOA exposures, VLDL clearance was not affected (Figure 5). The uptake of [3H]-TO, representing FFAs and TGs, was increased by the high dose dietary PFOA treatment as indicated by decreased plasma [3H]-TO at t = 15 min (−49%, p < .05; Figure 5A), resulting in a significantly decreased half-life time (−30%, p < .05; Figure 5B) relative to control. This effect was mainly due to increased [3H]-TO uptake by the liver (+54%, p < .05), sWAT (+180%, p < .01) and BAT (+90%, p < .01; Figure 5C). The uptake of [14C]-CO, representing cholesteryl oleate, was not affected by PFOA exposure (Figs. 5D–F).
Figure 5.
PFOA at high dose increases VLDL clearance mainly due to increased hepatic uptake. Mice received a Western-type diet without or with 10, 300 or 30 000 ng/g/d PFOA. After 4 weeks, 4-h fasted mice of experiment 2 were injected with glycerol tri[3H]-oleate ([3H]-TO) and [14C]-cholesteryl oleate ([14C]-CO)-labeled VLDL-like particles. [3H]-TO plasma decay was plotted (A) and used to calculate the rate of [3H]-TO uptake (B). Clearance of [3H]-TO in individual organs was determined (C). [14C]-CO plasma decay was plotted (D) and used to calculate the rate of [14C]-CO uptake (E). Clearance of [14C]-CO in individual organs was determined (F). Data are represented as mean ± SD (n = 6–7 per group).*p < .05, **p < .01, and ***p < .001 as compared with the control group. Abbreviations: BAT, brown adipose tissue; muscle, femoral muscle; sWAT, subcutaneous white adipose tissue; VLDL, very low-density lipoproteins.
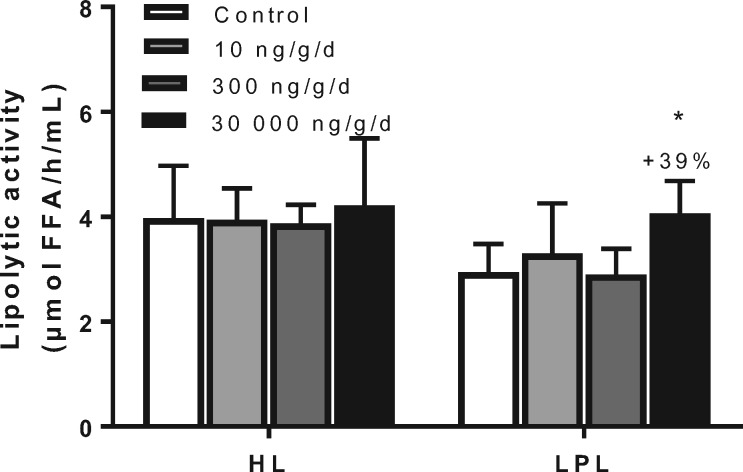
Because these data are consistent with increased lipolytic processing of VLDL particles, lipolytic activities (LPL and HL) were evaluated in experiment 1. After 5 weeks of PFOA exposure, mice were injected with heparin to liberate LPL from the endothelium, and LPL and HL activities were measured in plasma samples. HL activity was not affected by PFOA in any treatment group, but LPL activity was increased by the high PFOA dose at 30 000 ng/g/d (+39%, p < .05; Figure 6), providing an explanation for the increased FFA uptake by the liver and the adipose tissues.
Figure 6.
PFOA at high dose increases LPL activity. Mice received a Western-type diet without or with 10, 300 or 30 000 ng/g/d PFOA. After 5 weeks, 4-h fasted mice of experiment 1 were injected with heparin (0.1 U/g body weight) and postheparin plasma was collected. Plasma was incubated with a [3H]-TO-containing substrate mixture in the absence or presence of 1M NaCl, to estimate both the HL and LPL activity. Data are represented as mean + SD (n = 5–8 per group). *p < .05 as compared with the control group. Abbreviations: FFA, free fatty acids; HL, hepatic lipase; LPL, lipoprotein lipase; NaCl, sodium chloride.
Collectively, these data indicate that high PFOA dose at 30 000 ng/g/d increased the hepatic uptake of FFAs, which together with the reduced VLDL production contributed to the decreased plasma lipid levels.
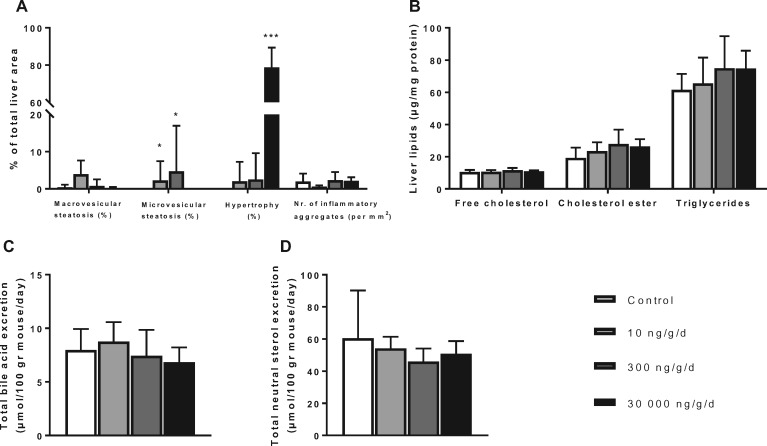
PFOA resulted in hepatic hypertrophy without altering hepatic lipid contents
Consistent with previous findings in rodents (Butenhoff et al., 2012; Yang et al., 2014) and monkeys (Butenhoff et al., 2002), high dose PFOA at 30 000 ng/g/d resulted in increased ALT (Table 2) and hepatic hypertrophy, which was absent in control mice and was minimally observed in the 2 lower PFOA dose groups (Figure 7A). Because the decreased VLDL-TG production rate and increased VLDL clearance may be the result of changes in hepatic lipid metabolism, we evaluated liver pathology and measured hepatic lipid content and fecal excretion of bile acids and neutral sterols. Microscopically, macrovesicular steatosis was not observed with PFOA exposure (Figure 7A). While there was no microvesicular steatosis present in livers of control mice or in mice administered with the high PFOA dose group at 30 000 ng/g/d, a low amount (<5% of total liver area) of the liver area consisted of microvesicular steatosis (2.3 ± 5.2% and 4.7 ± 12.3%) in mice treated with 10 and 300 ng/g/d PFOA, respectively. The number of inflammatory aggregates per mm2, defined as a cluster of ≥5 infiltrating inflammatory cells (Liang et al., 2015), was not affected by any of the dietary PFOA groups.
Figure 7.
PFOA at high dose induces hepatic hypertrophy. Mice received a Western-type diet without or with 10, 300 or 30 000 ng/g/d PFOA. After 4 weeks of PFOA exposure in experiment 2, livers were histologically analyzed for macrovesicular and microvesicular steatosis, hypertrophy and the number of inflammatory aggregates per mm2 (A), and hepatic lipid content per mg liver protein was measured (B). After 5 weeks of PFOA exposure in experiment 1, feces were collected per cage during a 48–72 h period at 2 consecutive time points, in which bile acid excretion (C) and neutral sterol excretion (D) were determined using gas chromatography. Data are represented as mean + SD (n = 6–7 per group and n = 6–8 collection points per group). *p < .05 and ***p < .001 as compared with the control group.
Hepatic FC, CE, and TG contents were also not altered by any of the PFOA doses (Figure 7B). Similarly, no changes were observed in the excretion of bile acids (which are solely produced by the liver) and neutral sterols (Figs. 7C and D). These data indicate that the decreased rate in VLDL-TG production is not explained by reduced availability of liver lipids for VLDL synthesis.
PFOA affected hepatic expression of genes involved in TG and cholesterol metabolism
To further investigate the mechanism by which PFOA affects lipid metabolism, gene expression of selected genes related to TG and cholesterol metabolism was determined in the liver as central organ in lipid metabolism. There were no significant changes in hepatic expression of lipid-related genes at the 2 lower PFOA dose groups. All significantly DEGs related to lipid metabolism in the liver mediated by the 30 000 ng/g/d PFOA dose group are depicted in Table 4. Although LPL expression in the liver is low as compared with heart, muscle, and adipose tissue, this PFOA exposure increased the expression of Lpl and decreased Apoc3 expression, which is in line with the increased LPL activity and VLDL-TG clearance. Genes involved in FA/TG synthesis and VLDL assembly (Scd3, Acss1, Scd2, Acsl3, Acsl4, Acsl5, Acsl1, Fasn, Acaca, Acss2, and Mttp), FA β-oxidation (Acss1, Ehhadh, Cpt1b, Acca1b, Acox1, Acsl3, Acsl4, Acsl5, Acsl1, Acca1a, Acss2), and uptake, transport, and binding of FAs (Slc27a1, Fabp4, Cd36, Slc27a4, Fabp1) were increased. Collectively, these data indicate that PFOA in the liver increases FA oxidation, binding and activation, and mobilizes FA for TG synthesis and secretion as VLDL. Most likely, the latter 2 pathways are surpassed by increased FA uptake and transport. Additionally, the expression of Apob was decreased, which provides an explanation for the decreased VLDL-ApoB formation.
Table 4.
The Effect of 30 000 ng/g/d PFOA Dose on Hepatic Expression of Genes Encoding Proteins and Transcription Factors Involved in TG and Cholesterol Metabolism
| TG metabolism | |||
|---|---|---|---|
| Protein | Gene | Fold change | padj |
| Lipolysis | |||
| LPL | Lpl | 4.3 | <.001 |
| HL | Lipc | 0.5 | <.001 |
| APOC3 | Apoc3 | 0.5 | <.001 |
| APOA5 | Apoa5 | 0.4 | <.001 |
| Fatty acid/TG synthesis | |||
| SCD | Scd3 | 157.6 | <.001 |
| ACS | Acss1 | 39.4 | <.001 |
| SCD | Scd2 | 18.4 | <.001 |
| ACS | Acsl3 | 2.5 | <.001 |
| ACS | Acsl4 | 2.3 | <.001 |
| ACS | Acsl5 | 2.3 | <.001 |
| ACS | Acsl1 | 2.1 | <.001 |
| FAS | Fasn | 2.1 | .001 |
| ACC | Acaca | 1.9 | .001 |
| ACS | Acss2 | 1.7 | .010 |
| DGAT1 | Dgat1 | 1.4 | .030 |
| ACS | Acsm3 | 0.7 | .020 |
| DGAT2 | Dgat2 | 0.7 | .030 |
| SREBP1a/c | Srebf1 | 0.7 | .040 |
| ACS | Acsm1 | 0.6 | <.001 |
| ACS | Acsm5 | 0.6 | .002 |
| ACS | Acsl6 | 0.1 | .006 |
| Beta oxidation | |||
| ACS | Acss1 | 39.4 | <.001 |
| Bifunctional enzyme | Ehhadh | 34.3 | <.001 |
| CPT1 | Cpt1b | 4.6 | <.001 |
| Thiolase | Acaa1b | 3.0 | <.001 |
| ACO | Acox1 | 2.6 | <.001 |
| ACS | Acsl3 | 2.5 | <.001 |
| ACS | Acsl4 | 2.3 | <.001 |
| ACS | Acsl5 | 2.3 | <.001 |
| ACS | Acsl1 | 2.1 | <.001 |
| Thiolase | Acaa1a | 1.7 | <.001 |
| ACS | Acss2 | 1.7 | .010 |
| ACO | Acox3 | 0.7 | .010 |
| ACS | Acsm3 | 0.7 | .020 |
| ACS | Acsm1 | 0.6 | <.001 |
| ACS | Acsm5 | 0.6 | .002 |
| PGC1alpha | Ppargc1a | 0.6 | .007 |
| ACS | Acsl6 | 0.1 | .006 |
| FA uptake, transport, binding | |||
| FATP | Slc27a1 | 9.8 | <.001 |
| FABP | Fabp4 | 8.6 | <.001 |
| CD36 | Cd36 | 7.5 | <.001 |
| FATP | Slc27a4 | 2.8 | <.001 |
| FABP | Fabp1 | 1.7 | .010 |
| FATP | Slc27a5 | 0.6 | <.001 |
| FABP | Fabp2 | 0.5 | <.001 |
| VLDL assemblage/formation | |||
| MTTP | Mttp | 1.5 | .020 |
| APOB | Apob | 0.8 | .050 |
| Cholesterol metabolism | |||
|---|---|---|---|
| Protein | Gene | Fold change | padj |
| HDL maturation | |||
| LCAT | Lcat | 0.6 | <.001 |
| HDL formation and remodeling/destabilization | |||
| APOA1 | Apoa1 | 0.2 | <.001 |
| PLPT | Plpt | 3.2 | <.001 |
| HL | Lipc | 0.5 | <.001 |
| HDL uptake | |||
| SRB1 | Scarb1 | 0.5 | <.001 |
| Synthesis | |||
| HMGCS1 | Hmgcs1 | 2.8 | <.001 |
| HMGCS2 | Hmgcs2 | 1.7 | .001 |
| Storage | |||
| ACAT2 | Acat2 | 1.6 | .001 |
| ACAT1 | Acat1 | 1.5 | .020 |
| Uptake | |||
| VLDLR | Vldlr | 32.0 | <.001 |
| LRP | Lrp11 | 2.5 | .002 |
| LRP | Lrp1 | 0.6 | .010 |
| SORT-1 | Sort1 | 0.5 | .004 |
| LRP | Lrp2 | 0.2 | .002 |
| Metabolism | |||
| BSEP | Abcb11 | 0.5 | <.001 |
| NTCP | Slc10a1 | 0.3 | <.001 |
| CYP7A | Cyp7a1 | 0.2 | <.001 |
| IBAT | Slc10a2 | 0.1 | .001 |
| Excretion | |||
| ABCG5 | Abcg5 | 0.6 | .010 |
|
Transcription factors | |||
| Protein | Gene | Fold change |
padj |
| HNF4A | Hnf4a | 1.6 | .001 |
| PGC1alpha | Ppargc1a | 0.6 | .007 |
| CAR | Nr1i3 | 0.6 | .009 |
Mice received a Western-type diet without or with 30 000 ng/g/d PFOA and livers were collected after a 4-h fast after the VLDL-clearance experiment (experiment 2) at t = 4 weeks. Total RNA was extracted from liver of individual mice (n = 8 mice per group) and gene expression analysis was performed using the Illumina Nextseq 500. A selection of genes involved in lipid metabolism is depicted with only those DEGs with an adjusted p-value <.05. Data represent fold change as compared with the control.
Abbreviations: FA, fatty acids; HDL, high-density lipoprotein; padj, adjusted p-value; TG, triglycerides; VLDL, very low-density lipoprotein.
PFOA affected hepatic expression of genes involved in HDL-C metabolism
High dose PFOA at 30 000 ng/g/d affected genes involved in HDL-C metabolism by decreasing the expression of Apoa1 (the major gene in the formation of HDL), Scarb1 (the principle gene in HDL-C clearance), and Lipc (plays a role in remodeling of HDL), and by increasing the expression of Pltp (which plays an important role in the remodeling of HDL by facilitating phospholipid transfer to HDL during its maturation from discoidal HDL into spherical HDL)(Table 4). Thus, together with the decreased CETP activity, changes in gene expression leading to reduced HDL-C uptake and formation of larger particles have contributed to the increased plasma HDL-C plasma levels and HDL size.
PFOA regulated pathways related to lipid and xenobiotic metabolism, coagulation, and inflammation
To further investigate the mechanism by which PFOA affects lipid metabolism and to explore its effect on other biological processes, pathway analysis was performed in the liver. The total number of DEGs was assessed (Supplementary Table 1) and used to identify overlap between the various treatments and PFOA-specific molecular responses.
There were no statistically significant changes in gene transcripts in the liver with the low PFOA dose group at 10 ng/g/d. In silico prediction of transcription factor activity in the liver (Table 5), based on the DEGs (padj <0.05), predicted activation of genes regulated by PPARα (p = 1E-75, Z-score 6.5) at 30 000 ng/g/d PFOA dose. This dose of PFOA also activated the transcription factor PXR (Nr1i2), a nuclear receptor that functions as sensor of endobiotic and xenobiotic substances. Analysis of the magnitude of the effect of PFOA on these 2 master regulators of PFOA-induced gene expression showed that these upstream regulators are activated in the order PPARα ≥ PXR.
In addition to modulation of lipid and xenobiotic metabolism, the 30 000 ng/g/d PFOA dose displayed significantly regulated pathways related to coagulation and inflammation (Supplementary Table 2). With the 300 ng/g/d PFOA dose group, there were also statistically significant changes in the gene transcripts related to hepatic activation of inflammation and immune responses (Supplementary Table 3). Because the biological processes of inflammation and immune response are complex (Parham, 2014) and involve various organs other than liver, the biological significance of these gene transcripts, as they relate to the assessment of cardiovascular risk, remains unclear.
Table 5.
In Silico Prediction of Transcription Factor Activity Based on the Expression Changes of Known Target Genes at 30 000 ng/g/d PFOA Dose
| Upstream regulator | Activation state | Z-score | p of overlap | |
|---|---|---|---|---|
| PPAR | Peroxisome proliferator-activated receptor α (PPARα) | activated | 6.5 | 1E-75 |
| HNF1A | Hepatocyte nuclear factor 1 homeobox A | inhibited | −3.6 | 1E-26 |
| HNF4A | Hepatocyte nuclear factor 4 alpha | inhibited | −2.4 | 1E-25 |
| ESR1 | Estrogen receptor 1 | activated | 2.4 | 1E-25 |
| NFE2L2 | Nuclear factor, erythroid 2 like 2 | activated | 3.3 | 1E-22 |
| NR1L2 | Pregnane X receptor (PXR) | activated | 5.4 | 1E-20 |
Mice received a Western-type diet without or with 30 000 ng/g/d PFOA, mRNA was isolated from liver tissue and gene expression analysis was performed using the Illumina Nextseq 500. To determine the activation status of transcription factors, an upstream regulator analysis was performed. A positive Z-score >2 indicates activation and a negative Z-score <−2 inhibition. All DEGs with an adjusted p-value <.05 were used for the analysis (n = 8 mice per group).
DISCUSSION
In this study, we have investigated the effects of 3 different doses of PFOA on plasma lipid levels and lipid metabolism in APOE*3-Leiden.CETP mice, a mouse model with a human-like lipoprotein metabolism (Princen et al., 2016). At the end of the study, the low dose group at 10 ng/g/d resulted in a mean plasma PFOA concentration of approximately 50 ng/ml, which is similar to the range reported in a community population in the mid-Ohio river valley area that had known PFOA exposure in its drinking water (median 27 ng/ml, mean 80 ng/ml; Steenland et al., 2009). Likewise, the mid-dose group at 300 ng/g/d resulted in a plasma concentration of approximately 1500 ng/ml which is within the range reported in different occupational studies among fluorochemical production workers (Costa et al., 2009; Olsen and Zobel, 2007; Olsen et al., 2003; Sakr et al., 2007a). At 30 000 ng/g/d PFOA achieved a plasma concentration that approached the level reported in the phase 1 clinical trial where a decrease in total cholesterol was clearly observed to occur (Convertino et al., 2018).
This differentiation of plasma PFOA concentrations is a major strength of this study to understand lipid-related associations, or lack of, in human observational and experimental studies. Plasma PFOA concentrations were translatable to the human exposure scenarios and we conclude that, using the APOE*3-Leiden.CETP mouse model, plasma PFOA levels at either 50 ng/ml (community drinking water exposed) or 1500 ng/ml (occupationally exposed) did not alter plasma lipids. PFOA exposure did decrease plasma TG, TC, and non-HDL-C levels and increased HDL-C level when plasma PFOA concentrations reached 90 663 ng/ml (at the end of the 4-week treatment) or 144 000 ng/ml (at the end of the 6-week treatment). We have demonstrated that environmentally and occupationally relevant PFOA exposures did not affect plasma lipids or lipoprotein metabolism using this mouse model. Our data are consistent with the findings by Convertino et al. (2018) that high serum or plasma PFOA levels resulted in lower cholesterol levels. Our current study data do not show an increase in cholesterol at environmental or occupational levels of PFOA exposure as shown in some observational epidemiological studies, suggesting these findings are likely associative rather than causal.
Consistent with our data, toxicological PFOA concentrations (>30 000 ng/g/d or 0.02% wt/wt) in mice and rats decreased plasma TC (Haughom and Spydevold, 1992; Loveless et al., 2006; Xie et al., 2003). While the major lipid in wild-type rodents are limited to HDL-C, it is interesting that cholesterol contained in the HDL-C fraction was reported to be increased in C57BL/6 and BALB/c mice fed a high cholesterol and high fat diet containing PFOA (560 μg/kg/d; Rebholz et al., 2016). However, given the inherent difference in rodent lipid metabolism (vs human), it is difficult to extrapolate these results to the human situation, emphasizing the importance to select an animal model resembling human lipid metabolism (Princen et al., 2016). The APOE*3-Leiden.CETP mouse used in our study is a well-characterized model for its human-like lipoprotein metabolism with delayed apoE-LDLR clearance and expression of CETP, and these characteristics are absent in wild-type rodents, including C57BL/6 and BALB/c mice.
Unlike wild-type rodents, nonhuman primates and humans express CETP. Butenhoff et al., (2002) found no effect on lipid levels when cynomolgus monkeys were administered with daily oral doses of PFOA for 6 months (Butenhoff et al., 2002). In that study, serum PFOA concentration approximated 158 000 ± 100 000 ng/ml; however, there was no distinction made between cholesterol in non-HDL-C or HDL-C hence precluding direct comparison with the present data. In humans, Convertino et al. (2018) reported a decline in TC and LDL-C with high (toxicological exposure) plasma concentrations of PFOA, however, unlike our study, they did not observe any change in HDL-C. This discrepancy could be due to the higher CETP activity measured in APOE*3-Leiden.CETP mouse plasma than human (Figure 3B). The concomitant increase in HDL-C observed in our study resulted from downregulation of CE transfer activity in plasma by PPARα activation (of which PFOA has been shown to be an activator), the strong downregulation of Scarb1 (the principle gene in HDL-C clearance) and Lipc (plays a role in remodeling of HDL), and by the increased expression of Pltp (resulting in formation of larger HDL particles)(Guerin et al., 1996; van der Hoogt et al., 2007; Wang et al., 2015).
Most reports studying the effects of PFOA in experimental animals do not provide a mechanistic explanation for the observed changes in lipoprotein metabolism. Our studies revealed that high PFOA exposure decreased (V)LDL levels by severely impairing the production of VLDL and increasing VLDL clearance by the liver through increased LPL-mediated lipolytic activity, accompanied by hepatomegaly with cellular hypertrophy. Gene expression and pathway analysis confirmed that lipid metabolism was regulated by PFOA mainly through activation of PPARα. Although not evaluated in this study, the hepatocellular hypertrophy is likely reversible, as has been reported in rats (Butenhoff et al., 2012) and monkeys (Butenhoff et al., 2002).
The increased VLDL clearance accompanied by augmented uptake of FFAs by the liver and LPL-expressing organs, such as sWAT and BAT, can be explained by upregulation of Lpl and downregulation of an inhibitor of LPL (Apoc3), resulting in increased plasma LPL activity. The strongly decreased VLDL-TG and VLDL-ApoB production rate (equally diminished by as much as 85%) is not caused by PPARα activation as fenofibrate enhances VLDL-TG secretion (Bijland et al., 2010) or by reduced availability of lipids for VLDL synthesis because hepatic lipid content was not reduced by PFOA. More likely, PFOA prevents VLDL particle formation and secretion from the liver by reduced ApoB de novo synthesis caused by decreased Apob mRNA expression. In line with this contention, treatment of cultured rat hepatocytes with PFOA decreased the VLDL secretion through disturbance of the association of ApoB48 with VLDL particles, a process independent of PPARα (Okochi et al., 1999), and toxicological PFOA exposure reduced Apob100 mRNA expression in BALB/c mice (Hui et al., 2017) as well. Therefore, we conclude that at toxicological PFOA exposure the decreased plasma TC and TG levels result from increased VLDL clearance and diminished VLDL-TG and VLDL-ApoB production, the latter caused by a reduced supply of ApoB substrate which is essential for the assemblage of the VLDL particles.
Fecal neutral sterol and bile acid excretion, the latter as marker of hepatic bile acid synthesis, were not affected by PFOA despite a decrease in Cyp7a1 mRNA expression, indicating that there is sufficient supply of substrate for bile acid synthesis. Consistently, we found no changes in hepatic lipid content, implying that despite reduced VLDL production, hepatic cholesterol and TG homeostasis is maintained. In contrast, the mass of adipose tissue was decreased in spite of enhanced plasma lipolytic activity. LPL-mediated delivery of VLDL-TG–derived FA is a strong determinant of WAT mass and obesity (Voshol et al., 2009). However, the reduced VLDL-TG production limits the availability of substrate for LPL on peripheral tissues, leading to less FA delivery to WAT and skeletal muscle. This can explain the reduced pWAT mass induced by toxicological PFOA exposure, accompanied by a reduction of plasma FFAs and glycerol that are mainly derived from TG lipolysis in adipose tissue.
Gene expression and pathway analysis revealed that FA oxidation and individual genes involved therein, all under control of PPARα, were enhanced, in line with previous reports in mice (Das et al., 2017), rat (Bjork et al., 2011) and human hepatocytes (Bjork et al., 2011; Buhrke et al., 2015). In silico prediction of transcription factor activity predicted PPARα as the principal transcription factor regulated by toxicological PFOA not only based on the regulation of lipid metabolism-related genes, but of all DEGs in the liver. Upstream regulator analysis also predicted the involvement of the xenosensor receptor PXR (Nr1l2) in the regulation of biological processes, whereas CAR (Nr1l3) mediated processes were affected to a much lesser extent, in line with literature (Elcombe et al., 2010). Next to its role in xenobiotic metabolism, PXR activation is also involved in lipid metabolism, as it reduces the hepatic expression of Apoa1, Lcat, and Hl, players in the formation, maturation, and remodeling of HDL-C in APOE*3-Leiden.CETP mice (de Haan et al., 2009) as also observed in the present study. While accumulation of TG and cholesterol in the liver can be induced by constitutive PXR expression (Zhou et al., 2006) or the PXR agonist PCN (de Haan et al., 2009), it was not observed in our study, most likely because the PXR-induced effect was negated by the strong PPARα activation, leading to decreased hepatic lipid content in APOE*3-Leiden.CETP mice (Bijland et al., 2010), provoked at the applied PFOA exposure at high (toxicological) level.
At 30 000 ng/g/d PFOA, inflammation-related gene pathways were observed to have statistically significant changes. Activation of both PPARα and PXR is known to attenuate the inflammatory response (Bougarne et al., 2018; Kleemann et al., 2003; Wallace et al., 2010), and toxicological exposure to PFOA has been reported to reduce inflammatory pathways and responses (Corsini et al., 2011; Guruge et al., 2006; Rosen et al., 2008, 2010). In contrast, PFOA at 300 ng/g/d upregulated pathways involved in inflammatory and immune response processes. The physiological consequences hereof, however, are unknown and require further research.
In conclusion, we have demonstrated that in APOE*3-Leiden.CETP mice, dietary PFOA exposure at 30 000 ng/g/d reduced plasma TG and TC levels by affecting VLDL-TG production through decreased apoB synthesis and by increasing VLDL clearance. This was not observed with lower PFOA doses. Our data confirmed the findings from a phase 1 clinical trial in humans that demonstrated high serum or plasma PFOA levels resulted in lower cholesterol levels. The study findings do not show an increase in cholesterol at environmental or occupational levels of PFOA exposure, thereby indicating these findings are associative rather than causal.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
This work was supported by 3M Company and S.C.C. and G.W.O. are employees of 3M. 3M was involved in the study design and preparation of the manuscript (S.C.C. and G.W.O.), but had no role in data collection apart from determination of dietary and serum concentrations of PFOA.
Supplementary Material
ACKNOWLEDGMENTS
We thank Nicole Worms, Nanda Keijzer, Anita van Nieuwkoop, Wim van Duyvenvoorden, Jessica Snabel, Christa de Ruiter, and Joline Attema for their excellent technical assistance.
REFERENCES
- Ason B., van der Hoorn J. W., Chan J., Lee E., Pieterman E. J., Nguyen K. K., Di M., Shetterly S., Tang J., Yeh W. C., et al. (2014). PCSK9 inhibition fails to alter hepatic LDLR, circulating cholesterol, and atherosclerosis in the absence of ApoE. J. Lipid Res. 55, 2370–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijland S., Pieterman E. J., Maas A. C., van der Hoorn J. W., van Erk M. J., van Klinken J. B., Havekes L. M., van Dijk K. W., Princen H. M., Rensen P. C. (2010). Fenofibrate increases very low density lipoprotein triglyceride production despite reducing plasma triglyceride levels in APOE*3-Leiden.CETP mice. J. Biol. Chem. 285, 25168–25175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijland S., Rensen P. C., Pieterman E. J., Maas A. C., van der Hoorn J. W., van Erk M. J., Havekes L. M., Willems van Dijk K., Chang S. C., Ehresman D. J., et al. (2011). Perfluoroalkyl sulfonates cause alkyl chain length-dependent hepatic steatosis and hypolipidemia mainly by impairing lipoprotein production in APOE*3-Leiden CETP mice. Toxicol. Sci. 123, 290–303. [DOI] [PubMed] [Google Scholar]
- Bjork J. A., Butenhoff J. L., Wallace K. B. (2011). Multiplicity of nuclear receptor activation by PFOA and PFOS in primary human and rodent hepatocytes. Toxicology 288, 8–17. [DOI] [PubMed] [Google Scholar]
- Bougarne N., Weyers B., Desmet S. J., Deckers J., Ray D. W., Staels B., De Bosscher K. (2018). Molecular actions of PPARalpha in lipid metabolism and inflammation. Endocr. Rev. 39, 760–802. [DOI] [PubMed] [Google Scholar]
- Buhrke T., Krüger E., Pevny S., Rößler M., Bitter K., Lampen A. (2015). Perfluorooctanoic acid (PFOA) affects distinct molecular signalling pathways in human primary hepatocytes. Toxicology 333, 53–62. [DOI] [PubMed] [Google Scholar]
- Butenhoff J., Costa G., Elcombe C., Farrar D., Hansen K., Iwai H., Jung R., Kennedy G. Jr., Lieder P., Olsen G., et al. (2002). Toxicity of ammonium perfluorooctanoate in male cynomolgus monkeys after oral dosing for 6 months. Toxicol. Sci. 69, 244–257. [DOI] [PubMed] [Google Scholar]
- Butenhoff J. L., Bjork J. A., Chang S. C., Ehresman D. J., Parker G. A., Das K., Lau C., Lieder P. H., van Otterdijk F. M., Wallace K. B. (2012). Toxicological evaluation of ammonium perfluorobutyrate in rats: Twenty-eight-day and ninety-day oral gavage studies. Reprod. Toxicol. 33, 513–530. [DOI] [PubMed] [Google Scholar]
- Convertino M., Church T. R., Olsen G. W., Liu Y., Doyle E., Elcombe C. R., Barnett A. L., Samuel L. M., MacPherson I. R., Evans T. R. J. (2018). Stochastic pharmacokinetic-pharmacodynamic modeling for assessing the systemic health risk of perfluorooctanoate (PFOA). Toxicol. Sci. 163, 293–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsini E., Avogadro A., Galbiati V., dell'Agli M., Marinovich M., Galli C. L., Germolec D. R. (2011). In vitro evaluation of the immunotoxic potential of perfluorinated compounds (PFCS). Toxicol. Appl. Pharmacol. 250, 108–116. [DOI] [PubMed] [Google Scholar]
- Costa G., Sartori S., Consonni D. (2009). Thirty years of medical surveillance in perfluooctanoic acid production workers. J. Occup. Environ. Med. 51, 364–372. [DOI] [PubMed] [Google Scholar]
- Das K. P., Wood C. R., Lin M. T., Starkov A. A., Lau C., Wallace K. B., Corton J. C., Abbott B. D. (2017). Perfluoroalkyl acids-induced liver steatosis: Effects on genes controlling lipid homeostasis. Toxicology 378, 37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan W., de Vries-van der Weij J., Mol I. M., Hoekstra M., Romijn J. A., Jukema J. W., Havekes L. M., Princen H. M., Rensen P. C. (2009). PXR agonism decreases plasma HDL levels in ApoE3-Leiden.CETP mice. Biochim. Biophys. Acta 1791, 191–197. [DOI] [PubMed] [Google Scholar]
- de Haan W., van der Hoogt C. C., Westerterp M., Hoekstra M., Dallinga-Thie G. M., Princen H. M., Romijn J. A., Jukema J. W., Havekes L. M., Rensen P. C. (2008). Atorvastatin increases HDL cholesterol by reducing CETP expression in cholesterol-fed APOE*3-Leiden.CETP mice. Atherosclerosis 197, 57–63. [DOI] [PubMed] [Google Scholar]
- de Vries-van der Weij J., de Haan W., Hu L., Kuif M., Oei H. L., van der Hoorn J. W., Havekes L. M., Princen H. M., Romijn J. A., Smit J. W., et al. (2009). Bexarotene induces dyslipidemia by increased very low-density lipoprotein production and cholesteryl ester transfer protein-mediated reduction of high-density lipoprotein. Endocrinology 150, 2368–2375. [DOI] [PubMed] [Google Scholar]
- Dewey F. E., Gusarova V., Dunbar R. L., O’Dushlaine C., Schurmann C., Gottesman O., McCarthy S., Van Hout C. V., Bruse S., Dansky H. M., et al. (2017). Genetic and pharmacologic inactivation of ANGPTL3 and cardiovascular disease. New Engl. J. Med. 377, 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehresman D. J., Froehlich J. W., Olsen G. W., Chang S. C., Butenhoff J. L. (2007). Comparison of human whole blood, plasma, and serum matrices for the determination of perfluorooctanesulfonate (PFOS), perfluorooctanoate (PFOA), and other fluorochemicals. Environ. Res. 103, 176–184. [DOI] [PubMed] [Google Scholar]
- Elcombe C. R., Elcombe B. M., Foster J. R., Farrar D. G., Jung R., Chang S. C., Kennedy G. L., Butenhoff J. L. (2010). Hepatocellular hypertrophy and cell proliferation in sprague-dawley rats following dietary exposure to ammonium perfluorooctanoate occurs through increased activation of the xenosensor nuclear receptors PPARalpha and car/pxr. Arch. Toxicol. 84, 787–798. [DOI] [PubMed] [Google Scholar]
- Eriksen K. T., Raaschou-Nielsen O., McLaughlin J. K., Lipworth L., Tjonneland A., Overvad K., Sorensen M. (2013). Association between plasma PFOA and PFOS levels and total cholesterol in a middle-aged Danish population. PLoS One 8, e56969.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisbee S. J., Shankar A., Knox S. S., Steenland K., Savitz D. A., Fletcher T., Ducatman A. M. (2010). Perfluorooctanoic acid, perfluorooctanesulfonate, and serum lipids in children and adolescents: Results from the C8 health project. Arch. Pediatr. Adolesc. Med. 164, 860–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier T., Tietge U. J., Boverhof R., Perton F. G., Le Guern N., Masson D., Rensen P. C., Havekes L. M., Lagrost L., Kuipers F. (2007). Hepatic lipid accumulation in apolipoprotein C-I-deficient mice is potentiated by cholesteryl ester transfer protein. J. Lipid Res. 48, 30–40. [DOI] [PubMed] [Google Scholar]
- Geerling J. J., Boon M. R., van der Zon G. C., van den Berg S. A. A., van den Hoek A. M., Lombès M., Princen H. M. G., Havekes L. M., Rensen P. C. N., Guigas B. (2014). Metformin lowers plasma triglycerides by promoting VLDL-triglyceride clearance by brown adipose tissue in mice. Diabetes 63, 880–891. [DOI] [PubMed] [Google Scholar]
- Geiger S. D., Xiao J., Ducatman A., Frisbee S., Innes K., Shankar A. (2014). The association between PFOA, PFOS and serum lipid levels in adolescents. Chemosphere 98, 78–83. [DOI] [PubMed] [Google Scholar]
- Guerin M., Bruckert E., Dolphin P. J., Turpin G., Chapman M. J. (1996). Fenofibrate reduces plasma cholesteryl ester transfer from HDL to VLDL and normalizes the atherogenic, dense LDL profile in combined hyperlipidemia. Arteriosclerosis Thrombosis Vasc. Biol. 16, 763–772. [DOI] [PubMed] [Google Scholar]
- Guruge K. S., Yeung L. W., Yamanaka N., Miyazaki S., Lam P. K., Giesy J. P., Jones P. D., Yamashita N. (2006). Gene expression profiles in rat liver treated with perfluorooctanoic acid (PFOA). Toxicol. Sci. 89, 93–107. [DOI] [PubMed] [Google Scholar]
- Haughom B., Spydevold O. (1992). The mechanism underlying the hypolipemic effect of perfluorooctanoic acid (PFOA), perfluorooctane sulphonic acid (PFOSA) and clofibric acid. Biochim. Biophys. Acta 1128, 65–72. [DOI] [PubMed] [Google Scholar]
- Hui Z., Li R., Chen L. (2017). The impact of exposure to environmental contaminant on hepatocellular lipid metabolism. Gene 622, 67–71. [DOI] [PubMed] [Google Scholar]
- ILAR. 2011. National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. National Academies Press (US), Washington, DC. [Google Scholar]
- Jain R. B., Ducatman A. (2018). Associations between lipid/lipoprotein levels and perfluoroalkyl substances among US children aged 6-11 years. Environ. Pollut. 243, 1–8. [DOI] [PubMed] [Google Scholar]
- Kleemann R., Gervois P. P., Verschuren L., Staels B., Princen H. M., Kooistra T. (2003). Fibrates down-regulate IL-1-stimulated C-reactive protein gene expression in hepatocytes by reducing nuclear p50-NFkappa B-C/EBP-beta complex formation. Blood 101, 545–551. [DOI] [PubMed] [Google Scholar]
- Kooistra T., Verschuren L., de Vries-van der Weij J., Koenig W., Toet K., Princen H. M., Kleemann R. (2006). Fenofibrate reduces atherogenesis in ApoE*3Leiden mice: Evidence for multiple antiatherogenic effects besides lowering plasma cholesterol. Arteriosclerosis Thrombosis Vasc. Biol. 26, 2322–2330. [DOI] [PubMed] [Google Scholar]
- Kühnast S., van der Hoorn J. W. A., Pieterman E. J., van den Hoek A. M., Sasiela W. J., Gusarova V., Peyman A., Schäfer H.-L., Schwahn U., Jukema J. W., et al. (2014). Alirocumab inhibits atherosclerosis, improves the plaque morphology, and enhances the effects of a statin. J. Lipid Res. 55, 2103–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühnast S., van der Tuin S. J., van der Hoorn J. W., van Klinken J. B., Simic B., Pieterman E., Havekes L. M., Landmesser U., Luscher T. F., Willems van Dijk K., et al. (2015). Anacetrapib reduces progression of atherosclerosis, mainly by reducing non-HDL-cholesterol, improves lesion stability and adds to the beneficial effects of atorvastatin. Eur. Heart J. 36, 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C., Anitole K., Hodes C., Lai D., Pfahles-Hutchens A., Seed J. (2007). Perfluoroalkyl acids: A review of monitoring and toxicological findings. Toxicol. Sci. 99, 366–394. [DOI] [PubMed] [Google Scholar]
- Liang W., Verschuren L., Mulder P., van der Hoorn J. W., Verheij J., van Dam A. D., Boon M. R., Princen H. M., Havekes L. M., Kleemann R., et al. (2015). Salsalate attenuates diet induced non-alcoholic steatohepatitis in mice by decreasing lipogenic and inflammatory processes. Br. J. Pharmacol. 172, 5293–5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H. S., Wen L. L., Chu P. L., Lin C. Y. (2018). Association among total serum isomers of perfluorinated chemicals, glucose homeostasis, lipid profiles, serum protein and metabolic syndrome in adults: NHANES, 2013-2014. Environ. Pollut. 232, 73–79. [DOI] [PubMed] [Google Scholar]
- Loveless S. E., Finlay C., Everds N. E., Frame S. R., Gillies P. J., O’Connor J. C., Powley C. R., Kennedy G. L. (2006). Comparative responses of rats and mice exposed to linear/branched, linear, or branched ammonium perfluorooctanoate (APFO). Toxicology 220, 203–217. [DOI] [PubMed] [Google Scholar]
- Nelson J. W., Hatch E. E., Webster T. F. (2010). Exposure to polyfluoroalkyl chemicals and cholesterol, body weight, and insulin resistance in the general U.S. Population. Environ. Health Perspect. 118, 197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okochi E., Nishimaki-Mogami T., Suzuki K., Takahashi A. (1999). Perfluorooctanoic acid, a peroxisome-proliferating hypolipidemic agent, dissociates apolipoprotein b48 from lipoprotein particles and decreases secretion of very low density lipoproteins by cultured rat hepatocytes. Biochim. Biophys. Acta 1437, 393–401. [DOI] [PubMed] [Google Scholar]
- Olsen G. W., Burris J. M., Burlew M. M., Mandel J. H. (2000). Plasma cholecystokinin and hepatic enzymes, cholesterol and lipoproteins in ammonium perfluorooctanoate production workers. Drug Chem. Toxicol. 23, 603–620. [DOI] [PubMed] [Google Scholar]
- Olsen G. W., Burris J. M., Burlew M. M., Mandel J. H. (2003). Epidemiologic assessment of worker serum perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) concentrations and medical surveillance examinations. J. Occup. Environ. Med. 45, 260–270. [DOI] [PubMed] [Google Scholar]
- Olsen G. W., Ehresman D. J., Buehrer B. D., Gibson B. A., Butenhoff J. L., Zobel L. R. (2012). Longitudinal assessment of lipid and hepatic clinical parameters in workers involved with the demolition of perfluoroalkyl manufacturing facilities. J. Occup. Environ. Med. 54, 974–983. [DOI] [PubMed] [Google Scholar]
- Olsen G. W., Zobel L. R. (2007). Assessment of lipid, hepatic, and thyroid parameters with serum perfluorooctanoate (PFOA) concentrations in fluorochemical production workers. Int. Arch. Occup. Environ. Health 81, 231–246. [DOI] [PubMed] [Google Scholar]
- Parham P. 2014. The Immune System. Ww Norton & Co., New York, New York, USA. [Google Scholar]
- Post S. M., de Crom R., van Haperen R., van Tol A., Princen H. M. (2003). Increased fecal bile acid excretion in transgenic mice with elevated expression of human phospholipid transfer protein. Arteriosclerosis Thrombosis Vasc. Biol. 23, 892–897. [DOI] [PubMed] [Google Scholar]
- Post S. M., Zoeteweij J. P., Bos M. H., de Wit E. C., Havinga R., Kuipers F., Princen H. M. (1999). Acyl-coenzyme A: Cholesterol acyltransferase inhibitor, avasimibe, stimulates bile acid synthesis and cholesterol 7alpha-hydroxylase in cultured rat hepatocytes and in vivo in the rat. Hepatology 30, 491–500. [DOI] [PubMed] [Google Scholar]
- Pouwer M. G., Pieterman E. J., Verschuren L., Caspers M. P. M., Kluft C., Garcia R. A., Aman J., Jukema J. W., Princen H. M. G. (2018). The BCR-ABL1 inhibitors imatinib and ponatinib decrease plasma cholesterol and atherosclerosis, and nilotinib and ponatinib activate coagulation in a translational mouse model. Front. Cardiovasc. Med. 5, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Princen H. M. G., Pouwer M. G., Pieterman E. J. (2016). Comment on “hypercholesterolemia with consumption of pfoa-laced western diets is dependent on strain and sex of mice” by Rebholz S.L. et al. Toxicol. Rep. 2016(3) 46-54. Toxicol. Rep. 3, 306–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raleigh K. K., Alexander B. H., Olsen G. W., Ramachandran G., Morey S. Z., Church T. R., Logan P. W., Scott L. L., Allen E. M. (2014). Mortality and cancer incidence in ammonium perfluorooctanoate production workers. Occup. Environ. Med. 71, 500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebholz S. L., Jones T., Herrick R. L., Xie C., Calafat A. M., Pinney S. M., Woollett L. A. (2016). Hypercholesterolemia with consumption of PFOA-laced western diets is dependent on strain and sex of mice. Toxicol. Rep. 3, 46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen M. B., Abbott B. D., Wolf D. C., Corton J. C., Wood C. R., Schmid J. E., Das K. P., Zehr R. D., Blair E. T., Lau C. (2008). Gene profiling in the livers of wild-type and PPARalpha-null mice exposed to perfluorooctanoic acid. Toxicol. Pathol. 36, 592–607. [DOI] [PubMed] [Google Scholar]
- Rosen M. B., Schmid J. R., Corton J. C., Zehr R. D., Das K. P., Abbott B. D., Lau C. (2010). Gene expression profiling in wild-type and PPARalpha-null mice exposed to perfluorooctane sulfonate reveals PPARalpha-independent effects. PPAR Res. 2010, 1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakr C. J., Kreckmann K. H., Green J. W., Gillies P. J., Reynolds J. L., Leonard R. C. (2007). Cross-sectional study of lipids and liver enzymes related to a serum biomarker of exposure (ammonium perfluorooctanoate or APFO) as part of a general health survey in a cohort of occupationally exposed workers. J. Occup. Environ. Med. 49, 1086–1096. [DOI] [PubMed] [Google Scholar]
- Sakr C. J., Leonard R. C., Kreckmann K. H., Slade M. D., Cullen M. R. (2007). Longitudinal study of serum lipids and liver enzymes in workers with occupational exposure to ammonium perfluorooctanoate. J. Occup. Environ. Med. 49, 872–879. [DOI] [PubMed] [Google Scholar]
- Staels B., Maes M., Zambon A. (2008). Fibrates and future PPARalpha agonists in the treatment of cardiovascular disease. Nat. Clin. Pract. Cardiovasc. Med. 5, 542–553. [DOI] [PubMed] [Google Scholar]
- Steenland K., Fletcher T., Savitz D. A. (2010). Epidemiologic evidence on the health effects of perfluorooctanoic acid (PFOA). Environ. Health Perspect. 118, 1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland K., Tinker S., Frisbee S., Ducatman A., Vaccarino V. (2009). Association of perfluorooctanoic acid and perfluorooctane sulfonate with serum lipids among adults living near a chemical plant. Am. J. Epidemiol. 170, 1268–1278. [DOI] [PubMed] [Google Scholar]
- Steenland K., Woskie S. (2012). Cohort mortality study of workers exposed to perfluorooctanoic acid. Am. J. Epidemiol. 176, 909–917. [DOI] [PubMed] [Google Scholar]
- Steenland K., Zhao L., Winquist A. (2015). A cohort incidence study of workers exposed to perfluorooctanoic acid (PFOA). Occup. Environ. Med. 72, 373–380. [DOI] [PubMed] [Google Scholar]
- Takacs M. L., Abbott B. D. (2007). Activation of mouse and human peroxisome proliferator-activated receptors (alpha, beta/delta, gamma) by perfluorooctanoic acid and perfluorooctane sulfonate. Toxicol. Sci. 95, 108–117. [DOI] [PubMed] [Google Scholar]
- van der Hoogt C. C., de Haan W., Westerterp M., Hoekstra M., Dallinga-Thie G. M., Romijn J. A., Princen H. M., Jukema J. W., Havekes L. M., Rensen P. C. (2007). Fenofibrate increases HDL-cholesterol by reducing cholesteryl ester transfer protein expression. J. Lipid Res. 48, 1763–1771. [DOI] [PubMed] [Google Scholar]
- van der Hoorn J. W., de Haan W., Berbee J. F., Havekes L. M., Jukema J. W., Rensen P. C., Princen H. M. (2008). Niacin increases HDL by reducing hepatic expression and plasma levels of cholesteryl ester transfer protein in APOE*3Leiden.CETP mice. Arteriosclerosis Thrombosis Vasc. Biol. 28, 2016–2022. [DOI] [PubMed] [Google Scholar]
- Voshol P. J., Rensen P. C., van Dijk K. W., Romijn J. A., Havekes L. M. (2009). Effect of plasma triglyceride metabolism on lipid storage in adipose tissue: Studies using genetically engineered mouse models. Biochim. Biophys. Acta 1791, 479–485. [DOI] [PubMed] [Google Scholar]
- Wallace K., Cowie D. E., Konstantinou D. K., Hill S. J., Tjelle T. E., Axon A., Koruth M., White S. A., Carlsen H., Mann D. A., et al. (2010). The PXR is a drug target for chronic inflammatory liver disease. J. Steroid Biochem. Mol. Biol. 120, 137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., van der Tuin S., Tjeerdema N., van Dam A. D., Rensen S. S., Hendrikx T., Berbee J. F., Atanasovska B., Fu J., Hoekstra M., et al. (2015). Plasma cholesteryl ester transfer protein is predominantly derived from kupffer cells. Hepatology 62, 1710–1722. [DOI] [PubMed] [Google Scholar]
- Xie Y., Yang Q., Nelson B. D., DePierre J. W. (2003). The relationship between liver peroxisome proliferation and adipose tissue atrophy induced by peroxisome proliferator exposure and withdrawal in mice. Biochem. Pharmacol. 66, 749–756. [DOI] [PubMed] [Google Scholar]
- Yang B., Zou W., Hu Z., Liu F., Zhou L., Yang S., Kuang H., Wu L., Wei J., Wang J., et al. (2014). Involvement of oxidative stress and inflammation in liver injury caused by perfluorooctanoic acid exposure in mice. Biomed Res. Int. 2014, 409837.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Zhai Y., Mu Y., Gong H., Uppal H., Toma D., Ren S., Evans R. M., Xie W. (2006). A novel pregnane x receptor-mediated and sterol regulatory element-binding protein-independent lipogenic pathway. J. Biol. Chem. 281, 15013–15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.