Summary
Multifocal papillary thyroid carcinoma (PTC) is common and the number of tumor foci rarely exceeds ten. The mechanism of multifocal disease is debated, with the two main hypotheses consisting of either intrathyroidal metastatic spread from a single tumor or independent multicentric tumorigenesis from distinct progenitor cells. We report the case of a 46-year-old woman who underwent total thyroidectomy and left central neck lymph node dissection after fine-needle aspiration of bilateral thyroid nodules that yielded cytological findings consistent with PTC. Final pathology of the surgical specimen showed an isthmic dominant 1.5 cm classical PTC and over 30 foci of microcarcinoma, which displayed decreasing density with increasing distance from the central lesion. Furthermore, all malignant tumors and lymph nodes harbored the activating BRAF V600E mutation. The present case highlights various pathological features that support a mechanism of intraglandular spread, namely a strategic isthmic location of the primary tumor, radial pattern of distribution and extensive number of small malignant foci and BRAF mutational homogeneity.
Learning points:
Multifocal papillary thyroid carcinoma (PTC) is commonly seen in clinical practice, but the number of malignant foci is usually limited to ten or less.
There is no clear consensus in the literature as to whether multifocal PTC arises from a single or multiple distinct tumor progenitor cells.
Strategic location of the dominant tumor in the thyroid isthmus may favor intraglandular dissemination of malignant cells by means of the extensive lymphatic network.
An important pathological finding that may be suggestive of intrathyroidal metastatic spread is a central pattern of distribution with a reduction in the density of satellite lesions with increasing distance from the dominant focus.
PTCs originating from the isthmus with intraglandular metastatic dissemination behave more aggressively. As such, a more aggressive treatment course may be warranted, particularly with regard to the extent of surgery.
Background
Multifocal papillary thyroid carcinoma (PTC) is common, with prevalence rates in the range of 18–87% (1). Recently reported series on multifocal PTC and microcarcinoma found a mean number of 2.7 (range: 2–8) (2) and 1.6 (range: 1–10) (3) foci, respectively. The highest number of tumor foci in a case series of PTC published in the literature is 125, although the corresponding pathological features of the lesions are not described (4).
The mechanism by which multifocality in PTC occurs remains unclear. There is controversy in the literature as to whether non-contiguous tumor foci originate independently as a result of distinct genetic mutations or arise from intrathyroidal dissemination of malignant cells from a single primary tumor, presumably via the lymphatic drainage system. Previous studies have used mutational analysis and X chromosome inactivation techniques to address this question, but taken collectively, the results have not clearly favored one theory over the other (1).
Case presentation
A previously healthy 46-year-old woman was referred to our thyroid clinic for evaluation of incidentally discovered bilateral thyroid nodules. She had no risk factors for thyroid cancer and was euthyroid. There was no reported family history of thyroid disease.
Investigation
Initial thyroid function tests were consistent with euthyroidism, with a normal TSH at 3.22 mIU/L (0.40–4.40). The measured level of anti-thyroid peroxidase antibodies (anti-TPO) at <0.35 IU/mL (0.0–9.0) was within normal limits.
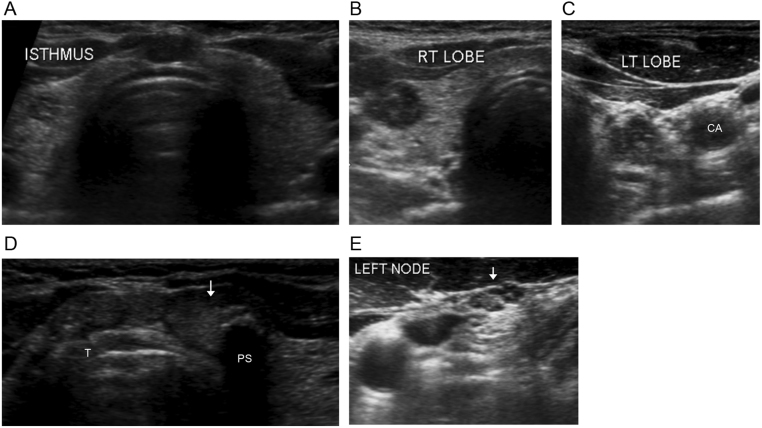
A thyroid ultrasound demonstrated the presence of six hypoechoic nodules, including three in the isthmus (Fig. 1). There were two nodules in the lower pole of the left lobe measuring 0.9 × 1.3 cm and 0.6 × 0.6 cm, containing cystic spaces and calcifications. There was one nodule at the upper pole of the right thyroid lobe measuring 0.8 × 1.0 cm with small cystic spaces. In addition, central neck lymph nodes containing cystic spaces and calcifications, suspicious for metastatic involvement, were seen. Given the highly suspicious sonographic features, ultrasound-guided fine-needle aspiration (FNA) of the dominant left- and right-sided nodules was carried out. Results of cytological analysis revealed atypia of undetermined significance (Bethesda category III) (5). FNA was therefore repeated, and cytology of the left nodule on this occasion was consistent with PTC (Bethesda category VI).
Figure 1.
Transverse views of preoperative neck ultrasound findings. (A) The isthmus is prominent and nodular and the hypoechoic right-sided nodule can be appreciated in this image showing the entire thyroid gland. Close-up views of the right (B) and left (C) thyroid lobes reveal the presence of hypoechoic nodules with cystic spaces. (C) Calcifications are present in the left-sided nodule. The carotid artery (CA) can be seen lateral to the nodule in the left lobe. (D) The isthmus, which lies anterior to the trachea (T), contains three discrete hypoechoic nodules. The largest nodule (arrow), located to the left of midline, contains a calcification with posterior shadowing (PS). (E) Left-sided lymph node (arrow) containing cystic spaces and calcifications, suspicious for metastatic involvement.
Treatment
The patient underwent total thyroidectomy with left central neck lymph node dissection. The postoperative course was uncomplicated, and she was started on levothyroxine at a dose of 150 µg daily thereafter.
Outcome and follow-up
Final pathology of the surgical specimen was that of an isthmic dominant 1.5 cm classical PTC (Figs 2 and 3) with minimal extrathyroidal extension, along with more than 30 foci of microcarcinoma, one of which showed lymphovascular invasion. Interestingly, there was a diminishing gradient of malignant foci from the isthmus to the lateral aspects of the thyroid lobes (Fig. 4). Seven harvested lymph nodes (six from the central compartment and one Delphian) had metastatic PTC (Fig. 5) The size of the largest metastatic focus was 2.4 cm and extranodal extension was present. This corresponds to a tumor stage of pT1bpN1a (6). Immunohistochemical staining for the activating BRAF V600E mutation was carried out, and all malignant foci were positive (Figs 2, 4 and 5).
Figure 2.
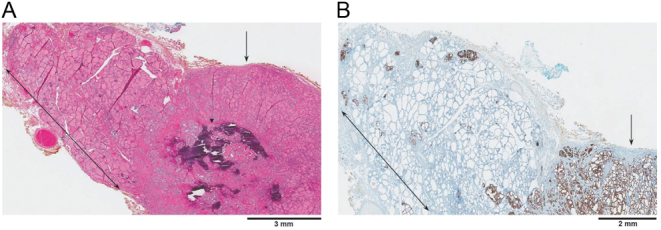
Microphotograph of the dominant 1.5 cm PTC in the isthmus (arrow). The tumor is well circumscribed and contains calcifications of non-psammomatous type (arrowhead). Thyroid tissue adjacent to the main tumor (double-headed arrow) shows multiple scattered foci of micro PTC. (A) Hematoxylin and eosin stain, X10. (B) BRAF V600E immunohistochemical stain, X10.
Figure 3.
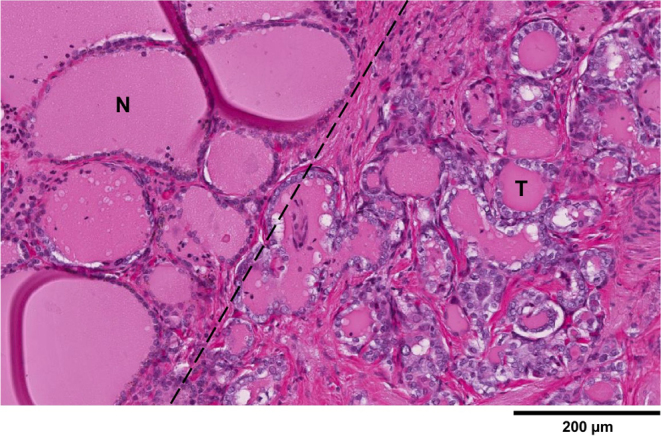
Isthmic tumor (T) cells, depicted in the follicles on the right, are lined by nuclei that are enlarged, clear, and overlapping with irregular contours. This is in contrast to cells in normal (N) follicles, seen on the left, which have smaller nuclei. Hematoxylin and eosin stain, X100.
Figure 4.
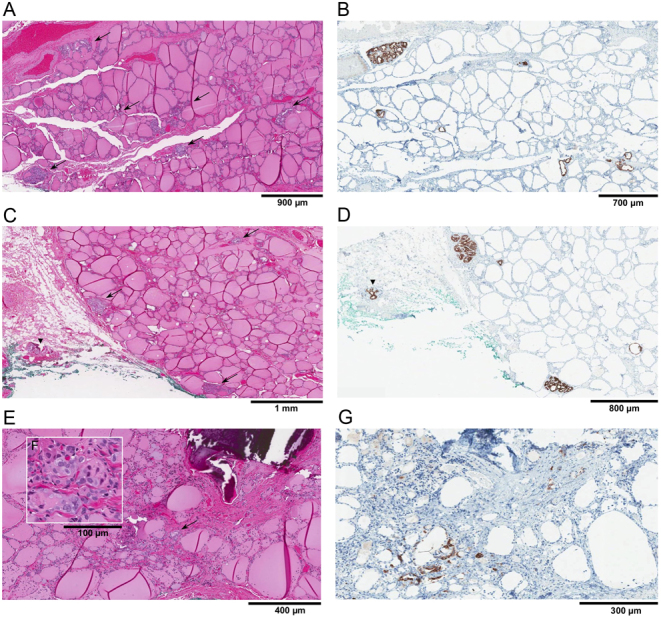
Decreasing density of malignant foci (arrows) with increasing distance from the central tumor. This phenomenon was observed in both thyroid lobes, but only representative images from the right side are depicted. Corresponding hematoxylin and eosin (A, C and E) and BRAF V600E immunohistochemistry (B, D and F) stains are shown. (A and B) Cuts from the lower right thyroid lobe close to the isthmus. (C and D) Cuts from the middle right thyroid lobe. Lymphovascular invasion (arrowhead) can be seen in extrathyroidal fat tissue. (E and G) Cuts from the superior right thyroid lobe, furthest away from the isthmic tumor. The inset (F) shows a higher magnification of malignant cells (hematoxylin and eosin, X200). (A, C and E) Hematoxylin and eosin stain, X20 (A and C), X40 (E). (B, D and G) BRAF V600E immunohistochemical stain, X20 (B and D), X40 (G).
Figure 5.
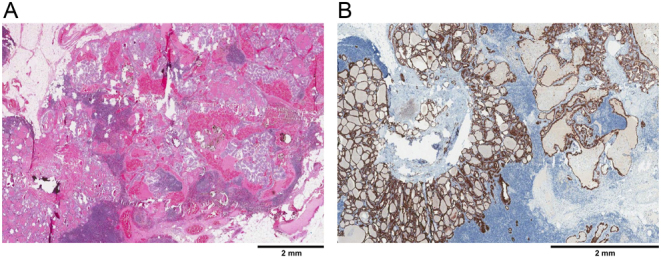
Lymph node showing metastatic PTC. (A) Hematoxylin and eosin stain, X20. (B) BRAF V600E immunohistochemical stain, X20.
The patient was placed on suppressive levothyroxine treatment post total thyroidectomy and she also received an ablative dose of 150 mCi of I-131 radioactive iodine under thyrogen stimulation.
Discussion
Multifocality in PTC is a common occurrence in clinical practice, but the clonal relationship between individual tumors remains uncertain. Multiple synchronous tumor foci might develop either through intrathyroidal metastasis of a single malignant clone or from independent progenitor clones (1).
One strategy employed in previous studies to better understand the clonal derivation of multiple tumors has been through BRAF V600E mutational analysis. The BRAF V600E mutation is a well-characterized oncogenic driver mutation and represents the most common genetic event in PTC. By establishing the presence or absence of the BRAF V600E mutation in individual tumor foci, one can determine if there is concordance or discordance among multiple tumors that arise from a single thyroid gland. In tumors that exhibit discordant BRAF V600E mutation status (i.e. at least one tumor focus harbors the mutation and at least one does not), it may be inferred that individual tumors arise independently. However, in the case of BRAF V600E concordance, one has to be more cautious in drawing conclusions concerning clonal origin as it is not possible to distinguish with certainty between a single clonal progenitor that has disseminated throughout the gland and concurrent, unrelated tumor clones that have independently acquired the BRAF V600E driver mutation (1, 7, 8, 9).
Published series to date using BRAF mutational analysis report discordance rates as high as 40% (7, 8). Of note, when reported, the majority of included cases consist of up to three tumor foci (7, 8, 9); the highest number of explicitly documented malignant nodules is nine. Interestingly, in a case series of 57 tumors from 27 patients with multifocal PTC, cases with at least four nodules tended to have concordant BRAF V600E mutation status (8 of 9, or 89%) as compared to those with three nodules or less (9 of 18, or 50%), although this did not reach statistical significance (9).
Our case is unique in displaying more than 30 diffusely spread foci of PTC with mutational homogeneity insofar that all foci harbored the activating BRAF V600E mutation.
A recent publication by Jin et al. described a case of multifocal PTC with a dominant 2.2 cm tumor located in the isthmus and scattered bilateral foci of micro-PTC distributed around the central lesion in a radial form, with lesion density decreasing with increasing distance from the isthmus. These pathological features are strikingly similar to those observed in our case. In the reported case, two out of three harvested lymph nodes were positive for metastatic PTC but results of BRAF mutational analysis were not available (10).
Several findings in our patient and in the recently reported case by Jin et al. are consistent with intrathyroidal metastasis from a primary dominant malignant focus. The isthmic location of the primary tumor provides optimal conditions for spread of tumor cells via the rich lymphatic system, as drainage between thyroid lobes occurs through the isthmus (11). The ‘fanning out’ pattern of distribution with tumor density decreasing with increasing distance from the central lesion further strengthens the argument for intraglandular spread by means of the lymphatic network. The presence of over 30 foci of microcarcinoma makes the possibility of a corresponding number of genetic events arising independently from one another highly unlikely. Furthermore, all intrathyroidal lesions and lymph node metastases harbored the BRAF V600E mutation. These observations are also in accordance with the known biological effects of the activating BRAF mutation, namely promoting regional lymph node metastases through the lymphatic system (12).
In conclusion, we report the case of multifocal PTC with over 30 tumor foci along with pathological features consistent with intrathyroidal metastatic dissemination of a single dominant focus originating in the isthmus. Recent published studies have emphasized that PTCs located in the isthmus are more aggressive with increased risks of multifocality, capsular invasion, extrathyroidal extension and central lymph node involvement (11, 13). The cascade of a PTC sited in the isthmus with ensuing intrathyroidal spread would enhance the aggressiveness of this phenotype. Based on our experience and review of the literature, we have created a list of parameters and potential hallmarks to facilitate the recognition of such multifocal PTCs (Table 1). Additional patients harboring large numbers of intrathyroidal malignant foci should be studied to validate and refine these proposed features, occurring singly or in various combinations.
Table 1.
Multifocal PTC: Proposed hallmarks of intrathyroidal metastatic dissemination.
| Features of multifocal PTC consistent with intraglandular metastasis from a single primary tumor |
| Isthmic or near-isthmic location of the primary focus |
| Large number of malignant foci |
| Predominance of microcarcinoma foci |
| Fanning-out pattern of malignant foci from the dominant tumor |
| Mutational homogeneity of the malignant PTC clones |
PTC, papillary thyroid carcinoma.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Patient consent
Written informed consent has been obtained from the patient for publication of the submitted article and accompanying images.
Author contribution statement
J How, E Mitmaker and R Tabah were directly involved in the management of the patient. O Ajise undertook the pathological analysis of the surgical specimen and provided corresponding images. J Pancer drafted the case report. All of the authors contributed to and approved the final draft of the report.
References
- 1.Iacobone M, Jansson S, Barczynski M, Goretzki P. Multifocal papillary thyroid carcinoma – a concensus report of the European Society of Endocrine Surgeons (ESES). Langenbeck’s Archives of Surgery 2014. 399 141–154. ( 10.1007/s00423-013-1145-7) [DOI] [PubMed] [Google Scholar]
- 2.Kim DW, Shin GW, Lee YJ, Jung SJ, Baek HJ, Kang T. Sonographic features of multifocal papillary thyroid carcinomas. Endocrine Practice 2018. 24 351–360. ( 10.4158/EP-2017-0205) [DOI] [PubMed] [Google Scholar]
- 3.So YK, Kim MW, Son YI. Multifocality and bilaterality of papillary thyroid microcarcinoma. Clinical and Experimental Otorhinolaryngology 2015. 8 174–178. ( 10.3342/ceo.2015.8.2.174) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katoh R, Sasaki J, Kurihara H, Suzuki K, Iida Y, Kawaoi A. Multiple thyroid involvement (intraglandular metastasis) in papillary thyroid carcinoma: a clinicopathologic study of 105 consecutive patients. Cancer 1992. 70 1585–1590. [DOI] [PubMed] [Google Scholar]
- 5.Cibas ES, Ali SZ. The 2017 Bethesda system for reporting thyroid cytopathology. Thyroid 2017. 27 1341–1346. ( 10.1089/thy.2017.0500) [DOI] [PubMed] [Google Scholar]
- 6.Tuttle RM, Haugen B, Perrier ND. The updated AJCC/TNM staging system for differentiated and anaplastic thyroid cancer (8th edition): what changed and why? Thyroid 2017. 27 751–756. ( 10.1089/thy.2017.0102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park SY, Park YJ, Lee YJ, Lee HS, Choi SH, Choe G, Jang HC, Park SH, Park DJ, Cho BY. Analysis of differential BRAFV600E mutational status in multifocal papillary thyroid carcinoma: evidence of independent clonal origin in distinct tumor foci. Cancer 2006. 107 1831–1838. ( 10.1002/cncr.22218) [DOI] [PubMed] [Google Scholar]
- 8.Giannini R, Ugolini C, Lupi C, Proietti A, Elisei R, Salvatore G, Berti P, Materazzi G, Miccoli P, Santoro M, et al The heterogeneous distribution of BRAF mutation supports the independent clonal origin of distinct tumor foci in multifocal papillary thyroid carcinoma. Journal of Clinical Endocrinology and Metabolism 2007. 92 3511–3516. ( 10.1210/jc.2007-0594) [DOI] [PubMed] [Google Scholar]
- 9.Kimbrell HZ, Sholl AB, Ratnayaka S, Japa S, Lacey M, Carpio G, Bhatia P, Kandil E. BRAF testing in multifocal papillary thyroid cancer. BioMed Research International 2015. 2015 486391 ( 10.1155/2015/486391) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin H, Yan H, Tang H, Zheng M, Wu C, Liu J. Internal spreading of papillary thyroid carcinoma: a case report and systemic review. Case Reports in Endocrinology 2018. 2018 7618456 ( 10.1155/2018/7618456) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vasileiadis I, Boutzios G, Karalaki M, Misiakos E, Karatzas T. Papillary thyroid carcinoma of the isthmus: total thyroidectomy or isthmusectomy? American Journal of Surgery 2018. 216 135–139. ( 10.1016/j.amjsurg.2017.09.008) [DOI] [PubMed] [Google Scholar]
- 12.Li C, Lee KC, Schneider EB, Zeiger MA. BRAF V600E mutation and its association with clinicopathological features of papillary thyroid cancer: a meta-analysis. Journal of Clinical Endocrinology and Metabolism 2012. 97 4559–4570. ( 10.1210/jc.2012-2104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pontiere G, Urselli F, Peschi L, Liccardi A, Ruggiero AR, Vergara E, Bellevicine C, Troncone G, De Palma M, Biondi B. Is the isthmus location an additional risk factor for indeterminate thyroid nodules? Case report and review of the literature. Frontiers in Endocrinology 2018. 9 750 ( 10.3389/fendo.2018.00750) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a