Abstract
Most neutralizing antibodies against highly pathogenic avian influenza A virus H5N1 recognize the receptor-binding site (RBS) on the globular head domain and the stem of H5N1 hemagglutinin (HA). Through comprehensive analysis of multiple human protective antibodies, we previously identified four vulnerable sites (VS1–VS4) on the globular head domain. Among them, the VS1, occupying the opposite side of the RBS on the same HA, was defined by the epitope of antibody 65C6. In this study, we report the crystal structures of two additional human H5N1 antibodies isolated from H5N1-infected individuals, 3C11 and AVFluIgG01, bound to the head at 2.33- and 2.30-Å resolution, respectively. These two new antibody epitopes have large overlap with and extend beyond the original VS1. Site-directed mutagenesis experiments identified eight pivotal residues (Ser-126b, Lys-165, Arg-166, Ser-167, Tyr-168, Asn-169, Thr-171, and Asn-172) critical for 65C6-, 3C11-, and AVFluIgG01-binding and neutralization activities. These residues formed a unique “Y”-shaped surface on H5N1 globular head and are highly conserved among H5N1 viruses. Our results further support the existence of a vulnerable site distinct from the RBS and the stem region of H5N1 HA, and future design of immunogens should take this particular site into consideration.
Keywords: influenza, influenza virus, monoclonal antibody, structural biology, X-ray crystallography, vaccine development, virology, mutagenesis, infectious disease, epitope, H5N1, HPAI, neutralizing antibody, vulnerable site
Introduction
The highly pathogenic avian influenza A virus H5N1, which is highly infectious and deadly for poultry, continues to disseminate across the globe and imposes a significant public health and economic burden on human society. The H5N1 virus was initially detected from geese in the Guangdong province of China in 1996. One year later, it emerged in humans in Hong Kong and re-emerged in Mainland China in 2003 (1–3). Close contact with infected fowls causes human H5N1 infections, and the patients demonstrate symptoms including fever, cough, headache, breathing problems, pneumonia, and even death. Until January 2019, 860 cases of H5N1 infection have been officially reported to the World Health Organization from 16 countries with the case-fatality rate among infected human estimated to be as high as 52% (https://www.who.int/influenza/human_animal_interface/2019_01_21_tableH5N1.pdf?ua=1, January 19, 2019).4 Moreover, only four amino acid substitutions in the hemagglutinin (HA)5 and one in the polymerase basic protein 2 appeared to be sufficient to generate airborne-transmitted variant in the ferret model (4). Also, another group reported that a reassortant virus with H5 HA obtaining four amino acid mutations from a 2009 pandemic H1N1 virus could be transmitted in the ferret model (5). The success of H5N1 virus in crossing species barrier from poultry to humans and its potential transmission between mammals imply the possibility of a H5N1 pandemic by rapid antigenic drift and shift through mutations and reassortment with other human epidemic strains.
Glycoprotein HA on the surface of influenza virus is responsible for recognizing the target cell through binding to the cellular receptor sialic acid and facilitating the entry of the viral genome into the target cell by mediating the fusion process of viral and host membranes. HA is encoded by the fourth segment of the viral genome and is produced as a homotrimeric precursor (HA0). In the viral life cycle, the cleavage of HA0 by protease from host cells into HA1 and HA2 subunits is critical for viral infection (6). The HA1 subunit binds to cellular receptor sialic acid, and the HA2 subunit mediates the subsequent membrane fusion process. The HA1 subunit is structurally characterized by the membrane-distal globular head domain containing the receptor-binding site (RBS), and the HA2 subunit is characterized by the membrane-proximal stem region (7).
HA is also a major target of neutralizing antibodies that prevent viral infection and slow disease progression (8, 9). Most highly potent neutralizing antibodies elicited by viral infection and vaccine immunization are against the globular head domain of HA1. Their epitope residues on HA1 are highly variable, and these antibodies are in general strain- or clade-specific (10, 11). In contrast, antibodies against the relatively conserved stem region in HA2 usually exhibit broad neutralizing breadth. Some of these stem-specific antibodies are highly potent, but they are rare compared with those against the head domain (12–15). Previous studies have defined five antigenic sites on the head domain for HAs from several subtypes, namely Sa, Sb, Ca1, Ca2, and Cb for H1 and sites A–E for H3 (16–18). We recently defined four vulnerable sites (VS1–VS4) on the head domain for H5 HA by investigating the epitopes of potent neutralizing antibodies 65C6, 100F4, and AVFluIgG03 (19, 20). These three antibodies, together with 3C11 and AVFluIgG01 studied here, were initially isolated from two patients (65C6, 100F4, and 3C11 from SZ06 patient and AVFluIgG03 and AVFluIgG01 from AH06 patient) who survived H5N1 infection in China (21–23).
Our previous study showed that during natural H5N1 infection, neutralizing antibodies recognizing the globular head domain played a dominant role in protective humoral immunity because the convalescent sera from patients contain high levels of neutralizing antibodies against the globular head domain rather than the stem region (19). It also indicated the existence of relatively conserved sites apart from the highly variable RBS. Identification of a highly conserved VS1 on the globular head is such an example. As shown previously, VS1 occupies the opposite side of the RBS, whereas VS2 corresponds to the RBS on the globular head domain (19). In this study, we further characterized the VS1 by structural and functional analysis of two additional human H5N1-neutralizing antibodies, 3C11 and AVFluIgG01, targeting the VS1. The epitopes of 3C11 and AVFluIgG01 significantly overlapped with the epitope of 65C6 and further extended beyond the original VS1. We further identified eight pivotal residues within the VS1 critical for antibody binding and neutralization. These residues constituted a unique Y-shaped surface within the VS1 and are highly conserved among 3758 full-length H5N1 HA sequences available in the current NCBI database. Taken together, these results further support the existence of an underappreciated vulnerable site distinct from the RBS and the stem region of H5N1 HA, and future efforts in designing immunogens should take this particular site into consideration.
Results
Crystal structures of 3C11 and AVFluIgG01 bound to the globular head of HA
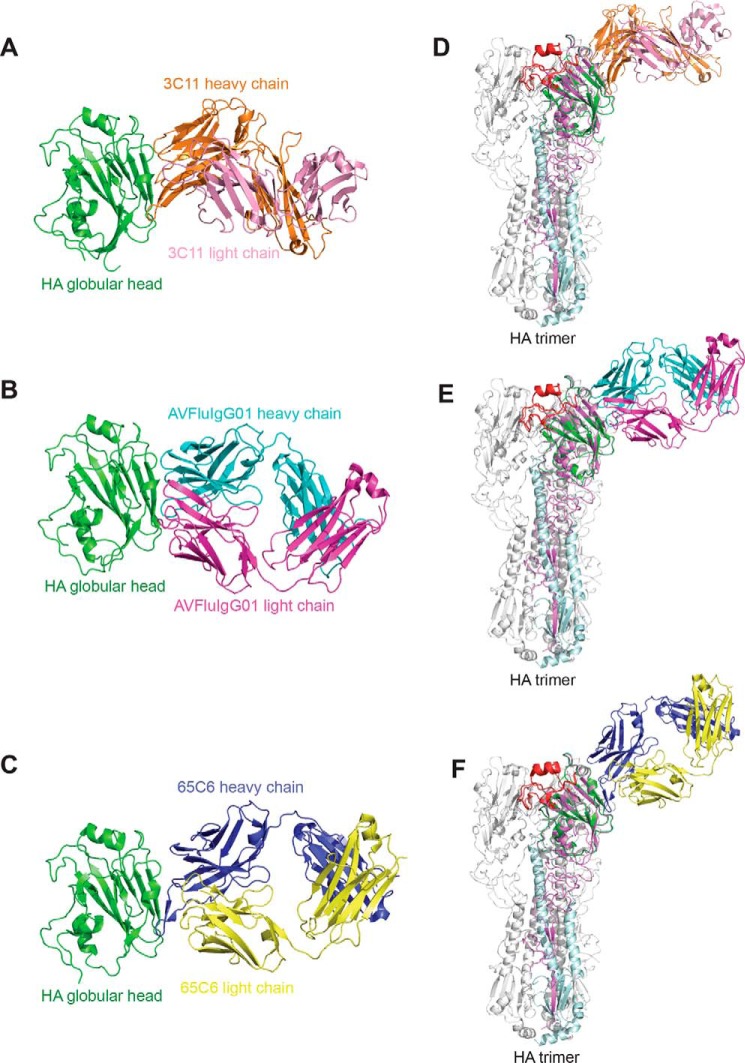
We reported previously that 3C11, 65C6, and AVFluIgG01 shared a similar neutralization profile against a panel of H5N1 pseudoviruses as mutations at a similar set of residues led to a similar trend of decrease in their neutralizing potencies (19). We also determined the crystal structure of one (65C6) of the antibodies complexed with the globular head of HA at a resolution of 3.0 Å and found that 65C6 recognized an epitope located on the back of the RBS (19). To precisely determine the epitope specificity of 3C11 and AVFluIgG01, we further obtained the complexes of the globular head of HA with the Fabs of 3C11 and AVFluIgG01 and determined their structures at resolutions of 2.33 and 2.30 Å, respectively (Table 1). Fig. 1, A and B, show the structures of the globular head (Asn-55–Glu-271) of the A/HongKong/156/1997 HA bound to the Fab of 3C11 and the globular head (Asp-55–Glu-271) of the A/Anhui/1/2005 HA bound to the Fab of AVFluIgG01, respectively. We superimposed these two complexes onto the HA trimer of A/Anhui/1/2005 virus together with the previously reported complex of the HA globular head bound to the 65C6 Fab (Fig. 1, C–F). 3C11, AVFluIgG01, and 65C6 were found to share similar epitope specificity (Fig. 1, D–F), confirming their similar neutralization profile against the panel of H5N1 pseudoviruses (19). Their epitopes are located on the back of the RBS on the same HA monomer, near the membrane-distal end of the HA spike (Fig. 1, D–F).
Table 1.
Data collection and refinement statistics
The values in parentheses represent the corresponding parameters of the highest resolution shell.
| 3C11 Fab–head | AVFluIgG01 Fab–head | |
|---|---|---|
| Data collection | ||
| Beamline | SSRF BL17U | SSRF BL17U |
| Wavelength (Å) | 0.9796 | 0.9796 |
| Space group | P21 | C2221 |
| Cell dimensions | ||
| a, b, c (Å) | 126.78, 62.24, 127.83 | 112.10, 150.05, 107.97 |
| α, β, γ (°) | 90, 117.22, 90 | 90, 90, 90 |
| Resolution (Å) | 50.0–2.33 (2.38–2.33) | 50.0–2.30 (2.35–2.30) |
| Rmergea | 0.086 (0.395) | 0.140 (0.957) |
| Rpimb | 0.034 (0.153) | 0.061 (0.382) |
| CC1/2c of the highest resolution shell | 0.957 | 0.838 |
| I/σI | 21.3 (6.1) | 13.8 (2.7) |
| Completeness (%) | 99.5 (99.1) | 99.0 (99.7) |
| Redundancy | 7.6 (7.6) | 6.7 (7.1) |
| Refinement | ||
| Resolution (Å) | 43.2–2.33 | 42.1–2.30 |
| No. reflections | 75,693 | 39,942 |
| Rwork/Rfreed (%) | 20.7/24.6 | 20.5/25.5 |
| No. atoms | ||
| Protein | 10,146 | 4,936 |
| Glycan | 76 | 52 |
| Water | 611 | 151 |
| Wilson B-factor (Å2) | 32 | 46 |
| Average B-factors (Å2) | 44 | 59 |
| Protein | 44 | 59 |
| Glycan | 65 | 65 |
| Water | 37 | 50 |
| Root mean square deviations | ||
| Bond lengths (Å) | 0.009 | 0.009 |
| Bond angles (°) | 1.1 | 1.1 |
| Ramachandran plot (%) | ||
| Favored | 96 | 92 |
| Allowed | 3.4 | 7.2 |
| Outlier | 0.39 | 0.94 |
aRmerge = ΣhklΣj|Ij(hkl) − 〈I(hkl)〉|/ΣhklΣjIj(hkl) where I is the intensity of reflection.
b Rpim = Σhkl[1/(N − 1)]1/2Σj|Ij(hkl) − 〈I(hkl)〉|/ΣhklΣjIj(hkl) where N is the redundancy of the data set.
c CC1/2 is the correlation coefficient of the half data sets.
d Rwork = Σhkl‖Fobs| − |Fcalc‖/Σhkl|Fobs| where Fobs and Fcalc are the observed and the calculated structure factors, respectively. Rfree is the cross-validation R factor for the test set of reflections (5% of the total) omitted in model refinement.
Figure 1.
Crystal structures of the HA globular head bound with 3C11, AVFluIgG01, and 65C6 Fabs. The HA globular head is colored in green. The heavy and light chains of 3C11 are colored in orange and pink (A), the heavy and light chains of AVFluIgG01 are colored in cyan and magenta (B), and the heavy and light chains of 65C6 are colored in blue and yellow (PDB code 5DUM) (C). D–F, the globular head and 3C11 Fab, AVFluIgG01 Fab, and 65C6 Fab complexes (PDB code 5DUM) are superimposed onto the A/Anhui/1/2005 HA trimer (PDB code 4KWM), respectively. HA1 is colored in violet, RBS is colored in red, and HA2 is colored in pale cyan on one of three monomers.
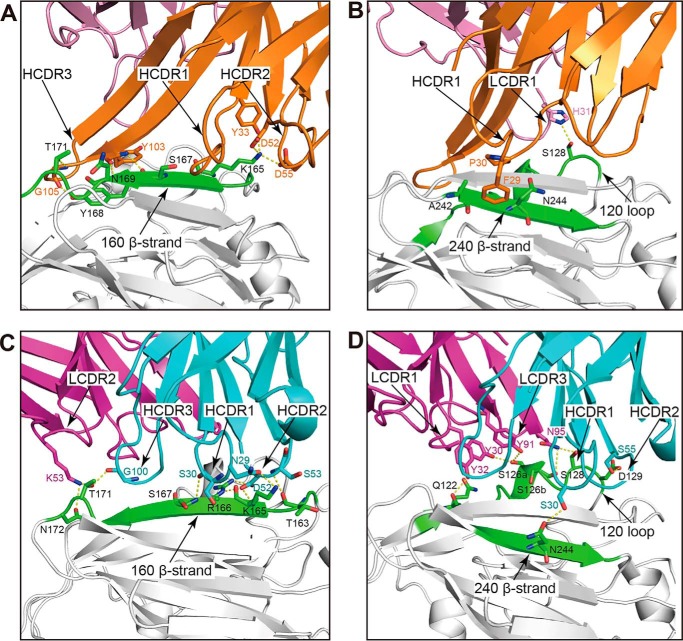
Like 65C6, the heavy chain of 3C11 dominates the binding to the globular head by contributing ∼85% of the buried surface on the antibody paratope. The 3C11 epitope included 19 residues from the 120 loop, 160 β-strand, 200 loop, and 240 β-strand of the globular head (Table S1). Also similar to 65C6, the long HCDR3 loop of 3C11 stretched parallel with the 120 loop (Ile-121–Asp-129) and the 160 β-strand (Pro-162–Asn-172) of the globular head (Fig. 2A). The hydrogen-bonding interactions involved Tyr-103 and Gly-105 of the HCDR3 and Ser-167, Tyr-168, Asn-169, and Thr-171 of the 160 β-strand (Fig. 2A). Additional hydrophilic interactions such as the salt bridge formed between Asp-52 and Asp-55 of the HCDR2 and Lys-165 of the 160 β-strand (Fig. 2A) and the hydrogen bonds formed between Tyr-33 of the HCDR1 and Lys-165 of the 160 β-strand were also identified (Fig. 2A). Besides, Phe-29 and Pro-30 of the HCDR1 interacted with Ala-242 and Asn-244 of the 240 β-strand mainly through van der Waals contacts (Fig. 2B). In contrast, the 3C11 light chain made a few contacts with the head domain through one hydrogen bond involving His-31 of the LCDR1 and Ser-128 of the 120 loop (Fig. 2B).
Figure 2.
Enlarged focused view of interactions between the globular head and 3C11 Fab and AVFluIgG01 Fab. In the four panels, the globular head is colored in gray, and the key structure elements (120 loop, 160 β-strand, and 240 β-strand) are colored in green. The heavy and light chains of 3C11 Fab are colored in orange and pink (A and B), and the heavy and light chains of AVFluIgG01 Fab are colored in cyan and magenta (C and D).
In contrast to 65C6 and 3C11, AVFluIgG01 utilized all six CDR loops for the binding to the 120 loop, 160 β-strand, and 240 β-strand of the head domain (Fig. 2, C and D). Such binding buried an 823-Å2 surface on the globular head domain, and 628- and 298-Å2 surfaces on the heavy and light chains of AVFluIgG01, respectively. The interface consisted of 19 residues from the head domain and 17 residues from AVFluIgG01 (Table S1). The HCDR1 and HCDR2 contacted the N-terminal part of the 160 β-strand (Fig. 2C). Specifically, residues Asn-29 and Ser-30 of the HCDR1 formed hydrogen bonds with Lys-165 and Ser-167 of the 160 β-strand, respectively (Fig. 2C). Residue Asp-52 of the HCDR2 interacted with Arg-166 through a salt bridge and with Lys-165 through a hydrogen bond (Fig. 2C). Furthermore, the HCDR3 and LCDR2 were close to the extending loop downstream of the 160 β-strand, enabling the HCDR3 Gly-100 and the LCDR2 Lys-53 to form hydrogen-bond interactions with Thr-171 and Asn-172, respectively (Fig. 2C). In addition, the HCDR1 made a few contacts with the 240 β-strand through one hydrogen bond involving Ser-30 of the HCDR1 and Asn-244 of the 240 β-strand (Fig. 2D). The HCDR2, LCDR1, and LCDR3 loops also made contacts with the 120 loop of the head domain (Fig. 2D). Residue Ser-55 of HCDR2 loop formed a hydrogen bond with Asp-129 (Fig. 2D), and residues Tyr-30 and Tyr-32 of LCDR1 loop formed hydrogen bonds with Ser-126a and Gln-122, respectively (Fig. 2D). Also, Tyr-91 and Asn-95 of the LCDR3 loop formed hydrogen bonds with Ser-126a, Ser-126b, and Ser-128, respectively (Fig. 2D).
Sequence and structural definition of a vulnerable site on the HA globular head recognized by 3C11, AVFluIgG01, and 65C6
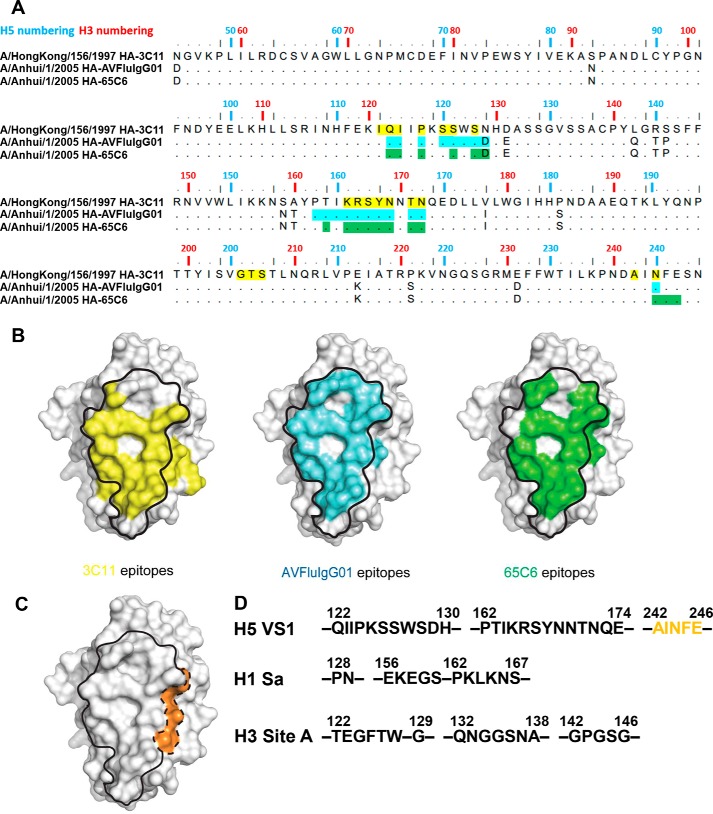
We have previously defined four vulnerable sites (VS1–VS4) on the H5N1 HA globular head based on crystal structures of four individual globular head and antibody complexes (65C6, 100F4, AVFluIgG03, and H5M9) as well as published epitope sequences of both human and mouse origins (19). Although the core of VS1 is represented by the 65C6 epitope, the external boundary of the VS1 was less clear. With the atomic structural information on the epitopes of 3C11 and AVFluIgG01, we could now be more precise in defining the sequence and spatial borderline of VS1 based on the three structures. Sequence analysis showed that the epitopes were largely located in two segments, one from Gln-122 to His-130 in the 120 loop and the other from Pro-162 to Glu-174 in the 160 β-strand (19) (Fig. 3A). In addition, whereas Asn-244 was a common residue recognized by all three antibodies, Phe-245 and Glu-246 were only recognized by 65C6, and Gly-205 to Ser-207 and Ala-242 were only recognized by 3C11 (Fig. 3A). Structurally speaking, the footprints of all three antibodies fit well with the previously defined VS1 (Fig. 3B). However, their structural superimposition further expanded VS1 beyond its original boundary with addition of the Ala-242–Glu-246 patch, forming an extended VS1 (Fig. 3C). In broad terms, the VS1 would correspond to Sa on the H1 and Site A on the H3, but clear differences existed among the three groups (Fig. 3D and Table S2) (16–19). For example, Glu-156–Ser-160 of H1 Sa, Gln-132–Ala-138 and Gly-142–Gly-146 of H3 Site A were not present in the VS1 of H5 (Fig. 3D). Conversely, the newly extended Ala-242–Glu-246 in the VS1 of H5 was not covered in the H1 Sa and H3 Site A (Fig. 3D).
Figure 3.
Epitopes (d ≤ 4 Å) recognized by 3C11, AVFluIgG01, and 65C6 define an optimized VS1 on H5N1 globular head. A, the sequence alignment of AH05 (A/Anhui/1/2005) and HK97 (A/HongKong/156/1997) HA globular heads. The epitopes (d ≤ 4 Å) recognized by 3C11, AVFluIgG01, and 65C6 are marked with yellow, cyan, and green. B, surface representation illustrates the 3C11 epitope (in yellow), AVFluIgG01 epitope (in cyan), and 65C6 epitope (in green) on the globular head, whereas the VS1 previously defined is circled by a solid line. C, surface representation illustrates previous VS1 circled by a solid line and the extended VS1 circled by a dashed line and colored in orange. D, the sequence comparison of the optimized H5 VS1 with H1 Sa site and H3 Site A. The residues (Ala-242–Glu-246) are colored in orange and correspond to the orange patch of C.
Validation of the VS1 by mutagenesis
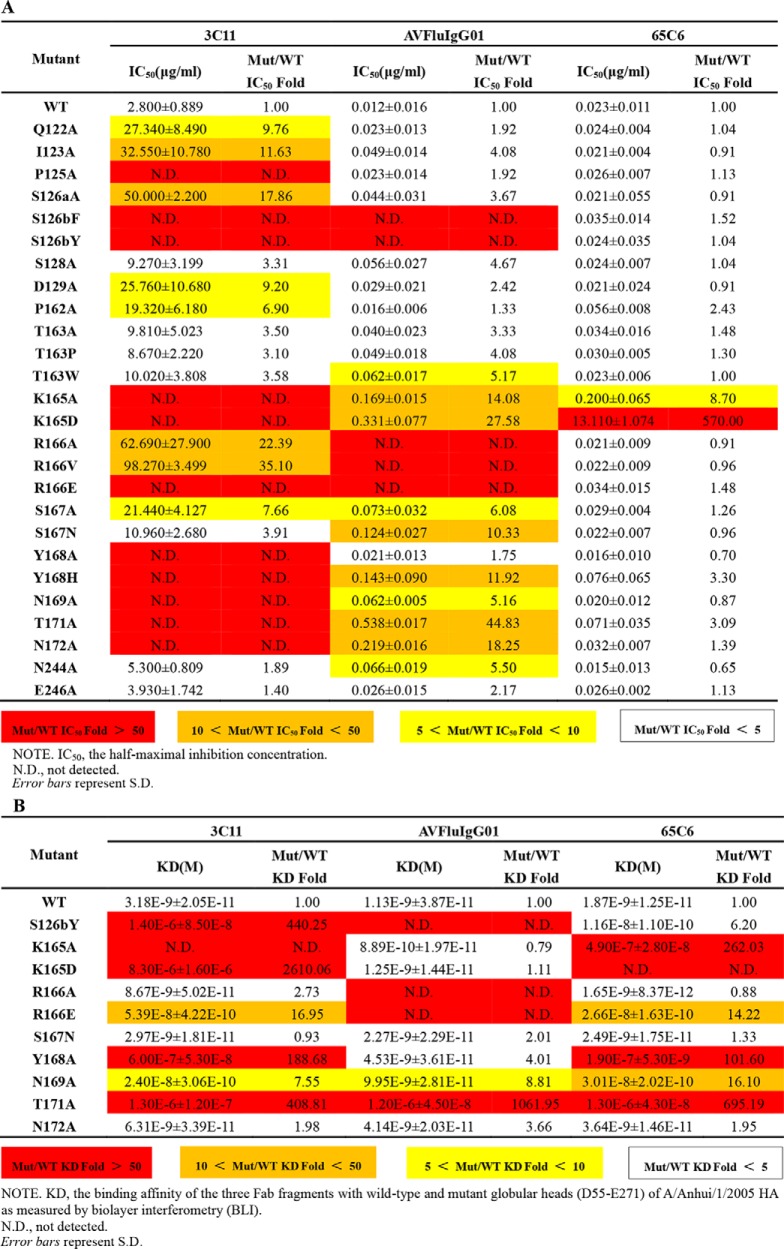
To study the impact of residues within the VS1 on antibody binding and neutralization, we generated a series of 26 single mutations on the interface of A/Anhui/1/2005 HA. Pseudoviruses bearing those mutations were then subjected to neutralization by 3C11, AVFluIgG01, and 65C6. As expected, different mutations resulted in different degrees of changes to antibody neutralization (Table 2A). For example, although the other 25 mutations had minimal effect on 65C6 neutralization, the K165D mutation increased its IC50 by 570-fold (Table 2A). In contrast, 11 (P125A, S126bF, S126bY, K165A, K165D, R166E, Y168A, Y168H, N169A, T171A, and N172A) of the 26 mutations severely compromised 3C11 neutralization, suggesting that 3C11 was much less tolerant of mutations than 65C6 (Table 2A). AVFluIgG01, however, was situated in between 65C6 and 3C11 in response to mutations. There were only five mutants (S126bF, S126bY, R166A, R166V, and R166E) capable of abolishing AVFluIgG01 neutralization, whereas the remaining 21 residues remained either minimally changed or moderately sensitive (Table 2A). Furthermore, such changes in antibody neutralizing activities were found to correlate with their reduced binding measured by biolayer interferometry assay (Table 2B and Fig. S1). Taken together, these results clearly validated the critical role of residues within the VS1 on antibody neutralization and supported the definition of the VS1 as one of the targets for neutralizing antibodies.
Table 2.
The comparison of neutralization potency (A) and binding affinity (B) of the three antibodies with wild-type and mutant A/Anhui/1/2005 H5N1 pseudoviruses and HA globular head
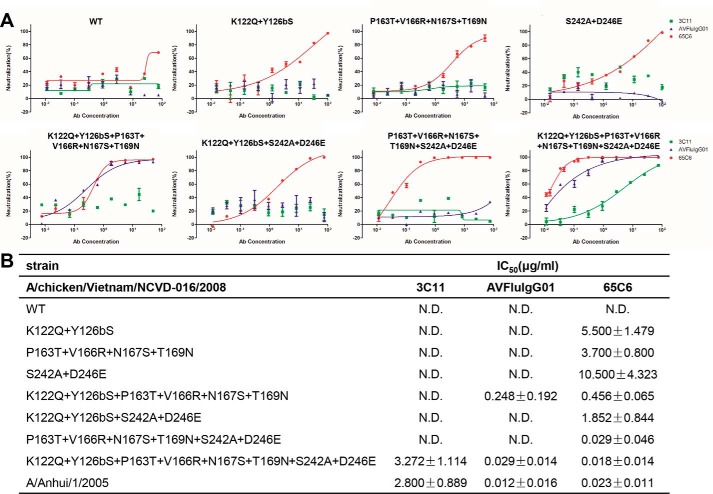
We further validated the critical role of residues within the VS1 by converting the resistant strains to sensitive strains. For instance, A/chicken/Vietnam/NCVD-03/2008 and A/chicken/Vietnam/NCVD-016/2008 in clade 7.1 were fully resistant to 3C11, AVFluIgG01, and 65C6, and eight residue differences were found within the VS1 from the other clades (Fig. S2) (19). These eight residues included Lys-122 and Tyr-126b in the 120 loop; Pro-163, Val-166, Asn-167, and Thr-169 in the 160 β-strand; and Ser-242 and Asp-246 in the 240 β-strand (Fig. S2). Mutating 2, 4, 6, or 8 of these residues to those of sensitive strain A/Anhui/1/2005 successfully transformed A/chicken/Vietnam/NCVD-016/2008 into a neutralization-sensitive strain, although different mutations or combination thereof resulted in different degrees of sensitivity. For example, although all of the mutant viruses became sensitive to 65C6, those with a larger number of mutations tended to be more sensitive (Fig. 4). The IC50 of 65C6 was about 5.50 μg/ml against the pseudovirus with two mutations (K122Q and Y126bS) in the 120 loop, whereas the IC50 decreased to 0.018 μg/ml against the pseudovirus with all eight mutations, equivalent to that toward the sensitive strain A/Anhui/1/2005 (Fig. 4). In contrast, only the virus with all eight mutations became sensitive to 3C11 and AVFluIgG01, whereas the virus with six mutations became sensitive to only AVFluIgG01 (Fig. 4). These results further confirmed the critical role of residues within the VS1 for the recognition of the three antibodies.
Figure 4.
Mutations within the VS1 convert A/chicken/Vietnam/NCVD-016/2008 (clade 7.1) from resistant to sensitive to the neutralization by 3C11, AVFluIgG01, and 65C6. A, neutralization graphs of 3C11, AVFluIgG01, and 65C6 on WT and multiple site mutants of A/chicken/Vietnam/NCVD-016/2008 (clade 7.1). B, summary of neutralization IC50 of 3C11, AVFluIgG01, and 65C6 on WT and multiple site mutants of A/chicken/Vietnam/NCVD-016/2008 (clade 7.1). N.D. means not detected. Error bars represent S.D.
Pivotal residues form a Y shape within the VS1
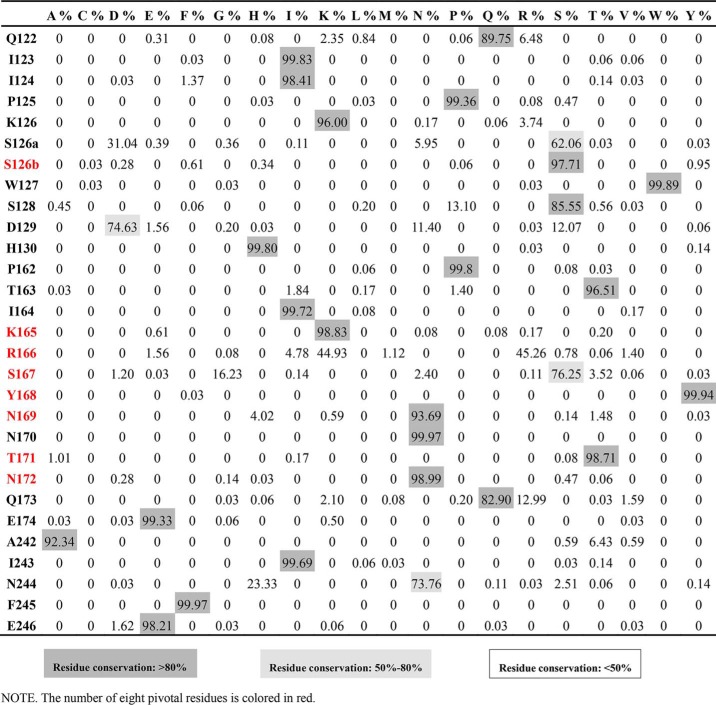
To further pinpoint the critical residues within the VS1 for antibody neutralization, we analyzed the mutations resulting in at least 5-fold reduction in IC50 for at least two of the three antibodies 3C11, AVFluIgG01, and 65C6 (Table 2A). A total of eight residues met this criterion, namely Ser-126b, Lys-165, Arg-166, Ser-167, Tyr-168, Asn-169, Thr-171, and Asn-172, and they formed a continuous Y-shaped region on the surface within the VS1 (Fig. 5). As shown in Table 2A, Lys-165 mutation dramatically reduced neutralization activity of all three antibodies, whereas the remaining seven mutations also had a profound but variable impact depending on the specific antibody. In addition, analysis of 3758 full-length H5N1 HA sequences available in the current NCBI database further demonstrated that the VS1 was well conserved in H5N1 subtype and revealed an exceptionally high degree of conservation of the eight residues (Table 3). Six of the eight residues (Ser-126b, Lys-165, Tyr-168, Asn-169, Thr-171, and Asn-172) demonstrated more than 93.7% identity, whereas Arg-166 and Lys-166 demonstrated up to 90.2% identity, and even the most divergent, Ser-167, showed up to 76.3% identity (Table 3). Variable neutralization potency and breadth observed for 3C11, AVFluIgG01, and 65C6 could therefore be partially explained by the degree of conservation in critical residues for three antibodies, respectively (Table 2A and Table 3). Taken together, in view of the vulnerability and conservation, the eight conserved residues within the VS1 formed a Y-shaped region and represent the key targets for neutralization of this class of antibodies (Fig. 5).
Figure 5.
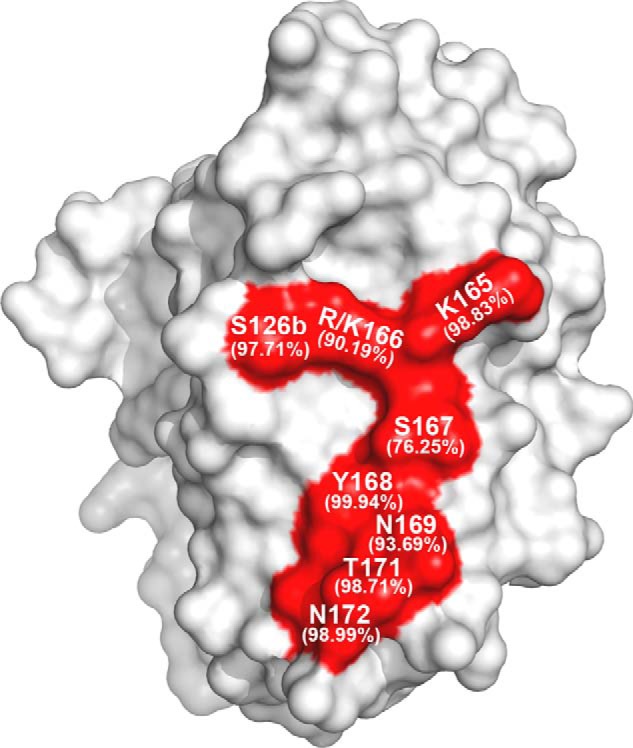
Eight pivotal residues form a Y-shaped region within the VS1 of H5N1 HA globular head. The surface view of the eight pivotal residues (red) on the HA globular head is shown with the residue conservation indicated in parentheses under the corresponding residue numbers.
Table 3.
The residue conservation of the VS1 within H5N1 subtype
Discussion
The zoonotic transmission of H5N1 virus to humans and potential human to human transmission remain a major public health threat as the case-fatality rate could be as high as 52% in humans. Understanding the epitopes and mechanisms of human neutralizing antibodies against H5N1 virus is an important step for the development of antibody therapies and structure-based vaccine design. Through comprehensive analysis of multiple human and mouse antibodies, we previously defined four vulnerable sites (VS1–VS4) on the globular head domain of H5N1 HA. In particular, the VS1 is located on the back of RBS and recognized by the representative antibody 65C6 isolated from H5N1-infected patients (19). In this study, we expanded the boundary of VS1 by determining the complex structures of the H5 globular head domain bound with two additional human neutralizing antibodies, 3C11 and AVFluIgG01. This result was further validated by site-directed mutagenesis and pseudovirus-based neutralization assays. Furthermore, within the VS1, we identified eight pivotal residues forming a Y-shaped surface critical for the recognition of targeting antibodies. These eight positions (Ser-126b, Lys-165, Arg-166, Ser-167, Tyr-168, Asn-169, Thr-171, and Asn-172) are highly conserved among H5N1 HAs available in the NCBI database. We believe we have identified a major vulnerable site distinct from the RBS and the stem of the HA. Such finding should provide us with a better and deeper understanding of the protective antibody immune response in humans as well as for the development of antibody-based interventions against H5N1 infection.
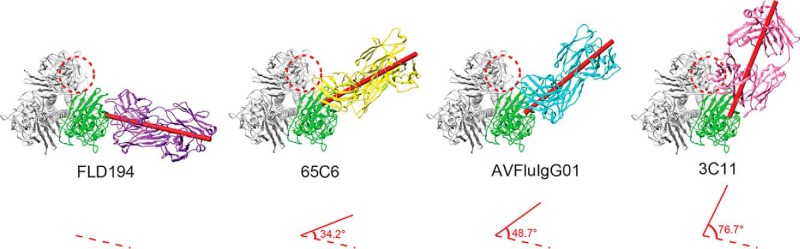
For this particular VS1, there are currently a total of four crystal structures resolved for four different antibody and antigen complexes, of which three (65C6, 3C11, and AVFluIgG01) are from our laboratories, whereas the remaining structure (FLD194) is from Xiong et al. (24) in the United Kingdom. It needs to be emphasized that despite their similarity in epitope footprint, their neutralizing activities vary considerably, perhaps due to their different angles and orientations approaching their epitopes and/or potential spatial interference with the neighboring RBS for receptor binding. For instance, among our three VS1-specific antibodies, 65C6 demonstrated the strongest and broadest activity, whereas AVFluIgG01 was the next strongest, and 3C11 was the weakest (19). FLD194 reported by Xiong et al. (24) was capable of neutralizing 13 tested pseudoviruses with an IC90 of 0.031 μg/ml. X-ray crystallography revealed that the epitope of FLD194 fell into our defined VS1 and recognized six of the eight pivotal residues identified in the current study (24) (Table 4). More importantly, head to head comparison among the four structure complexes revealed distinct differences among the four antibodies in their angles and orientations when approaching the HA trimer, resulting in varying levels of shielding of the RBS in the neighboring HA monomer (Fig. 6). For example, FLD194 bound to the contacting HA monomer in a nearly vertical direction and appeared not to block the RBS in the neighboring HA monomer (Fig. 6). Similarly, AVFluIgG01 and 65C6 approached HA like FLD194 and had limited interference with the RBS in the neighboring HA (Fig. 6). The angle and orientation of 3C11 approaching the HA, however, were significantly different from those of FLD194, AVFluIgG01, and 65C6 and resulted in extensive contact with the neighboring RBS (Fig. 6). However, the binding angle and orientation in potential interference with RBS could not fully explain the apparent differences in antibody neutralization. The most severe interference in RBS presumably came from 3C11, and yet its neutralizing activity was the weakest among the antibodies tested. In contrast, 65C6 demonstrated less interference in RBS but had the strongest and broadest neutralizing activity. These results indicated that antibodies targeting the VS1 must exert their neutralizing activity through other and different mechanisms in addition to receptor binding interference. Actually, we previously reported that antibody 65C6 exhibited weak inhibition of virus attachment to host cells and dramatically blocked low pH–triggered and HA-mediated fusion steps after attachment (25). Antibody AVFluIgG01 also inhibited both viral attachment and fusion steps after attachment (26). Antibody FLD194 neutralized viruses through block of receptor binding, shielding HAs from cellular receptor sialic acid by the Fc regions. EM micrographs revealed that FLD194 bound between two neighboring HAs, cross-linking them and forming a thick IgG layer, which restricted the attachment of the virus to cellular receptors (24). The neutralization mechanism of 3C11 has not been experimentally studied. Structural analysis revealed that a steric clash existed for receptor binding of 3C11 as the Fab inclined to the adjacent HA monomer, thereby shielding the RBS region and presumably blocking the binding of the sialic acid receptor. Therefore, we speculate that 3C11 may block receptor binding. Whether it is able to inhibit the postattachment fusion steps like 65C6 and AVFluIgG01 needs to be clarified in the future.
Table 4.
Summary of reported neutralizing antibodies of H5N1, H1N1 and H3N2 that target the VS1 or the position corresponding to VS1
Figure 6.
Different angles approaching the HA trimer of four VS1-specific antibodies. The complex structures of antibodies FLD194 (PDB code 5A3I), 65C6 (PDB code 5DUM), AVFluIgG01, and 3C11 are superimposed onto the A/Anhui/1/2005 HA trimer (PDB Code 4KWM) from the top view. HA1 is colored in green on one of three monomers and is in the same orientation in all panels. The RBS of the neighboring monomer is circled by a red dashed line. The Fabs of FLD194, 65C6, AVFluIgG01, and 3C11 are colored in purple, yellow, cyan, and pink, respectively. The long axes of the four Fabs are shown as red cylinders. The approaching angles of the antibodies are defined by the angle between the long axes of 65C6, AVFluIgG01, and 3C11 (red solid line) and the long axis of FLD194 (red dashed line), respectively.
The VS1 defined by investigating human H5N1 antibodies could be further expanded to H1N1 and H3N2 influenza viruses (19, 24, 27–36) (Table 4). For example, a recent study by Raymond et al. (35) reported a human clonal lineage antibody, CL6649, that recognized a panel of H1 HAs from strains isolated from 1977 to 2009. The epitope determined by the footprint of antibody Ab6649 pointed to the “lateral patch” of the H1 HA, including three regions (Glu-122–Asn-129, Ser-165–Glu-173, and Gly-240–Thr-242) that substantially overlapped with our defined VS1 (35) (Table 4). Meanwhile, these regions were almost invariant in human H1 isolates between 1977 and 2012 (35). Crystal structure determination revealed that another H1N1 human antibody, 2D1, also recognized epitopes corresponding to VS1 of H5 HA (34). Furthermore, an H3N2 human antibody D1-8 exhibited excellent neutralization potencies and breadth and could neutralize all tested representative influenza H3N2 virus strains with an average IC50 of 0.18 μg/ml (36). The escape mutants selected indicated that D1-8 targeted the conserved sites on H3N2 HA that also corresponded to the VS1 of H5 HA (36) (Table 4). Taken together, our study independently defines a conserved VS1 on the H5 HA that also exists in the H1 and H3 subtypes. The broad accessibility of the VS1 among highly pathogenic and seasonal influenza viruses highlights its distinction from the classical RBS and stem targets in the HA, and therefore, it represents a novel and potential target for developing antibody-based therapies and rational design of vaccines against influenza A virus in the future.
Experimental procedures
Monoclonal antibodies
Antibodies 65C6 and 3C11 were isolated from the SZ06 patient infected by H5N1 viruses. The SZ06 patient recovered by receiving convalescent serum of the AH06 patient, who also survived the H5N1 virus infection (22). Antibody AVFluIgG01 was isolated from an antibody phage library constructed from the recovered AH06 patient (23).
Production and purification of monoclonal antibodies
Both the variable heavy and light chain genes of each antibody were obtained by PCR and separately inserted into the expression vectors with the constant regions of human IgG1 (37). The whole human IgG1 was expressed in 293T cells obtained from ATCC (American Type Culture Collection) by transient transfection. Antibodies expressed in cell supernatant were purified by affinity chromatography using protein A beads (Pierce, Thermo) and titered using a BCA (bicinchoninic acid) Protein Assay kit (Thermo Scientific).
Generation of WT pseudoviruses and their mutants
WT pseudoviruses from all the strains of H5N1 (clades 0–9) in this study were generated as reported previously (22, 25). Pseudoviruses bearing single mutations were constructed by site-directed mutagenesis on the basis of clade 2.3.4 H5N1 strain (A/Anhui/1/2005), whereas pseudoviruses with multiple mutations were constructed in the context of clade 7.1 H5N1 strain (A/chicken/Vietnam/NCVD-016/2008). Site-directed mutagenesis was performed according to the QuikChange II Site-Directed Mutagenesis kit manual (Stratagene, catalog number 200523). All the HA genes used in this study were codon-optimized to increase the production of pseudoviruses.
Pseudovirus-based neutralization assay
Pseudovirus-based neutralization assays were carried out as described previously (22, 25). Each antibody was serially 3-fold diluted and incubated with pseudovirus at 37 °C for 1 h. Then 5 × 103–104 Madin-Darby canine kidney cells were added to each well containing antibody and virus mixture. After 48 h, the relative luciferase activity was measured using the BrightGlo luciferase assay (Promega). Titration curves were generated, and inhibitory concentration (IC50) values were calculated using sigmoid dose response of nonlinear fit from Prism.
Production of WT and mutant globular head, Fab, and complexes
The H5 globular head domain (Asn-55–Glu-271) of the A/HongKong/156/1997 HA and the globular head domain (Asp-55–Glu-271) of the A/Anhui/1/2005 HA were expressed in insect Spodoptera frugiperda (Sf9) cells via the Bac-to-Bac baculovirus expression system (Invitrogen). The DNA fragments encoding the globular head domain were inserted into the expression vector pFastBac-Dual (Invitrogen) with an N-terminal gp67 signal peptide for secretion and a C-terminal His6 tag to facilitate purification. The recombinant bacmid extracted from DH10Bac competent cells was transfected into Sf9 cells using Cellfectin II reagent (Invitrogen). The baculoviruses were acquired in cell supernatant after 7 days. The high-titer viruses were harvested after amplification and used to infect Sf9 cells at a density of 2 × 106/ml. After 48–72 h in culture, the cell supernatant containing secreted globular head was concentrated and exchanged to HBS buffer (10 mm HEPES, pH 7.2, 150 mm NaCl). The globular head was purified by affinity chromatography using nickel beads and then gel-filtration chromatography using a Superdex 200 10/300 column (GE Healthcare). Mutant globular heads bearing S126bY, K165A, K165D, R166A, R166E, S167N, Y168A, N169A, T171A, and N172A mutations were also expressed and purified as mentioned above. The purified 3C11 and AVFluIgG01 IgGs were digested by papain (Sigma-Aldrich, P3125) at 37 °C for 12 h. After loading onto protein A beads, the Fab fragment flowed through, and the Fc fragment was captured by the protein A beads. Then the Fab fragment was purified by gel-filtration chromatography (Superdex 200 10/300 column, GE Healthcare). Finally, the purified globular head and Fab were mixed at a molar ratio of 1:1, incubated on ice for 1 h, and then purified by gel-filtration chromatography on a Superdex 200 10/300 column (GE Healthcare) again.
Crystallization and data collection
The globular head–3C11 Fab complex was concentrated to 21 mg/ml for crystallization. Crystals were grown at 18 °C by vapor diffusion in sitting drops, which consisted of a mixture of equal volumes of protein solution and reservoir solution. The reservoir solution contained 2.0 m (NH4)2SO4, 0.1 m phosphate-citrate, pH 4.2. The globular head–AVFluIgG01 Fab complex was concentrated to 20 mg/ml for crystallization. Crystals were grown at 18 °C by vapor diffusion in sitting drops, which consisted of a mixture of equal volumes of protein solution and reservoir solution. The reservoir solution contained 25% (w/v) PEG 1500. All crystals for data collection were cryoprotected in reservoir solution plus 20% (v/v) glycerol and then quickly frozen in liquid nitrogen. Diffraction data were collected on the BL17U beamline at the Shanghai Synchrotron Research Facility (SSRF) (38) and indexed, integrated, and scaled with the software HKL2000 (39). All data collection and processing statistics are listed in Table 1.
Structural determination and refinement
The structures of complexes were determined by the molecular replacement method with the program PHASER in the CCP4 suite (40). The searching models were HA globular head of A/Anhui/1/2005 strain (PDB code 5DUT) and the variable and constant domains of heavy and light chains available in the Protein Data Bank with the highest sequence identities. Structural refinement was performed by iterative refinement with the program PHENIX and model building with the program Coot (41, 42). Structural validation was performed with the program MolProbity (43). All structural figures were generated with PyMOL (44). All refinement statistics are listed in Table 1. The atomic coordinates and structure factors (codes 6IUV and 6IUT) have been deposited in the Protein Data Bank.
Binding kinetics measurement using biolayer interferometry
Binding kinetics of 3C11, AVFluIgG01, and 65C6 to the WT and mutant globular head was measured by biolayer interferometry assay using an Octet Red96 instrument (FortéBio, Inc.). Antibodies at 10–20 μg/ml in kinetics buffer (10 mm HEPES, pH 7.2, 150 mm NaCl, 0.01% Tween 20) were loaded on protein A sensors and then incubated with serially diluted globular head in the same kinetics buffer. All the procedures were carried out with agitation set to 1000 rpm at room temperature (25 °C). The assays included six steps: 1) baseline (60 s), 2) antibody loading on protein A sensors for 120–200 s to reach a threshold of 1 or 3 nm, 3) second baseline (120 s), 4) association of the serially diluted globular head for measuring kon (60–300 s), 5) dissociation of the serially diluted globular head for measuring koff (60–300 s), and 6) sensor regeneration and neutralization. The protein A sensors were regenerated in buffer (10 mm glycine, pH 1.7) for 5 s and then neutralized in kinetics buffer for 5 s. This step was repeated three times. Data processing and analysis were carried out using Octet analysis software, version 9.0. Experimental curves were fitted using a 1:1 binding model. All sensorgrams used for fitting kon and koff and steady-state analysis curves are reported in Fig. S1.
Author contributions
P. W., Y. Z., L. Z., and X. W. conceptualization; P. W., Y. Z., J. S., T. Z., S. Z., S. G., and X. S. data curation; P. W., Y. Z., J. S., S. Z., S. G., and X. W. software; P. W., Y. Z., S. G., L. Z., and X. W. formal analysis; P. W., Y. Z., X. S., M. L., P. Z., L. Z., and X. W. validation; P. W., Y. Z., J. S., T. Z., S. Z., L. Z., and X. W. investigation; P. W., Y. Z., S. Z., S. G., X. S., L. Z., and X. W. visualization; P. W., Y. Z., J. S., T. Z., and S. Z. methodology; P. W., Y. Z., L. Z., and X. W. writing-original draft; P. W., Y. Z., M. L., P. Z., L. Z., and X. W. writing-review and editing; X. S., L. Z., and X. W. supervision; X. S., L. Z., and X. W. project administration; M. L., P. Z., and L. Z. resources; L. Z. and X. W. funding acquisition.
Supplementary Material
Acknowledgments
We are grateful to the staff scientists at the BL17U beamline of the Shanghai Synchrotron Research Facility (SSRF) and the X-ray crystallography platform of the Tsinghua University Technology Center for Protein Research for assistance with data collection and processing. We also thank Dr. Jia Zhang from FortéBio, Inc. for advice on biolayer interferometry assays.
This work was supported by Ministry of Science and Technology of the People's Republic of China (MOST) Grants 2018ZX10731101-002, 2017ZX10201101, 2016YFD0500307, and 2014CB542500-03 (to L. Z. and X. W.) and National Natural Science Foundation of China (NSFC) Grants 31470751, U1405228, 81530065, and 81661128042 (to L. Z. and X. W.). The authors declare that they have no conflicts of interest with the contents of this article.
This article was selected as one of our Editors' Picks.
This article contains Figs. S1 and S2 and Tables S1 and S2.
The atomic coordinates and structure factors (codes 6IUT and 6IUV) have been deposited in the Protein Data Bank (http://wwpdb.org/).
Please note that the JBC is not responsible for the long-term archiving and maintenance of this site or any other third party-hosted site.
- HA
- hemagglutinin
- RBS
- receptor-binding site
- VS
- vulnerable site
- NCBI
- National Center for Biotechnology Information
- CDR
- complementarity-determining region
- HCDR
- heavy chain complementarity-determining region
- LCDR
- light chain complementarity-determining region
- SSRF
- Shanghai Synchrotron Research Facility
- PDB
- Protein Data Bank.
References
- 1. Xu X., Subbarao, Cox N. J., and Guo Y. (1999) Genetic characterization of the pathogenic influenza A/Goose/Guangdong/1/96 (H5N1) virus: similarity of its hemagglutinin gene to those of H5N1 viruses from the 1997 outbreaks in Hong Kong. Virology 261, 15–19 10.1006/viro.1999.9820 [DOI] [PubMed] [Google Scholar]
- 2. Claas E. C., Osterhaus A. D., van Beek R., De Jong J. C., Rimmelzwaan G. F., Senne D. A., Krauss S., Shortridge K. F., and Webster R. G. (1998) Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet 351, 472–477 10.1016/S0140-6736(97)11212-0 [DOI] [PubMed] [Google Scholar]
- 3. Cowling B. J., Jin L., Lau E. H., Liao Q., Wu P., Jiang H., Tsang T. K., Zheng J., Fang V. J., Chang Z., Ni M. Y., Zhang Q., Ip D. K., Yu J., Li Y., et al. (2013) Comparative epidemiology of human infections with avian influenza A H7N9 and H5N1 viruses in China: a population-based study of laboratory-confirmed cases. Lancet 382, 129–137 10.1016/S0140-6736(13)61171-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Herfst S., Schrauwen E. J., Linster M., Chutinimitkul S., de Wit E., Munster V. J., Sorrell E. M., Bestebroer T. M., Burke D. F., Smith D. J., Rimmelzwaan G. F., Osterhaus A. D., and Fouchier R. A. (2012) Airborne transmission of influenza A/H5N1 virus between ferrets. Science 336, 1534–1541 10.1126/science.1213362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Imai M., Watanabe T., Hatta M., Das S. C., Ozawa M., Shinya K., Zhong G., Hanson A., Katsura H., Watanabe S., Li C., Kawakami E., Yamada S., Kiso M., Suzuki Y., et al. (2012) Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature 486, 420–428 10.1038/nature10831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schrauwen E. J., Herfst S., Leijten L. M., van Run P., Bestebroer T. M., Linster M., Bodewes R., Kreijtz J. H., Rimmelzwaan G. F., Osterhaus A. D., Fouchier R. A., Kuiken T., and van Riel D. (2012) The multibasic cleavage site in H5N1 virus is critical for systemic spread along the olfactory and hematogenous routes in ferrets. J. Virol. 86, 3975–3984 10.1128/JVI.06828-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yamada S., Suzuki Y., Suzuki T., Le M. Q., Nidom C. A., Sakai-Tagawa Y., Muramoto Y., Ito M., Kiso M., Horimoto T., Shinya K., Sawada T., Kiso M., Usui T., Murata T., et al. (2006) Haemagglutinin mutations responsible for the binding of H5N1 influenza A viruses to human-type receptors. Nature 444, 378–382 10.1038/nature05264 [DOI] [PubMed] [Google Scholar]
- 8. Chiu C., Ellebedy A. H., Wrammert J., and Ahmed R. (2015) B cell responses to influenza infection and vaccination. Curr. Top. Microbiol. Immunol. 386, 381–398 10.1007/82_2014_425 [DOI] [PubMed] [Google Scholar]
- 9. Sano K., Ainai A., Suzuki T., and Hasegawa H. (2017) The road to a more effective influenza vaccine: up to date studies and future prospects. Vaccine 35, 5388–5395 10.1016/j.vaccine.2017.08.034 [DOI] [PubMed] [Google Scholar]
- 10. Wrammert J., Koutsonanos D., Li G. M., Edupuganti S., Sui J., Morrissey M., McCausland M., Skountzou I., Hornig M., Lipkin W. I., Mehta A., Razavi B., Del Rio C., Zheng N. Y., Lee J. H., et al. (2011) Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J. Exp. Med. 208, 181–193 10.1084/jem.20101352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li Y., Myers J. L., Bostick D. L., Sullivan C. B., Madara J., Linderman S. L., Liu Q., Carter D. M., Wrammert J., Esposito S., Principi N., Plotkin J. B., Ross T. M., Ahmed R., Wilson P. C., et al. (2013) Immune history shapes specificity of pandemic H1N1 influenza antibody responses. J. Exp. Med. 210, 1493–1500 10.1084/jem.20130212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Corti D., Voss J., Gamblin S. J., Codoni G., Macagno A., Jarrossay D., Vachieri S. G., Pinna D., Minola A., Vanzetta F., Silacci C., Fernandez-Rodriguez B. M., Agatic G., Bianchi S., Giacchetto-Sasselli I., et al. (2011) A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science 333, 850–856 10.1126/science.1205669 [DOI] [PubMed] [Google Scholar]
- 13. Wu Y., Cho M., Shore D., Song M., Choi J., Jiang T., Deng Y. Q., Bourgeois M., Almli L., Yang H., Chen L. M., Shi Y., Qi J., Li A., Yi K. S., et al. (2015) A potent broad-spectrum protective human monoclonal antibody crosslinking two haemagglutinin monomers of influenza A virus. Nat. Commun. 6, 7708 10.1038/ncomms8708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Joyce M. G., Wheatley A. K., Thomas P. V., Chuang G. Y., Soto C., Bailer R. T., Druz A., Georgiev I. S., Gillespie R. A., Kanekiyo M., Kong W. P., Leung K., Narpala S. N., Prabhakaran M. S., Yang E. S., et al. (2016) Vaccine-induced antibodies that neutralize group 1 and group 2 influenza A viruses. Cell 166, 609–623 10.1016/j.cell.2016.06.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kallewaard N. L., Corti D., Collins P. J., Neu U., McAuliffe J. M., Benjamin E., Wachter-Rosati L., Palmer-Hill F. J., Yuan A. Q., Walker P. A., Vorlaender M. K., Bianchi S., Guarino B., De Marco A., Vanzetta F., et al. (2016) Structure and function analysis of an antibody recognizing all influenza A subtypes. Cell 166, 596–608 10.1016/j.cell.2016.05.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Caton A. J., Brownlee G. G., Yewdell J. W., and Gerhard W. (1982) The antigenic structure of the influenza virus A/PR/8/34 hemagglutinin (H1 subtype). Cell 31, 417–427 10.1016/0092-8674(82)90135-0 [DOI] [PubMed] [Google Scholar]
- 17. Wiley D. C., Wilson I. A., and Skehel J. J. (1981) Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature 289, 373–378 10.1038/289373a0 [DOI] [PubMed] [Google Scholar]
- 18. Wilson I. A., Skehel J. J., and Wiley D. C. (1981) Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 Å resolution. Nature 289, 366–373 10.1038/289366a0 [DOI] [PubMed] [Google Scholar]
- 19. Zuo T., Sun J., Wang G., Jiang L., Zuo Y., Li D., Shi X., Liu X., Fan S., Ren H., Hu H., Sun L., Zhou B., Liang M., Zhou P., et al. (2015) Comprehensive analysis of antibody recognition in convalescent humans from highly pathogenic avian influenza H5N1 infection. Nat. Commun. 6, 8855 10.1038/ncomms9855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zuo Y., Wang P., Sun J., Guo S., Wang G., Zuo T., Fan S., Zhou P., Liang M., Shi X., Wang X., and Zhang L. (2018) Complementary recognition of the receptor-binding site of highly pathogenic H5N1 influenza viruses by two human neutralizing antibodies. J. Biol. Chem. 293, 16503–16517 10.1074/jbc.RA118.004604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou B., Zhong N., and Guan Y. (2007) Treatment with convalescent plasma for influenza A (H5N1) infection. N. Engl. J. Med. 357, 1450–1451 10.1056/NEJMc070359 [DOI] [PubMed] [Google Scholar]
- 22. Hu H., Voss J., Zhang G., Buchy P., Zuo T., Wang L., Wang F., Zhou F., Wang G., Tsai C., Calder L., Gamblin S. J., Zhang L., Deubel V., Zhou B., et al. (2012) A human antibody recognizing a conserved epitope of H5 hemagglutinin broadly neutralizes highly pathogenic avian influenza H5N1 viruses. J. Virol. 86, 2978–2989 10.1128/JVI.06665-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sun L., Lu X., Li C., Wang M., Liu Q., Li Z., Hu X., Li J., Liu F., Li Q., Belser J. A., Hancock K., Shu Y., Katz J. M., Liang M., et al. (2009) Generation, characterization and epitope mapping of two neutralizing and protective human recombinant antibodies against influenza A H5N1 viruses. PLoS One 4, e5476 10.1371/journal.pone.0005476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xiong X., Corti D., Liu J., Pinna D., Foglierini M., Calder L. J., Martin S. R., Lin Y. P., Walker P. A., Collins P. J., Monne I., Suguitan A. L. Jr., Santos C., Temperton N. J., Subbarao K., et al. (2015) Structures of complexes formed by H5 influenza hemagglutinin with a potent broadly neutralizing human monoclonal antibody. Proc. Natl. Acad. Sci. U.S.A. 112, 9430–9435 10.1073/pnas.1510816112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Qian M., Hu H., Zuo T., Wang G., Zhang L., and Zhou P. (2013) Unraveling of a neutralization mechanism by two human antibodies against conserved epitopes in the globular head of H5 hemagglutinin. J. Virol. 87, 3571–3577 10.1128/JVI.01292-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cao Z., Meng J., Li X., Wu R., Huang Y., and He Y. (2012) The epitope and neutralization mechanism of AVFluIgG01, a broad-reactive human monoclonal antibody against H5N1 influenza virus. PLoS One 7, e38126 10.1371/journal.pone.0038126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kaverin N. V., Rudneva I. A., Ilyushina N. A., Varich N. L., Lipatov A. S., Smirnov Y. A., Govorkova E. A., Gitelman A. K., Lvov D. K., and Webster R. G. (2002) Structure of antigenic sites on the haemagglutinin molecule of H5 avian influenza virus and phenotypic variation of escape mutants. J. Gen. Virol. 83, 2497–2505 10.1099/0022-1317-83-10-2497 [DOI] [PubMed] [Google Scholar]
- 28. Kaverin N. V., Rudneva I. A., Govorkova E. A., Timofeeva T. A., Shilov A. A., Kochergin-Nikitsky K. S., Krylov P. S., and Webster R. G. (2007) Epitope mapping of the hemagglutinin molecule of a highly pathogenic H5N1 influenza virus by using monoclonal antibodies. J. Virol. 81, 12911–12917 10.1128/JVI.01522-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rudneva I. A., Kushch A. A., Masalova O. V., Timofeeva T. A., Klimova R. R., Shilov A. A., Ignatieva A. V., Krylov P. S., and Kaverin N. V. (2010) Antigenic epitopes in the hemagglutinin of Qinghai-type influenza H5N1 virus. Viral Immunol. 23, 181–187 10.1089/vim.2009.0086 [DOI] [PubMed] [Google Scholar]
- 30. Han T., Sui J., Bennett A. S., Liddington R. C., Donis R. O., Zhu Q., and Marasco W. A. (2011) Fine epitope mapping of monoclonal antibodies against hemagglutinin of a highly pathogenic H5N1 influenza virus using yeast surface display. Biochem. Biophys. Res. Commun. 409, 253–259 10.1016/j.bbrc.2011.04.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rockman S., Camuglia S., Vandenberg K., Ong C., Baker M. A., Nation R. L., Li J., and Velkov T. (2013) Reverse engineering the antigenic architecture of the haemagglutinin from influenza H5N1 clade 1 and 2.2 viruses with fine epitope mapping using monoclonal antibodies. Mol. Immunol. 53, 435–442 10.1016/j.molimm.2012.10.001 [DOI] [PubMed] [Google Scholar]
- 32. Du L., Jin L., Zhao G., Sun S., Li J., Yu H., Li Y., Zheng B. J., Liddington R. C., Zhou Y., and Jiang S. (2013) Identification and structural characterization of a broadly neutralizing antibody targeting a novel conserved epitope on the influenza virus H5N1 hemagglutinin. J. Virol. 87, 2215–2225 10.1128/JVI.02344-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gronsang D., Bui A. N., Trinh D. Q., Bui V. N., Nguyen K. V., Can M. X., Omatsu T., Mizutani T., Nagai M., Katayama Y., Thampaisarn R., Ogawa H., and Imai K. (2017) Characterization of cross-clade monoclonal antibodies against H5N1 highly pathogenic avian influenza virus and their application to the antigenic analysis of diverse H5 subtype viruses. Arch. Virol. 162, 2257–2269 10.1007/s00705-017-3350-0 [DOI] [PubMed] [Google Scholar]
- 34. Xu R., Ekiert D. C., Krause J. C., Hai R., Crowe J. E. Jr., and Wilson I. A. (2010) Structural basis of preexisting immunity to the 2009 H1N1 pandemic influenza virus. Science 328, 357–360 10.1126/science.1186430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Raymond D. D., Bajic G., Ferdman J., Suphaphiphat P., Settembre E. C., Moody M. A., Schmidt A. G., and Harrison S. C. (2018) Conserved epitope on influenza-virus hemagglutinin head defined by a vaccine-induced antibody. Proc. Natl. Acad. Sci. U.S.A. 115, 168–173 10.1073/pnas.1715471115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Benjamin E., Wang W., McAuliffe J. M., Palmer-Hill F. J., Kallewaard N. L., Chen Z., Suzich J. A., Blair W. S., Jin H., and Zhu Q. (2014) A broadly neutralizing human monoclonal antibody directed against a novel conserved epitope on the influenza virus H3 hemagglutinin globular head. J. Virol. 88, 6743–6750 10.1128/JVI.03562-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tiller T., Meffre E., Yurasov S., Tsuiji M., Nussenzweig M. C., and Wardemann H. (2008) Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning. J. Immunol. Methods 329, 112–124 10.1016/j.jim.2007.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang Q.-S., Zhang K.-H., Cui Y., Wang Z.-J., Pan Q.-Y., Liu K., Sun B., Zhou H., Li M.-J., Xu Q., Xu C.-Y., Yu F., and He J.-H. (2018) Upgrade of macromolecular crystallography beamline BL17U1 at SSRF. Nucl. Sci. Tech. 29, 68 10.1007/s41365-018-0398-9 [DOI] [Google Scholar]
- 39. Otwinowski Z., and Minor W. (1997) Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 10.1016/S0076-6879(97)76066-X [DOI] [PubMed] [Google Scholar]
- 40. McCoy A. J., Grosse-Kunstleve R. W., Adams P. D., Winn M. D., Storoni L. C., and Read R. J. (2007) Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 10.1107/S0021889807021206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Adams P. D., Grosse-Kunstleve R. W., Hung L. W., Ioerger T. R., McCoy A. J., Moriarty N. W., Read R. J., Sacchettini J. C., Sauter N. K., and Terwilliger T. C. (2002) PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr. D Biol. Crystallogr. 58, 1948–1954 10.1107/S0907444902016657 [DOI] [PubMed] [Google Scholar]
- 42. Emsley P., and Cowtan K. (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 10.1107/S0907444904019158 [DOI] [PubMed] [Google Scholar]
- 43. Chen V. B., Arendall W, B. 3rd, Headd J. J., Keedy D. A., Immormino R. M., Kapral G. J., Murray L. W., Richardson J. S., and Richardson D. C. (2010) MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D Biol. Crystallogr. 66, 12–21 10.1107/S0907444909042073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. DeLano W. L. (2015) The PyMOL Molecular Graphics System, version 1.8, Schrödinger, LLC, New York [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.