Abstract
A hallmark event in neurodegenerative diseases (NDs) is the misfolding, aggregation, and accumulation of proteins, leading to cellular dysfunction, loss of synaptic connections, and brain damage. Despite the involvement of distinct proteins in different NDs, the process of protein misfolding and aggregation is remarkably similar. A recent breakthrough in the field was the discovery that misfolded protein aggregates can self-propagate through seeding and spread the pathological abnormalities between cells and tissues in a manner akin to the behavior of infectious prions in prion diseases. This discovery has vast implications for understanding the mechanisms involved in the initiation and progression of NDs, as well as for the design of novel strategies for treatment and diagnosis. In this Review, we provide a critical discussion of the role of protein misfolding and aggregation in NDs. Commonalities and differences between distinct protein aggregates will be highlighted, in addition to evidence supporting the hypothesis that misfolded aggregates can be transmissible by the prion principle. We will also describe the molecular basis and implications for prion-like conformational strains, cross-interaction between different misfolded proteins in the brain, and how these concepts can be applied to the development of novel strategies for therapy and diagnosis.
NDs include highly debilitating illnesses, such as Alzheimer’s (AD), Parkinson’s disease (PD), amyotrophic lateral sclerosis, Huntington’s disease, spinocerebellar ataxias, frontotemporal dementia, corticobasal degeneration, progressive supranuclear palsy, chronic traumatic encephalopathy, multiple system atrophy, dementia with Lewy bodies, and prion diseases (PrD). Notwithstanding large differences in clinical manifestation and prevalence, NDs have many common features, including their chronic and progressive nature, increase of prevalence with age, destruction of neurons in specific areas of the brain, damage of the network of synaptic connections, and selective brain mass loss1. Another common event, which is thought to be at the root of these diseases, is the progressive accumulation of misfolded protein aggregates in well-ordered structures, usually referred to as amyloid1,2. Despite the fact that the protein aggregates involved in distinct NDs are different, the process of protein misfolding, its intermediates, end-products, and main features are remarkably similar2. In this article, we collectively review these commonalities and their impact for elucidating the underlying pathological mechanisms and how this knowledge has benefited the development of novel diagnostic tools and disease-modifying therapeutic strategies.
Misfolded protein aggregates as culprits in neurodegeneration
Compelling evidence coming from genetic, neuropathological, cellular, and biochemical studies, as well as from experiments with transgenic mouse models, have shown that protein misfolding, oligomerization, and accumulation in the brain are the main events triggering pathological abnormalities responsible for disease1–3. The proteins most commonly implicated in the accumulation of cerebral misfolded aggregates in NDs include: amyloid-beta (Aβ) in AD; tau in AD, frontotemporal dementia, corticobasal degeneration, progressive supranuclear palsy, argyrophilic grain disease, and chronic traumatic encephalopathy; alpha-synuclein (α-Syn) in PD, multiple system atrophy, and dementia with Lewy bodies; TAR DNA-binding protein 43 (TDP-43) in amyotrophic lateral sclerosis and frontotemporal dementia; and prion proteins in PrDs (i.e., Creutzfeldt–Jakob disease (CJD), bovine spongiform encephalopathy, chronic wasting disease, and scrapie).
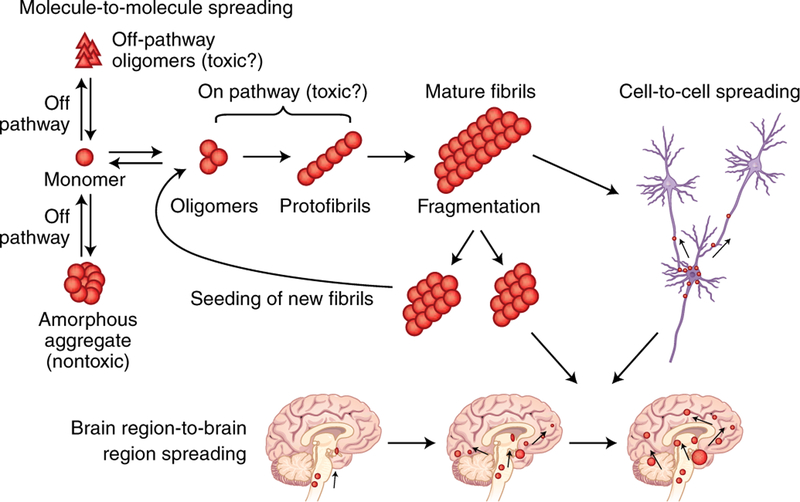
These disease-associated proteins do not exhibit obvious similarities in terms of sequence, size, structure, expression level, or function. Nonetheless, all these proteins undergo misfolding from their native states to form intermolecular β-sheet-rich structures, ranging from small oligomers to large fibrillar aggregates, in the diseased brain1,2. Amyloid are highly ordered aggregates, 100–200 Å in diameter, comprised of arrays of intermolecular β-sheets running parallel to the long axis of the fibrils, a structure known as cross-β4. The most routine technique used to recognize amyloids is staining with specific dyes, such as Congo red, thioflavin, and their derivatives5. Initially, it was thought that these large protein deposits were the neurotoxic species in the brain, but more recent evidence suggests that smaller, soluble misfolded oligomers, precursors of the fibrillar aggregates, appear to be the real culprits of neurodegeneration6–9. Misfolded oligomers are an ill-defined and heterogeneous group of species ranging from dimers to larger protofibrillar structures, likely composed of hundreds of monomers5,10,11. The oligomeric species are highly dynamic and exist in equilibrium with monomers and fibrils. Moreover, some oligomers are on-pathway intermediates for amyloid fibril formation, while others might be terminal off-pathway products, some of which could be highly toxic (Fig. 1)11,12. The large heterogeneity, rapid interconversion between species, and propensity to form higher-order aggregates have made it very difficult to obtain high-resolution structural information for misfolded oligomers, as well as to determine which are the most relevant oligomeric structures for the disease8,10,11.
Fig. 1 |. Protein aggregation and the prion principle of pathological transmission.
Monomeric proteins can misfold and aggregate. Spreading of protein misfolding operates at different levels during the pathogenesis of NDs, including molecule-to-molecule, cell-to-cell and brain region-to-brain region. In some specific cases, it may also operate to transmit the disease from individual to individual, as has been demonstrated for PrDs.
The mechanism of protein misfolding and aggregation is best described by the seeding-nucleation model, first proposed by Lansbury and colleagues13, which has been modeled kinetically in great detail14. During this process, a slow and thermodynamically unfavorable nucleation phase is followed by a rapid elongation stage13,15. In the nucleation phase, the rate-determining step is the formation of a stable seed or nucleus of polymerized protein. Once the seeds are formed, they rapidly grow by incorporating monomeric protein into the polymer13,15. Large polymers can fragment in a process not well-known in vivo to generate more seeds to propagate the reaction. A typical feature of the seeding–nucleation model is the ability of preformed seeds to greatly accelerate the aggregation process by recruiting the soluble normal protein into the growing aggregate13,15. From a biophysical viewpoint, the process of protein misfolding and aggregation involves rearranging the structure of the protein into a series of β-strands. These strands are stabilized by hydrogen bonding and hydrophobic interactions and open up ‘sticky’ ends for attracting molecules of the folded or partially unfolded protein, forcing its misfolding to fit into the cross-β polymeric structure. Although the primary scaffold of the misfolded aggregates is similar, the individual molecules can adopt many quite varied structures, which give rise to the possibility of conformational strains, as discussed below.
Despite the commonalities in the pathological mechanisms of NDs, there are some important differences among the distinct diseases: clinical symptoms, prevalence, risk factors, areas of the brain affected, cellular types injured, and genes implicated. In addition, each ND is usually associated with the misfolding and aggregation of a distinct protein that forms deposits that accumulate in diverse cellular locations, including the cytoplasm, nucleus, plasma membrane, or extracellular spaces. Finally, although the gross structural signature of the aggregates is similar (the cross-β conformation), their detailed structure is likely very different depending on the protein and the disease. The dissimilarities in the protein sequence, cellular location, and biophysical nature of the aggregates probably determine that the mechanisms of cellular toxicity are also different.
The prion principle and its role in neurodegenerative diseases
The seeding property, common to all misfolded protein aggregates, confers on them the inherent ability to spread the misfolding and aggregation process in a manner akin to infectious prion particles15,16. PrDs are the only NDs convincingly demonstrated to be transmissible by infection16,17. The infectious agent, termed a prion, is composed exclusively of misfolded prion protein (PrPSc) aggregates that self-replicate in the infected brain18,19. Disease transmission is mediated by prion aggregates acting as seeds to initiate the misfolding and aggregation of the native, monomeric prion protein in the host16. At some point, the long polymers undergo fragmentation to release more seeds, increasing the rate of prion propagation. In accordance with the seeding–nucleation model, PrPSc silently propagates for a long period of time until reaching the toxic threshold necessary for cellular dysfunction, brain damage, and clinical disease16.
The fact that seeding of protein aggregation is a common feature of all misfolded proteins implicated in NDs suggests that they have the potential to behave as prions15. This concept was initially taken with some reticence, despite the fact that it was supported by various previous articles showing evidence for pathological transmission of protein deposits in diverse forms of systemic amyloidosis20,21. In recent years, the concept that misfolded protein aggregates can spread pathologically by the prion principle has steadily gained acceptance in the field. Indeed, a series of reports have demonstrated that several NDs can be experimentally transmitted by a prion-like mechanism in various cellular and animal models of diverse diseases3,16,22,23. Studies with Aβ, tau, and α-Syn have shown that inoculation with tissue homogenates from patients affected by NDs or transgenic mouse models rich in protein aggregates results in the induction of disease pathology in the recipient cellular or animal models3,16,22,23. Moreover, in animals not genetically programmed to develop the disease spontaneously, pathological induction has been demonstrated to result in a completely de novo disease, more akin to infectious prions24–26. Pathological induction can be reduced by depleting the inoculum of protein aggregates27–29, and transmission has been achieved by adding misfolded protein aggregates prepared in vitro using synthetic or recombinant components30–33. However, in general, transmission using tissue homogenates is more efficient than with purified proteins, suggesting that other cellular cofactors may play a role in the pathological induction22,34. Accumulation of protein aggregates can be promoted by inoculation of small amounts of aggregated seeds35,36, and in some cases titration experiments have been done to show that the rate of induction is proportional to the amount of seed inoculated35. Finally, disease transmission has been observed even when seeds were administered systemically37,38. An open and controversial issue is whether spreading of protein misfolding is equivalent to spreading of disease. In some cases, the pathological induction is restricted to the accumulation of protein aggregates, and in others it is accompanied by tissue damage and clinical signs typical of the disease. These findings suggest that promoting protein misfolding not only leads to increased protein aggregation, but also accelerates the whole disease. It is also important to highlight that induction is not always expected to result in very obvious clinical signs leading to rapid death as in PrD. For example, in AD, the expected clinical phenotype is characterized by subtle memory and cognitive changes that can only be detected in rodents by sophisticated behavioral tests.
These findings support the concept that many of the hallmark properties of prions as infectious agents are shared by the main proteins involved in NDs. Still, the main controversial point is whether other misfolded proteins can act as infectious agents to transmit the disease among individuals under natural conditions17,39,40. However, it is important to note that even some typical PrDs are not naturally infectious and infectivity has only been supported by laboratory experiments41. Also, other PrDs are transmissible only in certain rare conditions, and tracking the infectious origin is often difficult because of the usually long time between infection and development of clinical symptoms16. Finally, it is important to highlight that transmission of biological information via prions by seeding of protein aggregation operates at multiple levels42,43 (Fig. 1). At the molecular level, the template-induced conversion of the natively folded protein by the polymeric misfolded protein leads to autocatalytic growth of protein aggregates. At the cellular level, the pathology spreads from cell to cell through the transfer of mis-folded protein aggregates between adjacent cells, leading to regional spreading of the abnormalities. At the organ level, the progressive spreading of the pathology among cells leads to tissue damage that can be transmitted to remote or distant areas of the brain, either by cell-to-cell contact or through biological fluids, such as interstitial fluid, cerebrospinal fluid, or blood. At the organismal level, exposure of a naive individual to misfolded aggregated seeds can initiate the process of protein misfolding, leading to disease in an infectious manner. It seems likely that in some NDs, the prion principle may operate only at the molecular, cellular, and tissue levels to spread the pathology, thus playing a key role in disease progression (Fig. 1). Currently, it is controversial whether transmission at all these levels is required before other misfolded proteins can be considered a ‘bona fide’ prion. In this sense, it might be necessary to update the definition of the word prion to refer to proteins able to adopt alternative conformations, some of which can self-propagate their folding in an autocatalytic seeding reaction that can be spread between cells and tissues.
The polymorphic nature of misfolded proteins and the concept of conformational strains
Misfolded protein aggregates consist of a heterogeneous mixture of different species, differing in size and structure12,44,45. In PrDs, the structural heterogeneity of protein aggregates has resulted in the ability of PrPSc to self-propagate distinct ‘conformational variants’ that can result in diseases with different characteristics. These conformational variants are often referred to as prion strains, analogous to strains of conventional infectious agents46–48. Different prion strains can perpetuate their properties indefinitely at the expense of the same normal prion protein, a process reproduced in a cell-free system in vitro49. The absence of high-resolution structural information for PrPSc has limited our understanding of the biophysical bases of prion strains50.
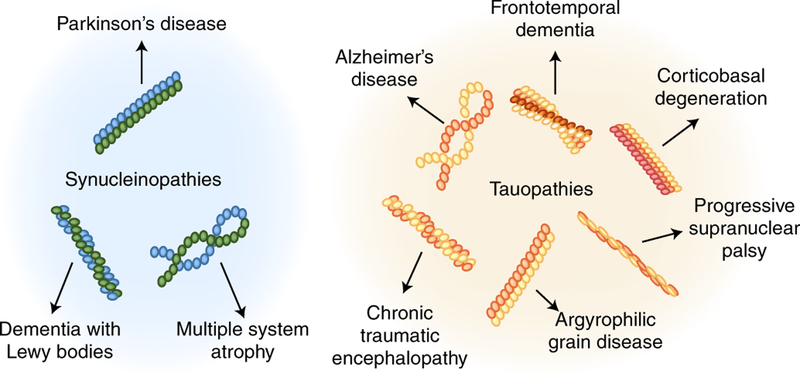
Several studies have reported evidence for the existence of conformational strains for misfolded aggregates composed of Aβ51–54, tau55–57, and α-Syn58–61. These findings may account for the large heterogeneity of AD and PD and may provide a molecular explanation for distinct tauopathies57 and synucleinopathies62 (Fig. 2). Indeed, there are at least seven different diseases associated with the accumulation of tau aggregates, including AD, frontotemporal dementia, progressive supranuclear palsy, corticobasal degeneration, argyrophilic grain disease, and chronic traumatic encephalopathy63, and three involving α-Syn deposition, including PD, multiple system atrophy, and Lewy body dementia64. As in PrDs caused by distinct prion strains, different tauopathies and synucleinopathies can be distinguished by the clinical symptoms, brain-region-specific pathology, and preference of the aggregates to accumulate in different cell types and/or by the distinct morphological and biophysical characteristics of the aggregates, their toxicity, and their seeding ability63–65.
Fig. 2 |. Conformational strains and their implications for the spectrum of synucleinopathies and tauopathies.
Various NDs are associated with the accumulation of tau and α-Syn aggregates, which are referred as tauopathies and synucleinopathies. Recent evidence suggests that aggregates adopting different structures, illustrated here as schematics, may be responsible for these diseases.
One study isolated and characterized 18 different tau strains in a cell culture model, each of which differed in various biochemical and biological properties55. Inoculation of transgenic mice with these strains produced strain-specific intracellular tau aggregates in distinct cell types and brain regions, which showed different rates of propagation55. These findings suggest that different tau species can self-propagate, leading to diverse neuropathological presentations, some reminiscent of those found in human tauopathies. In support of this conclusion, different tau strains were isolated from 29 patients affected by five distinct tauopathies, suggesting that diverse tauopathies are associated with different sets of conformational strains56. Cryo-electron microscopy has enabled the construction of atomic models of tau aggregates organized either as paired helical or straight filaments66. Filaments are made of two identical protofilaments spanning residues 306–378 of tau, which adopt a combined cross-β-β-helix structure. Paired helical and straight filaments differ in their inter-protofilament packing, providing a model to explain how the same protein can adopt different conformational variants.
Similarly, α-Syn assemblies displaying different structural characteristics have been shown to self-propagate in vivo, leading to distinct histopathological and behavioral phenotypes, some similar to those observed in different human synucleinopathies58,60,61. Inducing α-Syn aggregation in vitro in the presence of distinct concentrations of salts results in either cylindrical fibrils or flat, twisted ribbons61. These alternative structures were characterized in detail, showing profound differences with regards to proteolytic resistance, secondary structure, X-ray fiber-diffraction patterns, distribution of secondary structure elements determined by solid-state NMR, cellular toxicity, in vitro seeding, and propagation in mammalian cells.
In contrast to tau and α-Syn, which accumulate in diverse NDs, Aβ deposition occurs mostly in the brain of AD patients. Nevertheless, Aβ deposits are also highly heterogeneous, appearing in the form of mature dense-core plaques, diffuse deposits, cerebral amyloid angiopathy, inert deposits, and intracellular aggregates67. Several lines of evidence have shown that Aβ can also adopt different conformational strains, which may explain the heterogeneity observed in the patients’ brains. Studies by electron microscopy, atomic force microscopy, and solid-state NMR have revealed that Aβ can aggregate into multiple conformations in vitro52,68–70. High-resolution structural studies demonstrate that different experimental conditions can generate synthetic Aβ aggregates with substantially distinct structures52. Specifically, Aβ40 fibrils grown at 24 °C and pH 7.4 with gentle agitation have a predominantly ‘striated-ribbon’ morphology, whereas fibrils grown under the same conditions except without agitation have a predominantly ‘twisted’ morphology. The main biophysical difference between the two types of fibrils is their overall symmetry, with the striated ribbon filaments containing two cross-β subunits related by approximately two-fold rotational symmetry about the fibril growth axis and the twisted fibrils containing three cross-β units related by approximately three-fold rotational symmetry. These conformers were able to faithfully template their structure upon seeding of monomeric Aβ peptides in vitro over multiple rounds of self-propagation. In a similar manner, seeding experiments with Aβ aggregates obtained from the brains of patients affected by diverse clinicopathological AD phenotypes resulted in structurally distinct synthetic Aβ fibrils53,71, providing additional evidence for the existence of biologically relevant Aβ strains.
Molecular cross-talk among misfolded proteins through cross-seeding
Misfolded protein aggregates normally grow at the expense of proteins that can establish identical or highly complementary interactions and, thus, usually have the same or very similar amino acid sequence. However, misfolded aggregates can theoretically elongate by incorporating a distinct aggregation-prone protein if they share good conformational complementarity65. This process, often referred to as heterologous seeding or cross-seeding (Fig. 3), has been extensively described using pure preparations of proteins in test tube experiments72–78. The direct interaction leading to hybrid polymers initiated by seeds composed of one protein growing at expense of a second protein has been demonstrated by biophysical studies using immune-electron microscopy, co-immunoprecipitation, molecular modeling, and atomic force microscopy.
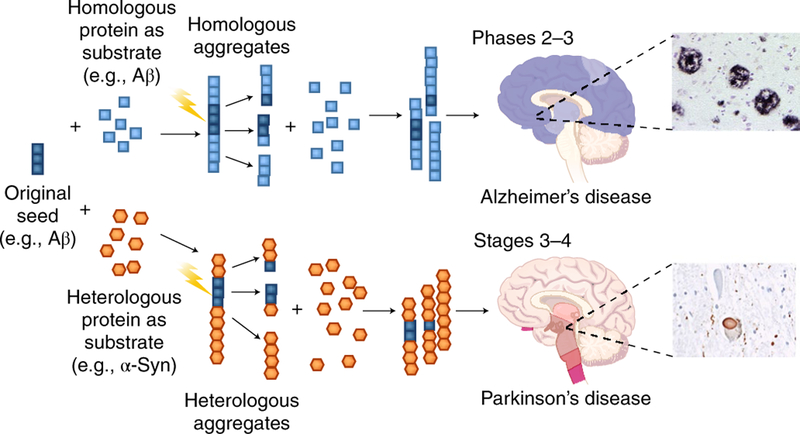
Fig. 3 |. Cross-seeding interactions between diverse misfolded protein aggregates.
In vitro and in vivo experiments have shown that aggregates composed of one protein usually seed the aggregation of the same protein (homologous seeding). However, in some circumstances, an aggregate may also seed the aggregation of a different protein, in a process termed heterologous seeding or cross-seeding. Cross-seeding events may explain the frequent finding of mixed pathologies in which more than one misfolded protein aggregate is found in a patient brain.
The co-existence of two or more different types of protein aggregates in various NDs has been extensively reported79–84. The archetypal case is AD, which simultaneously exhibits intracellular tau neurofibrillary tangles and extracellular Aβ amyloid plaques85. Although it is possible that tangles and plaques are formed independently, several studies have provided evidence for misfolded Aβ promoting tau abnormalities, perhaps by a direct protein-protein interaction86–90. Neuropathological studies have shown that nearly half of AD cases also display some α-Syn deposition82,91 and/ or TDP-43 aggregates92. In PD and related synucleinopathies, the frequency of mixed pathology is even higher, with approximately 80% of the cases showing detectable Aβ deposits, 50% showing tau aggregates, and 30% showing TDP-43 deposition82. The large pathological overlap between protein aggregates in the same brain complicates diagnosis and treatment and raises the question of which is the predominant disease. Based on pathological analysis and clinical progression, it seems that the disease is initiated by one type of protein aggregate, which acts as the driving force and defines the initial manifestation of the clinical phenotype, but later leads to the accumulation of other protein aggregates that come as secondary products and may change or expand the clinical picture82. An illustrative case for this concept is PD, which begins with classical motor symptoms, but over time a large proportion of the patients develop dementia93,94. It is tempting to speculate that the symptoms of dementia may be caused by the onset of AD-like protein aggregates95,96, but it is important to note that deposition of α-Syn aggregates in certain areas of the brain can also lead to dementia on its own, as happens in dementia with Lewy bodies97,98.
It is important to highlight that, although a direct interaction between misfolded proteins through cross-seeding is supported by in vitro experiments, there are various other alternative explanations for the synergistic interaction between diverse NDs. Alternative pathways to cross-seeding include enhancement of cellular vulnerability, impairments in clearance machinery, brain inflammation, and triggering of indirect signal transduction pathways resulting in increase of protein misfolding84. It is also important to consider that some properties attributed to seeding or cross-seeding, such as the stereotypical progression of pathology observed in some NDs, might be also explained by selective neuronal vulnerability99.
Protein aggregation in NDs might be also cross-seeded by seeds from systemic disorders associated with protein aggregation in peripheral tissues. Perhaps the best supported case for this mechanism is the interaction between AD and type-2 diabetes (T2D). T2D is associated with the pancreatic accumulation of the islet amyloid polypeptide (IAPP). Interestingly, T2D patients exhibit an increased risk of developing AD100,101, while approximately 80% of AD patients develop T2D or abnormalities in glucose metabolism102. Transgenic animals expressing both human Aβ and IAPP exhibit exacerbated AD-like pathology77. IAPP colocalizes with amyloid plaques in brain parenchymal deposits77,103, suggesting that these peptides may directly interact and aggravate the disease. Furthermore, inoculation of pancreatic IAPP aggregates into the brains of AD transgenic mice resulted in more severe AD pathology and substantially greater memory impairments than untreated animals77. The cross-seeding mechanism was supported by in vitro experiments showing that IAPP seeds can accelerate Aβ aggregation and that both peptides were found forming part of the same fibrils77,103.
Finally, an emerging possibility is that pathological aggregates responsible for NDs may be induced by seeds from ‘functional amyloids’16,104. In recent years, several proteins have been shown to naturally aggregate into nonpathogenic amyloid structures that contribute to modulating protein function or even acquire a new biological activity. These functional amyloids have been described in organisms ranging from bacteria to humans16,105–107, indicating that formation of these structures is not necessarily a pathological process. The possibility that protein misfolding and aggregation leading to NDs may be initiated by cross-seeding with functional amyloids has not been explored in detail16,104. However, a recent study reported that bacterial amyloids may play a role in α-Syn aggregation108.
Implications for therapy
Despite the extensive knowledge of the molecular mechanisms implicated in NDs, no cures or efficient treatments are yet available for these diseases. Misfolded protein aggregates are a primary target for therapeutic intervention. The recent discoveries of prion-like behavior, strain variability, and molecular cross-talk between different amyloidogenic proteins have uncovered both new therapeutic targets (Fig. 4) and potential unexpected difficulties.
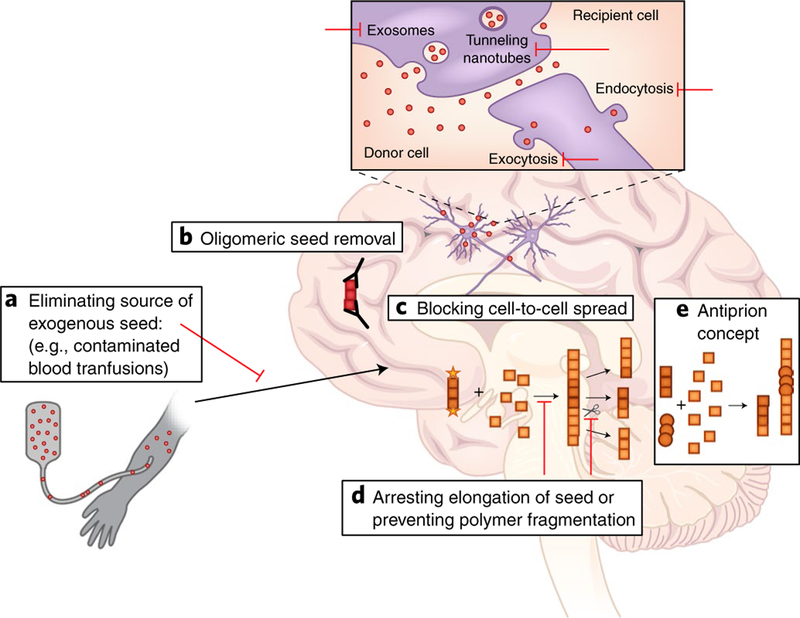
Fig. 4 |. Therapeutic strategies targeting the prion-like spread of misfolded proteins.
The recognition of the prion principle in NDs provides several opportunities for therapeutic intervention at different levels of the protein misfolding cascade. This picture illustrates some of these targets using strategies that are currently under development.
A primary strategy includes eliminating the source of exogenous seeds to which an individual may be exposed to (Fig. 4a). Although it is highly controversial whether NDs other than PrDs can be acquired by an external infectious process17,39,40,109, if this is proven for a portion of cases, reducing the risk of exposure will make a good strategy for preventing new cases. This approach has proven successful for PrDs. For example, the dramatic reduction of bovine spongiform encephalopathy by changing cattle feeding practices minimized human exposure and decreased the risk of variant CJD110.
Several approaches are under development to prevent the formation of or to remove misfolded aggregates (Fig. 4b). Targeting specifically the misfolded aggregates most competent for seeding might be a good approach for treatment, since these structures are likely less abundant than the normal protein or the fully aggregated material deposited in the brain. Various oligomer-specific antibodies and small molecules have already shown efficacy in animal models of diverse diseases (for review, see ref.111). Elucidation of the three-dimensional structure of oligomeric seeds may contribute substantially to the rational design of strategies targeting these species.
Cellular pathways implicated in the spreading of seeds can also be targeted (Fig. 4c). The exact mechanisms involved in the cell-to-cell spreading of misfolded seeds are not known, but several cellular pathways have been proposed112–114, including trans-synaptic transport, exocytosis and endocytosis, transfer through tunneling nanotubes, transport through exosomes, and direct protein–protein interactions at the cell surface. In theory, targeting various routes implicated in the transfer of seeds between cells might be an efficient approach for treatment. However, since these are general cellular processes, it is likely that manipulating them may produce side-effects.
The elongation and multiplication of seeds can also be arrested (Fig. 4d). The process of protein misfolding and aggregation depends on elongation and subsequent fragmentation of polymers to release more seeds. A good strategy for inhibiting elongation could be capping the seeds with molecules that prevent the incorporation of new monomers. The factors and forces involved in fragmentation of aggregates could also be manipulated to prevent the generation of additional seeds from an elongating protein aggregate. Although most fragmentation factors remain unknown, the yeast chaperone protein HSP104 has been shown to have this activity, and its inhibition cures yeast of prion infection115. Future research should aim to identify the HSP104-like factors operating in the human brain.
The prion principle can also be used to guide the development of antiprion therapeutic molecules (Fig. 4e). An important challenge for effectively attacking the prion-like spreading of protein misfolding and aggregation is that this process grows exponentially over time. We recently proposed utilizing the prion principle to generate a self-replicating therapy that could effectively outcompete with prion-like misfolded proteins116. The idea is to dissociate seeding from toxicity of the aggregated product, by generating a conformational strain that can efficiently seed and spread but result in the formation of innocuous material. Since both pathogenic seeds and therapeutic antiprions utilize the same monomeric protein to grow, antiprions will progressively deplete the substrate for seeding, thus delaying the accumulation of pathological misfolded proteins. A single prophylactic inoculation of prion-infected animals with an in vitro-generated antiprion delayed the onset of the disease and, in some animals, completely prevented the development of clinical symptoms and brain damage116. In this approach, the therapeutic molecule self-replicates in the body, outcompeting the pathogenic process. Extrapolation of this concept to other misfolded proteins may result in a universal approach for treatment of NDs by employing the prion principle to generate a self-replicating therapy targeted to each protein.
The recognition of the prion principle and its associated features poses some previously unappreciated difficulties for therapeutic interventions. For example, the large diversity of conformational strains that each misfolded protein can adopt make it challenging to identify molecules that will target all of them at the same time. Therefore, compounds may be efficient for only a subgroup of patients or a subset of pathological structures in the brain. Also, a well-established property of prion strains is their ability to change and mature over time, leading to changes in their properties47,117–119. In particular, it has been shown that prions can acquire drug resistance after prolonged treatment with a therapeutic molecule120,121. The phenomena of cross-seeding and mixed pathologies represent an additional difficulty for treatment. In fact, pharmacological inhibition of one misfolded protein aggregate may enhance cross-seeding events that result in the accumulation of a different type of aggregate. Thus, in patients harboring different types of protein aggregates in their brains, elimination of one of them may simply switch the clinical phenotype, but not eliminate the disease.
Implications for early diagnosis
Difficulties achieving therapeutic benefits in NDs can largely be attributed to the lack of diagnostic tools necessary for early identification of the disease before it destroys irreversibly the brain122. Today, all NDs are diagnosed by clinical examination with the help of imaging techniques123. The problem is that clear clinical symptoms are evident only after substantial damage to the brain, an organ that does not repair very well after injury. Extensive efforts are ongoing to identify biomarkers circulating in biological fluids that can be used for early, sensitive, objective, and noninvasive biochemical diagnosis of NDs124.
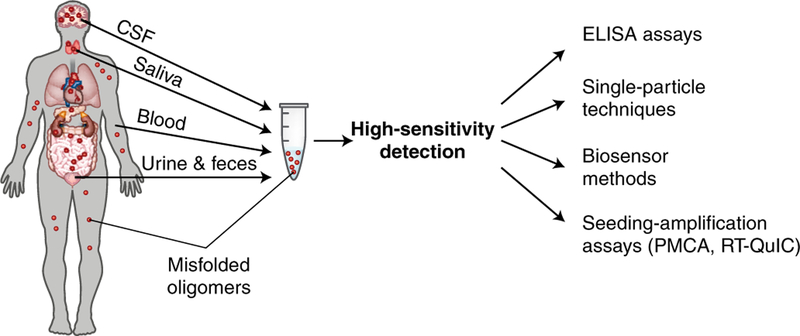
Several lines of evidence indicate that the process of misfolding and oligomerization in NDs begins years or even decades before these aggregates become massively deposited in the brain and induce the onset of brain damage and clinical symptoms125,126. Considering that soluble misfolded oligomers are the most likely culprits of neurodegeneration and pathological spreading, their sensitive detection might represent a great strategy for early and specific biochemical diagnosis of various NDs125. Moreover, various studies have shown that misfolded oligomers composed of different proteins are naturally secreted by cells and circulate in diverse biological fluids125,127,128. However, the challenge for detecting misfolded oligomers is that they are highly heterogeneous, are present in very low concentrations in biological fluids, and have the same sequence as the more abundant natively folded protein125. Nonetheless, various strategies have been proposed to specifically detect misfolded oligomeric forms of proteins associated to NDs (Fig. 5)125, such as enzyme-linked immunosorbent assay (ELISA)-based techniques in which oligomers are detected by using oligomer-specific conformational antibodies129; alternative ELISA strategies including conjugation with short oligonucleotides using the proximity-ligation assay130 or double usage of the same sequence-specific antibody twice in the system, for capturing as well as for detection131; methods for single-particle detection, such as fluorescence correlation spectroscopy132, flow cytometry133, and laser scanning microscopy134; and biosensor techniques employing surface plasmon resonance135 or electrochemical impedance spectroscopy136 sensors combined with oligomer-specific recognition methods.
Fig. 5 |. Disease diagnosis by sensitive detection of misfolded seeds in biological fluids.
The key role of misfolded protein oligomers in the prion-like spreading and neurodegeneration indicate that sensitive and specific detection of these structures in biological fluids might represent a good strategy for early biochemical diagnosis of NDs. Several strategies are under development for the detection of misfolded protein oligomers.
Another diagnostic strategy is to use the prion principle of spreading by seeding to amplify the misfolding and aggregation process in vitro. Two closely related seeding amplification assays have been employed for this purpose: protein misfolding cyclic amplification (PMCA)137,138 and real-time quaking-induced conversion (RT-QuIC)139. Both techniques use a system for cyclic amplification done in two phases. During the first phase, minute amounts of seeding-competent, misfolded oligomers from the patient’s samples are incubated with native protein substrate to induce the misfolding via polymer growth. In the second phase, the sample is subjected to mechanical fragmentation of the polymers (for example, sonication or strong shaking), multiplying the number of seeding-competent nuclei138. After each cycle, the number of seeds increases in an exponential fashion. The PMCA and RT-QuIC techniques were initially applied to amplify and detect PrPSc implicated in PrDs137,140. Using PMCA, the equivalent of a single particle of misfolded PrP oligomers can be detected141, and PrPSc can be identified in the blood and urine of people suffering from CJD142–144. PMCA and RT-QuIC are currently being routinely used in the USA and Europe to help in diagnosing CJD. Recently, the seeding amplification technology was extended to detect seeding-competent Aβ145, tau146, and α-Syn147,148, oligomers circulating in the cerebrospinal fluid of patients affected by AD, tauopathies, and PD, respectively. The technology successfully enabled detection with high sensitivity and specificity for patient samples as compared to controls affected by other NDs or neurological disorders. More research is necessary to evaluate the reproducibility, sensitivity, and specificity of seeding amplification assays and their application to monitor disease progression and preclinical diagnosis. Potential caveats of these technologies are the possibility for false-positive results due to contamination or cross-seeding events.
Future perspectives
Despite the impressive knowledge accumulated, NDs remain incurable. The prevalence of NDs continues to increase, and they have become one of the largest public health problems. There is a wide consensus that the key event common to all NDs is the misfolding, oligomerization, and progressive accumulation of proteins in the brain. A recent breakthrough was the discovery that misfolded protein aggregates can self-propagate their pathological properties using the prion principle of transmission of biological information by seeding of protein misfolding. This discovery has vast implications for understanding the mechanisms involved in the initiation and progression of NDs as well as for the design of novel strategies for treatment and diagnosis. It also sheds light on the great challenges that therapeutic strategies will face to produce a beneficial outcome for patients.
There are still many important open questions in relation to the role, mechanism, features, and implications of prion-like spreading of misfolded protein aggregates in NDs (Box 1). Considering the expansion of the prion concept in recent years, it may be necessary to implement a more modern definition of prions along the lines of ‘proteinaceous nucleating particles’22. In this article, we propose to define prions as proteins able to adopt alternative conformations, some of which can self-propagate their folding in an autocatalytic seeding reaction that can be spread between cells and tissues. This definition avoids the need for prions to be necessarily associated with infectious diseases, but captures the essential aspects of this important phenomenon, which represents a new biological framework with potentially important consequences to understand and treat many diseases.
Box 1 |. Open questions regarding the prion-like phenomenon in neurodegenerative diseases.
Role of prion-like propagation in NDs
Are misfolded protein aggregates the direct cause of NDs? What type(s) of misfolded, aggregated species are responsible for neurodegeneration? What is the role of prion-like spreading in the progression of NDs? How are the first seeds generated in the body? Can other NDs be transmitted by infection like PrDs? Does the prion principle operate in all diseases associated with misfolded protein aggregates as well as in functional amyloids?
Mechanisms of prion-like spreading
By which routes and practices can misfolded seeds be acquired in NDs? Is there any role for peripheral replication of prion-like proteins in NDs? Can other species of animals produce misfolded protein aggregates that can seed the pathological process in humans (similarly to how bovine spongiform encephalopathy triggers variant CJD in PrDs)? What are the cellular pathways implicated in prion-like spreading of protein aggregates? Which processes and factors are responsible for the fragmentation of polymers leading to multiplication ofseeds? What is the detailed molecular mechanism for templated conversion of the normal protein into the misfolded form? Which of the aggregated species are the most efficient in seeding? What is the three-dimensional structure of oligomeric seeds?
Conformational prion strains
How many conformational variants can a single misfolded protein adopt? Are there other factors necessary for the formation of prion-like strains? What are the structural differences between strains? How can different conformational strains target distinct areas of the brain? Do prion-like strains mature, change, and adapt depending on the conditions of the host? Can conformational variants undergo strain selection to develop resistance to drug treatment? Are different prion-like strains responsible for the diverse clinical phenotypes of NDs? Are different tauopathies and synucleinopathies caused by distinct conformational strains of tau and α-Syn? Are Aβ strains responsible for the heterogeneous accumulation of different types of protein deposits commonly observed in AD brains?
Cross-seeding and mixed pathology
What is the role of cross-seeding in the pathogenesis of NDs? Is mixed pathology caused by cross-seeding events? Which combination of misfolded proteins results in cross-seeding or cross-inhibition of protein misfolding? Can misfolded protein aggregates in the brain be promoted by cross-seeding from systemic amyloid diseases? Are functional amyloids involved in initiating ND pathology by cross-seeding?
Treatment and diagnosis
Can arresting prion-like spreading delay the progression of NDs? Is it possible to use the prion principle to develop a self-propagating therapy for NDs? Is detection of misfolded oligomers a good target for early diagnosis of NDs? Can the prion principle be used to amplify seeding-competent misfolded oligomers circulating in biological fluids to facilitate their detection?
Acknowledgements
The authors thank C. Mays for critical reading of the manuscript.
Footnotes
Competing interests
C.S. is the inventor of the PMCA technology and is currently the Founder, Chief Scientific Officer, and major shareholder of Amprion Inc., a biotech company aiming to develop PMCA and RT-QuIC seeding amplification assays for diagnosis of neurodegenerative diseases.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ross CA & Poirier MA Protein aggregation and neurodegenerative disease. Nat. Med 10(Suppl), S10–S17 (2004). [DOI] [PubMed] [Google Scholar]
- 2.Soto C Unfolding the role of protein misfolding in neurodegenerative diseases. Nat. Rev. Neurosci 4, 49–60 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Goedert M Neurodegeneration. Alzheimer’s and Parkinson’s diseases: The prion concept in relation to assembled Aβ, tau, and α-synuclein. Science 349, 1255555 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Fitzpatrick AW et al. Atomic structure and hierarchical assembly of a cross-β amyloid fibril. Proc. Natl Acad. Sci. USA 110, 5468–5473 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rambaran RN & Serpell LC Amyloid fibrils: abnormal protein assembly. Prion 2, 112–117 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caughey B & Lansbury PT Protofibrils, pores, fibrils, and neurodegeneration: separating the responsible protein aggregates from the innocent bystanders. Annu. Rev. Neurosci 26, 267–298 (2003). [DOI] [PubMed] [Google Scholar]
- 7.Gadad BS, Britton GB & Rao KS Targeting oligomers in neurodegenerative disorders: lessons from α-synuclein, tau, and amyloid-β peptide. J. Alzheimers Dis. 24(Suppl 2), 223–232 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Glabe CG Common mechanisms of amyloid oligomer pathogenesis in degenerative disease. Neurobiol. Aging 27, 570–575 (2006). [DOI] [PubMed] [Google Scholar]
- 9.Lesne S & Kotilinek L Amyloid plaques and amyloid-beta oligomers: an ongoing debate. J. Neurosci 25, 9319–9320 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benilova I, Karran E & De Strooper B The toxic Aβ oligomer and Alzheimer’s disease: an emperor in need of clothes. Nat. Neurosci 15, 349–357 (2012). [DOI] [PubMed] [Google Scholar]
- 11.Breydo L & Uversky VN Structural, morphological, and functional diversity of amyloid oligomers. FEBS Lett. 589(19 Pt A), 2640–2648 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Lesne SE Toxic oligomer species of amyloid-β in Alzheimer’s disease, a timing issue. Swiss Med. Wkly 144, w14021 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jarrett JT & Lansbury PT Jr. Seeding “one-dimensional crystallization” of amyloid: a pathogenic mechanism in Alzheimer’s disease and scrapie? Cell 73, 1055–1058 (1993). [DOI] [PubMed] [Google Scholar]
- 14.Meisl G et al. Scaling behaviour and rate-determining steps in filamentous self-assembly. Chem. Sci 8, 7087–7097 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soto C, Estrada L & Castilla J Amyloids, prions and the inherent infectious nature of misfolded protein aggregates. Trends Biochem. Sci 31, 150–155 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Soto C Transmissible proteins: expanding the prion heresy. Cell 149, 968–977 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aguzzi A & Lakkaraju AK Cell biology of prions and prionoids: a status report. Trends Cell Biol. 26, 40–51 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Prusiner SB Prions. Proc. Natl. Acad. Sci. USA 95, 13363–13383 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soto C Prion hypothesis: the end of the controversy? Trends Biochem. Sci 36, 151–158 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ganowiak K, Hultman P, Engstrom U, Gustavsson A & Westermark P Fibrils from synthetic amyloid-related peptides enhance development of experimental AA-amyloidosis in mice. Biochem. Biophys. Res. Commun 199, 306–312 (1994).This manuscript describes one of the earliest demonstrations of prion-like transmission of a nonprion protein misfolding disease.
- 21.Xing Y et al. Transmission of mouse senile amyloidosis. Lab. Invest 81, 493–499 (2001). [DOI] [PubMed] [Google Scholar]
- 22.Walker LC & Jucker M Neurodegenerative diseases: expanding the prion concept. Annu. Rev. Neurosci 38, 87–103 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stopschinski BE & Diamond MI The prion model for progression and diversity of neurodegenerative diseases. Lancet Neurol. 16, 323–332 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Morales R, Duran-Aniotz C, Castilla J, Estrada LD & Soto C De novo induction of amyloid-β deposition in vivo. Mol. Psychiatry 17, 1347–1353 (2012). [DOI] [PubMed] [Google Scholar]
- 25.Luk KC et al. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338, 949–953 (2012).This study reports the transmission of disease to wild-type, nontransgenic mice by a single intracerebral inoculation of synthetic α-Syn aggregates, leading to neurodegeneration and motor deficits.
- 26.Guo JL et al. Unique pathological tau conformers from Alzheimer’s brains transmit tau pathology in nontransgenic mice. J. Exp. Med 213, 2635–2654 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyer-Luehmann M et al. Exogenous induction of cerebral beta-amyloidogenesis is governed by agent and host. Science 313, 1781–1784 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Duran-Aniotz C et al. Aggregate-depleted brain fails to induce Aβ deposition in a mouse model of Alzheimer’s disease. PLoS One 9, e89014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tran HT et al. Alpha-synuclein immunotherapy blocks uptake and templated propagation of misfolded α-synuclein and neurodegeneration. Cell Rep. 7, 2054–2065 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stohr J et al. Purified and synthetic Alzheimer’s amyloid beta (Aβ) prions. Proc. Natl. Acad. Sci. USA 109, 11025–11030 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Volpicelli-Daley LA et al. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron 72, 57–71 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iba M et al. Synthetic tau fibrils mediate transmission of neurofibrillary tangles in a transgenic mouse model of Alzheimer’s-like tauopathy. J. Neurosci 33, 1024–1037 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luk KC et al. Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in mice. J. Exp. Med 209, 975–986 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Supattapone S Elucidating the role of cofactors in mammalian prion propagation. Prion 8, 100–105 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morales R, Bravo-Alegria J, Duran-Aniotz C & Soto C Titration of biologically active amyloid-β seeds in a transgenic mouse model of Alzheimer’s disease. Sci. Rep 5, 9349 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fritschi SK et al. Highly potent soluble amyloid-β seeds in human Alzheimer brain but not cerebrospinal fluid. Brain 137, 2909–2915 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eisele YS et al. Peripherally applied Abeta-containing inoculates induce cerebral beta-amyloidosis. Science 330, 980–982 (2010).This manuscript reports prion-like induction of protein aggregation by administration of seeds via peripheral routes.
- 38.Clavaguera F et al. Peripheral administration of tau aggregates triggers intracerebral tauopathy in transgenic mice. Acta Neuropathol. 127, 299–301 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walsh DM & Selkoe DJ A critical appraisal of the pathogenic protein spread hypothesis of neurodegeneration. Nat. Rev. Neurosci 17, 251–260 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Irwin DJ et al. Evaluation of potential infectivity of Alzheimer and Parkinson disease proteins in recipients of cadaver-derived human growth hormone. JAMA Neurol. 70, 462–468 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piccardo P, Manson JC, King D, Ghetti B & Barron RM Accumulation of prion protein in the brain that is not associated with transmissible disease. Proc. Natl. Acad. Sci. USA 104, 4712–4717 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moreno-Gonzalez I & Soto C Misfolded protein aggregates: mechanisms, structures and potential for disease transmission. Semin. Cell Dev. Biol 22, 482–487 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goedert M, Falcon B, Clavaguera F & Tolnay M Prion-like mechanisms in the pathogenesis of tauopathies and synucleinopathies. Curr. Neurol. Neurosci. Rep 14, 495 (2014). [DOI] [PubMed] [Google Scholar]
- 44.Hayden EY & Teplow DB Amyloid β-protein oligomers and Alzheimer’s disease. Alzheimers Res. Ther 5, 60 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eisenberg D & Jucker M The amyloid state of proteins in human diseases. Cell 148, 1188–1203 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aguzzi A, Heikenwalder M & Polymenidou M Insights into prion strains and neurotoxicity. Nat. Rev. Mol. Cell Biol 8, 552–561 (2007). [DOI] [PubMed] [Google Scholar]
- 47.Morales R Prion strains in mammals: Different conformations leading to disease. PLoS Pathog. 13, e1006323 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Poggiolini I, Saverioni D & Parchi P Prion protein misfolding, strains, and neurotoxicity: an update from studies on Mammalian prions. Int. J. Cell Biol 2013, 910314 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Castilla J et al. Cell-free propagation of prion strains. EMBO J. 27, 2557–2566 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Diaz-Espinoza R & Soto C High-resolution structure of infectious prion protein: the final frontier. Nat. Struct. Mol. Biol 19, 370–377 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heilbronner G et al. Seeded strain-like transmission of β-amyloid morphotypes in APP transgenic mice. EMBO Rep. 14, 1017–1022 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petkova AT et al. Self-propagating, molecular-level polymorphism in Alzheimer’s beta-amyloid fibrils. Science 307, 262–265 (2005).This study reports the generation of different polymorphic variants of amyloid-β aggregates and their detailed structural and biochemical characterizations.
- 53.Qiang W, Yau WM, Lu JX, Collinge J & Tycko R Structural variation in amyloid-β fibrils from Alzheimer’s disease clinical subtypes. Nature 541, 217–221 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watts JC et al. Serial propagation of distinct strains of Ap prions from Alzheimer’s disease patients. Proc. Natl. Acad. Sci. USA 111, 10323–10328 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaufman SK et al. Tau prion strains dictate patterns of cell pathology, progression rate, and regional vulnerability in vivo. Neuron 92, 796–812 (2016).This article describes the isolation and characterization of 18 tau strains in cells. Inoculation of transgenic mice with these strains causes strain-specific intracellular pathology in distinct cell types and brain regions.
- 56.Sanders DW et al. Distinct tau prion strains propagate in cells and mice and define different tauopathies. Neuron 82, 1271–1288 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Narasimhan S et al. Pathological tau strains from human brains recapitulate the diversity of tauopathies in nontransgenic mouse brain. J. Neurosci 37, 11406–11423 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo JL et al. Distinct α-synuclein strains differentially promote tau inclusions in neurons. Cell 154, 103–117 (2013).This study shows that different conformational strains differ in their cross-seeding activity.
- 59.Prusiner SB et al. Evidence for α-synuclein prions causing multiple system atrophy in humans with parkinsonism. Proc. Natl Acad. Sci. USA 112, E5308–E5317 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peelaerts W et al. α-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature 522, 340–344 (2015). [DOI] [PubMed] [Google Scholar]
- 61.Bousset L et al. Structural and functional characterization of two alpha-synuclein strains. Nat. Commun 4, 2575 (2013).This article reports a complete biochemical, biological, and structural characterization of α-Syn strains generated in vitro.
- 62.Melki R Role of different alpha-synuclein strains in synucleinopathies, similarities with other neurodegenerative diseases. J. Parkinsons Dis 5, 217–227 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Williams DR Tauopathies: classification and clinical update on neurodegenerative diseases associated with microtubule-associated protein tau. Intern. Med. J 36, 652–660 (2006). [DOI] [PubMed] [Google Scholar]
- 64.Goedert M, Jakes R & Spillantini MG The synucleinopathies: twenty years on. J. Parkinsons Dis 7(s1), S53–S71 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Melki R How the shapes of seeds can influence pathology. Neurobiol. Dis 109(Pt B), 201–208 (2018). [DOI] [PubMed] [Google Scholar]
- 66.Fitzpatrick AWP et al. Cryo-EM structures of tau filaments from Alzheimer’s disease. Nature 547, 185–190 (2017).This study describes atomic models for tau aggregates organized in different strains.
- 67.Condello C & Stoehr J Ap propagation and strains: Implications for the phenotypic diversity in Alzheimer’s disease. Neurobiol. Dis 109(Pt B), 191–200 (2018). [DOI] [PubMed] [Google Scholar]
- 68.Fandrich M, Meinhardt J & Grigorieff N Structural polymorphism of Alzheimer Abeta and other amyloid fibrils. Prion 3, 89–93 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goldsbury C, Frey P, Olivieri V, Aebi U & Muller SA Multiple assembly pathways underlie amyloid-beta fibril polymorphisms. J. Mol. Biol 352, 282–298 (2005). [DOI] [PubMed] [Google Scholar]
- 70.Elkins MR et al. Structural polymorphism of Alzheimer’s β-amyloid fibrils as controlled by an E22 switch: a solid-state NMR study. J. Am. Chem. Soc 138, 9840–9852 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lubomski M, Rushworth RL, Lee W, Bertram K & Williams DR A cross-sectional study of clinical management, and provision of health services and their utilisation, by patients with Parkinson’s disease in urban and regional Victoria. J. Clin. Neurosci 20, 102–106 (2013). [DOI] [PubMed] [Google Scholar]
- 72.O’Nuallain B, Williams AD, Westermark P & Wetzel R Seeding specificity in amyloid growth induced by heterologous fibrils. J. Biol. Chem 279, 17490–17499 (2004). [DOI] [PubMed] [Google Scholar]
- 73.Yan J et al. Cross-seeding and cross-competition in mouse apolipoprotein A-II amyloid fibrils and protein A amyloid fibrils. Am. J. Pathol 171, 172–180 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Krebs MR, Morozova-Roche LA, Daniel K, Robinson CV & Dobson CM Observation of sequence specificity in the seeding of protein amyloid fibrils. Protein Sci 13, 1933–1938 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ono K, Takahashi R, Ikeda T & Yamada M Cross-seeding effects of amyloid β-protein and α-synuclein. J. Neurochem 122, 883–890 (2012). [DOI] [PubMed] [Google Scholar]
- 76.Hu R, Zhang M, Chen H, Jiang B & Zheng J Cross-seeding interaction between β-amyloid and human islet amyloid polypeptide. ACS Chem. Neurosci 6, 1759–1768 (2015). [DOI] [PubMed] [Google Scholar]
- 77.Moreno-Gonzalez I et al. Molecular interaction between type 2 diabetes and Alzheimer’s disease through cross-seeding of protein misfolding. Mol. Psychiatry 22, 1327–1334 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morales R et al. Molecular cross talk between misfolded proteins in animal models of Alzheimer’s and prion diseases. J. Neurosci 30, 4528–4535 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Giasson BI, Lee VM & Trojanowski JQ Interactions of amyloidogenic proteins. Neuromolecular Med. 4, 49–58 (2003). [DOI] [PubMed] [Google Scholar]
- 80.Brown DF et al. Neuropathologic evidence that the Lewy body variant of Alzheimer disease represents coexistence of Alzheimer disease and idiopathic Parkinson disease. J. Neuropathol. Exp. Neurol 57, 39–46 (1998). [DOI] [PubMed] [Google Scholar]
- 81.Brown P et al. Coexistence of Creutzfeldt-Jakob disease and Alzheimer’s disease in the same patient. Neurology 40, 226–228 (1990). [DOI] [PubMed] [Google Scholar]
- 82.Spires-Jones TL, Attems J & Thal DR Interactions of pathological proteins in neurodegenerative diseases. Acta Neuropathol. 134, 187–205 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Clinton LK, Blurton-Jones M, Myczek K, Trojanowski JQ & LaFerla FM Synergistic Interactions between Abeta, tau, and alpha-synuclein: acceleration of neuropathology and cognitive decline. J. Neurosci 30, 7281–7289 (2010).This study shows that transgenic mice expressing four different mutant genes develop both Lewy bodies and AD pathologies, exhibiting accelerated cognitive decline associated with a dramatic enhancement of Aβ, tau, and α-Syn deposition.
- 84.Morales R, Moreno-Gonzalez I & Soto C Cross-seeding of misfolded proteins: implications for etiology and pathogenesis of protein misfolding diseases. PLoS Pathog. 9, e1003537 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dickson DW Neuropathology of Alzheimer’s disease and other dementias. Clin. Geriatr. Med 17, 209–228 (2001). [DOI] [PubMed] [Google Scholar]
- 86.Guo JP, Arai T, Miklossy J & McGeer PL Abeta and tau form soluble complexes that may promote self aggregation of both into the insoluble forms observed in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 103, 1953–1958 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lewis J et al. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science 293, 1487–1491 (2001). [DOI] [PubMed] [Google Scholar]
- 88.Götz J, Chen F, van Dorpe J & Nitsch RM Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science 293, 1491–1495 (2001).This study shows that intracerebral inoculation of Aβ aggregates in tau transgenic mice enhances the formation of neurofibrillary tangles, suggesting that amyloid induces tau pathology.
- 89.Pooler AM et al. Amyloid accelerates tau propagation and toxicity in a model of early Alzheimer’s disease. Acta Neuropathol. Commun 3, 14 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.He Z et al. Amyloid-β plaques enhance Alzheimer’s brain tau-seeded pathologies by facilitating neuritic plaque tau aggregation. Nat. Med 24, 29–38 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Uchikado H, Lin WL, DeLucia MW & Dickson DW Alzheimer disease with amygdala Lewy bodies: a distinct form of alpha-synucleinopathy. J. Neuropathol. Exp. Neurol 65, 685–697 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Josephs KA et al. Updated TDP-43 in Alzheimer’s disease staging scheme. Acta Neuropathol. 131, 571–585 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pigott K et al. Longitudinal study of normal cognition in Parkinson disease. Neurology 85, 1276–1282 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bosboom JL, Stoffers D & Wolters E Ch. Cognitive dysfunction and dementia in Parkinson’s disease. J. Neural Transm. (Vienna) 111, 1303–1315 (2004). [DOI] [PubMed] [Google Scholar]
- 95.Irwin DJ, Lee VM & Trojanowski JQ Parkinson’s disease dementia: convergence of α-synuclein, tau and amyloid-β pathologies. Nat. Rev. Neurosci 14, 626–636 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Buongiorno M, Compta Y & Marti MJ Amyloid-β and tau biomarkers in Parkinson’s disease-dementia. J. Neurol. Sci 310, 25–30 (2011). [DOI] [PubMed] [Google Scholar]
- 97.Iseki E Dementia with Lewy bodies: reclassification of pathological subtypes and boundary with Parkinson’s disease or Alzheimer’s disease. Neuropathology 24, 72–78 (2004). [DOI] [PubMed] [Google Scholar]
- 98.McKeith IG et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology 89, 88–100 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Saxena S & Caroni P Selective neuronal vulnerability in neurodegenerative diseases: from stressor thresholds to degeneration. Neuron 71, 35–48 (2011). [DOI] [PubMed] [Google Scholar]
- 100.Biessels GJ, Staekenborg S, Brunner E, Brayne C & Scheltens P Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 5, 64–74 (2006). [DOI] [PubMed] [Google Scholar]
- 101.Sims-Robinson C, Kim B, Rosko A & Feldman EL How does diabetes accelerate Alzheimer disease pathology? Nat. Rev. Neurol 6, 551–559 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Janson J et al. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes 53, 474–481 (2004). [DOI] [PubMed] [Google Scholar]
- 103.Oskarsson ME et al. In vivo seeding and cross-seeding of localized amyloidosis: a molecular link between type 2 diabetes and Alzheimer disease. Am. J. Pathol 185, 834–846 (2015).This article describes the in vivo cross-seeding between Aβ and islet amyloid polypeptide, providing a possible molecular explanation for the link between T2D and AD.
- 104.Friedland RP & Chapman MR The role of microbial amyloid in neurodegeneration. PLoS Pathog. 13, e1006654 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pham CL, Kwan AH & Sunde M Functional amyloid: widespread in Nature, diverse in purpose. Essays Biochem. 56, 207–219 (2014). [DOI] [PubMed] [Google Scholar]
- 106.Hammer ND, Wang X, McGuffie BA & Chapman MR Amyloids: friend or foe? J. Alzheimers Dis 13, 407–419 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fowler DM, Koulov AV, Balch WE & Kelly JW Functional amyloid-from bacteria to humans. Trends Biochem. Sci 32, 217–224 (2007). [DOI] [PubMed] [Google Scholar]
- 108.Chen SG et al. Exposure to the functional bacterial amyloid protein Curli enhances alpha-synuclein aggregation in aged Fischer 344 rats and Caenorhabditis elegans. Sci. Rep 6, 34477 (2016).This article describes the possibility that a functional bacterial amyloid may induce the aggregation of α-Syn in vivo.
- 109.Fernández-Borges N et al. Infectivity versus seeding in neurodegenerative diseases sharing a prion-like mechanism. Int. J. Cell Biol 2013, 583498 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Garske T & Ghani AC Uncertainty in the tail of the variant Creutzfeldt-Jakob disease epidemic in the UK. PLoS One 5, e15626 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Valera E, Spencer B & Masliah E Immunotherapeutic approaches targeting amyloid-β, α-synuclein, and tau for the treatment of neurodegenerative disorders. Neurotherapeutics 13, 179–189 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mohamed NV, Herrou T, Plouffe V, Piperno N & Leclerc N Spreading of tau pathology in Alzheimer’s disease by cell-to-cell transmission. Eur. J. Neurosci 37, 1939–1948 (2013). [DOI] [PubMed] [Google Scholar]
- 113.Costanzo M & Zurzolo C The cell biology of prion-like spread of protein aggregates: mechanisms and implication in neurodegeneration. Biochem. J 452, 1–17 (2013). [DOI] [PubMed] [Google Scholar]
- 114.Danzer KM et al. Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol. Neurodegener 7, 42 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wegrzyn RD, Bapat K, Newnam GP, Zink AD & Chernoff YO Mechanism of prion loss after Hsp104 inactivation in yeast. Mol. Cell. Biol 21, 4656–4669 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Diaz-Espinoza R et al. Treatment with a non-toxic, self-replicating anti-prion delays or prevents prion disease in vivo. Mol. Psychiatry 23, 777–788 (2018).This article reports the use of the prion principle to generate a self-replication protein therapy for prion diseases.
- 117.Li J, Mahal SP, Demczyk CA & Weissmann C Mutability of prions. EMBO Rep. 12, 1243–1250 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Telling GC Nucleic acid-free mutation of prion strains. Prion 4, 252–255 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li J, Browning S, Mahal SP, Oelschlegel AM & Weissmann C Darwinian evolution of prions in cell culture. Science 327, 869–872 (2010).This study shows that prion strains can mutate and selectively adapt to grow under different conditions and likely constitute an ensemble of substrains.
- 120.Oelschlegel AM & Weissmann C Acquisition of drug resistance and dependence by prions. PLoS Pathog. 9, e1003158 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ghaemmaghami S et al. Continuous quinacrine treatment results in the formation of drug-resistant prions. PLoS Pathog. 5, e1000673 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Anderson RM, Hadjichrysanthou C, Evans S & Wong MM Why do so many clinical trials of therapies for Alzheimer’s disease fail? Lancet 390, 2327–2329 (2017). [DOI] [PubMed] [Google Scholar]
- 123.Gomez-Rio M, Caballero MM, Gorriz Saez JM & Minguez-Castellanos A Diagnosis of neurodegenerative diseases: the clinical approach. Curr. Alzheimer Res 13, 469–474 (2016). [DOI] [PubMed] [Google Scholar]
- 124.Lewczuk P et al. Cerebrospinal fluid and blood biomarkers for neurodegenerative dementias: an update of the consensus of the Task Force on Biological Markers in Psychiatry of the World Federation of Societies of Biological Psychiatry. World J. Biol. Psychiatry 19, 244–328 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schuster J & Funke SA Methods for the specific detection and quantitation of amyloid-β oligomers in cerebrospinal fluid. J. Alzheimers Dis 53, 53–67 (2016). [DOI] [PubMed] [Google Scholar]
- 126.Bateman RJ et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N. Engl. J. Med 367, 795–804 (2012).This article describes studies in human AD patients to model the sequence of pathological changes over decades in cerebrospinal fluid biochemical markers, brain amyloid deposition, and brain metabolism, as well as progressive cognitive impairment.
- 127.Wegmann S, Nicholls S, Takeda S, Fan Z & Hyman BT Formation, release, and internalization of stable tau oligomers in cells. J. Neurochem 139, 1163–1174 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chai YJ et al. The secreted oligomeric form of α-synuclein affects multiple steps of membrane trafficking. FEBS Lett. 587, 452–459 (2013). [DOI] [PubMed] [Google Scholar]
- 129.Murakami K et al. Monoclonal antibody with conformational specificity for a toxic conformer of amyloid β42 and its application toward the Alzheimer’s disease diagnosis. Sci. Rep 6, 29038 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kamali-Moghaddam M et al. Sensitive detection of Aβ protofibrils by proximity ligation-relevance for Alzheimer’s disease. BMC Neurosci. 11, 124 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hölttä M et al. Evaluating amyloid-β oligomers in cerebrospinal fluid as a biomarker for Alzheimer’s disease. PLoS One 8, e66381 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pitschke M, Prior R, Haupt M & Riesner D Detection of single amyloid beta-protein aggregates in the cerebrospinal fluid of Alzheimer’s patients by fluorescence correlation spectroscopy. Nat. Med 4, 832–834 (1998). [DOI] [PubMed] [Google Scholar]
- 133.Santos AN et al. Detection of amyloid-beta oligomers in human cerebrospinal fluid by flow cytometry and fluorescence resonance energy transfer. J. Alzheimers Dis 11, 117–125 (2007). [DOI] [PubMed] [Google Scholar]
- 134.Funke SA, Wang L, Birkmann E & Willbold D Single-particle detection system for Abeta aggregates: adaptation of surface-fluorescence intensity distribution analysis to laser scanning microscopy. Rejuvenation Res. 13, 206–209 (2010). [DOI] [PubMed] [Google Scholar]
- 135.Haes AJ, Chang L, Klein WL & Van Duyne RP Detection of a biomarker for Alzheimer’s disease from synthetic and clinical samples using a nanoscale optical biosensor. J. Am. Chem. Soc 127, 2264–2271 (2005). [DOI] [PubMed] [Google Scholar]
- 136.Sierks MR et al. CSF levels of oligomeric alpha-synuclein and beta-amyloid as biomarkers for neurodegenerative disease. Integr. Biol. (Camb.) 3, 1188–1196 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Saborio GP, Permanne B & Soto C Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature 411, 810–813 (2001).This article reports the use of the prion principle to develop a PCR-like methodology for highly sensitive detection of prions that can also be used for other misfolded aggregates.
- 138.Soto C, Saborio GP & Anderes L Cyclic amplification of protein misfolding: application to prion-related disorders and beyond. Trends Neurosci. 25, 390–394 (2002). [DOI] [PubMed] [Google Scholar]
- 139.Orrù CD, Wilham JM, Vascellari S, Hughson AG & Caughey B New generation QuIC assays for prion seeding activity. Prion 6, 147–152 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Atarashi R et al. Simplified ultrasensitive prion detection by recombinant PrP conversion with shaking. Nat. Methods 5, 211–212 (2008). [DOI] [PubMed] [Google Scholar]
- 141.Saá P, Castilla J & Soto C Ultra-efficient replication of infectious prions by automated protein misfolding cyclic amplification. J. Biol. Chem 281, 35245–35252 (2006). [DOI] [PubMed] [Google Scholar]
- 142.Moda F et al. Prions in the urine of patients with variant Creutzfeldt-Jakob disease. N. Engl. J. Med 371, 530–539 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Concha-Marambio L et al. Detection of prions in blood from patients with variant Creutzfeldt-Jakob disease. Sci. Transl. Med 10.1126/scitranslmed.aaf6188 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bougard D et al. Detection of prions in the plasma of presymptomatic and symptomatic patients with variant Creutzfeldt-Jakob disease. Sci. Transl. Med 8, 370ra182 (2016). [DOI] [PubMed] [Google Scholar]
- 145.Salvadores N, Shahnawaz M, Scarpini E, Tagliavini F & Soto C Detection of misfolded Aβ oligomers for sensitive biochemical diagnosis of Alzheimer’s disease. Cell Rep. 7, 261–268 (2014). [DOI] [PubMed] [Google Scholar]
- 146.Saijo E et al. Ultrasensitive and selective detection of 3-repeat tau seeding activity in Pick disease brain and cerebrospinal fluid. Acta Neuropathol. 133, 751–765 (2017). [DOI] [PubMed] [Google Scholar]
- 147.Shahnawaz M et al. Development of a biochemical diagnosis of Parkinson disease by detection of α-synuclein misfolded aggregates in cerebrospinal fluid. JAMA Neurol. 74, 163–172 (2017). [DOI] [PubMed] [Google Scholar]
- 148.Fairfoul G et al. Alpha-synuclein RT-QuIC in the CSF of patients with alpha-synucleinopathies. Ann. Clin. Transl. Neurol 3, 812–818 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]