Summary
We report a case of rapid pleural effusion after discontinuation of lenvatinib. A 73-year-old woman was diagnosed with poorly differentiated thyroid cancer with right pleural metastasis. Weekly paclitaxel treatment was performed for 18 weeks, but it was not effective. Oral administration of lenvatinib, a multi-target tyrosine kinase inhibitor, reduced the size of cervical and thoracic tumors and lowered serum thyroglobulin levels. Lenvatinib was discontinued on day 28 because of Grade 2 thrombocytopenia and Grade 3 petechiae. Seven days after discontinuation of lenvatinib, the patient was hospitalized because of dyspnea and right pleural effusion. Pleural effusion rapidly improved with drainage and re-initiation of lenvatinib and did not recur. Anorexia caused by lenvatinib led to undernutrition, which resulted in death 13 months after initiation of lenvatinib. Autopsy revealed extensive necrosis with primary and metastatic lesions, suggesting that the patient responded to lenvatinib. Physicians should be aware of the possibility of flare-up in patients with thyroid cancer treated with lenvatinib.
Learning points:
Autopsy findings revealed that lenvatinib was efficacious in treating poorly differentiated thyroid cancer without primary lesion resection.
Flare-up phenomenon may occur in thyroid cancer treated with lenvatinib.
Attention should be paid to flare-up phenomenon within a few days of discontinuing lenvatinib.
Background
Poorly differentiated or anaplastic thyroid cancer has aggressive behavior and poor prognosis (1). In 2015, the SELECT study showed that lenvatinib, a multi-tyrosine kinase inhibitor (TKI), prolonged progression-free survival (PFS) of radioiodine-refractory differentiated thyroid cancer with primary tumor resection compared to placebo (2). The efficacy of lenvatinib was also confirmed in patients with poorly differentiated or anaplastic thyroid cancer (3). In some cases, rapid disease progression after TKI discontinuation was observed, which was called the flare-up phenomenon (4). No cases of flare-up phenomenon after discontinuation of lenvatinib in thyroid cancer have been reported. Here, we report rapid development of pleural effusion after discontinuation of lenvatinib in a patient with poorly differentiated thyroid cancer without primary tumor resection.
Case presentation
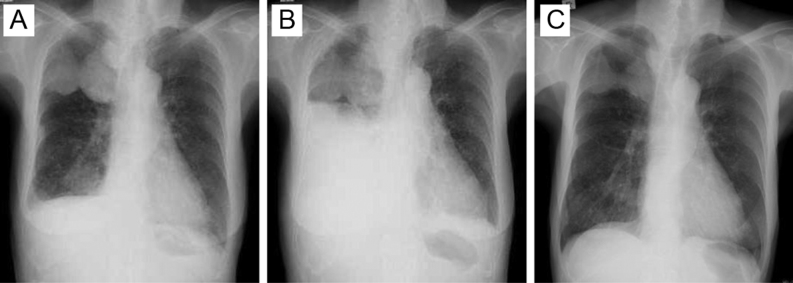
A 73-year-old woman was referred to our hospital because of abnormal shadows on chest X-ray (Fig. 1A). Computed tomography (CT) revealed solid tumors in the right anterior cervix, lungs and right pleura. Fine-needle aspiration biopsy of the thyroid and CT-guided biopsy of the pleural tumor showed thyroid tumor cells with limited evidence of follicular cell differentiation with an insular pattern and convoluted nuclei. The diagnosis was poorly differentiated thyroid cancer. The patient underwent weekly paclitaxel therapy (80 mg/m2) for 18 weeks. However, cervical tumors grew from 4.8 to 6.4 cm and serum thyroglobulin (Tg) levels increased from 2330 to 18 800 ng/mL. We chose to administer lenvatinib because of the multiple lung and pleural metastases and the lack of any emergent condition that would cause obstructive complications. Two weeks following treatment with daily oral lenvatinib (24 mg/day), cervical and pleural tumors decreased in size. Lenvatinib was discontinued for 1 week on day 28 because of Grade 2 thrombocytopenia (52 000/μL) and Grade 3 petechiae according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE, version 4.0). The patient was admitted to the hospital because of dyspnea 7 days after discontinuation of lenvatinib.
Figure 1.
Chest X-ray before and after re-starting lenvatinib. (A) Before discontinuation of lenvatinib. (B) Massive right pleural effusion 7 days after discontinuation of lenvatinib. (C) Smaller pleural effusion and lung shadows 6 months after re-starting lenvatinib.
Investigation
On examination, height was 151.7 cm and weight was 41.7 kg. Temperature was 37.7°C, blood pressure was 140/90 mmHg, heart rate was 110 beats per minute and respiratory rate was 36 breaths per minute. Arterial blood analysis showed hypoxia. We palpated thyroid tumors, but heard no sounds indicating airway obstruction. Auscultation revealed no breath sounds in the right lung field. Chest X-ray showed massive right pleural effusion (Fig. 1B). The pleural fluid was exudative with pleural Tg levels of 21 800 ng/mL, suggesting malignant pleural effusion from thyroid cancer.
Treatment
A chest tube was inserted to drain the pleural effusion and lenvatinib (20 mg/day) was re-started. Chest tube was removed shortly afterwards, with no pleural effusion recurrence after lenvatinib therapy was re-started (Fig. 1C).
Outcome and follow-up
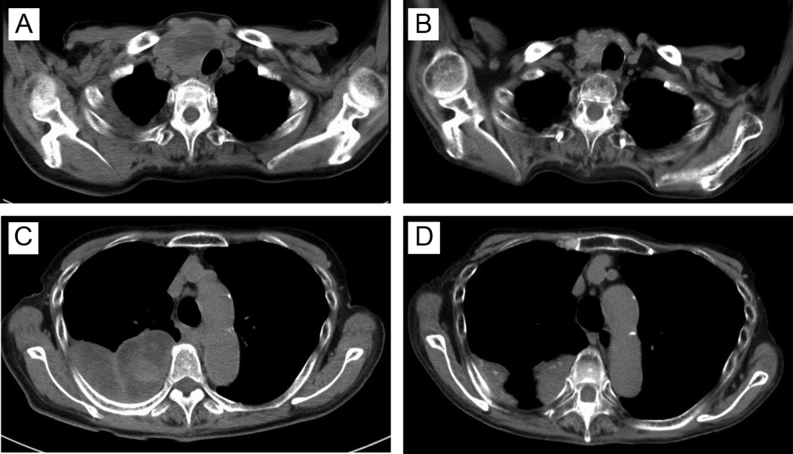
At 12 months after re-starting lenvatinib treatment, serum Tg levels decreased from 18 800 to 450 ng/mL. Simultaneous CT and positron emission tomography (PET-CT) revealed that the cervical tumor regressed from 6.4 to 4.0 cm with no evidence of new metastases (Fig. 2). Partial response based on the Response Evaluation Criteria in Solid Tumors (RECIST) was achieved. Thyroid function testing results before administration of lenvatinib were as follows: TSH 0.59 μU/mL, FT3 3.96 pg/mL and FT4 0.46 ng/dL. Hypothyroidism developed gradually during lenvatinib treatment. We prescribed levothyroxine, which was increased up to 150 μg per day. Cervical CT revealed shrinkage of thyroid tumors, which was consistent with hypothyroidism. Severe anorexia occurred as an adverse effect of lenvatinib. Neither dose reduction of lenvatinib (14 mg/day) nor administration of dexamethasone improved anorexia. Undernutrition and aspiration pneumonia led to poor systemic condition. Thirteen months after lenvatinib therapy began, the patient died because of respiratory and circulatory failure. Histopathology obtained during autopsy showed extensive necrosis in the primary lesion (80%) and metastatic lesions in the anterior mediastinum lymph nodes (60%) and the pleura (40%), consistent with effective treatment of thyroid cancer with pleural metastasis (Fig. 3).
Figure 2.
Computed tomography imaging before and after lenvatinib treatment. (A) Cervical tumor before administration of lenvatinib. (B) Shrinkage of cervical tumor 12 months after administration of lenvatinib. (C) Pleural metastases before administration of lenvatinib. (D) Size reduction of pleural metastases at 12 months after administration of lenvatinib.
Figure 3.
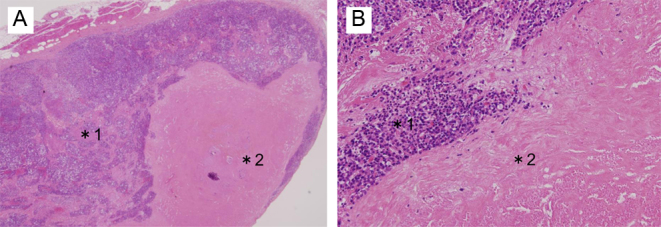
Histopathology of the pleural metastatic tumor obtained during autopsy showed tumor cells (*1) and extensive necrosis (*2). Hematoxylin-eosin staining, original magnification: ×20 (A), ×200 (B).
Discussion
Lenvatinib is a multi-TKI that targets vascular endothelial growth factor (VEGF) receptors 1–3, fibroblast growth factor receptors (FGFR) 1–4, RET, c-kit and platelet-derived growth factor receptor-alpha (PDGFRα). It inhibits tumor growth and angiogenesis. Lenvatinib was approved for the treatment of unresectable hepatocellular carcinoma and radioiodine-refractory differentiated thyroid cancer. Lenvatinib was shown to prolong progression-free survival (PFS) compared to sorafenib in patients with unresectable hepatocellular carcinoma (7.4 vs 3.7 months) in the REFLECT trial (5). Lenvatinib significantly prolonged PFS in patients with radioiodine-refractory differentiated thyroid cancer without primary lesion resection compared to placebo (18.3 vs 3.6 months) in the SELECT trial (2). Lenvatinib has also been prescribed for patients with poorly differentiated or anaplastic thyroid cancer. In patients with renal cell carcinoma with metastasis to the lung and pleura, rapid development of pleural effusion after discontinuation of sunitinib or sorafenib has been reported (6). Rapid disease progression after discontinuation of TKI is called the flare-up phenomenon (4); its mechanism is poorly understood. Tumor cells with hypersensitivity to TKI may proliferate rapidly after TKI discontinuation (6, 7). Experiments in mouse models of lung cancer suggest that VEGF plays an important role in the proliferation of pleural dissemination and formation of pleural effusion (8). Discontinuation of TKI enhances VEGF effects and increases angiogenesis, blood flow and vascular permeability in tumors (6). One report on the flare-up phenomenon in thyroid cancer is available (9); however, it has never been reported after discontinuation of lenvatinib. In lung cancer, the flare-up phenomenon occurs more commonly in patients with pleural lesions compared to patients without pleural lesions (7). In our case, right pleural effusion rapidly increased within 7 days after discontinuation of lenvatinib, but improved with re-starting lenvatinib, suggesting that thyroid cancer cells remained susceptible to lenvatinib. Autopsy findings showed that pleural lesions had widespread necrosis, consistent with the flare-up phenomenon induced by discontinuation of lenvatinib.
Adverse effects of lenvatinib include diarrhea (22.6%), hypertension (19.9%), proteinuria (18.8%) and decreased appetite (18.0%). It might reduce the quality of life. Temporary withholding, dose reduction or permanent discontinuation due to adverse effects of lenvatinib has been reported in 82.4, 67.8 and 14.2% of patients, respectively. In the middle of discontinuation, increases in tumor size and pleural effusion have been reported (4). In metastatic renal cell carcinoma, short-term intermittent administration of TKI was recommended (10). In our case, Grade 3 weight loss (−30%; 48 to 34 kg) secondary to anorexia occurred 5 months after administration of lenvatinib. Anorexia occurred not only due to the adverse effects of lenvatinib but also due to varicella zoster virus infection and post-herpetic neuralgia. We could not stop lenvatinib due to concerns about the flare-up phenomenon; thus, we continued lenvatinib at a lower dose (14 mg/day) over the next 8 months despite anorexia. Switching the TKI from lenvatinib to sorafenib was a therapeutic choice in our case, but we continued administration of lenvatinib because of its effectiveness against the patient’s tumors.
We described a patient with rapid accumulation of pleural effusion after discontinuation of lenvatinib. In TKI-responsive, poorly differentiated thyroid cancer with pleural metastasis, more attention should be paid to the flare-up phenomenon within a few days after discontinuation of lenvatinib.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Patient consent
Written informed consent was obtained from the patient’s husband.
Author contribution statement
T Uchida and H Yamaguchi wrote the case report. E Nakamura and Y Asada prepared and commented on the pathological findings. K Nagamine, T Yonekawa, N Shibata (oncologists), F Kawano (surgeon), and M Nakazato contributed to the clinical management of this patient.
References
- 1.Kepal N, Ashok R. Poorly differentiated and anaplastic thyroid cancer. Cancer Control 2006. 13 119–128. ( 10.1177/107327480601300206) [DOI] [PubMed] [Google Scholar]
- 2.Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, Habra MA, Newbold K, Shah MH, Hoff AO. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. New England Journal of Medicine 2015. 372 621–630. ( 10.1056/NEJMoa1406470) [DOI] [PubMed] [Google Scholar]
- 3.Tahara M, Kiyota N, Yamazaki T, Chayahara N, Nakano K, Inagaki L, Toda K, Enokida T, Minami H, Imamura Y, et al Lenvatinib for anaplastic thyroid cancer. Frontiers in Oncology 2017. 7 1–7. ( 10.3389/fonc.2017.00025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolter P, Beuselinck B, Pans S, Schöffski P. Flare up: an often unreported phenomenon nevertheless familiar to oncologists prescribing tyrosine kinase inhibitors. Acta Oncologica 2009. 4 621–624. ( 10.1080/02841860802609574) [DOI] [PubMed] [Google Scholar]
- 5.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018. 391 1163–1173. ( 10.1016/S0140-6736(18)30207-1) [DOI] [PubMed] [Google Scholar]
- 6.Desar IM, Mulder SF, Stillebroer AB, van Spronsen DJ, van der Graaf WT, Mulders PF, van Herpen CM. The reverse side of the victory: flare up of symptoms after discontinuation of sunitinib or sorafenib in renal cell cancer patients. A report of three cases. Acta Oncologica 2009. 48 927–931. ( 10.1080/02841860902974167) [DOI] [PubMed] [Google Scholar]
- 7.Chaft JE, Oxnard GR, Sima CS, Kris MG, Miller VA, Riely GJ. Disease flare after tyrosine kinase inhibitor discontinuation in patients with EGFR-mutant lung cancer and acquired resistance to erlotinib or gefitinib: implications for clinical trial design. Clinical Cancer Reserch 2011. 17 6298–6303. ( 10.1158/1078-0432.CCR-11-1468) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishii H, Yazawa T, Sato H, Suzuki T, Ikeda M, Hayashi Y, Takanashi Y, Kitamura H. Enhancement of pleural dissemination and lymph node metastasis of intrathoracic lung cancer cells by vascular endothelial growth factors (VEGFs). Lung Cancer 2004. 45 325–337. ( 10.1016/j.lungcan.2004.02.021) [DOI] [PubMed] [Google Scholar]
- 9.Yun KJ, Kim W, Kim EH, Kim MH, Lim DJ, Kang MI, Cha BY. Accelerated disease progression after discontinuation of sorafenib in a patient with metastatic papillary thyroid cancer. Endocrinology and Metabolism 2014. 29 388–393. ( 10.3803/EnM.2014.29.3.388) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grünwald V, Merseburger AS. The progression free survival-plateau with vascular endothelial growth factor receptor inhibitors – is there more to come. European Journal of Cancer 2013. 49 2504–2511. ( 10.1016/j.ejca.2013.03.022) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a