Abstract
Pemphigus vulgaris (PV) is a potentially lethal mucocutaneous blistering disease characterized by IgG autoantibodies (AuAbs) binding to epidermal keratinocytes and inducing this devastating disease. Here, we observed that non-desmoglein (Dsg) AuAbs in the sera of patients with Dsg1/3 AuAb–negative acute PV are pathogenic, because IgGs from these individuals induced skin blistering in neonatal mice caused by suprabasal acantholysis. Serum levels of AuAbs to desmocollin 3 (Dsc3), M3 muscarinic acetylcholine receptor (M3AR), and secretory pathway Ca2+/Mn2+-ATPase isoform 1 (SPCA1) correlated with the disease stage of PV. Moreover, AuAb absorption on recombinant Dsc3, M3AR, or SPCA1 both prevented skin blistering in the passive transfer of AuAbs model of PV in BALB/c mice and significantly decreased the extent of acantholysis in a neonatal mouse skin explant model. Although acantholytic activities of each of these immunoaffinity-purified AuAbs could not induce a PV-like phenotype, their mixture produced a synergistic effect manifested by a positive Nikolskiy sign in the skin of neonatal mice. The downstream signaling of all pathogenic non-Dsg AuAbs involved p38 mitogen-activated protein kinase (MAPK)–mediated phosphorylation and elevation of cytochrome c release and caspase 9 activity. Anti-Dsc3 and anti-SPCA1 AuAbs also activated SRC proto-oncogene, nonreceptor tyrosine kinase (SRC). Of note, although a constellation of non-Dsg AuAbs apparently disrupted epidermal integrity, elimination of a single pathogenic AuAb could prevent keratinocyte detachment and blistering. Therefore, anti-Dsg1/3 AuAb–free PV can be a model for elucidating the roles of non-Dsg antigen–specific AuAbs in the physiological regulation of keratinocyte cell–cell adhesion and blister development.
Keywords: autoimmune disease, antibody, antigen, pemphigus, apoptosis, acantholysis, cell–cell detachment, immune dysfunction, keratinocyte, non-desmoglein pemphigus antibodies
Introduction
Pemphigus vulgaris (PV)3 is a potentially lethal mucocutaneous blistering disease characterized by IgG autoantibodies (AuAbs) binding to epidermal keratinocytes and inducing a devastating blistering disease affecting oral and/or esophageal surfaces and, sometimes, also the skin. PV patients develop cell–cell detachment (acantholysis), blisters, and nonhealing erosions caused by suprabasal split within the epidermis. The majority of PV patients have AuAbs against desmoglein (Dsg) 3 ± 1, which are believed to cause acantholysis (reviewed in Refs. 1 and 2). However, on average 10–15% of acute PV patients with anti-keratinocyte AuAbs detectable by direct and/or indirect immunofluorescence are negative for Dsg1/3 ELISA (3–9). Although there are no known clinical and pathological differences between PV patients with versus without anti-Dsg AuAbs, the immunopathological mechanisms of acantholysis may be different.
In addition to the Dsg1 and Dsg3 antigens, recent proteomic analyses of large cohorts of pemphigus and normal control sera (10, 11) revealed reactivities with desmocollin (Dsc) 1 and 3, several muscarinic and nicotinic acetylcholine receptor (AR) subtypes, and some other intracellular proteins, including the secretory pathway Ca2+/Mn2+-ATPase isoform 1, (SPCA1), encoded by the ATP2C1 gene. The mechanisms of keratinocyte detachment and blister formation in the PV patients lacking anti-Dsg AuAbs remain practically unknown, except that anti-Dsc3 AuAbs have been shown to be pathogenic in PV patients (12, 13) and the Dsc3fl/fl/K14-Cre mice lacking Dsc3 develop PV-like phenotype (14). Also noteworthy is that one copy of ATP2C1 is mutated in patients with Hailey–Hailey disease (also known as familial benign chronic pemphigus), representing a nonimmune phenocopy of cutaneous lesions in PV (15, 16).
The purpose of the present study was to elucidate the pathogenic potential of the most common species of the non-Dsg AuAbs potentially involved in disease development and possible correlation of serum levels of such AuAbs with the clinical stage of disease in the anti-Dsg1/3 AuAb-negative PV patients. Herein, we demonstrate for the first time that (i) non-Dsg AuAbs developed by the Dsg1/3 AuAb-negative acute PV patients are pathogenic, because they induced acantholysis and epidermal split in the experimental models of PV in vitro and in vivo; (ii) anti-Dsc3, anti-M3 muscarinic AR (M3AR) anti-SPCA1 AuAbs synergize to cause acantholysis; and (iii) relative serum concentrations of these three pathogenic AuAbs correlate with the disease stage.
Results
Evaluation of pathogenic potential of AuAbs present in the Dsg1/3 AuAb-negative acute PV patients
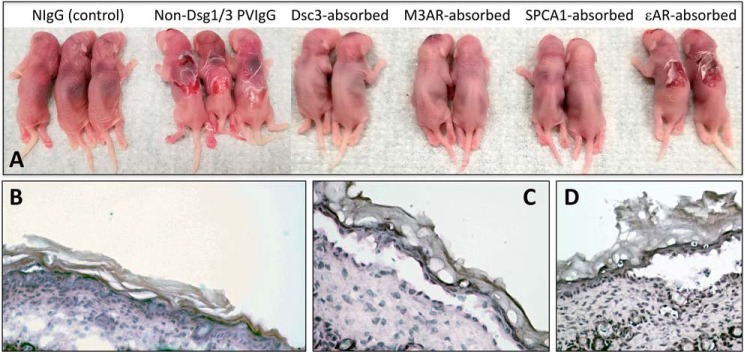
We hypothesized that serum IgG fraction of PV patients who do not develop either anti-Dsg1 or anti-Dsg3 AuAb can induce PV phenotype in neonatal mice, i.e. that non-Dsg AuAbs are pathogenic in vivo. To test this hypothesis, we isolated IgG fractions from sera of 12 anti-Dsg1/3 AuAb-negative acute PV patients and injected them intradermally to 1-day-old BALB/c mice. The pups were examined 24 h later for the presence of epidermal blistering. As expected, all tested IgG fractions of PV sera (PVIgGs), but not normal sera (NIgG), induced spontaneous skin blistering caused by suprabasal acantholysis (Fig. 1, A–C). These results demonstrated that non-Dsg AuAbs present in serum of the Dsg1/3 AuAb-negative acute PV patients are indeed pathogenic.
Figure 1.
Representative images of neonatal BALB/c mice and histopathology of their skin in the passive transfer of disease model of PV. Although the skin of neonatal BALB/c mice 24 h after a single injection of 25 μg/g of body weight of NIgG did not show any macro- (A) or microscopic (B) changes, the mice injected with the same dose of total IgG fraction of sera pooled from 12 anti-Dsg1/3 AuAb-negative acute PV patients demonstrated cutaneous blisters and erosions with loosely attached peripheral skin (A) and typical PV-like suprabasal acantholysis (C). Absorption of this PVIgG on the affinity purification columns containing recombinant Dsc3, M3AR, or SPCA1, but not ϵnAR, abolished its ability to induce skin blistering (A). Microscopic examination of the skin of mice injected with eluants from the Dsc3, M3AR, or SPCA1 columns did not reveal any pathologic changes (not shown), whereas skin of mice injected with PVIgG absorbed with ϵnAR demonstrated PV-like intraepidermal split (D).
Evaluation of AuAbs to specific non-Dsg antigens in sera of the Dsg1/3 AuAb-negative acute PV patients
Our previous proteomic studies have identified the most common antigenic specificities of AuAbs in acute PV patients with or without anti-Dsg1/3 AuAbs (10). We focused this study on self-antigens known to mediate, i.e. Dsc3, or regulate, i.e. M3AR and the ϵ-subunit of nicotinic ARs (ϵnAR) keratinocyte cell–cell adhesion or be altered in the chronic autosomal dominant disorder known as benign familial pemphigus, or Hailey–Hailey disease (HHD), in which patients develop skin erosions caused by suprabasal acantholysis, i.e. SPCA1. First, we determined whether serum levels of these non-Dsg AuAb species correlate with the Pemphigus Disease Area and Activity Index (PDAAI) in the Dsg1/3 AuAb-negative acute PV patients (Table 1). All patients were treated by our original multidrug protocol described elsewhere (17), and their serum samples were obtained prior to treatment and right after they reached complete remission (i.e. complete healing of erosions with or without secondary skin changes in the absence of new lesions and with negative Nikolskiy sign (i.e. when their PDAAI score went down to 0). The results demonstrated that all tested PV sera contained one or more of non-Dsg AuAbs against Dsc3, M3AR, ϵnAR, and SPCA1. The relative concentrations of all but anti-ϵnAR AuAb were always significantly (p < 0.05) higher before treatment, suggesting their role in disease pathophysiology.
Table 1.
Relative amounts of specific non-Dsg AuAbs and corresponding PDAAI scores before and after treatment of the Dsg1/3 AuAb-negative acute PV patients
| Patient's code | Anti-Dsc3 AuAba |
Anti-M3AR AuAb |
Anti-SPCA1 AuAb |
Anti-ϵAR AuAb% |
PDAAI score |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before Tx | After Tx | Before Tx | After Tx | Before Tx | After Tx | Before Tx | After Tx | Before Tx | After Tx | |
| % | ||||||||||
| A | <2b | <2 | 88.0 ± 5.6c | 6.3 ± 1.6d | <2 | <2 | 32.4 ± 7.1 | 48.3 ± 8.6 | 13 | 0 |
| B | 99.7 ± 6.7 | 10.9 ± 1.2d | 96.0 ± 1.5 | 14.3 ± 2.4d | 91.4 ± 4.5 | 5.2 ± 1.0d | <2 | <2 | 26 | 0 |
| C | 96.6 ± 12.1 | 11.2 ± 1.2d | <2 | <2 | <2 | <2 | <2 | <2 | 65.5 | 0 |
| D | 89.0 ± 3.8 | 16.4 ± 4.1d | <2 | <2 | 83.6 ± 5.11 | 20.0 ± 6.5d | <2 | <2 | 22.5 | 0 |
| E | <2 | <2 | 97.6 ± 3.1 | 12.6 ± 3.8d | <2 | <2 | 41.9 ± 6.7 | 45.9 ± 5.6 | 15.5 | 0 |
| F | 94.5 ± 12.6 | 11.8 ± 0.9d | <2 | <2 | 98.2 ± 4.3 | 15.3 ± 2.5d | 57.2 ± 10.0 | 36.5 ± 9.5 | 11 | 0 |
| G | 84.5 ± 4.8 | 6.6 ± 1.8 | 92.8 ± 5.0 | 14.3 ± 5.5d | <2 | <2 | <2 | <2 | 17.5 | 0 |
| H | <2 | <2 | <2 | <2 | 97.5 ± 4.4 | 21.9 ± 3.1d | <2 | <2 | 57.5 | 0 |
| I | 95.1 ± 7.5 | 10.6 ± 2.8d | 98.0 ± 3.5 | 24.5 ± 5.5d | 92.9 ± 2.4 | 16.0 ± 4.4d | <2 | <2 | 29 | 0 |
| J | 99.5 ± 5.3 | 16.0 ± 3.2d | <2 | <2 | <2 | <2 | <2 | <2 | 9.5 | 0 |
| K | <2 | <2 | 83.7 ± 7.4 | 10.7 ± 2.0 | 75.9 ± 4.3 | 10.8 ± 3.0d | <2 | <2 | 36.5 | 0 |
| L | 89.8 ± 1.4 | 14.4 ± 3.6d | 84.4 ± 5.8 | 5.8 ± 3.8d | 85.9 ± 5.1 | 9.6 ± 1.4d | <2 | <2 | 11.5 | 0 |
a The amount of affinity-purified specific AuAb is expressed relative to the maximum capacity of ELISA plates determined by NIgG binding and taken as 100% (see “Materials and methods”).
b The values below 2% were considered nonspecific, because eluants from the affinity purification columns treated with normal human IgGs produced the similar results (data not shown).
c The results are expressed as means ± S.D. of triplicate samples.
d Significantly different (p < 0.05) from the values before treatment; the rest are p > 0.05.
The effect of absorption of sera from the Dsg1/3 AuAb-negative acute PV patients with recombinant self-antigens on spontaneous skin blistering in the passive transfer of disease model of PV in BALB/c mice
To evaluate the potential role of each species of non-Dsg AuAbs in causing blisters, we determined whether their adsorption could affect the acantholytic effect of total IgG fraction of sera pooled from 12 anti-Dsg1/3 AuAb-negative acute PV patients. Adsorption with recombinant Dsc3, M3AR, and SPCA1 prevented both spontaneous blistering in BALB/c mice and the appearance of Nikolskiy sign (Fig. 1A). The acantholytic activity of preabsorbed PVIgGs could be restored by adding the eluted AuAb (data not shown). In marked contrast, absorption on recombinant ϵnAR did not affect the acantholytic activity of PVIgG, because all pups developed spontaneous skin blistering (Fig. 1A) caused by suprabasal acantholysis (Fig. 1D).
Measurements of acantholytic activity of PVIgG from the Dsg1/3 AuAb-negative acute PV patients in the NMSE model of PV after absorption with recombinant self-antigens
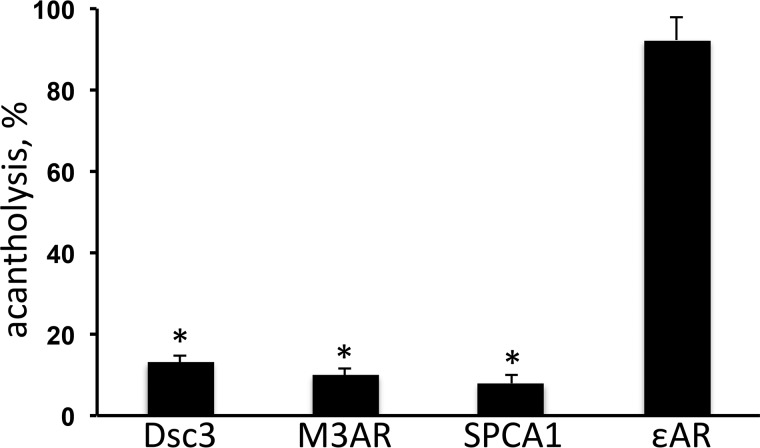
To determine the impact of elimination of AuAbs against Dsc3, M3AR, SPCA1, and ϵnAR on the disease-causing ability of PVIgG, we determined the extent of acantholysis in neonatal mouse skin explants (NMSEs) treated with preabsorbed PVIgG. Consistent with results of in vivo experiments, adsorption of AuAbs against Dsc3, M3AR, and SPCA1 in each case significantly (p < 0.05) decreased extent of acantholysis, whereas absorption on recombinant ϵnAR had no significant effect (p > 0.05) (Fig. 2). Thus, based on the results of both in vivo and in vitro absorption experiments, the AuAbs against Dsc3, M3AR, and SPCA1 could be considered pathogenic in PV.
Figure 2.
Morphometric analysis of extent of acantholysis in the NMSE model induced by total IgG fraction of sera pooled from the anti-Dsg1/3 AuAb-negative acute PV patients preabsorbed with recombinant Dsc3, M3AR, SPCA1, or ϵnAR. The NMSEs were incubated for 24 h in the presence of IgGs pooled from anti-Dsg1/3 AuAb-negative acute PV patients before (control, taken as 100%) and after preabsorption with recombinant Dsc3, M3AR, SPCA1, or ϵnAR, and the extent of acantholysis was determined using the morphometric analysis described under “Material and methods.” Each experiment was repeated at least three times. *, p < 0.05 compared with control.
Direct evaluation of acantholytic activities of anti-Dsc3, anti-M3AR, and anti-SPCA1 AuAbs in the in vitro and in vivo models of PV
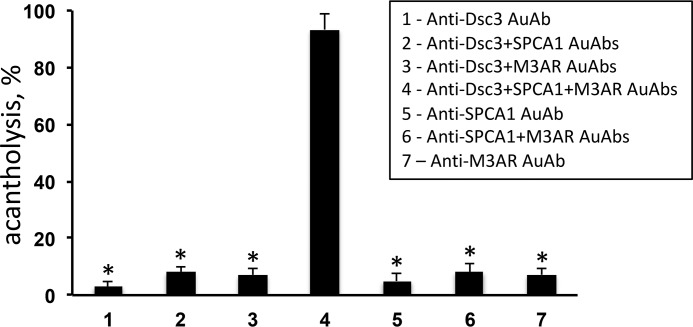
The extent of acantholysis was measured 24 h after addition to NMSEs of the pathophysiologically relevant anti-Dsc3, anti-M3AR, or anti-SPCA1 AuAbs immunoaffinity-purified from total serum IgG of the Dsg1/3 AuAb-negative acute PV patients who produced these AuAbs. When given alone, neither AuAb induced acantholysis (Fig. 3), suggesting that a simultaneous hit by non-Dsg AuAbs to different self-antigens was required to disrupt the integrity of epidermis. Therefore, we next analyzed the acantholytic activities of various combinations of anti-Dsc3, anti-M3AR, and anti-SPCA1 AuAbs seen in our Dsg1/3 AuAb-negative acute PV patients (Table 1). Among tested conditions, only the mixture anti-Dsc3 + M3AR + SPCA1 AuAbs reproduced the acantholytic effect of PVIgG (Fig. 3). This combination of AuAbs was present in three patients, i.e. patients “B”, “I,” and “L” (Table 1).
Figure 3.
Evaluation of acantholytic activities of immunoaffinity-purified anti-Dsc3, anti-M3AR, and anti-SPCA1 AuAbs in the NMSE model. The extent of acantholysis was measured 24 h after addition to NMSEs of individual or combinations of AuAbs immunoaffinity-purified from IgG fractions pooled from serum of PV patients producing these non-Dsg AuAbs. Each experiment was performed in triplicate. *, p < 0.05 compared with positive control, i.e. total PVIgG, taken as 100%.
To determine whether this AuAb combination is sufficient to disrupt the epidermal integrity in vivo, we employed the passive transfer of disease model of PV in BALB/c mice. Although none of the injected pups developed spontaneous skin blisters, all exhibited positive Nikolskiy sign, i.e. gentle rubbing of the skin at the injection site induced skin erosions caused by suprabasal acantholysis (Fig. 4).
Figure 4.
Evaluation of the synergistic acantholytic activity of a mixture of anti-Dsc3, anti-M3AR, and anti-SPCA1 AuAbs in the passive transfer of disease model of PV in BALB/c mice. Four 1-day-old pups were injected with a mixture of affinity-purified AuAbs to Dsc3, M3AR, and SPCA1 versus the same amount of NIgG (control, C) and subjected to gentle rubbing with a pencil eraser of their skin at the injection site 24 h later. All experimental mice showed skin detachment (i.e. positive Nikolskiy sign; left panel) caused by suprabasal acantholysis (right panel).
Taken together, these results suggested a synergy of the pathogenic actions of individual non-Dsg AuAbs. Although the combination anti-Dsc3 + M3AR + SPCA1 AuAbs did not match exactly the acantholytic effect of total PVIgG, because the mice did not develop spontaneous blistering, it did alter the ability of keratinocyte to maintain cell–cell adhesion to the clinically relevant degree. The reason for such discrepancy may be explained by the fact that in addition to these AuAbs, the Dsg1/3 AuAb-negative acute PV patients develop AuAbs to other self-antigens (10) that might be required for a complete constellation of pathogenic AuAbs capable of disrupting epidermal integrity.
Evaluation of the effects of immunoaffinity-purified anti-Dsc3, M3AR, and SPCA1 AuAbs on activities of Src, p38 MAPK, Cs9, and CytC release in keratinocytes
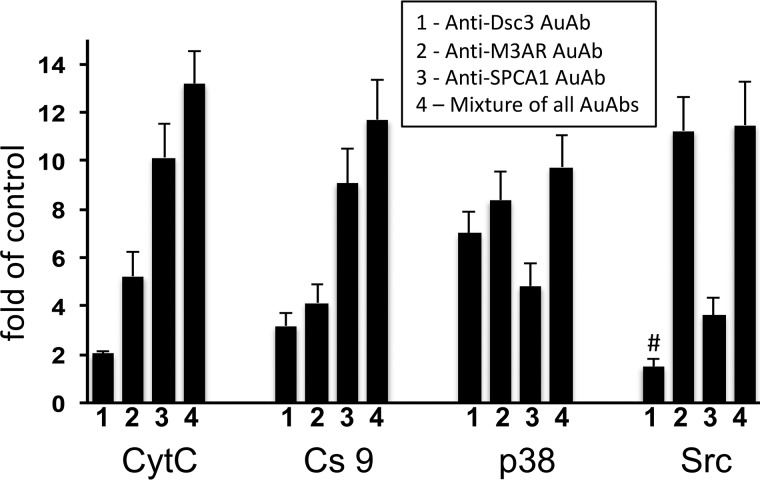
To obtain a first mechanistic insight on the pathophysiological pathways mediating the pro-acantholytic actions of each of the three pathogenic non-Dsg AuAbs under consideration, we measured their effects on the markers of apoptolysis—the paradigm of keratinocyte damage in PV underlying the suprabasal epidermal clefting in PV lesions (18)—in cell cultures of normal human epidermal keratinocytes. Previous studies convincingly demonstrated that pathogenic PVIgGs activate the signaling kinases Src and p38 MAPK, and the effectors of intrinsic apoptosis CytC and Cs9 (reviewed in Ref. 1 and 19). We found that each tested AuAb significantly (p < 0.05) increased activities of p38 MAPK and Cs9 and CytC release, whereas Src was activated only by anti-Dsc3 and anti-SPCA1 AuAbs (Fig. 5). Anti-M3AR AuAb produced the most prominent changes of p38 MAPK and Src, and anti-SPCA1 AuAb produced the most prominent changes of CytC and Cs9. Because the increase produced by a combination of AuAbs did not represent a sum of individual AuAb values, these results indicated that different non-Dsg AuAbs acted synergistically. Thus, each pathogenic non-Dsg AuAb may contribute to the disease process by eliciting the downstream signaling through a unique mechanism.
Figure 5.
Evaluation of the effects of immunoaffinity-purified anti-Dsc3, M3AR, and SPCA1 AuAbs on activities of Src, p38 MAPK, and Cs9, CytC release in keratinocytes. Normal human epidermal keratinocytes grown in culture to ∼80% confluence were incubated at 37 °C and 5% CO2 in the keratinocyte growth medium containing 1.6 mm Ca2+ in the presence of equal concentrations of immunoaffinity-purified anti-Dsc3, anti-M3AR, or anti-SPCA1 AuAbs and used to measure CytC release (after 2 h incubation) or the activities of Cs9 (16 h), p38 MAPK (4 h), or Src (1 h). These time points were chosen based on the highest elevation of test parameters in our previous time-course studies (24). The results are expressed as folds of mean control values determined in the keratinocyte monolayers exposed to the same amount of NIgG, taken as 1. #, p > 0.05 compared with control; the rest are p < 0.05.
Discussion
In this study, we demonstrate for the first time that non-Dsg AuAbs present in the serum of Dsg1/3 AuAb-negative acute PV patients are pathogenic, because patients' IgGs induced skin blistering in neonatal mice caused by suprabasal acantholysis. These PV patients had various combinations of anti-Dsc3, anti-M3AR, and anti-SPCA1 AuAbs, whose relative concentrations were always higher during the acute stage of PV than in remission, suggesting the role of these AuAbs in disease pathophysiology. Indeed, absorption on recombinant Dsc3, M3AR, or SPCA1 prevented both skin blistering in the passive transfer of AuAbs model of PV in BALB/c mice and significantly decreased extent of acantholysis in the NMSE model. On the other hand, a mixture of immunoaffinity-purified anti-Dsc3, M3AR, and SPCA1 AuAbs reproduced the acantholytic effect of PVIgG both in vitro and in vivo. Measurements of molecular markers of apoptolysis in human cultured keratinocytes treated with immunoaffinity-purified non-Dsg AuAbs provided a first mechanistic insight on the pathophysiological pathways mediating pathogenic actions of these AuAbs. Most importantly, whereas multiple hits sustained by a constellation of non-Dsg AuAbs appeared to be required to disrupt epidermal integrity, elimination of a single type of pathogenic AuAb was sufficient to prevent keratinocyte detachment and blistering.
Although the pathogenic role of anti-Dsg1/3 AuAbs in PV is widely accepted, very little is known about the antigen specificities of pathogenic AuAbs in the acute PV patients who do not produce anti-Dsg1/3 AuAbs. Proteomic studies have identified numerous types of non-Dsg AuAbs in PV (10, 11), some of which have been characterized regarding their roles in the physiology and cell adhesion of keratinocytes (reviewed in Ref. 20). The following reports testify to the fact that non-Dsg AuAbs developed by PV patients contribute to PV pathophysiology: (i) gross blisters were induced in mouse skin by the PVIgG depleted of anti-Dsg3 AuAb (21); (ii) passive transfer of PVIgG lacking anti-Dsg1 AuAb to neonatal Dsg3−/− mice induced PV-like phenotype featuring widespread blistering, which means that blisters were induced by targeting antigens other than Dsg1 and Dsg3 (22); (iii) preabsorption of PV sera with recombinant pemphaxin, a low affinity receptor for acetylcholine, eliminated acantholytic activity and eluted antibody immunoprecipitated native pemphaxin. The addition of anti-pemphaxin antibody back to the preabsorbed PVIgG fraction restored its acantholytic activity in neonatal mice (23); the anti-pemphaxin AuAb induced acantholysis in a keratinocyte monolayer (23); (iv) adsorption of anti-mitochondrial AuAbs abolished the ability of PVIgG to cause acantholysis both in vitro and in vivo (24); and (v) IgG antibodies directed against thyroid peroxidase induced keratinocyte fragmentation in vitro and caused a significant increase in p38 MAPK phosphorylation and increased [Ca2+]i concentrations, both of which are associated with the pathogenicity of PVIgG (25, 26).
The results of the present study identified the pathophysiological significance of AuAbs to Dsc3, M3AR, and SPCA1, which not only had serum levels correlated with the disease stage of PV but which also were indispensable for the ability of whole serum IgG from the Dsg1/3 AuAb-negative acute PV patients to induce acantholysis in experimental models of PV in vitro and in vivo. Although the acantholytic activities of each of these three pathogenic AuAbs were insufficient to overcome natural resistance of keratinocytes to dysadhesion, their mixture produced a synergistic effect manifested by inducible acantholysis, i.e. positive Nikolskiy sign in the skin of neonatal mice.
The principal mechanisms of keratinocyte damage by some known non-Dsg AuAbs can be deduced from reports about the biological significance of targeted antigens. The anti-Dsc3 AuAbs, which were first demonstrated in PV patients more than two decades ago (27, 28), may contribute to acantholysis by interference with desmosome formation/function caused by steric hindrance. Our findings that adsorption of anti-Dsc3 AuAb abolished acantholysis and skin blistering in neonatal mice extend previous observations made in in vitro experiments. In a case report of a 55-year-old female with the Dsg1/3 AuAb-negative PV affecting gingival mucosa, adsorption of anti-Dsc3 AuAb with the recombinant extracellular domain of Dsc3 abolished acantholytic activity of patient's IgGs in a primary human keratinocyte cell dissociation assay (12), and the other way around, a mAb targeting the extracellular domain of Dsc3 caused intraepidermal blistering in an in vitro model of human skin and a loss of intercellular adhesion in cultured keratinocytes (29). In the present study, however, immunoaffinity-purified anti-Dsc3 AuAb alone did not induce acantholysis in NMSE. The discrepancy may be explained by differences in the sensitivities between the in vitro models of PV used in two different studies.
Although the presence of anti-mAR AuAbs in PV patients has been known for 25 years (30), specific targeting of the M3AR subtype was discovered only recently in proteomic studies independently performed by our (10) and Dr. Sinha's groups (31). Yet another group most recently has demonstrated that anti-M3AR AuAb correlates with disease activity and its titer declines with therapy (32), which is consistent with our finding reported herein. The pathogenic activity of anti-M3AR AuAb apparently stems from phosphorylation of classical and desmosomal cadherins in keratinocytes, by analogy with that observed upon functional inactivation of M3AR through the pharmacological approach (reviewed in Ref. 33).
The last but not the least among the most common pathogenic non-Dsg AuAbs characterized in this study was anti-SPCA1 AuAb. Although SPCA1 is located intracellularly, it might be reached by an AuAb in a complex with FcRn (“neonatal” Fc receptor for IgG) that has been shown to bind PVIgGs on the cell membrane of keratinocytes and traffic them to intracellular targets (34). SPCA1 is encoded by the ATP2C1 gene, one copy of which is mutated in patients with HHD who exhibit the clinical and pathological phenocopy of PV (15, 16). Further, both keratinocytes from the skin of HHD patients (15, 36) and normal keratinocytes exposed to PVIgGs in culture (37) feature altered [Ca2+]i metabolism. SPCA1 pumps Ca2+ and Mn2+ from cytosol to the lumen of Golgi, thus playing a crucial role in setting up the [Ca2+]i oscillations that control many vital cell functions (reviewed in Ref. 38). Ablation or reduction in SPCA1 activity causes Golgi dysfunction and stress, as well as accumulation of Ca2+ and Mn2+ in the cytosolic space, which can trigger proapoptotic signaling (reviewed in Refs. 39–41). It is now widely accepted (42) that in addition to blocking function of adhesion molecules on keratinocyte cell membrane, PVIgGs elicit proapoptotic signaling events causing cell shrinkage, detachment from neighboring keratinocytes, and rounding up—the unique process of keratinocyte detachment and death termed apoptolysis (18). Indeed, specific signs of apoptosis were observed in experimental models of SPCA1 deficiency (43, 44).
The downstream signaling resulting from binding to keratinocytes of all pathogenic non-Dsg1/3 AuAbs under consideration involved p38 MAPK phosphorylation. Anti-Dsc3 and anti-SPCA1 AuAbs also activated Src. In the past, we (24, 45) and other workers (46–48) have demonstrated that PVIgGs can trigger these kinases regardless of the presence or absence of anti-Dsg AuAbs, indicating an overall significance of these pathways for keratinocyte detachment. Although Src activation is an early event in the PVIgG-triggered biochemical events, which may lead to transactivation of several downstream pathways, such as epidermal growth factor receptor (49) also known to play role in PV (50, 51), p38 MAPK activation is a late signaling step directly linked to keratinocyte apoptolysis (reviewed in Ref. 18). The p38 MAPK signaling pathway can be stimulated by cellular stress and proinflammatory cytokines and can be involved in cell death via apoptosis (reviewed in Ref. 52). Accordingly, all three pathogenic non-Dsg1/3 AuAbs characterized in the present study elevated the pro-apoptotic CytC and Cs9, albeit with different efficacy, with anti-SPCA1 AuAb being the most effective stimulus. This is in keeping with an important role of alterations of [Ca2+]i metabolism in triggering the intrinsic apoptotic pathway (reviewed in Ref. 53). Therefore, various AuAb species produced by different PV patients have both common and unique mechanisms of apoptolytic signaling.
Our findings support the notion that apoptolysis in PV is a complex process initiated by at least three classes of AuAbs directed against keratinocyte proteins that mediate intercellular adhesion, e.g. the desmosomal cadherin Dsc3; control vital cell functions, e.g. the metabotropic cholinergic receptor M3AR; and regulate [Ca2+]i levels, such as the Ca2+/Mn2+ ATPase SPCA1. The preferential signaling pathway downstream of targeted self-antigens is apparently determined by a unique composition of the pool of AuAbs produced by each PV patient. Different AuAb species apparently synergize to disrupt the epidermal cohesion and causes blistering in PV. Specific constellations of AuAbs developed by individual PV patients may therefore determine clinical severity of the disease, its natural course, and its response to treatment.
The role of anti-ϵ nAR AuAb in the Dsg1/3 AuAb-negative acute PV patients and its ability to contribute to apoptolysis remains to be determined. This AuAb may not be pathogenic, i.e. witnessing the disease rather than causing it, analogous to some AuAbs associated with connective tissue diseases (reviewed in Ref. 54), or exhibit an additive/synergistic action within a putative constellation of pathogenic PV AuAbs required to overcome the natural resistance of keratinocytes to dysadhesion.
Conclusions
The levels of pathogenic non-Dsg AuAbs in the Dsg1/3 AuAb-negative PV patient's serum correlate with the stage of disease, which may potentially allow to use certain type(s) of non-Dsg AuAbs as predictors of PV relapse. By analogy with synergy of anti-mitochondrial AuAbs and anti-Dsg3 AuAb in PV patients with conventional immunophenotype (34), in the anti-Dsg1/3 AuAb-negative PV patients, various non-Dsg AuAb species may concur to cause blistering by acting synergistically and thus imposing a simultaneous hit on keratinocytes affecting the intracellular homeostasis to the degree that it can alter the ability of keratinocyte to maintain the integrity of epidermis. The anti-Dsg1/3 AuAb-free PV may become a model for elucidation role of AuAbs to non-Dsg antigens in the physiological regulation of keratinocyte cell–cell adhesion and blister development. Because composition of the pool of the most common non-Dsg AuAbs appears to be similar among PV patients with or without anti-Dsg AuAbs, elucidation of the mechanisms of pathogenic actions of known non-Dsg AuAbs may also shed new light on the pathophysiology of conventional immunopathological variants of PV.
Materials and methods
Pemphigus sera
We used 12 serum specimens from patients with PV who did not develop anti-Dsg1 and anti-Dsg3 AuAbs detectable by either proteomic analysis (10) or the MESACUP Dsg-1 and Dsg-3 ELISA test system (MBL, Nagoya, Japan). This research had been approved by Institutional Review Boards of University of California–Irvine. The studies involving human subjects abided by the Declaration of Helsinki principles. The diagnosis of PV was made based on the results of comprehensive clinical and histological examinations, and immunological studies that included both direct immunofluorescence of skin biopsies and indirect immunofluorescence of the patients' sera on various epithelial substrates. The mucosal type of PV was diagnosed in seven patients, and the mucocutaneous type was diagnosed in five patients. Prior to initiation of treatment of our Dsg1/3 AuAb-negative acute PV patients with the multidrug protocol detailed elsewhere (17), the mean duration of the disease was 1.6 years. The titer of “intercellular” antibodies determined on monkey esophagus ranged from 1/320 to 1/5120. Three patients had refractory disease, and eight had a relapse. The severity of disease was determined using the PDAAI, described elsewhere (17).
Antibody adsorption and immunoaffinity purification experiments
The IgG fraction of PV sera and normal serum were isolated by FPLC protein G affinity chromatography using the FPLC system purchased from Amersham Biosciences Corp. (Waltham, MA) following the manufacturer's protocol (55). The pooled sera of healthy people was purchased from Bioreclamation, Inc. (Westbury, NY). All test sera were preincubated for 30 min at 56 °C to inactivate complement. For preadsorption of AuAbs from test PVIgGs, we used recombinant proteins immobilized on nickel–nitrilotriacetic acid resign and followed the protocol described by us elsewhere (23, 35, 56). Human recombinant His-tagged Dsc3 (AA 712–896) and M3AR (AA 253–491) expressed in SF9 insect cells, as well as SPCA1 (AA 313–699) and ϵnAR (AA 21–239) expressed in Escherichia coli, were purchased from Antibodies-online Inc. (Atlanta, GA). The PVIgGs from individual patients and NIgG were lyophilized and then reconstituted in PBS to the same protein concentration of 10 mg/ml and applied to the 10-ml nickel–nitrilotriacetic acid resigns containing immobilized recombinant antigens. Both the pass-through fraction and the immunoaffinity-purified fraction eluted from the columns were collected and used in experiments. The pass-through fraction was lyophilized and then reconstituted in PBS to the desired concentration for in vivo studies of acantholysis described below. The relative amounts of affinity-purified AuAbs were measured using the human total IgG platinum ELISA kit purchased from eBioscience (San Diego, CA) following the protocol provided by the manufacturer. The results were expressed relative to the maximum IgG binding capacity of the ELISA plates determined at the optical density of 450 nm and taken as 100%. To obtain this optical density value, the microwells precoated with anti-human total IgG antibody were saturated by an access of the human total IgG standard included in the assay kit. All eluted AuAbs were concentrated by lyophilization and resuspended in 100 μl of PBS prior to the assay. The aliquots of the obtained suspension were analyzed in triplicate. The value below 2% was considered negative or nonspecific, because eluants from the affinity purification columns treated with similarly prepared NIgG samples produced such values. The antigen specificity of AuAbs affinity-purified on each studied non-Dsg antigen was verified by visualizing a single protein band with expected molecular mass in immunoblots of homogenized monolayers of normal human keratinocytes following a standard protocol utilized by us in the past (35, 56) (data not shown).
Evaluation of acantholytic activities of non-Dsg AuAbs
For passive transfer experiments, we used 1-day-old pups delivered by BALB/c mice purchased from The Jackson Laboratory (Bar Harbor, ME). This study had been approved by the University of California–Irvine Institutional Animal Care and Use Committee. According to the protocol detailed by us elsewhere (35), the mice were injected intradermally through a 30-gauge needle with test IgG specimens (25 μg/g body weight) and examined 24 h later for the presence of skin erosions and blisters, as well as for Nikolskiy sign, i.e. appearance of skin erosions at the site of gentle rubbing with pensile eraser. The presence of suprabasal acantholysis in mouse skin lesions was confirmed by the light microscopic examination of skin samples stained with hematoxylin and eosin.
For morphometric assay of acantholysis in vitro, we used the NMSE model described by us in the past (34). Briefly, ∼3 × 3-mm pieces of trunkal skin of intact neonatal BALB/c mice were incubated at 37 °C and 5% CO2 for 24 h in PBS containing test IgG, after which the skin specimens were fixed, sectioned, and stained with hematoxylin and eosin. The images (×200) of six randomly selected areas in each specimen were photographed and printed. The extent of suprabasal split on each photograph was measured in μm along at least four basal cells by a researcher who was blind about the type of treatment, and the results are expressed as percentages of the total length of epidermis in the microscopic field, taken as 100%.
Keratinocyte cell culture experiments
The ELISA kits for measuring phosphorylated Src was purchased from EMD Biosciences, Inc. (La Jolla, CA). The enzymatic activity of Cs9, and concentrations of CytC and phosphorylated p38 were measured using kits purchased from R&D Systems, Inc. (Minneapolis, MN). Approximately 80% confluent monolayers of commercially obtained normal human keratinocytes (Invitrogen Life Technologies) grown in the serum-free keratinocyte growth medium containing 5 ng/ml epidermal growth factor and 50 mg/ml bovine pituitary extract (Gibco) in accordance the protocol provided by the vendor were exposed to test IgGs for the time periods specified under “Results” at 37 °C and 5% CO2, homogenized, and used in ELISA performed in accordance to the protocols provided by the manufacturers (24).
Statistical analysis
The data were analyzed using analysis of variance against an α level of 0.05 and are presented as means ± S.D. The graphs were created using GraphPad Prism 5.
Author contributions
A. C., K. T. A., A. F. A., C. W., and S. A. G. conceptualization; A. C., K. T. A., A. F. A., and C. W. formal analysis; A. C. and S. A. G. supervision; A. C., K. T. A., A. F. A., and S. A. G. funding acquisition; A. C. and S. A. G. validation; A. C., K. T. A., A. F. A., and C. W. methodology; A. C., K. T. A., A. F. A., and S. A. G. writing-original draft; A. C., K. T. A., and S. A. G. project administration.
This work was supported, in part, by a research grant from the International Pemphigus and Pemphigoid Foundation (to K. T. A.) and internal funds from the Department of Dermatology of University of California–Irvine. The authors declare that they have no conflicts of interest with the contents of this article.
- PV
- pemphigus vulgaris
- AuAb
- autoantibody
- Dsg
- desmoglein
- Dsc
- desmocollin
- AR
- acetylcholine receptor
- M3AR
- M3 muscarinic AR
- SPCA1
- secretory pathway Ca2+/Mn2+-ATPase isoform 1
- MAPK
- mitogen-activated protein kinase
- PVIgG
- IgG fraction of PV serum
- NIgG
- IgG fraction of normal serum
- ϵnAR
- ϵ-subunit of nicotinic AR
- HHD
- Hailey–Hailey disease
- PDAAI
- Pemphigus Disease Area and Activity Index
- NMSE
- neonatal mouse skin explant
- CytC
- cytochrome c
- AA
- amino acids
- PBS
- phosphate-buffered saline.
References
- 1. Ahmed A. R., Carrozzo M., Caux F., Cirillo N., Dmochowski M., Alonso A. E., Gniadecki R., Hertl M., López-Zabalza M. J., Lotti R., Pincelli C., Pittelkow M., Schmidt E., Sinha A. A., Sprecher E., et al. (2016) Monopathogenic vs. multipathogenic explanations of pemphigus pathophysiology. Exp. Dermatol. 25, 839–846 10.1111/exd.13106 [DOI] [PubMed] [Google Scholar]
- 2. Di Zenzo G., Amber K. T., Sayar B. S., Müller E. J., and Borradori L. (2016) Immune response in pemphigus and beyond: progresses and emerging concepts. Semin. Immunopathol. 38, 57–74 10.1007/s00281-015-0541-1 [DOI] [PubMed] [Google Scholar]
- 3. Sharma V. K., Prasad H. R., Khandpur S., and Kumar A. (2006) Evaluation of desmoglein enzyme-linked immunosorbent assay (ELISA) in Indian patients with pemphigus vulgaris. Int. J. Dermatol. 45, 518–522 10.1111/j.1365-4632.2006.02593.x [DOI] [PubMed] [Google Scholar]
- 4. Giurdanella F., Diercks G. F., Jonkman M. F., and Pas H. H. (2016) Laboratory diagnosis of pemphigus: direct immunofluorescence remains the gold standard. Br. J. Dermatol. 175, 185–186 10.1111/bjd.14408 [DOI] [PubMed] [Google Scholar]
- 5. Jamora M. J., Jiao D., and Bystryn J. C. (2003) Antibodies to desmoglein 1 and 3, and the clinical phenotype of pemphigus vulgaris. J. Am. Acad. Dermatol. 48, 976–977 10.1067/mjd.2003.438 [DOI] [PubMed] [Google Scholar]
- 6. Belloni-Fortina A., Faggion D., Pigozzi B., Peserico A., Bordignon M., Baldo V., and Alaibac M. (2009) Detection of autoantibodies against recombinant desmoglein 1 and 3 molecules in patients with pemphigus vulgaris: correlation with disease extent at the time of diagnosis and during follow-up. Clin. Dev. Immunol. 2009, 187864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cozzani E., Di Zenzo G., Riva S., Calabresi V., Sera F., Drosera M., and Parodi A. (2013) Are clinical phenotype and autoantibody profile always concordant in pemphigus?: a study in a cohort of pemphigus patients. Eur. J. Dermatol. 23, 40–48 [DOI] [PubMed] [Google Scholar]
- 8. Zagorodniuk I., Weltfriend S., Shtruminger L., Sprecher E., Kogan O., Pollack S., and Bergman R. (2005) A comparison of anti-desmoglein antibodies and indirect immunofluorescence in the serodiagnosis of pemphigus vulgaris. Int. J. Dermatol. 44, 541–544 10.1111/j.1365-4632.2004.02541.x [DOI] [PubMed] [Google Scholar]
- 9. Sardana K., Garg V. K., and Agarwal P. (2013) Is there an emergent need to modify the desmoglein compensation theory in pemphigus on the basis of Dsg ELISA data and alternative pathogenic mechanisms? Br. J. Dermatol. 168, 669–674 10.1111/bjd.12012 [DOI] [PubMed] [Google Scholar]
- 10. Kalantari-Dehaghi M., Anhalt G. J., Camilleri M. J., Chernyavsky A. I., Chun S., Felgner P. L., Jasinskas A., Leiferman K. M., Liang L., Marchenko S., Nakajima-Sasaki R., Pittelkow M. R., Zone J. J., and Grando S. A. (2013) Pemphigus vulgaris autoantibody profiling by proteomic technique. PLoS One 8, e57587 10.1371/journal.pone.0057587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sajda T., Hazelton J., Patel M., Seiffert-Sinha K., Steinman L., Robinson W., Haab B. B., and Sinha A. A. (2016) Multiplexed autoantigen microarrays identify HLA as a key driver of anti-desmoglein and -non-desmoglein reactivities in pemphigus. Proc. Natl. Acad. Sci. U.S.A. 113, 1859–1864 10.1073/pnas.1525448113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mao X., Nagler A. R., Farber S. A., Choi E. J., Jackson L. H., Leiferman K. M., Ishii N., Hashimoto T., Amagai M., Zone J. J., and Payne A. S. (2010) Autoimmunity to desmocollin 3 in pemphigus vulgaris. Am. J. Pathol. 177, 2724–2730 10.2353/ajpath.2010.100483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rafei D., Müller R., Ishii N., Llamazares M., Hashimoto T., Hertl M., and Eming R. (2011) IgG autoantibodies against desmocollin 3 in pemphigus sera induce loss of keratinocyte adhesion. Am. J. Pathol. 178, 718–723 10.1016/j.ajpath.2010.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen J., Den Z., and Koch P. J. (2008) Loss of desmocollin 3 in mice leads to epidermal blistering. J. Cell Sci. 121, 2844–2849 10.1242/jcs.031518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hu Z., Bonifas J. M., Beech J., Bench G., Shigihara T., Ogawa H., Ikeda S., Mauro T., and Epstein E. H. Jr. (2000) Mutations in ATP2C1, encoding a calcium pump, cause Hailey–Hailey disease. Nat. Genet. 24, 61–65 10.1038/71701 [DOI] [PubMed] [Google Scholar]
- 16. Sudbrak R., Brown J., Dobson-Stone C., Carter S., Ramser J., White J., Healy E., Dissanayake M., Larrègue M., Perrussel M., Lehrach H., Munro C. S., Strachan T., Burge S., Hovnanian A., et al. (2000) Hailey–Hailey disease is caused by mutations in ATP2C1 encoding a novel Ca2+ pump. Hum. Mol. Genet. 9, 1131–1140 10.1093/hmg/9.7.1131 [DOI] [PubMed] [Google Scholar]
- 17. Grando S. A. (2019) Retrospective analysis of a single-center clinical experience toward development of curative treatment of 123 pemphigus patients with a long-term follow-up: efficacy and safety of the multidrug protocol combining intravenous immunoglobulin with the cytotoxic immunosuppressor and mitochondrion-protecting drugs. Int. J. Dermatol. 58, 114–125 [DOI] [PubMed] [Google Scholar]
- 18. Grando S. A., Bystryn J. C., Chernyavsky A. I., Frusić-Zlotkin M., Gniadecki R., Lotti R., Milner Y., Pittelkow M. R., and Pincelli C. (2009) Apoptolysis: a novel mechanism of skin blistering in pemphigus vulgaris linking the apoptotic pathways to basal cell shrinkage and suprabasal acantholysis. Exp. Dermatol. 18, 764–770 10.1111/j.1600-0625.2009.00934.x [DOI] [PubMed] [Google Scholar]
- 19. Grando S. A. (2012) Pemphigus autoimmunity: hypotheses and realities. Autoimmunity 45, 7–35 10.3109/08916934.2011.606444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Amber K. T., Valdebran M., and Grando S. A. (2018) Non-desmoglein antibodies in patients with pemphigus vulgaris. Front. Immunol. 9, 1190 10.3389/fimmu.2018.01190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Amagai M., Karpati S., Prussick R., Klaus-Kovtun V., and Stanley J. R. (1992) Autoantibodies against the amino-terminal cadherin-like binding domain of pemphigus vulgaris antigen are pathogenic. J. Clin. Invest. 90, 919–926 10.1172/JCI115968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vu T. N., Lee T. X., Ndoye A., Shultz L. D., Pittelkow M. R., Dahl M. V., Lynch P. J., and Grando S. A. (1998) The pathophysiological significance of non-desmoglein targets of pemphigus autoimmunity: pemphigus vulgaris and foliaceus patients develop antibodies against keratinocyte cholinergic receptors. Arch. Dermatol. 134, 971–980 [DOI] [PubMed] [Google Scholar]
- 23. Nguyen V. T., Ndoye A., and Grando S. A. (2000) Pemphigus vulgaris antibody identifies pemphaxin: a novel keratinocyte annexin-like molecule binding acetylcholine. J. Biol. Chem. 275, 29466–29476 10.1074/jbc.M003174200 [DOI] [PubMed] [Google Scholar]
- 24. Marchenko S., Chernyavsky A. I., Arredondo J., Gindi V., and Grando S. A. (2010) Antimitochondrial autoantibodies in pemphigus vulgaris: a missing link in disease pathophysiology. J. Biol. Chem. 285, 3695–3704 10.1074/jbc.M109.081570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sajda T., Seiffert-Sinha K., and Sinha A. A. (2015) Anti-thyroid peroxidase antibodies induce similar effects on keratinocyte cell signaling as antibodies directed against desmoglein 3. J. Invest. Dermatol. 135, S12 (abstr. 68) [Google Scholar]
- 26. Sajda T., Seiffert-Sinha K., and Sinha A. A. (2014) Autoantibodies directed against thyroid peroxidase may contribute to blister formation in pemphigus vulgaris. J. Invest. Dermatol. 134, S13 (abstr. 74) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dmochowski M., Hashimoto T., Garrod D. R., and Nishikawa T. (1993) Desmocollins I and II are recognized by certain sera from patients with various types of pemphigus particularly Brazilian pemphigus foliaceus. J. Invest. Dermatol. 100, 380–384 10.1111/1523-1747.ep12471934 [DOI] [PubMed] [Google Scholar]
- 28. Dmochowski M., Hashimoto T., Chidgey M. A., Yue K. K., Wilkinson R. W., Nishikawa T., and Garrod D. R. (1995) Demonstration of antibodies to bovine desmocollin isoforms in certain pemphigus sera. Br. J. Dermatol. 133, 519–525 10.1111/j.1365-2133.1995.tb02698.x [DOI] [PubMed] [Google Scholar]
- 29. Spindler V., Heupel W. M., Efthymiadis A., Schmidt E., Eming R., Rankl C., Hinterdorfer P., Müller T., Drenckhahn D., and Waschke J. (2009) Desmocollin 3-mediated binding is crucial for keratinocyte cohesion and is impaired in pemphigus. J. Biol. Chem. 284, 30556–30564 10.1074/jbc.M109.024810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grando S. A., and Dahl M. V. (1993) Activation of keratinocyte muscarinic acetylcholine receptors reverses pemphigus acantholysis. J. Eur. Acad. Dermatol. Venereol. 2, 72–86 10.1111/j.1468-3083.1993.tb00016.x [DOI] [Google Scholar]
- 31. Sinha A. A. (2012) Constructing immunoprofiles to deconstruct disease complexity in pemphigus. Autoimmunity 45, 36–43 10.3109/08916934.2011.606445 [DOI] [PubMed] [Google Scholar]
- 32. Lakshmi M. J. D., Jaisankar T. J., Rajappa M., Thappa D. M., Chandrashekar L., Divyapriya D., Munisamy M., and Revathy G. (2017) Correlation of antimuscarinic acetylcholine receptor antibody titers and antidesmoglein antibody titers with the severity of disease in patients with pemphigus. J. Am. Acad. Dermatol. 76, 895–902 10.1016/j.jaad.2016.11.039 [DOI] [PubMed] [Google Scholar]
- 33. Grando S. A. (2012) Muscarinic receptor agonists and antagonists: effects on keratinocyte functions. Handb. Exp. Pharmacol. 2012, 429–450 [DOI] [PubMed] [Google Scholar]
- 34. Chen Y., Chernyavsky A., Webber R. J., Grando S. A., and Wang P. H. (2015) Critical role of the neonatal Fc receptor (FcRn) in the pathogenic action of antimitochondrial autoantibodies synergizing with anti-desmoglein autoantibodies in pemphigus vulgaris. J. Biol. Chem. 290, 23826–23837 10.1074/jbc.M115.668061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nguyen V. T., Ndoye A., Shultz L. D., Pittelkow M. R., and Grando S. A. (2000) Antibodies against keratinocyte antigens other than desmogleins 1 and 3 can induce pemphigus vulgaris-like lesions. J. Clin. Invest. 106, 1467–1479 10.1172/JCI10305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Behne M. J., Tu C. L., Aronchik I., Epstein E., Bench G., Bikle D. D., Pozzan T., and Mauro T. M. (2003) Human keratinocyte ATP2C1 localizes to the Golgi and controls Golgi Ca2+ stores. J. Invest. Dermatol. 121, 688–694 10.1046/j.1523-1747.2003.12528.x [DOI] [PubMed] [Google Scholar]
- 37. Seishima M., Esaki C., Osada K., Mori S., Hashimoto T., and Kitajima Y. (1995) Pemphigus IgG, but not bullous pemphigoid IgG, causes a transient increase in intracellular calcium and inositol 1,4,5-triphosphate in DJM-1 cells, a squamous cell carcinoma line. J. Invest. Dermatol. 104, 33–37 10.1111/1523-1747.ep12613469 [DOI] [PubMed] [Google Scholar]
- 38. Ferri K. F., and Kroemer G. (2001) Organelle-specific initiation of cell death pathways. Nat. Cell Biol. 3, E255–E263 10.1038/ncb1101-e255 [DOI] [PubMed] [Google Scholar]
- 39. Hirata Y., Furuta K., Miyazaki S., Suzuki M., and Kiuchi K. (2004) Anti-apoptotic and pro-apoptotic effect of NEPP11 on manganese-induced apoptosis and JNK pathway activation in PC12 cells. Brain Res. 1021, 241–247 10.1016/j.brainres.2004.06.064 [DOI] [PubMed] [Google Scholar]
- 40. Shull G. E., Miller M. L., and Prasad V. (2011) Secretory pathway stress responses as possible mechanisms of disease involving Golgi Ca2+ pump dysfunction. Biofactors 37, 150–158 10.1002/biof.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lehotsky J., Racay P., Pavlíková M., Tatarková Z., Urban P., Chomová M., Kovalská M., and Kaplán P. (2009) Cross-talk of intracellular calcium stores in the response to neuronal ischemia and ischemic tolerance. Gen. Physiol. Biophys. 28, F104–F114 [PubMed] [Google Scholar]
- 42. Madala J., Bashamalla R., and Kumar M. P. (2017) Current concepts of pemphigus with a deep insight into its molecular aspects. J. Oral Maxillofac. Pathol. 21, 260–263 10.4103/jomfp.JOMFP_143_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sepúlveda M. R., Vanoevelen J., Raeymaekers L., Mata A. M., and Wuytack F. (2009) Silencing the SPCA1 (secretory pathway Ca2+-ATPase isoform 1) impairs Ca2+ homeostasis in the Golgi and disturbs neural polarity. J. Neurosci. 29, 12174–12182 10.1523/JNEUROSCI.2014-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Okunade G. W., Miller M. L., Azhar M., Andringa A., Sanford L. P., Doetschman T., Prasad V., and Shull G. E. (2007) Loss of the Atp2c1 secretory pathway Ca2+-ATPase (SPCA1) in mice causes Golgi stress, apoptosis, and midgestational death in homozygous embryos and squamous cell tumors in adult heterozygotes. J. Biol. Chem. 282, 26517–26527 10.1074/jbc.M703029200 [DOI] [PubMed] [Google Scholar]
- 45. Chernyavsky A. I., Arredondo J., Kitajima Y., Sato-Nagai M., and Grando S. A. (2007) Desmoglein versus non-desmoglein signaling in pemphigus acantholysis: characterization of novel signaling pathways downstream of pemphigus vulgaris antigens. J. Biol. Chem. 282, 13804–13812 10.1074/jbc.M611365200 [DOI] [PubMed] [Google Scholar]
- 46. Jolly P. S., Berkowitz P., Bektas M., Lee H. E., Chua M., Diaz L. A., and Rubenstein D. S. (2010) p38MAPK signaling and desmoglein-3 internalization are linked events in pemphigus acantholysis. J. Biol. Chem. 285, 8936–8941 10.1074/jbc.M109.087999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mao X., Sano Y., Park J. M., and Payne A. S. (2011) p38 mitogen activated protein kinase (MAPK) activation is downstream of the loss of intercellular adhesion in pemphigus vulgaris. J. Biol. Chem. 286, 1283–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Spindler V., Vielmuth F., Schmidt E., Rubenstein D. S., and Waschke J. (2010) Protective endogenous cyclic adenosine 5′-monophosphate signaling triggered by pemphigus autoantibodies. J. Immunol. 185, 6831–6838 10.4049/jimmunol.1002675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Haas M., Wang H., Tian J., and Xie Z. (2002) Src-mediated inter-receptor cross-talk between the Na+/K+-ATPase and the epidermal growth factor receptor relays the signal from ouabain to mitogen-activated protein kinases. J. Biol. Chem. 277, 18694–18702 10.1074/jbc.M111357200 [DOI] [PubMed] [Google Scholar]
- 50. Frusic-Zlotkin M., Raichenberg D., Wang X., David M., Michel B., and Milner Y. (2006) Apoptotic mechanism in pemphigus autoimmunoglobulins-induced acantholysis: possible involvement of the EGF receptor. Autoimmunity 39, 563–575 10.1080/08916930600971836 [DOI] [PubMed] [Google Scholar]
- 51. España A., Mòdol T., Gil M. P., and López-Zabalza M. J. (2013) Neural nitric oxide synthase participates in pemphigus vulgaris acantholysis through upregulation of Rous sarcoma, mammalian target of rapamycin and focal adhesion kinase. Exp. Dermatol. 22, 125–130 10.1111/exd.12088 [DOI] [PubMed] [Google Scholar]
- 52. Cho S. G., and Choi E. J. (2002) Apoptotic signaling pathways: caspases and stress-activated protein kinases. J. Biochem. Mol. Biol. 35, 24–27 [DOI] [PubMed] [Google Scholar]
- 53. Carraro M., and Bernardi P. (2016) Calcium and reactive oxygen species in regulation of the mitochondrial permeability transition and of programmed cell death in yeast. Cell Calcium 60, 102–107 10.1016/j.ceca.2016.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Didier K., Bolko L., Giusti D., Toquet S., Robbins A., Antonicelli F., and Servettaz A. (2018) Autoantibodies associated with connective tissue diseases: what meaning for clinicians? Front. Immunol. 9, 541 10.3389/fimmu.2018.00541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Arredondo J., Chernyavsky A. I., Karaouni A., and Grando S. A. (2005) Novel mechanisms of target cell death and survival and of therapeutic action of IVIg in pemphigus. Am. J. Pathol. 167, 1531–1544 10.1016/S0002-9440(10)61239-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nguyen V. T., Ndoye A., and Grando S. A. (2000) Novel human a9 acetylcholine receptor regulating keratinocyte adhesion is targeted by pemphigus vulgaris autoimmunity. Am J. Pathol. 157, 1377–1391 10.1016/S0002-9440(10)64651-2 [DOI] [PMC free article] [PubMed] [Google Scholar]