Summary
Necrobiosis lipoidica diabeticorum (NLD) is a chronic granulomatous dermatitis generally involving the anterior aspect of the shin, that arises in 0.3–1.2% of patients with diabetes mellitus (1). The lesions are often yellow or brown with telangiectatic plaque, a central area of atrophy and raised violaceous borders (2). Similar to other conditions with a high risk of scarring including burns, stasis ulcers and lupus vulgaris, NLD provides a favourable environment for squamous cell carcinoma (SCC) formation (3). A number of cases of SCC from NLD have been recorded (3, 4, 5); however, our search of the literature failed to identify any cases of either metastatic or fatal SCC which developed within an area of NLD. This article describes a patient with established type 1 diabetes mellitus who died from SCC which developed from an area of NLD present for over 10 years. Currently, there are a paucity of recommendations in the medical literature for screening people with NLD for the early diagnosis of SCC. We believe that clinicians should regard non-healing ulcers in the setting of NLD with a high index of clinical suspicion for SCC, and an early biopsy of such lesions should be recommended.
Learning points:
Non-healing, recalcitrant ulcers arising from necrobiosis lipoidica diabeticorum, which fail to heal by conservative measures, should be regarded with a high index of clinical suspicion for malignancy.
If squamous cell carcinoma is suspected, a biopsy should be performed as soon as possible to prevent metastatic spread, amputation or even death.
Our literature search failed to reveal specific recommendations for screening and follow-up of non-healing recalcitrant ulcers in the setting of necrobiosis lipoidica diabeticorum.
Further research is required in this field.
Background
Our case describes a patient seen at a medical practice who died from metastatic squamous cell carcinoma (SCC), which developed from necrobiosis lipoidica diabeticorum (NLD). Similar to other conditions with a high risk of scarring, including burns, stasis ulcers and lupus vulgaris, NLD provides an ideal environment for SCC formation (3). To our knowledge, no cases of metastatic SCC arising from NLD resulting in a fatality have been recorded. This feature makes our case distinctive.
Our review of the literature failed to reveal specific recommendations for screening people with NLD for the early diagnosis of SCC. We believe that this article can form the basis of recommendations to regard chronic ulcers in NLD with a high index of clinical suspicion. A biopsy earlier than may otherwise have been performed, may help prevent metastatic spread, the need for radical treatment with limb amputations, and possibly even death.
Case presentation
Our report describes the case of a blonde, blue-eyed, 56-year-old British Caucasian gentleman with longstanding type 1 diabetes on insulin with an HbA1c of 5.5%. He formerly smoked, had developed stage IV chronic kidney disease (eGFR 24 mL/min/1.73 m2) as a result of diabetic nephropathy, and had NLD which had been present for over a decade. It is worth noting that in the presence of chronic kidney disease, an HbA1c of 5.5% does not necessarily reflect adequate glycaemic control.
Figure 1.
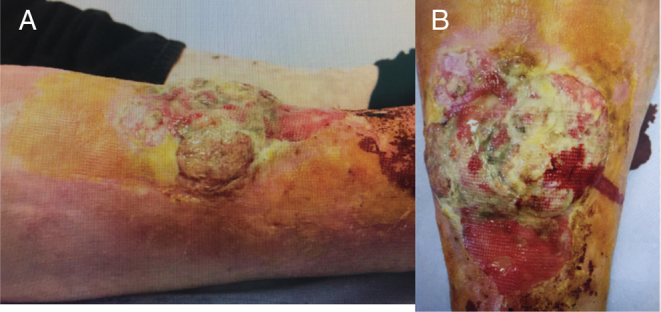
(A and B) Large ulcerating squamous cell carcinoma arising in an area of necrobiosis lipoidica diabeticorum.
He was referred to the dermatology outpatient clinic of a major metropolitan hospital by his general practitioner for investigation of an 8 × 5 cm fungating, erythematous lesion (photograph shown below) on the medial portion of his right leg. The lesion had been present for 3 months and was associated with ‘deep pain’, generalised malaise and unintentional weight loss.
Numerous courses of antibiotics prescribed by the general practitioner did not improve the lesion. Although the typically benign nature of NLD makes it reasonable for a conservative ‘wait and see’ management approach to be adopted, it is unsurprising that antibiotics did not improve the ulcerated lesion, given that they are not indicated as a treatment for the condition. The patient was not prescribed topical corticosteroids or any other medication to improve the appearance of the lesion.
Investigation
A subsequent excisional biopsy (10 × 5 × 4 mm, lesion embedded whole) confirmed the diagnosis of primary squamous cell carcinoma. The malignancy was classified as Clarke Level 4 and was moderately to poorly differentiated with no perineural or lymphovascular invasion. In addition to demonstrating extensive surface ulceration, the tumour had a prominent myxoid stroma and a pseudovascular pattern. It was difficult to measure the precise depth of the tumour given the extensive surface necrosis. However, the pathologist estimated that the tumour reached a depth of at least 12 mm and extended into the periosteum.
A staging CT demonstrated that the SCC involved 50% of the circumference of the leg, invading the anterior border of the tibia and through the fascia into the anterior and peroneal muscle compartment.
Treatment
Although surgeons considered performing a radical excision with reconstruction using bone and anterolateral thigh free flaps, the operation was deemed too risky because of a high likelihood of failure, incomplete excision and morbidity associated with T1DM and CKD. He underwent a successful below-knee amputation.
Outcome and follow-up
Five months after the operation, a large, fixed lymph node in the inguinal region (7 × 4 cm) was noted. Cytology from a fine-needle aspiration revealed metastatic SCC. He was treated with a right groin nodal dissection and post-operative external beam radiotherapy. A CT scan 4 weeks later revealed para-aortic and retroperitoneal lymphadenopathy consistent with distant metastases. Additional external beam radiotherapy to the para-aortic nodes was administered and an individual funding request for cetuximab was made. The oncologists preferred cetuximab to pembrolizumab in patients with T1DM; however, the funding request was ultimately rejected. Although the specific reason for this refusal remains unclear, it is likely due to the fact that cetuximab is currently licensed only for treatment of metastatic SCC arising from the head and neck. The patient was advised to forgo conventional chemotherapy given the presence of stage IV chronic kidney disease.
In the months that followed, the metastatic disease progressed. With the presence of significant back pain unable to be controlled by oral analgesia and corticosteroids, our patient was admitted to hospice care where he ultimately died as a result of his metastatic disease.
Discussion
The development of metastatic SCC from NLD is rare, however, even rarer is metastatic SCC from NLD resulting in a fatality. This feature, never before documented in the literature, makes this case distinctive.
Given that NLD belongs to the idiopathic cutaneous palisading granulomatous dermatitides that itself is characterised by collagen degradation, the development of SCC in areas of ulceration is rare, but possible. Ulceration occurs in approximately 30% of NLD cases, usually as a result of trauma (5).
At present, the evidence for treatment of NLD arises largely from small, uncontrolled trials and case reports (6). Most authors recommend high-potency topical corticosteroids as a first-line treatment; however, intralesional corticosteroid injections, topical retinoids and topical psoralen combined with ultraviolet A have had limited success in managing nonulcerative NLD (6). For ulcerative NLD, calcineurin inhibitors (7) and cyclosporine (2.5 mg/kg/day) (8) have been reported to successfully treat the lesions; however, none of these treatments have achieved a sustained effective response (6). It is worth noting that antibiotics, like our patient received, are not indicated for treatment of NLD.
In most cases of SCC arising from NLD, the risk of death from metastatic disease is low because less than 5% of cutaneous SCC metastasize (9). Factors which predict a poor prognosis include size >2 cm, depth >2 mm, invasion into subcutaneous fat or bone, poor differentiation, perineural invasion and anatomical location on the ear or nonglabrous lip (9). Our patient demonstrated the first four of these factors.
The pathogenesis of malignant transformation in NLD is not well understood although it is believed in part to be associated with reduced melanin pigment and subsequent loss of ultraviolet protection in addition to chronic trauma from an area with poor vascularization (4). A prospective study analysing the risk factors leading to poor prognosis of squamous cell carcinoma recommends following up high-risk SCC patients every 3 months with clinical investigation and ultrasound of the regional lymph nodes for 4 years after a diagnosis of SCC. It argues that ultrasound is cheap, safe and can detect the presence of early metastatic nodes more reliably than by palpation or CT scans (10).
Our review of the literature failed to reveal specific recommendations for screening people with NLD for (the early diagnosis of) squamous cell carcinoma. The British Association of Dermatology recognises that there is a small risk of squamous cell carcinoma developing in longstanding lesions to chronic NLD and recommends patients to consult their doctor if lesions are longstanding or ulceration occurs. The Australasian College of Dermatologists also acknowledges that there have been ‘rare reported cases of skin cancer developing in chronic lesions of necrobiosis lipoidica’ and suggest that persistent scabbing and ulceration should be investigated for SCC. Further, the Canadian Dermatology Association advises the SCC can commonly form in areas of ulceration. However, these bodies do not provide a timeline within which patients with ulcerated NLD should receive a biopsy for SCC or a recommendation regarding how often these patients should be monitored.
We believe that in the setting of NLD, non-healing, recalcitrant ulcers, which fail to improve with conservative measures should be regarded with a high index of clinical suspicion for SCC. Identifying SCC early by means of a biopsy may allow treatment to occur in time to prevent metastatic spread. This, in turn, may reduce the need for radical treatment with limb amputation and even possibly death.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Patient consent
Written, informed consent has been obtained from the patient.
Author contribution statement
The article was written by Yael Lefkovits. The article was edited by Amanda Adler.
Acknowledgement
Annie Girdwood for assistance with editing.
References
- 1.Shall L, Millard LG, Stevens A, Tattersall RB, Peacock I. Necrobiosis lipoidica: ‘the footprint not the footstep’. British Journal of Dermatology 1990. 123 47 ( 10.1111/j.1365-2133.1990.tb04456.x) [DOI] [Google Scholar]
- 2.Dissemond J. Images in clinical medicine. Necrobiosis lipoidica diabeticorum. New England Journal of Medicine 2012. 366 2502 ( 10.1056/NEJMicm1109700) [DOI] [PubMed] [Google Scholar]
- 3.Kossard S, Collins E, Wargon O, Downie D. Squamous carcinomas developing in bilateral lesions of necrobiosis lipoidica. Australasian Journal of Dermatology 1987. 28 14–17. ( 10.1111/j.1440-0960.1987.tb00321.x) [DOI] [PubMed] [Google Scholar]
- 4.Beljaards RC, Groen J, Starink TM. Bilateral squamous cell carcinomas arising in long-standing necrobiosis lipoidica. Dermatologica 1990. 180 96–98. ( 10.1159/000248001) [DOI] [PubMed] [Google Scholar]
- 5.Santos-Juanes J, Galache C, Curto JR, Carrasco MP, Ribas A, Sanchez del Rio J. Squamous cell carcinoma arising in long-standing necrobiosis lipoidica. Journal of the European Academy of Dermatology and Venereology 2004. 18 199–200. ( 10.1111/j.1468-3083.2004.00444.x) [DOI] [PubMed] [Google Scholar]
- 6.Grillo E, Rodriguez-Munoz D, Gonzalez-Garcia A, Jaen P. Necrobiosis lipoidica. Australian Family Physician 2014. 43 129–130. [PubMed] [Google Scholar]
- 7.Clayton TH, Harrison PV. Successful treatment of chronic ulcerated necrobiosis lipoidica with 0.1% topical tacrolimus ointment. British Journal of Dermatology 2005. 152 581–582. ( 10.1111/j.1365-2133.2005.06388.x) [DOI] [PubMed] [Google Scholar]
- 8.Stanway A, Rademaker M, Newman P. Healing of severe ulcerative necrobiosis lipoidica with cyclosporin. Australasian Journal of Dermatology 2004. 45 119–122. ( 10.1111/j.1440-0960.2004.00059.x) [DOI] [PubMed] [Google Scholar]
- 9.Ch’ng S, Clark JR, Brunner M, Palme CE, Morgan GJ, Veness MJ. Relevance of the primary lesion in the prognosis of metastatic cutaneous squamous cell carcinoma. Head and Neck 2013. 35 190–194. ( 10.1002/hed.22941) [DOI] [PubMed] [Google Scholar]
- 10.Brantsch KD, Meisner C, Schonfisch B, Trilling B, Wehner-Caroli J, Rocken M, Breuninger H. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: a prospective study. Lancet Oncology 2008. 9 713–720. ( 10.1016/S1470-2045(08)70178-5) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a