Abstract
Objective:
Although the incidence of lung cancer has decreased over the past decades, disparities in survival and treatment modalities have been observed for black and white patients with early stage non-small cell lung cancer (NSCLC), despite the fact that surgical resection has been established as the standard of care. Possible contributors to these disparities are stage at diagnosis, comorbidities, socio-economic factors, and patient preference. This study examines racial disparities in treatment, adjusting for clinicodemographic factors.
Methods:
The Surveillance, Epidemiology, and End Results (SEER)-Medicare dataset was queried to identify patients diagnosed with primary stage I NSCLC between 1992–2009. Multivariable logistic regressions were performed to assess the association between race and treatment modalities within 1 year of diagnosis, adjusted for clinical and demographic factors. Adjusted Cox proportional hazards models were performed to evaluate disparities in survival, accounting for mode of treatment.
Results:
We identified 22,724 patients; 21,230 (93.4%) white and 1,494 (6.6%) black. Black patients were less likely to receive treatment (Odds Ratio (OR)adj: 0.62, 95% Confidence Interval (CI): 0.53–0.73) and less likely to receive surgery only when treated (ORadj: 0.70, 95% CI: 0.61– 0.79). Although univariate survival for black patients was worse, when accounting for treatment mode, there was no difference in survival (Hazard Ratio (HR)adj: 0.97, 95% CI: 0.90–1.04 for all patients, HRadj: 0.98, 95% CI: 0.90–1.06 for treated patients).
Conclusions:
Treatment disparities persist, even when adjusting for clinical and demographic factors. However, when black patients receive similar treatment, survival is comparable to white patients.
Introduction
While the incidence of lung cancer has decreased over the past decades, disparities in survival and treatment have persisted amongst black and white patients with early-stage non-small cell lung cancer (NSCLC) with little change over this time period.1,2 These disparities have been attributed to a number of factors that may differ between Blacks and Whites, including stage at diagnosis, socioeconomic and/or insurance status, access to care, patient preference, and rates of surgical treatment.3–8
Advances in the management of early-stage NSCLC have established surgical resection, as the standard of care for operable patients.9,10 Additionally, mediastinal lymph node staging has been associated with improved survival, and studies have demonstrated that the number of lymph nodes examined is positively associated with more accurate lung cancer staging.11,12 Studies have shown that black patients were less likely to receive surgical treatment and had shorter overall survival for early-stage NSCLC compared to white patients.8,13 When analyzing survival rates for the subset of patients treated with surgery, survival rates did not differ by race.8 Black patients undergoing surgical resection were less likely to undergo lymph node examination and had fewer lymph nodes examined compared to Whites.12,14 No study to date has disentangled the effects of race, socioeconomic indicators, insurance, and clinical characteristics on treatment modalities in early stage lung cancer.
We evaluated racial differences in treatment and survival of early-stage NSCLC for patients in the Surveillance, Epidemiology and End Results (SEER)-Medicare database, looking at trends stratified by treatment modality and era. The primary aims of this analysis were to identify racial disparities in treatment and to determine if disparities in survival persisted when adjusting for confounding by treatment. Secondary aims were to examine racial disparities in node examination and reasons for not receiving surgery.
Materials and Methods
Data/Study population
The SEER-Medicare registry was queried for all patients diagnosed between 1992 and 2009. SEER encompasses 28% of the United States population, with an estimated 93% of the registry linked to Medicare claims.15–17 Medicare insures 97% of patients over 65 years old. Demographics (age at diagnosis, gender, race, marital status) and cancer characteristics (histology, tumor size, and date of diagnosis) were obtained from SEER. Medicare Provider Analysis and Review (MedPAR), outpatient, and physician claims [National Claims History (NCH)] files were used to define cofactors not available in SEER: Charlson comorbidity score, chemotherapy, extent of surgical resection, and pathologic lymph node evaluation. The dataset is de-identified and therefore the study was exempt from Institutional Review Board approval.
All black and white patients age 65 years and older whose first or only primary cancer was stage I pathologically-confirmed NSCLC were identified (n=41,431). Patients with missing date of diagnosis and those with a second primary cancer diagnosed within one year of the primary NSCLC diagnosis were excluded. Claims for comorbidity identification were available from 1991 and in order to allow for adequate follow-up after diagnosis, only patients diagnosed between 1992 to 2009 were included (n=36,740). Patients continuously enrolled in Medicare Part A and Part B and not a health maintenance organization for one year prior and one year following diagnosis (or until death) were included (n=25,280). Patients missing key covariates (zip code level socioeconomic data, marital status, comorbidities) were excluded as these were few in number and the proportions only differed between races for marital status (14% vs. 9%) and comorbidity score (4% vs. 3%). This resulted in a cohort of 22,724 patients (Online Supplemental Figure S1). The number of patients of other races (including non-Black Hispanics, Asians, and others) was 6.6% of the overall sample and was dispersed among many treatment approaches, limiting the ability to draw meaningful conclusions, and thus the study was limited to the two largest race cohorts, black and white patients.
Covariates
In addition to basic demographics, median income for the zip code of residence and percent of adults ≥ 25 years with less than a high school diploma in the zip code of residence were extracted from SEER as a proxy of socioeconomic status, as SEER-Medicare does not contain individual level socioeconomic data or exact treatment location. Stage was defined using the 3rd (1992–2003) and 6th (2004+) edition American Joint Committee on Cancer systems. Histology was classified according to the International Agency for Research on Cancer.18 All variables were measured at the time of diagnosis. As SEER includes only month and year of diagnosis, the date was recorded as the 1st of the month designated for each patient, to be able to capture all treatments that occurred in the month of diagnosis. All Medicare claims from the 12 months prior to the month of diagnosis were cross-referenced to calculate a Charlson comorbidity score for each patient (NCI: Charlson Comorbidity macro, 2014 version).19
Outcomes
The primary outcomes were receipt of treatment and mortality. Receipt of treatment included cancer-directed surgery, radiation therapy (RT), and chemotherapy. RT and chemotherapy were identified using published algorithms20,21 based on a combination of International Classification of Diseases, Ninth Revision, Clinical Modification(ICD-9-CM) diagnosis and procedure codes, Healthcare Common Procedure Coding System (HCPCS) /Current Procedural Terminology (CPT) codes, and revenue center codes. Cancer-directed surgery was identified using the ICD-9-CM procedure codes and CPT codes associated with lobectomy, sublobar resection, and pneumonectomy, supplemented by a published list of CPT codes associated with surgical resection22 (Online Supplemental Table S1).
Overall survival was analyzed as lung cancer-specific survival was only available through SEER and follow-up was shorter for that dataset. Overall 5-year survival was calculated from date of diagnosis to date of death or last follow-up, whichever came first. The dataset was granted in 2013 with diagnoses complete through 2009, Medicare claims complete through 2010, and Medicare date of death complete through 12/31/2011.
Secondary outcomes were type of treatment and, among those who received surgery, lymph node examination and number of lymph nodes examined.23 Recommendation of surgery and reasons for refusal of surgery were also analyzed. For patients who received surgery, the time until surgery was calculated as the number of days from the diagnosis until the date of admission to the hospital for surgery.
Statistical Analysis
Baseline characteristics were compared using χ2 tests for categorical variables and t-tests for continuous variables for the overall cohort and for the subset of patients who received treatment.
Multivariable logistic regressions were used to assess for associations between race and treatment. As the socioeconomic variables were measured at zip code level, they were included in the multivariable analyses but individual effects of these variables were not reported. The analysis was conducted for overall treatment (yes/no), and, among those treated, each treatment mode (yes/no): surgery only, RT only, chemotherapy only, and more than one treatment.
A single multinomial logistic regression was conducted among those who received treatment to assess the odds of receiving RT only, chemotherapy only, or more than one treatment, as compared to receiving surgery only, while adjusting for all other predictors. This analysis was stratified according to comorbidity score (0, 1, 2, ≥3).
Univariate analyses and multivariable logistic regressions were used to assess the odds of the following (yes/no) in black vs. white patients: lymph node examination among those who underwent surgery; ≥15 lymph nodes examined among those with lymph node examination; surgery recommendation among all patients; and surgery refusal among those recommended surgery. The possibility of an interaction between race and comorbidity score was explored by two methods: 1) conducting the multivariable analysis with and without inclusion of an interaction term for race and comorbidity score (race*comorbidity) for independent association with overall treatment and for surgery alone and 2) stratifying the analyses by comorbidity score.
Overall 5-year survival of black and white patients was assessed using the Kaplan-Meier method, and Cox proportional hazards models were created to assess the independent association between race and survival, adjusted for treatment and other clinical and demographic factors. For variables that violated the proportional hazards assumption, an interaction with log of survival time was included in the model.
All multivariable models included the following covariates: gender, age at diagnosis, marital status, comorbidity score, tumor histology, tumor size, zip code level income and education, and year of diagnosis, as these were considered clinically relevant and were significantly associated (p<0.05) with race or receipt of treatment. Models for all patients who underwent surgery also included type of surgery as a covariate. Statistical analysis was conducted using SAS software, version 9.4 (SAS Institute, Cary, NC).
Results
There were 21,230 (93.4%) white and 1,494 (6.6%) black patients. The median follow-up time was 34 months (range 0.1 – 60). On univariate analysis, black patients were younger, less often married, had higher comorbidity score, lower median zip-code level income, higher zip-code level percentage without high school diploma, and were more likely to have squamous cell carcinoma and larger tumor size (all p <.0001, Table 1). Similar differences were observed when the dataset was restricted to 20,555 patients (19,306 Whites, 1,249 Blacks) who received treatment (Table 2).
Table 1-.
Description of the population under study
| Variable | White (n=21230) | Black (n=1494) | p-valueǂ | |||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Treatment | No | 1924 | 9.06 | 245 | 16.40 | <.0001 |
| Yes | 19306 | 90.94 | 1249 | 83.60 | ||
| Gender | Male | 10494 | 49.43 | 728 | 48.73 | 0.60 |
| Female | 10736 | 50.57 | 766 | 51.27 | ||
| Age (years) | 65–69 | 4582 | 21.58 | 451 | 30.19 | <.0001 |
| 70–74 | 6258 | 29.48 | 469 | 31.39 | ||
| 75–79 | 5655 | 26.64 | 313 | 20.95 | ||
| 80–84 | 3308 | 15.58 | 191 | 12.78 | ||
| ≥85 | 1427 | 6.72 | 70 | 4.69 | ||
| Marital Status | Single | 1178 | 5.55 | 243 | 16.27 | <.0001 |
| Married | 12174 | 57.34 | 557 | 37.28 | ||
| Prior Marriage* | 7878 | 37.11 | 694 | 46.45 | ||
| Charlson Score | 0 | 8359 | 39.37 | 477 | 31.93 | <.0001 |
| 1 | 6739 | 31.74 | 433 | 28.98 | ||
| 2 | 3232 | 15.22 | 245 | 16.40 | ||
| ≥3 | 2900 | 13.66 | 339 | 22.69 | ||
| Histology | Squamous | 7097 | 33.43 | 592 | 39.63 | <.0001 |
| Adeno | 10367 | 48.83 | 634 | 42.44 | ||
| Large cell | 1497 | 7.05 | 101 | 6.76 | ||
| Other | 2269 | 10.69 | 167 | 11.18 | ||
| Tumor Size (mm) | 0–20 | 6915 | 32.57 | 442 | 29.59 | <.0001 |
| 21–30 | 6162 | 29.03 | 387 | 25.90 | ||
| 31–50 | 5712 | 26.91 | 441 | 29.52 | ||
| 51–70 | 1683 | 7.93 | 148 | 9.91 | ||
| ≥71 | 758 | 3.57 | 76 | 5.09 | ||
| Mean | Standard Error | Mean | Standard Error | |||
| Median Income ($)^ | 49552.6 | 135.1 | 34980.9 | 374.6 | <.0001 | |
| % less than High School^ | 17.8 | 0.07 | 28.4 | 0.31 | <.0001 | |
Divorced, Separated, Widowed
ZIP code level
χ2test for categorical variables, t-test for continuous variables
Table 2-.
Characteristics of patients who received treatment
| Variable | White (n=19,306) | Black (n=1,249) | p-valueǂ | |||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Radiation Therapy only | No | 17050 | 88.3 | 1030 | 82.5 | <.0001 |
| Yes | 2256 | 11.7 | 219 | 17.5 | ||
| Chemotherapy Only | No | 19055 | 98.7 | 1203 | 96.3 | <.0001 |
| Yes | 251 | 1.3 | 46 | 3.7 | ||
| Surgery Only | No | 6299 | 32.6 | 550 | 44.0 | <.0001 |
| Yes | 13007 | 67.4 | 699 | 56.0 | ||
| >1 Treatment | No | 15514 | 80.4 | 964 | 77.2 | 0.0064 |
| Yes | 3792 | 19.6 | 285 | 22.8 | ||
| Gender | Male | 9414 | 48.7 | 573 | 45.9 | 0.048 |
| Female | 9892 | 51.3 | 676 | 54.1 | ||
| Age (years) | 65–69 | 4262 | 22.1 | 404 | 32.4 | <.0001 |
| 70–74 | 5871 | 30.4 | 398 | 31.9 | ||
| 75–79 | 5174 | 26.8 | 263 | 21.1 | ||
| 80–84 | 2892 | 15.0 | 143 | 11.5 | ||
| ≥85 | 1107 | 5.7 | 41 | 3.3 | ||
| Marital Status | Single | 1037 | 5.4 | 204 | 16.3 | <.0001 |
| Married | 11289 | 58.4 | 478 | 38.3 | ||
| Prior Marriage* | 6980 | 36.2 | 567 | 45.4 | ||
| Charlson Score | 0 | 7609 | 39.4 | 394 | 31.6 | <.0001 |
| 1 | 6218 | 32.2 | 378 | 30.3 | ||
| 2 | 2910 | 15.1 | 196 | 15.7 | ||
| ≥3 | 2569 | 13.3 | 281 | 22.5 | ||
| Histology | Squamous | 6420 | 33.3 | 493 | 39.5 | <.0001 |
| Adeno | 9659 | 50.0 | 542 | 43.4 | ||
| Large cell | 1286 | 6.7 | 84 | 6.7 | ||
| Other | 1941 | 10.1 | 130 | 10.4 | ||
| Tumor size(mm) | 0–20 | 6408 | 33.2 | 386 | 30.9 | 0.0016 |
| 21–30 | 5616 | 29.1 | 329 | 26.3 | ||
| 31–50 | 5153 | 26.7 | 362 | 29.0 | ||
| 51–70 | 1469 | 7.6 | 110 | 8.8 | ||
| ≥71 | 660 | 3.4 | 62 | 5.0 | ||
| Mean | Standard Error | Mean | Standard Error | |||
| Median Income($)^ | 49886.4 | 143 | 35266.7 | 410.3 | <.0001 | |
| % less than high school^ | 17.6 | 0.1 | 28.3 | 0.3 | <.0001 | |
Divorced, Separated, Widowed;
ZIP code level;
χ2test for categorical variables, t-test for continuous
On multivariable analysis, black patients were significantly less likely to receive any treatment (ORadj: 0.62, 95% CI: 0.53–0.73, p-value<.0001) (Figure 1a). Other factors independently inversely associated with treatment were age (Odds Ratio (OR)adj:0.79, 95% Confidence Interval (CI): 0.68–0.91, ORadj:0.53, 95% CI: 0.46–0.61, ORadj:0.28, 95% CI: 0.24– 0.33 for patients aged 75–79, 80–84 and ≥85 years, compared to 65–69 years), marital status (ORadj: 0.59, 95% CI: 0.53–0.65 for previously married, ORadj: 0.61, 95% CI: 0.51–0.72 for single, compared to married), non-adenocarcinoma histology (ORadj:0.8, 95% CI: 0.72– 0.89 for squamous cell, ORadj:0.48, 95% CI: 0.41–0.57 for large cell and ORadj:0.57, 95% CI: 0.49–0.65 for other, compared to adenocarcinoma) and tumor size (ORadj :0.67, 95% CI; 0.57–0.79, ORadj :0.67, 95% CI: 0.53–0.84 for tumors 51–70 and 70+ mm versus tumors 0–20 mm) (Online Supplemental Table S2).
Figure 1:
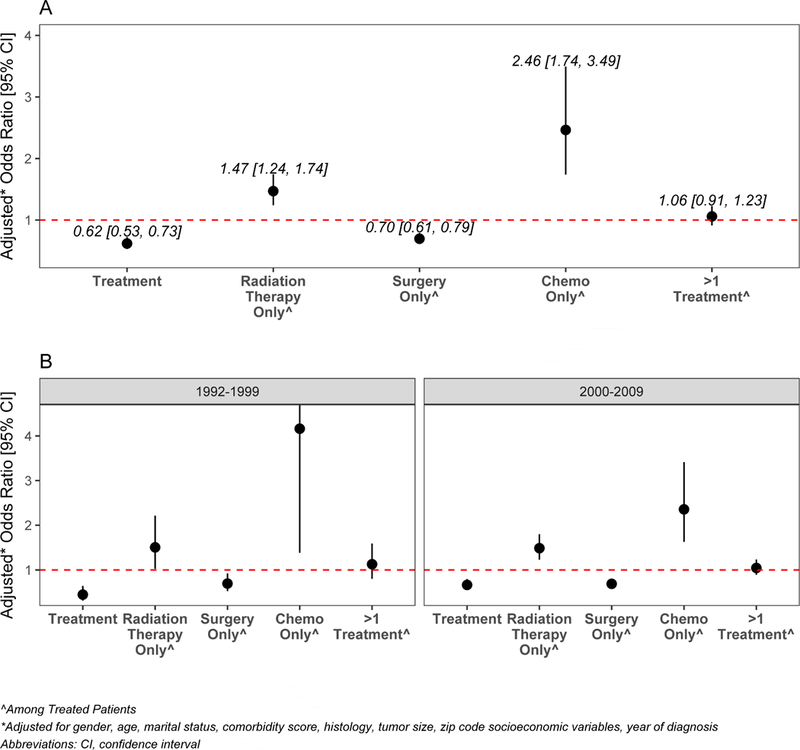
Adjusted odds of treatment in black vs. white patients by treatment category (a) overall and (b) stratified by year of diagnosis (1992–1999 and 200–2009). For individual treatment categories, patients who did not undergo any treatment were excluded.
In order to evaluate for potential non-measured confounders, a subset analysis was conducted comparing patients who were treated to those who were not, stratified by race. For both races, male gender, older age, and unmarried status were associated with not being treated. For white patients, additional cofactors associated with no treatment were higher comorbidity score, non-adenocarcinoma histology, increased tumor size, and both zip code socioeconomic variables. The race*comorbidity score interaction term was not significant in either multivariable model (p = 0.3954 and p = 0.3392, respectively), nor was it significant in the multinomial logistic regression model for all treatments (p = 0.1567). When stratified by comorbidity score, trends remained similar across scores.
Type of treatment and race:
On multivariable analysis of those who were treated, black patients were less likely to receive surgery only (ORadj:0.70, 95% CI: 0.61–0.79, p<.0001), more likely to receive RT only (ORadj: 1.47, 95% CI: 1.24–1.74, p<.0001) or chemotherapy only (ORadj: 2.46, 95%CI: 1.74–3.49, p<.0001). There was no racial difference in the odds of receiving a combination of treatments (ORadj for Blacks: 1.06, 95% CI: 0.91, 1.23, p=0.4482) (Figure 1a, Table 3, Online Supplemental Tables S3–S5). Similar direction of the associations was observed when the data were stratified according to year of diagnosis (Figure 1b) or according to number of comorbidities (data not shown). Among patients who received surgery or had documentation it was recommended (n=22,658), black patients were significantly less often offered surgery (ORadj:0.75, 95% CI: 0.65–0.86). Other factors independently associated with not being recommended surgery on multivariable analysis were older age, non-married status, higher comorbidity score, non-adenocarcinoma histology, and larger tumor size. Among patients who were offered surgery (n=18,313), black patients were significantly more likely than white patients to refuse surgery (ORadj:1.98, 95% CI: 1.40–2.81). Other factors independently associated with refusal of surgery on multivariable analysis were older age, non-married status, higher comorbidity score, non-adenocarcinoma histology, and larger tumor size. Among those who received surgery, there was no difference in the median time until surgery on univariate analysis (36 days in Whites vs. 37 days in Blacks, p = 0.3244).
Table 3-.
Univariate and multivariable odds of receipt of surgery alone (Yes vs. No), among treated patients
| Variable | Univariate | Multivariable | ||
|---|---|---|---|---|
| OR | 95% CI | OR* | 95% CI | |
| Race | ||||
| White | 1.00 | Ref | 1.00 | Ref |
| Black | 0.62 | 0.55–0.69 | 0.70 | 0.61–0.79 |
| Gender | ||||
| Male | 1.00 | Ref | 1.00 | Ref |
| Female | 1.12 | 1.06–1.19 | 0.98 | 0.92–1.05 |
| Age (years) | ||||
| 65–69 | 1.00 | Ref | 1.00 | Ref |
| 70–74 | 0.97 | 0.89–1.05 | 0.98 | 0.90–1.07 |
| 75–79 | 0.97 | 0.89–1.05 | 1.02 | 0.93–1.12 |
| 80–84 | 0.75 | 0.68–0.82 | 0.82 | 0.74–0.91 |
| ≥85 | 0.40 | 0.35–0.46 | 0.45 | 0.39–0.52 |
| Marital Status | ||||
| Married | 1.00 | Ref | 1.00 | Ref |
| Single | 0.85 | 0.75–0.96 | 0.93 | 0.82–1.07 |
| Prior Marriage^ | 0.87 | 0.82–0.93 | 0.95 | 0.88–1.02 |
| Charlson Score | ||||
| 0 | 1.00 | Ref | 1.00 | Ref |
| 1 | 0.73 | 0.68–0.79 | 0.74 | 0.68–0.80 |
| 2 | 0.66 | 0.61–0.72 | 0.71 | 0.65–0.78 |
| ≥3 | 0.54 | 0.49–0.59 | 0.60 | 0.54–0.66 |
| Histology | ||||
| Adenocarcinoma | 1.00 | Ref | 1.00 | Ref |
| Squamous | 0.59 | 0.55–0.63 | 0.72 | 0.67–0.77 |
| Large cell | 0.40 | 0.35–0.45 | 0.42 | 0.37–0.47 |
| Other | 0.29 | 0.26–0.32 | 0.36 | 0.33–0.40 |
| Tumor Size (mm) | ||||
| 0–20 | 1.00 | Ref | 1.00 | Ref |
| 21–30 | 0.59 | 0.55–0.64 | 0.60 | 0.55–0.65 |
| 31–50 | 0.36 | 0.33–0.39 | 0.37 | 0.34–0.41 |
| 51–70 | 0.24 | 0.22–0.27 | 0.24 | 0.22–0.27 |
| ≥71 | 0.21 | 0.18–0.25 | 0.20 | 0.17–0.24 |
Adjusted for all listed variables, zip code level socioeconomic status variables, and year of diagnosis
Divorced, separated, or widowed
n=20,555
Abbreviations: OR, odds ratio; CI, confidence interval
In patients who were treated with surgery and had known status regarding node examination (n=16,629), there was no association between race and pathologic node examination (yes/no) (ORadj:1.12, 95% CI: 0.91–1.39) in Blacks vs. Whites on multivariable analysis. Among patients who underwent surgery, black patients were more likely than Whites to undergo sublobar resection and there was a significant independent association between receiving a sublobar resection and lack of node examination, compared to lobectomy (ORadj 0.11, 95% CI: 0.10, 0.13). Other factors significantly independently inversely associated with node examination on multivariable analysis were increased age, non-married status, higher comorbidity score, large cell or other histology; larger tumor size was positively associated with node examination.
In those with node examination (n=14,161), there was a significant independent association between race and more nodes examined (ORadj: 0.81, 95% CI: 0.68–0.98 for ≥15 versus <15 nodes examined) in black vs. white patients. There was a significant independent association between sublobar resection (vs. lobectomy) and fewer number of nodes examined (ORadj:0.76, 95% CI: 0.68–0.84 for ≥15 nodes examined). Non-married status and higher comorbidity score were independently inversely associated, while larger tumor size was independently positively associated with more nodes examined.
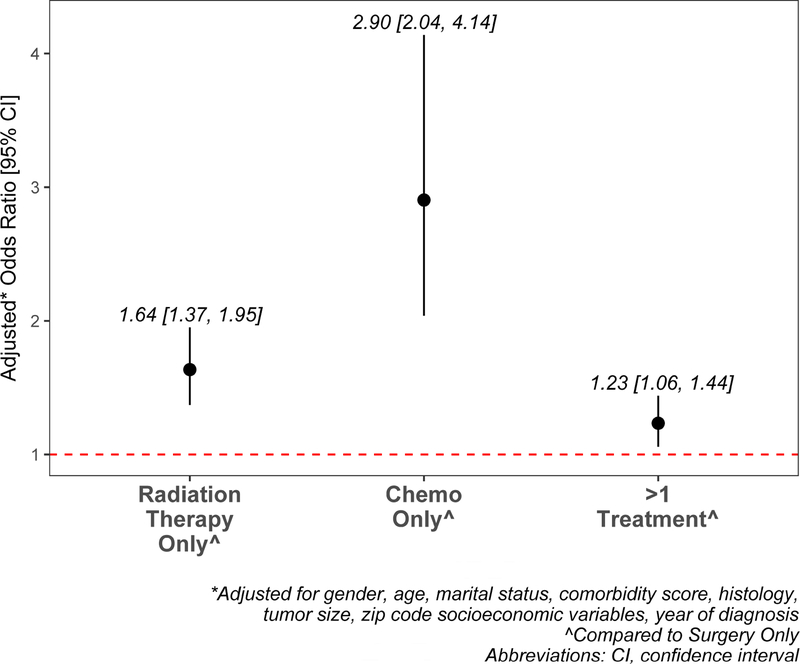
On multivariable analysis, Black race was associated with higher odds of being treated with RT alone (p<.0001), chemotherapy alone (p<.0001), or a combination of treatments (p=0.0077), compared to surgery alone (Figure 2). Other factors independently associated with receipt of RT alone on multivariable analysis were female gender, older age, non-married status, higher comorbidity score, non-adenocarcinoma histology, and larger tumor size. Factors independently associated with receipt of chemotherapy alone were older age, non-married status, higher comorbidity score, non-adenocarcinoma histology, and larger tumor size. Factors independently associated with receipt of more than 1 treatment were younger age, higher comorbidity score, larger tumor size, and non-adenocarcinoma histology (Online Supplemental Table S6).
Figure 2:
Adjusted relative odds of treatment, compared to surgery only in black vs. white patients.
Survival:
Univariate overall 5-year survival was lower for black than for white patients (Figure 3). When stratified by treatment type, however, those differences were not significant (Figure 4a–4d). When adjusted for confounding and accounting for mode of treatment, there was no significant association between race and overall survival; the Hazard Ratio (HR)adj for black patients was 0.97 (95% CI: 0.90–1.04) and HRadj 0.98 (95% CI: 0.90–1.06) among patients who received treatment. When multivariable models were stratified by treatment mode, there was no significant difference in survival for black and white patients (Online Supplemental Table S7). The analysis was repeated using lung-cancer specific survival and results were similar (data not shown).
Figure 3:
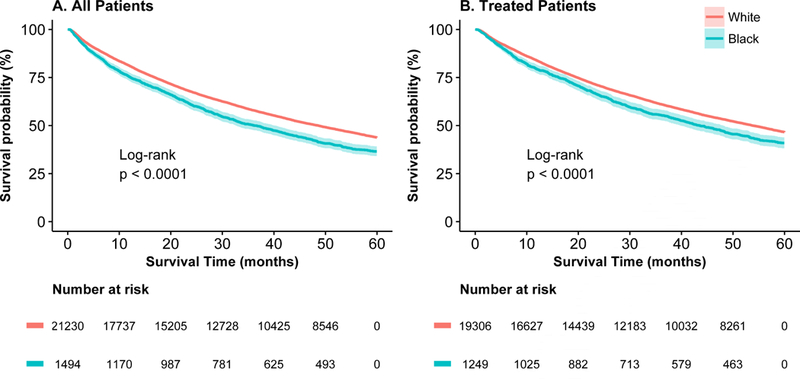
Five-year survival for black (blue line) and white (red line) patients for (a) all patients and (b) treated patients.
Figure 4:
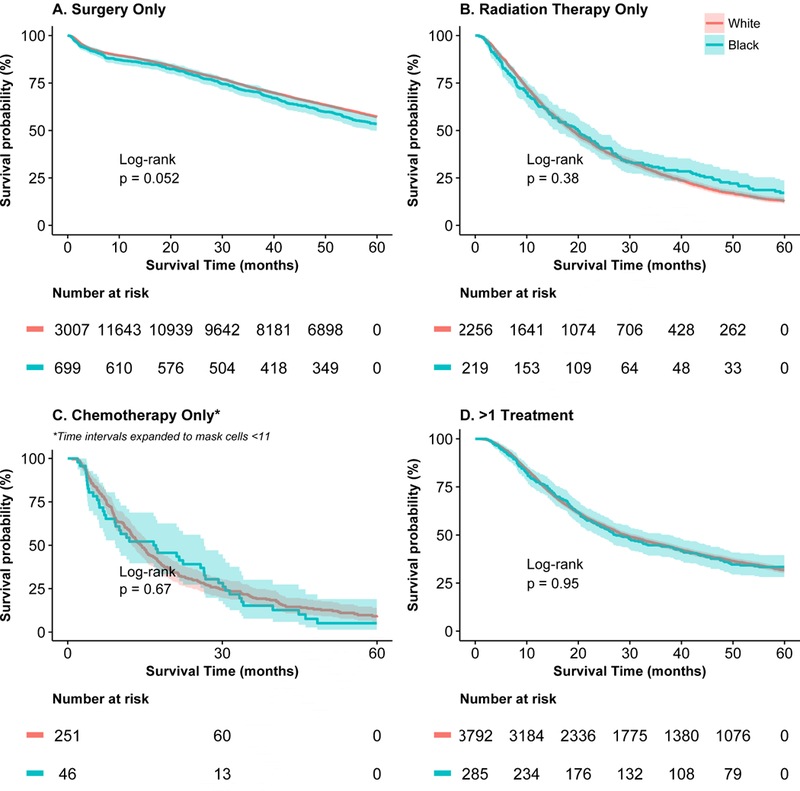
Five-year survival for black (blue line) and white (red line) patients for (a) patients undergoing surgery only, (b) radiation therapy only, (c) chemotherapy only, and (d)>1 treatment. For (c), time intervals were expanded to mask cells with <11 events.
Discussion
This study evaluated highlighted the racial disparities in the treatment of stage I NSCLC in a large series of patients: black patients with stage I NSCLC were less likely to receive surgery and more likely to be treated with RT and/or chemotherapy, frequently when the latter has not been considered standard of care. These differences were seen on univariate analysis and after adjustment for confounders that may have affected the choice of treatment, such as comorbidities and age. The SEER-Medicare dataset did not include details as to why individual patients received specific treatments (or no treatment) and the reason for receiving chemotherapy and/or radiation may have been mis-coding as stage I, adjuvant chemotherapy for large tumors, or receipt of non-standard treatment, but these are all factors that should not be related to race. Despite publication of a landmark paper highlighting racial disparities in 1999,8 no progress has occurred to improve the rate of appropriate therapy for black patients, as shown in Figure 1. Similar results were demonstrated in a previous study by our group24 and subsequently by others25,26 using the SEER registry and the Veterans Affairs (VA) database.27–29 More detailed comorbidity information in the SEER-Medicare database enabled the current study to conduct a more comprehensive adjustment for confounding, thus limiting selection bias due to comorbidities that might contraindicate surgery.
The persistent lower rate of delivery of standard of care to black lung cancer patients may result from hospital and patient-level factors, such as vulnerable socioeconomic status, lack of access to experienced surgeons/centers, misconceptions about surgery, and/or lack of adequate insurance coverage.30 A large study13 of 473,722 cancer patients from the SEER database showed that those with Medicaid coverage or without insurance were more likely to present with advanced disease, were less likely to receive cancer-directed surgery and/or RT, and had lower survival. A systematic review of insurance status and lung cancer outcome5 reported that patients with Medicaid or no insurance had worse outcomes; were diagnosed at a later stage; and were less likely to undergo treatment with surgery or RT, to be treated at a high-volume center, and/or to receive guideline-concordant care than those with private insurance. While these findings were important, neither study evaluated the role of race, interaction with insurance status, and the independent effect of race on outcomes. Our analysis independently evaluated patients covered by Medicare and, similar to the VA data,27–29 included patients covered by a single insurer, thus attenuating the contribution of type of insurance coverage to the disparities in treatment observed. Still, other financial factors such as copayment, ability to take off of work, and other non-covered cancer care costs may have contributed to treatment disparities.30
It is possible that racial disparities in appropriate treatment were affected by disparities in patient-surgeon interaction. In our analysis, black patients were less likely to be offered surgery, and among those who were recommended surgery, there was a higher rate of patient refusal. Tohme and coauthors reported similar results for early-stage pancreatic cancer using the National Cancer Data Base, finding an independent association between African American race and refusal of surgery on multivariable analysis.31 The current study corroborated a SEER analysis by Mehta et al. evaluating Stage I and II NSCLC patients diagnosed between 1988 and 2002, in which Black race was associated with higher odds of refusing surgery, adjusted for confounders.32 Further exploration of barriers to appropriate treatment in black lung cancer patients is needed.
The current study also noted racial differences in surgery and lymph node evaluation. Black patients were more likely than Whites to undergo sublobar resection instead of lobectomy, as previously reported by our group.33 Sublobar resection was associated with lower rates of nodal examination in general and, among those with nodes examined, fewer nodes evaluated. More importantly, Black race was independently associated with lower likelihood of having ≥ 15 nodes sampled, even when adjusted for extent of surgery. Osarogiagbon’s analysis of the SEER dataset found similar racial disparities.34 As lymph node staging and number of nodes examined may have impact on prognosis and survival,34,35 these differences may result in racial disparities in survival, although no such survival differences were found in our study for patients treated surgically. In the current study, among patients who underwent surgery, there was no significant difference in rate of lymph node staging related to race, similar to the conclusion of a retrospective analysis of NSCLC resections in the SEER database from 1998 to 2009.35
When long-term survival was assessed, there were no differences between black and white patients, as treatment was included in the adjusted models. This underscores the importance of providing appropriate care for all patients. When black patients with stage I NSCLC were treated with surgery (standard of care for operative candidates), survival was similar for both races. Other studies25 have had different results, but relied on SEER without linkage to Medicare, and analysis may have been confounded by insurance status and presence of comorbidities.
While the strengths of this study are the homogenous insurance status and more comprehensive adjustment for confounders, there are several limitations. Important variables not available in SEER-Medicare included: pulmonary function tests, academic/community status of the hospital, institution annual lung resection volume, and specialized (thoracic) training of the surgeon performing surgery. Additionally, stage reported in SEER-Medicare was based on pathologic stage when available, and clinical stage when it was not. This allowed for the possibility of understaging among patients not treated with surgery. As fewer black patients were treated with surgery, this may have partly confounded the univariate difference in survival. Assuming that some percentage of patients not receiving surgery may have had more advanced stage despite being clinically diagnosed as stage I, these patients were still offered therapies not currently considered standard of care for most patients with stage I lung cancer, and the existence of racial disparities in this regard is disturbing. Another limitation of the study was that there were disproportionately more black patients missing comorbidity score and/or marital status and therefore not included in the analysis. While this may have biased the results if these patients were not representative of the rest of the cohort, the effect was likely small given small percentages of the total sample and the effects observed for most outcomes still warrant attention.
Despite these limitations, the data and analysis of the current study of 22,724 patients demonstrated important findings. Despite nearly two decades of progress in cancer care since a landmark study highlighting racial disparities in lung cancer treatment, black patients still received a lower rate of appropriate treatment for stage I lung cancer. When provided equal treatment, there were no racial disparities in survival. Further evaluation of the socioeconomic, cultural, and possible bias-related causes of these differences must be evaluated to understand and reduce racial disparities to improve lung cancer care for all patients.
Supplementary Material
Selection criteria used for study sample and reasons for exclusion
Codes used to identify treatments in Medicare
Univariate and multivariable odds of receipt of treatment (Yes vs. No)
Univariate and multivariable odds of receipt of radiation therapy alone (Yes vs. No), among treated patients
Univariate and multivariable odds of receipt of chemotherapy alone (Yes vs. No), among treated patients
Univariate and multivariable odds of receipt of > 1 treatment (Yes vs. No), among treated patients
Multivariable relative odds of receipt of treatments (compared to surgery only), among treated patients
Adjusted hazard ratio of death for black vs. white patients
The video depicts a left VATS lower-lobe wedge resection and mediastinal lymph node sampling for a biopsy-proven left lower lobe adenocarcinoma in a patient with history of Bentall procedure for ascending aortic aneurysm and contralateral genetically different synchronous lung adenocarcinomas who had previously undergone right VATS upper and lower lobe wedge resections, mediastinal lymph node dissection and subsequent endobronchial ultrasound for preoperative lymph node staging. A ingle slice of the preoperative chest CT is shown at the start depicting the right lower lobe staple line (arrow) and the biopsy-proven metachronous left lower lobe adenocarcinoma (circle).
Central Figure abbreviated legend:
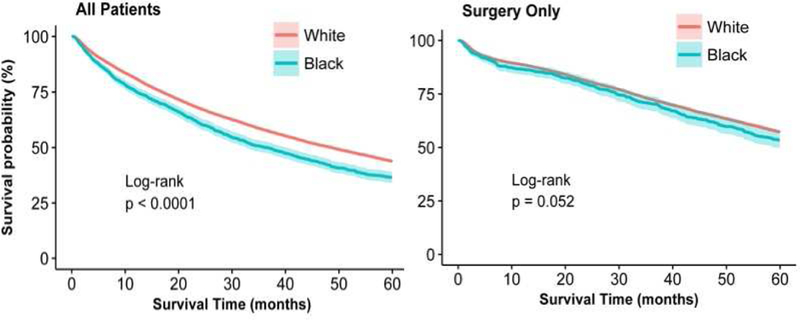
Survival for early stage lung cancer differed by race but not when treated with surgery.
Central message:
Racial disparities persist in lung cancer treatment, even when adjusting for clinical and demographic factors. When black patients receive similar treatment, survival is comparable to white patients.
Perspective statement:
We report on disparities in treatment and survival between black and white patients with early stage non-small cell lung cancer, and analyze factors contributing to disparity in 22,724 patients. Black patients were less likely to receive treatment and to receive surgery only when treated. When accounting for treatment mode, there was no difference in survival.
Acknowledgements
This study used the linked SEER-Medicare data base. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research program, NCI; the Office of Research, Development and Information, CMS; Information Management Service (IMS), and the Surveillance, Epidemiology and End-Results (SEER) Program tumor registries in the creation of the SEER-Medicare database. The authors would like to thank Mr. Larry Adel for patience and assistance with editing the video associated with this manuscript.
Funding: This grant was supported in part by the National Cancer Institute (P30CA196521)
Glossary of Abbreviations
- CI
Confidence Interval
- CPT
Current Procedural Terminology
- HR
Hazard Ratio
- HCPCS
Healthcare Common Procedure Coding System
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- MedPAR
Medicare Provider Analysis and Review
- NCH
National Claims History
- NSCLC
Non-small cell lung cancer
- OR
Odds Ratio
- RT
Radiation Therapy
- SEER
Surveillance, Epidemiology, and End Results
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have no conflicts of interest to declare
References
- 1.Siegel RL, Miller MD, Jemal A. Cancer Statistics, 2018. CA Cancer J Clin 2018; 68 (1): 7–30 [DOI] [PubMed] [Google Scholar]
- 2.Noone AM, Howlader N, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975–2015, National Cancer Institute; Bethesda, MD, https://seer.cancer.gov/csr/1975_2015/, based on November 2017 SEER data submission, posted to the SEER web site, April 2018. [Google Scholar]
- 3.Esnaola NF, Gebregziabher M, Knott K, et al. Underuse of Surgical Resection for Localized Non-Small Cell Lung Cancer Among Whites and African Americans in South Carolina. Ann Thorac Surg 2008; 86 (1): 220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehta RS, Lenzner D, Argiris A. Race and Health Disparities in Patient Refusal of Surgery for Early-Stage Non-Small Cell Lung Cancer: A SEER Cohort Study. Ann Surg Oncol 2012; 19(3): 722–7 [DOI] [PubMed] [Google Scholar]
- 5.Slatore CG, Au DH, Gould MK; American Thoracic Society Disparities in Healthcare Group. An official American Thoracic Society systematic review: insurance status and disparities in lung cancer practices and outcomes. Am J Respir Crit Care Med 2010;182(9):1195–205 [DOI] [PubMed] [Google Scholar]
- 6.Virgo KS, Little AG, Fedewa SA, Chen AY, Flanders WD, Ward EM. Safety-Net Burden Hospitals and Likelihood of Curative-Intent Surgery for Non-Small Cell Lung Cancer. J Am Coll Surg 2011; 213 (5): 633–43 [DOI] [PubMed] [Google Scholar]
- 7.Lieberman-Cribbin W, Liu B, Leonine E, Flores R, Taioli E. Temporal trends in centralization and racial disparities in utilization of high-volume hospitals for lung cancer surgery. Medicine (Baltimore) 2017; 96 (16): e6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bach PB, Cramer LD, Warren JL, Begg CB. Racial differences in the treatment of early-stage lung cancer. N Engl J Med 1999;341(16):1198–205. [DOI] [PubMed] [Google Scholar]
- 9.Altorki NK, Yip R, Hanaoka T, et al. Sublobar resection is equivalent to lobectomy for clinical stage 1A lung cancer in solid nodules. J Thorac Cardiovasc Surg 2014; 147 (2):754–62. [DOI] [PubMed] [Google Scholar]
- 10.Taioli E, Yip R, Olkin I, et al. Brief Report: Survival after sub-lobar resection for early stage lung cancer - Methodological obstacles in comparing the efficacy to lobectomy. J Thorac Oncol 2016; 11(3):400–406. [DOI] [PubMed] [Google Scholar]
- 11.Ettinger DS, Akerley W, Borghaei H, et al. (National Comprehensive Cancer Network). Non-small cell lung cancer. J Natl Compr Canc Netw 2012;10(10):1236–71. [DOI] [PubMed] [Google Scholar]
- 12.Krantz SB, Lutfi W, Kuchta K, Wang CH, Kim KW, Howington JA. Improved Lymph Node Staging in Early-Stage Lung Cancer in the National Cancer Database. Ann Thorac Surg 2017;104(6):1805–1814. [DOI] [PubMed] [Google Scholar]
- 13.Walker GV, Grant SR, Guadagnolo BA, et al. Disparities in stage at diagnosis, treatment, and survival in nonelderly adult patients with cancer according to insurance status. J Clin Oncol 2014;32(28):3118–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tantraworasin A, Taioli E, Liu B, Kaufman AJ, Flores RM. Underperformance of Mediastinal Lymph Node Evaluation in Resectable Non-Small Cell Lung Cancer. Ann Thorac Surg 2018;105(3):943–949. [DOI] [PubMed] [Google Scholar]
- 15.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Medical care 2002;40:IV-3–18. [DOI] [PubMed] [Google Scholar]
- 16.Paul S, Isaacs AJ, Treasure T, Altorki NK, Sedrakyan A. Long term survival with thoracoscopic versus open lobectomy: propensity matched comparative analysis using SEER-Medicare database. BMJ 2014;349:g5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Surveillance, Epidemiology and End Results program Vol 2017. [Google Scholar]
- 18.Egevad LHM, Berney D, Fleming K, Ferlay J Chapter 4: Histological groups. In: Curado MPEB, Shin HR, Storm H, Ferlay J, Heanue M, Boyle P, eds. Cancer Incidence in Five Continents Vol IX Lyon, France: IARC Scientific Publications No. 160; 2007:61–66. [Google Scholar]
- 19.NCI: Charlson Comorbidity Macro, 2014. Version
- 20.Virnig BA, Warren JL, Cooper GS, Klabunde CN, Schussler N, Freeman J. Studying Radiation Therapy Using SEER-Medicare-Linked Data. Medical Care 2002:40:IV-49–54. [DOI] [PubMed] [Google Scholar]
- 21.Warren JL, Harlan LC, Fahey A, et al. Utility of the SEER-Medicare Data to identify Chemotherapy use. Medical Care 2002:40:IV-55–61. [DOI] [PubMed] [Google Scholar]
- 22.Cooper GS, Zhong Y, Strange KC, Dennis LK, Amini SB, Rimm AA. Agreement of Medicare Claims and Tumor Registry Data for Assessment of Cancer-Related Treatment. Medical Care 2000. 38(4):411–421. [DOI] [PubMed] [Google Scholar]
- 23.Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: Results of the ACOSOG Z0030 Trial. J Thorac Cardiovasc Surg 2011; 141(3): 662–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taioli E, Flores R. Appropriateness of Surgical Approach in Black Patients with Lung Cancer-15 Years Later, Little Has Changed. J Thorac Oncol 2017;12(3):573–577. [DOI] [PubMed] [Google Scholar]
- 25.Soneji S, Tanner NT, Silvestri GA, Lathan CS, Black W. Racial and Ethnic Disparities in Early-Stage Lung Cancer Survival. Chest 2017;152(3):587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmed Z, Kujtan L, Kennedy KF, Davis JR, Subramanian J. Disparities in the Management of Patients With Stage I Small Cell Lung Carcinoma (SCLC): A Surveillance, Epidemiology and End Results (SEER) Analysis. Clinical Lung Cancer 2017: 18(5) e315–25. [DOI] [PubMed] [Google Scholar]
- 27.Jazieh AR, Kyasa MJ, Sethuraman G, Howington J. Disparities in surgical resection of early-stage non–small cell lung cancer. J Thorac Ca rdiovasc Surg 2002;123(6):1173–6 [DOI] [PubMed] [Google Scholar]
- 28.Ganti AK, Subbiah SP, Kessinger A, Gonsalves WI, Silberstein PT, Loberiza FR Jr. Association Between Race and Survival of Patients With Non Small-Cell Lung Cancer in the United States Veterans Affairs Population. Clinical Lung Cancer 2014. ;15(2):152–8. [DOI] [PubMed] [Google Scholar]
- 29.Williams CD, Salama JK, Moghanaki D, Karas TZ, Kelley MJ. Impact of Race on Treatment and Survival among U.S. Veterans with Early-Stage Lung Cancer. J Thorac Oncol 2016;11(10):1672–81. [DOI] [PubMed] [Google Scholar]
- 30.Esnaola NF, Ford ME. Racial differences and disparities in cancer care and outcomes: where’s the rub? Surg Oncol Clin N Am 2012;21(3):417–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tohme S, Kaltenmeier C, Bou-Samra P, Varley PR, Tsung A. Race and Health Disparities in Patient Refusal of Surgery for Early-Stage Pancreatic Cancer: An NCDB Cohort Study. Annals of surgical oncology 2018;25:3427–35. [DOI] [PubMed] [Google Scholar]
- 32.Mehta RS, Lenzner D, Argiris A. Race and health disparities in patient refusal of surgery for early-stage non-small cell lung cancer: a SEER cohort study. Annals of surgical oncology 2012;19:722–7. [DOI] [PubMed] [Google Scholar]
- 33.Taioli E, Liu B, Nicastri DG, Lieberman-Cribbin W, Leoncini E, Flores RM. Personal and hospital factors associated with limited surgical resection for lung cancer, in-hospital mortality and complications in New York State. Journal of surgical oncology 2017;116:471–81. [DOI] [PubMed] [Google Scholar]
- 34.Osarogiagbon RU, Ogbata O, Yu X. Number of Lymph Nodes Associated with maximal Reduction of Long-term Mortality Risk in Pathologic Node-Negative Non-Small Cell Lung Cancer. Ann Thorac Surg 2014: 97(2):385–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Osarogiagbon RU, Yua X. Nonexamination of Lymph Nodes and Survival After Resection of Non-Small Cell Lung Cancer. Ann Thorac Surg 2013;96(4): 1178–1189. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Selection criteria used for study sample and reasons for exclusion
Codes used to identify treatments in Medicare
Univariate and multivariable odds of receipt of treatment (Yes vs. No)
Univariate and multivariable odds of receipt of radiation therapy alone (Yes vs. No), among treated patients
Univariate and multivariable odds of receipt of chemotherapy alone (Yes vs. No), among treated patients
Univariate and multivariable odds of receipt of > 1 treatment (Yes vs. No), among treated patients
Multivariable relative odds of receipt of treatments (compared to surgery only), among treated patients
Adjusted hazard ratio of death for black vs. white patients
The video depicts a left VATS lower-lobe wedge resection and mediastinal lymph node sampling for a biopsy-proven left lower lobe adenocarcinoma in a patient with history of Bentall procedure for ascending aortic aneurysm and contralateral genetically different synchronous lung adenocarcinomas who had previously undergone right VATS upper and lower lobe wedge resections, mediastinal lymph node dissection and subsequent endobronchial ultrasound for preoperative lymph node staging. A ingle slice of the preoperative chest CT is shown at the start depicting the right lower lobe staple line (arrow) and the biopsy-proven metachronous left lower lobe adenocarcinoma (circle).