Abstract
Background
Traumatic peripheral nerve injury (TPI) is a major medical problem without effective treatment options. There is no way to diagnose or treat an incomplete injury and delays contribute to morbidity. We examined 4-aminopyridine (4-AP), a potassium-channel blocker as a possible treatment for TPI.
Methods
We used standard mouse models of TPI with functional outcomes including sciatic-functional-index, sensory indices, and electrodiagnostics; in addition to standard immunohistochemical, and electron microscopic correlates of axon and myelin morphology.
Results
Sustained early 4-AP administration increased the speed and extent of behavioral recovery too rapidly to be explained by axonal regeneration. 4-AP also enhanced recovery of nerve conduction velocity, promoted remyelination, and increased axonal area post-injury. 4-AP treatment also enabled the rapid distinction between incomplete and complete nerve lesions.
Conclusion
4-AP singularly provides both a new potential therapy to promote durable recovery and remyelination in acute peripheral nerve injury and a means of identifying lesions in which this therapy would be most likely to be of value. The ability to distinguish injuries that may respond to extended therapy without intervention can offer benefit to wounded soldiers.
Keywords: Peripheral nerve injury, trauma, compression, gap
INTRODUCTION
Acute peripheral nerve injuries represent a major problem in the care of trauma patients. Most of these injuries result from either acute blunt trauma or acute compression.1 The reasons for the resulting loss of function in a traumatized extremity are complex and often include some mixture of axonal transection, myelin destruction, and compression neuropathy. Traumatic nerve injuries can result in partial or complete functional loss, which can be permanent or transient. In a traumatized limb with complete functional loss – there is currently no method to prognosticate functional return with clinicians adopting a posture of monitoring return of function to diagnose a partial injury. One example of this paradigm is the current treatment of distal humeral fractures with radial nerve injury, where it is recommended to adopt a watchful waiting approach to nerve recovery before undertaking surgical exploration.2 These recommendations largely come from the inability to diagnose a partial from a complete nerve injury based on function in the limb even with the aid of electrodiagnostic testing, which often only becomes clinically relevant weeks after the injury. The diagnosis of the extent of a nerve injury has made little progress in three decades.3
This difficulty in diagnosis notwithstanding, even greater hurdles exist in the treatment of nerve trauma. If some part of a nerve is known to be spared in an “incomplete” injury, then nothing can be done to speed the course of recovery of function lost. When added to the recent advances in the care of traumatized patients in the surgical and intensive care setting, peripheral neurotrauma can often be the hardest consequence of trauma to treat because of the unpredictability of returning function. Severe injuries offer little hope for functional recovery, especially in populations with comorbidities (elderly, multiply injured, etc.). Much prognostic value is given to the extent of recovery that occurs in the few weeks after an injury.4,5 During all waiting periods for spontaneous recovery, there is an added concern that the speed of recovery will be insufficient to outpace the natural muscle atrophy and changes at the neuromuscular synapse which may render nerve recovery moot if too slow.6
Nerve crush injuries are more common than nerves completely severed by trauma and can often be the result of indirect shockwaves through nerve tissue after gunshot wounds.1 Within the crushed nerve, incompletely disrupted fibers may still exist. However, the cellular organization of the fibers that includes myelin and Schwann cells can be disrupted to the point where function is lost even in the setting of an intact axon. This may be why loss of function as evaluated by the clinician can be variably reversible. No adjuvants exist to make distinctions regarding residual nerve continuity to be made by the clinician and no treatments exist to speed or alter such recovery, even when the nerve is known to be not severed.
In this way trauma to peripheral nerves including crush, acute compression and blunt soft-tissue damage present distinct diagnostic and treatment challenges.
We reasoned that there might be a distinct sub-population of neurons spared complete disruption in a standard model used in the study of peripheral nerve crush injury. If these neurons suffered injury to myelin but not the axons themselves, then drugs used to treat disorders of systemic demyelination may be clinically useful. We chose 4-aminopyridine (4-AP) for its longstanding use in multiple sclerosis patients, in whom it is believed to prolong action potentials and amplify neurotransmitter release which both contribute to systemic functional improvement in multiple sclerosis,7 spinal cord injury,8 myasthenia gravis,9 and Lambert-Eaton syndrome.10 We formed the hypothesis that 4-AP would help to diagnose the presence of incompletely injured fibers and that the actions of 4-AP would be evident with local administration at the site of a single calibrated peripheral nerve crush injury. Finally, we hypothesized that persistent treatment at the site of injury would lead to reliable improvements in the extent and speed of recovery which could be measured functionally and using electrodiagnostic means.
MATERIALS AND METHODS
Reagents and Antibodies
4-Aminopyridine, Rhodamine-B, Poly (D,L-lactide-co-glycolide) (50:50, acid terminated, average Mw 38,000–54,000) were purchased from Sigma Aldrich. Antibodies used were: anti-protein zero (P0) monoclonal antibodies were purchased from Aves Labs Inc.; anti-β-actin monoclonal antibodies were purchased from Santa Cruz Biotechnology; Hydrogel poly(ethylene glycol) dimethacrylate (PEGDM) kindly provided by Danielle S.W. Benoit (University of Rochester).
Systemic 4-AP Dosing
Dosing for 4-AP was administered to the intraperitoneal (ip) compartment in different experiments at doses ranging from 10 to 50 μg. This dose, based on a range at or below the mass-adjusted values for human use was administered ip based on the recommendation of the institutional animal use committee. Previous work, in our laboratory had also utilized subcutaneous administration with similar results (excluded from this manuscript). Methods for administration of 4-AP in PLGA vehicles locally at the site of injury and characterization of the in vitro release characteristics of 4-AP from those carriers for systemic administration are described in the results section below. The only systemic application route used in this manuscript was ip.
Mouse Model of Peripheral Nerve Injury
All animal experiments described in this report were reviewed and approved by the University Committee on Animal Resources at the University of Rochester Medical Center. Ten-week-old female (for the experiments presented here, note that these experiments were repeated in animals from both genders, per NIH guidelines for Rigor and Reproducibility) C57BL6 mice weighing 20–25 g were anesthetized with an intraperitoneal injection of ketamine (60 mg/kg) and xylazine (4 mg/kg). A lateral skin incision along the length of the femur was made and a direct lateral approach through the iliotibial band was performed to expose the sciatic nerve lying directly posterior to the femur. The sciatic nerve was bluntly exposed, and then the mice underwent one of the following randomly assigned procedures: (1) wound closure without manipulation of the nerve (sham-surgery group), (2) a sciatic nerve crush injury of 30 seconds duration (crush-injury group). Injury was created proximal to the nerve trifurcation using smooth forceps with a calibration jig.11 Buprenorphine (0.05 mg/kg) was used for postoperative analgesia on the day of surgery and was given every 12 hours until the mice walked without pain.
About 5 mg (4-AP)- poly(lactic-co-glycolic acid) (PLGA) films (containing 300 μg 4-AP) or 10 mg (4-AP)-PLGA particles (containing ~10 μg 4-AP) in 20 μL polyethylene glycol (PEG) hydrogel were placed onto crushed sciatic nerve immediately after the surgery. The dosages translated to current human usage for 4-AP. Films were shredded evenly to around 1 mm × 3 mm size and placed at the nerve crush site of sciatic nerve followed by wound closure procedure. Five milligrams of (4-AP)-PLGA particles were suspended in 20 μL PEG hydrogel then placed and photopolymerized in a plastic tube mold (0.02 inches in diameter) to form a “(4-AP)-PLGA particles/PEG hydrogel noodle” (see details of photopolymerization in PEG hydrogel section). The noodle was placed at the nerve crush site of the injured nerve immediately after the surgery.
Fabrication of (4-AP)-PLGA Carriers
For water/oil/water double emulsion: 6 mg of 4-AP was dissolved in 0.3 mL of deionized water and the drug solution was added to 3 mL of 5% w/v PLGA in dichloromethane (DCM) and emulsified with a high-speed homogenizer (Polytron) at 21,000 rpm for 90 seconds to yield a w/o emulsion. Next, the water/oil primary emulsion was added to 10 mL PVA aqueous solution (2.5%) and further emulsified at 13,500 rpm for 90 sec. The organic solvent evaporated (ambient temperature) by stirring at 1,000 rpm for 18 hours and microparticles were collected by centrifugation at 8,000 rpm for 10 minutes and washed twice with deionized water.12 The morphology of (4-AP)-PLGA films assessed by scanning electron microscopy (FIB- SEM, Zeiss Auriga) with samples prepared by substrate air-drying and gold sputter-coating (Denton Vacuum Desk II).
For Solvent Casting
About 50 mg of 50:50 PLGA polymer and 3 mg of 4-AP were dissolved in 1 mL of dichloromethane, with solvent casting on either glass coverslips or syringes. Samples were placed within a chemical hood with constant air flow at ambient temperature until the dichloromethane was completely evaporated. Thus, the amount of 4-AP enclosed in PLGA bricks had a theoretical encapsulating capacity equal to the maximum solubility of 4-AP in dichloromethane. The morphology of (4-AP)-PLGA films was assessed identically as with particles as noted above.
Rhodamine-labeled PLGA particles were prepared identically to 4-AP particles above using water/oil/water double emulsion. About 6 mg of 4-AP was dissolved in 0.3 mL of deionized water and the drug solution was added to 3 mL of 5% w/v PLGA and 10 mg of rhodamineB in DCM and emulsified with a high-speed homogenizer (Polytron) at 21,000 rpm for 90 seconds to yield a w/o emulsion. Next, the primary emulsion was added to 10 mL PVA aqueous solution (2.5%) and further emulsified at 13,500 rpm for 90 seconds. Organic solvent evaporation and microparticle preparation were identical to that described above.
Polyethylene glycol hydrogel polymerization was performed using 5 mg of (4-AP)-PLGA particles suspended with 20 μL of PEG hydrogels consisting of 10 wt.% PEGDM with 0.05 wt.% lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) as a photoinitiator and were formed in a plastic tube mold (0.02 inches in diameter) via photopolymerization using long-wavelength 365 nm light (∼5 mW cm2 intensity) for 10 minutes.13
The assessment of motor function recovery was performed by calculating the sciatic function index (SFI). Walking track analysis was performed according to a published model that quantifies sciatic nerve function performance.14 Assessment of gait in this model is widely used by our laboratory and others.15 Briefly, individual mouse footprints were obtained by painting each foot prior to mice walking a 50 cm path down a narrow corridor lined with paper. Gait was measured from the metrics of resulting footprints: (1) toe spread (TS) (first through fifth toes), (2) print length (PL), and (3) intermediate toe spread (ITS) (second, third, and fourth toes) of both limbs. All three measurements from three clearly inked randomly chosen footprints per trial were taken from the normal (N) and experimental (E) sides, and the SFI was calculated using the following formula:
where E is the injured limb and N is the control limb as in previous studies.16 All analysis was performed by trained and blinded reviewers.
Electrophysiology
Nerve conduction studies were performed by electrical stimulation of a nerve and recording the compound muscle action potential (CMAP) from needle electrodes overlying a muscle supplied by that nerve. Electromyography was performed by means of subdermal stainless steel needle electrode placed into the hind limbs (6 V, 0.1 ms, 1 Hz, 5–15 mA). All experiments, in this and other sections, were repeated at least three times, with a minimum of five (and usually eight) mice per treatment group. Stimulating electrode was placed in resting muscle on gluteal fold to obtain the first CMAP. Then the stimulating electrode was moved to popliteal fossa with a 10 mm fixed distance from gluteal fold to get the second record of CMAP. The nerve conduction velocity (NCV) was determined from the latencies of the potentials and the distance between two stimulating positions (10 mm). The muscle was stimulated by a monopolar needle electrode, with a fixed frequency of 1 Hz. Recording electrode was inserted in the tibialis anterior muscle approximately 3 mm above the heel. The reference recording electrode was inserted into the plantar aspect of the foot, and the reference stimulating electrode was inserted into the ipsilateral lumbar paraspinal muscles.11,17
Transmission Electron Microscopy
The sciatic nerves were immersion fixed overnight at 4°C in 2.5% glutaraldehyde and 4.0% paraformaldehyde in 0.1 M sodium cacodylate buffer. The nerves were rinsed in 0.1 M sodium cacodylate buffer and post-fixed for 1 hour in 1.0% osmium tetroxide combined with 1.0% potassium ferrocyanide. After rinsing in distilled water, the sections were dehydrated in a graded series of ethanol to 100% three times, transitioned into propylene oxide followed by Epon/Araldite epoxy resin overnight and finally embedded and polymerized at 60°C for 48 hours. Using an ultramicrotome and a diamond knife, thin-sections (70 nm) were collected onto 150 mesh nickel grids and stained with uranyl acetate and lead citrate. The stained grids were examined using a Hitachi 7650 TEM and photographed using an attached Gatan Erlangshen 11 megapixel digital camera system.
Myelin thickness was assessed by two blinded reviewers each counting at least five unique axons for each animal (four animals for each experimental groups; one for control group); six thicknesses of myelin sheath were measured on each axon. Axonal area/Myelin area/garea-ratio was measured using 40 axons in each animal. All selections were made and analyzed randomly by blinded reviewers.
Immunofluorescence
The experimental and contralateral (uninjured) sciatic nerves from each test group were harvested at specific time points during healing and recovery. The nerves were completely harvested by blunt dissection from the dorsal root ganglion to a point distal to the peroneal-tibial nerve divisions. All nerves were fixed in 4% paraformaldehyde solution for 3 hours and embedded in paraffin to evaluate cross-sections. Slides were pretreated with 0.01 M citrate buffer (pH 6.0) for antigen retrieval. Nonspecific blocking was performed with 1:20 diluted serum for 30 min. Sequentially sectioned slides were incubated with a primary antibody overnight, followed by incubation with a fluorescent-labeled secondary antibody for 1 hour.
Immunoblotting
The crushed site of the sciatic nerve at 21 days post-injury was collected and lysed in cell extraction buffer (Invitrogen). The 2 mm length of crushed sciatic nerve was placed in a 1.5 mL microtube with 100 μL cell extraction buffer. The tissue was frozen in liquid nitrogen and thawed repetitively for three times. After that, the tissue was grinded by pestle till no chunks could be visualized. Samples were resolved on sodium dodecyl sulfate polyacrylamide gel electrophoresis gels and transferred to polyvinylidene difluoride membranes (PerkinElmer Life Science, Wellesley, MA, USA). After being blocked in 5% bovine serum albumin in phosphate-buffered saline (PBS) containing 0.1% Tween 20, membranes were incubated with a primary antibody, followed by incubation with a horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology). Membranes were visualized using Western Blotting Luminol Reagent (Santa Cruz Biotechnology) and imaging system.
Image Analysis
Images of cross-sectioned sciatic nerve taken by TEM were processed by ImageJ (US National Institutes of Health, Bethesda, Maryland, USA) to determine myelin area, myelin thickness, and gArea-ratio on myelinated axonal fibers. Axonal area, axonal circularity, and the number of myelinated and total axon were also counted. Immunofluorescent images of P0 expression in cross-sectioned sciatic nerve were analyzed by ImageJ to determine the average fluorescence intensity of axons-associated P0 labeling.
Statistics
Normally distributed data were tested in using student’s t-tests where appropriate (two tailed, unpaired). None of our data was found to be non-normally distributed and no categorical data are here presented. Our statistical analysis was reviewed by a qualified departmental bio-statistician.
RESULTS
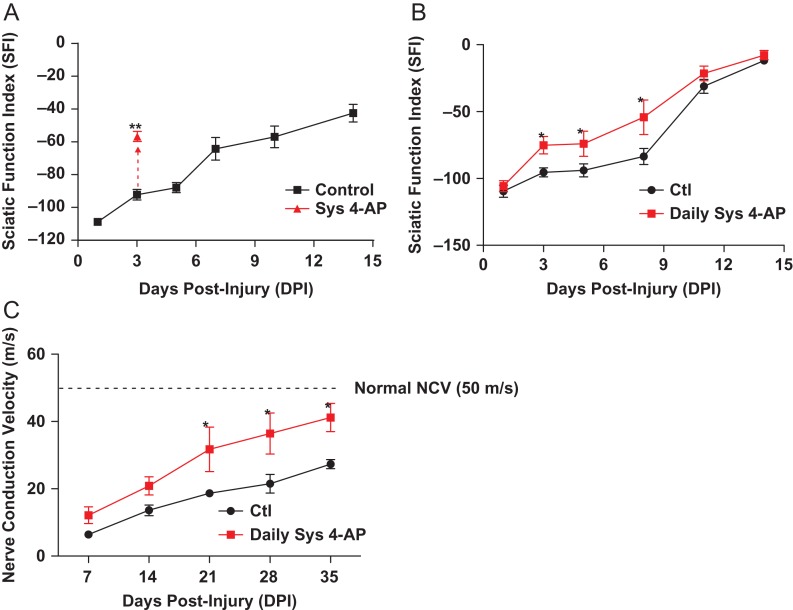
1. Single dose 4-AP administration can revive crushed nerve functionality for immediately diagnostic of incomplete nerve injury. In this experiment, the temporary effect of 4-AP treatment was investigated at day 3 post-injury by administering 4-AP and assessing function 15 minutes post administration.
4-AP treatment allows immediate diagnosis of remaining functionality in incomplete nerve injury. Motor function as assessed with standard SFI measurements demonstrated that 4-AP drove remarkable improvement on gait function within minutes with single dose administration of 4-AP (50 μg for a mouse) (Fig. 1A). Since this assessment is based on the ability of a newly introduced drug (4-AP) to alter functional gait, these animals were assessed within 15 minutes of dosing systemically to evaluate this “diagnostic effect” of 4-AP treatment. Improvement was large (40%) and lasted for four hours (data not shown). Immunofluorescence analyses of crushed and healthy nerves confirmed that the structure of axons (neurofilament+) and myelin sheaths (P0+) was heavily destroyed by experimental crush (not shown). Therefore, this large transient improvement on SFI after 4-AP administration may be evidence of remaining intact but demyelinated axonal fibers able to conduct impulses at a time post-injury as activated by 4-AP when control animals still demonstrated severe conduction dysfunction. Single dose 4-AP treatment in this setting was therefore immediately diagnostic of incomplete injury. After administration, control animals and single dose treated animals recovered along the same time course (not shown).
FIGURE 1.
Systemic 4-AP administration transiently enhances sciatic nerve motor function after crush injury and enhances function over time after nerve injury. (A) At 3 days post-injury, 50 μg of systemic 4-AP (ip) transiently enhanced SFI by 40%, as measured 1 hr after treatment and where 0 = normal function. (p < 0.01) (B) Daily systemic 4-AP administration (10 μg/day, ip) improves functional recovery of crushed sciatic nerve. Daily 10 μg 4-AP enhanced recovery of sciatic nerve motor function as compared with treatment with vehicle at 3 dpi through 8 dpi. (p < 0.05) (C) Daily 10 μg 4-AP administration also enhanced recovery of nerve conduction velocity (NCV) as observed beginning at 21 days post-injury, eventually restoring NCV to nearnormal values while NCV in vehicle-treated mice remained less than half that of uninjured animals (p < 0.05 at 21, 28 and 35 dpi). *p < 0.05. (D) Local administration of 4-AP in PLGA films enhances functional and (E) electrophysiological recovery after sciatic nerve crush. Local 4-AP treated crushed sciatic nerve (black: vehicle PLGA films; red: (4-AP)-PLGA films) regained partial walking ability as early as 3 days post-injury compared to vehicle-treated group. (*p < 0.05; **p < 0.01, N = 8 per group).
2. Daily 4-AP treatment accelerates crush nerve injury recovery. In this experiment function was assessed almost a full day after administration of 4-AP using the same sciatic nerve crush injury to examine any enduring effects of 4-AP treatment on injury recovery, while at the same time re-affirming the transient diagnostic effect noted previously.
Animals with nerve crush were dosed with human-mass equivalent doses of 4-AP (0.5 mg/kg/day) and tested 20 hours after renal clearance of 4-AP. Functional walking track analysis (by SFI) of animals dosed daily 4-AP in this manner showed similar transient improvements as ween in the demonstration of the “diagnostic effect” (Fig. 1A) which wore off after four hours (not shown), However, SFI at 23 hours post 4-AP resulted in enduring improvement (Fig. 1B) – even when 4-AP had been cleared from the animal’s system. Electrodiagnostic NCV improvements soon followed so that by 35 days post-injury, 4-AP-treated animals had nearly double the NCV of untreated controls (Fig. 1C).
Immunofluoresecence analysis of myelinated fibers (P0) was measured weekly out to 21 days post-injury to estimate the extent of structural recovery. Animals dosed daily with systemic 4-AP showed marked increases in P0 immunostaining (Fig. 2A) on cross-sections of sciatic nerve, in addition to increased P0 protein levels (Fig. 2B) and increased numbers of myelinated but not total axon numbers (Fig. 2C,D). In this way, treatment with 4-AP had either myeloprotective or myeloregenerative effects or both.
FIGURE 2.
Long-term/local 4-AP treatment promoted remyelination and increased the number of myelinated axons. (A) Increases in P0 protein over time also were observed by immunofluorescence analysis at different time points, with P0 protein expression increasing to a greater extent in nerves of mice treated with 4-AP. (B) 4-AP treated nerves also showed increases in the levels of P0 protein as detected by western blot analysis. (C, D) 4-AP treatment increased the number of myelinated axons (p < 0.01, N = 8 per group). Even though the 4-AP treated group also exhibited a greater number of total axons, this difference was not statistically significant.
3. Localized treatment with 4-AP results in durable improvement in function.
Given the positive effects of continuous 4-AP treatment on a localized traumatic lesion of an important peripheral nerve, the possibility of localized administration was examined. Biomaterial encapsulation with Poly lactic-co-glycolic acid (PLGA, 50:50) was chosen to deliver sustained doses of 4-AP locally at the site of injury. Two distinctly engineered forms of encapsulation were used with different geometries, loading capacities, and release rates: Microparticles (Supplementary Figure S1A, panels i,ii) and single-layered films (Supplementary Figure S1B, i,ii) (see methods).
To test the degradation rate of implanted PLGA vehicles, and the consistency of location after implantation, particles were labeled with rhodamine and implanted directly onto the crush-injured sciatic nerve of mice. Fluorescence intensity changes were monitored using In Vivo Imaging System (Supplementary Figure S2) and slowly fell over 3 weeks post implantation – during which time, beads stayed at the injury site. Residual fabricated vehicle was recoverable from the site at 21 days post-injury despite detection falling below whole animal soft-tissue thresholds at that point.
In vitro experiments were performed on engineered carriers prior to use in vivo (Supplementary Figure S1). Testing was also performed to ensure that the packaged 4-AP would retain activity even after the turbulent emulsion process used to construct carriers. To evaluate the bioactivity of packaged 4-AP released over time in vitro by incubating particles and films in vitro for 1 month in PBS under mild physical agitation, we isolated 4-AP released from each vehicle using spectrophotometry to measure relative concentrations. Spectrophotometric evaluations were performed on in vitro agitated PLGA-4-AP polymers incubated in sterile saline. These preparations had samples taken from them every day for 30 days, which were tested for spectrophotometric absorbance. Then based on these relative measurements, samples were compared to control dilutions of 4-AP in saline to get appropriate estimates of 4-AP that had eluted from the PLGA-4-AP polymers. These were then used to prepare polymer-derived 4-AP for testing at doses appropriate for use in animals (based on mass-adjusted human dosing). Since the only bioassay available for 4-AP quickly applicable in a live animal is the response to a dose after crush injury (see results section below), we applied the “diagnostic effect” described int he results section as a bioassay for eluted 4-AP activity. These doses were given systemically to animals with nerve crush injuries, which resulted in predictable and reproducible improvements in gait function after dosing (Supplementary Figure S3).
The results of treatment with localized administration of 4-AP showed improvement in SFI (Fig. 1F), and nerve conduction velocity (Fig. 1G) (data for films, please see Supplementary Figure S4 for comparison between particles and films). Localized treatment using doses well below systemic dose levels resulted in reproducible, clinically relevant improvements in functional and tissue parameters of nerve recovery.
DISCUSSION
In severe extremity limb trauma, multiple tissue types are often affected. Peripheral nerve components of this mixed injury type are often the most difficult to diagnose and treat, and the morbidity attributable to peripheral nerve injury is likely to outlast injuries to bone or other tissues. Two distinct reasons for this are the difficulty in distinguishing partial from complete injuries without surgical exploration and the unpredictable healing of nerve tissue. This may be due to the nature of nerve injuries which variably affect neurons or Schwann cells in the zone of trauma. If such injuries spare some neurons but injure the Schwann cells or myelin, then the function of the nerve may be indistinguishable from scenarios where nerves are completely severed. The hypothesis for this work rests on the notion that some portion of traumatic demyelination may be amenable to treatment with agents used in systemic demyelinating disease.
In the present study and our accompanying work (reproduced with permission for this review),18 we found two novel and beneficial clinical applications of 4-AP on peripheral nerve injury treatment. First, single dose systemic 4-AP administration temporarily restores activity of demyelinated axons within crushed nerve to improve the diagnosis of remaining intact axons within the injured nerve. Second, continuous 4-AP administration accelerates nerve injury recovery by improving function, NCV, and myelination. Additionally, sustained and local delivery of 4-AP provides functional improvement at doses lower than those used currently in humans. Interestingly, 4-AP applied at doses comparatively smaller than systemic dosing at the site of the nerve injury was similar in efficacy at improving functional outcome when compared to systemic dosing.
4-AP has been used for decades to enhance nerve activity in the setting of systemic neuropathic diseases like multiple sclerosis and myasthenia gravis.9,19 In these settings, the effect of 4-AP is believed to be immediate and directed chiefly at the demyelinated axon by bolstering excitability. The picture which emerges from the current treatment paradigm for these and other systemic demyelinating disorders is one where 4-AP, a known blocker of potassium channels, is that of transient electrical stabilization of neurons denuded of myelin. Indeed, our results in Figure 1 seem to support this idea – a severely damaged nerve with little intact myelin conducts impulses to support function with treatment, albeit transiently.
There is however reason to believe that longer term effects may underlie 4-AP mediated improvement. Previous studies link electrical stimulation in the setting of both central and peripheral nerve injury to improvement in axonal regeneration, remyelination, and reinnervation.20–23 We tested these effects in a rodent sciatic nerve crush injury experiment with daily 4-AP treatment, which resulted in marked nerve structural improvement which could not be attributed to the short-term effects of 4-AP on axons. When function and electrodiagnostic parameters are assessed well after 4-AP has been cleared from the system, continued functional improvement was found. We have published these findings along with ultrastructural evidence of myelination.18 Taken together, these two findings underline one way where 4-AP may be valid to address both the difficulties in diagnostic (short-term) and treatment (long-term) problems associated with clinical peripheral nerve crush injury.
If 4-AP is to be used to diagnose or treat peripheral nerve crush injuries, then a key hurdle will be to produce a product that can be administered to the site of injury. Here, we present an approach utilizing a PLGA delivery system to release sustained local doses of 4-AP directly onto a known crush-injured nerve. The essential elements required for (4-AP)-PLGA carriers to deliver local doses relevant for treatment are: (1) a sufficient amount of encapsulated 4-AP in the PLGA vehicle for a long-term treatment and (2) the controllable release rate of the encapsulated 4-AP. Both requirements were met with two different vehicles translatable into a usable product in wounded patients.
In vitro data showed that both carriers could slowly and sustainably release 4-AP up to a month after implantation, all the while remaining at the site of injury at doses far below the systemically allowable level.
As a companion to this work, nerves from this experiment were subjected to formal electron microscopic analysis.18 In that work, the morphologic structure of the crushed sciatic nerve revealed a clear increase in the average thickness of myelin sheaths, myelin protein (P0) content and even unmyelinated neuron counts at injury sites. This paralleled the significant electrodiagnostic improvement in the two weeks after implantation.
Taken together, 4-AP may be a reasonable systemic and local candidate treatment in nerve injury. In translation of this work to human studies, which is currently underway, efforts should be made to independently consider the utility of 4-AP in the diagnosis of incomplete injuries and the potential for 4-AP to alter the myelination or remyelination of the regenerating nerve after severe trauma.
Supplementary Material
Supplementary Material
Supplementary material is available at Military Medicine online.
Previous Presentation
Presented as a poster at the MHSRS Conference 2017, Orlando, FL. Experimental results from this work, published by our group (18) were reproduced with permission.
Funding
This work was supported by grants from the National Institutes of Health (NIH) (K08 AR060164-01A) and the Department of Defense (DOD) (W81XWH-16-1-0725), an American Society for Surgery of the Hand (ASSH) Hand Surgeon Scientist Award grant. Additionally, institutional support was provided by the University of Rochester and Pennsylvania State University. This supplement was sponsored by the Office of the Secretary of Defense for Health Affairs.
References
- 1. Feliciano DV, Moore EE, Mattox KL: Trauma, Ed 3, Norwalk, Conn, Appleton & Lange, 1996. [Google Scholar]
- 2. Niver GE, Ilyas AM: Management of radial nerve palsy following fractures of the humerus. Orthop Clin North Am 2013; 44: 419–24. [DOI] [PubMed] [Google Scholar]
- 3. Trumble T: Principles of Hand Surgery and Therapy. Philadelphia, PA, Saunders/Elsevier, 2010. [Google Scholar]
- 4. Novak CB, Anastakis DJ, Beaton DE, Katz J: Patient-reported outcome after peripheral nerve injury. J Hand Surg Am 2009; 34: 281–7. [DOI] [PubMed] [Google Scholar]
- 5. Novak CB, Anastakis DJ, Beaton DE, Mackinnon SE, Katz J: Biomedical and psychosocial factors associated with disability after peripheral nerve injury. J Bone Joint Surg Am 2011; 93: 929–36. [DOI] [PubMed] [Google Scholar]
- 6. Kang JR, Zamorano DP, Gupta R: Limb salvage with major nerve injury: current management and future directions. J Am Acad Orthop Surg 2011; 19(Suppl 1): S28–34. [DOI] [PubMed] [Google Scholar]
- 7. Davis FA, Stefoski D, Rush J: Orally administered 4-aminopyridine improves clinical signs in multiple sclerosis. Ann Neurol 1990; 27: 186–92. [DOI] [PubMed] [Google Scholar]
- 8. Hansebout RR, Blight AR, Fawcett S, Reddy K: 4-Aminopyridine in chronic spinal cord injury: a controlled, double-blind, crossover study in eight patients. J Neurotrauma 1993; 10: 1–18. [DOI] [PubMed] [Google Scholar]
- 9. Lundh H, Nilsson O, Rosen I: Effects of 4-aminopyridine in myasthenia gravis. J Neurol Neurosurg Psychiatry 1979; 42: 171–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sanders DB, Massey JM, Sanders LL, Edwards LJ: A randomized trial of 3,4-diaminopyridine in Lambert-Eaton myasthenic syndrome. Neurology 2000; 54: 603–7. [DOI] [PubMed] [Google Scholar]
- 11. Geary MB, Li H, Zingman A, et al. : Erythropoietin accelerates functional recovery after moderate sciatic nerve crush injury. Muscle Nerve 2017; 56: 143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chaisri W, Hennink WE, Okonogi S: Preparation and characterization of cephalexin loaded PLGA microspheres. Curr Drug Deliv 2009; 6: 69–75. [DOI] [PubMed] [Google Scholar]
- 13. Hoffman MD, Van Hove AH, Benoit DS: Degradable hydrogels for spatiotemporal control of mesenchymal stem cells localized at decellularized bone allografts. Acta Biomater 2014; 10: 3431–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Medinaceli L, Freed WJ, Wyatt RJ: An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Exp Neurol 1982; 77: 634–43. [DOI] [PubMed] [Google Scholar]
- 15. Elfar JC, Jacobson JA, Puzas JE, Rosier RN, Zuscik MJ: Erythropoietin accelerates functional recovery after peripheral nerve injury. J Bone Joint Surg Am 2008; 90: 1644–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gladman SJ, Huang W, Lim SN, et al. : Improved outcome after peripheral nerve injury in mice with increased levels of endogenous omega-3 polyunsaturated fatty acids. J Neurosci 2012; 32: 563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gupta R, Steward O: Chronic nerve compression induces concurrent apoptosis and proliferation of Schwann cells. J Comp Neurol 2003; 461: 174–86. [DOI] [PubMed] [Google Scholar]
- 18. Tseng KC, Li H, Clark A, et al. : 4-aminopyridine promotes functional recovery and remyelination in acute peripheral nerve injury. EMBO Mol Med 2016; 8: 1409–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hayes KC: The use of 4-aminopyridine (fampridine) in demyelinating disorders. CNS Drug Rev 2004; 10: 295–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gordon T, Brushart TM, Amirjani N, Chan KM: The potential of electrical stimulation to promote functional recovery after peripheral nerve injury--comparisons between rats and humans. Acta Neurochir Suppl 2007; 100: 3–11. [DOI] [PubMed] [Google Scholar]
- 21. Vivo M, Puigdemasa A, Casals L, Asensio E, Udina E, Navarro X: Immediate electrical stimulation enhances regeneration and reinnervation and modulates spinal plastic changes after sciatic nerve injury and repair. Exp Neurol 2008; 211: 180–93. [DOI] [PubMed] [Google Scholar]
- 22. Wan L, Xia R, Ding W: Short-term low-frequency electrical stimulation enhanced remyelination of injured peripheral nerves by inducing the promyelination effect of brain-derived neurotrophic factor on Schwann cell polarization. J Neurosci Res 2010; 88: 2578–87. [DOI] [PubMed] [Google Scholar]
- 23. Wake H, Lee PR, Fields RD: Control of local protein synthesis and initial events in myelination by action potentials. Science 2011; 333: 1647–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.