Abstract
Objective:
To develop a pharmacokinetic-pharmacogenomic (PK-PG) population model of morphine in critically ill children with acute respiratory failure.
Design:
Prospective PK-PG observational study.
Setting:
13 pediatric intensive care units across the United States
Patients:
Pediatric subjects (n=66) mechanically ventilated for acute respiratory failure, weight greater than or equal to 7 kg, receiving morphine and/or midazolam continuous infusions.
Interventions:
Serial blood sampling for drug quantification and a single blood collection for genomic evaluation.
Measurements and Main Results:
Concentrations of morphine, the two main metabolites, morphine-3-glucuronide (M3G), and morphine-6-glucuronide (M6G) were quantified by high performance liquid chromatography tandem mass spectroscopy. Subjects were genotyped using the Illumina HumanOmniExpress genome-wide SNP chip. Nonlinear mixed effects modeling was performed to develop the PK-PG model. A two-compartment model with linear elimination and two individual compartments for metabolites best describes morphine disposition in this population. Our analysis demonstrates that body weight and post-menstrual age are relevant predictors of PK parameters of morphine and its metabolites. Furthermore, our research shows that a duration of mechanical ventilation ≥ 10 days reduces metabolite formation and elimination upwards of 30%. However, due to the small sample size and relative heterogeneity of the population, no heritable factors associated with UGT2B7 metabolism of morphine were identified.
Conclusions:
The results provide a better understanding of the disposition of morphine and its metabolites in critically ill children with acute respiratory failure requiring mechanical ventilation due to non-heritable factors. It also provides the groundwork for developing additional studies to investigate the role of heritable factors.
Keywords: morphine, pediatric, pharmacokinetics, pharmacogenomics, acute respiratory failure, dose adjustment
INTRODUCTION
Mechanical ventilation is a common intervention used in critically ill children with respiratory failure, which require sedative for pain and agitation prevention [1]. Nearly a third of the top 25 medications commonly prescribed in the pediatric intensive care unit (PICU) are sedatives [2]. Traditionally, deep levels of sedation were considered optimal, but it is now apparent that oversedation contributes to worse patient outcomes [1, 3, 4].
Morphine is administered as an analgesic sedative for mechanical ventilation for children experiencing acute respiratory failure. Morphine is associated with a variety of adverse effects, including pruritus, nausea, emesis, and respiratory depression [5–7]. Morphine acts on mu opioid receptors and is extensively metabolized via glucuronidation to form two main metabolites, morphine-3-glucuronide (M3G), and the pharmacologically active morphine-6-glucuronide (M6G). Critical illness affects all the major organ systems and can have profound effects on morphine pharmacokinetics (PK) and disposition [8–12]. Glucuronidation occurs through the uridine diphosphate glucuronyl transferase (UGT) 2B7 isoform [13], and UGT2B7 genetic polymorphisms have been shown to affect both PK and pharmacodynamics (PD) of morphine [14, 15]. There is a gap in knowledge of how much these factors affect the PK and disposition of morphine and its metabolites. Therefore, it is crucial to understand the impact of critical illness on morphine PK in this population.
To our knowledge, there are limited studies on morphine PK in the PICU setting that account for changes in organ dysfunction due to critical illness or mechanical ventilation as well as genetic polymorphisms, especially in children [16, 17]. The aim of this study was to develop a population PK model to quantify the effects of these factors on morphine PK in critically ill children.
METHODS
Study Overview
Data was collected from subjects enrolled in the Pharmacologic Impact on Sedation Assessments study (PISA, NCT01105663, R01 HL098087). PISA was approved by the Children’s Hospital of Philadelphia (CHOP) Institutional Review Board and consent was obtained by parents/guardians before enrollment into the study. PISA was an ancillary study to the Randomized Evaluation of Sedation Titration fOr Respiratory failure trial (RESTORE, NCT00814009, U01 HL086622), which was described previously [18]. Briefly, pediatric subjects (aged 0.47 to 17.6 years) who were mechanically ventilated for acute respiratory failure were enrolled and followed until 72 hours after opioids were discontinued, 28 days, or hospital discharge. Additional PISA inclusion criteria included weight greater than or equal to 7 kg and receiving morphine and/or midazolam continuous infusions or oral administration. Demographic information is provided in Table 1.
Table 1.
Baseline characteristics of the study population
| Demographic | Number (n=66) |
|---|---|
| Age (years) | 6.1 (0.47 – 17.6) |
| Weight (kg) | 21 (7.2 – 99.0) |
| Height (cm) | 111.8 (66.0–177.0) |
| Serum Creatinine (mg/dL) | 0.30 (0.10 – 3.1) |
| eGFR (mL/min/1.73m2) | 168.7 (40.0 – 566.5) |
| ALT (U/L) b | 27.0 (6.0–193.0) |
| Duration on study > 10 days | |
| yes | 34 (52%) |
| no | 32 (48%) |
| Gender | |
| Male | 41 (62%) |
| Female | 25 (38%) |
| Race | |
| Caucasian | 53 (80%) |
| African American | 6 (9%) |
| Multiracial | 5 (8%) |
| Asian | 2 (3%) |
| Ethnicity | |
| Not Hispanic | 42 (64%) |
| Hispanic | 24 (36%) |
| Received concomitant midazolam | |
| yes | 64 (97%) |
| no | 2 (3%) |
Values are expressed as either median (range) or as a count (percentage). ALT = Alanine aminotransferase; eGFR = estimated glomerular filtration rate determined by the Schwartz equation.
A total of 3 subjects were missing height information and heights were imputed
A total of 18 subjects were missing ALT information
Drug administration
Morphine was administered as standard of care as continuous intravenous infusions and boluses or enteral doses. All doses were recorded, including start and stop time of infusions, infusion titrations, route of administration and bolus doses. Enteral dosing was administered after completion of the infusion as children were transitioned from intravenous to enteral dosing. These doses were administered for the prevention/treatment of narcotic withdrawal and were not scheduled.
PK Sampling
The number of PK samples was weight based, with a maximum sample volume of 3 mL/kg. Subjects were divided into cohorts based on the following weights: (i) 7–9.9 kg, (ii) 10–11.9 kg, (iii) 12–15.9 kg, and (iv) ≥ 16 kg, in order to determine the number of samples and blood drawn from the subject based on body weight. PK samples were taken daily, every other day, or every third day (weight based) during the continuous infusion. Upon discontinuation of the infusion, PK samples were obtained to define the terminal decline of drug concentration and subsequent additional samples were obtained 1-, 3-, 5-, 7-, and/or 10 hours after the end of infusion. In the event that a subject demonstrated either pain or withdrawal after the infusion was discontinued, additional doses of morphine (intravenous and/or enteral) were administered at the discretion of the clinical team. PK sampling was not modified based on the administration of enteral dosing, but all enteral doses were captured and incorporated into the model. Blood (1 mL for each sample) was centrifuged, plasma separated and stored at −80 degrees until shipped on dry ice. PK samples were analyzed to quantify morphine, M3G, and M6G using a validated high performance liquid chromatography tandem mass spectrometry (HPLC-MS/MS) in the Bioanalytical Core within the Center for Clinical Pharmacology at CHOP [19, 20]. More detailed information about the analytical assay can be found in the supplemental document.
Population Pharmacokinetic Modeling
Analysis data sets and exploratory graphics were programmed from source data using the R programming language (www.r-project.org) [21]. Data were excluded for the following reasons: samples drawn from the dosing catheter immediately after dosing, missing observation times, or missing dose times. Plasma drug concentrations that were below the analytical assay’s limit of quantification were not reported, but the sampling times were flagged and retained in the analysis data set. Patient-specific covariates, including height and ALT, were obtained from the source data and imputed if missing. A population PK model was developed to simultaneously characterize the PK disposition of morphine, M3G, and M6G (NONMEM v. 7.3.0 software, ICON Development Solutions, Ellicott City, MD). Models were specified using a matrix exponential method for general linear systems of differential equations (NONMEM’s ADVAN 5) with Conditional Laplacian estimation utilizing the M3 method to account for data below the limit of quantitation [22, 23]. Model evaluation/selection was based on successful convergence, precision of the parameter estimates, the Akaike Information Criteria (AIC), and comparison of goodness-of-fit diagnostic plots.
Various compartmental disposition models were investigated for the parent drug, morphine, including one-, two-, and three-compartment models. A relative bioavailability parameter was included in the model to account for the differences between doses administered parenterally and orally. Random inter-individual variability was described by an exponential variance model, with individual random effects for all parameters assumed to follow a multivariate-normal covariance structure. Given the multivariate nature of the repeated-measures parent and metabolites observations, residual errors across the analytes were also described with a multivariate-normal covariance matrix, but with a proportional error model structure. The typical values of clearance (CL) and intercompartmental clearance (Q) were allometrically scaled with body weight: , where TVP is the typical value of the parameter, WT is the weight of each subject i and a reference weight, which was set at 70kg. The impact of size is represented by θallometric, which is a power parameter fixed at 0.75 for CL and Q and 1 for volumes [24].
Metabolites were modeled as additional compartments in the general linear system of differential equations. In order to ensure mathematical identifiability of metabolite parameters, two approaches were explored: (i) fixing the fraction of morphine metabolized to M3G, M6G, and other metabolites to previously reported values (60%, 10%, and 30%, respectively) [25–27], and (ii) fixing the volumes of the M3G and M6G metabolite compartments to previously reported values (23 L and 29 L, respectively) [16, 28–30].
After finalizing the parent-metabolite model, the impact of covariates on model parameters was assessed. The first step was to assess maturation of the UGT and renal clearance pathways for both morphine and its metabolites. The sigmoidal Hill equation was used to describe the maturation process of the UGT enzyme and renal clearance of metabolites. This equation uses post-menstrual age (PMA), maturation half time (TM50), and an exponential coefficient (γ). PMA is defined as the age at enrollment plus gestational age, which is assumed to be 0.767 years (40 weeks) and added to the model as a continuous variable. TM50 described the maturation half time in years and the coefficient is the slope of the maturation profile.
The second step was to assess the impact of pre-specified covariates. These included sex, age, weight, height, duration of enrollment in RESTORE as a measure of duration of mechanical ventilation, alanine aminotransferase (ALT) as a surrogate for hepatic function, and estimated glomerular filtration rate (eGFR) as a measure of renal function as calculated by the Schwartz equation [31]. Duration of enrollment in RESTORE was used to differentiate children whose duration of mechanical ventilation was short versus those who were sicker and remained ventilated for longer periods of time. Renal function was normalized to 90 mL/min/1.73m2, with any normalized value above 1 set equal to 1. ALT was normalized to the upper limit of normal (55 U/L), and any normalized value below 1 was set equal to 1. Missing values for hepatic function and height were imputed based on the remaining observed data in this population.
Pharmacogenomics
Genomic data for this analysis was received directly from the CHOP Center for Applied Genomics (CAG). Data summaries and preliminary genomic information searches were done using the R software, and associated packages (metrumrg 5.55, NCBI2R, and rsnps) [21, 32]. NCBI2R GetSNPInfo and rsnps NCBI snp query commands were used to retrieve SNP information from various data bases on the web: openSNP, NBCI’s dbSNP database, and Broad Institute SNP Annotation & Proxy Search. Genotypes for UGT2B7 for each SNP were determined by imputation methods similar to those described by Howie et al. [33].
Only single nucleotide polymorphisms (SNPs) in the UGT2B7 gene (n=139) were considered. Genotype probabilities were assigned to the genotype categories: 0, 1 or 2, with 1 being heterozygous, and 0 or 2 being homozygous to the minor or major allele. Genotype continuous probabilities were binned into categories where p(0 ≤ x ≤ 0.2) represented homozygous for the minor allele, p(0.9 ≤ x ≤ 1.1) represented heterozygous alleles, and p(1.8 ≤ x ≤ 2) represented homozygous for the major allele. Probabilities outside of these bins were excluded from the analysis. Variability criteria were set to determine the number of subjects within each genotype group per SNP. Subjects within each bin group were tallied and SNPs with 5 or more subjects in any two of the three bin groups were kept for further evaluation. SNPs with fewer than 5 subjects in any two of the three bin groups were not included in the analysis.
The potential impact of each SNP on metabolite formation was evaluated by exploring the relationships between SNPs and remaining unexplained inter-subject variability in formation clearance for each metabolite. A Kruskal-Wallis test with a Bonferroni correction (p ≤ 0.00061) was utilized to determine if any of the candidate SNPs were potentially associated with morphine metabolism.
SNPs that represented heterozygous or homozygous minor alleles were included in the population PK model to assess their impact on M6G formation (CL14) compared to subjects with SNPs coding for homozygous major alleles. The pharmacogenomic effect of either the heterozygous and homozygous minor genotypes were coded as binary covariates and calculated as shown: where θhetero and θhomoMinor were coded as 0 or 1 to represent the estimation of the heterozygous or homozygous minor effects as compared to homozygous major as the baseline value. This value was then included in the final parent-metabolite PK model as shown: , where PG represents the genetic effect on the typical value of clearance (TVCL).
RESULTS
Of the children (n = 173) recruited into the PISA study, 39% (n=68) received continuous infusions of morphine. However, samples for one patient were never received and another patient had samples drawn exclusively from the dosing catheter, so these patients were excluded from final analysis. Additionally, 21% of morphine observations (n=102), 1% of M3G metabolite observations (n=5), and 8% of M6G metabolite observations (n=39) were flagged due to being below the limit of quantification. Therefore, the final morphine PK dataset contained 66 subjects with 1364 observation records, including 386 morphine observations, 506 M3G observations and 472 M6G observations. Subject characteristics are summarized in Table 1. Genotype information was available for 88% (n=58) of the subjects with PK information. Inclusion of subjects and data is summarized in Table 1.
A linear two-compartment model for morphine with single compartments for the M3G and M6G metabolites (Figure 1) best fit the data. Since two of the subjects received 41 doses of morphine orally, relative bioavailability (F = 0.24) was fixed in the model to account for the differences between doses administered parenterally and orally [34]. Total morphine clearance was described as a sum of the formation clearances into M3G (CL13) and M6G (CL14), and the non-glucuronidated elimination of morphine (CL10). Additionally, the model did not support estimation of separate individual random effects on M3G and M6G clearances, so a shared scaled random effect was estimated. To ensure mathematical identifiability of parameters, metabolite volumes of distribution were fixed to previously described volumes of distribution determined by single occasion metabolite studies in adults [30, 35].
Figure 1.
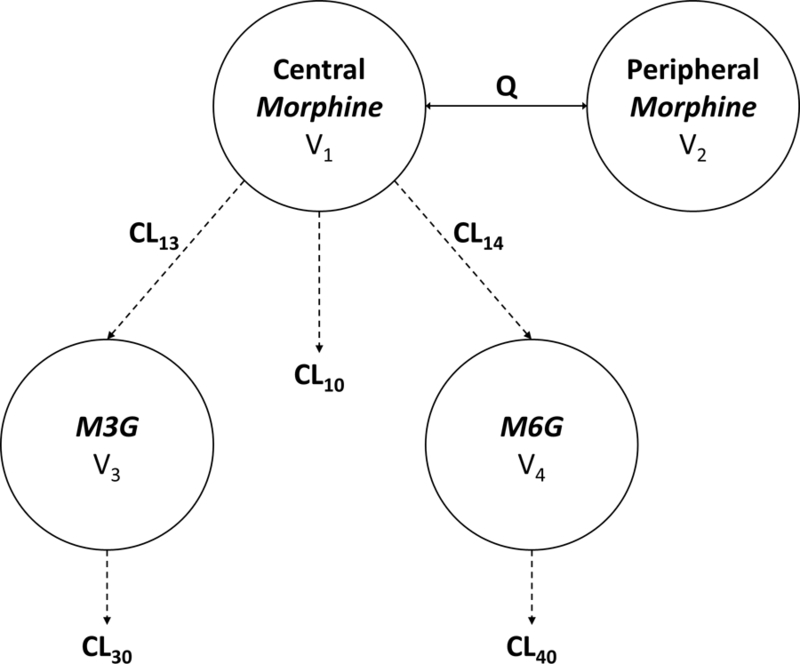
Schematic of pharmacokinetics of morphine and its metabolites. CL is clearance with the respective routes of elimination. V is the volume of the respective compartments. Q is the intercompartmental clearance. Morphine clearance and metabolite formation is CL10 + CL13 + CL14. Morphine metabolite clearances are defined as CL30 and CL40.
After finalizing the base parent-metabolite model, the effects of covariates were assessed. The first step was to set the maturation processes associated with hepatic UGT and renal elimination of morphine and its metabolites. The data did not support full estimation of maturation processes (TM50 or γ), therefore these values were fixed to prior reported values [36, 37]. Including maturation processes as a covariate in addition to allometrically scaling weight improved model performance, lowering AIC by 112 points.
Next, the parent-metabolite model with fixed maturation processes was expanded to assess the impact of covariates of critical illness, including eGFR, ALT, and duration of RESTORE enrollment. The effects of eGFR and ALT were not precisely estimated and did not improve goodness of fit. Incorporating duration of RESTORE enrollment into the model improved model performance and reduced the inter-individual variability observed with metabolite formation and elimination clearances. The final model demonstrated that duration of RESTORE enrollment greater than 10 days reduced M3G formation and elimination (by 33% and 34 respectively), and also reduced M6G formation and elimination (by 7% and 12%, respectively). Final parameter estimates are shown in Table 2. Observed concentrations of morphine and its metabolites are compared against individual and population predicted concentrations overall (Figure 3) and for each subject (Supplemental Figure 1) showing that the model adequately describes the data.
Table 2.
Population PK-PG model parameter estimates and model description. Parameter estimates and 95% confidence intervals (CI) are shown for each PK parameter. Point estimates and 95% CI are shown for evaluated SNPs. SNPs were evaluated for their relative effects on the formation CL of M6G (CL14) using the homozygous major SNP as the reference value. In this parameterization, a value of 1 indicates no difference from the reference. PG= (gHetero**Hetero(0/1)*(gMinor**hMinor(0/1). Next, The PG effect was added to the determination of CL14 by the following equation: CL14 = TVCL14*PG. NA indicates that for the rs62296959 there were less than 5 subjects that expressed the homozygous minor SNP.
| Parameter | Parameter Estimate | 95% CI |
|---|---|---|
| Total morphine CL (L/h) | 72.1 | -- |
| CL13 (L/h) | 13.2 | (10.4 , 16.0) |
| CL14 (L/h) | 3.3 | (2.6 , 3.9) |
| CL10 (L/h) | 55.6 | (48.2 , 62.9) |
| CL30 (L/h) | 3.6 | (2.9 , 4.2) |
| CL40 (L/h) | 4.9 | (4.0 , 5.9) |
| Q (L/h) | 53.2 | (41.9 , 64.4) |
| V1 (L) | 22.9 | (16.8 , 29.0) |
| V2 (L) | 172.0 | (158.6 , 185.5) |
| Estimated variance scale factor on shared eta | 1.01 | (0.97 , 1.1) |
| Effect of duration of stay on formation of M3G | 0.67 | (0.49 , 0.86) |
| Effect of duration of stay on formation of M6G | 0.93 | (0.69 , 1.2) |
| Effect of duration of stay on M3G CL | 0.66 | (0.49 , 0.82) |
| Effect of duration of stay on M6G CL | 0.88 | (0.64 , 1.1) |
| V3 (L) | 23.0 FIXED | -- |
| V4 (L) | 29.0 FIXED | -- |
| F1, relative bioavailability | 0.24 FIXED | -- |
| TM50 UGT maturation (years) | 1.04 FIXED | -- |
| γ UGT maturation | 3.92 FIXED | -- |
| TM50 renal maturation (years) | 0.92 FIXED | -- |
| γ renal maturation | 3.4 FIXED | -- |
| Interindividual Variability in CL10 | 0.48 | (0.28 , 0.68) |
| Interindividual Variability in CL13 | 0.63 | (0.39 , 0.87) |
| Interindividual Variability in CL14 | 0.54 | (0.31 , 0.77) |
| Interindividual Variability in Metabolite CL (CL30 + CL40) | 0.49 | (0.28 , 0.72) |
| Residual Variability for Morphine | 0.22 | (0.20 , 0.25) |
| Residual Variability for M3G | 0.089 | (0.082 , 0.096) |
| Residual Variability for M6G | 0.11 | (0.10 , 0.12) |
| Heterozygous SNP rs28375964 | 1.03 | (0.92,1.14) |
| Homozygous SNP rs28375964 | 1.04 | (0.83, 1.25) |
| Heterozygous SNP rs10006452 | 0.99 | (0.92,1.14) |
| Homozygous SNP rs10006452 | 1.04 | (0.83, 1.25) |
| Heterozygous SNP rs62296959 | 0.99 | (0.77,1.29) |
| Homozygous SNP rs62296959 | NA | NA |
PK = pharmacokinetic; PG = pharmacogenomic; CI = confidence interval (lower bound, upper bound); M3G = morphine-3-glucuronide metabolite; M6G = morphine-6-glucuronide metabolite; WT= weight; TM50 = time to reach 50% of a completely mature clearance; PMA = post-menstrual age; θMV = effect of mechanical ventilation greater than 10 days. Correlation for parameters of interindividual and residual variability random-effect matrices were in the range of (−46%, 99%) and (62%, 87%), respectively. SNP = single nucleotide polymorphism
Figure 3.
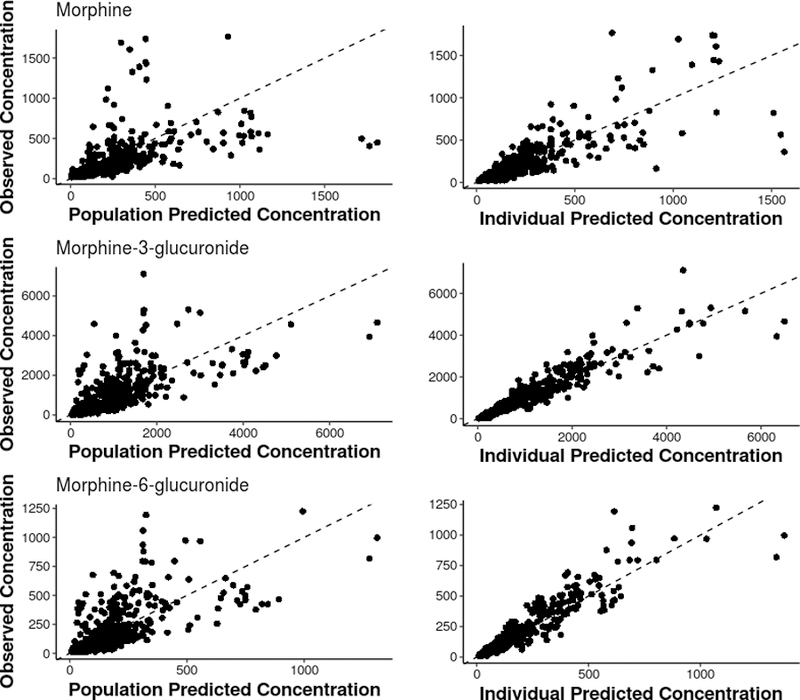
Observed concentrations of morphine and its metabolites compared against the model-based population and individual predicted concentrations.
Of the 66 subjects with PK information, 58 (88%) subjects provided genotype information which was analyzed using the Illumina HumanOmniExpress genome-wide SNP chip. Of the 139 SNP surveyed, 82 SNPs (59%) met the variability criteria. However, none of these SNPs met the corrected significance criteria. A total of 3 SNPs were potentially associated with M6G formation (CL14) at an uncorrected p value (p ≤ 0.05) and were further evaluated in the population PK model as a hypothesis generating exercise. In the population PK model context, the point estimates and 95% confidence intervals for the estimates for all three of these SNP effects were consistent with no effect on the formation CL of M6G (e.g. all 95% CI were within +/− 29% of the null value of 1). This exploratory analysis suggests that the polymorphisms in theses UGT2B7 SNPs do not impact metabolism of morphine to M6G in this particular study population. Therefore, the final prediction model only included PK parameter estimates and did not include PG estimates (Table 2).
DISCUSSION
The PISA data set contains PK samples from mechanically ventilated children over a considerably wide range of time (1 day to 39 days). One to six samples were obtained prior to and up to 10 hours after the last dose (median 2.7 hours) for 75% of the population. The main objective of the population PK analysis was to determine the overall effect of maturation of renal function, maturation of UGT metabolism, post-menstrual age, degree of critical illness, pharmacogenomics, and weight on morphine disposition. The study found that the estimated population PK model using two-compartment with single compartments for the morphine metabolites and allometrically scaled weight, referenced to 70kg, fit the data well.
The final parent-metabolite model was able to characterize the compartmental structure and disposition of morphine and its metabolites in this population. Using previously published maturation values, the model established a reduction in clearance of morphine and its metabolites in children with longer periods of mechanical ventilation. Other studies in critically ill children estimated similar values for clearances [29, 38]. When adjusted for body size, our results indicate that duration of mechanical ventilation greater than 10 days is associated with a reduction in M3G formation and clearance by up to 30% in children, which follows a similar pattern to that seen in critically ill adults, who experience up to a 76% decrease in clearance compared to healthy adults [16]. Model-based simulations conducted by Ahlers et. al. indicated a 33% reduction in the maintenance dose would result in morphine serum concentrations equal to those in healthy volunteers versus intensive care patients [16]. It is difficult to ascertain the magnitude of the pharmacodynamics effect of these findings since M3G formation and clearance are both reduced. It is noteworthy however that, since M3G may be implicated in the antagonism of morphine-induced analgesia/sedation, clinicians should consider alternate sedative medications for children with prolonged critical illness. When the median PMA and weight are incorporated into the clearance equations provided in Table 2, the total clearance of morphine into its metabolites is 32.5 L/h when the subject has been on mechanical ventilation for more than 10 days. This is over a 50% reduction to the normal clearance value of 72.1 L/h when a subject has been on mechanical ventilation for less than 10 days. Along with these previous studies, the results of this study support that increased duration of critical illness reduces clearance of morphine and its metabolites, which suggests that doses should be reduced or monitored.
While our exploratory model-based analysis revealed no impact of heritable factors on the disposition of morphine and its metabolites after adjusting for other patient-specific covariates, several morphine studies have linked SNPs to meaningful PK and PD outcomes. A study by Bastami et al., 2014 concluded there was a clear and significant relationship between homozygosity for UGT2B7 802C and the dose of morphine required for pain relief [14]. Studies demonstrated that SNPs in UGT2B7*2 and ABCB1 were associated with significantly lower frequency of fatigue, nausea, and vomiting [15]. However, three studies conducted in cancer patients found no correlations between polymorphisms and morphine or morphine metabolite concentrations. A study conducted by Holthe et al. published in 2002, evaluated 70 cancer patients with normal renal and hepatic function using slow release morphine [39]. They found no statistically significant differences in plasma ratios between genotypes and concluded that this polymorphism cannot account for the considerable variation in metabolite to morphine serum ratios. A subsequent study conducted by Holthe et al. published in 2003, evaluated 10 UGT2B7 polymorphisms in 175 Norwegian cancer patients, with normal renal and hepatic function, that received slow-release morphine orally [13]. The authors again found no correlation between UGT2B7 genotypes or haplotypes and metabolite to morphine serum ratios. Finally, the third study conducted by Ross et al. evaluated 4 UGT2B7 polymorphisms in 172 Caucasian cancer patients [40]. The control group consisted of 117 patients who responded to morphine and an alternative group, referred to as switchers, consisted of 39 patients who did not tolerate morphine and were switched to alternative opioids. The authors found no significant differences in genotype or allelic frequencies and found no correlation between genotype and morphine or morphine metabolite concentration levels. Therefore, it would be worthwhile to study the impact of pharmacogenomics on the disposition of morphine and its metabolites in a larger trial in critically ill children.
One limitation of this research was the relatively small sample size. Small sample sizes present distinct issues during analysis, including the methodology of how to deal with missing covariates. Since this cohort was relatively small, we strived to include all patients who had PK samples drawn to prevent the sample size decreasing any further and losing important PK information. Another issue is that small sample sizes can inflate the variability in parameter estimates and reduce the model’s ability to precisely predict the concentrations of morphine or its metabolites in future populations. However, the large interindividual variability for morphine in this model (52%) is consistent with the interindividual variability in other published models (48%−78%) in children [28, 29]. Additionally, many pharmacogenomic studies require a large sample size to see an effect. Studying genetic diversity in small more homogeneous populations could lead to a better understanding of genetics and PK. Furthermore, the relatively small sample size and reliance on sparse sampling required that this analysis fixed certain values. The fixed values for volumes of distribution were similar across children and adults as demonstrated in the literature. The fixed values of the fraction of morphine metabolized to M3G, M6G, and other metabolites was taken from studies completed in adults. However, as demonstrated by Choonara et al., the ratio of M3G to M6G metabolites was similar between neonates and children [41], therefore it was assumed to be similar to adults for the convergence of the model in this analysis. This assumption could be a limitation to this analysis as the fraction of morphine metabolized to each metabolite could differ based on age and disease state and should therefore be investigated in future, larger cohorts to confirm these ratios. Additionally, the small sample size and the missing ALT and height values could have limited our ability to precisely quantify the impact of hepatic (ALT) and renal (eGFR calculated with the Schwartz equation using height) on the disposition of morphine and its metabolites. Both of these covariates should be considered in future, larger trials in this population. Finally, it should be noted that the majority of the 102 samples where morphine was below the limit of quantitation had measurable amounts of metabolites. Therefore, the reason for having 102 samples with morphine below the limit of quantitation is most likely due to a combination of the lower sensitivity of our assay compared to previously published assays as well as patient specific issues, such as metabolism.
CONCLUSION
Overall, this study provides a model of morphine and its metabolites in critically ill children with acute respiratory failure requiring mechanical ventilation. Our analysis demonstrates that body weight and post-menstrual age are relevant predictors of PK parameters of morphine and its metabolites. Exploratory model-based analyses revealed no impact of heritable factors associated with UGT2B7 metabolism of morphine after adjusting for other patient-specific covariates. Furthermore, our research shows that duration of mechanical ventilation ≥10 days reduces metabolite formation and elimination upwards of 30%. Clinically, our analysis suggests that clinicians should closely monitor either the therapeutic drug levels or clinical outcomes of morphine administered in this population due to a reduction in morphine clearance by up to 50%, which could impact the level of sedation these children experience.
Supplementary Material
Figure 2.

Flowchart of data included in the final model.
ACKNOWLEDGMENTS:
PISA site PI’s and RCs: Heidi Fiori and Julie Simone (Children’s Hospital and Research Center at Oakland); Santiago Borasino and Rachel Martin Bush (Children’s Hospital of Alabama);, Sheila McGowan (Children’s Hospital of Philadelphia); David Steinhorn and Lauren Sorce (Children’s Memorial Hospital-Chicago); Mary Jo Grant and Emily Howe (Primary Children’s Medical Center); Alan Doctor and Bert Hicks (St. Louis Children’s Hospital); E. Vincent S/ Fausino and Joana Tala (Children’s Hospital – Yale New Haven Health); Adam Schwarz, Jennifer Cohen and Stephanie Osborne (Children’s Hospital Orange County); Edward Truemper and Machelle Zink (Children’s Hospital and Medical Center, Nebraska); James Schneider and Gulru Sharifova (Cohen’s Children’s Medical Center); Jeanette Green, Aaron Godshall and Amber Kaman (Florida Hospital for Children); Marc Berg, Claire Wells and Jennifer Deschenes (University Medical Center Tucson Arizona); and G. Kris Bysani and Tatiana Khorokhorina (Medical City Dallas).
Financial Support:
Zuppa: 1 R01 HL098087-01 - Impact of Pharmacology on the Duration of Ventilation in Pediatric Patients with Respiratory Failure NHLBI
Hakon: eMERGE consortium grant 1U01HG006830 from the NHGRI.
Zane: Supported by a NIGMS T32 Clinical Pharmacology Fellowship (2T32GM008562-21)
Footnotes
Disclosures: The authors declare no conflict of interest.
Copyright form disclosure: Drs. Zuppa, Zane, Hakonarson, and Moorthy received support for article research from the National Institutes of Health (NIH). Dr. Curley’s institution received funding from National Heart, Lung, and Blood Institute. Dr. Moorthy’s institution received funding from the NIH. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Reade MC, Finfer S: Sedation and delirium in the intensive care unit. The New England journal of medicine 2014, 370(5):444–454. [DOI] [PubMed] [Google Scholar]
- 2.Zuppa AF, Adamson PC, Mondick JT, et al. : Drug utilization in the pediatric intensive care unit: monitoring prescribing trends and establishing prioritization of pharmacotherapeutic evaluation of critically ill children. J Clin Pharmacol 2005, 45(11):1305–1312. [DOI] [PubMed] [Google Scholar]
- 3.Riker RR, Fraser GL: Altering intensive care sedation paradigms to improve patient outcomes. Crit Care Clin 2009, 25(3):527–538, viii-ix. [DOI] [PubMed] [Google Scholar]
- 4.Shehabi Y, Bellomo R, Mehta S, et al. : Intensive care sedation: the past, present and the future. Crit Care 2013, 17(3):322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walder B, Schafer M, Henzi I, et al. : Efficacy and safety of patient-controlled opioid analgesia for acute postoperative pain. A quantitative systematic review. Acta anaesthesiologica Scandinavica 2001, 45(7):795–804. [DOI] [PubMed] [Google Scholar]
- 6.Sinatra RS, Jahr JS, Reynolds LW, et al. : Efficacy and safety of single and repeated administration of 1 gram intravenous acetaminophen injection (paracetamol) for pain management after major orthopedic surgery. Anesthesiology 2005, 102(4):822–831. [DOI] [PubMed] [Google Scholar]
- 7.Anand KJ, Willson DF, Berger J, et al. : Tolerance and withdrawal from prolonged opioid use in critically ill children. Pediatrics 2010, 125(5):e1208–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zuppa AF, Barrett JS: Pharmacokinetics and pharmacodynamics in the critically ill child. Pediatr Clin North Am 2008, 55(3):735–755, xii. [DOI] [PubMed] [Google Scholar]
- 9.Hites M, Dell’Anna AM, Scolletta S, et al. : The challenges of multiple organ dysfunction syndrome and extra-corporeal circuits for drug delivery in critically ill patients. Adv Drug Deliv Rev 2014, 77:12–21. [DOI] [PubMed] [Google Scholar]
- 10.Singer M, De Santis V, Vitale D, et al. : Multiorgan failure is an adaptive, endocrine-mediated, metabolic response to overwhelming systemic inflammation. Lancet 2004, 364(9433):545–548. [DOI] [PubMed] [Google Scholar]
- 11.Thakkar N, Salerno S, Hornik CP, et al. : Clinical Pharmacology Studies in Critically Ill Children. Pharm Res 2016. [DOI] [PMC free article] [PubMed]
- 12.Hartman ME, McCrory DC, Schulman SR: Efficacy of sedation regimens to facilitate mechanical ventilation in the pediatric intensive care unit: a systematic review. Pediatr Crit Care Med 2009, 10(2):246–255. [DOI] [PubMed] [Google Scholar]
- 13.Holthe M, Rakvag TN, Klepstad P, et al. : Sequence variations in the UDP-glucuronosyltransferase 2B7 (UGT2B7) gene: identification of 10 novel single nucleotide polymorphisms (SNPs) and analysis of their relevance to morphine glucuronidation in cancer patients. Pharmacogenomics J 2003, 3(1):17–26. [DOI] [PubMed] [Google Scholar]
- 14.Bastami S, Gupta A, Zackrisson AL, et al. : Influence of UGT2B7, OPRM1 and ABCB1 gene polymorphisms on postoperative morphine consumption. Basic Clin Pharmacol Toxicol 2014, 115(5):423–431. [DOI] [PubMed] [Google Scholar]
- 15.Fujita K, Ando Y, Yamamoto W, et al. : Association of UGT2B7 and ABCB1 genotypes with morphine-induced adverse drug reactions in Japanese patients with cancer. Cancer Chemother Pharmacol 2010, 65(2):251–258. [DOI] [PubMed] [Google Scholar]
- 16.Ahlers SJ, Valitalo PA, Peeters MY, et al. : Morphine Glucuronidation and Elimination in Intensive Care Patients: A Comparison with Healthy Volunteers. Anesth Analg 2015, 121(5):1261–1273. [DOI] [PubMed] [Google Scholar]
- 17.Peters JW, Anderson BJ, Simons SH, et al. : Morphine pharmacokinetics during venoarterial extracorporeal membrane oxygenation in neonates. Intensive care medicine 2005, 31(2):257–263. [DOI] [PubMed] [Google Scholar]
- 18.Curley MA, Wypij D, Watson RS, et al. : Protocolized sedation vs usual care in pediatric patients mechanically ventilated for acute respiratory failure: a randomized clinical trial. Jama 2015, 313(4):379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moorthy GS, Jogiraju H, Vedar C, et al. : Development and validation of a sensitive assay for analysis of midazolam, free and conjugated 1-hydroxymidazolam and 4-hydroxymidazolam in pediatric plasma: Application to Pediatric Pharmacokinetic Study. J Chromatogr B Analyt Technol Biomed Life Sci 2017, 1067:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahsman MJ, van der Nagel BC, Mathot RA: Quantification of midazolam, morphine and metabolites in plasma using 96-well solid-phase extraction and ultra-performance liquid chromatography-tandem mass spectrometry. Biomed Chromatogr 2010, 24(9):969–976. [DOI] [PubMed] [Google Scholar]
- 21.Bergsma TT, Knebel W, Fisher J, et al. : Facilitating pharmacometric workflow with the metrumrg package for R. Comput Methods Programs Biomed 2013, 109(1):77–85. [DOI] [PubMed] [Google Scholar]
- 22.Ahn JE, Karlsson MO, Dunne A, et al. : Likelihood based approaches to handling data below the quantification limit using NONMEM VI. J Pharmacokinet Pharmacodyn 2008, 35(4):401–421. [DOI] [PubMed] [Google Scholar]
- 23.Beal SL: Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn 2001, 28(5):481–504. [DOI] [PubMed] [Google Scholar]
- 24.Anderson BJ, Allegaert K, Holford NH: Population clinical pharmacology of children: modelling covariate effects. Eur J Pediatr 2006, 165(12):819–829. [DOI] [PubMed] [Google Scholar]
- 25.Lotsch J, Stockmann A, Kobal G, et al. : Pharmacokinetics of morphine and its glucuronides after intravenous infusion of morphine and morphine-6-glucuronide in healthy volunteers. Clinical pharmacology and therapeutics 1996, 60(3):316–325. [DOI] [PubMed] [Google Scholar]
- 26.Ohno S, Kawana K, Nakajin S: Contribution of UDP-glucuronosyltransferase 1A1 and 1A8 to morphine-6-glucuronidation and its kinetic properties. Drug metabolism and disposition: the biological fate of chemicals 2008, 36(4):688–694. [DOI] [PubMed] [Google Scholar]
- 27.Hasselstrom J, Sawe J: Morphine pharmacokinetics and metabolism in humans. Enterohepatic cycling and relative contribution of metabolites to active opioid concentrations. Clinical pharmacokinetics 1993, 24(4):344–354. [DOI] [PubMed] [Google Scholar]
- 28.Knosgaard KR, Foster DJ, Kreilgaard M, et al. : Pharmacokinetic models of morphine and its metabolites in neonates:: Systematic comparisons of models from the literature, and development of a new meta-model. Eur J Pharm Sci 2016, 92:117–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bouwmeester NJ, Anderson BJ, Tibboel D, et al. : Developmental pharmacokinetics of morphine and its metabolites in neonates, infants and young children. Br J Anaesth 2004, 92(2):208–217. [DOI] [PubMed] [Google Scholar]
- 30.Penson RT, Joel SP, Clark S, et al. : Limited phase I study of morphine-3-glucuronide. Journal of pharmaceutical sciences 2001, 90(11):1810–1816. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz GJ, Work DF: Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol 2009, 4(11):1832–1843. [DOI] [PubMed] [Google Scholar]
- 32.Fladvad T, Klepstad P, Langaas M, et al. : Variability in UDP-glucuronosyltransferase genes and morphine metabolism: observations from a cross-sectional multicenter study in advanced cancer patients with pain. Pharmacogenetics and genomics 2013, 23(3):117–126. [DOI] [PubMed] [Google Scholar]
- 33.Howie BN, Donnelly P, Marchini J: A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet 2009, 5(6):e1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoskin PJ, Hanks GW, Aherne GW, et al. : The bioavailability and pharmacokinetics of morphine after intravenous, oral and buccal administration in healthy volunteers. British journal of clinical pharmacology 1989, 27(4):499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lotsch J, Geisslinger G: Morphine-6-glucuronide: an analgesic of the future? Clinical pharmacokinetics 2001, 40(7):485–499. [DOI] [PubMed] [Google Scholar]
- 36.Rhodin MM, Anderson BJ, Peters AM, et al. : Human renal function maturation: a quantitative description using weight and postmenstrual age. Pediatr Nephrol 2009, 24(1):67–76. [DOI] [PubMed] [Google Scholar]
- 37.Anderson BJ, Holford NH: Mechanistic basis of using body size and maturation to predict clearance in humans. Drug metabolism and pharmacokinetics 2009, 24(1):25–36. [DOI] [PubMed] [Google Scholar]
- 38.Knibbe CA, Krekels EH, van den Anker JN, et al. : Morphine glucuronidation in preterm neonates, infants and children younger than 3 years. Clinical pharmacokinetics 2009, 48(6):371–385. [DOI] [PubMed] [Google Scholar]
- 39.Holthe M, Klepstad P, Zahlsen K, et al. : Morphine glucuronide-to-morphine plasma ratios are unaffected by the UGT2B7 H268Y and UGT1A1*28 polymorphisms in cancer patients on chronic morphine therapy. European journal of clinical pharmacology 2002, 58(5):353–356. [DOI] [PubMed] [Google Scholar]
- 40.Ross JR, Rutter D, Welsh K, et al. : Clinical response to morphine in cancer patients and genetic variation in candidate genes. Pharmacogenomics J 2005, 5(5):324–336. [DOI] [PubMed] [Google Scholar]
- 41.Choonara IA, McKay P, Hain R, et al. : Morphine metabolism in children. British journal of clinical pharmacology 1989, 28(5):599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


