Abstract
Introduction
Acute appendicitis is a common and serious situation during pregnancy, because of the increased risk of fetal loss and perforation in the third trimester, as well as a diagnostic difficulty. During recent years laparoscopic approach has been introduced to clinical practice with encouraging results. The purpose of this meta-analysis is to compare the surgical and obstetrical outcomes between laparoscopic and open appendectomy during pregnancy.
Materials and methods
MEDLINE, SCOPUS, Clinicaltrials.gov, CENTRAL and Google Scholar were searched for studies reporting on postoperative outcomes between laparoscopic and open appendectomy during pregnancy. The random effects model (DerSimonian–Laird) was used to calculate pooled effect estimates when high heterogeneity was encountered, otherwise the fixed-effects (Mantel–Haenszel) model was implemented.
Results
Twenty-one studies that enrolled 6276 pregnant women are included in the present meta-analysis. Of these women, 1963 underwent laparoscopic appendectomy and 4313 underwent an open appendectomy. Women who underwent laparoscopic appendectomy demonstrated an increase in fetal loss risk, while neonates of women that underwent open appendectomy presented decreased Apgar score at five minutes after birth. All the rest outcomes were similar between the two groups. The time that each study took place seemed to affect the comparison of birth weight and postoperative hospital stay between the two groups.
Conclusion
Laparoscopic appendectomy seems to be a relatively safe therapeutic option in pregnancy when it is indicated. Thus, it should be implemented in clinical practice, always considering the experience of the surgeon in such procedures. Nevertheless, the need of new studies to enhance this statement remains crucial.
Keywords: Laparoscopy, Appendicitis, Pregnancy
Introduction
During pregnancy, appendicitis is a common non-obstetric emergency that may entail surgery. It is observed in approximately 1/1500 pregnancies,1 representing 25% of non-obstetric operations performed in the antenatal period.2 Incidence varies throughout the pregnancy course, ranging from 19% to 36% during the first trimester, from 27% to 60% during the second trimester and from 15% to 33% during the last three months of pregnancy.3 Despite the fact that acute appendicitis is more common during the first and second trimesters, perforation is more common in the third trimester.4
Pregnant women who undergo surgery, including appendectomy, are at a higher risk of fetal loss, particularly when the operation takes place during early pregnancy and if the mother fails to receive immediate and appropriate medical attention.5 Accurate diagnosis becomes a problem, because appendicitis is complicated by the physiological and anatomical changes that occur during pregnancy. In several cases, the signs and symptoms are similar to those of pregnancy or the onset of labour and include loss of appetite, nausea and vomiting and lower abdominal pain.3 This can delay diagnosis and increase the risk of morbidity for both the mother and the fetus.6
Pregnancy was a relative contraindication to laparoscopy until recently because of the belief that the procedure would decrease uterine and fetal blood flow; thus there were concerns that it could result in miscarriage or could possibly influence fetal development.7 Although laparoscopic appendectomy has become more popular in daily practice, some controversy still exists regarding its everyday practice in pregnancy.8 Current data suggest that laparoscopy can be performed without complications during the three trimesters,7,9 although the procedure may become particularly difficult at term gestation as the operating field is obstructed by the gravid uterus.10 Since then, several studies have been published in this field that add new data, thus rendering necessary the conduct of a new meta-analysis.
In the present meta-analysis, our aim was to assess the safety and feasibility of laparoscopic appendectomy in pregnant women and to compare the perioperative surgical as well as the perinatal outcomes between laparoscopic and open appendectomy.
Materials and methods
The present meta-analysis was designed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and the protocol of has been registered with PROSPERO (CRD42018087261).11
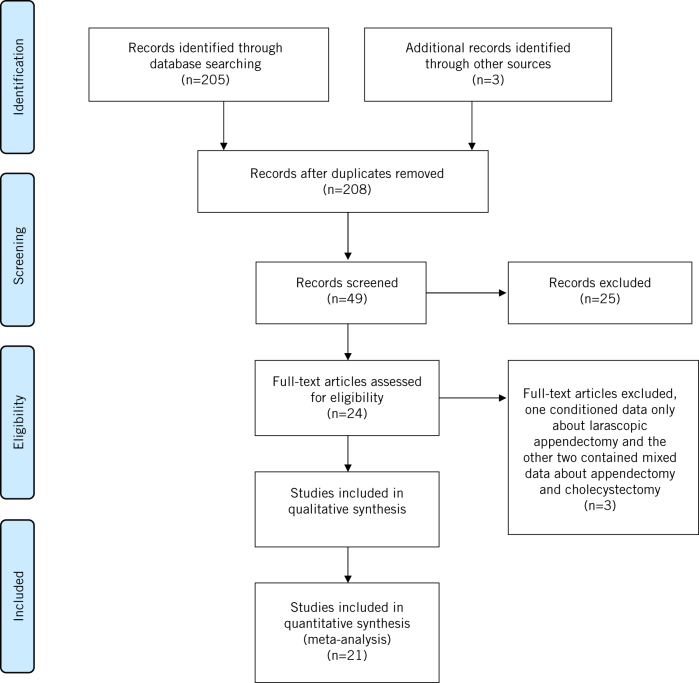
We used the Medline (1966–2018), Scopus (2004–2018), Clinicaltrials.gov (2008–2018), EMBASE (1980–2018), the Cochrane Central Register of Controlled Trials CENTRAL (1999–2018) and Google Scholar (2004–2017) databases in our primary search, together with the reference lists of electronically retrieved full-text papers. The date of our last search was 25 March 2018. Our search strategy included the text words ‘laparoscopic’, ‘laparoscopy’, ‘appendectomy’, ‘appendicitis’ and ‘pregnancy’ and is schematically presented in the PRISMA flow diagram (Fig 1).
Figure 1.
The PRISMA flow chart of study selection.
The studies were selected in three consecutive stages. Following deduplication, the titles and abstracts of all electronic articles were screened by two authors (MF and CN) to assess their eligibility. The decision for inclusion of studies in the present meta-analysis was taken after retrieving and reviewing the full text of articles that were held potentially eligible. Potential discrepancies in this latter stage were resolved by the consensus of all authors.
Types of studies and patients
The eligibility criteria for the inclusion of studies were predetermined. All observational studies and randomised trials that assessed the perioperative outcomes and antenatal/perinatal outcomes of pregnant women who had laparoscopic or open appendectomy during their pregnancy course (irrespective of the trimester of pregnancy) were considered eligible for inclusion. Case reports, experimental animal studies and reviews were not considered eligible for inclusion. In addition, studies that demonstrated mixed surgical and obstetric outcomes after laparoscopic appendectomy and cholecystectomy in combination with studies that presented outcomes only after laparoscopic appendectomy during pregnancy, without comparison to the open technique, were excluded from our meta-analysis (Fig 1).
Outcome measures
Outcome measures were predefined during the design of the present meta-analysis. Fetal loss during the antenatal period was defined as the primary outcome, whereas birth weight, preterm birth, intraoperative duration of appendectomy, Apgar score at one and five minutes, duration of postoperative hospitalisation and wound infection rates were predefined as secondary outcomes.
Data tabulation
Data on variable of interest were tabulated in four structured forms. Table 1 presents the basic study characteristics, the trimesters of pregnancy, the continent of each study and diagnosis of acute appendicitis. Table 2 summarises patient characteristics: maternal age, gestational age at the time of surgery, maternal age at time of delivery, time from the start of symptoms until reaching the emergency department, laboratory findings (e.g. fever and leucocytosis) and trimester of pregnancy of each group of women. Table 3 presents the obstetric outcomes: Apgar scores at one and five minutes, birth weights, preterm birth rates, miscarriage rates, caesarean section frequency and fetal loss rates. Table 4 contains the intraoperative and postoperative parameters: interval from presentation to surgery, wound infection rates, abscess formation, intraoperative time, postoperative hospital stay, overall complications rates and histopathological type of appendix, divided into normal, phlegmonus or complicated.
Table 1.
Study characteristics
| Study | Type of study | Patients (n) | Age (years) | Trimesters of pregnancy | Continent of origin | Diagnosis |
| Curet 199644 | Retrospective | 16 vs 18 | 23.8 vs 22.4 | 1st, 2nd | USA | N/A |
| Gurbuz 199722 | Retrospective | 5 vs 4 | 24.5 vs 24.5 | 1st, 2nd, 3rd | USA | Clinical manifestation, lab. findings (WBC) |
| Conron 199943 | Retrospective | 12 vs 9 | 24.5 ± 1.8 vs 23.7 ± 1.6 | 1st, 2nd ,3rd | USA | N/A |
| Affleck 199916 | Retrospective | 22 vs 18 | N/A | 1st, 2nd, 3rd | USA | N/A |
| Lyass 200125 | Prospective | 11 vs 11 | 27 vs 30 | 1st, 2nd, 3rd | Asia | Clinical examination, laparoscopy |
| Carver 200517 | Retrospective | 17 vs 11 | 23 ± 5 vs 24 ± 7 | 1st, 2nd | USA | N/A |
| McGory 200726 | Retrospective | 454 vs 2.679 | N/A | 1st, 2nd, 3rd | USA | N/A |
| Upadhyay 200730 | Retrospective | 4 vs 2 | 27 vs 27.5 | 1st, 2nd, 3rd | USA | Clinical examination, ultrasound |
| Kirshtein 200923 | Retrospective | 23 vs 19 | 29.8 vs 26.8 | 1st, 2nd | Asia | Clinical examination, ultrasound |
| Sadot 200928 | Retrospective | 48 vs 17 | 29.79 ± 6.2 vs 28.76 ± 5.1 | 1st, 2nd, 3rd | USA | Imaging studies, operative intervention |
| Corneille 201020 | Retrospective | 9 vs 40 | 24 ± 8 vs 26 ± 6 | 1st, 2nd, 3rd | USA | Clinical examination, operative intervention |
| de Bakker 201133 | Retrospective | 17 vs 3 | 32 vs 35 | 1st, 2nd, 3rd | Europe | N/A |
| Eom 201234 | Retrospective | 15 vs 28 | N/A | 1st, 2nd, 3rd | Asia | N/A |
| Kapan 201335 | Retrospective | 10 vs 10 | 27.1 vs 25 | 1st, 2nd, 3rd | Europe | Clinical examination, lab. findings (WBC, NEU), ultrasound |
| Chung 201319 | Retrospective | 22 vs 39 | 29.3 ± 3.1 vs 31.4 ± 4.3 | 1st, 2nd, 3rd | Asia | Clinical examination, ultrasound |
| Peled 201427 | Retrospective | 26 vs 59 | 29.2 ± 4.9 vs 27.6 ± 4.7 | 2nd | Asia | Clinical examination, lab. findings (WBC) |
| Cheng 201518 | Retrospective | 128 vs 653 | 27.28 vs 27.36 | N/A | Asia | N/A |
| Cox 201621 | Retrospective | 894 vs 441 | 27.7 ± 6.2 vs 28.2 ± 6.3 | N/A | USA | N/A |
| Laustsen 201624 | Retrospective | 19 vs 25 | 30.5 vs 30.5 | 1st, 2nd, 3rd | Europe | Clinical examination, ultrasound |
| Segev 201629 | Retrospective | 50 vs 42 | 28 vs 29 | 1st, 2nd, 3rd | Asia | Clinical examination, ultrasound, MRI |
| Winter 201731 | Retrospective | 125 vs 93 | 27 vs 28 | 1st, 2nd, 3rd | Australia | Clinical examination, Operative intervention |
| Yoo 201632 | Retrospective | 24 vs 56 | 30.2 vs 31 | 1st, 2nd, 3rd | Asia | Clinical examination, ultrasound, MRI |
| Karaman 201636 | Retrospective | 12 vs 36 | 27.08 ± 5.48 vs 28.81 ± 8.35 | 1st, 2nd, 3rd | Europe | Clinical examination, ultrasound, lab. findings (WBC) |
lab., laboratory; MRI, magnetic resonance image; N/A, not available; NEU, neutrophil count; vs, compared with; WBC, white blood cell count.
Table 2.
Patient characteristics
| Study | Age (years) | Gestational age (weeks) | Patient delay (hours)a | Fever (oC) | Leucocytosis (WBC > 10 × 109/l) | Trimester | |||
| At surgery | At delivery | 1st | 2nd | 3rd | |||||
| Curet 199644 | 23.8 vs 22.4 | N/A | 38.2 vs 39.2 | N/A | N/A | N/A | 7 vs 8 | 9 vs 10 | None |
| Gurbuz 199722 | 24.5 vs 24.5 | 26 vs 17 | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Conron 199943 | 24.5 ± 1.8 vs 23.7 ± 1.6 | 11.5 ± 2.1 vs 24.9 ± 1.9 | 39.2 ± 0.7 vs 38.4 ± 0.6 | N/A | N/A | N/A | N/A | N/A | N/A |
| Affleck 199916 | N/A | N/A | N/A | N/A | N/A | N/A | 6 vs N/A | 9 vs N/A | 4 vs N/A |
| Lyass 200125 | 27 vs 30 | 16 vs 24 | 40 vs 40 | N/A | N/A | N/A | 5 vs 2 | 4 vs 4 | 2 vs 5 |
| Carver 200517 | 23 ± 5 vs 24 ± 7 | 14 ± 5 vs 14 ± 6 | 38 ± 1 vs 39 ± 1 | N/A | N/A | N/A | 5 vs 4 | 12 vs 7 | None |
| McGory 200726 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Upadhyay 200730 | 27 vs 27.5 | N/A | 31 vs 34 | N/A | N/A | N/A | 0 vs 0 | 0 vs o | 4 vs 2 |
| Kirshtein 200923 | 29.8 vs 26.8 | 12 ± 5 vs 16.2 ± 7.2 | 39.3 ± 1.2 vs 38.9 ± 2.6 | 35.9 ± 29.5 vs 24.2 ± 1 9.3 | 37.2 vs 37.2 | 11.800 vs 13.100 | 23 (total) | 19 (total) | 0 vs 0 |
| Sadot 200928 | 29.79 ± 6.2 vs 28.76 ± 5.1 | 18.1 ± 7.4 vs 24.3 ± 6.7 | 37.9 ± 3.5 vs 38.54 ± 1.48 | 34.4 ± 18.4 vs 33.7 ± 15.8 | 36.96 ± 0.58 vs 36.67 ± 0.82 | 14.220 ± 4.500 vs 13.660 ± 4000 | 14 vs 0 | 32 vs 12 | 2 vs 5 |
| Corneille 201020 | 24 ± 8 vs 26 ± 6 | 11 ± 6 vs 17 ± 9 | 38 ± 5 vs 38 ± 3 | N/A | N/A | N/A | 6 vs 19 | 3 vs 12 | 0 vs 9 |
| de Bakker 201133 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Eom 201234 | N/A | 15 vs 17 | 38 vs 39 | N/A | 0 vs 3 | 15 vs 28 | N/A | N/A | N/A |
| Kapan 201335 | 27,1 vs 25 | 17,8 vs 17,5 | N/A | N/A | N/A | 13.410 vs 14.430 | N/A | N/A | N/A |
| Chung 201319 | 29.3 ± 3.1 vs 31.4 ± 4.3 | 16.4 ± 5.7 vs 16.7 ± 4.8 | 37.1 ± 1.7 vs 38.2 ± 3.5 | N/A | N/A | N/A | 6 vs 8 | 13 vs 20 | 3 vs 11 |
| Peled 201427 | 29.2 ± 4.9 vs 27.6 ± 4.7 | 14.6 ± 6.9 vs 19.3 ± 7.7 | 37.8 ± 2.8 vs 38.6 ± 2.5 | 1.9 ± 1.9 vs 2.3 ± 2.6 | N/A | 13.100 ± 5.300 vs 13.900 ± 3.800 | N/A | N/A | N/A |
| Cheng 201518 | 27.28 vs 27.36 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Cox 201621 | 27.7 ± 6.2 vs 28.2 ± 6.3 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Laustsen 201624 | 30.5 vs 30.5 | 16 vs 22 | N/A | N/A | N/A | N/A | 8 vs 0 | 7 vs 20 | 4 vs 5 |
| Segev 201629 | 28 vs 29 | 16 vs 24 | 38 vs 39 | 24 vs 24 | 5 vs 7 (No) | 38 vs 34 (No) | 20 vs 6 | 29 vs 16 | 1 vs 20 |
| Winter 201731 | 27 vs 28 | 13 vs 22 | N/A | N/A | N/A | N/A | 67 vs 13 | 54 vs 58 | 4 vs 22 |
| Yoo 201632 | 30.2 vs 31 | 19.1 vs 20.7 | 38.4 vs 38.5 | N/A | 37.3 vs 37.1 | 14.700 vs 14.100 | 7 vs 14 | 15 vs 29 | 2 vs 13 |
| Karaman 201636 | 27.08 ± 5.48 vs 28.81 ± 8.35 | 22.42 ± 8.25 vs 25.67 ± 6.57 | 37.25 ± 3.41 vs 36.72 ± 4.84 | N/A | N/A | 13.92 ± 5.10 vs 13.62 ± 5.40 | 1 vs 2 | 7 vs 12 | 4 vs 22 |
N/A, not available; vs, compared with.
a Patient delay: interval from the beginning of symptoms to theatre.
Table 3.
Obstetric parameters
| Study | Apgar | Weight (g) | Preterm labour | Miscarriage | Caesarean section | Fetal loss | |
| 5 minutes | 1 minute | ||||||
| Curet 199644 | 9 vs 9 | 7 vs 8 | 3.290 vs 3.293 | 0 vs 1 | 0 vs 0 | N/A | 0 vs 0 |
| Gurbuz 199722 | N/A | N/A | N/A | 0 vs 0 | 0 vs 0 | N/A | 0 vs 0 |
| Conron 199943 | 9 ± 0.2 vs 8.8 ± 0.1 | 7.4 ± 0.3 vs 7.6 ± 0.3 | 3.360 ± 140 vs 3.270 ± 180 | 0 vs 0 | 0 vs 1 | N/A | 1 vs 0 |
| Affleck 199916 | N/A | N/A | N/A | 3 vs 2 | 0 vs 0 | N/A | 0 vs 0 |
| Lyass 200125 | No statistical difference | No statistical difference | No statistical difference | 0 vs 0 | 0 vs 0 | N/A | 0 vs 0 |
| Carver 200517 | 9 ± 0.6 vs 9 ± 0.5 | 8 ± 2 vs 7 ± 1 | 3.500 ± 500 vs 3.500 ± 400 | 0 vs 0 | 0 vs 0 | 2 vs 3 | 2 vs 0 |
| McGory 200726 | N/A | N/A | N/A | 1 vs 216 | N/A | N/A | 31 vs 88 |
| Upadhyay 200730 | N/A | N/A | N/A | 1 VS 0 | 0 vs 0 | 0 vs 0 | 0 vs 0 |
| Kirshtein 200923 | 10 vs 10 | 8.9 vs 8.7 | 3.165 ± 413 vs 3.118 ± 600 | 0 vs 0 | 1 vs 1 | 6 vs 3 | 0 vs 0 |
| Sadot 200928 | 8.97 ± 0.17 vs 9.06 ± 0.25 | 8.91 ± 0.29 vs 9.0 ± 0 | 3.298 ± 544 vs 3.311 ± 340 | 12 vs 3 | 0 vs 0 | 12 vs 4 | 1 vs 0 |
| Corneille 201020 | 9 ± 2 vs 9 ± 2 | 8 ± 2 vs 8 ± 2 | N/A | 1 vs 5 | 0 vs 3 | N/A | N/A |
| de Bakker 201133 | N/A | N/A | N/A | 1 vs1 | N/A | N/A | 1 vs 0 |
| Eom 201234 | N/A | N/A | 3125 vs 2780 | 0 vs 3 | 1 vs 2 | 0 vs 4 | 0 vs 0 |
| Kapan 201335 | N/A | N/A | N/A | 0 vs 0 | 0 vs 0 | N/A | 0 vs 0 |
| Chung 201319 | 9.8 ± 0.2 vs 9.9 ± 0.1 | 9.2 ± 0.1 vs 9.3 ± 0.2 | 2.810 ± 293 vs 2.790 ± 312 | 2 vs 4 | 0 vs 0 | 6 vs 14 | 0 vs 0 |
| Peled 201427 | 10 ± 0 vs 9.9 ± 0.1 | 8.9 ± 0.2 vs 8.7 ± 1.0 | 3.075 ± 479 vs 3.115 ± 636 | 5 vs 14 | 1 vs 0 | 7 vs 18 | 0 vs 0 |
| Cheng 201518 | N/A | N/A | N/A | 7 vs 74 | 7 vs 37 | 52 vs 258 | N/A |
| Cox 201621 | N/A | N/A | N/A | 0 vs 0 | N/A | N/A | N/A |
| Laustsen 201624 | 9.7 vs 9.3 | 8.7 vs 8.2 | 3.458 vs 3.366 | 3 vs 2 | 0 vs 0 | N/A | 0 vs 0 |
| Segev 201629 | 10 vs 10 | 9 vs 9 | 3.000 vs 3.400 | 5 vs 3 | 0 vs 0 | N/A | 2 vs 2 |
| Winter 201731 | N/A | N/A | N/A | 8 vs 8 | 0 vs 0 | N/A | 7 vs 0 |
| Yoo 201632 | N/A | N/A | 2.860 vs 2.840 | 2 vs 4 | 0 vs 0 | 13 vs 18 | 3 vs 4 |
| Karaman 201636 | N/A | 8.42 ± 1.08 vs 8.11 ± 1.62 | 3030 ± 744 vs 2944 ± 664 | 3 vs 9 | 1 vs 0 | 4 vs 11 | 1 vs 1 |
N/A, not available; vs, compared with.
Table 4.
Surgery and postoperative parameters
| Study | Surgery delay (hours) | Wound Infection | Abscess | Surgery time (minutes) | Length of Stay (days) | Overall complications | Pathology | ||
| Normal | Phlegmonus | Complicated | |||||||
| Curet 199644 | N/A | N/A | N/A | 82 vs 49 | 1.5 vs 2.8 | 6 vs 3 | N/A | N/A | N/A |
| Gurbuz 199722 | N/A | N/A | N/A | 64 vs 58 | 1.2 vs 1.8 | N/A | N/A | N/A | 0 vs 0 |
| Conron 199943 | N/A | N/A | N/A | 51.3 ± 5.5 vs 63.5 ± 7.5 | 1.41 ± 0.15 vs 3.78 ± 0.74 | 1 vs 1 | N/A | N/A | N/A |
| Affleck 199916 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Lyass 200125 | N/A | N/A | N/A | 60 vs 40 | 3.6 vs 5.2 | 1 vs 1 | 1 vs 6 | 10 vs 5 | 0 vs 0 |
| Carver 200517 | N/A | 0 vs 0 | N/A | N/A | 2.6 ± 1.6 vs 2.4 ± 1.4 | 0 vs 0 | N/A | N/A | 0 vs 1 |
| McGory 200726 | N/A | N/A | N/A | N/A | N/A | 32 vs 304 | 245 vs 480 | 122 vs 1507 | 86 vs 697 |
| Upadhyay 200730 | N/A | N/A | N/A | N/A | N/A | 1 vs 1 | 1 vs 0 | 2 vs 2 | 1 vs 0 |
| Kirshtein 200923 | 9.8 ± 7.2 vs 7.6 ± 4.6 | N/A | N/A | 29.9 ± 6.3 vs 28.9 ± 9.2 | 2.4 ± 1.7 vs 1.4 ± 0.5 | 0 vs 0 | 4 vs 1 | 12 vs 17 | 7 vs 1 |
| Sadot 200928 | 11.28 ± 9.55 vs 13.23 ± 7.41 | 1 vs 0 | 0 vs 1 | 54 ± 34 vs 55 ± 25 | 3.44 ± 5.4 vs 4.2 ± 2.1 | 2 vs 1 | 12 vs 3 | 32 vs 10 | 4 vs 4 |
| Corneille 201020 | N/A | N/A | N/A | N/A | 5 ± 4 vs 4 ± 3 | 1 vs 6 | N/A | N/A | N/A |
| de Bakker 201133 | N/A | N/A | N/A | 68 vs 59 | N/A | 1 vs 1 | 3 vs 0 | 8 vs 2 | 1 vs 1 |
| Eom 201234 | N/A | 0 vs 0 | 0 vs 1 | 27.5 vs 55 | 4 vs 5 | 1 vs 7 | 0 vs 0 | 11 vs 2 | 4 vs 6 |
| Kapan 201335 | 6.3 vs 5.7 | N/A | N/A | 59.5 VS 48.2 | 1.1 VS 1.1 | N/A | 3 vs 0 | 7 vs 10 | 0 vs 0 |
| Chung 201319 | N/A | 0 vs 1 | 1 vs 1 | 44.2 ± 16.4 vs 47.3 ± 14.7 | 4.2 ± 2.9 vs 6.9 ± 3.7 | 1 vs 2 | 2 vs 4 | 19 vs 29 | 1 vs 6 |
| Peled 201427 | N/A | N/A | N/A | N/A | 3.7 ± 1.1 vs 3.8 ± 1.2 | 1 vs 15 | 5 vs 10 | 19 vs 37 | 1 vs 10 |
| Cheng 201518 | N/A | N/A | N/A | N/A | 3.8 vs 5.5 | N/A | N/A | N/A | 12 vs 109 |
| Cox 201621 | N/A | 6 vs 17 | 0 vs 0 | 47.1 ± 20.2 vs 52.1 ± 25.1 | 2.3 ± 5.8 vs 3.3 ± 2.5 | 36 vs 31 | 0 vs 0 | 823 vs 392 | 71 vs 49 |
| Laustsen 201624 | N/A | 1 vs 6 | 0 vs 2 | 69 vs 49 | 2.6 vs 5.5 | 1 vs 9 | 3 vs 13 | 13 vs 7 | 3 vs 5 |
| Segev 201629 | 13 vs 12 | 0 vs 5 | 0 vs 0 | 57 vs 60 | 3 vs 5 | 4 vs 10 | 9 vs 11 | 36 vs 23 | 4 vs 7 |
| Winter 201731 | N/A | N/A | N/A | N/A | 3.7 vs 4.5 | N/A | N/A | N/A | N/A |
| Yoo 201632 | N/A | 3 vs 2 | 1 vs 4 | 52.8 vs 53.9 | 5.1 vs 8.1 | 4 vs 6 | 0 vs 0 | 13 vs 42 | 11 vs 14 |
| Karaman 201636 | 18.42 ± 17.15 vs 13.83 ± 13.45 | 0 vs 1 | 0 vs 1 | 49.42 ± 11.38 vs 38.61 ± 11.50 | 3.25 ± 2.45 vs 4.28 ± 3.31 | 0 vs 1 | N/A | N/A | N/A |
N/A, not available; vs, compared with.
Statistical analysis
Statistical meta-analysis was performed using RevMan 5.3 software. Confidence intervals (CI) were set at 95%. We calculated pooled odds ratios (OR), mean differences (MD) and 95% CI with the DerSimonian–Laird random effect model due to the significant heterogeneity in the methodological characteristics of included studies.12 Publication bias was assessed with the funnel plot method in cases of variables that included outcomes from at least 10 studies.13
Sensitivity analysis
Sensitivity analyses were considered, taking into account the fact that each study may have significantly altered the results of the meta-analysis. The fixed effects model results in narrower CI and these might have accounted for non-significance. The idea behind changing the selected model is to evaluate whether, in an ideal world of no heterogeneity, the results of included studies would have been significant. Consequently, leave-one-out meta-analysis was performed to rule out the potential effect of individual studies on the outcomes of the primary meta-analysis. Furthermore, to study the effect of time (year of publication) we performed cumulative meta-analysis and meta-regression analysis. In addition, meta-regression analysis was performed to investigate the effect of the origin of each study on the obstetric and surgical outcomes after appendectomy. Although these analyses do not have a reference to adhere to, we believe that they are rational and that they provide a spherical approach in the field of this meta-analysis. The forest plots of the ‘leave one out’, cumulative meta-analysis and meta-regression analysis were produced with the Open-Meta Analyst statistical software.14
Quality assessment
We evaluated the quality of included studies using the risk of bias in non-randomised studies (ROBINS-I) assessment tool. The tool briefly assesses the possibility of bias that may arise due to confounding, selection, classification, attrition or selective reporting (Table 5).15
Table 5.
Risk of bias in non-randomised studies of interventions (ROBINS-I) tool
| Study | Bias due to confounding | Bias in selection of participants into the study | Bias in classification of interventions | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall bias |
| Curet 199644 | Serious | Low | Low | Moderate | Low | Low | Moderate |
| Gurbuz 199722 | Serious | Low | Low | Low | Low | Low | Moderate |
| Conron 199943 | Serious | Low | Low | Low | Low | Low | Moderate |
| Affleck 199916 | Serious | Low | Low | Moderate | Low | Low | Moderate |
| Lyass 200125 | Moderate | Low | Low | Low | Low | Low | Low |
| Carver 200517 | Moderate | Low | Low | Low | Low | Low | Low |
| McGory 200726 | Moderate | Moderate | Low | Low | Low | Low | Moderate |
| Upadhyay 200730 | Moderate | Low | Low | Low | Low | Low | Low |
| Kirshtein 200923 | Moderate | Low | Low | Low | Low | Low | Low |
| Sadot 200928 | Low | Low | Low | Low | Low | Low | Low |
| Corneille 201020 | Serious | Low | Low | Low | Low | Low | Moderate |
| de Bakker 201133 | Moderate | Low | Low | Low | Low | Low | Low |
| Eom 201234 | Serious | Moderate | Low | Low | Low | Low | Moderate |
| Kapan 201335 | Moderate | Moderate | Low | Low | Low | Moderate | Moderate |
| Chung 201319 | Low | Low | Low | Low | Low | Low | Low |
| Peled 201427 | Low | Low | Low | Low | Low | Low | Low |
| Cheng 201518 | Low | Low | Low | Low | Low | Low | Low |
| Cox 201621 | Moderate | Low | Low | Moderate | Low | Low | Moderate |
| Laustsen 201624 | Moderate | Low | Low | Low | Low | Low | Moderate |
| Segev 201629 | Low | Low | Low | Low | Low | Low | Low |
| Winter 201731 | Low | Low | Low | Low | Low | Low | Low |
| Yoo 201632 | Moderate | Moderate | Low | Moderate | Low | Low | Moderate |
| Karaman 201636 | Low | Moderate | Low | Low | Low | Moderate | Moderate |
Results
Included studies
Twenty-one studies are included in the present meta-analysis,16–36 which presented the postoperative and obstetric outcomes of 6276 pregnant women who underwent appendectomy under the suspicion of acute appendicitis. A total of 1963 pregnant women underwent laparoscopic appendectomy, while 4313 pregnant women underwent an open appendectomy. The enrolled patients, who were divided into two groups (‘laparoscopic’ and ‘open’), according to the surgical technique they underwent, presented no difference in terms of age, gestational age at the operation time, gestational age at the delivery time, delay until they were taken to theatre, fever, leucocytosis and trimester of pregnancy (Table 2).
Outcome of interest
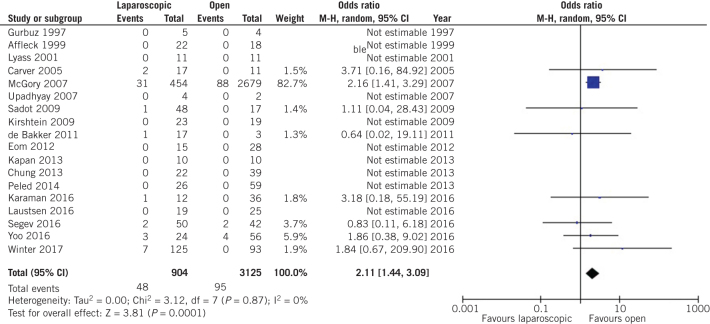
Our analysis outlined a statistically significant increase in fetal loss rates during laparoscopic appendectomy compared with open appendectomy (OR 2.11, 95% CI 1.44–3.09, P = 0.0001; Fig 2).
Figure 2.
Odds ratio according to fetal loss rate. The overall effect was statistically significant (P < 0.0001); vertical line, no difference point between two groups; squares, odds ratios; diamonds, pooled odds ratio for all studies; horizontal lines, 95% confidence interval, CI; M-H, Mantel–Haenszel model.
Secondary outcomes
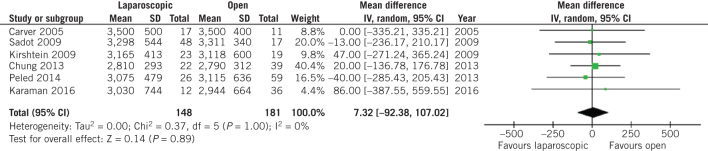
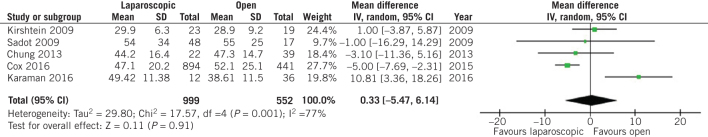
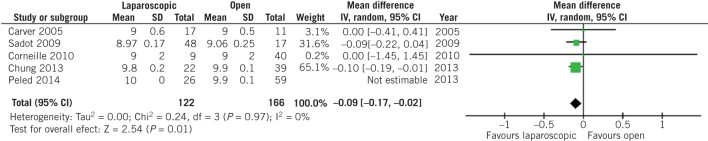
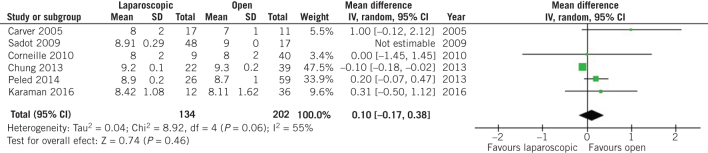
On the other hand, no difference was observed in terms of birth weight at the time of delivery (MD 7.32, 95% CI –92.38 to 107.02, P = 0.89; Fig 3) and preterm birth rates (OR 0.72, 95% CI 0.40 to 1.29, P = 0.27; Fig 4) between patients who underwent open and laparoscopic appendectomy. Intraoperative times did not differ between open and laparoscopic appendectomy (MD 0.33, 95% CI –5.47 to 6.14, P = 0.91; Fig 5). There was a statistically significant decrease in Apgar score at five minutes after delivery in the babies of women who had undergone open appendectomy compared with those of women who had undergone laparoscopic appendectomy during pregnancy (MD –0.09, 95% CI –0.17 to –0,02, P = 0.01; Fig 6). On the other hand, Apgar score at one minute after delivery showed no difference (MD 0.10, 95% CI –0.17 to 0.38, P = 0.46; Fig 7).
Figure 3.
Odds ratio according to neonatal birth weight at time of delivery. The overall effect was not statistically significant (P > 0.05); vertical line, no difference point between two groups; squares, odds ratios; diamonds, pooled odds ratio for all studies; horizontal lines, 95% confidence interval, CI; IV, weighted mean difference.
Figure 4.
Odds ratio according to preterm birth rate. The overall effect was not statistically significant (P > 0.05); vertical line, no difference point between two groups; squares, odds ratios; diamonds, pooled odds ratio for all studies; horizontal lines, 95% CI.
Figure 5.
Odds ratio according to intraoperative duration during appendectomy. The overall effect was not statistically significant (P > 0.05); vertical line, no difference point between two groups; squares, odds ratios; diamonds, pooled odds ratio for all studies; horizontal lines, 95% confidence interval, CI; IV, weighted mean difference.
Figure 6.
Odds ratio according to Apgar score five minutes after delivery. The overall effect was statistically significant (P = 0.01); vertical line, no difference point between two groups; squares, odds ratios; diamonds, pooled odds ratio for all studies; horizontal lines, 95% confidence interval, CI; IV, weighted mean difference.
Figure 7.
Odds ratio according to Apgar score one minute after delivery. The overall effect was not statistically significant (P > 0.05); vertical line, no difference point between two groups; squares, odds ratios; diamonds, pooled odds ratio for all studies; horizontal lines, 95% confidence interval, CI; IV, weighted mean difference.
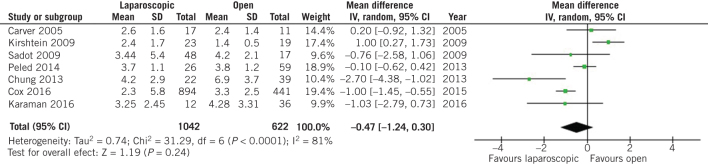
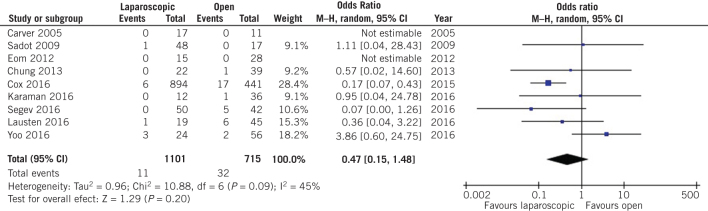
The surgical parameters after appendectomy were similar between the two groups two. More specifically, no difference was observed in terms of postoperative hospital stay duration (MD –0.47, 95% CI –1.24 to 0.30, P = 0.24; Fig 8) or wound infection rates (OR 0.47, 95% CI 0.15 to 1.48, P = 0.20; Fig 9) after open and laparoscopic appendectomy during pregnancy.
Figure 8.
Odds ratio according to postoperative interval of hospital stay. The overall effect was not statistically significant (P > 0.05); vertical line, no difference point between two groups; squares, odds ratios; diamonds, pooled odds ratio for all studies; horizontal lines, 95% confidence interval, CI; IV, weighted mean difference.
Figure 9.
Odds ratio according to wound infection rate after appendectomy. The overall effect was not statistically significant (P > 0.05); vertical line, no difference point between two groups; squares, odds ratios; diamonds, pooled odds ratio for all studies; horizontal lines, 95% confidence interval, CI; M-H, Mantel–Haenszel model.
Sensitivity analysis
A cumulative effect was observed that tended to stabilise the results of our study in terms of OR and 95% CI after 2012, indicating that current knowledge has been established since that time, and that the latter studies do not tend to significantly alter the results of the cumulative analysis (Suppl. figure 1).
Leaving one out meta-analysis outlined that Peled et al27 [MD –0.09, 95% CI –0.17 to –0.02, P = 0.011] affected significantly the comparison of Apgar score at five minutes between women who had undergone open appendectomy during pregnancy compared with those who had undergone laparoscopic appendectomy (Suppl. figure 2). In addition, Sadot et al28 (OR 0.66, 95% CI 0.45 to 0.98, P = 0.039), Laustsen et al24 (OR 0.67, 95% CI 0.46 to 0.99, P = 0.043) and Segev et al29 (OR 0.67, 95% CI 0.45 to 0.99, P = 0.044) could influence the comparison of preterm labour rates between the two groups of patients ( (Suppl. figure 3). All studies that provide data about fetal loss rates could affect the comparison of that outcome between women who underwent open and laparoscopic appendectomy during pregnancy (P < 0.001),16, 17, 19, 22–25, 27–36 except from Mc Gory et al26 (OR 1,58, 95% CI 0.75 to 3.33, P = 0.23; Suppl. figure 2). Yoo et al32 had a strong effect on the comparison of wound infection rates after open and laparoscopic appendectomy during pregnancy (OR 0.25, 95% CI 0.12 to 0.52, P < 0.001; Suppl. figure 2). Finally, Kirshtein et al23 influenced the comparison of postoperative duration of hospital stay (MD –0.73, 95% CI –1.40 to –0.05, P = 0.035; Suppl. figure 2).
Meta-regression analysis outlined that passing years only affected the comparison of neonatal birth weight (P = 0.011) and the comparison of postoperative hospital stay after open and laparoscopic appendectomy during pregnancy (P = 0.044; Suppl. figure 2). The continent of origin of the studies did not affect any comparison between the two groups of patients.
Quality assessment
According to the ROBINS-I tool, 7 studies presented a low possibility of bias due to confounding, 10 studies presented moderate possibility and 6 studies presented a serious possibility of bias; 18 studies presented a low possibility of bias in selection of participants into the study, while 5 presented a moderate possibility. All 23 studies presented a low possibility of bias in classification of interventions and in measurements of outcomes. In addition, 19 studies presented a low possibility of bias due to missing data, while 4 presented a moderate possibility; 21 studies had a low possibility of bias in selection of the reported result, while 2 had a moderate possibility. Finally, 11 studies presented a low possibility of overall bias, while 12 studies presented a moderate possibility of overall bias (Suppl. figure 4).
Discussion
Emergency surgical procedures such us appendectomy or cholecystectomy during pregnancy remained a concern for surgeons and gynaecologists for many years. The overlapping symptoms of these two conditions with the early symptoms of pregnancy and the possible serious complications of a late diagnosis form the necessity for a safe and immediate surgical intervention, without considering the trimester of the pregnant woman or the preferred surgical procedure, open or laparoscopic, as a contraindication. Our meta-analysis outlines that women who had undergone laparoscopic appendectomy during pregnancy, had almost double the risk of fetal loss during delivery compared with women who underwent open appendectomy, regardless of the trimester of pregnancy. Nevertheless, the higher rate of complicated appendicitis among patients who underwent laparoscopic appendectomy could serve as a confounding factor, which possibly led to a weighted prognosis both for the mother and the fetus, resulting in increased fetal loss rate for the laparoscopic group. In addition, open appendectomy during pregnancy seems to be associated with a lower Apgar score at five minutes after the delivery of the neonate compared with the laparoscopic appendectomy. However, despite the fact that the difference in five-minute Apgar score after birth between the groups of women was statistically significant, it seemed to be clinically insignificant, as this difference is quite small and only one study with 60 patients analysed the five-minute Apgar score and presented a statistically significant decrease for the open group.19
On the other hand, all the remaining obstetric outcomes at delivery, such as Apgar score at one minute after delivery, birth weight of the neonatal and preterm birth rates, as well as all surgical outcomes during and after appendectomy, such as wound infection rates, intraoperative duration and postoperative hospital stay duration, seemed to present no correlation with the type of surgical. Furthermore, the passage of time was an important factor that affected the comparison of neonatal birth weight and postoperative hospital stay interval between the two groups, while the continent of origin of each study had no effect.
Our findings are in accordance with previous studies that examined the outcomes after laparoscopic appendectomy in pregnancy. In a previous meta-analysis, Bakker et al demonstrated an increase in fetal loss rate for pregnant women who underwent laparoscopic appendectomy compared with those who had open surgery, while all other obstetric and surgical outcomes (preterm labour rate, postoperative hospital stay, wound infection rates, neonatal birth weight, operation time and Apgar scores) were similar between the two groups.37 In addition, Won et al indicated that despite the fact that pregnant women had higher negative appendectomy rates and a lower possibility of undergoing laparoscopic appendectomy, there was no difference in surgical complications that were not related to pregnancy and, while there was an increase in preterm labour risk during surgery, that risk diminished over time.38 Furthermore, Walker et al outlined the lack of strong evidence between laparoscopic and open appendectomy during pregnancy, nevertheless a slight increase in fetal loss rates for those who underwent laparoscopic appendectomy was demonstrated.39 Preterm labour was the only finding in 2 of 11 pregnant women who underwent laparoscopic appendectomy, as Kocael et al described, but no complications in terms of uterine injury, fetal death or maternal mortality were observed.40 Finally, Park et al reported no maternal or fetal mortality or morbidity, no conversion to laparotomy and no uterine injury in eight pregnant women who underwent laparoscopic appendectomy.41
Our study presents certain methodological strengths. First of all, our protocol has been registered to the international database PROSPERO. In addition, our study group conducted comprehensive literature search following rigorous and systematic methodology and detailed data extraction with pre-piloted forms. Furthermore, the eligible studies underwent standardised quality assessment using the well-validated ROBINS-I tool, which is indicated for assessing non-randomised trials. In comparison with the previous meta-analysis,37 in terms of the issue we investigate, our study includes a larger amount of eligible studies, as many studies have been published since 2012 (the year of publication of the previous meta-analysis), while our study presents more outcomes that underwent more extensive analysis, such us sensitivity analysis.
On the other hand, the present paper has some limitations. As with any systematic review and meta-analysis, certain studies did not report on all outcomes of interest and all statistical analyses were performed using available data. The non-randomised nature, as the most studies are retrospective, and the small number of cases of the majority of the included studies constitute another important limitation of our study, because of the possible implementation of certain selection bias in the study design process. However, the possibility of bias for each particular included study has been interpreted by the ROBINS-I tool, which demonstrated low and moderate possibility of bias for the majority of the included studies.
In addition, it was not possible to perform a multivariate analysis to investigate whether the severity of appendicitis or the surgical technique could affect fetal loss rates as independent factors, due to the fact that the present study is a meta-analysis. Finally, the lack of classification of the obstetric and surgical outcomes according to the trimester of pregnancy from the eligible studies meant that we were unable to investigate the correlation between the postoperative outcomes of laparoscopic and open appendectomy with the trimester of pregnancy in which the operation was conducted.
During the past 20 years that laparoscopy has been implemented many surgical procedures, designing and conducting a randomised trial to compare laparoscopic and open appendicitis during pregnancy seemed a difficult issue, because of the emergent nature of acute appendicitis and the high mortality rate that accompanies it during pregnancy.4 Moreover, conducting a randomised trial is now more difficult, as the findings of recent studies, like ours, demonstrate an increased risk in certain obstetric complications after laparoscopic appendectomy compared with the open procedure, almost double the risk of fetal loss in our study. Nevertheless, all the available outcomes so far have been based on low-quality, non-randomised retrospective studies, so large-scale prospective randomised trials need to be designed and conducted to evaluate which surgical procedure overmatches for managing acute appendicitis in pregnancy. Considering the fact that laparoscopic appendectomy during pregnancy would be an innovative procedure to a novel group of patients, future randomised trials should be programmed according to IDEAL stage 3 framework for surgical innovation, which is the stage of assessment of a novel surgical procedure in a specific patient group.42
In addition, there are indications that the outcomes of each procedure, laparoscopic or open appendectomy, depend on the trimester of pregnancy; thus, in future studies need to be stratified into postoperative and obstetric outcomes according to the trimester of pregnancy, in order to conclude in specified guidelines according to the trimester of pregnancy. On the other hand, gynaecologists should inform pregnant women of the risks of appendicitis and raise awareness of recognising symptoms early, as they overlap the symptoms of pregnancy in its early stages, to avoid complicated appendicitis with generalised peritonitis, which is related to increased morbidity and mortality for mother and fetus. Undoubtedly, targeted training of gynaecologists and general surgeons in laparoscopic surgery is a fundamental requirement to improve the postoperative outcomes of laparoscopic appendectomy and its implementation in clinical practice.
Conclusion
Laparoscopic approach for the management of acute appendicitis in pregnancy does not seem to have worse postoperative surgical and obstetric outcomes than open approach, except for a slight increase in the risk of fetal loss at the time of delivery. Consequently, laparoscopic appendectomy in pregnancy seems to be a relatively safe choice, which presents few differences compared with the open procedure when it is indicated, always considering the experience of the surgeon in such procedures. The design and accomplishment of future studies should guide to this direction, in accordance with all the other factors that would outline the superiority of laparoscopic approach.
References
- 1.Mourad J, Elliott JP, Erickson L, Lisboa L. Appendicitis in pregnancy: new information that contradicts long-held clinical beliefs. Am J Obstet Gynecol 2000; (5): 1,027–1,029. [DOI] [PubMed] [Google Scholar]
- 2.Gilo NB, Amini D, Landy HJ. Appendicitis and cholecystitis in pregnancy. Clin Obstet Gynecol 2009; (4): 586–596. [DOI] [PubMed] [Google Scholar]
- 3.Pastore PA, Loomis DM, Sauret J. Appendicitis in pregnancy. J Am Board Fam Med 2006; (6): 621–626. [DOI] [PubMed] [Google Scholar]
- 4.Reedy MB, Kallen B, Kuehl TJ. Laparoscopy during pregnancy: a study of five fetal outcome parameters with use of the Swedish Health Registry. Am J Obstet Gynecol 1997; (3): 673–679. [DOI] [PubMed] [Google Scholar]
- 5.Andersen B, Nielsen TF. Appendicitis in pregnancy: diagnosis, management and complications. Acta Obstet Gynecol Scand 1999; (9): 758–762. [PubMed] [Google Scholar]
- 6.Stepp K, Falcone T. Laparoscopy in the second trimester of pregnancy. Obstet Gynecol Clin North Am 2004; (3): 485–496. [DOI] [PubMed] [Google Scholar]
- 7.Machado NO, Grant CS. Laparoscopic appendicectomy in all trimesters of pregnancy. JSLS 2009; (3): 384–390. [PMC free article] [PubMed] [Google Scholar]
- 8.Wu JM, Chen KH, Lin H et al. Laparoscopic appendectomy in pregnancy. J Laparoendosc Adv Surg Tech A 2005; (5): 447–450. [DOI] [PubMed] [Google Scholar]
- 9.Weiner E, Mizrachi Y, Keidar R et al. Laparoscopic surgery performed in advanced pregnancy compared to early pregnancy. Arch Gynecol Obstet 2015; (5): 1,063–1,068. [DOI] [PubMed] [Google Scholar]
- 10.Lachman E, Schienfeld A, Voss E et al. Pregnancy and laparoscopic surgery. J Am Assoc Gynecol Laparosc 1999; (3): 347–351. [DOI] [PubMed] [Google Scholar]
- 11.Liberati A, Altman DG, Tetzlaff J et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009; (10): e1–e34. [DOI] [PubMed] [Google Scholar]
- 12.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials 2007; (2): 105–114. [DOI] [PubMed] [Google Scholar]
- 13.Souza JP, Pileggi C, Cecatti JG. Assessment of funnel plot asymmetry and publication bias in reproductive health meta-analyses: an analytic survey. Reprod Health 2007; : 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wallace BC, Dahabreh IJ, Trikalinos T et al. Closing the gap between methodologists and end-users: r as a computational back-end. J Stat Software 2012; (5): 1–15. [Google Scholar]
- 15.Al-Moghrabi D, Salazar FC, Pandis N, Fleming PS. Compliance with removable orthodontic appliances and adjuncts: a systematic review and meta-analysis. Am J Orthodont Dentofac Orthop 2017; (1): 17–32. [DOI] [PubMed] [Google Scholar]
- 16.Affleck DG, Handrahan DL, Egger MJ, Price RR. The laparoscopic management of appendicitis and cholelithiasis during pregnancy. Am J Surg 1999; (6): 523–529. [DOI] [PubMed] [Google Scholar]
- 17.Carver TW, Antevil J, Egan JC, Brown CV. Appendectomy during early pregnancy: what is the preferred surgical approach? Am Surg 2005; (10): 809–812. [PubMed] [Google Scholar]
- 18.Cheng HT, Wang YC, Lo H et al. Laparoscopic appendectomy versus open appendectomy in pregnancy: a population-based analysis of maternal outcome. Surg Endosc 2015; (6): 1,394–1,399. [DOI] [PubMed] [Google Scholar]
- 19.Chung JC, Cho GS, Shin E et al. Clinical outcomes compared between laparoscopic and open appendectomy in pregnant women. Can J Surg 2013; (5): 341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corneille MG, Gallup TM, Bening T et al. The use of laparoscopic surgery in pregnancy: evaluation of safety and efficacy. Am J Surg 2010; (3): 363–367. [DOI] [PubMed] [Google Scholar]
- 21.Cox TC, Huntington CR, Blair L et al. Laparoscopic appendectomy and cholecystectomy versus open: a study in 1999 pregnant patients. Surg Endosc 2016; (2): 593–602. [DOI] [PubMed] [Google Scholar]
- 22.Gurbuz AT, Peetz ME. The acute abdomen in the pregnant patient: Is there a role for laparoscopy? Surg Endosc 1997; (2): 98–102. [DOI] [PubMed] [Google Scholar]
- 23.Kirshtein B, Perry ZH, Avinoach E et al. Safety of laparoscopic appendectomy during pregnancy. World J Surg 2009; (3): 475–480. [DOI] [PubMed] [Google Scholar]
- 24.Laustsen JF, Bjerring OS, Johannessen O, Qvist N. Laparoscopic appendectomy during pregnancy is safe for both the mother and the fetus. Dan Med J 2016; (8): pii: A5259. [PubMed] [Google Scholar]
- 25.Lyass S, Pikarsky A, Eisenberg V et al. Is laparoscopic appendectomy safe in pregnant women? Surg Endosc 2001; (4): 377–379. [DOI] [PubMed] [Google Scholar]
- 26.McGory ML, Zingmond DS, Tillou A et al. Negative appendectomy in pregnant women is associated with a substantial risk of fetal loss. J Am Coll Surg 2007; (4): 534–540. [DOI] [PubMed] [Google Scholar]
- 27.Peled Y, Hiersch L, Khalpari O et al. Appendectomy during pregnancy: is pregnancy outcome depending by operation technique? J Matern Fetal Neonatal Med 2014; (4): 365–367. [DOI] [PubMed] [Google Scholar]
- 28.Sadot E, Telem DA, Arora M et al. Laparoscopy: a safe approach to appendicitis during pregnancy. Surg Endosc 2010; (2): 383–389. [DOI] [PubMed] [Google Scholar]
- 29.Segev L, Segev Y, Rayman S et al. Appendectomy in pregnancy: appraisal of the minimally invasive approach. J Laparoendosc Adv Surg Tech A 2016; (11): 893–897. [DOI] [PubMed] [Google Scholar]
- 30.Upadhyay A, Stanten S, Kazantsev G et al. Laparoscopic management of a nonobstetric emergency in the third trimester of pregnancy. Surg Endos 2007; (8): 1,344–1,348. [DOI] [PubMed] [Google Scholar]
- 31.Winter NN, Guest GD, Bozin M et al. Laparoscopic or open appendicectomy for suspected appendicitis in pregnancy and evaluation of foetal outcome in Australia. A N Z J Surg 2017; (5): 334–338. [DOI] [PubMed] [Google Scholar]
- 32.Yoo KC, Park JH, Pak K et al. Could laparoscopic appendectomy in pregnant women affect obstetric outcomes? A multicenter study. Int J Colorectal Dis 2016; (8): 1,475–1,481. [DOI] [PubMed] [Google Scholar]
- 33.de Bakker JK, Dijksman LM, Donkervoort SC. Safety and outcome of general surgical open and laparoscopic procedures during pregnancy. Surg Endosc 2011; (5): 1,574–1,578. [DOI] [PubMed] [Google Scholar]
- 34.Eom JM, Hong JH, Jeon S et al. Safety and clinical efficacy of laparoscopic appendectomy for pregnant women with acute appendicitis. Ann Acad Med Singapore 2012; (2): 82–86. [PubMed] [Google Scholar]
- 35.Kapan S, Bozkurt MA, Turhan A et al. Management of acute appendicitis in pregnancy. Ulus Travma Acil Cerrahi Derg 2013; (1): 20–24. [DOI] [PubMed] [Google Scholar]
- 36.Karaman E, Aras A, Cim N et al. Maternal and fetal outcomes after laparoscopic vs. open appendectomy in pregnant women: data from two tertiary referral centers. Ginekol Pol 2016; (2): 98–103. [DOI] [PubMed] [Google Scholar]
- 37.Bakker OJ. Systematic review and meta-analysis of safety of laparoscopic versus open appendicectomy for suspected appendicitis in pregnancy. Br J Surg 2012; : 1,470–1,478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Won RP, Friedlander S, Lee SL. Management and outcomes of appendectomy during pregnancy. Am Surg 2017; (10): 1,103–1,107. [PubMed] [Google Scholar]
- 39.Walker HG, Al Samarae A, Mills SJ, Kalbassi MR. Laparoscopic appendicectomy in pregnancy: a systematic review of the published evidence. Int J Surg 2,014; (11): 1,235–1,241. [DOI] [PubMed] [Google Scholar]
- 40.Kocael PC, Simsek O, Saribeyoglu K et al. Laparoscopic surgery in pregnant patients with acute abdomen. Ann Ital Chir 2015; (2): 137–142. [PubMed] [Google Scholar]
- 41.Park SH, Park MI, Choi J et al. Laparoscopic appendectomy performed during pregnancy by gynecological laparoscopists. Eur J Obstet Gynecol Reprod Biol 2010; (1): 44–48. [DOI] [PubMed] [Google Scholar]
- 42.Cook JA, McCulloch P, Blazeby J et al. IDEAL framework for surgical innovation 3: randomised controlled trials in the assessment stage and evaluations in the long term study stage. BMJ 2013; : f2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conron RW Jr, Abbruzzi K, Cochrane S et al. Laparoscopic procedures in pregnancy. Am Surg 1999; (3): 259–263. [PubMed] [Google Scholar]
- 44.Curet MJ, Allen D, Josloff R et al. Laparoscopy during pregnancy. Arch Surg 1996; (5): 546–551. [DOI] [PubMed] [Google Scholar]