The World Health Organization estimates that global vaccination programs save 2–3 million lives per year by priming the immune system to protect against pathogenic threats that pose significant global health and economic burdens1. Besides individual protection, vaccination programs also rely on population or “herd” immunity: the immunization of large portions of the population to protect the unvaccinated, immunocompromised, and immunologically naïve by reducing the number of susceptible hosts to a level below the threshold needed for transmission. For example, immunization of >80% of the global population against smallpox virus reduced transmission rates to uninfected individuals to a point low enough to achieve eradication of the virus2. Similarly, poliovirus is now targeted for eradication, with only Pakistan, Afghanistan, and Nigeria documenting endemic viral infections1.
Despite the success of select vaccination programs, societal and biological factors (Figure 1), including the inability of population groups to generate protective immunity in response to vaccination is a challenge for achieving herd immunity. The unvaccinated, including the very young, aged, and those who are immunocompromised due to infection, congenital conditions or medical history, must be protected by passive immunity. For example, unvaccinated females benefit from human papillomavirus (HPV) vaccination of their male partners3,4, as HPV strains 16 and 18 are linked to 70–80% of cases of cervical cancer4. While both males and females can be infected with HPV, females are more likely to experience detrimental oncogenic effects, including loss of reproductive capabilities. The vaccination of females and males in young populations with attenuated vaccines of the circulating HPV strains (6, 11, 16, and 18) has decreased transmission of these specific HPV viruses to young unvaccinated females in Scotland3. Thus, vaccination of the potential carriers within the population can promote disease prevention in the unvaccinated.
Figure 1.
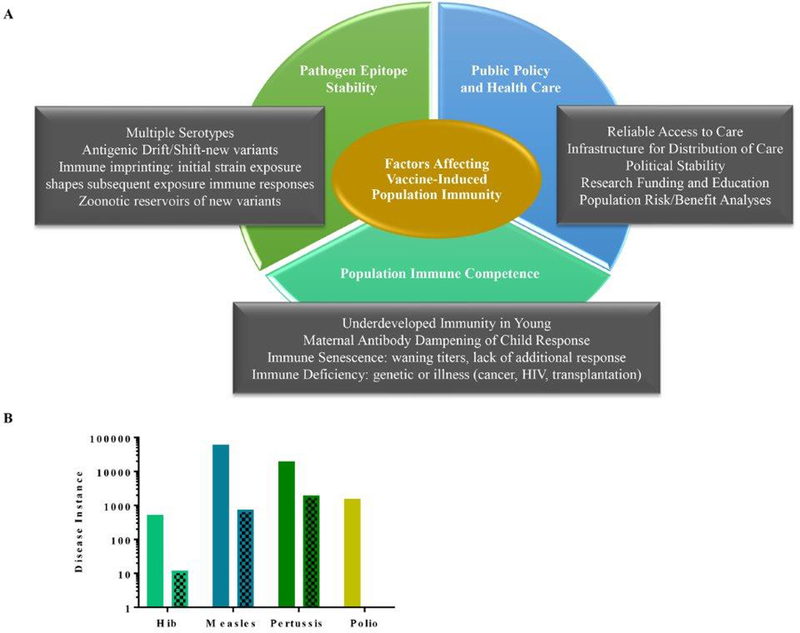
Biological and societal factors affecting vaccine-induced population immunity.
Immune senescence, immaturity, and imprinting limit human vaccine responses. These issues pose challenging obstacles to the development of vaccine-induced broadly protective long-lasting immunity. Immunosenescence refers to the progressive loss of responsiveness to a pathogen, resulting in decreased antibody titer or cellular responses, requiring booster vaccinations to restore immunity. In the late 20th century, measles was nearly eradicated in China, Korea and the United States as the result of early-age vaccination and boosters with attenuated virus5,6. However, re-introduction of the wild-type virus into these localities has caused spikes of infection in immunized middle-aged adults and has been linked to the wild-type measles virus’ capacity to overcome reduced antibody titers in these more aged populations5,6, indicating a prospective need for a tertiary booster in adults to bolster neutralization titers.
The breadth and length of duration of herd immunity are also dependent upon characteristics of the pathogen and its interactions with the immune system. Antigenic drift, antigenic shift, and recombination result in antigenic diversity within a pathogen pool, and a viral species can consist of multiple variants that are antigenically distinct from each other. The lack of a proof-reading function associated with most RNA polymerases makes RNA viruses particularly adept at generating strain diversity. For example, norovirus, the leading cause of acute gastroenteritis, has >30 genotypes. The GII.4 genotype evolves rapidly at surface epitopes that are key targets of protective immunity/neutralizing antibodies7. Despite maintaining 93.9% amino acid sequence homology between the earliest and most recent known pandemic strains, evolution at hypervariable epitopes correlates with pandemic bursts of disease approximately every 2–5 years. The immunocompromised, immunosenescent, and young children have been postulated to be norovirus reservoirs7. In these populations, a suppressed or naïve immune system may not fully neutralize every viral variant. Lower immune pressure on the virus, along with an error-prone RNA polymerase, allows for mutated variants to emerge and escape neutralization.
Pathogen-immune response interactions also shape Dengue and influenza virus immunity and subsequent vaccine design. Dengue circulates as four serotypes, (DENV1–4), which have roughly 70% sequence homology. Infection with one serotype induces production of both cross-reactive and serotype-specific neutralizing antibodies during initial infection8. Upon infection with a second serotype, the original memory immune response is preferentially stimulated. Due to antigenic differences between the primary and secondary viruses, pre-existing antibody is unable to effectively neutralize the second virus, allowing immune evasion through immune imprinting (original antigenic sin/antigenic seniority)8,9. Importantly, depending on the level and neutralization capacity of the cross-reactive pre-existing antibodies, a secondary infection can cause severe disease10. Similarly, the immune system preferentially recalls memory antibody responses to the earliest infecting strains of influenza A and norovirus and shows weaker consecutive responses to infection with subsequent serotypes, respectively 11,13. As the herd is composed of individuals from many different age groups, and pandemic Influenza and norovirus strains change over time, effective vaccine development that induces broad protection is highly complicated by age and pre-exposure history. Conserved epitopes are potential vaccine targets for these and other pathogens that are susceptible to antigenic drift/shift and recombination7. While progress toward a universal influenza vaccine is being made, protection currently relies on regular vaccination with multiple attenuated circulating strains to reduce transmission and mitigate disease severity11. Despite a global surveillance network, predicting future flu epidemic strains for vaccine production is high risk, as evidence by influenza vaccine-contemporary strain mismatches in both 2014–2015 and 2017–2018 14.
Norovirus, dengue, and influenza illustrate challenges in eliciting broad immunity through vaccination with a single serotype of attenuated virus. While vaccination would protect against the vaccinated serotype, it has the potential to increase the severity of a secondary infection by inducing a dampened response to later variants based on antigenic variation, imprinting, and timing between inoculation and secondary infection and/or immune enhancement which occurs in Dengue virus secondary infections. Research focusing on protective immunity correlates, particularly cross-reactive neutralizing antibody titers, and chimeric serotype attenuated-viruses to elicit broad immunity is underway7,8. Understanding and optimizing these factors may induce broad immunity across populations.
As vaccines that can overcome the challenges of poor immune responsiveness in hosts and antigenic diversity in pathogens are developed, public policy plays a critical role in achieving the high population vaccination rate needed to achieve herd immunity and freedom from disease. Universal access to affordable health care is of the highest priority, as individuals of lower socioeconomic status often forgo vaccinations due to accessibility reasons12, which can leave large pockets of non-vaccinated, susceptible people who not only may suffer disease themselves but can also facilitate transmission to others who are unable to get immunized, thus breaking the protective barrier herd immunity provides. The observed spike in incidents of pertussis and measles in children and adults in the United States15, indicates a need for effective public education to counter anti-vaccination proponents whose fear of vaccine side-effects form pockets of susceptibility and opportunity for spread of pathogens among those who are immunocompromised or unable to receive a vaccine due to age requirements. The more accessible we make vaccination through education programs, minute clinics, job-site resources, and lowered costs, the more effective vaccination programs will be12. To reach the goal of protection from disease and eradication of pathogens through vaccination, governments, scientists, and citizens will have to commit resources equivalent to the diversity and abundance of the pathogens we fight.
Acknowledgements:
This work was supported by grants from the National Institutes of Health, Allergy and Infectious Diseases (R56AI106006 and U19 AI109761 CETR) and the Wellcome Trust [203268/Z/16/Z].
References
- 1.“Immunization Coverage.” World Health Organization: Media Centre, World Health Organization, July 2017, http://www.who.int/mediacentre/factsheets/fs378/en/. [Google Scholar]
- 2.Brilliant LB, and Hodakevič LN. “Certification of Smallpox Eradication.” Bulletin of the World Health Organization 565 (1978): 723–733. Print. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2395660/ [PMC free article] [PubMed] [Google Scholar]
- 3.Cameron RL, Kavanagh K, Pan J, et al. Human Papillomavirus Prevalence and Herd Immunity after Introduction of Vaccination Program, Scotland, 2009–2013. Emerging Infectious Diseases 2016;22(1):56–64. 10.3201/eid2201.150736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Machalek Dorothy A., et al. for the IMPACT and IMPRESS Study Groups; Human Papillomavirus Prevalence in Unvaccinated Heterosexual Men After a National Female Vaccination Program, The Journal of Infectious Diseases, Volume 215, Issue 2, 15 January 2017, Pages 202–208, 10.1093/infdis/jiw530 [DOI] [PubMed] [Google Scholar]
- 5.He Hanqing et al. “Waning Immunity to Measles in Young Adults and Booster Effects of Revaccination in Secondary School Students.” Amsterdam, Netherlands: Elsevier, 313 (2013): 533–537. Web. 14 Nov. 2017. 10.1016/j.vaccine.2012.11.014 [DOI] [PubMed] [Google Scholar]
- 6.Kang Hae Ji et al. “An Increasing, Potentially Measles-Susceptible Population over Time after Vaccination in Korea.” Amsterdam, Netherlands: Elsevier 3533 (2017): 4126–4132. Web. 14 Nov. 2017. 10.1016/j.vaccine.2017.06.058 [DOI] [PubMed] [Google Scholar]
- 7.Debbink Kari et al. “Within-Host Evolution Results in Antigenically Distinct GII.4 Noroviruses.” Ed. Dermody TS. Journal of Virology 8813 (2014): 7244–7255. PMC. Web. 17 Nov. 2017. 10.1128/JVI.00203-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katzelnick Leah C. et al. “Neutralizing Antibody Titers against Dengue Virus Correlate with Protection from Symptomatic Infection in a Longitudinal Cohort.” Proceedings of the National Academy of Sciences of the United States of America 1133 (2016): 728–733. PMC. Web. 14 Nov. 10.1073/pnas.1522136113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Midgley Claire M. et al. “An In-Depth Analysis of Original Antigenic Sin in Dengue Virus Infection.” Journal of Virology 851 (2011): 410–421. PMC. Web. 14 Nov. 2017. 10.1128/JVI.01826-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Alwis Ruklanthi, et al. “Dengue Viruses Are Enhanced by Distinct Populations of Serotype Cross-Reactive Antibodies in Human Immune Sera.” Ed. Gack Michaela U.. PLoS Pathogens 1010 (2014): e1004386 PMC. Web. 17 Nov. 2017. 10.1371/journal.ppat.1004386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lessler Justin et al. “Evidence for Antigenic Seniority in Influenza A (H3N2) Antibody Responses in Southern China.” Ed. Basler Christopher F.. PLoS Pathogens 87 (2012): e1002802 PMC. Web. 11 Dec. 2017. 10.1371/journal.ppat.1002802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer Michael (2017) “The Ethics of Universal Health Care In The United States,” Reflections on Healthcare Management: Vol. 1: Iss. 1, 10.6083/M4SN082Q [DOI] [Google Scholar]
- 13.Lindesmith Lisa C. et al. “Broad Blockade Antibody Responses in Human Volunteers after Immunization with a Multivalent Norovirus VLP Candidate Vaccine: Immunological Analyses from a Phase I Clinical Trial.” Ed. Lopman Benjamin. PLoS Medicine 123 (2015): e1001807 PMC. Web. 19 Dec. 2017. 10.1371/journal.pmed.1001807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Nicholas C. et al. “A Structural Explanation for the Low Effectiveness of the Seasonal Influenza H3N2 Vaccine.” Ed. Palese Peter. PLoS Pathogens 1310 (2017): e1006682 PMC. Web. 8 Jan. 2018. 10.1371/journal.ppat.1006682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phadke Varun K. et al. “Association Between Vaccine Refusal and Vaccine-Preventable Diseases in the United States: A Review of Measles and Pertussis.” JAMA 31511 (2016): 1149–1158. PMC. Web. 8 Jan. 2018. 10.1001/jama.2016.1353 [DOI] [PMC free article] [PubMed] [Google Scholar]


