Abstract
The National Cancer Institute and the National Institute for Diabetes and Digestive and Kidney Diseases initiated the Consortium for the study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer (CPDPC) in 2015 (the CPDPC’s origin, structure, governance and research objectives are described in another article in this journal). One of the key objectives of CPDPC is to assemble a cohort of 10,000 subjects ≥50 years with new-onset diabetes, called the NOD cohort. Using a DEF (Define, Enrich and Find) early detection approach, the aims of the NOD study are to: (a) estimate the 3-year probability of pancreatic ductal adenocarcinoma (PDAC) in NOD (Define); (b) establish a bio-bank of clinically annotated bio-specimens, from pre-symptomatic PDAC and control new-onset type 2 diabetes mellitus subjects; (c) conduct Phase 3 validation studies of promising biomarkers for identification of incident PDAC in NOD patients (Enrich); and (d) provide a platform for development of a future interventional screening protocol for early detection of PDAC in patients with NOD that incorporates imaging studies and/or clinical algorithms (Find). It is expected that 85-100 incidences of PDAC will be diagnosed during the study period in this cohort of 10,000 patients.
Keywords: screening, sporadic pancreatic cancer, diabetes, high-risk group, biomarker
BACKGROUND AND SIGNIFICANCE
Among the most compelling needs to impact the dismal mortality associated with PDAC cases today is a rational, evidence-based strategy to detect the cancer at an early stage when it is still resectable, thereby permitting therapeutic options that result in long term survival. Greater than 90% of PDAC is sporadic (i.e. not arising in high risk cohorts of patients with an underlying germline mutation), and 85% of such sporadic PDAC patients present at an advanced stage, rapidly progressing to death.1 Some of the principal challenges to developing an early detection program for sporadic PDAC are the lack of an ascertainable high-risk group for sporadic PDAC; limited availability of high quality bio-specimens from pre-symptomatic cancer patients for study of biomarkers; a dearth of sensitive and specific biomarkers of early PDAC; and the inability of conventional imaging (and absence of novel imaging techniques) to identify early PDAC.1
A high-risk group for sporadic PDAC has been identified as subjects ≥50 years who have newly develop diabetes mellitus (NOD).2 Chari and colleagues first described in a retrospective population-based study of 2122 NOD subjects, 18 (0.85%) were diagnosed with PDAC within 3 years of meeting criteria for NOD,3 resulting in a 6-8-fold higher risk for PDAC than general population.3, 4 Small prospective screening studies for PDAC in NOD have also shown a 3-10% prevalence of PDAC in NOD.5-7 Conversely, ~25% of PDAC patients develop NOD between 6 and 24 months prior to cancer diagnosis.8 A recent study showed hyperglycemia precedes PDAC diagnosis by ~36 months, providing a possible window of opportunity for the early detection of pancreatic cancer in new-onset diabetes patients.9 The National Cancer Institute (NCI) has acknowledged that studying the relationship between DM and PDAC is one of the highest research priorities in PDAC research.10 To respond to the challenges posed by early detection of sporadic PDAC, we developed the following research aims.
Specific Aims
We will prospectively develop a cohort of 10,000 subjects’ age ≥50 and ≤85 years with NOD to:
Estimate the probability of PDAC in the NOD Cohort,
Establish a biobank of clinically annotated bio-specimens including a reference set of bio-specimens from pre-symptomatic PDAC and controls new-onset type 2 DM,
Conduct Phase 3 validation studies11 of promising biomarkers for identification of occult PDAC in NOD patients and,
Provide a platform for development of a future interventional screening protocol for early detection of PDAC in NOD patients that also incorporates imaging studies and clinical algorithms.
One key to accurately estimating the risk of harboring an occult PDAC in patients with NOD is to identify DM within a short time frame from first meeting biochemical criteria (defined as <90 days). In a pilot study at Mayo Clinic Rochester, investigators have been able to successfully identify incident DM subjects using surveillance of electronic databases and focused chart review (Examination of the Pancreas in New-onset Diabetes [EXPAND] trial; NCT0200133).
Rationale for NOD as a High-risk Group
The primary aim of NOD study is to estimate the incidence of PDAC in subjects with NOD. Based on previously published data, it is estimated that in the NOD cohort as defined, approximately 85 cases of incident PDAC will be diagnosed during the three years of study follow-up after meeting NOD study criteria, assuming the 1-year, 2-year, and 3-year cumulative incidence rate of PDAC is 0.63%, 0.73%, and 0.85%, respectively.12, 13 If diabetes in the study subjects is not due to a manifestation of occult PDAC, then the incidence rate of PDAC should be similar to the risk in the general population which is about 0.04% per patient year. Conservatively, we use 0.1%, 0.2%, and 0.3% for 1-year, 2-year, and 3-year cumulative risk, respectively, as null hypotheses. We will reject the null hypothesis of no association between NOD and PDAC if, one of the three observed proportions is significantly higher than the null hypothesis value, using an exact binomial test at a 2-sided nominal level 0.05/3. The power will be >90% to reject the null hypothesis if the true 1-year, 2-year, and 3-year cumulative incidence rate is at least 0.43%, 0.64%, and 0.85%, respectively, i.e. 50%, 75%, and 100% of underlying PDACs will be clinically diagnosed during 1-year, 2-year, and 3-year since baseline.
STUDY DESIGN SUMMARY
Assemble a Cohort of Subjects With NOD
A prospective cohort of 10,000 subjects will be enrolled with each subject participating for up to 3 years from the date they meet criteria for NOD (Table 1). A collaboration of CPDPC clinical centers, described in the CPDPC introductory paper in this journal and NCI Community Oncology Research Program (NCORP) sites will enroll subjects (Table 2).
TABLE 1.
Study Definitions and Criteria Used in the NOD Study
| Terms | Study Definitions |
|---|---|
| Glycemic Parameters | Only the following glycemic parameters measured in outpatient setting are to be assessed: • Fasting blood glucose (FBG) • Random (non-fasting) glucose • Glycosylated hemoglobin (HbA1c) • 2h blood glucose following 75 gm glucose load (oral glucose tolerance test [OGTT]) The following glucose measurements are not included: urine glucose, other body fluid glucose, blood glucose measured in emergency department, urgent care, or inpatient setting |
| Parameters of Diabetes Mellitus (PDM) | • Fasting blood glucose (≥126 mg/dl) • Random blood glucose (≥200 mg/dl) • Glycosylated hemoglobin (HbA1c) (≥6.5%) • 2h post oral glucose load (PG) ≥200 mg/dl during OGTT |
| Diagnosis of Diabetes (DxDM) | Any (1) of the following: • Any (2) PDMs present on consecutive or simultaneous testing • On anti-diabetic medications preceded by at least (1) PDM* |
| Date of meeting criteria for DM (DtDM) | Whichever of the following is earliest: • Date of first of (2) consecutive or simultaneous measured PDMs • Date of first PDM if patient is started on anti-DM medications following this measurement |
| New-onset DM | Meets all the following criteria: • Age ≥50 and ≤85 years • Date of meeting criteria for DM ≤90 days prior to enrollment • Have had glycemic parameter(s) measured in the 3-18 months prior to screening (demonstrating absence of PDM) |
| PDAC Definition |
• Definite: Requires biopsy proof of pancreatic adenocarcinoma on autopsy, biopsy, or surgical resection of pancreatic lesion or adenocarcinoma on biopsy of a metastatic lesion with imaging evidence of pancreatic mass • Probable: presence of one of the following: 1. Obstructive jaundice and low-density pancreatic head mass, OR 2. Low density pancreatic mass and elevated serum CA 19-9 level • Possible: Jaundice, anorexia, and/or weight loss associated with an indeterminate pancreatic lesion or normal pancreatic imaging |
If patient has (1) PDM and has started anti-diabetic medication within 3 months; repeat value may or may not meet PDM, patient is still eligible.
TABLE 2.
Study Sites Participating in NOD Study
| Site/Group | Site Name |
|---|---|
| Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer (CPDPC) Clinical Centers | Baylor College of Medicine Cedars-Sinai Medical Center Mayo Clinic, Minnesota The Ohio State University Wexner Medical Center University of Florida University of Pittsburgh Medical Center Indiana University, Indianapolis Stanford University Medical Center |
| NCI Community Oncology Research Program (NCORP) | St. Joseph Mercy Health System Kaiser Permanente Northern California Multicare Health System |
| Other | Kaiser Permanente Southern California |
Develop a Biobank Containing Longitudinal Samples From Subjects With NOD
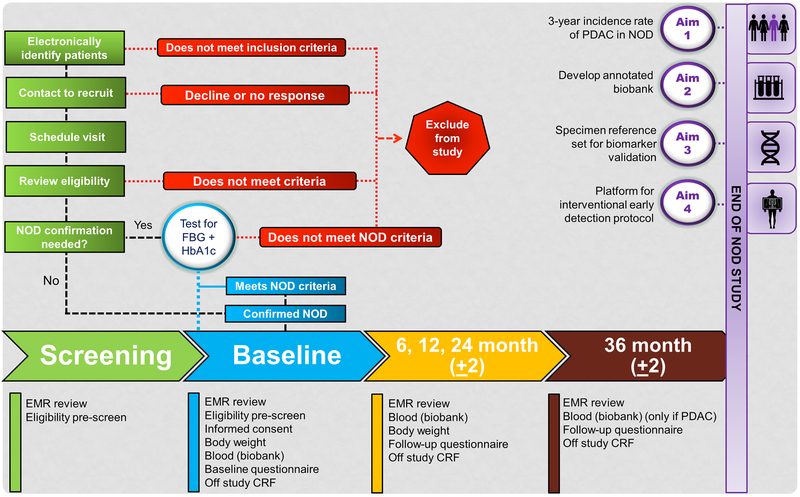
All enrolled subjects will be passively followed for collection of clinical data and bio-specimen samples at baseline (i.e., at the time of recruitment), and subsequently at 6, 12, and 24 months (Figure 1). Blood (processed into aliquots of plasma, serum, buffy coat) stored will be stored for future studies.
FIGURE 1.
Overview of activities of the new-onset diabetes study.
Determine the Incidence of PDAC in a Prospective Cohort With NOD
The 1-year, 2-year, and 3-year incidence rates of PDAC during passive surveillance will be calculated using health records patient interviews.
Establish a Specimen Reference Set for High-risk Individuals
Future promising biomarkers for early detection of PDAC will be evaluated using this specimen reference set to calculate testing characteristics (sensitivity, specificity, positive predictive value [PPV], and negative predictive value [NPV]) for predicting 1-year, 2-year, and 3-year PDAC risk. These tests will then be useful clinically to stratify subsets of NOD that would benefit from early diagnostic work-up.
Develop a Platform for the Development of a Future Interventional Protocol for Early Detection of PDAC
Following development of predictive models and/or biomarkers of PDAC in NOD we will subsequently design a protocol to prospectively evaluate different screening protocols for PDAC in NOD patients.
DEFINITIONS AND ELIGIBILITY CRITERIA
Glycemic Definition of NOD
Parameters of DM (PDM) will be used to define the NOD state as described in Table 1. A strict biochemical definition of DM using American Diabetes Association (ADA) guidelines is being used to avoid delays that commonly occur in care teams formally making the diagnosis of diabetes.
Inclusion and Exclusion Criteria
An overview of inclusion and exclusion criteria is outlined in Table 3. The inclusion criteria will be used for invitation to the study. The study is designed to include women and minorities, but not to measure differences among them. No exclusion to this study will be based on race. Minorities will actively be recruited to participate.
TABLE 3.
Overview of Eligibility Criteria for New-Onset Diabetes (NOD) Study
| Category | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Age Consent | Willing to provide and sign informed consent form ≥50 and ≤85 years | |
| DM-related | • Must have at least (1) PDM in past 90 days measured in outpatient setting: • FBG≥126mg/dl;HbA1c≥6.5%; RBG≥200mg/dl; 2h post-glucose≥200mg/dl(OGTT) • Must have had glycemic parameter measured in 3-18 months prior to screening without meeting DM criteria • Must not be on anti-DM medications prior to meeting study entry criteria |
• patient met criteria for DM>90 days prior to enrollment, carried a physician DM diagnosis or used anti-DM medications in 3-18 months prior to enrollment • Has had no glycemic parameter measured within 3-18 months prior to enrollment |
| Bio-specimen/Questionnaire | Patient must be willing to provide blood sample (fasted) at baseline and complete a detailed questionnaire For subsequent serial bio-sampling patient must meet criteria for NOD (Table 1) and willing to provide blood samples/complete questionnaires (Figure 1) |
|
| Personal history of cancer | Should not have a prior history of pancreatic cancer No history of other active cancers (excluding basal cell carcinoma or carcinoma in situ of cervix) |
Must not be on active cancer treatment, carry a current cancer diagnosis, and/or be investigated for suspicion of past cancer recurrence Any past history of pancreatic cancer |
| Steroid use or acute illness blood draw | Current chronic or acute oral steroid Recent (<1 week) intra-articular steroid injections (allowed: nasal, topical, oral budesonide) Blood sugar measured in urgent care, emergency visit, or as inpatient |
|
| Chronic severe illness | In physician’s judgement patients co-morbidities do not limit patient’s participation in study interventions | Patients must not have any significant medical illness that in the investigator’s opinion cannot be adequately controlled with appropriate therapy or would compromise the patient’s ability to tolerate study interventions. |
PDAC Definition
All PDAC cases will be defined as definite, probable and possible (Table 1). Only cases classified as definite or probable will be used for final analyses and biomarker discovery efforts.
SUBJECT ENROLLMENT PROCEDURES
The coordination and compliance activities of the NOD cohort will be managed by the NCI’s Early Detection and Research Network (EDRN) funded Data Management Coordination Center (DMCC), based in the Fred Hutchinson Cancer Research Center (Seattle, Wash). Subjects will provide written consent and HIPAA authorization prior to initiating study activities. All participating institutions will have access to a centralized data management using Validation Study Information Management System (VSIMS),14 a web-based system designed by EDRN to automate the flow to maintain and quality control all data for: consent and HIPAA waiver, systematic review to confirm eligibility and other regulatory documents.
STUDY VISITS AND SPECIMEN COLLECTION
An overview of the activities is summarized below (Fig. 1):
Pre-screening/screening
Potential participants will be identified through searches of electronic medical records (EMRs), and/or through physician or self-referral. Once identified, the EMR will be reviewed to confirm NOD status and the patient will be invited to participate. Patients agreeing to participate will check-in to the approaching study site within 21 days of initial contact.
Baseline
Fasting (>8 hours) blood samples drawn for bio-banking and body weight will be obtained. For patients needing confirmation of NOD criteria, additional blood will be drawn for measuring fasting blood glucose (FBG) and glycosylated hemoglobin (HbA1c). If a patient meets NOD criteria on confirmatory testing, the participant and their primary care physician will be notified of the patient’s laboratory values and will be contacted to schedule the 6-month follow-up visit.
Follow-up
Patients meeting NOD criteria at baseline will be asked to return for follow-up visits at 6, 12, and 24 months. Follow-up questionnaires, blood (fasting), and body weights will be collected at each visit. If a subject misses a follow-up visit, the EMR will be reviewed to determine if they have developed interval PDAC.
End point follow-up (36 ± 2 months)
At 36-month follow-up, the subject will be contacted to determine if they have developed PDAC or other cancers. All subjects with PDAC will be invited to return to the study site for a final blood draw (fasting) for biobanking. For subjects indicating no development of PDAC, their follow-up will be closed. The EMR will be reviewed for any subjects who are lost to follow-up for development of PDAC or other cancers.
Specimen Collection
At the baseline time point, 40 mL of blood will be drawn for patients with confirmed NOD criteria while those needing NOD confirmation, and additional 10mL of whole blood will be collected for measuring FBG and HbA1c (Fig. 1). For all patients, 40 mL of blood will be drawn at each subsequent follow-up visit. All patients with PDAC will be invited to return to the study site for a final blood draw. All blood specimens will be labeled and shipped to a central biorepository at NCI Frederick for long-term storage and use.
Criteria for Subject Removal From Study
A subject will be removed from study if: they develop PDAC or any other type of cancer (except basal cell carcinoma or in situ carcinoma of the cervix); completion of 3-year follow-up from the date they first met criteria for DM or the study ends; the patient withdraws from the study at any time for any reason; and medical or psychiatric illness which in the investigators judgement renders the subject incapable for further participation.
BIOMARKERS FOR EARLY DETECTION OF PDAC
Currently, there are no validated biomarkers for early PDAC due to lack of samples from pre-symptomatic PDAC patients. Therefore, a key aim of NOD study is to establish a biobank of clinically annotated bio-specimens in pre-symptomatic PDAC. To evaluate a future promising biomarker (or biomarker panel), we assume that the cutoff for the biomarker and the combination rule of the panel under evaluation has been pre-fixed in preliminary studies, so the NOD specimen reference set is used as a validation test set for its potential clinical utility of “rule-in” high risk patients for PDAC diagnostic workup (e.g. CT, MRI). Since the NOD population has high risk, PDAC is very lethal, and the workup is only moderately harmful in invasiveness and cost, the primary performance criteria should focus on high sensitivity for predicting 3-year risk of PDAC after baseline. The NOD study is designed to have the capacity to validate the sensitivity of a new biomarker in predicting PDAC within 1-year, 2-year, or 3-year after blood collection, since the lead time varies. Estimating development of 85 incident PDAC cases in this study, the half- length of 2-sided 95% confidence interval for sensitivity ranges from 6.4% - 9.7% if the true sensitivity ranges from 90% - 70%.
The study sample size is primarily powered to study and validate a future biomarker as having a higher sensitivity compared to carbohydrate antigen (CA) 19-9, which is currently leading PDAC biomarker. CA 19-9 has not been evaluated in the NOD population for its sensitivity or specificity, but it is known that CA 19-9 has low sensitivity for PDAC at asymptomatic stage.15 We will assume 50% sensitivity for CA 19-9 on PDAC in NOD population. With 85 PDACs, the study will have at least 86% power if the sensitivity for the new test is at least 70%, assuming the new test and CA 19-9 is independent. However, they are likely to be positively correlated making the true study power to be greater than 90%. The evaluation of specificity has ample power due to large number of controls. The half-length of 95% confidence interval for specificity will be <1% if true specificity is at least 50%.
With the 3-year cumulative incidence of 0.85%, the anticipated sensitivity >70% for a new test will have PPV of at least 2%, 2.9%, and 5.7%, if the specificity is at least 70%, 80%, and 90%, respectively, as compared to the background incidence rate 0.85%. This puts the yield of cancer in the test positive group compatible to that for colorectal cancer screening with fecal occult-blood tests for people aged >50 (PPV, 2.5-5.0%; sensitivity, 69-79%),16 or that for lung cancer LDCT screening for heavy smokers (PPV, 3.6%).17 Therefore, this NOD cohort is powered to validate a new test with clinical useful performance as a second sieve for PDAC early detection following the identification of NOD as the first sieve.
Developing an effective strategy for early detection of finding PDAC is essential to result in improvements in survival. Diagnosis of pre-symptomatic PDAC is anticipated to result in a cancer stage shift that would allow patients access to potential cures or delayed disease progression. The NOD study will provide key results that will advance the field of early detection of PDAC through accurately describing the incidence of PDAC in this high-risk population, defining subsets of NOD at highest risk, and providing a platform for validation of promising, early, diagnostic biomarkers.
Acknowledgments
Grant Support:
Research reported in this publication was supported by the National Cancer Institute and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) under award numbers: U01DK108288: Mayo Clinic; U01DK108300: Stanford University; U01DK108306: University of Pittsburgh; U01DK108314: Cedars-Sinai Medical Center; U01DK108320: University Of Florida; U01DK108323: Indiana University; U01DK108326: Baylor College of Medicine; U01DK108327: Ohio State University; U01DK108332: Kaiser Foundation Research Institute; U01DK108328: University of Texas MD Anderson Cancer Center.
Abbreviations:
- DM
diabetes mellitus
- NOD
new onset diabetes
- PDAC
pancreatic ductal adenocarcinoma
Footnotes
Conflicts of interest/disclosures: No conflicts of interest exist.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Chari ST, Kelly K, Hollingsworth MA, et al. Early detection of sporadic pancreatic cancer: summative review. Pancreas. 2015;44:693–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pannala R, Basu A, Petersen GM, et al. New-onset diabetes: a potential clue to the early diagnosis of pancreatic cancer. Lancet Oncol. 2009;10:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chari ST, Leibson CL, Rabe KG, et al. Probability of pancreatic cancer following diabetes: a population-based study. Gastroenterology. 2005;129:504–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sah RP, Nagpal SJS, Mukhopadhyay D, et al. New insights into pancreatic cancer-induced paraneoplastic diabetes. Nat Rev Gastroenterol Hepatol. 2013;10:423–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Damiano J, Bordier L, Le Berre JP, et al. Should pancreas imaging be recommanded in patients over 50 years when diabetes is discovered because of acute symptoms? Diabetes Metab. 2004;30:203–207. [DOI] [PubMed] [Google Scholar]
- 6.Illés D, Terzin V, Holzinger G, et al. New-onset type 2 diabetes mellitus--A high-risk group suitable for the screening of pancreatic cancer? Pancreatology. 2016;16:266–271. [DOI] [PubMed] [Google Scholar]
- 7.Ogawa Y, Tanaka M, Inoue K, et al. A prospective pancreatographic study of the prevalence of pancreatic carcinoma in patients with diabetes mellitus. Cancer. 2002;94:2344–2349. [DOI] [PubMed] [Google Scholar]
- 8.Aggarwal G, Rabe KG, Petersen GM, et al. New-onset diabetes in pancreatic cancer: a study in the primary care setting. Pancreatology. 2012;12:156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma A, Smyrk TC, Levy MJ, et al. Fasting Blood Glucose Levels Provide Estimate of Duration and Progression of Pancreatic Cancer before Diagnosis. Gastroenterology. 2018;155:490–500.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scientific Framework for Pancreatic Ductal Adenocarcinoma (PDAC). National Cancer Institute; 2014. Available from: http://deainfo.nci.nih.gov/advisory/ctac/workgroup/pc/pdacframework.pdf. Accessed July 10, 2018.
- 11.Pepe MS, Etzioni R, Feng Z, et al. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst. 2001;93:1054–1061. [DOI] [PubMed] [Google Scholar]
- 12.Gullo L, Pezzilli R, Morselli-Labate AM, et al. Diabetes and the risk of pancreatic cancer. N Engl J Med. 1994;331:81–84. [DOI] [PubMed] [Google Scholar]
- 13.Chari ST, Klee GG, Miller LJ, et al. Islet amyloid polypeptide is not a satisfactory marker for detecting pancreatic cancer. Gastroenterology. 2001;121:640–645. [DOI] [PubMed] [Google Scholar]
- 14.Winget M, Kincaid H, Lin P, et al. A web-based system for managing and co-ordinating multiple multisite studies. Clin Trials. 2005;2:42–49. [DOI] [PubMed] [Google Scholar]
- 15.Nolen BM, Brand RE, Prosser D, et al. Prediagnostic serum biomarkers as early detection tools for pancreatic cancer in a large prospective cohort study. PLoS One. 2014;9:e94928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allison JE, Tekawa IS, Ransom LJ, et al. A comparison of fecal occult-blood tests for colorectal-cancer screening. N Engl J Med. 1996;334:155–159. [DOI] [PubMed] [Google Scholar]
- 17.National Lung Screening Trial Research Team, Aberle DR, Berg CD, et al. The National Lung Screening Trial: overview and study design. Radiology. 2011;258:243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]