Abstract
Neuroblastoma cells highly express the disialoganglioside GD2, a tumor-associated carbohydrate antigen, which is only sparsely expressed on healthy tissue. GD2 is a primary target for the development of immunotherapy for neuroblastoma. Immunotherapy with monoclonal anti-GD2 antibodies has proven safety and efficacy in clinical trials and is included in the standard treatment for children with high-risk neuroblastoma. Strategies to modulate GD2 expression in neuroblastoma could further improve anti-GD2–targeted immunotherapy. Here, we report that the cellular sialylation pathway, as well as epigenetic reprogramming, strongly modulates GD2 expression in human and mouse neuroblastoma cell lines. Recognition of GD2 by the 14G2a antibody is sialic acid–dependent and was blocked with the fluorinated sialic acid mimetic Ac53FaxNeu5Ac. Interestingly, sialic acid supplementation using a cell-permeable sialic acid analogue (Ac5Neu5Ac) boosted GD2 expression without or with minor alterations in overall cell surface sialylation. Furthermore, sialic acid supplementation with Ac5Neu5Ac combined with various histone deacetylase (HDAC) inhibitors, including vorinostat, enhanced GD2 expression in neuroblastoma cells beyond their individual effects. Mechanistic studies revealed that Ac5Neu5Ac supplementation increased intracellular CMP–Neu5Ac concentrations, thereby providing higher substrate levels for sialyltransferases. Furthermore, HDAC inhibitor treatment increased mRNA expression of the sialyltransferases GM3 synthase (ST3GAL5) and GD3 synthase (ST8SIA1), both of which are involved in GD2 biosynthesis. Our findings reveal that sialic acid analogues and HDAC inhibitors enhance GD2 expression and could potentially be employed to boost anti-GD2 targeted immunotherapy in neuroblastoma patients.
Keywords: neuroblastoma, sialic acid, histone deacetylase inhibitor (HDAC inhibitor) (HDI), sialyltransferase, cancer, glycosyltransferase, disialoganglioside, GD2
Introduction
Neuroblastoma is a childhood malignancy that originates in the sympathetic nervous system and is responsible for 12% of cancer-related deaths in children younger than 15 years (1). Although, the prognosis of high-risk neuroblastoma patients has improved over the last decades, their long-term survival still remains poor despite the intensive multimodal treatment (2). All neuroblastomas express high levels of GD2, a ganglioside composed of a ceramide anchored in the outer leaflet of the plasma membrane and a glycan moiety extending into the extracellular milieu. The glycan consists of glucose, galactose, GalNAc, and two sialic acid residues (Neu5Ac) that branch from the galactose residue (Fig. 1A). GD2 is produced from its precursor gangliosides GM3 and GD3 by sequential activity of the glycosyltransferases GM3 synthase (ST3GAL5), GD3 synthase (ST8SIA1) and GD2 synthase (B4GALNT1), respectively (Fig. 1A) (3). GD2 is homogenously and highly expressed by neuroblastoma cells, whereas in healthy tissues it is expressed only by neurons, skin melanocytes, and peripheral pain fibers (4–6). This preferential expression on neuroblastoma cells makes GD2 a key target for the development of immunotherapy for neuroblastoma, ranked 12th of 75 of all important tumor antigens identified by the National Cancer Institute (7).
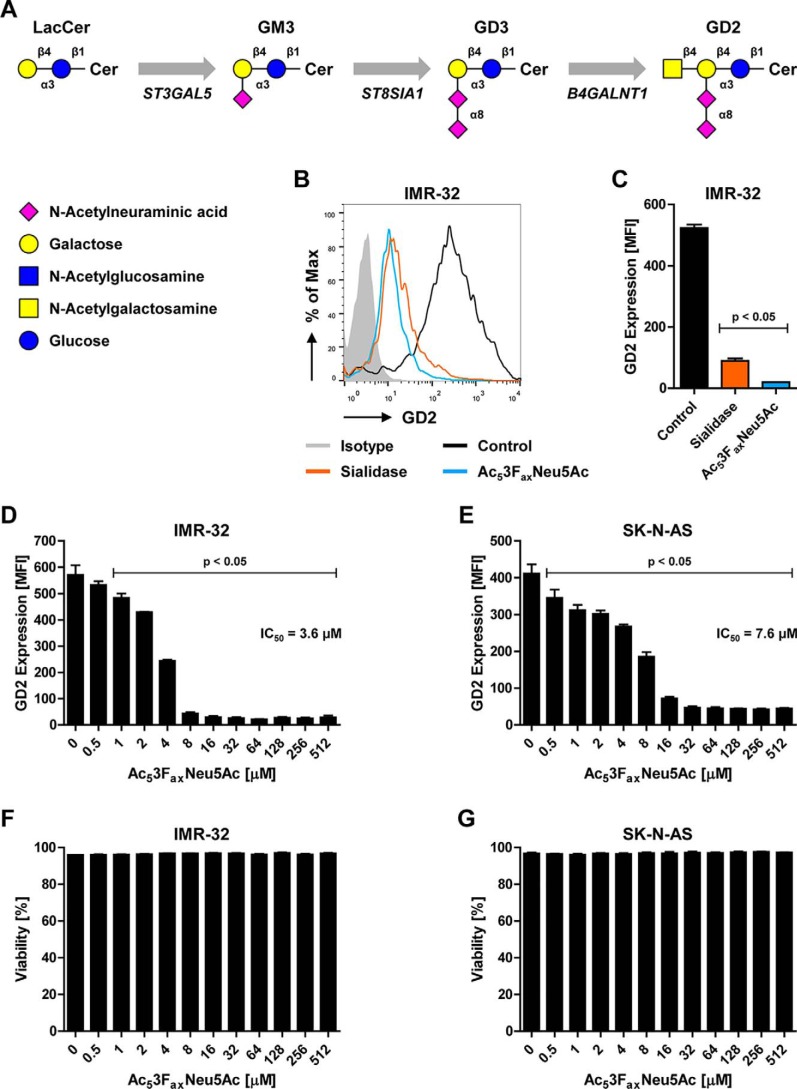
Figure 1.
Recognition of GD2 by the 14G2a antibody is sialic acid dependent. A, schematic representation of the GD2 biosynthesis pathway. B and C, binding of the anti-GD2 antibody 14G2a to control or sialidase- or Ac53FaxNeu5Ac-treated IMR-32 cells. A representative histogram shows GD2 expression as detected by flow cytometry (B), and the bar diagram shows GD2 expression as mean fluorescence intensity ± S.E. of three independent experiments (C). D and E, effect of metabolic sialic acid inhibition on GD2 expression. Bar diagrams show GD2 expression as mean fluorescence intensity ± S.E. on IMR-32 cells (D) and SK-N-AS cells (E) treated with 0–512 μm Ac53FaxNeu5Ac for 3 days (n = 3). F and G, effect of Ac53FaxNeu5Ac on cell viability. Bar diagrams show mean percentage of viable cells ± S.E. in the IMR-32 (F) and SK-N-AS (G) cultures after treatment with 0–512 μm Ac53FaxNeu5Ac for 3 days (n = 3).
Over the past 3 decades, GD2 has been used as the primary target for the development of immunotherapeutic monoclonal antibodies. Monoclonal anti-GD2 antibodies effectively mediate the lysis of neuroblastoma cells via antibody-dependent cell-mediated cytotoxicity (ADCC)2 involving natural killer cells and granulocytes as well as complement-dependent cytotoxicity (6, 8–12). Anti-GD2 antibodies, e.g. dinutuximab, proved safe and efficacious in clinical trials and are therefore included in the routine treatment of high-risk neuroblastoma (5, 13–18). More recently, GD2 has also been explored as a target for T-cell immunotherapy by incorporating the antibody specificity into chimeric antigen receptor (CAR) T cells. In a small patient cohort, GD2-specific CAR-T cell administration was well-tolerated and was associated with tumor regression and necrosis in half of the patients (19). The long-term follow-up showed low-level persistence of CAR T cells, which was associated with clinical benefit, including three complete responses (20). Based on these encouraging results, several clinical phase I trials are currently testing third- and fourth-generation GD2-specific CAR T cells, including combinations with immune checkpoint–blocking antibodies (21, 22). Next to monoclonal antibodies and CAR T cells, GD2 is also a potential target for carbohydrate-based neuroblastoma vaccines (23, 24).
Despite these advances in neuroblastoma immunotherapy, still around half of the patients eventually show progressive disease (25). Combining immunotherapy with other tumor-targeting therapies could further improve the treatment of neuroblastoma. We have recently reported that histone deacetylase (HDAC) inhibitors could be successfully applied together with anti-GD2 antibody as immune-combination therapy in a preclinical model (26, 27). The HDAC family controls gene expression at the epigenetic level by removing acetyl groups from histones and from nonhistone proteins (28). HDAC inhibitors are emerging as potent anticancer drugs that induce cell cycle arrest and differentiation in neuroblastoma and other cancer types (29, 30). Using a murine neuroblastoma model resembling the immunobiology of human neuroblastoma, our group recently reported that the pan-HDAC inhibitor vorinostat synergized with anti-GD2 mAb therapy in reducing neuroblastoma tumor growth (27). Vorinostat created a more immunopermissive tumor microenvironment, but it also enhanced GD2 expression on neuroblastoma cells by increasing GD2 synthase (B4GALNT1) protein but not mRNA levels.
Here, we report that the fluorinated sialic acid analogue Ac53FaxNeu5Ac potently blocked GD2 expression, whereas the cell-permeable, acetylated sialic acid Ac5Neu5Ac boosted GD2 expression on neuroblastoma cells. In view of the total cellular sialylation pathway, the GD2 biosynthesis pathway in neuroblastoma cells appeared highly sensitive to the effects of the sialic acid analogues. Moreover, we found that sialic acid supplementation combined with various HDAC inhibitors strongly increased GD2 expression. As a result of Ac5Neu5Ac addition, intracellular CMP–Neu5Ac levels, the substrate for sialyltransferases, increased strongly. In addition, HDAC inhibitor treatment increased the expression of sialyltransferases involved in GD2 biosynthesis, thereby providing mechanistic insights into the strong combination effect of Ac5Neu5Ac and HDAC inhibitors. In conclusion, this study provides a rationale for boosting GD2-targeted neuroblastoma immunotherapy through sialic acid engineering and/or HDAC inhibition.
Results
Ac53FaxNeu5Ac blocks GD2 expression in neuroblastoma cell lines
The tumor antigen GD2 carries two sialic acid residues, one that is linked via an α2,3 linkage to galactose and a second one that is linked to the sialic acid via an α2,8 linkage (Fig. 1A). These linkages are produced by GM3 synthase (ST3GAL5) and GD3 synthase (ST8SIA1), two sialyltransferases that transfer sialic acids to the GD2 precursors GM3 and GD3, respectively (Fig. 1A). The availability of GM3 and GD3 is therefore rate-limiting for GD2 synthase activity and the production of GD2 (3, 31). We and others have shown previously that a cell-permeable fluorinated sialic acid analogue, Ac53FaxNeu5Ac, inhibits sialyltransferases and blocks α2,3 and α2,6 sialylation in human cells (32–35). Here we investigated whether Ac53FaxNeu5Ac is capable of blocking the production of the disialoganglioside GD2 in human neuroblastoma cell lines. Human IMR-32 cells were treated with the sialic acid inhibitor Ac53FaxNeu5Ac (100 μm) for 3 days or with Clostridium perfringens sialidase for 1 h after which membrane GD2 expression was measured by flow cytometry using the anti-GD2 antibody 14G2a. Both enzymatic removal of sialic acid and metabolic sialic acid blockade with Ac53FaxNeu5Ac almost completely abolished GD2 staining on IMR-32 cells (Fig. 1, B and C). To determine the effective dose of the sialic acid inhibitor, increasing concentrations of Ac53FaxNeu5Ac were added to IMR-32 and SK-N-AS cells, and the expression of GD2 was analyzed. GD2 expression in both cell lines was reduced in a dose-dependent manner with an IC50 of 3.6 μm for IMR-32 cells and 7.6 μm for SK-N-AS cells (Fig. 1, D and E). Higher concentrations, around 8 μm for IMR-32 and 32 μm for SK-N-AS, reduced GD2 expression by more than 90%. Importantly, at all concentrations used, the fluorinated sialic acid showed no effect on cell viability in either of the cell lines (Fig. 1, F and G). These results confirm that GD2 recognition by the anti-GD2 antibody 14G2a depends on sialic acid residues and identify Ac53FaxNeu5Ac as a potent metabolic inhibitor of GD2 expression.
Cell-permeable sialic acid enhances GD2 expression
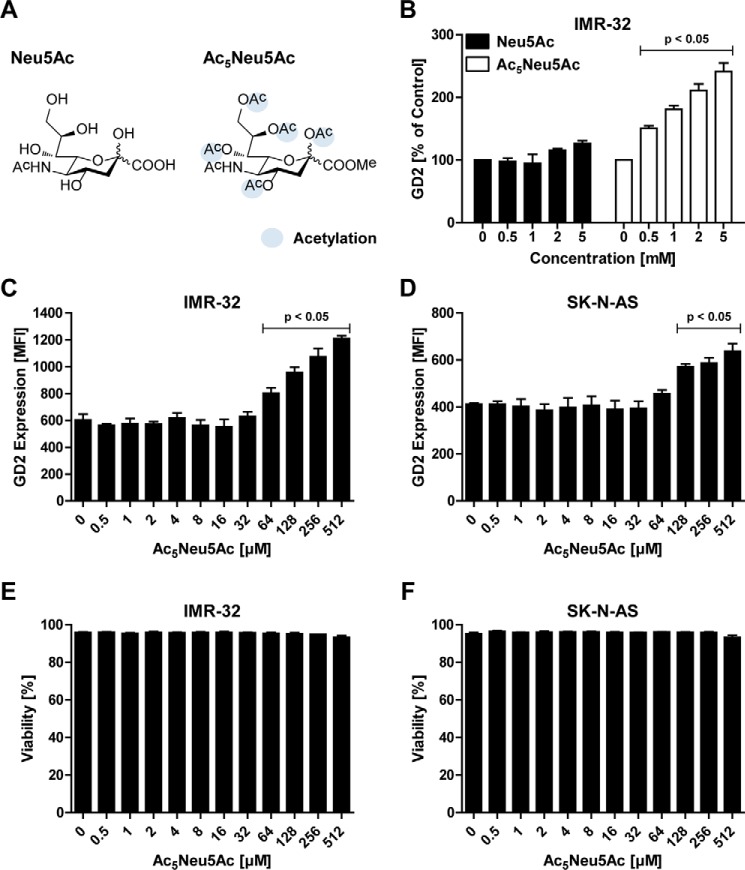
Having shown that GD2 expression can be blocked with the metabolic inhibitor of sialylation Ac53FaxNeu5Ac, we next investigated whether GD2 expression can be influenced by supplementing the sialylation pathway with exogenous sialic acid. Physiologically, sialic acids are poorly cell-permeable because of their negative charge and hydrophilic properties. In addition, mammalian cells lack an active uptake system for sialic acids (36). To overcome this obstacle, ester-linked acetyl groups were added to the sialic acid sugar, yielding a peracetylated sialic acid, Ac5Neu5Ac, which is cell-permeable (Fig. 2A). Inside the cell, the acetyl groups are removed by cytoplasmic esterases and the sialic acid enters the sialylation pathway (37, 38). To test the effect of control Neu5Ac and Ac5Neu5Ac on GD2 expression, IMR-32 cells were incubated for 3 days with increasing concentrations of the sialic acids and analyzed by flow cytometry. Although the addition of up to 5 mm Neu5Ac showed no significant effect on GD2 expression, peracetylated sialic acid Ac5Neu5Ac significantly enhanced GD2 expression at concentrations of 0.5 mm and higher (Fig. 2B). Subsequently, the titration of Ac5Neu5Ac into IMR-32 and SK-N-AS cell cultures confirmed that GD2 expression was increased in a dose-dependent manner without affecting cell viability (Fig. 2, C–F). In IMR-32 cells, the GD2 expression was doubled by adding Ac5NeuAc concentrations above 128 μm, and in SK-N-AS expression levels could be increased about 1.5 times. Similar results were obtained using N-acetylmannosamine (ManNAc) supplementation, the biological precursor of sialic acid (Fig. S1). Altogether, these results indicate that increased intracellular sialic acid availability leads to higher GD2 expression in neuroblastoma cells.
Figure 2.
Peracetylated sialic acid enhances GD2 expression. A, structural representation of Neu5Ac (left) and the peracetylated form Ac5Neu5Ac (right). B, effect of sialic acid supplementation on GD2 expression of IMR-32 cells. The cells were cultured for 3 days with 0–5 mm Neu5Ac or Ac5Neu5Ac, and GD2 expression was assessed by flow cytometry. The bar diagram shows the mean percentage of GD2 expression ± S.E. of IMR-32 cells treated with Neu5Ac or Ac5Neu5Ac normalized to control (n = 3). C and D, IMR-32 and SK-N-AS cells were cultured for 3 days with 0–512 μm Ac5Neu5Ac and stained with anti-GD2 antibody. Bar diagrams show GD2 expression of IMR-32 cells (C) and SK-N-AS cells (D) as mean fluorescence intensity ± S.E. of three independent experiments. E and F, effect of Ac5Neu5Ac on cell viability. Bar diagrams show mean percentage of viable IMR-32 cells (E) and SK-N-AS cells (F) ± S.E. in culture after treatment with 0–512 μm Ac5Neu5Ac for 3 days (n = 3).
GD2 expression is sensitive to sialic acid modulation relative to overall sialylation
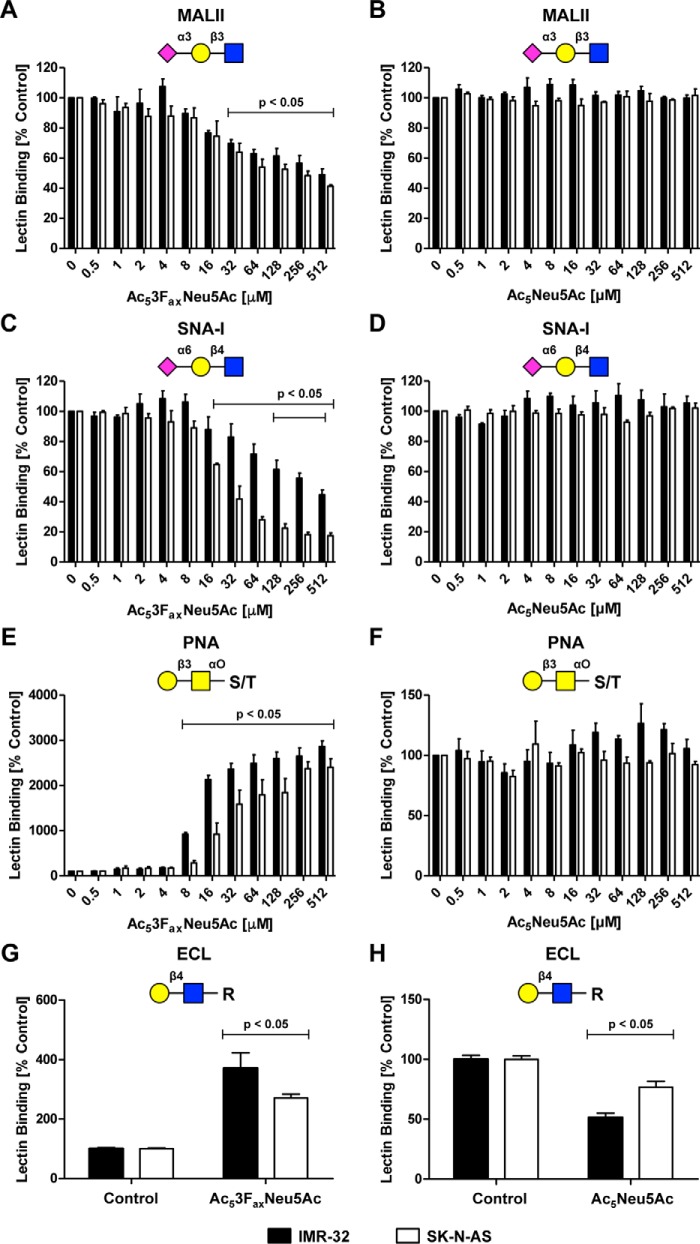
To determine the effect of sialic acid analogues on total cell surface sialylation, neuroblastoma cells were incubated for 3 days with increasing concentrations of the sialic acid inhibitor Ac53FaxNeu5Ac or supplemented with cell-permeable Ac5Neu5Ac. Cell surface sialylation was analyzed by flow cytometry using carbohydrate-binding lectins. IMR-32 and SK-N-AS cells expressed high levels of α2,3-linked and α2,6-linked sialic acids as detected with the lectins MALII and SNA-I, respectively, and uncapped glycans were barely detected using PNA lectin (Fig. S2). The sialic acid inhibitor Ac53FaxNeu5Ac reduced α2,3 and α2,6 sialylation in a dose-dependent manner in both cell lines (Fig. 3, A and C). Consistent with decreased sialic acid expression, exposure of terminal β1–3– and β1–4–linked galactose residues increased as reflected by enhanced binding of the lectins PNA and ECL, respectively (Fig. 3, E and G). The maximum level of inhibition observed with 512 μm Ac53FaxNeu5Ac was 50 and 60% for α2,3-linked sialic acids and 50 and 80% for α2,6-linked sialic in IMR-32 and SK-N-AS cells, respectively. In contrast, GD2 expression was almost completely lost at concentrations as low as 16 μm, a concentration at which the overall cell surface sialylation was only slightly reduced.
Figure 3.
Effect of sialic acid analogues on overall cell surface sialylation. A–F, IMR-32 cells and SK-N-AS cells were cultured for 3 days with 0–512 μm Ac53FaxNeu5Ac or Ac5Neu5Ac, and cell surface sialylation was assessed by flow cytometry using fluorescent lectins. The lectins recognize α2,3-linked sialic acids (MALII), α2,6-linked sialic acids (SNA-I), or terminal β-galactose (PNA), respectively. Bar diagrams show average binding ± S.E. normalized to control of MALII (A and B), SNA-I (C and D), or PNA (E and F) on IMR-32 cells and SK-N-AS cells treated with Ac53FaxNeu5Ac or Ac5Neu5Ac (n = 3). G–H, IMR-32 and SK-N-AS cells were cultured for 3 days with Ac53FaxNeu5Ac (32 μm) or Ac5Neu5Ac (2 mm). Cells were stained with the ECL lectin, which recognizes uncapped terminal Galβ1–4GlcNAc residues. Bar diagrams show average binding ± S.E. normalized to control of ECL on IMR-32 cells and SK-N-AS cells treated with Ac53FaxNeu5Ac (G) or Ac5Neu5Ac (H) (n = 2).
Ac5Neu5Ac supplementation did not result in significant changes in total cell surface sialylation as detected by MALII and SNA-I, even at a concentration of 512 μm (Fig. 3, B, D, and F, and Fig. S2). Only ECL staining revealed a significantly lower exposure of terminal β1–4–linked galactose residues upon treatment with a high concentration (2 mm) of Ac5Neu5Ac (Fig. 3H). Overall glycosylation was not altered upon Ac5Neu5Ac supplementation (Fig. S3, A–C).
In contrast, Ac5Neu5Ac concentrations from 128 μm and higher significantly increased GD2 expression (Fig. 2). Collectively, these findings suggest that the GD2 biosynthesis pathway is highly sensitive to both sialic acid inhibition and supplementation as compared with overall cell surface sialylation of neuroblastoma cells.
Combined effect of sialic acid analogues and HDAC inhibitors on GD2 expression
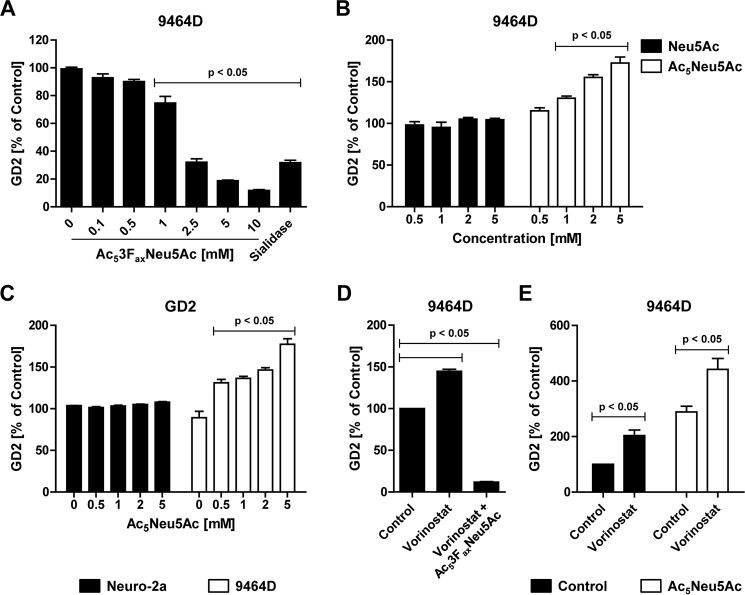
Previously, we reported that the pan-HDAC inhibitor vorinostat enhances GD2 expression in the 9464D neuroblastoma mouse tumor model in vitro and in vivo. The enhanced GD2 expression induced by vorinostat resulted in improved anti-GD2 antibody–mediated ADCC and reduced tumor growth (27); the vorinostat-increased GD2 expression in 9464D neuroblastoma cells was caused by increased GD2 synthase protein but not mRNA levels. These findings prompted us to assess the combined effect of sialic acid analogues and HDAC inhibitors on GD2 expression in murine 9464D and the human IMR-32 and SK-N-AS cell lines. First, we confirmed the effects of the sialic acid analogues in the murine neuroblastoma cells. 9464D cells were cultured for 3 days with 0–10 mm Ac53FaxNeu5Ac or were treated for 1 h with C. perfringens sialidase. Ac53FaxNeu5Ac inhibited GD2 expression in this murine neuroblastoma cell line, although higher concentrations were needed compared with the human cells (Fig. 4A). Similar to the human neuroblastoma cells, the addition of peracetylated sialic acid Ac5Neu5Ac, but not the nonpermeable sialic acid Neu5Ac, significantly enhanced GD2 expression at concentrations of 1 mm and higher (Fig. 4B). Importantly, Ac5Neu5Ac supplementation did not induce GD2 expression in GD2 synthase negative Neuro-2a neuroblastoma cells, in various nonneuroblastoma tumor cell lines, or in noncancerous human or mouse cells (Figs. 4C and S4). These data confirm that the increased availability of sialic acids enhances GD2 biosynthesis also in murine neuroblastoma cells.
Figure 4.
Sialic acid analogues and vorinostat modulate GD2 expression in murine 9464D cells. A, effect of Ac53FaxNeu5Ac on GD2 expression in 9464D cells. 9464D cells were cultured for 3 days with 0–10 mm Ac53FaxNeu5Ac or treated for 1 h with C. perfringens sialidase, and GD2 expression was determined by flow cytometry. The bar diagram shows the mean percentage of GD2 expression ± S.E. normalized to control (n = 3). B, effect of sialic acid supplementation on GD2 expression of 9464D cells. The cells were cultured for 3 days with 0–5 mm Neu5Ac or Ac5Neu5Ac, and GD2 expression was assessed by flow cytometry. The bar diagram shows the mean percentage of GD2 expression ± S.E. of 9464D cells treated with Neu5Ac or Ac5Neu5Ac normalized to control (n = 3). C, Neuro-2a or 9464D cells were cultured for 3 days with 0–5 mm Ac5Neu5Ac and stained with anti-GD2 antibody. Bar diagrams show the mean percentage of GD2 expression ± S.E. (n = 3). D, Ac53FaxNeu5Ac blocks vorinostat-mediated up-regulation of GD2. 9464D cells were cultured for 3 days with 5 mm Ac53FaxNeu5Ac, and during the last 24 h of the culture 256 nm vorinostat was added. The bar diagram shows the mean percentage of GD2 expression ± S.E. normalized to control (n = 3). E, Ac5Neu5Ac and vorinostat boost GD2 expression. 9464D cells were treated for 3 days with 2 mm Ac5Neu5Ac. On the last 2 days of the culture, 256 nm vorinostat was added, and GD2 expression was assessed. The bar diagram shows the mean percentage of GD2 expression ± S.E. normalized to control (n = 2).
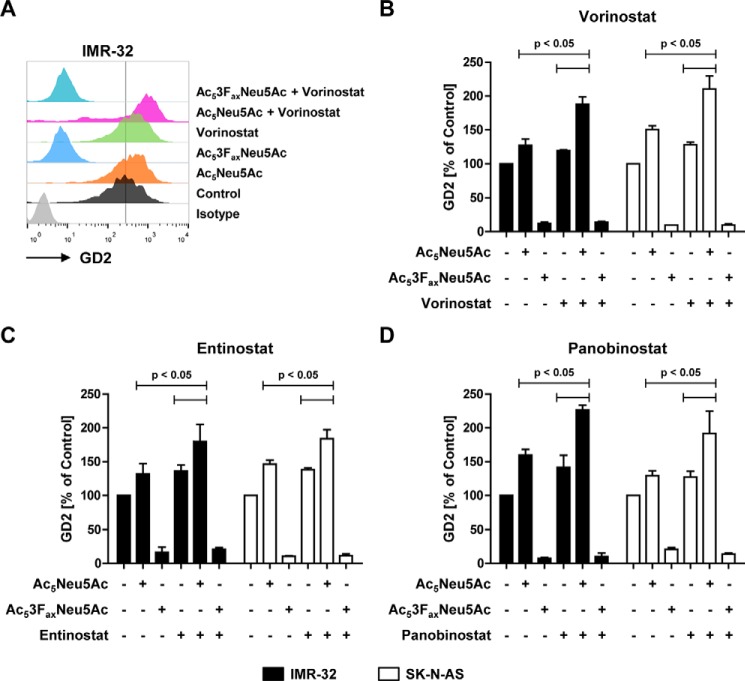
Next, we assessed the combined effect of HDAC inhibitors and the sialic acid analogues on GD2 expression. Murine 9464D and human IMR-32 and SK-N-AS neuroblastoma cells were treated for 48 h with control or Ac53FaxNeu5Ac and for another 24 h with vorinostat, after which GD2 expression was analyzed. In line with our previous study, vorinostat treatment enhanced GD2 expression in all three neuroblastoma cell lines. Cells treated with Ac53FaxNeu5Ac lost expression of GD2 even in the presence of vorinostat, confirming that the HDAC inhibitor–induced up-regulation of GD2 is also sialic acid–dependent (Figs. 4D and 5, A and B). Subsequently, neuroblastoma cells were cultured with the cell-permeable sialic acid Ac5Neu5Ac followed by vorinostat. Strikingly, this combination significantly increased GD2 expression in both murine and human neuroblastoma cell lines beyond the effect of the individual compounds (Figs. 4E and 5, A and B). Whereas Ac5Neu5Ac and vorinostat alone each enhanced GD2 expression about 1.5-fold, the combined treatment resulted in more than 2-fold higher GD2 expression. Notably, vorinostat treatment showed no significant effect on overall cell surface sialylation or overall glycosylation (Fig. S3). Furthermore, the class I HDAC inhibitor entinostat and the pan-HDAC inhibitor panobinostat also increased GD2 expression to a similar extend as vorinostat (Fig. 5, C and D). In conclusion, these data show that the combination of sialic acid supplementation and HDAC inhibition results in an even stronger up-regulation of GD2 expression in neuroblastoma cells.
Figure 5.
Combined effect of Ac5Neu5Ac and HDAC inhibitors on GD2 expression in human neuroblastoma cells. A–D, IMR-32 and SK-N-AS cells were cultured for 2 days with control, Ac53FaxNeu5Ac (32 μm), or Ac5Neu5Ac (2 mm) and for another day with or without HDAC inhibitors; GD2 expression was determined by flow cytometry. A representative histogram shows GD2 expression of IMR-32 cells treated with sialic acid analogues alone or in combination with 32 nm vorinostat (A). Bar diagrams show the mean GD2 expression ± S.E. normalized to control of IMR-32 and SK-N-AS cells incubated with Ac53FaxNeu5Ac or AcNeu5Ac in combination with 32 nm vorinostat (B), 32 nm entinostat (C), or 1 nm panobinostat (D). Data from three independent experiments are shown.
Ac5Neu5Ac increases intracellular CMP–Neu5Ac levels, and vorinostat induces expression of sialyltransferases involved in GD2 biosynthesis
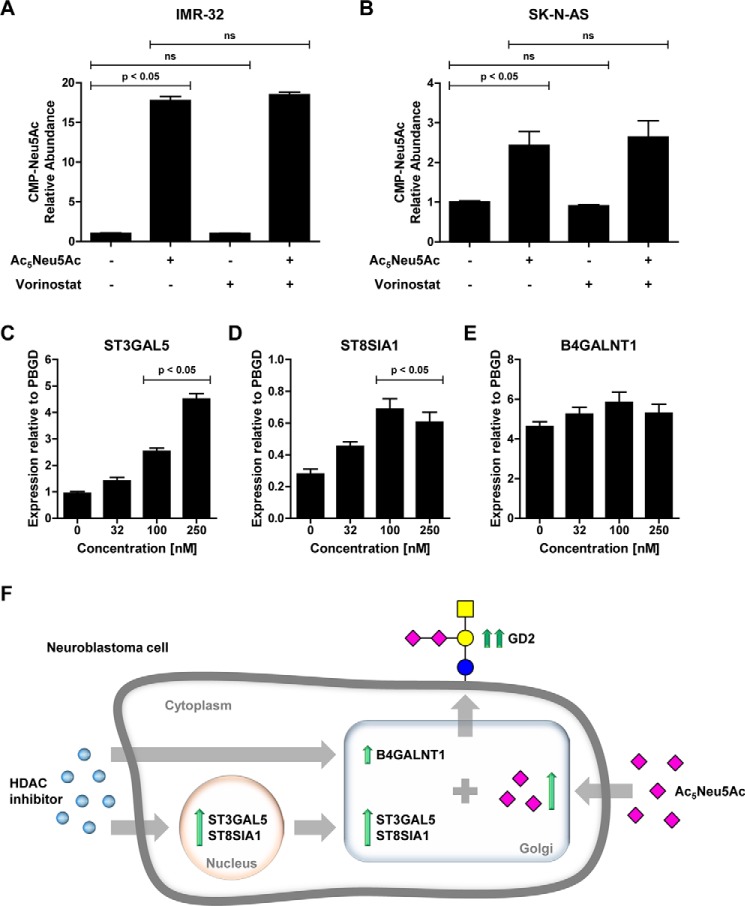
To investigate the effect of sialic acid supplementation on substrate availability for sialyltransferases, we assessed intracellular CMP–Neu5Ac levels in IMR-32 and SK-N-AS cells treated with Ac5Neu5Ac, vorinostat, or a combination of the two. We found that supplementation with Ac5Neu5Ac increased CMP–Neu5Ac levels around 20-fold in IMR-32 cells and around 3-fold in SK-N-AS cells. Vorinostat alone did not affect CMP–Neu5Ac abundance and had no additional effect on the Ac5Neu5Ac–induced increase (Fig. 6, A and B). These results show that Ac5Neu5Ac supplementation increases intracellular CMP–Neu5Ac levels, thereby enhancing substrate availability for sialyltransferases.
Figure 6.
Ac5Neu5Ac increases intracellular CMP–Neu5Ac levels, and vorinostat induces sialyltransferase expression. A and B, Ac5Neu5Ac increases intracellular CMP–Neu5Ac levels in IMR-32 cells (A) and SK-N-AS cells (B). Cells were cultured for 2 days with control or Ac5Neu5Ac (2 mm) and for another day with or without vorinostat (32 nm); intracellular CMP–Neu5Ac levels were determined by MS. Relative abundance ± S.E. relative to control is presented. C–E, vorinostat induces sialyltransferase expression in IMR-32 cells. Cells were treated with 0–250 nm vorinostat for 5 h, and expression of glycosyltransferase genes involved in GD2 biosynthesis was determined by quantitative PCR. Mean expression values ± S.E. relative to PBGD of ST3GAL5 (C), ST8SIA1 (D), and B4GALNT1 (E) are presented from three independent experiments. F, schematic model showing the proposed mechanism of enhanced GD2 expression induced by HDAC inhibitors and peracetylated sialic acid.
We reported earlier that vorinostat increases GD2 synthase protein levels without affecting mRNA expression. Others have reported that GD2 expression also depends strongly on GM3 and GD3 synthase expression (27, 39). Therefore, we investigated whether vorinostat induces changes in the expression of genes involved in GD2 biosynthesis. IMR-32 cells were treated with 0–250 nm vorinostat for 5 h, and expression of glycosyltransferase was determined by quantitative PCR. In line with our previous findings, vorinostat had no significant effect on B4GALNT1 expression but up-regulated ST3GAL5 and ST8SIA1 expression in a dose-dependent manner (Fig. 6, C–E). These results show that vorinostat mainly affects ST3GAL5 and ST8SIA1 expression, sialyltransferases that generate GM3 and GD3 gangliosides, the precursors for GD2 synthase. Notably, expression of GD1b synthase (B3GALT4), which converts GD2 to GD1b, was not detected in IMR-32 cells (data not shown). Altogether, these findings show that the combination of increased intracellular CMP–sialic acid levels and increased ST3GAL5 and ST8SIA1 expression induced by Ac5Neu5Ac and HDAC inhibition, respectively, results in increased expression of GD2 on neuroblastoma cells.
Discussion
In this study, we investigated the possibility of using sialic acid analogues alone or in combination with HDAC inhibitors to increase GD2 expression in human and mouse neuroblastoma cells. First, we found that the fluorinated sialic acid analogue Ac53FaxNeu5Ac potently inhibited GD2 expression in human neuroblastoma cells in the low micromolar range. Second, we demonstrated that increasing intracellular CMP–sialic acid levels enhanced GD2 expression on cells, whereas overall cell surface sialylation remained largely unaltered. Third, a combination treatment of Ac5Neu5Ac with different HDAC inhibitors showed an additive effect and strongly boosted GD2 expression. This effect was strongly associated with enhanced expression of the sialyltransferases ST3GAL5 and ST8SIA1 upon HDAC inhibitor treatment.
GD2 expression depends on the availability of GM3 and GD3, two sialic acid–carrying gangliosides that are produced by the sialyltransferases GM3 synthase and GD3 synthase, respectively (3, 31). Previous studies by other groups and our own group have shown that the fluorinated sialic acid analogue Ac53FaxNeu5Ac blocks sialic acid expression with high efficiency and selectivity (32–35). In the present study we found that this fluorinated sialic acid analogue is also a potent inhibitor of GD2 expression, consistent with previous data using sialidase (40). The fluorinated sialic acid analogue almost completely blocked GD2 expression in both human neuroblastoma cell lines at concentrations in the low micromolar range. This effect of Ac53FaxNeu5Ac is most likely attributable to inhibition of the two sialyltransferases ST3GAL5 and ST8SIA1 that generate GM3 and GD3, respectively, thereby depleting the substrate for GD2 synthase. Murine 9464D cells were less sensitive to inhibition of GD2 expression with Ac53FaxNeu5Ac, which may be caused by the differences in esterase expression required to remove the acetyl moieties. Neu5Ac carries a natural acetyl group on C-5, and four acetyl groups were added to C-1, C-7, C-8, and C-9 to increase cell permeability. These acetyl groups need to be removed, at least partially, by esterases before the supplemented sialic acid can act as a substrate or inhibitor. Interestingly, next to the natural C-5 acetyl modification, sialic acids can be 9-O–acetylated inside the cell, and this form of sialic acid is incorporated into GD2 (41, 42). The 14G2a antibody used recognizes both GD2 forms, and it has been suggested that O-acetyl–GD2 is expressed on neuroblastoma cells but not on peripheral nerves. Therefore, anti-O-acetyl–GD2 antibodies might show higher tumor specificity and less adverse effects in future (43). In addition, unlike human cells, mouse cells can produce N-glycolylneuraminic acid (Neu5Gc), a hydroxylated form of Neu5Ac (36, 44, 45). Possibly, this difference could account for the lower sensitivity of the murine cells to manipulation with Neu5Ac analogues. Overall the influence of the diverse sialic acid modifications in GD2 biology needs to be investigated further. Interestingly, the low micromolar concentrations used in the human cell lines caused only a mild reduction of total α2,3-/α2,6-sialylation. These findings indicate that the ganglioside synthesis pathway resulting in GD2 expression is more sensitive to metabolic sialic acid blockade compared with the synthesis pathway of other sialoglycans as detected using MALII and SNA-I lectin.
Although sialic acid blockade abrogates GD2 expression, the addition of Neu5Ac analogues could enhance GD2 expression in neuroblastoma cells by increasing intracellular CMP–sialic acid levels. Importantly, at the effective concentrations no change in overall sialylation was detected, indicating that the GD2 biosynthesis pathway in neuroblastoma cells is relatively more sensitive to changes in intracellular sialic acid levels compared with overall cell surface sialylation. This finding is particularly important, as sialic acids have been shown to promote tumor growth and suppress the immune system (46–49). The IMR-32 and SK-N-AS cells we used are naturally highly sialylated as evidenced by strong binding of MALII and SNA-I lectin and almost no binding of PNA lectin. Possibly, overall sialylation could not be enhanced by Ac5Neu5Ac treatment because of the high endogenous sialylation of the neuroblastoma cell lines and the absence of uncapped glycans, which could serve as a substrate for sialyltransferases. Only exposure of terminal β1–4–linked galactose residues showed a significant decrease upon treatment with high Ac5Neu5Ac concentrations as detected by the lectin ECL. The decrease in ECL binding could be explained by sialylation of lipids, like GD2, and/or glycoproteins. GD2 expression, however, could be readily enhanced by sialic acid supplementation suggesting that this pathway is not saturated in the tested neuroblastoma cell lines. Presumably, the increased bioavailability of CMP–Neu5Ac for sialyltransferases facilitates GM3 and GD3 production and thus raises substrate levels for GD2 synthase. Further research is needed to investigate the precise relationship between intracellular sialic acid levels and disialoganglioside synthesis.
Previously, we showed that the HDAC inhibitor vorinostat enhanced GD2 expression in neuroblastoma cells in vitro as well as in vivo. Treatment with vorinostat and anti-GD2 antibody synergistically reduced neuroblastoma growth in mice (27). We now showed here that the combination of Ac5Neu5Ac with three different HDAC inhibitors, vorinostat, entinostat, and panobinostat, had an additive effect on GD2 expression. No induction of GD2 by this combination treatment was observed in neuroblastoma cells, nonneuroblastoma tumor cell lines, or normal cells negative for GD2. These findings suggest that sialic acid supplementation and HDAC inhibition act only on cells with endogenous GD2 synthesis capacity. Furthermore, HDAC inhibition showed no significant effect on CMP–sialic acid levels or overall cell surface glycosylation suggesting that they increase GD2 expression by a different mechanism. Suzuki et al. (50) have provided evidence that the expression of GD3 synthase, and especially GD2 synthase, is regulated at the epigenetic level. We have observed previously that the treatment of neuroblastoma cells with vorinostat enhances GD2 synthase protein levels but not mRNA expression in 9464D cells (27). In line with these findings, vorinostat had no significant effect on GD2 synthase (B4GALNT1) mRNA expression in human neuroblastoma cells or on CMP–Neu5Ac levels, but it strongly up-regulated GM3 synthase (ST3GAL5) and GD3 synthase (ST8SIA1) gene expression. The additive effect of peracetylated sialic acid and HDAC inhibition on GD2 expression is therefore the overall result of (i) increased intracellular CMP–sialic acid levels, (ii) up-regulated sialyltransferase expression, and (iii) higher GD2 synthase protein expression levels (as shown schematically in Fig. 6F). Further research is needed to investigate how HDAC inhibition regulates GD2 synthase stability as well as sialyltransferase expression. Moreover, this combination treatment could also influence the expression levels of other glycosphingolipids that are synthesized by GD2 synthase and sialyltransferases or that are derived from GD2 (e.g. GD1b) (3). Mass spectrometry analysis would be useful in further detailing the effects of the combination treatment presented here on glycosylation, including glycosphingolipid expression.
Current immunotherapy efforts in neuroblastoma, like mAb and CAR-T cell therapy and carbohydrate vaccines, rely on the recognition of GD2 on the neuroblastoma cells. Increasing GD2 expression in neuroblastoma cells by using engineered sialic acid supplementation and HDAC inhibition may further increase the efficacy of current and future GD2-targeted immunotherapy in neuroblastoma patients.
Experimental procedures
Reagents and antibodies
C. perfringens sialidase, Neu5Ac, ManNAc, SYBR Green, and primers were purchased from Sigma-Aldrich. Ac5Neu5Ac and Ac53FaxNeu5Ac were synthesized as described previously and dissolved in PBS (51). Mouse anti-GD2 antibody (clone 14G2a) was purified from a hybridoma cell line (obtained from Dr. Reisfeld, Scripps, La Jolla, CA) (52). Purified mouse IgG2a isotype antibody, PE-conjugated goat anti-mouse Ig, and streptavidin–PE were purchased from BD Biosciences (BD PharmingenTM) and eFluor 780 viability dye from eBioscience, Inc. (San Diego, CA). The HDAC inhibitors vorinostat (suberoylanilide hydroxamic acid, MK0683), panobinostat (LBH589), and entinostat (MS-275) were purchased from Selleckchem (Houston, TX) and dissolved in DMSO. Carbo-Free blocking solution and biotin–MALII (Maackia amurensis lectin II), biotin–SNA-I (Sambucus nigra I agglutinin), biotin–PNA (peanut agglutinin), biotin–GSL-I (Griffonia simplicifolia I), biotin–LCA (Lens culinaris), biotin–PHA-L (Phaseolus vulgaris), and biotin–ECL (Erythrina crista-galli) were obtained from Vector Laboratories Inc. (Burlingame, CA).
Cell culture
Human IMR-32 (ATCC, CCL-127) neuroblastoma cells and the murine neuroblastoma cell lines 9464D (obtained from Dr. Orentas, National Institutes of Health, Bethesda, MD) and Neuro-2a (ATCC, CCL-131) were cultured in Dulbecco's modified Eagle's medium—GlutaMAX (Gibco/Invitrogen) with 10% FBS (Greiner Bio-One), 1% nonessential amino acids (Gibco), 50 μm 2-mercaptoethanol (Sigma-Aldrich), and 1% antibiotic–antimycotic solution (Gibco). Human SK-N-AS neuroblastoma (ATCC, CRL-2137) cells were cultured in Dulbecco's modified Eagle's medium—GlutaMAX (Gibco) with 10% FBS (Gibco), 1% nonessential amino acids (Gibco), 2 mm glutamine (Lonza, Walkersville, MD), and 1% antibiotic–antimycotic solution. B16-F10 (ATCC, CRL-6475) melanoma cells were cultured in minimum essential medium (MEM; Gibco) containing 5% FBS, 1% MEM nonessential amino acids, 0.15% sodium bicarbonate (Gibco), 1 mmol/liter sodium pyruvate (Gibco), 1.5% MEM vitamins (Gibco), and 1% antibiotic–antimycotic solution. 3T3 fibrosarcoma cells (ATCC, CRL-1658) were cultured in Dulbecco's modified Eagle's medium—GlutaMAX with 10% FBS, 1 mmol/liter sodium pyruvate, and 1% antibiotic–antimycotic solution. HeLa cells (ATCC, CCL-2) were cultured in Dulbecco's modified Eagle's medium—GlutaMAX with 10% FBS, 1% nonessential amino acids, 1 mmol/liter sodium pyruvate, and 1% antibiotic–antimycotic solution. Mouse mammary gland (NMuMG) epithelial cells (ATCC, CRL-1636) were cultured in Dulbecco's modified Eagle's medium—GlutaMAX with 10% FBS, 1 mmol/liter sodium pyruvate, 2 mm glutamine, 10 μg/ml insulin (Sigma-Aldrich), and 1% antibiotic-antimycotic solution. The murine fibroblast cell line B6MEC was cultured in Iscove's modified Dulbecco's medium (Gibco) with 5% FBS (Greiner Bio-One), 50 μm 2-mercaptoethanol (Sigma-Aldrich), and 1% antibiotic–antimycotic solution. Human primary dermal fibroblasts were kindly provided by the Department of Dermatology, Radboud University Medical Center, Nijmegen, the Netherlands. Primary dermal fibroblasts were isolated from skin tissue biopsies taken from patients undergoing elective surgery after written consent and were maintained in Dulbecco's modified Eagle's medium—GlutaMAX with 10% FBS, 1 mmol/liter sodium pyruvate, and 1% antibiotic–antimycotic solution. Cryopreserved cells were resuscitated and used within 3 month after thawing. All cells were incubated in a humidified CO2 incubator at 37 °C in 5% CO2 and tested regularly for mycoplasma contamination using a mycoplasma detection test (Lonza, Basel, Switzerland).
Sialic acid analogue and HDAC inhibitor treatment
To inhibit sialic acid expression, IMR-32, SK-N-AS, or 9464D cells were cultured for 3 days with increasing concentrations of Ac53FaxNeu5Ac. For enzymatic removal of sialic acid expression, the cells were incubated for 1 h with 200 milliunits/ml C. perfringens sialidase in serum-free medium at 37 °C. To assess the effect of Neu5Ac, Ac5Neu5Ac, and ManNAc on GD2 expression and total sialylation, the cells were cultured for 3 days in the presence of different concentrations of these sialic acid analogues. To study the effect of HDAC inhibitors on GD2 expression, cells were treated with different concentrations of vorinostat, entinostat, panobinostat, or DMSO control for 24 h. In addition, the cells were cultured for 48 h with 32 μm (IMR-32 or SK-N-AS), 5 mm (9464D) Ac53FaxNeu5Ac, or 2 mm Ac5Neu5Ac before the HDAC inhibitors were added to the culture medium for 24 h. For IMR-32 and SK-N-AS cells, 32 nm vorinostat, 32 nm entinostat, or 1 nm panobinostat were used, and for 9464D cells 256 nm vorinostat was used. B16-F10, 3T3, NMuMG, B6MEC, and HeLa cells and primary dermal fibroblasts were cultured for 48 h with 2 mm Ac5Neu5Ac and for another 24 h in combination with 32 or 256 nm vorinostat.
Antibody staining, lectin staining, and flow cytometry
To detect GD2 expression, IMR-32 and SK-N-AS cells treated with sialic acid analogues, sialidase, or HDAC inhibitors were stained for 20 min at 4 °C with mouse anti-GD2 antibody or isotype antibody in PBA (1× PBS, 1% BSA, and 0.02% sodium azide). The cells were washed with PBA and incubated for 20 min at 4 °C with PE-conjugated goat anti-mouse IgG antibody. After extensive washing, the cells were resuspended in PBA for flow cytometry analysis. Additionally, cell viability was determined by staining the cells with eFluor 780 viability dye following the manufacturer's instructions. Cell surface sialylation was assessed by staining the cells with the biotinylated lectins MALII (α2,3-linked sialic acids), SNA-I (α2,6-linked sialic acids), PNA (β-galactose), GSL-I (GalNAc and galactose), LCA (branched (N-)glycans and mannose), PHA-L (complex branched glycans), and ECL (uncapped terminal Galβ1–4GlcNAc residues). The cells were washed in 1× Carbo-Free blocking solution containing 1 mm CaCl and 1 mm MgCl and incubated for 45 min at 4 °C with biotinylated MALII (5 μg/ml), SNA-I (1 μg/ml), PNA (5 μg/ml), GSL-I (5 μg/ml), PHA-L (5 μg/ml) and LCA (5 μg/ml) or for 30 min at 4 °C with biotinylated ECL (0.5 μg/ml). The cells were washed with PBA and incubated for 20 min at 4 °C with streptavidin—PE and then washed and resuspended in PBA for analysis. Cell fluorescence was measured using a CyAn ADP flow cytometer (Beckman Coulter), and the data were analyzed using FlowJo software (Tree Star Inc., Ashland, OR). IC50 values for the inhibition of GD2 expression with Ac53FaxNeu5Ac were calculated using Prism 5 software (GraphPad, Inc., La Jolla, CA).
CMP–Neu5Ac quantification
IMR-32 and SK-N-AS cells were cultured on 6-well plates for 72 h with or without 2 mm Ac5Neu5Ac, and for the last 24 h of the culture 32 nm vorinostat or DMSO control was added. Cells were washed twice with ammonium carbonate buffer (pH 7.4) and snap-frozen in liquid nitrogen. Cell extracts were prepared by incubating the wells twice for 3 min with cold extraction buffer (acetonitrile:methanol:water, 2:2:1 (v/v/v)). The samples were centrifuged for 3 min at 13,000 rpm and vacuum-dried. The extracts were reconstituted in 100 μl of MilliQ and analyzed by reversed-phase ion pair chromatography (Agilent Technologies 1290 Infinity LC) coupled to a triple quadrupole mass spectrometer operating in negative ion mode (Agilent Technologies 6490 Triple Quad MS). CMP–sialic acid levels were expressed as the relative ratio of all nucleotide sugars.
RNA isolation and quantitative PCR
IMR-32 cells were treated for 5 h with 0, 32, 100, or 250 nm vorinostat, and total RNA was isolated from neuroblastoma cells using an RNA isolation kit (Zymo Research, Irvine, CA) following the manufacturer's instructions. RNA samples were in-column DNase I—treated, and concentrations were quantified by spectrophotometry. cDNA was synthesized using random hexamers and Moloney murine leukemia virus reverse transcriptase (Invitrogen). mRNA levels for the genes of interest were determined with a Bio-Rad CFX96 sequence detection system (Bio-Rad) with SYBR Green. The reaction mixtures and program conditions used were as recommended by the manufacturer; data were analyzed with the CFX Manager V1.6 (Bio-Rad) and checked for correct amplification and dissociation of the products. mRNA expression was determined relative to the housekeeping gene porphobilinogen deaminase (PBGD). Primer sequences as follows were derived from the Harvard University PrimerBank: ST3GAL5_FW_TCCCTGCAATGGTACACCC; ST3GAL5_RV_ACTTGGGACGACATTCCTTCT; ST8SIA1_FW_GTCCTCTGTTGGCTCTACATCT; ST8SIA1_RV_CCCCGTCATACCACATGCTC; B4GALNT1_FW_CAGAAACAAGTCCGAGCTATTGA; B4GALNT1_RV_GAGGGGCTGAACTTCCACAC.
Statistical analysis
Significance between two groups was calculated using a Student's t test, and comparisons between multiple groups were made using one-way analysis of variance followed by Bonferroni's correction using Prism 5 software (GraphPad Inc.). p values < 0.05 were considered significant.
Author contributions
R. J. E. v. d. B., M. K., T. J. B., M. H. d. B., C. B., and G. J. A. conceptualization; R. J. E. v. d. B., M. W., I. C. B., E. D. K.-R., and C. B. data curation; R. J. E. v. d. B., M. K., M. W., I. C. B., E. D. K.-R., M. v. S., D. J. L., and C. B. investigation; R. J. E. v. d. B., M. K., T. H., M. v. S., D. J. L., T. J. B., M. H. d. B., and C. B. methodology; R. J. E. v. d. B. and C. B. writing-original draft; M. K., L. B., T. H., T. J. B., M. H. d. B., P. M. H., and G. J. A. writing-review and editing; L. B., T. H., and T. J. B. resources; M. H. d. B., P. M. H., and G. J. A. project administration; P. M. H. and G. J. A. supervision.
Supplementary Material
This work was supported by Grant KUN2015-7604 (to G. J. A., T. J. B., and C. B.) from the KWF Kankerbestrijding (Dutch Cancer Society) and by continuous financial support from the Villa Joep Foundation (to P. M. H. and G. J. A.) and Stichting Vrienden KOC Nijmegen (to P. M. H. and G. J. A.). The authors declare no potential conflicts of interest.
This article contains Figs. S1–S4.
- ADCC
- antibody-dependent cell-mediated cytotoxicity
- CAR
- chimeric antigen receptor
- HDAC
- histone deacetylase
- PNA
- peanut agglutinin
- ECL
- Erythrina crista-galli lectin
- SNA-I
- Sambucus nigra I lectin
- MALII
- Maackia amurensis lectin II
- GSL-I
- Griffonia simplicifolia I
- PHA-L
- Phaseolus vulgaris L
- LCA
- Lens culinaris
- PE
- phycoerythrin
- FBS
- fetal bovine serum
- MEM
- minimum essential medium.
References
- 1. Maris J. M., Hogarty M. D., Bagatell R., and Cohn S. L. (2007) Neuroblastoma. Lancet 369, 2106–2120 10.1016/S0140-6736(07)60983-0 [DOI] [PubMed] [Google Scholar]
- 2. Matthay K. K., Reynolds C. P., Seeger R. C., Shimada H., Adkins E. S., Haas-Kogan D., Gerbing R. B., London W. B., and Villablanca J. G. (2009) Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: A children's oncology group study. J. Clin. Oncol. 27, 1007–1013 10.1200/JCO.2007.13.8925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schnaar R. L., and Kinoshita T. (2015) Glycosphingolipids, in Essentials of Glycobiology (Varki A., Cummings R. D., Esko J. D., Stanley P., Hart G. W., Aebi M., Darvill A. G., Kinoshita T., Packer N. H., Prestegard J. H., Schnaar R. L., and Seeberger P. H., eds) 3rd Ed., pp. 125–135, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY: [PubMed] [Google Scholar]
- 4. Svennerholm L., Boström K., Fredman P., Jungbjer B., Lekman A., Månsson J. E., and Rynmark B. M. (1994) Gangliosides and allied glycosphingolipids in human peripheral nerve and spinal cord. Biochim. Biophys. Acta 1214, 115–123 10.1016/0005-2760(94)90034-5 [DOI] [PubMed] [Google Scholar]
- 5. Mackall C. L., Merchant M. S., and Fry T. J. (2014) Immune-based therapies for childhood cancer. Nat. Rev. Clin. Oncol. 11, 693–703 10.1038/nrclinonc.2014.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cheung N. K., and Dyer M. A. (2013) Neuroblastoma: developmental biology, cancer genomics and immunotherapy. Nat. Rev. Cancer 13, 397–411 10.1038/nrc3526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheever M. A., Allison J. P., Ferris A. S., Finn O. J., Hastings B. M., Hecht T. T., Mellman I., Prindiville S. A., Viner J. L., Weiner L. M., and Matrisian L. M. (2009) The prioritization of cancer antigens: A National Cancer Institute pilot project for the acceleration of translational research. Clin. Cancer Res. 15, 5323–5337 10.1158/1078-0432.CCR-09-0737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheung N. K., Cheung I. Y., Kramer K., Modak S., Kuk D., Pandit-Taskar N., Chamberlain E., Ostrovnaya I., and Kushner B. H. (2014) Key role for myeloid cells: phase II results of anti-G(D2) antibody 3F8 plus granulocyte-macrophage colony-stimulating factor for chemoresistant osteomedullary neuroblastoma. Int. J. Cancer 135, 2199–2205 10.1002/ijc.28851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tarek N., Le Luduec J. B., Gallagher M. M., Zheng J., Venstrom J. M., Chamberlain E., Modak S., Heller G., Dupont B., Cheung N. K., and Hsu K. C. (2012) Unlicensed NK cells target neuroblastoma following anti-GD2 antibody treatment. J. Clin. Invest. 122, 3260–3270 10.1172/JCI62749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sorkin L. S., Otto M., Baldwin W. M. 3rd, Vail E., Gillies S. D., Handgretinger R., Barfield R. C., Ming Yu H., and Yu A. L. (2010) Anti-GD(2) with an FC point mutation reduces complement fixation and decreases antibody-induced allodynia. Pain 149, 135–142 10.1016/j.pain.2010.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fukuda M., Horibe K., and Furukawa K. (1998) Enhancement of in vitro and in vivo anti-tumor activity of anti-GD2 monoclonal antibody 220–51 against human neuroblastoma by granulocyte-macrophage colony-stimulating factor and granulocyte colony-stimulating factor. Int. J. Mol. Med. 2, 471–475 [DOI] [PubMed] [Google Scholar]
- 12. Kroesen M., Nierkens S., Ansems M., Wassink M., Orentas R. J., Boon L., den Brok M. H., Hoogerbrugge P. M., and Adema G. J. (2014) A transplantable TH-MYCN transgenic tumor model in C57Bl/6 mice for preclinical immunological studies in neuroblastoma. Int. J. Cancer 134, 1335–1345 10.1002/ijc.28463 [DOI] [PubMed] [Google Scholar]
- 13. Mora J. (2016) Dinutuximab for the treatment of pediatric patients with high-risk neuroblastoma. Expert. Rev. Clin. Pharmacol. 9, 647–653 10.1586/17512433.2016.1160775 [DOI] [PubMed] [Google Scholar]
- 14. Yu A. L., Gilman A. L., Ozkaynak M. F., London W. B., Kreissman S. G., Chen H. X., Smith M., Anderson B., Villablanca J. G., Matthay K. K., Shimada H., Grupp S. A., Seeger R., Reynolds C. P., Buxton A., et al. (2010) Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N. Engl. J. Med. 363, 1324–1334 10.1056/NEJMoa0911123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cheung N. K., Saarinen U. M., Neely J. E., Landmeier B., Donovan D., and Coccia P. F. (1985) Monoclonal antibodies to a glycolipid antigen on human neuroblastoma cells. Cancer Res. 45, 2642–2649 [PubMed] [Google Scholar]
- 16. Mujoo K., Cheresh D. A., Yang H. M., and Reisfeld R. A. (1987) Disialoganglioside GD2 on human neuroblastoma cells: Target antigen for monoclonal antibody-mediated cytolysis and suppression of tumor growth. Cancer Res. 47, 1098–1104 [PubMed] [Google Scholar]
- 17. Ahmed M., and Cheung N. K. (2014) Engineering anti-GD2 monoclonal antibodies for cancer immunotherapy. FEBS Lett. 588, 288–297 10.1016/j.febslet.2013.11.030 [DOI] [PubMed] [Google Scholar]
- 18. Matthay K. K., George R. E., and Yu A. L. (2012) Promising therapeutic targets in neuroblastoma. Clin. Cancer Res. 18, 2740–2753 10.1158/1078-0432.CCR-11-1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pule M. A., Savoldo B., Myers G. D., Rossig C., Russell H. V., Dotti G., Huls M. H., Liu E., Gee A. P., Mei Z., Yvon E., Weiss H. L., Liu H., Rooney C. M., Heslop H. E., and Brenner M. K. (2008) Virus-specific T cells engineered to coexpress tumor-specific receptors: Persistence and antitumor activity in individuals with neuroblastoma. Nat. Med. 14, 1264–1270 10.1038/nm.1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Louis C. U., Savoldo B., Dotti G., Pule M., Yvon E., Myers G. D., Rossig C., Russell H. V., Diouf O., Liu E., Liu H., Wu M. F., Gee A. P., Mei Z., Rooney C. M., et al. (2011) Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood 118, 6050–6056 10.1182/blood-2011-05-354449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gargett T., Yu W., Dotti G., Yvon E. S., Christo S. N., Hayball J. D., Lewis I. D., Brenner M. K., and Brown M. P. (2016) GD2-specific CAR T cells undergo potent activation and deletion following antigen encounter but can be protected from activation-induced cell death by PD-1 blockade. Mol. Ther. 24, 1135–1149 10.1038/mt.2016.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dobrenkov K., and Cheung N. K. (2014) GD2-targeted immunotherapy and radioimmunotherapy. Semin. Oncol. 41, 589–612 10.1053/j.seminoncol.2014.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fest S., Huebener N., Weixler S., Bleeke M., Zeng Y., Strandsby A., Volkmer-Engert R., Landgraf C., Gaedicke G., Riemer A. B., Michalsky E., Jaeger I. S., Preissner R., Förster-Wald E., Jensen-Jarolim E., and Lode H. N. (2006) Characterization of GD2 peptide mimotope DNA vaccines effective against spontaneous neuroblastoma metastases. Cancer Res. 66, 10567–10575 10.1158/0008-5472.CAN-06-1158 [DOI] [PubMed] [Google Scholar]
- 24. Kushner B. H., Cheung I. Y., Modak S., Kramer K., Ragupathi G., and Cheung N. K. (2014) Phase I trial of a bivalent gangliosides vaccine in combination with β-glucan for high-risk neuroblastoma in second or later remission. Clin. Cancer Res. 20, 1375–1382 10.1158/1078-0432.CCR-13-1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Louis C. U., and Shohet J. M. (2015) Neuroblastoma: Molecular pathogenesis and therapy. Annu. Rev. Med. 66, 49–63 10.1146/annurev-med-011514-023121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kroesen M., Lindau D., Hoogerbrugge P., and Adema G. J. (2012) Immunocombination therapy for high-risk neuroblastoma. Immunotherapy 4, 163–174 10.2217/imt.11.169 [DOI] [PubMed] [Google Scholar]
- 27. Kroesen M., Büll C., Gielen P. R., Brok I. C., Armandari I., Wassink M., Looman M. W., Boon L., den Brok M. H., Hoogerbrugge P. M., and Adema G. J. (2016) Anti-GD2 mAb and vorinostat synergize in the treatment of neuroblastoma. Oncoimmunology 5, e1164919 10.1080/2162402X.2016.1164919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Haberland M., Montgomery R. L., and Olson E. N. (2009) The many roles of histone deacetylases in development and physiology: Implications for disease and therapy. Nat. Rev. Genet. 10, 32–42 10.1038/nrg2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marks P., Rifkind R. A., Richon V. M., Breslow R., Miller T., and Kelly W. K. (2001) Histone deacetylases and cancer: Causes and therapies. Nat. Rev. Cancer 1, 194–202 10.1038/35106079 [DOI] [PubMed] [Google Scholar]
- 30. Glozak M. A., and Seto E. (2007) Histone deacetylases and cancer. Oncogene 26, 5420–5432 10.1038/sj.onc.1210610 [DOI] [PubMed] [Google Scholar]
- 31. Sphyris N., Sarkar T. R., Battula V. L., Andreeff M., and Mani S. A. (2015) GD2 and GD3 synthase: Novel drug targets for cancer therapy. Mol. Cell. Oncol. 2, e975068 10.4161/23723556.2014.975068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rillahan C. D., Antonopoulos A., Lefort C. T., Sonon R., Azadi P., Ley K., Dell A., Haslam S. M., and Paulson J. C. (2012) Global metabolic inhibitors of sialyl- and fucosyltransferases remodel the glycome. Nat. Chem. Biol. 8, 661–668 10.1038/nchembio.999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Büll C., Collado-Camps E., Kers-Rebel E. D., Heise T., Søndergaard J. N., den Brok M. H., Schulte B. M., Boltje T. J., and Adema G. J. (2016) Metabolic sialic acid blockade lowers the activation threshold of moDCs for TLR stimulation. Immunol. Cell Biol. 95, 408–415 [DOI] [PubMed] [Google Scholar]
- 34. Büll C., Heise T., Beurskens D. M., Riemersma M., Ashikov A., Rutjes F. P., van Kuppevelt T. H., Lefeber D. J., den Brok M. H., Adema G. J., and Boltje T. J. (2015) Sialic acid glycoengineering using an unnatural sialic acid for the detection of sialoglycan biosynthesis defects and on-cell synthesis of siglec ligands. ACS Chem. Biol. 10, 2353–2363 10.1021/acschembio.5b00501 [DOI] [PubMed] [Google Scholar]
- 35. Burkart M. D., Vincent S. P., and Wong C. H. (1999) An efficient synthesis of CMP-3-fluoroneuraminic acid. Chem. Commun. 16, 1525–1526 [Google Scholar]
- 36. Varki A., Schnaar R. L., and Schauer R. (2015) Sialic acids and other nonulosonic acids, in Essentials of Glycobiology (Varki A., Cummings R. D., Esko J. D., Stanley P., Hart G. W., Aebi M., Darvill A. G., Kinoshita T., Packer N. H., Prestegard J. H., Schnaar R. L., and Seeberger P. H., eds) 3rd Ed., pp. 179–195, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY: [PubMed] [Google Scholar]
- 37. Wang Z., Du J., Che P. L., Meledeo M. A., and Yarema K. J. (2009) Hexosamine analogs: From metabolic glycoengineering to drug discovery. Curr. Opin. Chem. Biol. 13, 565–572 10.1016/j.cbpa.2009.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cheng B., Xie R., Dong L., and Chen X. (2016) Metabolic remodeling of cell-surface sialic acids: Principles, applications, and recent advances. Chembiochem 17, 11–27 10.1002/cbic.201500344 [DOI] [PubMed] [Google Scholar]
- 39. Battula V. L., Shi Y., Evans K. W., Wang R. Y., Spaeth E. L., Jacamo R. O., Guerra R., Sahin A. A., Marini F. C., Hortobagyi G., Mani S. A., and Andreeff M. (2012) Ganglioside GD2 identifies breast cancer stem cells and promotes tumorigenesis. J. Clin. Invest. 122, 2066–2078 10.1172/JCI59735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yamaguchi H., Furukawa K., Fortunato S. R., Livingston P. O., Lloyd K. O., Oettgen H. F., and Old L. J. (1990) Human monoclonal antibody with dual GM2/GD2 specificity derived from an immunized melanoma patient. Proc. Natl. Acad. Sci. U.S.A. 87, 3333–3337 10.1073/pnas.87.9.3333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sjoberg E. R., Manzi A. E., Khoo K. H., Dell A., and Varki A. (1992) Structural and immunological characterization of O-acetylated GD2: Evidence that GD2 is an acceptor for ganglioside O-acetyltransferase in human-melanoma cells. J. Biol. Chem. 267, 16200–16211 [PubMed] [Google Scholar]
- 42. Sjoberg E. R., and Varki A. (1993) Kinetic and Spatial Interrelationships between ganglioside glycosyltransferases and O-acetyltransferase(s) in human-melanoma cells. J. Biol. Chem. 268, 10185–10196 [PubMed] [Google Scholar]
- 43. Alvarez-Rueda N., Desselle A., Cochonneau D., Chaumette T., Clemenceau B., Leprieur S., Bougras G., Supiot S., Mussini J. M., Barbet J., Saba J., Paris F., Aubry J., and Birklé S. (2011) A monoclonal antibody to O-acetyl-GD2 ganglioside and not to GD2 shows potent anti-tumor activity without peripheral nervous system cross-reactivity. PloS ONE 6, e25220 10.1371/journal.pone.0025220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brinkman-Van der Linden E. C., Sjoberg E. R., Juneja L. R., Crocker P. R., Varki N., and Varki A. (2000) Loss of N-glycolylneuraminic acid in human evolution: Implications for sialic acid recognition by siglecs. J. Biol. Chem. 275, 8633–8640 10.1074/jbc.275.12.8633 [DOI] [PubMed] [Google Scholar]
- 45. Davies L. R., and Varki A. (2015) Why Is N-glycolylneuraminic acid rare in the vertebrate brain? Top. Curr. Chem. 366, 31–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Büll C., den Brok M. H., and Adema G. J. (2014) Sweet escape: Sialic acids in tumor immune evasion. Biochim. Biophys. Acta 1846, 238–246 [DOI] [PubMed] [Google Scholar]
- 47. Büll C., Stoel M. A., den Brok M. H., and Adema G. J. (2014) Sialic acids sweeten a tumor's life. Cancer Res. 74, 3199–3204 10.1158/0008-5472.CAN-14-0728,10.1158/1538-7445.AM2014-3199 [DOI] [PubMed] [Google Scholar]
- 48. Pinho S. S., and Reis C. A. (2015) Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 15, 540–555 10.1038/nrc3982 [DOI] [PubMed] [Google Scholar]
- 49. Büll C., Boltje T. J., Balneger N., Weischer S. M., Wassink M., van Gemst J. J., Bloemendal V. R., Boon L., van der Vlag J., Heise T., den Brok M. H., and Adema G. J. (2018) Sialic acid blockade suppresses tumor growth by enhancing T cell-mediated tumor immunity. Cancer Res. 78, 3574–3588 10.1158/1538-7445.AM2018-3574 [DOI] [PubMed] [Google Scholar]
- 50. Suzuki Y., Yanagisawa M., Ariga T., and Yu R. K. (2011) Histone acetylation-mediated glycosyltransferase gene regulation in mouse brain during development. J. Neurochem. 116, 874–880 10.1111/j.1471-4159.2010.07042.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Büll C., Boltje T. J., Wassink M., de Graaf A. M., van Delft F. L., den Brok M. H., and Adema G. J. (2013) Targeting aberrant sialylation in cancer cells using a fluorinated sialic acid analog impairs adhesion, migration, and in vivo tumor growth. Mol. Cancer Ther. 12, 1935–1946 10.1158/1535-7163.MCT-13-0279 [DOI] [PubMed] [Google Scholar]
- 52. Mujoo K., Kipps T. J., Yang H. M., Cheresh D. A., Wargalla U., Sander D. J., and Reisfeld R. A. (1989) Functional properties and effect on growth suppression of human neuroblastoma tumors by isotype switch variants of monoclonal antiganglioside GD2 antibody 14.18. Cancer Res. 49, 2857–2861 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.