Abstract
Aim
We aimed to assess the role of 21-gene recurrence score (RS) in the decision-making for surgical treatment in early stage breast cancer and compared the outcomes between breast-conserving surgery (BCS) and mastectomy (MAST) among various 21-gene RS groups.
Methods
We included patients with stage T1-2M0M0 and estrogen receptor-positive breast invasive ductal carcinoma who underwent BCS + radiotherapy or MAST between 2004 and 2012 as part of the Surveillance, Epidemiology, and End Results program. Data were analyzed using binomial logistic regression, multivariate Cox proportional hazards models, and propensity score matching (PSM).
Results
We enrolled 34,447 patients including 22,681 (65.8%) and 11,766 (34.2%) who underwent BCS and MAST, respectively. Patients with high-risk RS were more likely to receive MAST. Multivariate analysis indicated that patients with intermediate-risk (P<0.001) and high-risk (P<0.001) RS had poor breast cancer-specific survival (BCSS), as compared to those with low-risk RS. Moreover, patients who underwent MAST also exhibited poor BCSS (P<0.001), as compared to those who underwent BCS. In low-risk (P<0.001) and intermediate-risk (P=0.020) RS groups, patients who underwent MAST had poor BCSS, as compared to those treated with BCS. However, BCSS was comparable between patients who underwent MAST and BCS (P=0.952); similar trends were also observed after PSM.
Conclusion
The 21-gene RS may impact the decision-making for surgery in early stage breast cancer. Our study provides additional support for a shared decision-making process for BCS when both local management options are appropriate choices regardless of the 21-gene RS.
Keywords: breast cancer, oncotype, mastectomy, breast conserving treatment, survival
Introduction
Breast cancer is the most common malignant tumor in women: ~2 million breast cancer cases are diagnosed worldwide annually.1–3 At present, surgery, including breast-conserving surgery (BCS) or mastectomy (MAST), remains the basic method for the local management of breast cancer. Several previous trials have shown similar long-term outcomes between BCS + radiotherapy (RT) and MAST in early stage breast cancer.4–6 Results from recent studies also showed better survival outcomes in patients who received BCS + RT, as compared to those who underwent MAST.7–9 Patients who underwent BCS also had better psychosocial well-being, a higher level of satisfaction with life, and better psychological health compared to patients treated with MAST.10,11
Several factors, including clinicopathological, individual, and physician factors may affect the decision-making for surgery in early stage breast cancer.12 Moreover, the fear of cancer recurrence is an important factor driving the decision for MAST, which reportedly reduces the risk and avoids the need for repeated surgery or RT associated with BCS.13 A recent meta-analysis indicated that ~25% of patients were more likely to choose BCS rather than MAST, if a decision aid was used.14 The Oncotype DX Breast Cancer Assay (GenomicHealth®) is a 21-gene assay used to calculate a recurrence score (RS), which serves as an assessment of the probability of distant recurrence. Several studies have confirmed that there is a correlation between the 21-gene RS and the response to adjuvant chemotherapy.15,16 In addition, a higher RS was also found to be associated with a higher risk of locoregional or distant relapse.16–21 However, the role of the 21-gene RS in surgical decision-making remains unclear. Therefore, in the present study, we aimed to assess the role of the 21-gene RS on decision-making for surgical treatment in early stage breast cancer, and compared the outcomes between BCS and MAST among different 21-gene RS groups.
Materials and methods
Patients
We linked to the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program (SEER) 18 Regs (Excl AK) Custom Data Malignant Breast (with Oncotype DX and Additional Treatment Fields) to obtain patient demographics, clinicopathological variables, vital status, and results of the 21-gene RS testing of breast cancer patients.22 In particular, we included patients with stage tumor (T)1–2 node (N)0 metastasis (M)0, estrogen receptor (ER)-positive breast invasive ductal carcinoma who received BCS + RT or MAST between 2004 and 2012. We excluded patients without a positive pathology diagnosis, unknown race/ethnicity, unknown tumor grade, unknown progesterone receptor (PR) status, as well as those who underwent RT after MAST. The use of data from SEER is exempt from the approval process by institutional review boards due to the de-identified nature of patient information.
Variables
Patient characteristics including age, race/ethnicity, tumor grade, T stage, PR status, surgical procedure, history of chemotherapy, and the 21-gene RS classification were assessed. Based on the 21-gene RS, patients were classified as “low-risk” (score: <18), “intermediate-risk” (score: 18–30), or “high-risk” (score: >30).23 The primary survival outcome included breast cancer-specific survival (BCSS), calculated as the time from initial diagnosis to the date of breast cancer-related death or final follow-up.
Statistical analysis
Chi-squared tests were performed to compare the difference in patient characteristics between the two surgical treatment arms. The predictors of undergoing surgery were evaluated using binomial logistic regression. We used a 1:1 propensity score matching (PSM) method to balance the patient characteristics and reduce potential selection bias.24,25 BCSS curves were plotted using the Kaplan–Meier method and then compared using log-rank tests. Multivariate Cox proportional hazards models were established using the Backward Wald Method to evaluate the independent prognostic indicators associated with BCSS. All statistical analyses were conducted using IBM SPSS version 22.0 (IBM Corporation, Armonk, NY, USA), and P-values <0.05 were considered significant.
Results
We enrolled 34,447 patients, including 22,681 (65.8%) and 11,766 (34.2%) who underwent BCS and MAST, respectively. Table 1 lists the patient characteristics. Approximately 75% of patients were aged ≥50 years, were of Non-Hispanic White ethnicity, and had not undergone chemotherapy. Moreover, 81.2% (n=27,974) of patients exhibited T1 stage disease, and 89.9% (n=30,962) of patients exhibited PR-positive disease. The median RS was 16 (range, 0–59), and 18,664 (54.2%), 12,693 (36.8%), and 3,090 (9.0%) of patients were assigned to the low-, intermediate-, and high-risk RS groups, respectively. The human epidermal growth factor receptor-2 (HER2) status was routinely registered after 2010 in SEER database. A total of 17,810 patients had the results of HER2 status, and 97.4% (n=17,353) of them had HER2-negative disease.
Table 1.
Patient characteristics
| Variables | n | BCS (%) | MAST (%) | P-value |
|---|---|---|---|---|
|
| ||||
| Age (years) | <0.001 | |||
| <50 | 8,943 | 5,148 (22.7) | 3,795 (32.3) | |
| ≥50 | 25,504 | 17,533 (77.3) | 7,971 (67.7) | |
| Race/ethnicity | <0.001 | |||
| Non-Hispanic White | 26,417 | 17,607 (77.6) | 8,810 (74.9) | |
| Non-Hispanic Black | 2,481 | 1,617 (7.1) | 864 (7.3) | |
| Hispanic (all races) | 2,578 | 1,684 (7.4) | 894 (7.6) | |
| Other | 2,971 | 1,773 (7.8) | 1,198 (10.2) | |
| Grade | <0.001 | |||
| Well differentiated | 9,400 | 6,503 (28.7) | 2,897 (24.6) | |
| Moderately differentiated | 18,510 | 12,137 (53.5) | 6,373 (54.2) | |
| Poorly/undifferentiated | 6,537 | 4,041 (17.8) | 2,496 (21.2) | |
| Unknown | ||||
| Tumor stage | <0.001 | |||
| T1 | 27,974 | 19,131 (84.3) | 8,843 (75.2) | |
| T2 | 6,473 | 3,550 (15.7) | 2,923 (24.8) | |
| PR status | <0.001 | |||
| Negative | 3,485 | 2,189 (9.7) | 1,296 (11.0) | |
| Positive | 30,962 | 20,492 (90.3) | 10,470 (89.0) | |
| Chemotherapy | 0.002 | |||
| No/unknown | 26,512 | 17,569 (77.5) | 8,943 (76.0) | |
| Yes | 7,935 | 5,112 (22.5) | 2,823 (24.0) | |
| 21-gene recurrence score | <0.001 | |||
| Low risk | 18,664 | 12,441 (54.9) | 6,223 (52.9) | |
| Intermediate risk | 12,693 | 8,423 (37.1) | 4,270 (36.3) | |
| High risk | 3,090 | 1,817 (8.0) | 1,273 (10.8) | |
Abbreviations: MAST, mastectomy; PR, progesterone receptor; T, tumor; BCS, breast-conserving surgery.
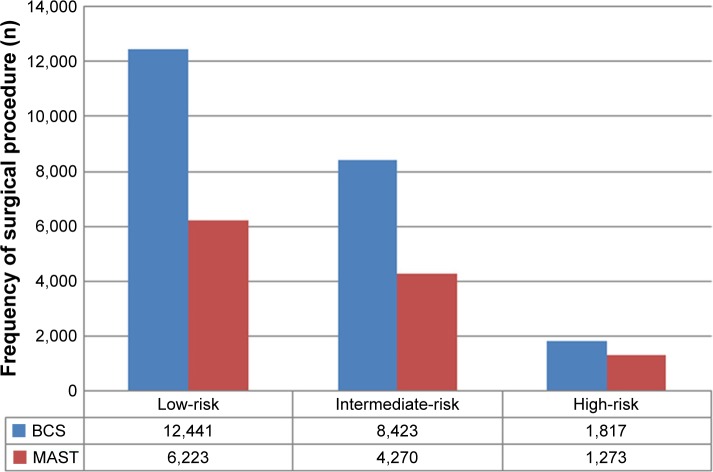
There was a significant difference between patients who underwent BCS and MAST (Table 1). Binomial regression analysis indicated that younger age, other race/ethnicity, higher tumor grade, T2 stage, PR-negative disease, no history of chemotherapy, and high-risk RS were the independent indicators associated with the decision for MAST (Table 2). The proportion of various procedures among the different RS groups is presented in Figure 1. A total of 66.7% (n=12,441), 66.4% (n=8,423), and 58.8% (n=1,817) patients with low-, intermediate-, and high-risk RS underwent BCS, respectively.
Table 2.
Factors predicting the surgical procedure performed (mastectomy vs breast conserving surgery)
| Variables | OR | 95% CI | P-value |
|---|---|---|---|
|
| |||
| Age (years) | |||
| <50 | 1 | ||
| ≥50 | 0.585 | 0.556–0.616 | <0.001 |
| Race/ethnicity | |||
| Non-Hispanic White | 1 | ||
| Non-Hispanic Black | 0.997 | 0.913–1.088 | 0.942 |
| Hispanic (all races) | 0.981 | 0.900–1.070 | 0.669 |
| Other | 1.237 | 1.143–1.339 | <0.001 |
| Grade | |||
| Well differentiated | 1 | ||
| Moderately differentiated | 1.138 | 1.077–1.201 | <0.001 |
| Poorly/undifferentiated | 1.239 | 1.151–1.334 | <0.001 |
| Unknown | |||
| Tumor stage | |||
| T1 | 1 | ||
| T2 | 1.768 | 1.671–1.870 | <0.001 |
| PR status | |||
| Negative | 1 | ||
| Positive | 0.833 | 0.771–0.900 | <0.001 |
| Chemotherapy | |||
| No/unknown | 1 | ||
| Yes | 0.844 | 0.793–0.899 | <0.001 |
| 21-gene recurrence score | |||
| Low risk | 1 | ||
| Intermediate risk | 1.004 | 0.952–1.059 | 0.883 |
| High risk | 1.286 | 1.167–1.417 | 0 |
Abbreviations: N, node; OR, odds radio; PR, progesterone receptor; T, tumor.
Figure 1.
The proportion of surgical procedures in different 21-gene recurrence score groups.
Abbreviations: MAST, mastectomy; BCS, breast-conserving surgery.
The median follow-up duration of this study was 65 months (range, 0–143 months). Multivariate Cox analysis indicated that the 21-gene RS was an independent factor for BCSS. Patients with intermediate-risk RS (HR, 2.284; 95% CI, 1.826–2.858; P<0.001) and high-risk RS (HR, 4.902; 95% CI, 3.790–6.340; P<0.001) had poor BCSS, as compared to those with low-risk RS. The 5-year BCSS was 99.5%, 98.7%, and 95.4% in patients with low-, intermediate-, and high-risk RS, respectively (log-rank test, P<0.001). In addition, patients who underwent MAST were more likely to exhibit poor BCSS (HR, 1.385; 95% CI, 1.162–1.650; P<0.001) as compared to those who had undergone BCS. The 5-year BCSS was 99.0% and 98.5% in patients who had undergone BCS and MAST, respectively (log-rank test, P<0.001). The tumor grade, T stage, and age at diagnosis were also independent factors for BCSS.
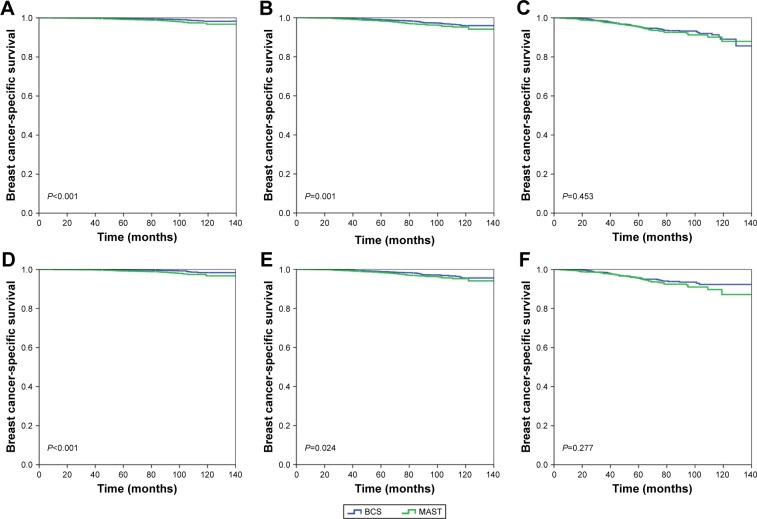
Furthermore, we analyzed the effect of the surgical procedure on BCSS in different 21-gene RS groups (Table 3). Following adjustment by age, race/ethnicity, tumor grade, T stage, PR status, and history of chemotherapy in patients with low- (HR, 2.043; 95% CI, 1.422–2.935; P<0.001) and intermediate-risk (HR, 1.368; 95% CI, 1.051–1.781; P=0.020) RS groups, we found that those who underwent MAST had poorer BCSS than those who underwent BCS. However, the BCSS was comparable between patients who underwent MAST and BCS (HR, 1.009; 95% CI, 0.740–1.377; P=0.952) in high-risk RS cohort. The BCSS curves between the two treatment arms according to the 21-gene RS group are shown in Figure 2A–C.
Table 3.
Multivariate analysis of prognostic factors of breast cancer-specific survival in different 21-gene recurrence score groups
| Variables | Low-risk
|
Intermediate-risk
|
High-risk
|
|||
|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
|
| ||||||
| Age (years) | ||||||
| <50 | 1 | 1 | 1 | |||
| ≥50 | 2.156 (1.318–3.528) | 0.002 | 1.160 (0.854–1.577) | 0.342 | 1.043 (0.723–1.505) | 0.822 |
| Race/ethnicity | ||||||
| Non-Hispanic White | 1 | 1 | 1 | |||
| Non-Hispanic Black | 1.719 (0.979–3.020) | 0.059 | 1.220 (0.782–1.904) | 0.381 | 1.247 (0.766–2.209) | 0.374 |
| Hispanic (all races) | 0.779 (0.341–1.480) | 0.553 | 0.794 (0.460–1.370) | 0.407 | 1.246 (0.727–2.134) | 0.423 |
| Other | 0.604 (0.264–1.382) | 0.232 | 0.822 (0.499–1.355) | 0.443 | 1.097 (0.650–1.851) | 0.728 |
| Grade | ||||||
| Well differentiated | 1 | 1 | 1 | |||
| Moderately differentiated | 2.290 (1.416–3.704) | 0.001 | 1.925 (1.206–3.073) | 0.006 | 3.343 (0.816–13.691) | 0.093 |
| Poorly/undifferentiated | 2.771 (1.472–5.214) | 0.002 | 3.848 (2.391–6.192) | <0.001 | 3.565 (0.878–14.471) | 0.075 |
| Tumor stage | ||||||
| T1 | 1 | 1 | 1 | |||
| T2 | 1.437 (0.916–2.185) | 0.089 | 2.307 (1.752–3.039) | <0.001 | 1.843 (1.360–2.497) | 1.843 |
| PR status | ||||||
| Negative | 1 | 1 | 1 | |||
| Positive | 0.672 (0.327–1.380) | 0.279 | 1.354 (0.897–2.043) | 0.149 | 0.879 (0.641–1.205) | 0.422 |
| Chemotherapy | ||||||
| No/unknown | 1 | 1 | 1 | |||
| Yes | 1.575 (0.891–2.783) | 0.118 | 0.832 (0.631–1.095) | 0.189 | 0.733 (0.533–1.010) | 0.058 |
| Surgical procedure | ||||||
| BCS | 1 | 1 | 1 | |||
| MAST | 2.043 (1.422–2.935) | <0.001 | 1.368 (1.051–1.781) | 0.020 | 1.009 (0.740–1.377) | 0.952 |
Abbreviations: MAST, mastectomy; PR, progesterone receptor; T, tumor; BCS, breast-conserving surgery.
Figure 2.
Kaplan–Meier curves of breast cancer-specific survival between breast-conserving surgery and mastectomy according to 21-gene RS groups before (A, low-risk; B, intermediate-risk; C, high-risk) and after (D, low-risk; E, intermediate-risk; F, high-risk) propensity score matching.
Abbreviations: MAST, mastectomy; BCS, breast-conserving surgery; RS, recurrence score.
Finally, we used PSM to reduce the potential bias in patient selection. A total of 6,109, 4,123, and 1,116 pairs of patients were completely matched in the low-, intermediate-, and high-risk RS groups, respectively. The results after PSM also showed that, in patients with low-risk (HR, 2.611; 95% CI, 1.644–4.146; P<0.001) and intermediate-risk (HR, 1.420; 95% CI, 1.048–1.926; P=0.024) RS groups, treatment with MAST was associated with a poorer BCSS as compared to treatment with BCS. However, the BCSS was comparable between patients who received MAST and BCS in the high-risk RS cohort (HR, 1.196; 95% CI, 0.836–1.710; P=0.327). The BCSS curves between the two treatment arms after PSM according to the 21-gene RS group are shown in Figure 2D–F.
Discussion
In the present study, we assessed the effect of the 21-gene RS on decision-making for surgical treatment in early stage breast cancer and compared the survival outcomes between BCS and MAST in different 21-gene RS groups. Our results showed that patients with high-risk RS were more likely to receive MAST and among those with low- and intermediate-risk RS, the treatment with BCS was associated with better BCSS, as compared to the treatment with MAST. In addition, breast-conserving treatment is safe in patients with a high-risk RS.
Several studies have confirmed the superiority of BCS over MAST or at least equal outcomes between BCS and MAST.8,9,26–28 However, a recent study using large national databases indicated that higher proportions of BCS-eligible patients chose MAST in recent years, and marked trends were observed in patients with node-negative or in situ disease.29 These findings may be related to the concern among patients regarding tumor recurrence.13,30 Our study was conducted during the contemporary chemo-endocrine therapy era, and the absolute benefit of BCS was 0.5%, as compared to MAST (99.0% vs 98.5%). Furthermore, our study confirmed that the outcomes of BCS were not inferior to those of MAST and that BCS can be used as a standard surgical treatment for early breast cancer.
Several studies have found that the 21-gene RS can affect not only distant recurrence but also locoregional recurrence (LRR) after BCS + RT.16–21 Trials such as the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14 and NSABP B-20 that included node-negative breast cancer patients who underwent BCS + RT indicated a 10-year LRR of 4.3%, 7.2%, and 15.8% in low-, intermediate-, and high-risk RS groups, respectively.19 In addition, the NSABP B-28 study that included node-positive patients undergoing BCS + RT showed a 10-year LRR of 3.0%, 8.7%, and 11.0% in low-, intermediate-, and high-risk RS groups, respectively.21 Although we were unable to obtain LRR data from the SEER database, our study also found that the 21-gene RS is an independent prognostic indicator of BCSS. Thus, the high-risk RS cohort may have a high risk of LRR and distant metastasis after RT, which could affect the efficacy of breast-conserving treatment. However, to our knowledge, no study has assessed the effect of the surgical procedure on outcomes according to different 21-gene RS groups.
The 21-gene RS has been shown to indicate whether patients receive a survival benefit from chemotherapy,15,16 and recent studies indicated that the 21-gene RS can predict the benefits of postoperative RT.31,32 In the present study, patients from the high-risk RS cohort were less likely to undergo BCS, which may be related to the concern for potential tumor recurrence after BCS. However, we found that BCS was not inferior to MAST, regardless of the 21-gene RS status. The increased use of contemporary chemo-endocrine therapy regimens for better disease control may have contributed to this lack of inferiority in BCSS among patients undergoing BCS in our study. Since the clinical role of BCS has been validated in early stage breast cancer, it remains difficult to conduct clinical trials to assess the survival outcomes between BCS and MAST in different 21-gene RS groups. Our study provides additional information on the local treatment in high-risk RS patients.
In the present study, younger age, higher tumor grade, T2 stage, and PR-negative disease were independent indicators for undergoing MAST, consistent with that observed in previous studies.33 Several prior studies also showed that surgeon recommendation was an important factor in decision-making for the surgical procedure.34,35 In addition, access to health care is a major determinant of choice for the selected breast cancer surgical procedure.36 Patients who underwent BCS had good psychosocial well-being, higher level of satisfaction with life, and better psychological health than those treated with MAST.10,11 In order to achieve an advantageous long-term outcome, it is necessary to inform patients in a clear manner regarding the advantages and disadvantages through an appropriate shared decision-making process. Although patients with high-risk RS may have biologically aggressive cancers,16–21 the 21-gene RS should not be used to impact treatment decisions for surgery, based on our findings; in fact, patients with high-risk RS are also suitable candidates for BCS.
Limitations
This study is limited by the inherent bias associated with any observational study; in fact, there were significant differences in several baseline characteristics between the two treatment arms. However, our study was conducted using real-world data and provided additional information related to the effects of different surgical procedures in a large cohort of patients. Moreover, we use PSM to balance the clinicopathological features of patients, although potentially significant confounders such as comorbidities and compliance to recommended therapy remain inevitable. Second limitation is the limited follow-up time of <6 years in our study because the relapses of breast cancer in early stage disease are not rare even 10–20 years after diagnosis and treatment.37 Third, the relatively small absolute differences were found even though statistically significant because of such a large sample size between the treatment arms. In addition, in the newer prospective data from Trial Assigning Individualized Options for Treatment (TAILORx) trail, 21-gene RS cutoffs have led to regrouping the RS risk groups, 0–10, 11–25, and >25 and that results in our study may not be applicable to the new RS groups.38 Finally, the patterns of disease recurrence were unavailable in the SEER database. However, the present study is the first population-based study to investigate the effect of the 21-gene RS in the decision for surgery in early breast cancer.
Conclusion
Our study suggests that the 21-gene RS may impact the decision for surgery in early stage breast cancer. Our results could provide additional support for a shared decision-making process for BCS when both local management options are appropriate choices regardless of the 21-gene RS.
Acknowledgments
This work was partly supported by the National Natural Science Foundation of China (81872459; 81803050), the Natural Science Foundation of Fujian Province (2016J01635), the Social Science and Technology Development Major Project of Dongguan (2018507150241630), the Natural Science Foundation of Guangdong Province (2018A030313666), and the Science and Technology Planning Projects of Xiamen Science & Technology Bureau (3502Z20174070).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Ginsburg O, Bray F, Coleman MP, et al. The global burden of women’s cancers: a grand challenge in global health. Lancet. 2017;389(10071):847–860. doi: 10.1016/S0140-6736(16)31392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harbeck N, Gnant M. Breast cancer. Lancet. 2017;389(10074):1134–1150. doi: 10.1016/S0140-6736(16)31891-8. [DOI] [PubMed] [Google Scholar]
- 4.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 5.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347(16):1227–1232. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 6.Litière S, Werutsky G, Fentiman IS, et al. Breast conserving therapy versus mastectomy for stage I–II breast cancer: 20 year follow-up of the EORTC 10801 phase 3 randomised trial. Lancet Oncol. 2012;13(4):412–419. doi: 10.1016/S1470-2045(12)70042-6. [DOI] [PubMed] [Google Scholar]
- 7.van Maaren MC, de Munck L, de Bock GH, et al. 10 year survival after breast-conserving surgery plus radiotherapy compared with mastectomy in early breast cancer in the Netherlands: a population-based study. Lancet Oncol. 2016;17(8):1158–1170. doi: 10.1016/S1470-2045(16)30067-5. [DOI] [PubMed] [Google Scholar]
- 8.de Boniface J, Frisell J, Bergkvist L, Andersson Y. Breast-conserving surgery followed by whole-breast irradiation offers survival benefits over mastectomy without irradiation. Br J Surg. 2018;105(12):1607–1614. doi: 10.1002/bjs.10889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lagendijk M, van Maaren MC, Saadatmand S, et al. Breast conserving therapy and mastectomy revisited: breast cancer-specific survival and the influence of prognostic factors in 129,692 patients. Int J Cancer. 2018;142(1):165–175. doi: 10.1002/ijc.31034. [DOI] [PubMed] [Google Scholar]
- 10.Cipora E, Konieczny M, Karwat ID, Roczniak W, Babuśka-Roczniak M. Surgical method of treatment and level of satisfaction with life among women diagnosed with breast cancer, according to time elapsed since performance of surgery. Ann Agric Environ Med. 2018;25(3):453–459. doi: 10.26444/aaem/91586. [DOI] [PubMed] [Google Scholar]
- 11.Klassen AF, Pusic AL, Scott A, Klok J, Cano SJ. Satisfaction and quality of life in women who undergo breast surgery: a qualitative study. BMC Womens Health. 2009;9:11. doi: 10.1186/1472-6874-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu J, Groot G. Creation of a new clinical framework – why women choose mastectomy versus breast conserving therapy. BMC Med Res Methodol. 2018;18(1):77. doi: 10.1186/s12874-018-0533-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu J, Groot G, Holtslander L, Engler-Stringer R. Understanding women’s choice of mastectomy versus breast conserving therapy in early-stage breast cancer. Clin Med Insights Oncol. 2017;11:117955491769126. doi: 10.1177/1179554917691266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waljee JF, Hawley S, Alderman AK, Morrow M, Katz SJ. Patient satisfaction with treatment of breast cancer: does surgeon specialization matter? J Clin Oncol. 2007;25(24):3694–3698. doi: 10.1200/JCO.2007.10.9272. [DOI] [PubMed] [Google Scholar]
- 15.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24(23):3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 16.Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11(1):55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dowsett M, Cuzick J, Wale C, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol. 2010;28(11):1829–1834. doi: 10.1200/JCO.2009.24.4798. [DOI] [PubMed] [Google Scholar]
- 18.Turashvili G, Chou JF, Brogi E, et al. 21-gene recurrence score and locoregional recurrence in lymph node-negative, estrogen receptor-positive breast cancer. Breast Cancer Res Treat. 2017;166(1):69–76. doi: 10.1007/s10549-017-4381-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mamounas EP, Tang G, Fisher B, et al. Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: results from NSABP B-14 and NSABP B-20. J Clin Oncol. 2010;28(10):1677–1683. doi: 10.1200/JCO.2009.23.7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solin LJ, Gray R, Goldstein LJ, et al. Prognostic value of biologic subtype and the 21-gene recurrence score relative to local recurrence after breast conservation treatment with radiation for early stage breast carcinoma: results from the Eastern Cooperative Oncology Group E2197 study. Breast Cancer Res Treat. 2012;134(2):683–692. doi: 10.1007/s10549-012-2072-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mamounas EP, Liu Q, Paik S, et al. 21-gene recurrence score and locoregional recurrence in Node-Positive/ER-Positive breast cancer treated with chemo-endocrine therapy. J Natl Cancer Inst. 2017;109(4) doi: 10.1093/jnci/djw259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence – SEER 18 Regs (Excl AK) Custom Data Malignant Breast (with Oncotype DX and Additional Treatment Fields), Nov 2017 Sub 2004–2015 – Linked to County Attributes – Total U.S., 1969–2016 Counties, National Cancer Institute DCCPS, Surveillance Research Program, released April 2018 based on the November 2017 submission. [Accessed January 3, 2019]. Available from: https://seer.cancer.gov/seerstat/databases/oncotype-dx/index.html.
- 23.Wong WB, Ramsey SD, Barlow WE, Garrison LP, Veenstra DL. The value of comparative effectiveness research: projected return on investment of the RxPONDER trial (SWOG S1007) Contemp Clin Trials. 2012;33(6):1117–1123. doi: 10.1016/j.cct.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39(1):33–38. [Google Scholar]
- 25.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agarwal S, Pappas L, Neumayer L, Kokeny K, Agarwal J. Effect of breast conservation therapy vs mastectomy on disease-specific survival for early-stage breast cancer. JAMA Surg. 2014;149(3):267–274. doi: 10.1001/jamasurg.2013.3049. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Wang S, Tang Y, et al. Comparison of treatment outcomes with breast-conserving surgery plus radiotherapy versus mastectomy for patients with stage I breast cancer: a propensity score-matched analysis. Clin Breast Cancer. 2018;18(5):e975–e984. doi: 10.1016/j.clbc.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Thöne K, Rudolph A, Obi N, Chang-Claude J, Flesch-Janys D. Prognostic impact of surgery for early-stage invasive breast cancer on breast cancer-specific survival, overall survival, and recurrence risk: a population-based analysis. Breast Cancer Res Treat. 2018;170(2):381–390. doi: 10.1007/s10549-018-4754-6. [DOI] [PubMed] [Google Scholar]
- 29.Kummerow KL, du L, Penson DF, Shyr Y, Hooks MA. Nationwide trends in mastectomy for early-stage breast cancer. JAMA Surg. 2015;150(1):9–16. doi: 10.1001/jamasurg.2014.2895. [DOI] [PubMed] [Google Scholar]
- 30.Cao JQ, Olson RA, Tyldesley SK. Comparison of recurrence and survival rates after breast-conserving therapy and mastectomy in young women with breast cancer. Curr Oncol. 2013;20(6):593–601. doi: 10.3747/co.20.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jayasekera J, Schechter CB, Sparano JA, et al. Effects of radiotherapy in early-stage, Low-Recurrence risk, hormone-sensitive breast cancer. J Natl Cancer Inst. 2018;110(12):1370–1379. doi: 10.1093/jnci/djy128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goodman CR, Seagle B-LL, Kocherginsky M, et al. 21-gene recurrence score assay predicts benefit of post-mastectomy radiotherapy in T1-2 N1 breast cancer. Clin Cancer Res. 2018;24(16):3878–3887. doi: 10.1158/1078-0432.CCR-17-3169. [DOI] [PubMed] [Google Scholar]
- 33.Gu J, Groot G, Boden C, et al. Review of factors influencing women’s choice of mastectomy versus breast conserving therapy in early stage breast cancer: a systematic review. Clin Breast Cancer. 2018;18(4):e539–e554. doi: 10.1016/j.clbc.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 34.Molenaar S, Oort F, Sprangers M, et al. Predictors of patients’ choices for breast-conserving therapy or mastectomy: a prospective study. Br J Cancer. 2004;90(11):2123–2130. doi: 10.1038/sj.bjc.6601835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Temple WJ, Russell ML, Parsons LL, et al. Conservation surgery for breast cancer as the preferred choice: a prospective analysis. J Clin Oncol. 2006;24(21):3367–3373. doi: 10.1200/JCO.2005.02.7771. [DOI] [PubMed] [Google Scholar]
- 36.Bellavance EC, Kesmodel SB. Decision-making in the surgical treatment of breast cancer: factors influencing women’s choices for mastectomy and breast conserving surgery. Front Oncol. 2016;6:74. doi: 10.3389/fonc.2016.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan H, Gray R, Braybrooke J, et al. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med. 2017;377(19):1836–1846. doi: 10.1056/NEJMoa1701830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sparano JA, Gray RJ, Makower DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379(2):111–121. doi: 10.1056/NEJMoa1804710. [DOI] [PMC free article] [PubMed] [Google Scholar]