Abstract
Background:
The Foundation for the National Institutes of Health Sarcopenia Project validated cutpoints for appendicular lean mass (ALM) to identify individuals at risk for functional impairment. Recognizing possible underlying mechanisms between adipose tissue and muscle, we sought to apply the recent definitions and determine the relationship with markers of glucose homeostasis and inflammation in individuals with sarcopenia and sarcopenic obesity.
Methods:
The National Health and Nutrition Examination Surveys 1999–2004 were used to identify 4,984 adults aged ≥60 years with DEXA measures. Sarcopenia was defined using ALM (men<19.75 kg, women<15.02 kg) and ALM adjusted for body mass index (BMI; men<0.789 kg/m2, women<0.512 kg/m2). Sarcopenic obesity was defined as subjects fulfilling the criteria for sarcopenia and obesity by body fat (men ≥25%, women ≥35%). We assessed the association between ALM and ALM:BMI with inflammatory and markers of glucose homestasis, both as continuous variables but also classifying as having sarcopenic obesity or not after adjusting for confounding variables including pro-inflammatory chronic diseases such as diabetes and cancer.
Results:
Mean age was 71.1 years (56.5%) females. Prevalence of sarcopenia and sarcopenic obesity were (ALM definition: 29.9 and 24.4%; ALM:BMI definition: 23.0 and 22.7%). There were significant associations with ALM and ln C-reactive protein (β=0.0287;p=0.001), fibrinogen (β=0.519;p<0.001), and HOMA-IR (β=0.359;p<0.001). Using ALM:BMI, significant associations were observed with ln CRP (β=−2.58;p=0.001), fibrinogen (β=−124.2;p<0.001), and HOMA-IR (β=−6.63;p<0.001). Sarcopenic obesity using the ALM:BMI definition demonstrated significant associations with CRP (β=0.422;p<0.001), fibrinogen (β=22.5;p<0.001), but not HOMA-IR (β=1.19;p=0.13). Strong associations with seen with increased levels of fibrinogen and CRP with sarcopenic obesity (ALM:BMI definition) that persisted after adjusting for diabetes and cancer.
Conclusions:
Biologically plausible associations exist between ALM:BMI and inflammation and HOMA-IR that were not observed when using ALM alone. Future study should validate each of these definitions to prevent disparate results from being determined.
Keywords: sarcopenia, insulin resistance, inflammation, obesity, diagnostic accuracy, body mass index, body fat, epidemiology
INTRODUCTION
The changing aging demographic and problematic obesity epidemic[1] has led to a group of older adults with obesity at a high risk of adverse outcomes. Sarcopenia is a natural phenomenon of aging defined by the loss of muscle mass and strength[2] that can be accelerated by co-morbid disease states. Individuals with both sarcopenia and obesity portend worse outcomes than either state alone[3]. Although there are clear relationships between the adipocyte and muscle, the underlying mechanisms leading to functional decline have yet to be fully elucidated.
A pro-inflammatory state exists in individuals with obesity that is highlighted by studies demonstrating a relationship between higher degrees of fat and inflammatory markers such as C-reactive protein, fibrinogen[4, 5] and markers of insulin resistance, such as homeostatic model assessment (HOMA) insulin resistance and sensitivity (HOMA-S)[6]. Concomitantly, the aging process also leads to increasing levels of serological biomarkers which are strongly associated with functional decline, frailty and institutionalization[7, 8].
Much of the sarcopenia literature has focused on defining cutpoints for identifying this geriatric syndrome in a clinical setting[9]. Recently, the Foundation for the National Institutes of Health proposed definitions of sarcopenia that could assist in identifying cohorts of subjects[2]. This important advance has allowed researchers to begin characterizing the underlying mechanisms important in ascertaining this phenotype. The purpose of this study was to apply these newly standardized definitions to a representative cohort of older United States adults and determine the relationship between the different definitions of muscle mass and markers of inflammation. We hypothesize that individuals with sarcopenia and sarcopenic obesity, irrespective of the given sarcopenia definition, have strong associations with pro-inflammatory markers.
METHODS
Study Design and Population
Using cross-sectional data from the 1999–2004 National Health and Nutrition Surveys (NHANES), we performed a secondary analysis of data. The survey uses a multistage, complex, stratified probability sampling design that oversamples minorities and older adults and is representative of non-institutionalized adults in the United States, providing excellent external validity. The survey contents and procedure manuals are available online at http://www.cdc.gov/nchs/nhanes.htm (accessed September 2015) and has been conducted and managed by the Centers for Disease Control and Prevention since 1971. This study was exempt from local Institutional Review Board review due to the de-identified data analyzed.
Of the 38,077 subjects screened, 31,125 were interviewed, and ultimately 29,402 were examined in a mobile examination center. We restricted our sample to individuals aged 60 and older with body composition assessed (see below) as the prevalence of sarcopenia and sarcopenic obesity are much less prevalent in younger populations[9]. We ultimately included 4,984 subjects in our study.
Body Composition Variables
All body composition measures were assessed using a dual energy x-ray absorptiometry QDR-4500 Hologic scanner (Bedford, MA). Individuals whose height was >192.5cm or whose weight was >136.4kg were excluded from this assessment. Metal objects (except false teeth and hearing aids) were removed. Each participant had fat, lean muscle, and appendicular mass measured. The report also provided total body fat and lean mass percent. Each NHANES cycle consisted of similar operations procedures.
Appendicular lean mass (ALM) was defined as the sum of the muscle mass of both legs and arms. Two FNIH definitions exist defined as participants with an ALM <19.75kg and <15.02kg in men and women, respectively, and those defined as the ratio of ALM and BMI, with cutpoints of <0.789 and <0.512, respectively in each sex. We defined individuals with obesity as having a body fat of ≥25% in men, and ≥35% in females, as used in our previous studies[9, 10]. Individuals were classified as having sarcopenic obesity if they fulfilled criteria for both obesity and sarcopenia (dependent on the sarcopenia definition used) and defined as sarcopenic obesity ALM or ALM:BMI.
Covariates
Demographic (age, sex, race), socioeconomic (education, smoking) and co-morbidities were assessed using a self-reported questionnaire. All races were included (non-Hispanic White, non-Hispanic black, Hispanic, and other), and individuals were additionally classified by age group (60–69.9, 70–79.9, and ≥ 80years). Education level was reported as years of schooling and ultimately grouped as having completed 12 years of schooling or not. Smoking status was categorized as never smoked, former smokers or current smokers of cigarettes, in addition to all co-morbid conditions were based on self-reported questionnaires.
Measurements were all performed to the nearest tenth of a centimeter, except where amputations, casts and other factors prevented the assessment, on the right side of the body. An electronic digital scale, calibrated in kilograms assessed weight, and a stadiometer measured height after deep inhalation. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. A high BMI was classified if individuals had a BMI ≥30kg/m2. Waist circumference (WC) was measured using a tape placed around the trunk, measured standing at the iliac crest, crossing at the mid-axillary line. A high WC was classified based on the ACC/AHA guidelines (≥88cm in females; ≥102cm in males)[11].
Cardiometabolic Variables
The NHANES examination consisted of a trained medical provider performing blood pressure measurements using a mercury sphyngomanometer in triplicate. The mean value reported was used for our analysis. All techniques in NHANES followed the guidelines put forth by the American Heart Association. We assessed glucose using a routine biochemistry profile using the glucose hexokinase methods for both fasting and non-fasting samples, measured spectrophotometrically. Cholesterol was measured enzymatically in a series of coupled reactions and the color intensity and absorbance was measured at 500nm. HDL was measured using two methods. A heparin-manganese precipitation method and a direct immunoassay technique were used. C-Reactive Protein was quantified using a latex-enhanced Behring nephelometry and concentrations were calculated using a calibration curve. Fibrinogen was quantitatively determined using the Clauss clotting method, measuring the rate of fibrinogen to fibrin conversion in the diluted sample under the influence of excess thrombin. A fibrinogen standard curve allowed for determination of the fibrinogen concentration. Notably, this variable was measured only in NHANES 1999–2002. Fasting samples were processed, stored and shipped to the University of Missouri-Columbia for analysis. The Homeostasis Model Assessment (HOMA) was used to estimate insulin resistance (HOMA-IR) and beta cell function (HOMA-B)[6]. These measures correspond well, but are not necessarily equivalent, to non-steady state estimates of beta cell function and insulin sensitivity derived from stimulatory models such as the hyperinsulinaemic clamp, the hyperglycaemic clamp, the intravenous glucose tolerance test (acute insulin response, minimal model), and the oral glucose tolerance test (0–30 delta I/G). The recalibrated model was used to calculate these variables. All other methods are described online at http://www.cdc.gov/nchs/nhanes.
Statistical Analyses:
All data was downloaded, merged according to NHANES guidelines and analyzed incorporating weights, primary sampling unit and strata as supplied by NHANES. Separate weights were used for the non-fasting and fasting variables. Continuous variables are represented as means ± standard error and categorical variables as count (percent). Logarithmic transformation was performed where needed. Individuals were classified as having sarcopenia (yes/no) dependent on the definition with and without obesity. Point prevalence rates were ascertained using weighted estimates.
Initially, we determined the association in separate multivariable linear regression models between individual cardiometabolic variables (outcome), with ALM or ALM:BMI as primary predictors representing the β ± standard error and associated p-value for each model. Three separate models were performed for each primary predictor model (ALM or ALM:BMI). We first adjusted for age and sex; added race, education, smoking, and arthritis to our models; we then adjusted for cancer and diabetes and body fat (in separate modeling). We separately tested for an interaction between obesity (based on body fat) and ALM or ALM:BMI. We also present multivariable linear regression models for each of the four definitions (SALM, ALM:BMI, sarcopenic obesity ALM, sarcopenic obesity ALM:BMI, each yes/no) each as a separate model.
As an exploratory analysis, we stratified key mediators of systemic inflammation and glucose homeostasis into quartiles and ascertained the prevalence of sarcopenia and sarcopenic obesity. We created multivariable logistic regression analyses to assess the relationship between quartile of the inflammatory marker and presence/absence of sarcopenia or sarcopenic obesity. Model 1 adjusted for age, sex, race, education, smoking status and arthritis. Model 2 additionally adjusted for body fat. All analyses were performed using STATA v.13 (College Station, TX). A two-sided p-value of 0.05 was considered statistically significant.
RESULTS
There were 4,984 in the cohort, of which 29.9% and 23.0% were classified as having sarcopenia based on the ALM and ALM:BMI definition, and 24.4% and 22.7% classified as having sarcopenic obesity, respectively. The mean age of the included cohort was 71.1± 0.19 years (56.5% female). Baseline characteristics otherwise are represented in Table 1. C-reactive protein was lower in those with sarcopenic obesity based on definition 2. We observed higher levels of fibrinogen in those with sarcopenic obesity, irrespective of the definition used as compared to those without sarcopenic obesity. HOMA-IR was higher in those with the ALM definition, as were insulin levels.
Table 1:
Demographic Characteristics of Cohort
| Overall | Sarcopenic Obesity | ||
|---|---|---|---|
| Variable | Cohort | ALM Definition | ALM:BMI Definition |
| N=4,984 | N=1223 | N=1276 | |
| Age, years | 71.1 ± 0.19 | 73.7±0.29 | 72.7±0.26 |
| Age Category | |||
| 60–70 years | 2176 (46.6) | 376 (16.5) | 459 (17.0) |
| 70–80 years | 1635 (35.2) | 405 (27.0) | 459 (26.1) |
| 80+ years | 1173 (18.2) | 442(40.5) | 358 (31.9) |
| Female Sex (%) | 2531 (56.5) | 859 (77.6) | 574 (47.6) |
| Race | |||
| Non-Hispanic White (%) | 2846 (81.2) | 717 (34.3) | 677 (22.4) |
| Non-Hispanic Black (%) | 811 (8.3) | 57 (7.9) | 55 (7.4) |
| Mexican American (%) | 1202 (7.3) | 405 (24.7) | 513(41.5) |
| Other (%) | 125 (3.2) | 44 (36.6) | 31 (28.2) |
| Socioeconomic | |||
| Smoking | |||
| Never (%) | 2327 (46.7) | 662 (28.5) | 594 (22.7) |
| Former (%) | 2035 (41.4) | 421 (19.8) | 542 (23.4) |
| Current (%) | 611 (11.9) | 137(24.0) | 139 (20.3) |
| Education | |||
| <12 years (%) | 3301 (59.4) | 878 (27.0) | 934 (25.5) |
| >12 years (%) | 1676(40.6) | 342 (20.6) | 341 (18.8) |
| Comorbidities | |||
| Diabetes Mellitus (%) | 1060 (18.3) | 206 (18.3) | 317 (28.4) |
| Arthritis (%) | 2379 (50.2) | 597 (25.6) | 638 (24.1) |
| Coronary Artery Disease (%) | 870 (18.3) | 205 (24.3) | 267 (32.0) |
| Cancer (%) | 916 (21.7) | 222 (23.2) | 206 (20.7) |
| Anthropometrics | |||
| Weight, kg | 77.7±0.30 | 62.7±0.32 | 79.0±0.64 |
| Body Mass Index, kg/m2 | 28.2±0.10 | 25.0±0.13 | 30.5±0.20 |
| Body Mass Index >30kg/m2 (%) | 1466 (31.7) | 91 (4.7) | 557 (34.2) |
| Waist Circumference, cm | 100.1±0.22 | 91.3±0.40 | 105.2±0.55 |
| High Waist Circumference, cm | 2906 (65.1) | 591 (19.3) | 894 (26.5) |
| % Body Fat | 37.2±0.11 | 39.7±0.18 | 40.3±0.25 |
| % with High Body Fat | 4,195 (88.2) | ||
| Lean mass % | 60.4±0.01 | 58.0±0.01 | 57.4±0.24 |
| Appendicular Lean Mass, kg | 19.7±0.09 | 14.4±0.07 | 18. ±0.19 |
| ALM/BMI | 0.706±0.003 | 0.584±0.003 | 0.61±0.005 |
| Prevalence (%) | --- | 1223 (24.4) | 1276 (22.7) |
| Hypertension | |||
| Systolic, mmHg | 138.1±0.57 | 142.2±0.91 | 140.1±1.04 |
| Diastolic , mmHg | 68.1±0.37 | 66.5±0.67 | 67.0±0.59 |
| Lipids | |||
| Total Cholesterol, mg/dL | 210.5±0.72 | 216.6±1.70 | 208.4±1.79 |
| HDL-C, mg/dL | 54.2±0.40 | 60.7±0.65 | 52.0±0.55 |
| LDL-C, g/dL | |||
| Non-Fasting Triglycerides, mg/dL | 151.5±2.18 | 139.0±3.27 | 160.7±3.47 |
| Non-Fasting Glucose , mg/dL | 104.2±0.68 | 100.7±1.09 | 108.9±1.50 |
| Inflammatory Variables | |||
| CRP, mg/dL | 0.53±0.02 | 0.55±0.04 | 0.63±0.04 |
| Fibrinogen, mg/dL | 391.4±2.93 | 396.8±4.66 | 407.0±4.15 |
| Fasting Subsample | |||
| HOMA-IR | 3.80±0.13 | 2.77±0.12 | 4.72±0.68 |
| HOMA-B | 106.8±5.03 | 96.0±3.86 | 104.2±16.95 |
| Glucose, mg/dL | 110.7±1.34 | 105.7±1.97 | 115.8±3.24 |
| Triglycerides, mg/dL | 157.4±2.75 | 150.0±4.82 | 168.8±5.40 |
| Insulin μU/mL | 13.1±0.28 | 10.2±0.34 | 15.3±1.66 |
| LDL-C, mg/dL | 126.5±1.45 | 128.4±2.79 | 125.0±2.58 |
All values represented are weighted means ± standard error, or counts (weighted prevalence). Rates in each definition column (ALM or ALM:BMI) are weighted and percentages are in relation to those without sarcopenia. Sarcopenic Obesity (ALM definition) is defined as an appendicular lean mass <19.75 in men, or <15.02 in females; Sarcopenic Obesity using the ALM:BMI definition is defined as ALM:BMI ratio <0.789 and <0.512. Obesity is defined as subjects fulfilling criteria for elevated body fat (≥25% in men, or ≥35% in females) in both definitions. High Waist circumference is defined as ≥88cm in females and ≥102cm in males. Natural log transformation was used for triglycerides and C-reactive protein
Abbreviations: ALM: appendicular lean mass; BMI: body mass index; HDL-C: high density lipoprotein; HOMA-B: Homeostatic model assessment beta sensitivity; HOMA-IR: Homeostatic model assessment insulin resistance; LDL-C: low density lipoprotein.
ALM and ALM:BMI regression models are presented in Table 2. Using ALM, inflammatory mediators and HOMA-IR were positively associated with measures of muscle mass. C-reactive protein, HOMA-IR continued to be significant after adjusting for diabetes and cancer. We observed a fat/muscle interaction for HOMA-IR alone. Using ALM:BMI, we observed a significant relationship in systolic blood pressure, HDL-cholesterol, ln CRP and triglycerides, fibrinogen, and HOMA-IR that persisted even after adjusting for diabetes and cancer. Muscle/fat interaction in the C-reactive protein, fibrinogen and HOMA-IR models were significant.
Table 2 –
Multivariable Regression coefficients for Measures of Sarcopenia used continuously (ALM and ALM:BMI)
| ALM | ALM:BMI | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Ix | Model 1 | Model 2 | Ix | |||||
| Variable | β ± se | p | β ± se | p | β ± se | p | β ± se | p | p | |
| Non-Fasting | ||||||||||
| Systolic | −0.224±0.091 | 0.18 | −0.209±0.09 | 0.03 | 0.008 | −14.1±3.895 | 0.001 | −14.22±3.92 | 0.001 | 0.13 |
| Diastolic | 0.053±0.078 | 0.83 | 0.140±0.076 | 0.07 | 0.47 | 5.57±2.81 | 0.053 | 3.42±2.77 | 0.22 | 0.85 |
| Cholesterol | ||||||||||
| Total | −0.644±0.209 | 0.001 | −0.462±0.229 | 0.05 | 0.002 | −7.35±9.17 | 0.43 | −10.47±9.00 | 0.25 | 0.36 |
| HDL-C | −1.056±0.079 | 0.85 | −0.979±0.083 | <0.001 | <0.001 | 8.24±2.69 | 0.004 | 5.75±2.63 | 0.03 | 0.07 |
| Triglycerides | 3.462±0.469 | 0.79 | 2.70±0.489 | <0.001 | <0.001 | −81.7±13.6 | <0.001 | −62.45±14.93 | <0.001 | 0.02 |
| ln triglycerides | 0.0231±0.003 | 0.29 | 0.0192±0.003 | <0.001 | <0.001 | −0.575±0.083 | <0.001 | −0.48±0.089 | <0.001 | 0.001 |
| Glucose | 0.965±0.199 | .018 | 0.292±0.165 | 0.08 | <0.001 | −30.0±7.83 | <0.001 | −15.39±6.62 | 0.03 | 0.22 |
| Inflammatory | ||||||||||
| C-reactive protein | −0.0028±0.005 | <0.001 | −0.0044±0.0051 | 0.39 | 0.05 | −0.897±0.150 | <0.001 | −0.881±0.153 | <0.001 | 0.007 |
| ln CRP | 0.0287±0.006 | 0.001 | 0.027±0.006 | <0.001 | 0.62 | −2.58±0.26 | <0.001 | −2.57±0.272 | <0.001 | 0.03 |
| Fibrinogen | 0.519±0.486 | <0.001 | 0.108±0.475 | 0.82 | 0.14 | −124.2±22.1 | <0.001 | −117.03±22.2 | <0.001 | 0.004 |
| Fasting Variables | ||||||||||
| HOMA-IR | 0.359±0.057 | 0.002 | 0.305±0.055 | <0.001 | <0.001 | −6.63±1.99 | 0.002 | −6.22±1.73 | 0.001 | 0.001 |
| HOMA-B | 7.15±1.28 | 0.73 | 7.58±1.42 | <0.001 | 0.07 | −34.5±88.6 | 0.70 | −36.68±84.73 | 0.67 | 0.03 |
| Triglycerides | 2.81±1.03 | 0.74 | 2.343±0.97 | 0.02 | 0.009 | −60.4±18.2 | 0.002 | −55.62±17.4 | 0.003 | 0.003 |
| Ln trig | 0.0171±0.006 | 0.86 | 0.0147±0.006 | 0.02 | 0.03 | −0.38±0.096 | <0.001 | −0.355±0.093 | 0.001 | 0.002 |
| Insulin | 1.064±0.167 | <0.001 | 0.963±2.06 | 0.001 | <0.001 | −22.2±6.14 | 0.001 | −21.3±5.60 | 0.001 | 0.001 |
| LDL-Cholesterol | −0.063±0.393 | 0.12 | 0.112±0.402 | 0.78 | 0.38 | −3.77±13.9 | 0.79 | −5.03±13.07 | 0.70 | 0.91 |
All values represented are from multivariable linear regression models (β coefficient ± standard error) with p-values.
Values bolded are considered statistically significant
Model 1: adjusted for age, sex race, education, smoking status, arthritis
Model 2: Model 1 co-variates, cancer and diabetes
Interaction (Ix): interaction term obesity (Body Fat) term x ASM with model 1 co-variates
Abbreviations: ALM: Appendicular lean mass; BMI: body mass index; HDL-C: high density lipoprotein cholesterol; HOMA: homeostatic assessment model; IR: insulin resistance; Ix: interaction; LDL-C: low density lipoprotein cholesterol; S-sensitivity; s.e.: standard error.
Natural log transformation was used for triglycerides and C-reactive protein
We present estimates with sarcopenic obesity (ALM or ALM:BMI) as the primary predictor (Table 3) and observed differences in the significance of given metabolic outcomes. Sarcopenic obesity using ALM demonstrated significance in HDL-C, ln triglycerides, HOMA-IR and HOMA-B. All remained significant except HOMA-B after adjusting for diabetes and cancer. Using ALM:BMI sarcopenic obesity was associated with systolic blood pressure, ln triglycerides, glucose, C-reactive protein and fibrinogen. Notably, the directionality of the relationships differed dependent on the definition used.
Table 3 –
Multivariable Regression coefficients for Sarcopenic Obesity (high/low)
| Sarcopenic Obesity (ALM definition) Present/Absent | Sarcopenic Obesity (ALM:BMI definition) Present/Absent | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 | |||||||
| Outcome Variable | β ± se | p | β ± se | p | β ± se | p | β ± se | p | β ± se | p | β ± se | p |
| Non-Fasting | ||||||||||||
| Systolic | 1.73±1.06 | 0.11 | 2.13±1.03 | 0.05 | 2.17±1.03 | 0.04 | 2.22±0.88 | 0.02 | 2.12±0.93 | 0.03 | 2.01±0.95 | 0.04 |
| Diastolic | 0.092±0.76 | 0.91 | 0.21±0.74 | 0.78 | −0.033±0.72 | 0.96 | −0.46±0.64 | 0.48 | −0.41±0.65 | 0.53 | −0.132±0.66 | 0.84 |
| Cholesterol | ||||||||||||
| Total | 4.16±2.33 | 0.08 | 4.44±2.33 | 0.06 | 3.55±2.43 | 0.15 | 0.20±2.04 | 0.92 | 0.485±2.03 | 0.81 | 0.87±1.97 | 0.66 |
| HDL-C | 5.23±0.78 | <0.001 | 5.86±0.76 | <0.001 | 5.56±0.75 | <0.001 | −1.89±0.53 | 0.001 | −1.06±0.55 | 0.06 | −0.674±0.54 | 0.22 |
| Triglycerides | −14.4±3.35 | <0.001 | −16.7±3.33 | <0.001 | −14.7±3.12 | <0.001 | 14.2±3.54 | <0.001 | 10.8±3.57 | 0.004 | 7.80±3.78 | 0.05 |
| Ln triglycerides | −0.083±0.02 | <0.001 | −0.101±0.021 | <0.001 | −0.090±0.02 | <0.001 | 0.099±0.02 | <0.001 | 0.074±0.021 | 0.001 | 0.058±0.022 | 0.01 |
| Glucose | −3.03±1.59 | 0.06 | −2.59±1.65 | 0.12 | −0.673±1.37 | 0.63 | 6.35±1.52 | <0.001 | 6.34±1.58 | <0.001 | 4.14±1.32 | 0.003 |
| Inflammatory | ||||||||||||
| C-reactive protein | 0.026±0.04 | 0.51 | 0.044±0.04 | 0.29 | 0.050±0.041 | 0.23 | 0.15±0.04 | <0.001 | 0.164±0.04 | <0.001 | 0.16±0.04 | <0.001 |
| Ln crp | −0.061±0.05 | 0.23 | −0.035±0.051 | 0.50 | −0.022±0.05 | 0.67 | 0.406±0.052 | <0.001 | 0.422±0.052 | <0.001 | 0.42±0.05 | <0.001 |
| Fibrinogen | 0.83±4.28 | 0.85 | 1.42±4.6 | 0.76 | 2.57±4.65 | 0.59 | 21.3±4.71 | <0.001 | 22.5±4.82 | <0.001 | 21.26±4.77 | <0.001 |
| Fasting Variables | ||||||||||||
| HOMA-IR | −1.16±0.23 | <0.001 | −1.13±0.21 | <0.001 | −1.03±0.19 | <0.001 | 1.22±0.75 | 0.11 | 1.19±0.76 | 0.13 | 1.14±0.71 | 0.12 |
| HOMA-B | −14.3±6.6 | 0.04 | −11.4±8.00 | 0.17 | −12.05±7.69 | 0.13 | −1.51±16.68 | 0.93 | −1.67±16.9 | 0.92 | −1.72±16.4 | 0.92 |
| Triglycerides | −8.86±6.28 | 0.17 | −13.8±6.06 | 0.03 | −12.72±5.64 | 0.03 | 16.8±6.85 | 0.02 | 10.6±6.92 | 0.14 | 10.0±6.66 | 0.14 |
| Ln triglycerides | −0.043±0.033 | 0.20 | −0.074±0.03 | 0.02 | −0.07±0.03 | 0.02 | 0.104±0.038 | 0.011 | 0.066±0.036 | 0.08 | 0.064±0.035 | 0.08 |
| Insulin | −3.27±0.64 | <0.001 | −3.33±0.60 | <0.001 | −3.15±0.54 | <0.001 | 3.04±1.89 | 0.12 | 2.92±1.90 | 0.14 | 2.76±1.78 | 0.13 |
| LDL-C | 2.03±0.3.03 | 0.51 | 2.28±3.10 | 0.47 | 1.89±2.92 | 0.52 | −1.07±2.84 | 0.71 | −0.82±2.97 | 0.79 | −1.04±2.71 | 0.71 |
All values represented are from multivariable linear regression models (β coefficient ± standard error) with p-values.
Values bolded are considered statistically significant
Model 1: Adjusted for age, sex
Model 2: Model 1 co-variates, race, education, smoking status, arthritis
Model 3: Model 2 co-variates, diabetes, cancer
Abbreviations: ALM: Appendicular lean mass; BMI: body mass index; HDL-C: high density lipoprotein cholesterol; HOMA: homeostatic assessment model; IR: insulin resistance; LDL-C: low density lipoprotein cholesterol; S-sensitivity; s.e.: standard error.
Natural log transformation was used for triglycerides and C-reactive protein
As an exploratory analysis, we stratified C-reactive protein, fibrinogen, HOMA-IR and HOMA-S by quartile. A clear inverse and positive relationship between rate of sarcopenia and sarcopenic obesity using both definitions exist for HOMA-IR (Figure 1 and Appendix). Sarcopenia and Sarcopenic obesity based on ALM drops is inversely related to increasing HOMA-B and HOMA-IR. For ALM:BMI, we observed clear positive trends in rates of sarcopenia and sarcopenic obesity with increasing C-reactive protein, fibrinogen, and HOMA-IR. Our multivariable models suggested positive associations with high quartile of C-reactive protein with sarcopenia and sarcopenic obesity based on ALM:BMI, and fibrinogen.
Figure 1:
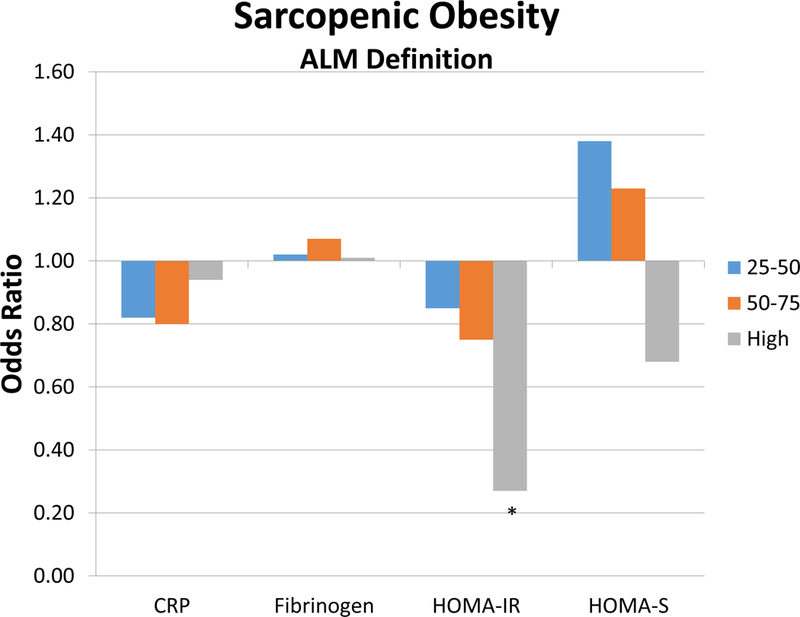
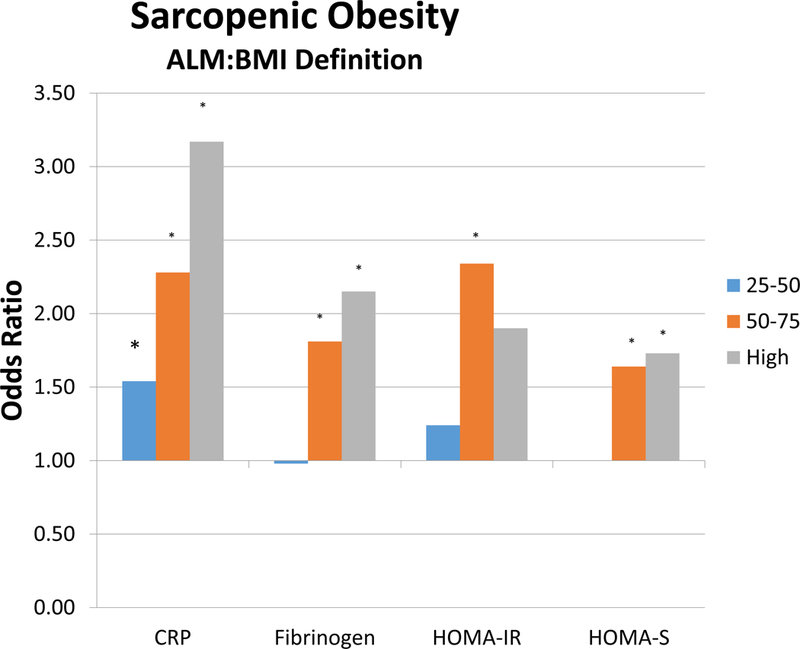
Figure represents the odds ratios within each variable (C-reactive protein, fibrinogen, HOMA-IR, and HOMA-B) according to quartile. Figure A represents sarcopenic obesity based on the ALM definition, and Figure B represents sarcopenic obesity based on ALM:BMI.
DISCUSSION
Our findings suggest that there may be an association between insulin resistance and systemic inflammation with sarcopenic obesity. However, this relationship is likely highly dependent on the definition of sarcopenia used.
To our knowledge, our study is the first to apply the new FNIH definition of ALM on the NHANES cohort combined with obesity based on body composition. Our conflicting results parallel those observed by others[12–15]. A number of explanations could account for these contradictory results. First, we were parsimonious in selecting our potential co-variates in our modeling. Barzilay et al (2009) demonstrated in their findings that the relationship changes dependent on the variables incorporated in their models. Second, the ALM definition did not account for weight or height; a phenomenon partially accounted for using ALM:BMI that adjusts for BMI. The different observations and the direction of the associations can partially be explained by the variable that standardizes ALM. Third, while DEXA is increasingly used in research centers, it can overestimate muscle mass due to hydration or intramuscular fat deposition, which will be detected as increases in lean tissue. Fourth, our findings did not account for weight change or loss of ALM[13], which could modify not only the ratio between lean mass and adipose tissue, but could modify its association with inflammatory cytokines[16].
Obesity is likely to play a significant role in our findings. We purposefully created modeling that adjusted for body fat, and it notably attenuated our results. Previous authors have demonstrated that the distribution of fat mass is much more important than generic overall obesity. The examined cohort notably had low levels of central adiposity, irrespective of the sarcopenia definition used. This is an important observation in that central adiposity drives visceral adipocyte cytokine production, which directly impacts insulin resistance and physical function, and accelerates body composition changes with aging. Central obesity leads to a more pro-inflammatory state than subcutaneous fat[17]. Our findings parallel those observed by Cesari in the INChianti study that demonstrated fat-adjusted ALM led to persistent significance of inflammatory markers[18]. Additionally, the ALM:BMI definition incorporates body fat which normally is higher in females and can, in part, account for the body composition differences, rates and results observed using this definition.
Both diabetes and cancer are considered pro-inflammatory states. The results presented suggest that the relationships, generally, are unaltered after adjusting for diabetes and cancer. Importantly, persons with diabetes are believed to have an accelerated aging process leading to disability and frailty and that sarcopenia is known to be associated with losing muscle mass[19, 20]. Hyperglycemia directly impairs skeletal muscle contractility and leads to insulin resistance. We believe that independent of this process, the results suggest that the combination of sarcopenia and obesity is strongly associated with systemic inflammation and inversely associated with insulin resistance.
We believe that our findings using ALM:BMI are biologically plausible. A cross-talk between adipocyte and myocyte mass is partially mediated by TNF-α, adiponectin and leptin[21]. While NHANES does not have a full compendium of these biomarkers, leptin itself can stimulate muscle catabolism. This promotes a vicious cycle that leads to accelerated sarcopenia by promoting skeletal muscle fiber diameter and protein degradation, gain in fat and ultimately physical disability[22, 23]. With additional fat, leptin levels do increase peripherally, and can possibly lead to leptin resistance, thus preventing the stimulation of lipolysis and reducing insulin sensitivity. This may in part be a factor in increased risk of morbidity and mortality, particularly in older adults[24]. Additionally, the aging process leads to impairments in insulin’s ability to stimulate muscle protein synthesis and inhibit protein breakdown subsequently leading to insulin resistance[25, 26]. Further, higher HOMA-IR levels represent increased insulin resistance and perhaps a specific type of muscle may mediate this physiological response. The ALM:BMI ratio may account for this phenomenon since BMI also accounts for total body muscle mass. In both models the implications of a muscle/fat interaction is important as CRP is mediated by both of these tissue.
Our exploratory analysis provides additional insight into the relationship between inflammatory and insulin resistance markers that are highly dependent on the sarcopenia definitions. While the FNIH definitions advance the science of sarcopenia and allow researchers to plan possible interventions, a continued lack of consensus persists in the manner in which sarcopenic obesity is identified as evidenced by the discrepancies observed in this study. The deliberate use of body fat composition thresholds prevents the need from using BMI, an inaccurate measure of fat that has been proven to have poor sensitivity, incorrectly assessing adiposity in older subjects[27]. We acknowledge that our findings are subject to the limits of mathematical thresholds that have a propensity for both over- and underdiagnosis of clinical conditions, and hence, used both the ALM and ALM:BMI definitions as continuous variables to account for this phenomenon. However, our results actually parallel a study ascertaining the prevalence of sarcopenia and sarcopenic obesity[9] and its relationship to functional disability where using the ALM:BMI definition demonstrated stronger strengths of associations[28].
We acknowledge a number of important limitations in this current analysis. First, our data does not suggest causality as the study is cross-sectional in nature. Any associations presented are based on a point in time and we cannot be certain whether a predictor led to the outcome or vice-a-versa. The dataset has a limited set of survey variables. For instance, this iteration of NHANES does not contain any muscle strength data which is imperative when discussing sarcopenia. Only non-institutionalized adults are included in this analysis and the results can only be applied to this population. By including nursing home patients, who may have higher degrees of sarcopenia or obesity[29, 30], our estimates are prone to changing and perhaps are conservative. Weight-cycling may also impact inflammatory variables. We did not adjust for cardiovascular fitness or physical activity which are known to dampen pro-inflammatory responses. Lastly, we relied on the self-reported data used in NHANES.
Future prospective studies should focus on changes in weight and inflammatory markers on muscle and fat mass and further the existing but limited longitudinal studies. Importantly, muscle strength should additionally be integrated in the identification of these at risk subjects. Understanding the underlying mechanisms and interplay between types of adipose tissue, muscle mass and strength will further advance the ability to limit progression of those with sarcopenia or sarcopenic obesity at risk, but importantly target clinical interventions to reduce the burden of disability downstream.
CONCLUSIONS
The association of inflammation and insulin homeostasis is highly dependent on the definition of sarcopenia used. Our results suggest that muscle distribution may impact the degree of inflammation and insulin homeostasis, but biomarkers of inflammation may be associated with sarcopenic obesity.
Acknowledgments
FINANCIAL DISCLOSURE
Dr. Batsis receives funding from Health Resources Services Administration (UB4HP19206–01-00) for medical geriatric teaching, the Junior Faculty Career Development Award, the Department of Medicine, Dartmouth-Hitchcock Medical Center, and the Dartmouth Centers for Health and Aging
Dr. Bartels receives funding from the National Institute of Mental Health (K12 HS0217695 (AHRQ), NIMH: T32 MH073553, R01 MH078052, R01 MH089811; R24 MH102794 CDC U48DP005018
Dr. Mackenzie: n/a
Dr. Lopez: none
Support was also provided by the Dartmouth Health Promotion and Disease Prevention Research Center supported by Cooperative Agreement Number U48DP005018 from the Centers for Disease Control and Prevention. The findings and conclusions in this journal article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
ABBREVIATIONS
- ALM
appendicular lean mass
- BMI
body mass index
- CRP
C-reactive protein
- DEXA
Dual energy x-ray absorptiometry
- FNIH
Foundation for the National Institutes of Health
- HOMA-B
Homeostatic Model Assessment beta sensitivity
- HOMA-IR
Homeostatic Model Assessment, Insulin resistance
- NHANES
National Health and Nutrition Examination Survey
- WC
Waist circumference
APPENDIX: Baseline Characteristics of Cohort by Sarcopenia Definition
| Overall | Sarcopenia | ||
|---|---|---|---|
| Variable | Cohort | ALM | ALM:BMI |
| N=4,984 | N=1487 | N=1296 | |
| Age, years | 71.1 ± 0.19 | 73.7±0.27 | 72.7±0.27 |
| Age Category | |||
| 60–70 years | 2176 (46.6) | 441 (20.2) | 466 (17.3) |
| 70–80 years | 1635 (35.2) | 495 (32.6) | 465 (26.3) |
| 80+ years | 1173 (18.2) | 551 (50.7) | 365 (32.5) |
| Female Sex (%) | 2531 (56.5) | 1028 (76.8) | 577 (47.3) |
| Race | |||
| Non-Hispanic White (%) | 2846 (81.2) | 885 (30.0) | 685 (22.6) |
| Non-Hispanic Black (%) | 811 (8.3) | 82 (11.2) | 56 (7.5) |
| Mexican American (%) | 1202 (7.3) | 462 (41.6) | 521 (42.3) |
| Other (%) | 125 (3.2) | 58 (47.8) | 34 (30.0) |
| Socioeconomic | |||
| Smoking | |||
| Never (%) | 2327 (46.7) | 779 (33.5) | 600 (22.8) |
| Former (%) | 2035 (41.4) | 506 (24.1) | 553 (24.0) |
| Current (%) | 611 (11.9) | 199(35.3) | 142 (20.7) |
| Education | |||
| <12 years (%) | 3301 (59.4) | 1070 (33.4) | 953 (26.0) |
| >12 years (%) | 1676(40.6) | 413 (24.7) | 342 (18.8) |
| Comorbidities | |||
| Diabetes Mellitus (%) | 1060 (18.3) | 235 (21.1) | 320 (28.5) |
| Arthritis (%) | 2379 (50.2) | 696 (29.9) | 644 (24.3) |
| Coronary Artery Disease (%) | 870 (18.3) | 246 (29.6) | 269 (32.0) |
| Cancer (%) | 916 (21.7) | 270 (29.0) | 208 (21.0) |
| Anthropometrics | |||
| Weight, kg | 77.7±0.30 | 60.6±0.31 | 78.6±0.65 |
| Body Mass Index, kg/m2 | 28.2±0.10 | 24.1±0.12 | 30.4±0.20 |
| Body Mass Index >30kg/m2 (%) | 1466 (31.7) | 91 (4.7) | 557 (34.2) |
| Waist Circumference, cm | 100.1±0.22 | 89.0±0.35 | 104.9±0.57 |
| High Waist Circumference, cm | 2906 (65.1) | 605 (19.7) | 894 (26.5) |
| % Body Fat | 37.2±0.11 | 37.6±0.19 | 40.1 ± 0.26 |
| % with High Body Fat | 4,195 (88.2) | ||
| Lean mass % | 60.4±0.01 | 60.0±0.18 | 57.6±0.25 |
| Appendicular Lean Mass, kg | 19.7±0.09 | 14.4±0.07 | 18.4±0.19 |
| ALM/BMI | 0.706±0.003 | 0.61±0.003 | 0.61±0.005 |
| Prevalence (%) | --- | 1487 (29.9) | 1296 (23.0) |
| Hypertension | |||
| Systolic, mmHg | 138.1±0.57 | 142.1±0.84 | 140.0±1.05 |
| Diastolic , mmHg | 68.1±0.37 | 66.4±0.65 | 67.1±0.58 |
| Lipids | |||
| Total Cholesterol, mg/dL | 210.5±0.72 | 216.0±1.49 | 208.4±1.80 |
| HDL-C, mg/dL | 54.2±0.40 | 61.4±0.67 | 52.2±0.56 |
| LDL-C, g/dL | |||
| Non-Fasting Triglycerides, mg/dL | 151.5±2.18 | 135.8±3.13 | 159.9±3.42 |
| Non-Fasting Glucose , mg/dL | 104.2±0.68 | 99.4±0.90 | 108.7±1.48 |
| Inflammatory Variables | |||
| CRP, mg/dL | 0.53±0.02 | 0.54±0.03 | 0.62±0.04 |
| Fibrinogen, mg/dL | 391.4±2.93 | 396.4±4.43 | 406.5±4.18 |
| Fasting Subsample | |||
| HOMA-IR | 3.80±0.13 | 2.56±010 | 4.69±0.67 |
| HOMA-B | 106.8±5.03 | 91.9±2.90 | 103.8±16.85 |
| Glucose, mg/dL | 110.7±1.34 | 104.0±1.53 | 115.7±3.22 |
| Triglycerides, mg/dL | 157.4±2.75 | 146.9±4.7 | 168.5±5.42 |
| Insulin μU/mL | 13.1±0.28 | 9.53±0.29 | 15.2±1.65 |
| LDL-C, mg/dL | 126.5±1.45 | 127.1±2.30 | 125.0±2.56 |
All values represented are weighted means ± standard error, or counts (weighted prevalence). Sarcopenia using the ALM definition is defined as an appendicular lean mass <19.75 in men, or <15.02 in females; Sarcopenia using the ALM:BMI ratio is defined as <0.789 and <0.512. Obesity is defined as subjects fulfilling criteria for elevated body fat (≥25% in men, or ≥35% in females) in both definitions. High Waist circumference is defined as ≥88cm in females and ≥102cm in males. Natural log transformation was used for triglycerides and C-reactive protein
Abbreviations: ALM: appendicular lean mass; BMI: body mass index; HDL-C: high density lipoprotein; HOMA-B: Homeostatic model assessment beta sensitivity; HOMA-IR: Homeostatic model assessment insulin resistance; LDL-C: low density lipoprotein.
Appendix Table 2.
Multivariable Regression Coefficients for ALM and ALM:BMI
| ALM | ALM | ALM:BMI | ||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 3 | Model1 | Model 3 | |||||
| Variable | β ± se | p | β ± se | p | β ± se | p | β ± se | p |
| Non-Fasting | ||||||||
| Systolic | −0.152±0.057 | <0.001 | −0.266±0.096 | 0.02 | −13.1±3.73 | 0.001 | −19.9±4.38 | <0.001 |
| Diastolic | 0.075±0.077 | 0.34 | 0.064±0.086 | 0.50 | 5.74±2.76 | 0.04 | 9.42±3.64 | 0.013 |
| Cholesterol | ||||||||
| Total | −0.634±0.181 | 0.001 | −0.811±0.246 | 0.004 | −5.87±8.72 | 0.50 | 1.08±11.15 | 0.10 |
| HDL-C | −0.885±0.079 | <0.001 | −0.928±0.08 | <0.001 | 13.1±2.69 | <0.001 | −13.1±3.30 | <0.001 |
| Triglycerides | 2.775±0.403 | <0.001 | 2.89±0.480 | <0.001 | −100.6±14.26 | <0.001 | −31.3±22.5 | 0.17 |
| ln triglycerides | 0.0183±0.12 | <0.001 | 0.018±0.003 | <0.001 | −0.722±0.079 | <0.001 | −0.099±0.106 | 0.35 |
| Glucose | 0.980±0.168 | <0.001 | 0.454±0.108 | <0.001 | −29.0±7.34 | <0.001 | −15.7±10.6 | 0.15 |
| Inflammatory | ||||||||
| C-reactive protein | 0.0011±0.0045 | 0.82 | −0.010±0.005 | 0.58 | −0.77±0.14 | <0.001 | −0.675±0.237 | 0.007 |
| ln CRP | 0.0312±0.0052 | <0.001 | 0.0031±0.006 | <0.001 | −2.40±0.247 | <0.001 | −0.914±0.335 | 0.009 |
| Fibrinogen | 0.779±0.456 | 0.09 | −0.775±0.504 | 0.30 | −107.0±20.0 | <0.001 | −58.0±29.5 | 0.06 |
| Fasting Variables | ||||||||
| HOMA-IR | 0.324±0.055 | <0.001 | 0.120±0.020 | <0.001 | −6.59±1.88 | 0.001 | −0.205±3.09 | 0.95 |
| HOMA-B | 6.86±1.20 | <0.001 | 1.947±1.03 | <0.001 | −32.8±83.7 | 0.70 | 147.4±104.4 | 0.17 |
| Triglycerides | 1.47±1.07 | 0.18 | 1.13±0.406 | 0.01 | −93.4±19.2 | <0.001 | −13.7±32.4 | 0.68 |
| Ln trig | 0.0085±0.006 | 0.19 | 0.0127±0.006 | 0.01 | −0.585±0.108 | <0.001 | 0.012±0.19 | 0.95 |
| Insulin | 0.937±0.169 | <0.001 | 0.846±0.180 | <0.001 | −21.9±5.99 | 0.001 | −0.218±8.6 | 0.98 |
| LDL-Cholesterol | 0.008±0.35 | 0.98 | −0.370±0.417 | 0.87 | −2.11±12.8 | 0.87 | 27.3±21.9 | 0.22 |
All values represented are from multivariable linear regression models (β coefficient ± standard error) with p-values.
Values bolded are considered statistically significant
Model 1: adjusted for age, sex race, education, smoking status, arthritis
Model 2: Model 1 co-variates, cancer and diabetes
Interaction (Ix): interaction term obesity (Body Fat) term x ASM with model 1 co-variates
Abbreviations: ALM: Appendicular lean mass; BMI: body mass index; HDL-C: high density lipoprotein cholesterol; HOMA: homeostatic assessment model; IR: insulin resistance; Ix: interaction; LDL-C: low density lipoprotein cholesterol; S-sensitivity; s.e.: standard error.
Natural log transformation was used for triglycerides and C-reactive protein
Appendix Table 3A –
Multivariable Regression coefficients for ALM-defined Sarcopenia (high/low)
| ALM Sarcopenia Present/Absent | ||||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
| β ± se | p | β ± se | p | β ± se | p | β ± se | p | |
| Non-Fasting | ||||||||
| Systolic | 1.74±1.08 | 0.12 | 2.15±1.10 | 0.06 | 2.36±1.11 | 0.04 | 2.13±1.09 | 0.06 |
| Diastolic | 0.00003±0.705 | 1.00 | 0.233±0.70 | 0.74 | −0.0078±0.046 | 0.87 | −0.084±0.68 | 0.90 |
| Cholesterol | ||||||||
| Total | 3.82±2.23 | 0.09 | 4.02±2.35 | 0.09 | 4.75±2.63 | 0.08 | 3.11±2.41 | 0.20 |
| HDL-C | 7.16±0.83 | <0.001 | 7.93±0.81 | <0.001 | 7.04±0.80 | <0.001 | 7.56±0.79 | <0.001 |
| Triglycerides | −21.0±3.51 | <0.001 | −23.6±3.55 | <0.001 | −19.8±3.89 | <0.001 | −20.69±3.29 | <0.001 |
| Ln triglycerides | −0.13±0.021 | <0.001 | −0.151±0.022 | <0.001 | −0.118±0.023 | <0.001 | −0.135±0.02 | <0.001 |
| Glucose | −5.43±1.55 | 0.001 | −5.09±1.71 | 0.005 | −3.66±1.79 | 0.05 | −2.34±1.41 | 0.10 |
| Inflammatory | ||||||||
| C-reactive protein | 0.014±0.04 | 0.72 | 0.027±0.04 | 0.50 | 0.065±0.04 | 0.11 | 0.033±0.04 | 0.41 |
| Ln crp | −0.154±0.045 | 0.001 | −0.14±0.044 | 0.003 | 0.0057±0.046 | 0.90 | −0.126±0.04 | 0.007 |
| Fibrinogen | −0.164±4.26 | 0.97 | −0.238±4.31 | 0.96 | 6.70±4.57 | 0.15 | 1.62±4.37 | 0.71 |
| Fasting Variables | ||||||||
| HOMA-IR | −1.63±0.27 | <0.001 | −1.64±0.25 | <0.001 | −1.19±0.27 | <0.001 | −1.43±0.23 | <0.001 |
| HOMA-B | −23.2±5.57 | <0.001 | −20.3±6.84 | 0.006 | −11.8±5.68 | 0.05 | −21.3±6.04 | 0.001 |
| Triglycerides | −15.0±6.9 | 0.04 | −20.9±6.54 | 0.003 | −17.2±6.4 | 0.01 | −19.3±6.14 | 0.004 |
| Ln trig | −0.079±0.042 | 0.07 | −0.12±0.04 | 0.006 | 0.01±0.003 | 0.002 | −0.110±0.036 | 0.005 |
| Insulin | −4.65±0.72 | <0.001 | −4.83±0.67 | <0.001 | −3.27±0.67 | <0.001 | −4.41±0.59 | <0.001 |
| LDL-C | 0.43±3.07 | 0.89 | 0.75±3.28 | 0.82 | 2.46±3.34 | 0.47 | 0.407±3.25 | 0.90 |
Values bolded are considered statistically significant
Model 1: adjusted for age, sex race, education, smoking status, arthritis
Model 2: Model 1 co-variates, cancer and diabetes
Interaction (Ix): interaction term obesity (Body Fat) term x ASM with model 1 co-variates
Abbreviations: ALM: Appendicular lean mass; BMI: body mass index; HDL-C: high density lipoprotein cholesterol; HOMA: homeostatic assessment model; IR: insulin resistance; Ix: interaction; LDL-C: low density lipoprotein cholesterol; S-sensitivity; s.e.: standard error.
Natural log transformation was used for triglycerides and C-reactive protein
Appendix Table 3B –
Multivariable Regression coefficients for ALM:BMI defined Sarcopenia (high/low)
| ALM:BMI defined Sarcopenia Present/Absent | ||||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | |||||
| β ± se | p | β ± se | p | β ± se | p | β ± se | p | |
| Non-Fasting | ||||||||
| Systolic | 2.13±0.90 | 0.023 | 2.01±0.95 | 0.04 | 1.97±0.97 | 0.05 | 1.90±0.98 | 0.06 |
| Diastolic | −0.38±0.63 | 0.55 | −0.32±0.64 | 0.62 | −0.373±0.73 | 0.61 | −0.059±0.65 | 0.93 |
| Cholesterol | ||||||||
| Total | 0.33±2.03 | 0.87 | 0.63±2.03 | 0.76 | −0.65±1.86 | 0.73 | 0.97±1.97 | 0.62 |
| HDL-C | −1.61±0.56 | 0.006 | −0.76±0.59 | 0.21 | 1.90±0.61 | 0.003 | −0.390±0.57 | 0.50 |
| Triglycerides | 13.2±3.53 | 0.001 | 9.75±3.53 | 0.008 | 0.50±3.41 | 0.89 | 6.91±3.73 | 0.07 |
| Ln triglycerides | 0.093±0.020 | <0.001 | 0.067±0.021 | 0.002 | −0.010±0.021 | 0.62 | 0.052±0.021 | 0.019 |
| Glucose | 6.12±1.51 | <0.001 | 6.05±1.57 | <0.001 | 3.59±1.74 | 0.05 | 4.00±1.31 | 0.004 |
| Inflammatory | ||||||||
| C-reactive protein | 0.15±0.04 | <0.001 | 0.161±0.04 | <0.001 | 0.105±0.045 | 0.02 | 0.16±0.04 | <0.001 |
| Ln crp | 0.392±0.053 | <0.001 | 0.409±0.053 | <0.001 | 0.128±0.05 | 0.01 | 0.403±0.054 | <0.001 |
| Fibrinogen | 20.7±4.65 | <0.001 | 21.9±4.80 | <0.001 | 10.8±4.8 | 0.03 | 20.7±4.74 | <0.001 |
| Fasting Variables | ||||||||
| HOMA-IR | 1.19±0.74 | 0.12 | 1.15±0.75 | 0.14 | 0.307±0.87 | 0.73 | 1.11±0.705 | 0.13 |
| HOMA-B | −2.05±16.6 | 0.90 | −2.33±16.9 | 0.89 | −21.6±15.7 | 0.18 | −2.47±16.4 | 0.88 |
| Triglycerides | 16.4±6.88 | 0.02 | 9.92±6.93 | 0.16 | 3.23±7.70 | 0.68 | 9.50±6.67 | 0.17 |
| Ln trig | 0.10±0.04 | 0.01 | 0.063±0.036 | 0.10 | 0.012±0.04 | 0.77 | 0.061±0.35 | 0.10 |
| Insulin | 2.94±1.88 | 0.13 | 2.80±1.89 | 0.15 | −0.26±2.05 | 0.90 | 2.68±1.77 | 0.14 |
| LDL-C | −1.14±2.82 | 0.69 | −0.88±2.97 | 0.77 | −4.010±3.22 | 0.21 | −1.17±2.70 | 0.67 |
Values bolded are considered statistically significant
Model 1: adjusted for age, sex race, education, smoking status, arthritis
Model 2: Model 1 co-variates, cancer and diabetes
Interaction (Ix): interaction term obesity (Body Fat) term x ASM with model 1 co-variates
Abbreviations: ALM: Appendicular lean mass; BMI: body mass index; HDL-C: high density lipoprotein cholesterol; HOMA: homeostatic assessment model; IR: insulin resistance; Ix: interaction; LDL-C: low density lipoprotein cholesterol; S-sensitivity; s.e.: standard error.
Natural log transformation was used for triglycerides and C-reactive protein
Appendix Table 4A:
Prevalence and Relationship of Sarcopenia and Sarcopenic Obesity by Quartile of Variable: ALM-definition
| ALM-defined Sarcopenia | ALM-defined Sarcopenic Obesity | ||||||
|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 1 | Model 3 | |||
| Variable & Quartile | Rate (%) | Odds Ratio [95%CI] | Odds Ratio [95%CI] | Odds Ratio [95%CI] | Rate (%) | Odds Ratio [95%CI] | Odds Ratio [95%CI] |
| C-reactive protein | p=0.02 | p=0.42 | |||||
| Low | 33.8 | referent | referent | referent | 25.5 | referent | referent |
| 25–50 | 26.8 | 0.64 [0.51,0.81] | 0.77 [0.62,0.98] | 0.65 [0.52,0.82] | 22.6 | 0.82 [0.65,1.04] | 0.83 [0.65,1.06] |
| 50–75 | 23.7 | 0.58 [0.45,0.74] | 0.75 [0.58,0.98] | 0.59 [0.46,0.76] | 23.6 | 0.80 [0.61,1.05] | 0.82 [0.63,1.08] |
| High | 31.7 | 0.79 [0.64,0.97] | 1.08 [0.87,1.35] | 0.81 [0.66,1.01] | 26.1 | 0.94 [0.74,1.21] | 0.99 [0.77,1.26] |
| Fibrinogen | p=0.21 | p=0.19 | |||||
| Low | 26.5 | referent | referent | referent | 21.3 | referent | referent |
| 25–50 | 28.6 | 1.00 [0.73,1.38] | 1.14 [0.84,1.56] | 1.03 [0.73,1.44] | 23.3 | 1.02 [0.72,1.45] | 1.06 [0.74,1.52] |
| 50–75 | 29.8 | 1.01 [0.75, 1.37] | 1.26 [0.95,1.68] | 1.04 [0.76,1.44] | 24.5 | 1.07 [0.78,1.46] | 1.09 [0.79,1.52] |
| High | 32.8 | 0.98 [0.71,1.34] | 1.23 [0.88,1.71] | 1.04 [0.75,1.43] | 26.3 | 1.01 [0.73,1.38] | 1.05 [0.76,1.45] |
| HOMA-IR | p<0.001 | p=0.003 | |||||
| Low | 50.4 | referent | referent | referent | 31.3 | referent | referent |
| 25–50 | 31.8 | 0.44 [0.31,0.63] | 0.55 [0.37,0.81] | 0.44 [0.31,0.62] | 27.1 | 0.85 [0.57,1.28] | 0.85 [0.57,1.27] |
| 50–75 | 27.7 | 0.35 [0.21,0.57] | 0.50 [0.29,0.84] | 0.34 [0.21,0.55] | 24.7 | 0.75 [0.44,1.28] | 0.74 [0.44,1.26] |
| High | 11.5 | 0.10 [0.05,0.21] | 0.14 [0.07,0.31] | 0.10 [0.05,0.19] | 11.1 | 0.27 [0.13,0.53] | 0.24 [0.12,0.49] |
| HOMA-B | P<0.001 | p=0.03 | |||||
| Low | 37.3 | referent | referent | referent | 22.8 | referent | referent |
| 25–50 | 37.2 | 0.98 [0.63,1.51] | 1.14 [0.73,1.79] | 0.88 [0.57,1.35] | 29.0 | 1.38 [0.90,2.11] | 1.36 [0.87,2.12] |
| 50–75 | 28.8 | 0.70 [0.42,1.16] | 0.95 [0.54,1.67] | 0.63 [0.36,1.08] | 25.4 | 1.23 [0.65,2.31] | 1.18 [0.61,2.29] |
| High | 18.3 | 0.35 [0.22,0.56] | 0.53 [0.33,0.87] | 0.32 [0.19,0.53] | 16.3 | 0.68 [0.35,1.33] | 0.66 [0.33,1.33] |
All values represented are odds ratios [95% confidence intervals).
Each variable (C-reactive protein, fibrinogen, HOMA-IR and HOMA-S) were stratified by quartile. Rate represents the proportion of individuals within the quartile (low, 25–50, 50–75, or high) that fulfill criteria for sarcopenia or sarcopenic obesity definitions. The primary outcome is presence/absence of sarcopenia or sarcopenic obesity and the primary predictor is quartile of the variable.
Model 1: age, sex, race, education, smoking, arthr
Model 2: Model 1 co-variates and body fat
Model 3: Model 1 co-variates with diabetes and cancer
Appendix Table 4B:
Prevalence and Relationship of Sarcopenia and Sarcopenic Obesity by Quartile of Variable: ALM:BMI definition
| ALM:BMI defined Sarcopenia | ALM:BMI defined Sarcopenic Obesity | |||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | ||
| Variable & Quartile | Odds Ratio [95%CI] | Odds Ratio [95%CI] | Odds Ratio [95%CI] | Rate (%) | Odds Ratio [95%CI] | Odds Ratio [95%CI] |
| C-reactive protein | p<0.001 | |||||
| Low | referent | referent | referent | 15.1 | referent | referent |
| 25–50 | 1.51 [1.20,1.88] | 0.95 [0.74,1.24] | 1.50 [1.20,1.87] | 20.3 | 1.54 [1.23,1.92] | 1.53 [1.23,1.91] |
| 50–75 | 2.21 [1.73,2.82] | 1.24 [0.98,1.56] | 2.19 [1.71,2.80] | 25.9 | 2.28 [1.80,2.91] | 2.26 [1.76,2.89] |
| High | 3.06 [2.28,4.11] | 1.51 [1.10,2.07] | 3.06 [2.27,4.13] | 30.3 | 3.17 [2.37,4.24] | 3.17 [2.36,4.25] |
| Fibrinogen | p=0.007 | |||||
| Low | referent | referent | referent | 17.6 | referent | referent |
| 25–50 | 0.95 [0.73,1.25] | 0.71 [0.54,0.94] | 0.96 [0.73,1.26] | 17.2 | 0.98 [0.74,1.30] | 0.99 [0.74,1.31] |
| 50–75 | 1.81 [1.27,2.58] | 1.09 [0.75,1.57] | 1.80 [1.27,2.55] | 27.1 | 1.81 [1.26,2.61] | 1.81 [1.26,2.59] |
| High | 2.11 [1.43,3.13] | 1.27 [0.86,1.86] | 2.06 [1.39,3.06] | 29.9 | 2.15 [1.44,3.21] | 2.09 [1.40,3.13] |
| HOMA-IR | p=0.02 | |||||
| Low | referent | referent | referent | 14.7 | referent | referent |
| 25–50 | 1.18 [0.65,2.14] | 0.61 [0.30,1.24] | 1.17 [0.64,2.13] | 18.9 | 1.24 [0.67,2.32] | 1.24 [0.66,2.32] |
| 50–75 | 2.21 [1.09,4.48] | 0.69 [0.30,1.57] | 2.25 [1.10,4.59] | 29.9 | 2.34 [1.13,4.82] | 2.37 [1.14,4.94] |
| High | 1.79 [0.84, 3.80] | 0.48 [0.20,1.17] | 1.80 [0.83,3.90] | 25.8 | 1.90 [0.87,4.14] | 1.90 [0.86,4.20] |
| HOMA-B | p=0.23 | |||||
| Low | referent | referent | referent | 19.4 | referent | referent |
| 25–50 | 0.95 [0.61,1.47] | 0.60 [0.32,1.15] | 0.97 [0.64,1.49] | 18.9 | 1.00 [0.63,1.58] | 1.03 [0.66,1.62] |
| 50–75 | 1.57 [0.95,2.58] | 0.74 [0.40,1.38] | 1.62 [0.99,2.65] | 25.5 | 1.64 [1.00,2.70] | 1.71 [1.05,2.80] |
| High | 1.64 [1.08,2.50] | 0.49 [0.27,0.88] | 1.65 [1.09,2.49] | 26.0 | 1.73 [1.12,2.65] | 1.75 [1.14,2.67 |
All values represented are odds ratios [95% confidence intervals).
Each variable (C-reactive protein, fibrinogen, HOMA-IR and HOMA-S) were stratified by quartile. Rate represents the proportion of individuals within the quartile (low, 25–50, 50–75, or high) that fulfill criteria for sarcopenia or sarcopenic obesity definitions. The primary outcome is presence/absence of sarcopenia or sarcopenic obesity and the primary predictor is quartile of the variable.
Model 1: age, sex, race, education, smoking, arthr
Model 2: Model 1 co-variates and body fat
Model 3: Model 1 co-variates with diabetes and cancer
Footnotes
There are no conflicts of interest pertaining to this manuscript
REFERENCES
- [1].Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 2014;311:806–14. 10.1001/jama.2014.732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci 2014;69:547–58. 10.1093/gerona/glu010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE. Sarcopenic obesity predicts Instrumental Activities of Daily Living disability in the elderly. Obes Res 2004;12:1995–2004. 10.1038/oby.2004.250 [DOI] [PubMed] [Google Scholar]
- [4].Levine M, Crimmins E. The Impact of Insulin Resistance and Inflammation on the Association between Sarcopenic Obesity and Physical Functioning. Obesity (Silver Spring) 2012 10.1038/oby.2012.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Schrager MA, Metter EJ, Simonsick E, Ble A, Bandinelli S, Lauretani F, et al. Sarcopenic obesity and inflammation in the InCHIANTI study. J Appl Physiol 2007;102:919–25. 10.1152/japplphysiol.00627.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care 1998;21:2191–2 [DOI] [PubMed] [Google Scholar]
- [7].Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Current Opinion in Clinical Nutrition and Metabolic Care 2008;11:693–700. 10.1097/MCO.0b013e328312c37d [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Stenholm S, Rantanen T, Heliovaara M, Koskinen S. The mediating role of C-reactive protein and handgrip strength between obesity and walking limitation. J Am Geriatr Soc 2008;56:462–9. 10.1111/j.1532-5415.2007.01567.x [DOI] [PubMed] [Google Scholar]
- [9].Batsis JA, Barre LK, Mackenzie TA, Pratt SI, Lopez-Jimenez F, Bartels SJ. Variation in the prevalence of sarcopenia and sarcopenic obesity in older adults associated with different research definitions: dual-energy x-ray absorptiometry data from the national health and nutrition examination survey 1999–2004. J Am Geriatr Soc 2013;61:974–80. 10.1111/jgs.12260 [DOI] [PubMed] [Google Scholar]
- [10].Batsis JA, Mackenzie TA, Barre LK, Lopez-Jimenez F, Bartels SJ. Sarcopenia, sarcopenic obesity and mortality in older adults: results from the National Health and Nutrition Examination Survey III. Eur J Clin Nutr 2014;68:1001–7. 10.1038/ejcn.2014.117 [DOI] [PubMed] [Google Scholar]
- [11].Batsis JA, Nieto-Martinez RE, Lopez-Jimenez F. Metabolic syndrome: from global epidemiology to individualized medicine. Clin Pharmacol Ther 2007;82:509–24. 10.1038/sj.clpt.6100355 [DOI] [PubMed] [Google Scholar]
- [12].Kim TN, Yang SJ, Yoo HJ, Lim KI, Kang HJ, Song W, et al. Prevalence of sarcopenia and sarcopenic obesity in Korean adults: the Korean sarcopenic obesity study. Int J Obes 2009;33:885–92. 10.1038/ijo.2009.130 [DOI] [PubMed] [Google Scholar]
- [13].Lee CG, Boyko EJ, Strotmeyer ES, Lewis CE, Cawthon PM, Hoffman AR, et al. Association between insulin resistance and lean mass loss and fat mass gain in older men without diabetes mellitus. J Am Geriatr Soc 2011;59:1217–24. 10.1111/j.1532-5415.2011.03472.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lim S, Kim JH, Yoon JW, Kang SM, Choi SH, Park YJ, et al. Sarcopenic Obesity: Prevalence and Association With Metabolic Syndrome in the Korean Longitudinal Study on Health and Aging (KLoSHA). Diabetes Care 2010;33:1652–4. 10.2337/dc10-0107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Srikanthan P, Karlamangla AS. Relative muscle mass is inversely associated with insulin resistance and prediabetes. Findings from the third National Health and Nutrition Examination Survey. J Clin Endocrinol Metab 2011;96:2898–903. 10.1210/jc.2011-0435 [DOI] [PubMed] [Google Scholar]
- [16].Schaap LA, Pluijm SM, Deeg DJ, Harris TB, Kritchevsky SB, Newman AB, et al. Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci 2009;64:1183–9. 10.1093/gerona/glp097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab 1998;83:847–50. 10.1210/jcem.83.3.4660 [DOI] [PubMed] [Google Scholar]
- [18].Cesari M, Pahor M, Lauretani F, Zamboni V, Bandinelli S, Bernabei R, et al. Skeletal muscle and mortality results from the InCHIANTI Study. J Gerontol A Biol Sci Med Sci 2009;64:377–84. 10.1093/gerona/gln031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Park SW, Goodpaster BH, Strotmeyer ES, de Rekeneire N, Harris TB, Schwartz AV, et al. Decreased muscle strength and quality in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes 2006;55:1813–8. 10.2337/db05-1183 [DOI] [PubMed] [Google Scholar]
- [20].Park SW, Goodpaster BH, Strotmeyer ES, Kuller LH, Broudeau R, Kammerer C, et al. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes Care 2007;30:1507–12. 10.2337/dc06-2537 [DOI] [PubMed] [Google Scholar]
- [21].Peppa M, Koliaki C, Nikolopoulos P, Raptis SA. Skeletal muscle insulin resistance in endocrine disease. J Biomed Biotechnol 2010;2010:527850. 10.1155/2010/527850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hotamisligil GS. The role of TNFalpha and TNF receptors in obesity and insulin resistance. J Intern Med 1999;245:621–5 [DOI] [PubMed] [Google Scholar]
- [23].Roubenoff R, Freeman LM, Smith DE, Abad LW, Dinarello CA, Kehayias JJ. Adjuvant arthritis as a model of inflammatory cachexia. Arthritis Rheum 1997;40:534–9 [DOI] [PubMed] [Google Scholar]
- [24].Batsis JA, Sahakyan KR, Singh P, Bartels SJ, Somers VK, Lopez-Jimenez F. Leptin, adiposity, and mortality: results from the National Health and Nutrition Examination Survey III, 1988 to 1994. Mayo Clin Proc 2015;90:481–91. 10.1016/j.mayocp.2015.01.023 [DOI] [PubMed] [Google Scholar]
- [25].Guillet C, Zangarelli A, Gachon P, Morio B, Giraudet C, Rousset P, et al. Whole body protein breakdown is less inhibited by insulin, but still responsive to amino acid, in nondiabetic elderly subjects. J Clin Endocrinol Metab 2004;89:6017–24. 10.1210/jc.2003-031323 [DOI] [PubMed] [Google Scholar]
- [26].Rasmussen BB, Fujita S, Wolfe RR, Mittendorfer B, Roy M, Rowe VL, et al. Insulin resistance of muscle protein metabolism in aging. FASEB J 2006;20:768–9. 10.1096/fj.05-4607fje [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Batsis JA, Mackenzie TA, Bartels SJ, Sahakyan KR, Somers VK, Lopez-Jimenez F. Diagnostic accuracy of body mass index to identify obesity in older adults: NHANES 1999–2004. Int J Obes (Lond) 2015 10.1038/ijo.2015.243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Batsis JA, Mackenzie TA, Lopez-Jimenez F, Bartels SJ. Sarcopenia, sarcopenic obesity, and functional impairments in older adults: National Health and Nutrition Examination Surveys 1999–2004. Nutr Res 2015;35:1031–9. 10.1016/j.nutres.2015.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Landi F, Liperoti R, Fusco D, Mastropaolo S, Quattrociocchi D, Proia A, et al. Sarcopenia and mortality among older nursing home residents. J Am Med Dir Assoc 2012;13:121–6. 10.1016/j.jamda.2011.07.004 [DOI] [PubMed] [Google Scholar]
- [30].Zizza CA, Herring A, Stevens J, Popkin BM. Obesity affects nursing-care facility admission among whites but not blacks. Obes Res 2002;10:816–23. 10.1038/oby.2002.110 [DOI] [PubMed] [Google Scholar]


