Highlights
-
•
ADHD affects functional connectivity in a brain network for vigilant attention (VA).
-
•
First evidence showing the VA network is intrinsically coupled in older children.
-
•
ADHD patients showed less consistent network connectivity, forming 2 subnetworks.
-
•
Lower network integrity in ADHD is due to both weaker and stronger connections.
-
•
Aberrant connectivity between several regions is linked to ADHD symptomatology.
Keywords: ADHD, Resting-state fMRI, Sustained attention, Alertness, Children, Adolescents
Abstract
The ability to maintain attention to simple tasks (i.e., vigilant attention, VA) is often impaired in attention-deficit/hyperactivity disorder (ADHD), but the underlying pathophysiological mechanisms at the brain network level are not clear yet. We therefore investigated ADHD-related differences in resting-state functional connectivity within a meta-analytically defined brain network of 14 distinct regions subserving VA (comprising 91 connections in total), as well as the association of connectivity with markers of behavioural dysfunction in 17 children (age range: 9–14 years) with a diagnosis of ADHD and 21 age-matched neurotypical controls. Our analyses revealed selective, rather than global, differences in the intrinsic coupling between nodes of the VA-related brain network in children with ADHD, relative to controls. In particular, ADHD patients showed substantially diminished intrinsic coupling for 7 connections and increased coupling for 4 connections, with many differences involving connectivity with the anterior insula. Moreover, connectivity strength of several aberrant connections was found to be associated with core aspects of ADHD symptomatology, such as poor attention, difficulties with social functioning, and impaired cognitive control, attesting to the behavioural relevance of specific connectivity differences observed in the resting state.
1. Introduction
Attention-deficit/hyperactivity disorder (ADHD) is one of the most commonly diagnosed disorders in child and adolescent psychiatry, and is characterized by developmentally inappropriate levels of attention, impulsivity and hyperactivity (Biederman & Faraone, 2005). As many activities of daily living require attention for a period of more than a few seconds, a capacity for sustaining attention over time is required (Langner & Eickhoff, 2013). Amongst other attentional deficits, children and adolescents with ADHD are known to show decreased performance in tasks requiring sustained attention, which contributes to problems experienced in everyday life (Baroni and Castellanos, 2015, Greimel et al., 2008, Rubia et al., 2009).
Compared to situations that are cognitively demanding but interesting, maintaining attention in monotonous or boring situations is often challenging, or maybe even onerous (Langner and Eickhoff, 2013, Manly et al., 2003). In this context, a critical attentional function that enables prolonged engagement in intellectually uninteresting and unchallenging situations has been defined as vigilant attention (VA; Robertson & Garavan, 2004). Models of VA assume that these attentional processes are generated without being exogenously triggered by novelty (Robertson, Ridgeway, Greenfield, & Parr, 1997), and that inhibitory control is critical to prevent other mental schemata from interfering with the VA-related task at hand (Stuss, Shallice, Alexander, & Picton, 1995). Research has identified brain regions involved in tasks taxing VA. In a recent neuroimaging meta-analysis, 14 distinct regions were found to be consistently activated during VA tasks (Langner & Eickhoff, 2013). These particular regions included the dorsomedial, mid- and ventrolateral prefrontal cortex, the anterior insula, different parietal areas (intraparietal sulcus, temporoparietal junction), and subcortical regions (cerebellar vermis, thalamus, midbrain). From a clinical point of view, children and adolescents with ADHD show marked deficits in VA-related tasks (Günther et al., 2011, Manly et al., 2003, Rubia et al., 2009). In line with this, functional abnormalities in these particular brain areas have previously been associated with ADHD symptoms (Arnsten, 2009a, Arnsten, 2009b, Baroni and Castellanos, 2015, Kasparek et al., 2015).
To date, neuroimaging research of brain networks involved in VA has been conducted using functional magnetic resonance imaging (fMRI) techniques that involve the examination of hemodynamic activity during specific attention-task performance. To date, no studies have examined spontaneous activity in the above-mentioned VA-network in young people at rest while in the fMRI environment, as well as correlations amongst spontaneous activity across the various regions implicated in the study of VA-related tasks. Such “resting state” analyses provide insights into the functional connectivity (FC) of the brain regions implicated in prior task based fMRI studies. FC analyses of resting-state fMRI data aim to detect temporal correlations between the spontaneous hemodynamic activity profiles of spatially distinct brain regions in the absence of specific attention tasks. Highly similar spontaneous activity patterns (i.e., strong intrinsic coupling), in turn, are taken to reflect intense interregional communication and, ultimately, collaboration.
As a result, analyses of resting-state FC (RS-FC) may shed light onto the intrinsic organization of functional brain networks and their aberrations in different populations, such as individuals with psychiatric disorders, with findings being uncontaminated by “noise” introduced by extrinsic task demands (Greicius, 2008). In this regard, RS-fMRI can be seen as a complementary assessment of brain network characteristics besides task-related neuroimaging, allowing for the comparative examination of VA network integrity in neurotypical (NT) controls versus patients with ADHD.
In the past decade, different RS-FC studies have examined brain network changes in patients with ADHD in an attempt to achieve a deeper understanding of the neurobiological correlates of this particular disorder. Previous research has focused primarily on the default-mode network (DMN), other networks involved in cognitive control, or cortico-striato-thalamo-cortical (CSTC) neurocircuitries, which play a major role in the “dual-pathway theory” of ADHD (Sonuga-Barke, 2003). In short, this theory suggests that ADHD-related symptoms can be interpreted as the outcome of two dysfunctional psycho-physiological pathways, namely the executive and the reward circuit. Reviewing the pertinent literature, three major findings can be described: First, the DMN, a network associated with introspective mental processes, mind-wandering and rumination, is functionally less connected in patients with ADHD when compared to NT controls (Kessler et al., 2014, Konrad et al., 2006, Posner et al., 2014). Second, anti-correlations between the DMN and task-positive networks such as cognitive control networks were either reduced or absent in patients with an ADHD diagnosis (Kessler et al., 2014, Mattfeld et al., 2014, Posner et al., 2014). Notably, these cognitive control networks are typically engaged during tasks taxing executive functioning. Failure to suppress or deactivate DMN activity while processing external task demands is typically associated with lapses in attention (Weissman, Roberts, Visscher, & Woldorff, 2006), suggesting inappropriate persistence of DMN activity (Konrad et al., 2006, Posner et al., 2014, Sonuga-Barke, 2003). Third, RS-FC studies have detected reduced coupling in cognitive and limbic CSTC neurocircuitries in patients with ADHD compared to NT controls (Baroni and Castellanos, 2015, Posner et al., 2014, Rubia et al., 2009). In this context, persistent DMN activity has also been found to predict errors in the stop-signal task (Li, Yan, Bergquist, & Sinha, 2007), a well-established measure of response inhibition, which is often found to be impaired in patients with ADHD. These findings might explain why patients with ADHD often experience difficulties with tasks such as waiting one’s turn, or not remaining seated when required.
However, to our knowledge, there have been no studies related to the intrinsic organization of the network subserving VA in children and adolescents with ADHD, a group of patients who are significantly affected by deficits in sustained attention (Baroni and Castellanos, 2015, Epstein et al., 2009, Greimel et al., 2008, Günther et al., 2011, Rubia et al., 2009). Identifying ADHD-related differences in FC between brain regions involved in VA may help to further disentangle the pathophysiology underlying ADHD, and could provide a potential avenue for new intervention strategies linked to VA and related processes. We therefore investigated FC patterns in the previously described VA network in children and adolescents with ADHD and compared them to FC patterns of NT participants of the same age group.
As a secondary aim, we examined the relationship between FC in the VA network and a range of temperamental and cognitive performance indicators. To move beyond the syndromic nosology relied upon by psychiatry, a clearer understanding of neurobiologically informed dimensions of functioning related to mental disorders is required. This concern was reflected in the National Institutes of Mental Health Research Domain Criteria initiative (Insel, 2014). As VA reflects a key candidate domain, we aimed to examine the correlation of FC in the VA network with a number of candidate measures of theoretically implicated constructs that are supposed to reflect ADHD and related symptomatology as a syndrome, including changes in persistence, impulsivity, novelty seeking, and inhibitory control. Given the exploratory nature of the current study, and because of the lack of empirical literature exploring FC and behavioural symptoms in children with ADHD in a VA-related context, we did not propose specific hypotheses.
2. Methods
2.1. Participants
Seventeen male patients with ADHD (age range: 9–14 years) were recruited from a major mental health service (Clinic for Child and Adolescent Psychiatry, Psychosomatics and Psychotherapy, RWTH Aachen University Hospital, Germany) via advertisements (see Table 1 for demographic details and also Bubenzer-Busch et al. 2016, for a more detailed description of the study sample and recruitment procedure). For this study, only male participants were recruited as per protocol to provide a homogeneous sample for analysis, and to avoid putative gender effects. Furthermore, 21 NT controls of a similar age range were recruited from the local community, also via advertisements. Due to excessive motion during the fMRI scanning, 2 NT participants were excluded, yielding a final group of 19 NT participants for analysis (see Table 1 for demographic data).
Table 1.
Sample characteristics.
| ADHD (n = 17) M (SD) |
Controls (n = 19) M (SD) |
Statistics | p | |
|---|---|---|---|---|
| Demographic data | ||||
| Age (years) | 11.5 (1.0) | 11.0 (1.3) | t = 1.27 | 0.212 |
| Age range (min-max) | 9.9–13.4 | 9.1–14.0 | ||
| IQ | 111.0 (17.5) | 120.0 (16.6) | t = −1.68 | 0.102 |
| IQ range (min-max) | 90.0–150.0 | 96.0–162.0 | ||
| Questionnaire data | ||||
| SBQ – ADHD | 3.8 (1.2) | 2.2 (0.8) | t = 4.77 | < 0.001 |
| CBCL – Attentional Problems | 8.1 (3.8) | 1.84 (2.5) | U = 23.5 | < 0.001 |
| JTCI – NS1 (Exploratory Excitability) | 8.7 (2.8) | 7.8 (3.0) | t = 0.83 | 0.415 |
| JTCI – NS2 (Impulsiveness) | 8.1 (2.8) | 6.2 (2.1) | t = 2.40 | 0.022 |
| JTCI – HA4 (Fatigability) | 3.2 (2.2) | 3.6 (2.5) | U = 150.0 | 0.731 |
| JTCI – PS1 (Eagerness of Effort) | 4.5 (3.3) | 6.5 (2.8) | t = −1.93 | 0.063 |
| JTCI – PS2 (Work Hardened) | 6.5 (4.6) | 7.7 (2.9) | t = −0.92 | 0.365 |
| APSD – Impulsivity | 58.5 (12.2) | 46.6 (9.1) | t = 3.32 | 0.002 |
| IVE – Impulsivity | 9.7 (3.7) | 7.7 (3.8) | t = 1.56 | 0.128 |
| Neuropsychological data | ||||
| Alertness (mean RT, in ms) | 271.9 (36.8) | 278.2 (37.2) | t = −0.50 | 0.618 |
| Alertness (RT-SD, in ms) | 60.9 (37.8) | 64.6 (49.0) | U = 158.0 | 0.925 |
| Go/No-go (mean RT, in ms) | 384.6 (81.4) | 372.2 (78.7) | t = 0.46 | 0.646 |
| Go/No-go (RT-SD, in ms) | 115.4 (45.9) | 113.1 (49.8) | U = 147.0 | 0.661 |
| Go/No-go error rate | 6.9 (3.3) | 7.2 (4.1) | t = 0.22 | 0.831 |
Note. Significant group differences are marked in italics.
ADHD/DBD = Attention Deficit Hyperactivity Disorder; SBQ = Social Behavior Questionnaire; CBCL = Child Behavior Checklist; JTCI = Junior Temperament and Character Inventory (NS = Novelty Seeking, HA = Harm Avoidance, PS = Persistence); APSD = Antisocial Process Screening Device; IVE = Impulsiveness–Venturesomeness–Empathy questionnaire; RT-SD = intraindividual standard deviation of response times; t = t-value of independent-sample t-test; U = U-value of Mann–Whitney test.
To confirm (ADHD group) or exclude (NT control group) the diagnosis of ADHD and to control for comorbidities, all parents participated in a structured clinical interview (K-DIPS; Schneider, Unnewehr & Margraf, 2008) that was performed by a trained psychologist. ADHD as a diagnosis was established in accordance with the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV; American Psychiatric Association, 1994) and the International Classification of Diseases, Version 10 (ICD-10; World Health Organization, 1993). Exclusion criteria for both groups included an IQ below 85, neurodevelopmental problems/disorders other than ADHD, psycho-organic syndromes, schizophrenia and affective psychotic disorders, current or past substance abuse, severe somatic disorders, and problems with the German language (in order to ensure that all participants and their families understood the study protocol, the task instructions, and the questionnaires). All participants were right-handed and free of chronic physical illness (e.g. asthma, diabetes, or obesity) as well as (other) neuropsychiatric disorders (e.g. substance abuse). Conduct disorder was tolerated as a comorbid diagnosis in the ADHD group. The most common comorbidities for the participants with ADHD were oppositional defiant disorder (F91.3; n = 8); specific reading disorder (F81.0; n = 4); and conduct disorder confined to the family context (F91.0; n = 3). Patients under continuous treatment with methylphenidate (n = 13, detailed dose information available for n = 10 participants: average dose was 34.3 mg of methylphenidate per day) discontinued their medication two days prior to their study day (see Bubenzer-Busch et al., 2016, for a more detailed description), with the permission of their parents and in close consultation with the clinician in charge of treatment.
The study was approved by the Ethics Committee of the RWTH Aachen University Hospital, and was carried out in accordance with the Declaration of Helsinki. Verbal and written informed consent was obtained from all participants and their legal guardian. Compensation for participation was provided by means of cinema vouchers.
2.2. Behavioural tests and questionnaire measures
Fluid intelligence was assessed using the Culture Fair Intelligence Test (CFT-20R), a German adaptation of the Cattell Culture Fair Intelligence Test, Scale 1 (Weiß & Weiß, 2008). Briefly, in this subtest participants were required to complete a series of geometric progressions.
Variables that were used in the following analyses were considered to best describe ADHD symptoms and / or related constructs (in the following referred to as ADHD-relevant traits). Participants completed the ‘intrinsic alertness’ and ‘go/no-go’ tasks of the Test Battery of Attentional Performance (TAP; Zimmermann & Fimm, 2002). The ‘intrinsic alertness’ component is a simple visual reaction time task in which a cross is displayed in the middle of the screen after variable, unpredictable delays, and participants must respond to the stimulus occurrence via a button press with their dominant hand as quickly as possible. The go/no-go task of the TAP was used to assess attentional selectivity and inhibitory control. In this version, participants were instructed to provide (“go”) or inhibit (“no go”) a button-press response with their dominant hand, depending on the appearance of one of two symbols (e.g. “+” and “x”). Stimuli appeared in random order for 200 ms, with 50% go trials. Intervals between stimuli were variable to a maximum lag of 1600 ms. Young people with ADHD have been shown to perform poorly compared to NT children, with no gender differences observed (Günther, Knospe, Herpertz-Dahlmann, & Konrad, 2015).
To assess relevant aspects of ADHD, parents completed the following questionnaires relating to their child: the Child Behavior Checklist (CBCL; Achenbach, 1991), the Junior Temperament and Character Inventory (JTCI; Goth & Schmeck, 2009), the Social Behavior Questionnaire (SBQ; Tremblay et al., 1991), and a study version of the Antisocial Process Screening Device (APSD; Vitacco, Rogers, & Neumann, 2003). In addition, participants (both patients and NT controls) completed an impulsivity questionnaire (IVE; Stadler, Janke, & Schmeck, 2004).
The CBCL (Achenbach, 1991) is a widely-used standardized parent-report scale measuring a range of children’s emotional and behavioural problems. The CBCL employs a three-point Likert response scale (0 = not true; 1 = somewhat or sometimes true; 2 = very true or often true). These items are often reduced to eight syndromes, all of which have been empirically validated: Withdrawn, Anxious/Depressed, Somatic Complaints, Thought Problems, Social Problems, Attention Problems, Delinquent Problems, and Aggressive Behavior, and three Competence scales: Activities, Social, and School. In this study, we examined only the Attention Problems syndrome. This scale has been shown to be a useful screening instrument for ADHD (Chen et al., 1994, Hudziak et al., 2004). The SBQ (Tremblay et al., 1991) is a parent-reported instrument that uses a three-point Likert scale (1 = never; 2 = sometimes; 3 = often) to assess the prevalence of behaviours during the last 6 months. Sample ADHD items include “could not sit still, was restless or hyperactive” and “acted without thinking.”
The JTCI (Goth & Schmeck, 2009) was inspired by Cloninger’s biosocial model of temperament (Cloninger, Svrakic, & Przybeck, 1993) and the Temperament and Character Inventory (designed for adult populations). The JTCI includes 84 items, which assess four temperament dimensions (Novelty Seeking [i.e., rigid vs. impulsive], Harm Avoidance [risk-taking vs. anxious], Reward Dependence [aloof vs approval seeking], and Persistence [over-achieving vs under-achieving]), and three components of character (Cooperativeness, Self-Directedness, and Self-Transcendence). For this study, we focused on aspects of temperament with clear application to ADHD, and assessed two subcomponents of Novelty Seeking (Exploratory Excitability [NS1] and Impulsiveness [NS2]), two subcomponents of Persistence (Eagerness of Effort [PS1] and Work Hardened [PS2]), and an aspect of Harm Avoidance, Fatigability (HA4).
The Antisocial Process Screening Device (APSD; Vitacco et al., 2003) is a 20-item parent-report measure of psychopathy with established child and adolescent norms. Factor analyses indicate a 3-factor structure including callousness/unemotionality, impulsivity, and narcissism. Only the impulsivity items were used for the current study. Finally, the Impulsiveness-Venturesomeness-Empathy questionnaire (IVE; Stadler et al., 2004) is a 3-subscale self-report measure. Here, we only analysed its impulsiveness subscale, which was derived from the I-6 Impulsiveness Questionnaire (Eysenck, Easting, & Pearson, 1984). Concurrent validity of the IVE impulsiveness subscale was established for children aged 9–11 years via use of a go/no-go paradigm (Stadler & Janke, 2003).
The time between the related clinical and psychological assessments and the fMRI session was short, with all test batteries and questionnaires administered approximately 1–2 weeks prior to the fMRI measurement. Demographic variables, test scores and questionnaire data were analyzed using the software package SPSS 21 (IBM, Boston, MA, USA). Where Kolmogorov–Smirnov tests indicated that the data were normally distributed, independent-sample t-tests were employed to compare means between both groups. Otherwise, Mann–Whitney U-tests were used.
2.3. RS-FC analyses
2.3.1. Definition of seed regions
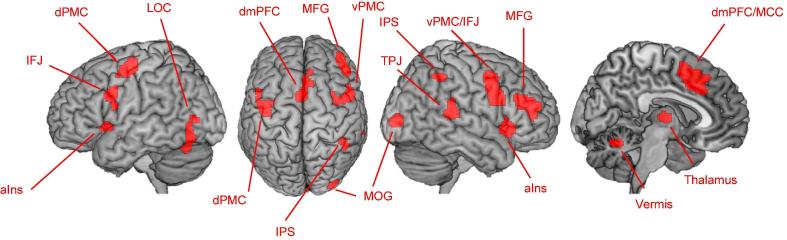
Seed regions for RS-FC analyses were derived from a large-scale neuroimaging meta-analysis on VA (Langner & Eickhoff, 2013). More specifically, we used the 14 significant clusters of voxels as obtained from the VA main effect (Fig. 1): bilateral anterior insula (aIns), bilateral inferior frontal junction (IFJ) extending to ventral premotor cortex on the right, bilateral thalamus, dorsomedial prefrontal cortex (dmPFC) extending to anterior midcingulate cortex (MCC), cerebellar vermis, right middle frontal gyrus (MFG), right intraparietal sulcus (IPS), right temporoparietal junction (TPJ), right middle occipital gyrus (MOG), left lateral occipital cortex (LOC), and left dorsal premotor cortex (dPMC). In total, this VA-related functional network comprised 91 edges.
Fig. 1.
Regions of the vigilant-attention network (Langner & Eickhoff, 2013) used as seeds for the resting-state FC analysis. aIns = anterior insula, dmPFC = dorsomedial prefrontal cortex, dPMC/vPMC = dorsal/ventral premotor cortex, IFJ = inferior frontal junction, IPS = intraparietal sulcus, LOC = lateral occipital cortex, MCC = midcingulate cortex, MFG = middle frontal gyrus, MOG = middle occipital gyrus, TPJ = temporoparietal junction.
2.3.2. fMRI data acquisition
Resting-state fMRI data were obtained using a 3-T Siemens Magnetom Trio scanner with a 12-channel head coil at the Research Centre Jülich. To record spontaneous blood oxygenation level–dependent (BOLD) signal changes, we employed a T2∗-weighted gradient-echo echo-planar imaging (EPI) sequence (repetition time = 2210 ms, echo time = 30 ms, field of view = 200 mm, flip angle = 80°, 36 slices, in-plane resolution 3 × 3 mm2, slice thickness = 3.0 mm, slice gap = 25%, interleaved slice acquisition order), including an algorithm reducing motion artifacts by prospective motion correction through the realignment of the acquired volumes (Thesen, Heid, Mueller, & Schad, 2000). Resting-state fMRI data were collected during a period of five minutes. Participants were instructed to focus their gaze on a white cross in the centre of a black screen and to let their mind wander without falling asleep.
2.3.3. fMRI data analysis
Data were preprocessed using SPM8 (www.fil.ion.ucl.ac.uk/spm). Prior to analysis, four dummy scans, which preceded image acquisition to allow for magnetic field saturation, were discarded. The remaining EPI volumes were first corrected for head movements by affine registration using a two-pass procedure in which images were initially realigned to the first volume and subsequently to the mean of the previously realigned volumes. Each participant's mean image was then spatially normalized to the Montreal Neurological Institute (MNI) single-subject template brain included in SPM8 using the “unified segmentation” approach (Ashburner & Friston, 2005). The ensuing deformation was then applied to all individual EPI volumes, resampling the images at 1.5 × 1.5 × 1.5 mm3 voxel size. Afterwards, images were spatially smoothed by a 5-mm full-width at half-maximum Gaussian kernel to increase the signal-to-noise ratio and compensate for remaining differences in individual anatomy.
RS-FC measures can be affected by several confounds such as head movements and non-neural physiological processes (e.g., fluctuations due to cardiac and respiratory cycles; cf. Fox, Zhang, Snyder, & Raichle, 2009). To reduce spurious correlations, variance explained by the following nuisance variables was removed from each voxel’s BOLD signal time series (cf. Satterthwaite et al., 2013): (i) the six motion parameters obtained from image realignment; (ii) the first derivatives of the six motion parameters, (iii) mean white-matter and cerebrospinal-fluid signal intensity obtained by averaging, at each time point, across all voxels attributed to the respective tissue class (according to the SPM8 segmentation). All nuisance variables were entered into the regression model as first-order terms, and the motion parameters and their derivatives were additionally entered as second-order terms, resulting in a total of 26 nuisance regressors. After confound removal, data were band-pass filtered, and frequencies between 0.01 and 0.08 Hz were retained. The rationale for this is that meaningful resting-state correlations are typically found in this range, given that the BOLD response acts as a low-pass filter (Greicius, Krasnow, Reiss, & Menon, 2003).
The time course of each seed region’s BOLD signal was extracted for each participant as the first eigenvariate of activity in all voxels of the given seed. For each participant, the time-series data of all seed regions were correlated with one another, and the resulting Pearson correlation coefficients were transformed into Fisher’s Z scores. Subsequently, the influence of age was partialled out of both the RS correlations and the covariates of interest (i.e., performance and questionnaire data). Main effects of RS-FC were tested separately in each subgroup using one-sample t-tests against the null hypothesis of no association, applying a significance threshold of p < .05 (adjusted for multiple comparisons by false discovery rate [FDR] correction). Mean RS-FC in patients with ADHD and NT controls was compared via a non-parametric approach using 10,000 realizations of the null hypothesis (group-label exchangeability) in a Monte-Carlo simulation to create an empirical null distribution of group differences (posterior-probability significance threshold: P > .95, uncorrected). Additionally, we applied effect-size criteria: First, differences were only considered potentially important if the RS-FC main effect in either group (or in both) corresponded to a small effect at least, according to Cohen’s categorization (i.e., r ≥ 0.10). Second, the between-group RS-FC mean differences themselves needed to correspond at least to a medium-sized effect (i.e., Cohen’s d ≥ 0.50). Finally, the behavioural relevance of observed ADHD-related differences in interregional coupling was further examined by rank-correlating those Fisher-Z-transformed RS-FC values that differed between groups according to the above criteria with performance and questionnaire scores across the entire sample. The results of these Spearman correlation analyses were regarded significant at p < .05 (uncorrected). Again, an effect-size criterion was applied, considering correlations relevant only if they were of at least medium size according to Cohen’s categorization (i.e., r ≤ –0.24 or r ≥ 0.24).
3. Results
3.1. Subjective and behavioural measures
As summarized in Table 1, the ADHD and NT groups did not significantly differ with respect to age or fluid intelligence. With respect to the measures assessing ADHD symptomatology, there were significant differences between the NT and ADHD groups on the SBQ, CBCL Attentional Problems Subscale, and the Impulsivity Scales of the JTCI-NS2 and the APSD. No significant group differences were observed on the IVE impulsiveness subscale.
Although previous research indicated that children with ADHD show significantly more variability in attentional performance than NT controls, no differences were observed with respect to reaction time or error rate in the alertness and go/no-go tasks of the TAP. In the group of patients with ADHD, scores for baseline aspects of impulsivity as assessed by the IVE were not significantly correlated with reaction time, error rate and omissions assessed by the go/no-go task of the TAP. Similar findings were found for the control group. The lack of group differences may be due to the patients still being on medication during the time that behavioural tasks were being completed. Of note, the IVE in the version for children and adolescents captures more general features of baseline impulsivity in terms of motivational and cognitive aspects, whereas the go/no-go task in the TAP was designed to test for the ability to suppress non-adequate motor reactions.
3.2. FC in the NT control group
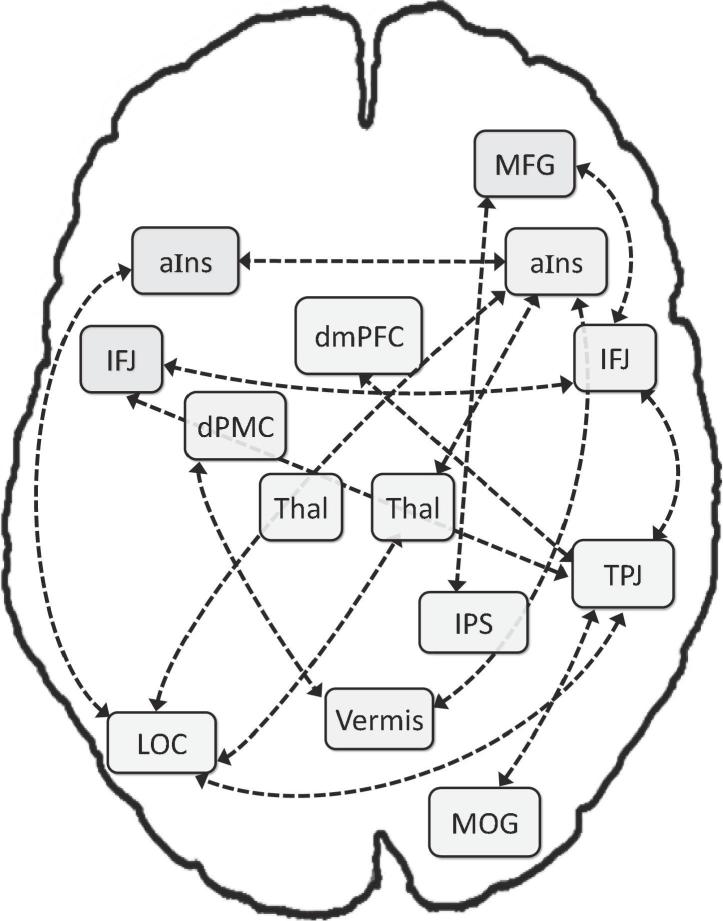
In the NT group, substantial positive RS-FC was found for fourteen connections between nodes of the VA network (see Fig. 2 and Table 2): right aIns with (a) left aIns, (b) left LOC, (c) vermis, and (d) right thalamus; (e) left aIns with left LOC; (f) right thalamus with left LOC; right TPJ with (g) left LOC, (h) right MOG, (i) left and (j) right IFJ, and (k) dmPFC; (l) right IPS with right MFG; and right IFJ with (m) left IFJ and (n) right MFG. As visualized in Fig. 2, these 14 connections link 13 nodes, with only the left thalamus not functionally connected to other regions in the task-unconstrained state.
Fig. 2.
Resting-state FC in NT control children. Thal = thalamus; for further abbreviations, see Fig. 1.
Table 2.
Functional connectivity (FC) between brain regions of the VA-related network in neurotypical (NT) controls: Differences indicate lower FC in patients with attention-deficit/hyperactivity disorder (ADHD) versus controls.
| Pair of regions | NT Group | Between-Group Differences: FCADHD < FCNT |
|||||
|---|---|---|---|---|---|---|---|
| z | Fisher’s ZNT | Pearson rNT | Fisher’s ZADHD | Pearson rADHD | Cohen’s d | P | |
| r aIns – l LOC | 3.12 | 0.597 | 0.535 | 0.136 | 0.135 | 0.87 | 0.99 |
| r aIns – l aIns | 3.02 | 0.888 | 0.710 | 0.198 | 0.195 | 0.66 | 0.97 |
| r aIns – vermis | 2.09 | 0.442 | 0.415 | −0.063 | −0.063 | 0.82 | 0.99 |
| r aIns – r Thal | 1.76 | 0.385 | 0.367 | −0.051 | −0.051 | 0.66 | 0.98 |
| l aIns – l LOC | 2.97 | 0.551 | 0.501 | −0.070 | −0.070 | 1.11 | 1.00 |
| r Thal – l LOC | 2.86 | 0.422 | 0.399 | 0.065 | 0.065 | 0.80 | 0.99 |
| r TPJ – l LOC | 2.83 | 0.336 | 0.324 | −0.089 | −0.089 | 0.99 | 1.00 |
| r TPJ – r MOG | 1.79 | ||||||
| r TPJ – l IFJ | 2.25 | ||||||
| r TPJ – r IFJ | 1.88 | ||||||
| r TPJ – dmPFC | 1.90 | ||||||
| r IPS – r MFG | 2.78 | ||||||
| r IFJ – l IFJ | 2.80 | ||||||
| r IFJ – r MFG | 2.15 | ||||||
Note: z (column 2) reflects t-test-derived z-scores of NT-group average FC as significantly different from zero. FC is presented per group as Fisher’s Z scores and Pearson r for ease of interpretation. Group differences in FC were tested via Monte Carlo simulation of the null hypothesis (k = 10,000). Cohen’s d = effect size of the group difference; P = posterior probability (inverse significance value) of the group difference. Only significant differences (P ≥ 0.95) of at least medium size (Cohen’s d ≥ 0.50) are shown.
r = right; l = left; Thal = thalamus; for further abbreviations of brain regions, please see legend of Fig. 1.
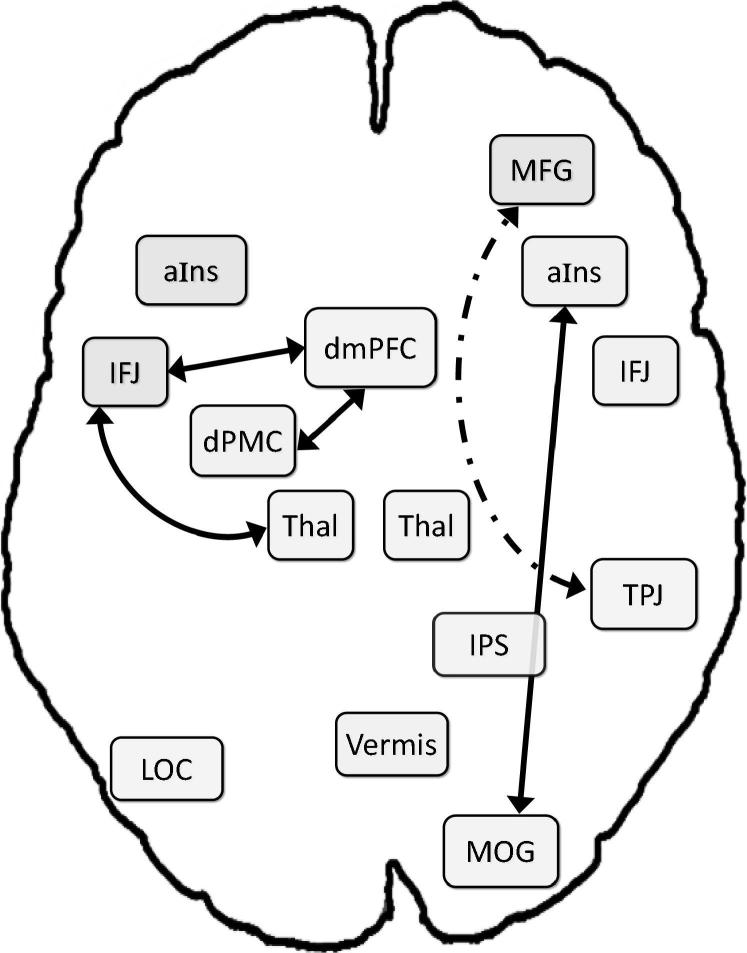
3.3. FC in patients with ADHD
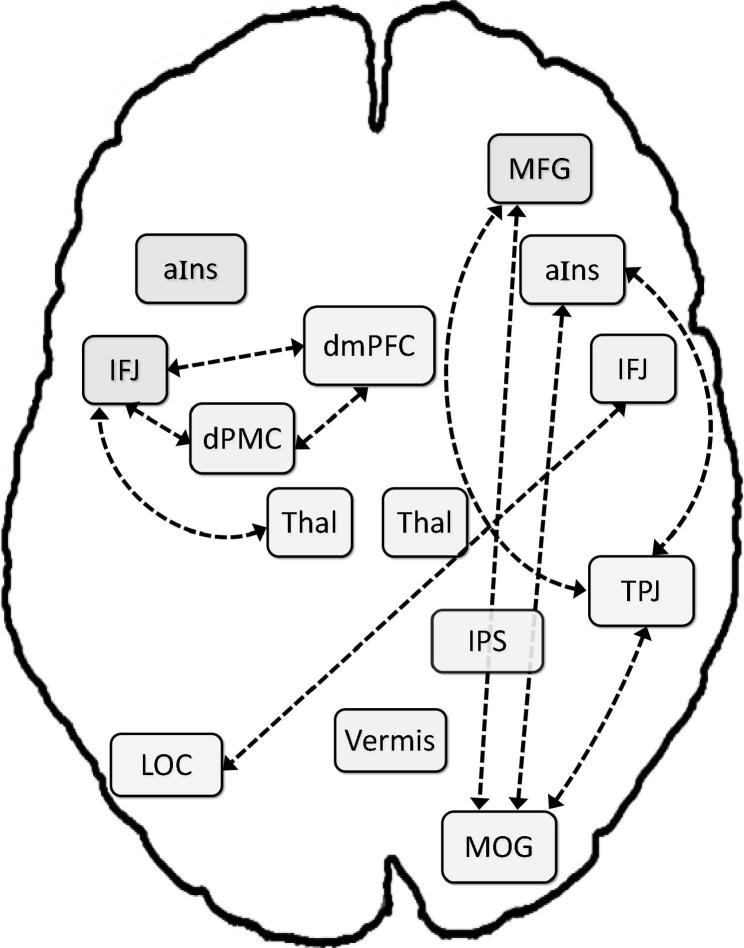
In the ADHD group, our analysis revealed 7 positive connections between VA network nodes (see Table 3 and Fig. 3): right aIns with (a) right MOG and (b) right TPJ; (c) left thalamus with left IFJ; (d) right TPJ with right MOG; left IFJ with (e) dmPFC and (f) left dPMC; and (g) dmPFC with left dPMC. Further, we observed negative RS-FC (i.e., anticorrelations) between MFG and TPJ, MFG and MOG, as well as LOC and right IJF. Thus, unlike the NT group, for whom all nodes were connected with other nodes, except for the left thalamus, seven regions were not significantly coupled to any other node: vermis, left aIns, LOC, right thalamus, right IFJ, MFG, and IPS. Moreover, the RS-FC pattern for patients with ADHD indicated two rather isolated sub-networks: a left-hemisphere network, including IFJ, dPMC, dmPFC, and thalamus; and a right-lateralized sub-network, linking TPJ, MOG, and aIns.
Table 3.
Functional connectivity (FC) between brain regions of the VA-related network in patients with attention-deficit/hyperactivity disorder (ADHD): Differences indicate greater FC in patients versus neurotypical (NT) controls.
| Pair of regions | ADHD Group | Between-Group Differences: FCADHD > FCNT |
|||||
|---|---|---|---|---|---|---|---|
| z | Fisher’s ZNT | PearsonrNT | Fisher’s ZADHD | Pearson rADHD | Cohen’s d | P | |
| r aIns – r MOG | 2.51 | −0.056 | −0.056 | 0.543 | 0.495 | 0.76 | 0.99 |
| l Thal – l IFJ | 2.29 | −0.075 | −0.075 | 0.346 | 0.333 | 0.89 | 0.99 |
| l IFJ - dmPFC | 2.98 | 0.312 | 0.302 | 0.786 | 0.656 | 0.57 | 0.95 |
| dmPFC – l dPMC | 2.32 | 0.157 | 0.156 | 0.643 | 0.567 | 0.58 | 0.96 |
| r TPJ – r MFG | −2.78 | −0.062 | −0.062 | −0.383 | −0.365 | 0.71 | 0.98 |
| r aIns – r TPJ | 1.92 | ||||||
| r TPJ – r MOG | 2.77 | ||||||
| r MFG - r MOG | −1.89 | ||||||
| r IFJ – l LOC | −3.00 | ||||||
| l IFJ – l dPMC | 2.05 | ||||||
Note: z (column 2) reflects t-test-derived z-scores of ADHD-group average FC as significantly different from zero. FC is presented per group as Fisher’s Z scores and Pearson r for ease of interpretation. Group differences in FC were tested via Monte Carlo simulation of the null hypothesis (k = 10,000). Cohen’s d = effect size of the group difference; P = posterior probability (inverse significance value) of the group difference. Only significant differences (P ≥ 0.95) of at least medium size (Cohen’s d ≥ 0.50) are shown.
r = right; l = left; Thal = thalamus; for further abbreviations of brain regions, please see legend of Fig. 1.
Fig. 3.
Resting-state FC in children with attention-deficit/hyperactivity disorder (ADHD). Dashed lines reflect positive correlations; dash-dot lines reflect anticorrelations. Thal = thalamus; for further abbreviations, see Fig. 1.
3.4. FC differences between NT controls and patients with ADHD
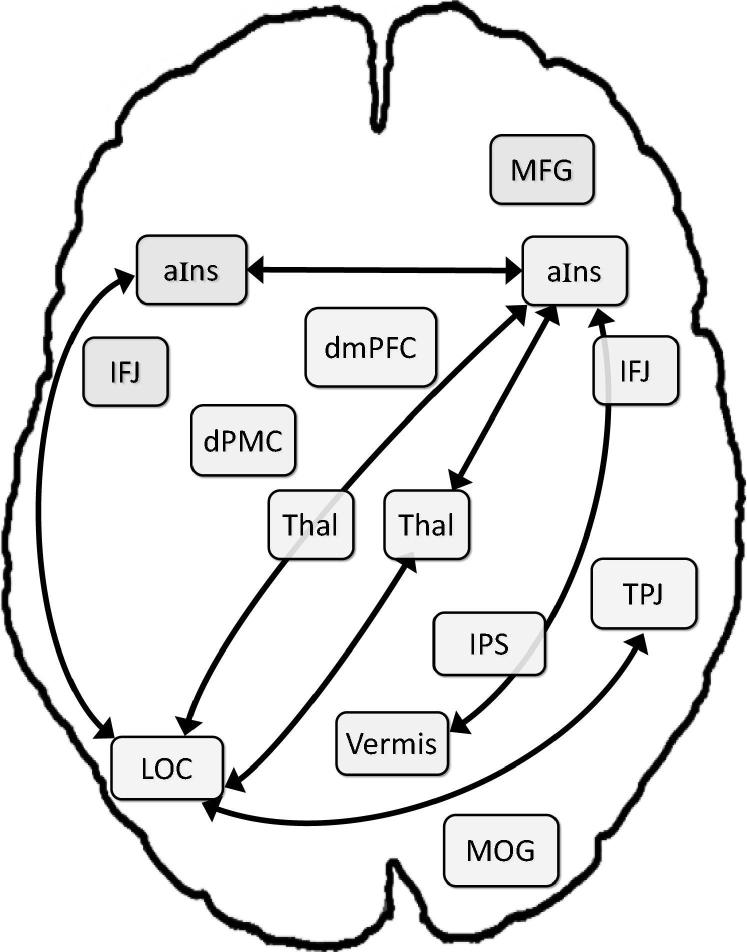
The comparison of RS-FC in the VA network between both groups revealed 7 connections where patients with ADHD showed significantly diminished FC values that met our effect size criterion (i.e., Cohen’s d ≥ 0.50; see Table 2 and Fig. 4): the right aIns with (a) left aIns, (b) left LOC, (c) vermis, and (d) right thalamus; left LOC with (e) left aIns, (f) right thalamus, and (g) right TPJ. Conversely, significantly greater FC in patients with ADHD was found for 4 connections that met our effect size criterion (see Table 3 and Fig. 5): (a) right aIns with right MOG; left IFJ with (b) left thalamus and (c) dmPFC; and (d) dmPFC with left dPMC. In addition, we observed stronger decoupling in ADHD patients (vs. NT controls) between right TPJ and right MFG.
Fig. 4.
Significant between-group (ADHD vs. NT children) differences in resting-state FC, with greater connectivity amongst NT controls. Thal = thalamus; for further abbreviations, see Fig. 1.
Fig. 5.
Significant between-group (ADHD vs. NT children) differences in resting-state FC, with greater connectivity amongst ADHD patients. Solid lines reflect positive correlations; dashed line represents stronger anticorrelation. Thal = thalamus; for further abbreviations, see Fig. 1.
3.5. Correlations between RS-FC and phenotypical measures
Correlational analyses (k = 168) revealed that 4 of the above 12 connections showing relevant group differences also were significantly associated with some of the 14 ADHD-relevant behavioural traits (see Table 4 and Fig. S1 in the Supplementary Material): Higher connectivity between right and left aIns was associated with lower overall ADHD symptomatology (SBQ-ADHD) and lower scores in the Impulsiveness subscale of the JTCI novelty seeking scale (NS2). Connectivity between right aIns and cerebellar vermis was anti-correlated with overall ADHD symptomatology (SBQ-ADHD) and positively correlated with go/no-go error rate, a measure of inhibitory control (failure). Further, FC between left aIns and left LOC was anti-correlated with the CBCL Attentional Problems score. Finally, another anti-correlation was observed between FC of left thalamus and left IFJ and scores on the Eagerness of Effort subscale of the JTCI Persistence scale (PS1).
Table 4.
Correlation of functional connectivity (FC) between brain regions of the VA-related network and ADHD-related behavioural measures.
| Pair of regions | Score | rs |
|---|---|---|
| r aIns – l aIns | SBQ – ADHD | −0.46 |
| r aIns – l aIns | JTCI – NS2 (Impulsiveness) | −0.34 |
| r aIns – vermis | SBQ – ADHD | −0.33 |
| r aIns – vermis | Go/no-go error rate | 0.40 |
| l aIns – l LOC | CBCL – Attentional Problems | −0.34 |
| l Thal – l IFJ | JTCI – PS1 (Eagerness of Effort) | −0.39 |
Note: rs = Spearman’s Rho: only significant correlations (p < .05, uncorrected) with an at least medium effect size are reported. ADHD = attention-deficit/hyperactivity disorder; r = right; l = left; SBQ = Social Behavior Questionnaire; JTCI = Junior Temperament and Character Inventory (NS = Novelty Seeking; PS = Persistence); CBCL = Child Behavior Checklist; Thal = thalamus; for further abbreviations of brain regions, please see legend of Fig. 1.
4. Discussion
Deficits in maintaining focus and attention to uninteresting stimuli or tasks (i.e., vigilant attention) are commonly observed in patients with ADHD. Our study examined FC in the neural networks involved in VA amongst young people with ADHD and NT controls of the same age. We used resting-state fMRI to assess FC between brain regions previously implicated in VA, drawn from a comprehensive meta-analysis of these neural networks (Langner & Eickhoff, 2013). As hypothesized, RS-FC between several regions of the VA-related network was significantly different in the ADHD group compared to NT controls, and several of the connections showing group differences in intrinsic coupling were associated with ADHD-relevant measures of dysfunctional behaviour.
Our analyses revealed a more robustly integrated VA-related network in NT controls, relative to patients with ADHD. For the NT group, the correlated activity formed a single network of 13 interconnected nodes, with the right TPJ serving a central role, with unique connections to four other regions. By contrast, RS-FC in the network of patients with ADHD consisted of two unconnected, and much smaller, sub-networks. Seven nodes that were well integrated in the NT group’s network were completely unconnected functionally to any other nodes in the ADHD group. Importantly, the present findings draw attention to the enhanced, potentially maladaptive, left-hemispheric FC of patients with ADHD relative to NT controls. These data also complement the well-established literature implicating right-hemispheric dominance in attentional processes (García-Sánchez et al., 1997, Langner and Eickhoff, 2013, Sandson et al., 2000, Shulman et al., 2010), and right-hemispheric dysfunction in ADHD (Bellgrove et al., 2009). The full significance of the group differences in the FC network architecture requires further investigation. It is worth noting, however, that this is the third neural network to be identified as showing poor intrinsic connectivity amongst patients with ADHD, the other two being the DMN (Kessler et al., 2014, Konrad et al., 2006, Posner et al., 2014) and the CSTC neurocircuitries (Baroni and Castellanos, 2015, Posner et al., 2014, Rubia et al., 2009).
The clinical significance of the observed poor connectivity within the VA network is not yet clear, but it is plausible that poor intrinsic coupling may contribute to problems in sustained activation of the VA system in young people with ADHD. Specifically, if the system is functionally dysconnected in a task-unconstrained state (rest), activation of any one node during tasks may fail to quickly activate or synchronize other nodes, thereby resulting in a failure to recruit the VA system when required. Indeed, activation networks often mirror resting network connectivity (Smith et al., 2009). By reducing the “noise” introduced by variance in task-based fMRI (e.g., differences in effort, strategy, and other aspects of the task itself; Fox & Greicius, 2010), our RS-FC analyses may provide an indication of spontaneous co-activations in the VA network and differences therein between young people with ADHD and NT controls. Our findings further support the hypothesis of hypo-connectivity of the VA network in the right hemisphere in young patients with ADHD, as compared to NT controls (Arnsten, 2009a, Arnsten, 2009b), consistent with the literature. These findings could have potential therapeutic implications, and future research could explore whether aspects of neuromodulation targeting right-hemispheric activity, such as neurofeedback (Zilverstand et al., 2017), are associated with clinically significant reductions in ADHD symptoms. The possibility of targeted interventions could be considered of further value if the present findings on the VA network in patients with ADHD could be detected and replicated, particularly the relationship between FC and neuropsychological measures assessing ADHD symptoms. Replication of the current study paradigm should also involve a larger sample that includes both males and females.
Results also showed that there were 4 connections where FC was significantly greater in young people with ADHD compared to NT controls. These connections comprised (1) right aIns and right MOG; (2) left thalamus and left IFJ; (3) left IFJ and dmPFC, and (4) dmPFC and left dPMC. These brain regions have been implicated in a range of social and executive functioning skills, suggesting that FC differences between the patient and control groups may reflect an impact on such skills, in line with ADHD symptomatology. Briefly, the insula is implicated in the integration of cognitive, affective, sensory and autonomic information. Literature has shown that interoceptive awareness and emotional decision making is impacted when insular lesions are present (Jones, Ward, & Critchley, 2010). Specifically, lesions involving the aIns result in disrupted affective responses and language functions. The right aIns is important in guiding ‘second order’ cognitive representations of bodily arousal states. More generally, the insulae are implicated in detecting incongruities in predicted and actual emotional states (Jones et al., 2010). The absence of a robust connection of right aIns in our ADHD participants might underlie or contribute to compromised social functioning, difficulties that are well-documented in children and adolescents with ADHD (Biederman et al., 1993, Hoy et al., 1978).
Notably, the present study showed differences in RS-FC between right and left aIns for patients with ADHD versus the NT group. Specifically, connectivity between these two regions was prominent amongst NT controls, but absent amongst the ADHD sample, and was inversely correlated with ADHD symptoms generally, and with perceived impulsivity in particular. This suggests that FC between the anterior insulae may be particularly important for regulating risk-taking behaviours associated with hyperactivity. For patients with ADHD, right aIns was connected instead with right MOG and with right TPJ. The former connection was not observed amongst NT controls, and the latter was indirect for NT participants, mediated in the network via the left occipital cortex. Based on the right TPJ’s role in stimulus-driven attentional capture (Corbetta & Shulman, 2002), enhanced FC between these regions might contribute to increased distractibility and dysfunctional salience processing in ADHD. Further research with younger children could shed light on the development of aIns connectivity and whether this is a congenital architectural difference or an adaptation due to ADHD.
While the right TPJ is implicated in shifting attention to unexpected stimuli, the right MFG is involved in action control and monitoring (e.g., Cieslik et al., 2013), as well as complex non-motor tasks such as decision making, discrimination, computation, and reasoning (Tanji & Mushiake, 1996). Our results showed that while the right TPJ and MFG were basically uncoupled in the NT control group (with FC values close to zero), they were substantially anti-correlated in patients with ADHD. Children and adolescents with ADHD are known to have deficits in the areas of attention, decision making, and reasoning. As such, it is possible that their poorer executive functioning skills may be, at least in part, mediated by an aberrant disconnection between these two areas.
Increased FC between the IFJ and the dmPFC, and between the dmPFC and the left dPMC, were observed for patients with ADHD compared to the NT sample. The IFJ is implicated in cognitive control (task shifting, set switching; Brass, Derrfuss, Forstmann, & von Cramon, 2005). The dmPFC subserves complex cognitive processes (Gusnard, Akbudak, Shulman, & Raichle, 2001). The dPMC is involved in selection of motor acts based on visual stimuli (Grafton, Fadiga, Arbib, & Rizzolatti, 1997).
Findings indicate that another area of potential significance is the vermis of the cerebellum. Notably, this region was strongly connected in NT participants; however, there were no connections seen in patients with ADHD. The cerebellar vermis is important for sustaining attention and alertness, and more specifically, response readiness under circumstances of time uncertainty (Langner et al., 2012). Absent connectivity in young patients with ADHD could potentially explain part of the known difficulties in maintaining focused attention over time.
We also examined whether the strength of FC between VA-related regions was significantly associated with individual differences in measures linked to ADHD symptomatology. Indeed, there were significant associations between intra-network FC and such measures. Five of the six significant associations involved aIns connectivity, both cross-hemispheric (the right to left aIns) and connectivity with the vermis. As noted above, both the right–left aIns connection and the right aIns–vermis connection were significantly weaker in patients with ADHD. Similarly, left aIns–LOC connectivity, which showed significant anti-correlations with CBCL-assessed attentional problems, was also less strong for patients with ADHD. The associations with markers of behavioural dysfunction linked to ADHD demonstrate that strong connectivity between these particular regions is associated with fewer attentional and behavioural problems, corroborating the behavioural relevance of ADHD-related RS-FC aberrations involving the vermis and the aIns.
Although we did not forward spatially specific hypotheses, the significant association between go/no-go error rate and right aIns–vermis connectivity was unexpected. A more likely expectation may have been a negative association, especially in light of the finding that strong right aIns–vermis connectivity was linked to lower scores on the SBQ-ADHD scale. Such findings raise questions as to why a strong aIns–vermis FC may be associated with benefits for attentiveness but with a negative impact on inhibitory control (as assessed by go/no-go errors). It may be possible that the right aIns–vermis connection supports heightened alertness that may result in more errors stemming from inhibitory failure in the go/no-go task. However, it is also important to note that go/no-go error rates did not differ between the two groups. Furthermore, the NT control group had slightly higher rates of error than the ADHD group. Thus, replication of this finding is required to avoid over-interpretation of a potentially spurious association.
Finally, stronger RS-FC between the left IFJ and the left thalamus was associated with lower scores on the Eagerness of Effort subscale of the JTCI. Interestingly, connectivity between these two regions was essentially non-existent in the NT group but was significantly greater in the ADHD group. Together, the results indicate a dysfunctional hyper-connectivity at rest, which might negatively impact on the willingness or ability to exert mental effort over time to stay on a “job”, even if it is boring. In this context, it is still unclear if increased RS-FC in subjects with ADHD represents a compensatory mechanism resulting from hypo-connectivity of the VA-based network, or if it needs to be considered as being a more proximal disorder-related finding. Thus, the implications of the present findings must be interpreted with some caution. On a very speculative note, the tighter intrinsic coupling between the IFJ and the thalamus in ADHD may reflect a dysfunctional inhibitory system; for example, the attentional gating function of the thalamus may be too easily overcome by IFJ signals that promote a shift of the current task set. Notably, however, this subscale’s score was not significantly different between the two groups, which in turn suggests that patients with ADHD can, at least to some degree, compensate the putative negative effect of this hyper-connectivity on effort regulation.
It is also worth mentioning that given the short time period between clinical-behavioural assessment and the RS-fMRI experiment, our RS-FC results may be considered as reflective of the current clinical status of the sample at the time of investigation. Notably, however, interpretations of the aforementioned results are somewhat speculative, which is a characteristic of brain research assessing associations between FC and behavioural observations. The outlined explanations and interpretations are based on putative links between clinical scales, symptoms and RS-FC data, potentially reflecting disorder-related processes. As such, explanations should be considered hypothetical, and each of these will require careful evaluation in future studies.
4.1. Limitations and future outlook
In the context of the current study, several limitations warrant mention. It is important to note that all medical diagnoses and most psychiatric comorbidities were in the exclusion criteria, thereby limiting subject recruitment and the opportunity for investigating a larger sample. Similarly, patients on methylphenidate were required to discontinue their treatment two days prior to the study, which is not a suitable option for many patients with ADHD. By excluding these variables, however, we can be sure that our results are not due to confounding effects of methylphenidate or other medication, and that the intrinsic organization of neural networks implicated in VA is predominately representative of ADHD rather than other psychiatric, medical or neurodevelopmental disorders. Nevertheless, a large proportion of participants were on medication when completing the behavioural tasks, as screening took place approximately 1–2 weeks prior to the fMRI scans. This may be a potential contributor to the observed lack of correlation between questionnaires and tasks measuring ADHD symptomatology, and also the lack of differences in task performance between the ADHD and the NT group.
Another potential limitation may be our non-standard approach to the multiple-comparison issue in the correlational analysis, using an effect-size criterion rather than a traditional p-value correction. We chose this approach for three major reasons: First of all, we used correlation analysis as a kind of post hoc test aiming to further characterise significant FC differences between patients with ADHD and NT controls. Second, the non-independence between several of the phenotypical measures would not have been taken into account when using standard methods like Bonferroni correction. Third, the standard approach to testing correlation coefficients for significance, which cannot rely on a distribution of values, already is very conservative, especially given the moderate sample size. In essence, future research may determine whether these results are replicable in a larger, gender-mixed sample, since our study only included male participants as a precaution against a potential gender confound.
Finally, our study design does not allow for causal inferences as to why the integrity of the neural network subserving VA is altered in ADHD; aberrant connectivity could be organic in origin, or the result of compensatory neural reorganization. In addition, we focused on an a priori defined VA-related network, which was motivated by a better functional interpretability of our findings at the expense of taking a whole-brain perspective. This, in turn, precluded us from investigating ADHD-related differences in inter-network FC such as with the DMN, which may contribute to ADHD symptomatology. Mapping the intrinsic organization of these neural networks longitudinally will help shed light on this question. Additionally, using a repeated-measures design, where the neural network underlying VA is examined at three time points (e.g., while on methylphenidate, following a washout period, and upon recommencement of methylphenidate therapy), future work could also explore whether ADHD-specific functional circuitries during pharmacotherapy are more reminiscent of NT controls. Such data may provide valuable insights into the clinical utility of different treatment approaches.
5. Conclusion
In summary, to our knowledge, this is the first study to examine FC in the neural network subserving VA in young patients with ADHD and NT controls of the same age. We observed significant differences in RS-FC between the two subgroups for several, but not all, regions of the VA network. A network hub that is most strongly affected in its FC by ADHD appears to be the anterior insula. The behavioural relevance of several of these differences was corroborated by meaningful associations of RS-FC with ADHD symptomatology and ADHD-related behavioural traits.
Acknowledgments
This work was supported by the Excellence Initiative of the German federal and state governments, the Deutsche Forschungsgemeinschaft (LA 3071/3-1 to R.L. & S.B.E.; EI 816/4-1 to S.B.E.), the National Institute of Mental Health (R01-MH074457 to S.B.E.), the Helmholtz Portfolio Theme “Supercomputing and Modeling for the Human Brain” (S.B.E.), and the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 604102 (S.B.E.).
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.bandc.2017.10.005.
Contributor Information
Florian D. Zepf, Email: florian.zepf@uwa.edu.au, http://www.cap.uwa.edu.au.
Robert Langner, Email: robert.langner@uni-duesseldorf.de.
Appendix A. Supplementary material
References
- Achenbach T.M. University of Vermont; Burlington, VT: 1991. Manual for the Child behavior checklist/4-18. [Google Scholar]
- American Psychiatric Association . American Psychiatric Association; Washington, DC: 1994. Diagnostic and statistical manual of mental disorders (DSM-IV) [Google Scholar]
- Arnsten A.F. The emerging neurobiology of attention deficit hyperactivity disorder: the key role of the prefrontal association cortex. Journal of Pediatrics. 2009;154(5):I–S43. doi: 10.1016/j.jpeds.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten A.F. Toward a new understanding of attention-deficit hyperactivity disorder pathophysiology. CNS Drugs. 2009;23(Suppl. 1):33–41. doi: 10.2165/00023210-200923000-00005. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Unified segmentation. NeuroImage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Baroni A., Castellanos F.X. Neuroanatomic and cognitive abnormalities in attention-deficit/hyperactivity disorder in the era of ‘high definition’neuroimaging. Current Opinion in Neurobiology. 2015;30:1–8. doi: 10.1016/j.conb.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellgrove M.A., Johnson K.A., Barry E., Mulligan A., Hawi Z., Gill M. Dopaminergic haplotype as a predictor of spatial inattention in children with attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 2009;66(10):1135–1142. doi: 10.1001/archgenpsychiatry.2009.120. [DOI] [PubMed] [Google Scholar]
- Biederman J., Faraone S.V. Attention-deficit hyperactivity disorder. Lancet. 2005;366(9481):237–248. doi: 10.1016/S0140-6736(05)66915-2. [DOI] [PubMed] [Google Scholar]
- Biederman J., Faraone S.V., Chen W.J. Social adjustment inventory for children and adolescents: Concurrent validity in ADHD children. Journal of the American Academy of Child & Adolescent Psychiatry. 1993;32(5):1059–1064. doi: 10.1097/00004583-199309000-00027. [DOI] [PubMed] [Google Scholar]
- Brass M., Derrfuss J., Forstmann B., von Cramon D.Y. The role of the inferior frontal junction area in cognitive control. Trends in Cognitive Sciences. 2005;9(7):314–316. doi: 10.1016/j.tics.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Bubenzer-Busch S., Herpertz-Dahlmann B., Kuzmanovic B., Gaber T., Helmbold K., Ullisch M.G. Neural correlates of reactive aggression in children with attention-deficit/hyperactivity disorder and comorbid disruptive behaviour disorders. Acta Psychiatrica Scandinavica. 2016;133(4):310–323. doi: 10.1111/acps.12475. [DOI] [PubMed] [Google Scholar]
- Chen W.J., Faraone S.V., Biederman J., Tsuang M.T. Diagnostic accuracy of the Child Behavior Checklist scales for attention-deficit hyperactivity disorder: A receiver-operating characteristic analysis. Journal of Consulting and Clinical Psychology. 1994;62(5):1017. doi: 10.1037/0022-006X.62.5.1017. [DOI] [PubMed] [Google Scholar]
- Cieslik E.C., Zilles K., Caspers S., Roski C., Kellermann T.S., Jakobs O. Is there “one” DLPFC in cognitive action control? Evidence for heterogeneity from co-activation-based parcellation. Cerebral Cortex. 2013;23(11):2677–2689. doi: 10.1093/cercor/bhs256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger C.R., Svrakic D.M., Przybeck T.R. A psychobiological model of temperament and character. Archives of General Psychiatry. 1993;50(12):975–990. doi: 10.1001/archpsyc.1993.01820240059008. [DOI] [PubMed] [Google Scholar]
- Corbetta M., Shulman G.L. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Epstein J.N., DelBello M.P., Adler C., Altaye M., Kramer M., Mills N. Differential patterns of brain activation over time in adolescents with and without attention deficit hyperactivity disorder (ADHD) during performance of a sustained attention task. Neuropediatrics. 2009;40(01):1–5. doi: 10.1055/s-0029-1220686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck S.B., Easting G., Pearson P.R. Age norms for impulsiveness, venturesomeness and empathy in children. Personality and Individual Differences. 1984;5(3):315–321. [Google Scholar]
- Fox M.D., Greicius M. Clinical applications of resting state functional connectivity. Frontiers in Systems Neuroscience. 2010;4:19. doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Zhang D., Snyder A.Z., Raichle M.E. The global signal and observed anticorrelated resting state brain networks. Journal of Neurophysiology. 2009;101(6):3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Sánchez C., Estévez-González A., Suárez-Romero E., Junqué C. Right hemisphere dysfunction in subjects with attention-deficit disorder with and without hyperactivity. Journal of Child Neurology. 1997;12(2):107–115. doi: 10.1177/088307389701200207. [DOI] [PubMed] [Google Scholar]
- Goth K., Schmeck K. Hogrefe; Göttingen: 2009. Das Junior Temperament und Charakter Inventar (JTCI): Eine Inventarfamilie zur Erfassung der Persönlichkeit vom Kindergarten- bis zum Jugendalter nach Cloningers biopsychosozialem Persönlichkeitsmodell. [Google Scholar]
- Grafton S.T., Fadiga L., Arbib M.A., Rizzolatti G. Premotor cortex activation during observation and naming of familiar tools. NeuroImage. 1997;6(4):231–236. doi: 10.1006/nimg.1997.0293. [DOI] [PubMed] [Google Scholar]
- Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Current Opinion in Neurology. 2008;21(4):424–430. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- Greicius M.D., Krasnow B., Reiss A.L., Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the USA. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greimel E., Herpertz-Dahlmann B., Günther T., Vitt C., Konrad K. Attentional functions in children and adolescents with attention-deficit/hyperactivity disorder with and without comorbid tic disorder. Journal of Neural Transmission. 2008;115(2):191–200. doi: 10.1007/s00702-007-0815-4. [DOI] [PubMed] [Google Scholar]
- Günther T., Knospe E.L., Herpertz-Dahlmann B., Konrad K. Sex differences in attentional performance in a clinical sample with ADHD of the combined subtype. Journal of Attention Disorders. 2015;19(9):764–770. doi: 10.1177/1087054712461176. [DOI] [PubMed] [Google Scholar]
- Günther T., Konrad K., De Brito S.A., Herpertz-Dahlmann B., Vloet T.D. Attentional functions in children and adolescents with ADHD, depressive disorders, and the comorbid condition. Journal of Child Psychology and Psychiatry. 2011;52(3):324–331. doi: 10.1111/j.1469-7610.2010.02320.x. [DOI] [PubMed] [Google Scholar]
- Gusnard D.A., Akbudak E., Shulman G.L., Raichle M.E. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proceedings of the National Academy of Sciences of the USA. 2001;98(7):4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy E., Weiss G., Minde K., Cohen N. The hyperactive child at adolescence: Cognitive, emotional, and social functioning. Journal of Abnormal Child Psychology. 1978;6(3):311–324. doi: 10.1007/BF00924734. [DOI] [PubMed] [Google Scholar]
- Hudziak J.J., Copeland W., Stanger C., Wadsworth M. Screening for DSM-IV externalizing disorders with the Child Behavior Checklist: A receiver-operating characteristic analysis. Journal of Child Psychology and Psychiatry. 2004;45(7):1299–1307. doi: 10.1111/j.1469-7610.2004.00314.x. [DOI] [PubMed] [Google Scholar]
- Insel T.R. The NIMH research domain criteria (RDoC) project: Precision medicine for psychiatry. American Journal of Psychiatry. 2014;171(4):395–397. doi: 10.1176/appi.ajp.2014.14020138. [DOI] [PubMed] [Google Scholar]
- Jones C.L., Ward J., Critchley H.D. The neuropsychological impact of insular cortex lesions. Journal of Neurology, Neurosurgery & Psychiatry. 2010;81(6):611–618. doi: 10.1136/jnnp.2009.193672. [DOI] [PubMed] [Google Scholar]
- Kasparek T., Theiner P., Filova A. Neurobiology of ADHD from childhood to adulthood: Findings of imaging methods. Journal of Attention Disorders. 2015;19(11):931–943. doi: 10.1177/1087054713505322. [DOI] [PubMed] [Google Scholar]
- Kessler D., Angstadt M., Welsh R.C., Sripada C. Modality-spanning deficits in attention-deficit/hyperactivity disorder in functional networks, gray matter, and white matter. Journal of Neuroscience. 2014;34(50):16555–16566. doi: 10.1523/JNEUROSCI.3156-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad K., Neufang S., Hanisch C., Fink G.R., Herpertz-Dahlmann B. Dysfunctional attentional networks in children with attention deficit/hyperactivity disorder: Evidence from an event-related functional magnetic resonance imaging study. Biological Psychiatry. 2006;59(7):643–651. doi: 10.1016/j.biopsych.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Langner R., Eickhoff S.B. Sustaining attention to simple tasks: A meta-analytic review of the neural mechanisms of vigilant attention. Psychological Bulletin. 2013;139(4):870–900. doi: 10.1037/a0030694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner R., Kellermann T., Eickhoff S.B., Boers F., Chatterjee A., Willmes K. Staying responsive to the world: Modality-specific and-nonspecific contributions to speeded auditory, tactile, and visual stimulus detection. Human Brain Mapping. 2012;33(2):398–418. doi: 10.1002/hbm.21220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.S., Yan P., Bergquist K.L., Sinha R. Greater activation of the “default” brain regions predicts stop signal errors. Neuroimage. 2007;38:640–648. doi: 10.1016/j.neuroimage.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manly T., Owen A.M., McAvinue L., Datta A., Lewis G.H., Scott S.K. Enhancing the sensitivity of a sustained attention task to frontal damage: convergent clinical and functional imaging evidence. Neurocase. 2003;9(4):340–349. doi: 10.1076/neur.9.4.340.15553. [DOI] [PubMed] [Google Scholar]
- Mattfeld A.T., Gabrieli J.D., Biederman J., Spencer T., Brown A., Kotte A. Brain differences between persistent and remitted attention deficit hyperactivity disorder. Brain. 2014;137(Pt 9):2423–2428. doi: 10.1093/brain/awu137. [DOI] [PubMed] [Google Scholar]
- Posner J., Park C., Wang Z. Connecting the dots: A review of resting connectivity MRI studies in attention-deficit/hyperactivity disorder. Neuropsychology Review. 2014;24(1):3–15. doi: 10.1007/s11065-014-9251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson I.H., Garavan H. Vigilant Attention. In: Gazzaniga M.S., editor. The cognitive neurosciences. MIT Press; Cambridge, MA: 2004. pp. 631–640. [Google Scholar]
- Robertson I.H., Ridgeway V., Greenfield E., Parr A. Motor recovery after stroke depends on intact sustained attention: A 2-year follow-up study. Neuropsychology. 1997;11(2):290. doi: 10.1037//0894-4105.11.2.290. [DOI] [PubMed] [Google Scholar]
- Rubia K., Halari R., Cubillo A., Mohammad A.-M., Brammer M., Taylor E. Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naive children with ADHD during a rewarded continuous performance task. Neuropharmacology. 2009;57(7):640–652. doi: 10.1016/j.neuropharm.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Sandson T.A., Bachna K.J., Morin M.D. Right hemisphere dysfunction in ADHD: Visual hemispatial inattention and clinical subtype. Journal of Learning Disabilities. 2000;33(1):83–90. doi: 10.1177/002221940003300111. [DOI] [PubMed] [Google Scholar]
- Satterthwaite T.D., Elliott M.A., Gerraty R.T., Ruparel K., Loughead J., Calkins M.E. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. NeuroImage. 2013;64:240–256. doi: 10.1016/j.neuroimage.2012.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S., Unnewehr S., Margraf J. 2nd ed. Springer; 2008. Kinder-DIPS: Diagnostisches Interview bei psychischen Störungen im Kindes-und Jugendalter [Children-DIPS: Diagnostic Interview for Psychological Disorders in Childhood and Adolescence] [Google Scholar]
- Shulman G.L., Pope D.L., Astafiev S.V., McAvoy M.P., Snyder A.Z., Corbetta M. Right hemisphere dominance during spatial selective attention and target detection occurs outside the dorsal frontoparietal network. Journal of Neuroscience. 2010;30(10):3640–3651. doi: 10.1523/JNEUROSCI.4085-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Fox P.T., Miller K.L., Glahn D.C., Fox P.M., Mackay C.E. Correspondence of the brain's functional architecture during activation and rest. Proceedings of the National Academy of Sciences of the USA. 2009;106(31):13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke E.J. The dual pathway model of AD/HD: An elaboration of neuro-developmental characteristics. Neuroscience & Biobehavioral Reviews. 2003;27(7):593–604. doi: 10.1016/j.neubiorev.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Stadler C., Janke W. Concurrent validity of the German version of SB Eysenck’s impulsiveness questionnaire for children. Personality and Individual Differences. 2003;35(1):51–58. [Google Scholar]
- Stadler C., Janke W., Schmeck K. Hogrefe; Göttingen: 2004. IVE: Inventar zur Erfassung von Impulsivität, Risikoverhalten und Empathie bei 9- bis 14-jährigen Kindern. [Google Scholar]
- Stuss D.T., Shallice T., Alexander M.P., Picton T.W. A multidisciplinary approach to anterior attentional functions. Annals of the New York Academy of Sciences. 1995;769(1):191–212. doi: 10.1111/j.1749-6632.1995.tb38140.x. [DOI] [PubMed] [Google Scholar]
- Tanji J., Mushiake H. Comparison of neuronal activity in the supplementary motor area and primary motor cortex. Cognitive Brain Research. 1996;3(2):143–150. doi: 10.1016/0926-6410(95)00039-9. [DOI] [PubMed] [Google Scholar]
- Thesen S., Heid O., Mueller E., Schad L.R. Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magnetic Resonance in Medicine. 2000;44(3):457–465. doi: 10.1002/1522-2594(200009)44:3<457::aid-mrm17>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Tremblay R.E., Loeber R., Gagnon C., Charlebois P., Larivee S., LeBlanc M. Disruptive boys with stable and unstable high fighting behavior patterns during junior elementary school. Journal of Abnormal Child Psychology. 1991;19(3):285–300. doi: 10.1007/BF00911232. [DOI] [PubMed] [Google Scholar]
- Vitacco M.J., Rogers R., Neumann C.S. The antisocial process screening device an examination of its construct and criterion-related validity. Assessment. 2003;10(2):143–150. doi: 10.1177/1073191103010002005. [DOI] [PubMed] [Google Scholar]
- Weiß, R., & Weiß, B. (2008). CFT 20-R mit WS/ZF-R: Grundintelligenztest Skala 2-Revision (CFT 20-R) mit Wortschatztest und Zahlenfolgentest-Revision (WS/ZF-R): Hogrefe, Göttingen.
- Weissman D.H., Roberts K., Visscher K., Woldorff M. The neural bases of momentary lapses in attention. Nature Neuroscience. 2006;9(7):971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- World Health Organization (1993). The ICD-10 classification of mental and behavioural disorders: diagnostic criteria for research, WHO, Geneva.
- Zilverstand A., Sorger B., Slaats-Willemse D., Kan C.C., Goebel R., Buitelaar J.K. FMRI neurofeedback training for increasing anterior cingulate cortex activation in adult attention deficit hyperactivity disorder. An exploratory randomized, single-blinded study. PLoS ONE. 2017;12(1):e0170795. doi: 10.1371/journal.pone.0170795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P., Fimm B. A test battery for attentional performance. In: Leclercq M., Zimmermann P., editors. Applied neuropsychology of attention: Theory, diagnosis and rehabilitation. Psychology Press; London: 2002. pp. 110–151. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.