Abstract
Objective
To investigate the incidence and predictors of dyspnea on exertion among subjects with rheumatoid arthritis (RA).
Methods
We investigated dyspnea on exertion using a prospective cohort, the Brigham RA Sequential Study (BRASS). Clinically significant dyspnea on exertion was defined as a score of ≥ 3 (unable to ambulate without breathlessness or worse) on the validated Medical Research Council (MRC) scale (range 0‐5). We analyzed subjects with MRC score < 3 at BRASS baseline and at ≥ 1 year of follow‐up. The MRC scale was administered annually. We determined the incidence rate (IR) of dyspnea on exertion. We used Cox regression to estimate the hazard ration (HR) for dyspnea on exertion occurring one year after potential predictors were assessed.
Results
We analyzed 829 subjects with RA and no clinically significant dyspnea on exertion during a mean follow‐up period of 3.0 years (SD 1.9). At baseline, mean age was 55.7 years (SD 13.6), 82.4% of subjects were female, and median RA duration was 8 years. During follow‐up, 112 subjects (13.5%) developed incident dyspnea on exertion during 2476 person‐years of follow‐up (incidence rate 45.2 per 1000 person‐years). Independent predictors of incident dyspnea on exertion were older age (HR 1.03 per year, 95% CI, 1.01‐1.04), female sex (HR 2.22, 95% CI, 1.14‐4.29), mild dyspnea (HR 2.62, 95% CI, 1.60‐4.28), and worsened Multi‐Dimensional Health Assessment Questionnaire score (HR 2.36 per unit, 95% CI, 1.54‐3.60). Methotrexate use, RA disease activity, and seropositivity were not associated with incident dyspnea on exertion after accounting for other dyspnea risk factors.
Conclusion
Dyspnea on exertion occurred commonly in patients with RA. Older women with impaired physical function were especially vulnerable to developing dyspnea on exertion.
Introduction
Patients with rheumatoid arthritis (RA) are known to be at a markedly increased risk for respiratory and cardiovascular morbidity/mortality than the general population in relation to autoimmunity, immunosuppression, and chronic inflammation 1, 2. In addition to these RA factors, lifestyle and clinical factors such as smoking, elevated body mass index (BMI), and impaired functional status could also contribute. While clinicians are increasingly aware of this RA respiratory and cardiovascular burden, clinical workup is usually initiated once symptoms develop. Previous studies of patients with RA have focused on symptoms of chest pain, but no previous study has investigated incidence and predictors of dyspnea 2, 3.
Dyspnea on exertion, defined as breathlessness with activity, is a potentially serious symptom often related to cardiopulmonary and functional status. In previous studies of patients without RA, clinically significant dyspnea on exertion was a powerful predictor of disability and early mortality 4, 5. Therefore, understanding the incidence and identifying RA‐related and modifiable risk factors for developing dyspnea on exertion is important in the clinical care of RA patients.
Significance & Innovations.
Patients with rheumatoid arthritis (RA) may be susceptible to developing dyspnea on exertion related to disease progression, drug toxicity, or respiratory/cardiovascular morbidities.
We investigated incidence and predictors of dyspnea on exertion using a prospective cohort of patients with RA in which dyspnea was assessed annually using a validated scale.
Among 829 patients with RA, 112 developed incident dyspnea on exertion (13.5%), for an incidence rate of 45.2 per 1000 person‐years. Older age, female sex, mild dyspnea, and impaired physical function were identified as significant predictors.
These results quantified the burden of dyspnea on exertion for RA patients and identified risk factors to allow clinicians to risk‐stratify patients who may be susceptible.
Our aim was to quantify the incidence and identify predictors of dyspnea on exertion among patients with RA using a prospective cohort. We hypothesized that RA disease factors, multimorbidities, and unhealthy lifestyle factors would be associated with increased risk for developing dyspnea on exertion.
Methods
Study population
We analyzed data from the Brigham RA Sequential Study (BRASS), a single‐center research registry of patients with RA at Brigham and Women's Hospital in Boston, Massachusetts. All subjects in BRASS have RA as diagnosed by the treating physician and according to accepted criteria. Subjects are surveyed semiannually on a variety of domains, including sociodemographics, symptoms, quality of life, pain, function, and sleep. In addition, validated measures of disease activity, RA characteristics, multimorbidities, and more detailed measures of symptoms are assessed annually at a study visit. Data from the electronic medical record are used to supplement diagnoses and medications. All aspects of this study were approved by the Partners HealthCare IRB.
Study design
We performed a prospective cohort study among subjects in BRASS to investigate incidence and predictors of clinically significant dyspnea on exertion. We excluded subjects who had clinically significant dyspnea on exertion at baseline, missing data for any predictor, and less than one year of follow‐up. Figure 1 shows a flow diagram illustrating the analyzed study sample.
Figure 1.
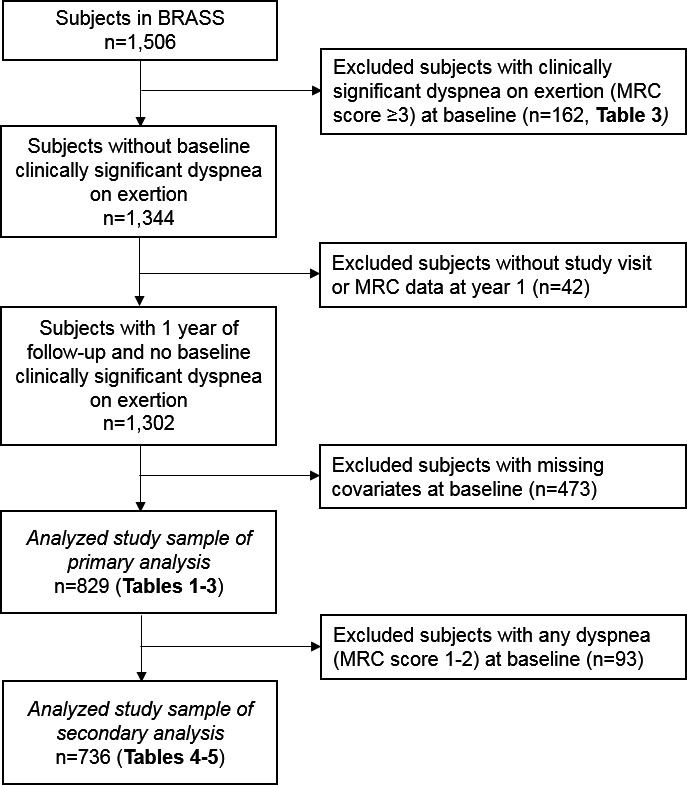
Flow diagram for the analyzed study sample. BRASS, Brigham Rheumatoid Arthritis Sequential Study; MRC, Medical Research Council.
Identification of incident dyspnea on exertion
We used the previously validated Medical Research Council (MRC) dyspnea scale 6. The MRC dyspnea scale rates breathlessness by the following responses: 0 = “I am never breathless or short of breath;” 1 = “I only get breathless with strenuous exercise;” 2 = “I get short of breath when hurrying on the level or up a slight hill;” 3 = “I walk slower than people of the same age on the level because of breathlessness or have to stop for breath when walking at my own pace on the level;” 4 = “I stop for breath after walking 100 yards or after a few minutes on the level;” or 5 = “I am too breathless to leave the house.” As in previous studies, we defined MRC scores of ≥ 3 as clinically significant dyspnea on exertion, 1‐2 as mild dyspnea, and 0 as no dyspnea 4, 7. The MRC dyspnea scale was administered at baseline and annually using a structured in‐person interview. For this study, we only analyzed subjects with MRC dyspnea scores < 3 at BRASS baseline and who had at least one year of follow‐up data.
Potential predictors of dyspnea
We considered sociodemographic predictors including age (continuous in years), sex, race, and education. We dichotomized race as white vs nonwhite and education as received college degree vs less education.
All variables were time‐updated with each annual questionnaire. BMI was calculated from self‐reported height and weight. We categorized subjects into underweight (<18.5 kg/m2), normal weight (18.5‐24.9 kg/m2), overweight (25‐29.9 kg/m2), or obese (≥30 kg/m2). Self‐reported smoking was categorized as never, past, or current. We used standard methods to quantify self‐reported physical activity in metabolic equivalent of task in hours per week (MET‐hours/week) 8. Subjects self‐reported cough. Anxiety was measured using the anxiety component of the Multidimensional Health Assessment Questionnaire (MDHAQ), which rates how feelings of anxiety or nervousness affect abilities on a scale from 0 (not at all) to 3 (unable to do) 9. We dichotomized anxiety as present (≥1) or not (=0), as very few subjects responded with scores of 2 or 3. We considered respiratory diseases likely to be associated with dyspnea symptoms, including self‐reported asthma, bronchitis, emphysema, and history of pneumonia. Interstitial lung disease (ILD) was identified by two pulmonologists and one radiologist reviewing clinically obtained chest computed tomography imaging and all agreeing on the diagnosis 10. We used a previously validated multimorbidity index specific for patients with RA using billing codes from a total of 40 conditions using data obtained from the electronic medical record 11, 12. We removed asthma and chronic obstructive pulmonary disease from this index, because these diseases were already included in the respiratory morbidity variable.
We also considered RA characteristics as predictors of incident dyspnea on exertion, including RA duration and seropositivity (defined as positive rheumatoid factor or cyclic citrullinated peptide). We categorized medications as glucocorticoids, methotrexate, other nonbiologic disease‐modifying antirheumatic drugs (DMARDs), or biologic DMARDs. The disease activity score using 28 joints and C‐reactive protein (DAS28‐CRP3) was dichotomized as remission/low (≤3.2) vs moderate/high (>3.2) 13. MDHAQ is a validated measure of functional status 9. Finally, we considered the presence of bone erosions or radiographic changes and rheumatoid nodules.
Statistical analysis
We reported descriptive characteristics at baseline overall and stratified by whether dyspnea on exertion occurred during follow‐up.
We used Cox proportional hazard models to estimate hazard ratios (HR) and 95% confidence intervals (CI) for developing dyspnea on exertion for each potential predictor. Person‐time accrued from the index date of baseline to censoring variables as of the following date, whichever came first: outcome (MRC score ≥ 3), loss to follow‐up, death, or end of study. All potential predictors were time‐updated so that the predictor was assessed one year before the MRC dyspnea scale was administered. We initially performed unadjusted models for each potential predictor. We then performed a series of multivariable models for each domain (sociodemographic, lifestyle and clinical, RA characteristics) to display the independent effect of each variable, including age and sex in all models. Because there was no previous literature investigating dyspnea on exertion among patients with RA, we used a backward selection process to choose variables for our final multivariable model (entry criterion was P < 0.1 in the unadjusted model). The final model included age, sex, smoking status, pulmonary morbidity, multimorbidity count, cough, mild dyspnea (vs no dyspnea), binary DAS28‐CRP3, and MDHAQ.
We performed two secondary analyses to further investigate dyspnea on exertion in our study and establish the robustness of our findings. Subjects who were susceptible to clinically significant dyspnea on exertion may have already had this outcome at the baseline of BRASS, because some patients had longstanding RA and were excluded from the primary analysis. Therefore, we compared the study sample of subjects in the primary analysis to those we excluded because of prevalent clinically significant dyspnea (MRC score ≥ 3, n = 162). We reported descriptive statistics of the predictors considered in the primary analysis and tested for differences using bivariate analyses. We obtained P values using t tests, Wilcoxon rank‐sum tests, chi‐squared tests, or Fisher's exact tests, as appropriate for each predictor variable.
Subjects with mild dyspnea on exertion at baseline may be more prone to developing clinically significant dyspnea on exertion during follow‐up than those who reported no dyspnea at baseline. Therefore, we performed analyses using an alternate study sample that comprised only subjects without any dyspnea (MRC score = 0) at the baseline visit. We used similar methods as the primary analysis for this study sample to evaluate the robustness of those findings. Finally, because mild dyspnea on exertion is intermediate between no dyspnea and clinically significant dyspnea on exertion, we performed an analysis investigating the outcome of incident mild dyspnea on exertion (MRC score = 1 or 2) among the subgroup who reported no dyspnea at baseline.
For all analyses using Cox regression, we verified that the proportional hazards assumption was met by including an interaction term between time since baseline and each exposure and determining there was no statistically significant interaction. We considered a two‐sided P value of <0.05 as significant in all analyses.
Results
Baseline characteristics
In this primary analysis, we analyzed 829 subjects with RA and no significant dyspnea (MRC score < 3) at baseline. Mean age was 55.7 years (SD 13.6), 82.4% were women, and 93.2% were white (Table 1). Subjects who developed dyspnea on exertion were older than those who never developed dyspnea (mean 60.1 years [SD 11.8] vs 55.0 [SD 13.8]). Those who developed clinically significant dyspnea on exertion (MRC score ≥ 3) were more likely to be past smokers and have obesity. Patients who developed clinically significant dyspnea on exertion were more likely to have longer RA duration (median 11 years vs 7 years), moderate/high disease activity (64.3% vs 50.6%), and worse functional status by MDHAQ. More subjects who developed clinically significant dyspnea on exertion reported pulmonary morbidities (67.0%) than did those who did not develop this level of dyspnea (41.1%).
Table 1.
Baseline characteristics of subjects with rheumatoid arthritis overall, and according to those with and without incident clinically significant dyspnea on exertion (MRC score ≥ 3) during follow‐up (n = 829)
| Study sample of primary analysis (n = 829) | Had dyspnea (MRC score ≥ 3) during follow‐up (n = 112) | No dyspnea (MRC score < 3) during follow‐up (n = 717) | |
|---|---|---|---|
| Sociodemographics | |||
| Mean age, y (SD) | 55.7 (13.6) | 60.1 (11.8) | 55.0 (13.8) |
| Female, n (%) | 683 (82.4) | 101 (90.2) | 582 (81.2) |
| White race, n (%) | 773 (93.2) | 100 (89.3) | 673 (93.9) |
| College degree, n (%) | 498 (60.1) | 58 (51.8) | 440 (61.4) |
| Lifestyle and clinical | |||
| Body mass index category, n (%) | |||
| Underweight | 10 (1.2) | 1 (0.9) | 9 (1.3) |
| Normal | 360 (43.4) | 35 (31.3) | 325 (45.3) |
| Overweight | 263 (31.7) | 39 (34.8) | 224 (31.2) |
| Obesity | 196 (23.6) | 37 (33.0) | 159 (22.2) |
| Smoking status, n (%) | |||
| Never | 446 (53.8) | 46 (41.1) | 400 (55.8) |
| Past | 327 (39.5) | 61 (54.5) | 266 (37.1) |
| Current | 56 (6.8) | 5 (4.5) | 51 (7.1) |
| Mean MET‐hours/week (SD) | 5.5 (5.7) | 4.5 (4.9) | 5.6 (5.8) |
| Pulmonary morbidities, n (%) | |||
| Asthma | 123 (14.8) | 24 (21.4) | 99 (13.8) |
| Bronchitis | 250 (30.2) | 57 (50.9) | 193 (26.9) |
| Emphysema | 13 (1.6) | 5 (4.5) | 8 (1.1) |
| Pneumonia | 177 (21.4) | 34 (30.4) | 143 (19.9) |
| Interstitial lung disease | 2 (0.2) | 1 (0.9) | 1 (0.1) |
| Any of the above | 370 (44.6) | 75 (67.0) | 295 (41.1) |
| Mean multimorbidity index counta (SD) | 0.6 (1.2) | 1.0 (1.5) | 0.6 (1.1) |
| Multimorbidity index counta, n (%) | |||
| 0 | 567 (68.4) | 64 (57.1) | 503 (70.2) |
| 1 | 129 (15.6) | 18 (16.1) | 111 (15.5) |
| >1 | 133 (16.0) | 30 (26.8) | 103 (14.4) |
| Mild dyspnea (MRC score=1 or 2), n (%) | 93 (11.2) | 26 (23.2) | 67 (9.3) |
| Cough | 194 (23.4) | 42 (37.5) | 152 (21.2) |
| Anxiety | 316 (38.1) | 53 (47.3) | 263 (36.7) |
| RA Characteristics | |||
| Median RA duration, years (IQR) | 8 (3‐20) | 11 (3‐23.5) | 7 (2‐19) |
| Mean DAS28‐CRP3 score (SD) | 3.6 (1.6) | 3.8 (1.5) | 3.5 (1.6) |
| Moderate/high disease activity, n (%) | 435 (52.5) | 72 (64.3) | 363 (50.6) |
| Seropositive, n (%) | 547 (66.0) | 77 (68.8) | 470 (65.6) |
| Mean MDHAQ score (SD) | 0.53 (0.47) | 0.74 (0.48) | 0.50 (0.46) |
| Methotrexate use, n (%) | 433 (52.2) | 54 (48.2) | 379 (52.9) |
| Non‐biologic DMARD use, n (%) | 608 (73.3) | 81 (72.3) | 527 (73.5) |
| Biologic DMARD use, n (%) | 339 (40.9) | 55 (49.1) | 284 (39.6) |
| Glucocorticoid use, n (%) | 230 (27.7) | 39 (34.8) | 191 (26.6) |
| Erosion/radiographic changes present, n (%) | 418 (50.4) | 60 (53.6) | 358 (49.9) |
| Rheumatoid nodule present, n (%) | 218 (26.3) | 42 (37.5) | 176 (24.6) |
Excluding asthma and chronic obstructive pulmonary disease.
DAS28‐CRP3 = disease activity score with 28 joints and C‐reactive protein; DMARD = disease‐modifying antirheumatic drug; IQR = interquartile range; MDHAQ = multi‐dimensional health assessment questionnaire; MET‐hours/week = metabolic equivalent of task in hours/week; MRC = Medical Research Council; RA = rheumatoid arthritis; SD = standard deviation.
Dyspnea on exertion outcomes
During 2476 person‐years of follow‐up, we identified 112 subjects (13.5%) who developed clinically significant dyspnea on exertion during mean follow‐up of 3.0 years (SD 1.9). Accounting for censoring factors, the cumulative incidence at 6 years after baseline was 22.5%. The overall incidence rate for dyspnea on exertion was 45.2 per 1000 person‐years.
Cox regression results for the primary analysis
Table 2 shows HRs for dyspnea on exertion. In unadjusted Cox proportional hazard models, several predictors were significantly associated with dyspnea on exertion, including increasing age (HR 1.03, 95% CI, 1.01‐1.05), female sex (HR 1.92, 95% CI, 1.02‐3.60), having a college degree (HR 0.68, 95% CI, 0.47‐1.00), past smoking (HR 1.80, 95% CI, 1.21‐2.67, reference: never smoking), cough (HR 2.02, 95% CI, 1.36‐3.00), anxiety (HR 1.75, 95% CI, 1.19‐2.56) mild dyspnea (HR 2.90, 95% CI, 1.83‐4.59), DAS28‐CRP3 (HR 1.15 per unit, 95% CI, 1.02‐1.30), longer RA duration (HR 1.02, 95% CI, 1.00‐1.03), worse MDHAQ (HR 3.00, 95% CI, 2.10‐4.30), glucocorticoid use (HR 1.57, 95% CI, 1.04‐2.37), and rheumatoid nodules (HR 1.63, 95% CI, 1.10‐2.41). Methotrexate use, other types of DMARDs, and seropositivity were not associated with dyspnea.
Table 2.
Primary analysis: Hazard ratios for incident clinically significant dyspnea on exertion (MRC score ≥ 3) by sociodemographic, lifestyle, clinical, and RA characteristics (n = 829; number of outcomes = 112)
| Time‐updated predictor | Unadjusted HR (95% CI) | Sociodemographics model HR (95% CI) | Lifestyle and clinical model HR (95% CI) | RA characteristics model HR (95% CI) | Final multivariable HR (95% CI) |
|---|---|---|---|---|---|
| Sociodemographics | |||||
| Age (per year) | 1.03 (1.01‐1.05) | 1.04 (1.02‐1.05) | 1.03 (1.01‐1.05) | 1.03 (1.02‐1.05) | 1.03 (1.01‐1.04) |
| Female sex (vs male) | 1.92 (1.02‐3.60) | 2.35 (1.23‐4.49) | 2.65 (1.37‐5.14) | 2.21 (1.15‐4.26) | 2.22 (1.14‐4.29) |
| White race (vs nonwhite) | 0.56 (0.30‐1.04) | 0.50 (0.27‐0.95) | – | – | – |
| College degree (vs less education) | 0.68 (0.47‐1.00) | 0.83 (0.56‐1.23) | – | – | – |
| Lifestyle and clinical | |||||
| Body mass index category | – | – | – | ||
| Underweight/normal | 1.00 (Ref) | 1.00 (Ref) | |||
| Overweight | 1.67 (1.06‐2.63) | 1.45 (0.90‐2.34) | |||
| Obesity | 2.07 (1.28‐3.34) | 1.83 (1.10‐3.03) | |||
| Smoking status | – | – | |||
| Never | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | ||
| Past | 1.80 (1.21‐2.67) | 1.39 (0.93‐2.10) | 1.44 (0.96‐2.17) | ||
| Current | 1.24 (0.52‐2.97) | 1.05 (0.43‐2.56) | 0.93 (0.38‐2.26) | ||
| Physical activity (per MET‐hour/week) | 0.97 (0.94‐1.01) | – | 1.00 (0.96‐1.04) | – | – |
| Pulmonary morbidity (vs none)a | 2.07 (1.33‐3.24) | – | 1.40 (0.87‐2.26) | – | 1.40 (0.87‐2.26) |
| Multimorbidity index count | – | – | |||
| 0 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | ||
| 1 | 1.46 (0.88‐2.42) | 1.41 (0.84‐2.37) | 1.30 (0.77‐2.19) | ||
| >1 | 2.17 (1.37‐3.42) | 1.56 (0.96‐2.53) | 1.46 (0.89‐2.38) | ||
| Cough (vs none) | 2.02 (1.36‐3.00) | – | 1.45 (0.95‐2.20) | – | 1.45 (0.95‐2.20) |
| Mild dyspnea, MRC score = 1 or 2 (vs MRC score = 0) | 2.90 (1.83‐4.59) | – | 2.40 (1.48‐3.90) | – | 2.62 (1.60‐4.28) |
| Anxiety (vs none) | 1.75 (1.19‐2.56) | 1.56 (1.05‐2.32) | 1.19 (0.79‐1.81) | ||
| RA characteristics | |||||
| DAS28‐CRP3 continuous (per unit) | 1.15 (1.02‐1.30) | – | – | – | – |
| Moderate/high DAS28‐CRP (vs low/remission) | 1.65 (1.12‐2.43) | – | – | 1.12 (0.73‐1.73) | 1.05 (0.69‐1.60) |
| RA duration (per year) | 1.02 (1.00‐1.03) | – | – | 1.00 (0.98‐1.02) | – |
| Seropositive (vs seronegative) | 0.89 (0.59‐1.35) | – | – | 0.74 (0.46‐1.19) | – |
| MDHAQ (per unit) | 3.00 (2.10‐4.30) | – | – | 2.62 (1.74‐3.95) | 2.36 (1.54‐3.60) |
| Methotrexate use (vs not) | 0.78 (0.53‐1.14) | – | – | 0.63 (0.38‐1.04) | – |
| Non‐biologic DMARD use (vs not) | 0.94 (0.62‐1.43) | – | – | 1.50 (0.85‐2.64) | – |
| Biologic DMARD use (vs not) | 1.35 (0.92‐1.98) | – | – | 1.65 (1.08‐2.52) | – |
| Glucocorticoid use (vs not) | 1.57 (1.04‐2.37) | – | – | 1.11 (0.72‐1.71) | – |
| Erosion present (vs not) | 1.06 (0.72‐1.55) | – | – | 0.71 (0.45‐1.13) | – |
| Rheumatoid nodule present (vs not) | 1.63 (1.10‐2.41) | – | – | 1.45 (0.90‐2.32) | – |
Pulmonary morbidities were asthma, bronchitis, emphysema, pneumonia, or interstitial lung disease. Bold values indicate P < 0.05.
CI = confidence interval; DAS28‐CRP3 = disease activity score with 28 joints and C‐reactive protein; DMARD = disease‐modifying antirheumatic drug; HR = hazard ration; MDHAQ = multi‐dimensional health assessment questionnaire; MET‐hours/week = metabolic equivalent of task in hours/week; MRC = Medical Research Council; RA = rheumatoid arthritis.
In the model adjusting for all lifestyle and clinical factors, obesity (HR 1.76, 95% CI, 1.07‐2.92, reference: normal/overweight), multimorbidity index count > 1 (HR 1.61, 95% CI, 1.00‐2.61, reference: count of 0), anxiety (HR 1.56, 95% CI, 1.05‐2.32), and mild dyspnea (HR 2.49, 95% CI, 1.53‐4.04) were independent predictors of incident clinically significant dyspnea on exertion. In the model adjusting for all RA characteristics, MDHAQ was strongly associated with incident clinically significant dyspnea on exertion (HR 2.62, 95% CI, 1.74‐3.95). Besides biologic DMARD use (HR 1.65, 95% CI, 1.08‐2.52), there were no other RA characteristics significantly associated with dyspnea on exertion development in that model.
In the final multivariable model, the following variables were statistically significant predictors of incident clinically significant dyspnea on exertion: older age (HR 1.03 per year, 95% CI, 1.01‐1.04), female sex (HR 2.22, 95% CI, 1.14‐4.29), mild dyspnea (HR 2.62, 95%, CI 1.60‐4.28), and worse MDHAQ (HR 2.36 per unit increase, 95% CI, 1.54‐3.60). These results were independent of RA disease activity, smoking status, presence of pulmonary morbidity, anxiety, cough, and the multimorbidity index count.
Prevalent clinically significant dyspnea on exertion at baseline
Of the 1506 subjects in BRASS, 162 (10.8%) reported MRC scores ≥ 3 at baseline, indicating clinically significant dyspnea on exertion. Table 3 shows the baseline characteristics of these subjects who had prevalent clinically significant dyspnea on exertion (MRC score ≥ 3) compared with the characteristics of those without this level of dyspnea in the primary analysis. Subjects with prevalent clinically significant dyspnea on exertion had many statistically significant differences in covariates. Compared with those without clinically significant dyspnea on exertion, these patients were more likely to be older, less educated, obese, smokers, and less physically active. They were more likely to have excess multimorbidities and to report cough and anxiety. These subjects tended to have higher MDHAQ scores, had more frequent use of DMARDs and glucocorticoids and a higher proportion had rheumatoid nodules compared to those who were not excluded.
Table 3.
Baseline characteristics of subjects by those with and without incident clinically significant dyspnea on exertion
| Study sample of primary analysis (MRC score < 3 at baseline) (n = 829) | Prevalent clinically significant dyspnea on exertion (MRC score ≥ 3 at baseline) (n = 162) | P value | |
|---|---|---|---|
| Sociodemographics | |||
| Mean age, y (SD) | 55.7 (13.6) | 60.4 (13.0) | <0.001 |
| Female, n (%) | 683 (82.4) | 135 (83.3) | 0.77 |
| White race, n (%) | 773 (93.2) | 143 (89.9) | 0.14 |
| College degree, n (%) | 498 (60.1) | 55 (36.4) | <0.001 |
| Lifestyle and clinical | |||
| Body mass index category, n (%) | <0.001 | ||
| Underweight | 10 (1.2) | 2 (1.2) | |
| Normal | 360 (43.4) | 40 (24.7) | |
| Overweight | 263 (31.7) | 44 (27.2) | |
| Obesity | 196 (23.6) | 64 (39.5) | |
| Smoking status, n (%) | <0.001 | ||
| Never | 446 (53.8) | 47 (34.1) | |
| Past | 327 (39.5) | 75 (54.3) | |
| Current | 56 (6.8) | 16 (11.6) | |
| Mean MET‐hours/week (SD) | 5.5 (5.7) | 2.9 (3.2) | <0.001 |
| Pulmonary morbidities, n (%) | |||
| Asthma | 123 (14.8) | 41 (25.5) | <0.001 |
| Bronchitis | 250 (30.2) | 77 (47.8) | <0.001 |
| Emphysema | 13 (1.6) | 13 (8.1) | <0.001 |
| Pneumonia | 177 (21.4) | 62 (44.3) | <0.001 |
| Interstitial lung disease | 2 (0.2) | 0 (0.0) | 0.53 |
| Any of the above | 370 (44.6) | 42 (25.9) | 0.003 |
| Mean multimorbidity index count (SD)a | 0.6 (1.2) | 2.6 (1.5) | <0.001 |
| Multimorbidity index counta, n (%) | <0.001 | ||
| 0 | 567 (68.4) | 0 (0.0) | |
| 1 | 129 (15.6) | 42 (25.9) | |
| >1 | 133 (16.0) | 120 (74.1) | |
| Cough | 194 (23.4) | 45 (27.8) | 0.01 |
| Anxiety (vs none) | 316 (38.1) | 176 (54.3) | <0.001 |
| RA characteristics | |||
| Median RA duration, y (IQR) | 8 (3‐20) | 11 (4‐24) | <0.001 |
| Mean DAS28‐CRP3 score (SD) | 3.6 (1.6) | 4.2 (1.6) | <0.001 |
| Moderate/high disease activity, n (%) | 435 (52.5) | 111 (68.5) | <0.001 |
| Seropositive, n (%) | 547 (66.0) | 103 (63.6) | 0.56 |
| Mean MDHAQ score (SD) | 0.53 (0.47) | 1.10 (0.60) | <0.001 |
| Methotrexate use, n (%) | 433 (52.2) | 118 (72.8) | <0.001 |
| Non‐biologic DMARD use, n (%) | 608 (73.3) | 149 (92.0) | <0.001 |
| Biologic DMARD use, n (%) | 339 (40.9) | 86 (53.1) | 0.004 |
| Glucocorticoid use, n (%) | 230 (27.7) | 144 (88.9) | <0.001 |
| Erosion/radiographic changes present, n (%) | 418 (50.4) | 86 (53.1) | 0.54 |
| Rheumatoid nodule present, n (%) | 218 (26.3) | 53 (32.7) | 0.09 |
Excluding asthma and chronic obstructive pulmonary disease
DAS28‐CRP3 = disease activity score with 28 joints and C‐reactive protein; DMARD = disease‐modifying antirheumatic drug; IQR = interquartile range; MDHAQ = multi‐dimensional health assessment questionnaire; MET‐hours/week = metabolic equivalent of tast in hours per week; MRC = Medical Research Council; RA, rheumatoid arthritis; SD = standard deviation.
Secondary analyses excluding subjects with mild dyspnea at baseline
These analyses consisted of the 736 subjects who reported no dyspnea (MRC score = 0) after excluding 93 subjects with mild dyspnea (MRC scores of 1 or 2) at baseline. Table 4 shows the results for risk of incident clinically significant dyspnea on exertion (MRC score ≥ 3). There were 86 subjects who had the outcome in this analysis. Overall, results were similar to the primary analysis. In the final multivariable model, MDHAQ remained strongly associated with incident clinically significant dyspnea on exertion with a similar point estimate (HR 2.48, 95% CI, 1.56‐3.94). Older age was also statistically associated with incident dyspnea on exertion as in the primary analysis. While female sex did not reach statistical significance (HR 2.03, 95% CI, 0.98‐4.20), the point estimate was similar to the primary analysis.
Table 4.
Secondary analysis: Hazard ratios for incident clinically significant dyspnea on exertion (MRC score ≥ 3) by sociodemographic, lifestyle, clinical, and RA characteristics among subjects with no dyspnea (MRC score = 0) at baseline (n = 736, number of outcomes = 86)
| Time‐updated predictor | Unadjusted HR (95% CI) | Sociodemographics model HR (95% CI) | Lifestyle and clinical model HR (95% CI) | RA characteristics model HR (95% CI) | Final multivariable HR (95% CI) |
|---|---|---|---|---|---|
| Sociodemographics | |||||
| Age (per year) | 1.03 (1.01‐1.05) | 1.04 (1.02‐1.06) | 1.03 (1.01‐1.05) | 1.03 (1.01‐1.06) | 1.03 (1.00‐1.05) |
| Female sex (vs male) | 1.78 (0.88‐3.59) | 2.21 (1.08‐4.53) | 2.46 (1.19‐5.09) | 2.14 (1.03‐4.45) | 2.03 (0.98‐4.20) |
| White race (vs nonwhite) | 0.55 (0.27‐1.13) | 0.49 (0.23‐1.00) | – | – | – |
| College degree (vs less education) | 0.67 (0.43‐1.03) | 0.85 (0.54‐1.33) | – | – | – |
| Lifestyle and clinical | |||||
| Body mass index category | – | – | – | ||
| Underweight/normal | 1.00 (Ref) | 1.00 (Ref) | |||
| Overweight | 1.73 (1.03‐2.89) | 1.53 (0.90‐2.61) | |||
| Obesity | 2.36 (1.36‐4.07) | 2.21 (1.24‐3.92) | |||
| Smoking status | – | – | |||
| Never | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | ||
| Past | 1.76 (1.12‐2.75) | 1.38 (0.87‐2.18) | 1.48 (0.94‐2.35) | ||
| Current | 1.38 (0.53‐3.59) | 1.15 (0.43‐3.05) | 1.13 (0.42‐3.00) | ||
| Physical activity (per MET‐hour/week) | 0.95 (0.90‐0.99) | – | 0.98 (0.93‐1.02) | – | – |
| Pulmonary morbidity (vs none)a | 1.95 (1.16‐3.28) | – | 1.51 (0.88‐2.61) | – | 1.46 (0.85‐2.53) |
| Multimorbidity index count | – | – | |||
| 0 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | ||
| 1 | 1.62 (0.93‐2.83) | 1.46 (0.83‐2.58) | 1.38 (0.78‐2.44) | ||
| >1 | 2.21 (1.30‐3.76) | 1.60 (0.92‐2.80) | 1.55 (0.88‐2.71) | ||
| Cough (vs none) | 1.57 (0.98‐2.51) | – | 1.18 (0.72‐1.94) | – | 1.27 (0.78‐2.08) |
| Anxiety (vs none) | 1.66 (1.07‐2.57) | 1.57 (1.00‐2.46) | 1.16 (0.72‐1.85) | ||
| RA characteristics | |||||
| DAS28‐CRP3 continuous (per unit) | 1.13 (0.98‐1.30) | – | – | – | – |
| Moderate/high DAS28‐CRP (vs low/remission) | 1.44 (0.93‐2.23) | – | – | 0.94 (0.57‐1.53) | 0.96 (0.59‐1.54) |
| RA duration (per year) | 1.02 (0.99‐1.03) | – | – | 1.00 (0.97‐1.02) | – |
| Seropositive (vs seronegative) | 0.99 (0.61‐1.59) | – | – | 0.83 (0.48‐1.44) | – |
| MDHAQ (per unit) | 3.08 (2.07‐4.57) | – | – | 2.73 (1.72‐4.32) | 2.48 (1.56‐3.94) |
| Methotrexate use (vs not) | 0.77 (0.50‐1.19) | – | – | 0.66 (0.37‐1.16) | – |
| Non‐biologic DMARD use (vs not) | 0.88 (0.54‐1.41) | – | – | 1.30 (0.68‐2.48) | – |
| Biologic DMARD use (vs not) | 1.38 (0.89‐2.14) | – | – | 1.63 (1.01‐2.63) | – |
| Glucocorticoid use (vs not) | 1.70 (1.07‐2.68) | – | – | 1.11 (0.72‐1.71) | – |
| Erosion present (vs not) | 0.99 (0.64‐1.52) | – | – | 0.64 (0.37‐1.08) | – |
| Rheumatoid nodule present (vs not) | 1.73 (1.11‐2.70) | – | – | 1.63 (0.95‐2.80) | – |
Pulmonary morbidities were asthma, bronchitis, emphysema, pneumonia, or interstitial lung disease. Bold values indicate P < 0.05.
CI = confidence interval; DAS28‐CRP3 = disease activity score with 28 joints and C‐reactive protein; DMARD = disease‐modifying antirheumatic drug; HR = hazard ration; MDHAQ = multi‐dimensional health assessment questionnaire; MET‐hours/week = metabolic equivalent of tast in hours per week; MRC = Medical Research Council; RA = rheumatoid arthritis.
Table 5 shows results analyzing the same sample without dyspnea but with any incident dyspnea (MRC score > 1) as the outcome. There were 148 subjects who reported incident dyspnea in this analysis. Again, MDHAQ was strongly associated with the development of any dyspnea (HR 1.94, 95% CI, 1.33‐2.82). Older age (HR 1.01 per year, 95% CI, 1.00‐1.03) and presence of pulmonary morbidities (HR 2.26, 95% CI, 1.45‐3.54) were also associated with incident dyspnea in this analysis. After accounting for other variables, no other RA‐specific factors besides MDHAQ were associated with dyspnea in this analysis.
Table 5.
Secondary analysis: Hazard ratios for incident any dyspnea (MRC score ≥1) by sociodemographic, lifestyle, clinical, and RA characteristics among subjects without dyspnea (MRC score = 0) at baseline (n = 736, number of outcomes = 148)
| Time‐updated predictor | Unadjusted HR (95% CI) | Sociodemographics model HR (95% CI) | Lifestyle and clinical model HR (95% CI) | RA characteristics model HR (95% CI) | Final multivariable HR (95% CI) |
|---|---|---|---|---|---|
| Sociodemographics | |||||
| Age (per year) | 1.03 (1.01‐1.03) | 1.03 (1.01‐1.04) | 1.01 (1.00‐1.03) | 1.02 (1.00‐1.04) | 1.01 (1.00‐1.03) |
| Female sex (vs male) | 1.49 (0.90‐2.48) | 1.75 (1.04‐2.95) | 1.80 (1.05‐3.06) | 1.63 (0.96‐2.76) | 1.58 (0.93‐2.69) |
| White race (vs non‐white) | 0.74 (0.40‐1.38) | 0.68 (0.36‐1.28) | – | – | – |
| College degree (vs less education) | 0.83 (0.59‐1.16) | 0.98 (0.69‐1.40) | – | – | – |
| Lifestyle and clinical | |||||
| Body mass index category | – | – | – | ||
| Underweight/normal | 1.00 (Ref) | 1.00 (Ref) | |||
| Overweight | 1.56 (1.06‐2.28) | 1.41 (0.94‐2.10) | |||
| Obesity | 1.55 (0.97‐2.42) | 1.46 (0.91‐2.32) | |||
| Smoking status | – | – | |||
| Never | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | ||
| Past | 1.56 (1.10‐2.19) | 1.32 (0.93‐1.88) | 1.37 (0.96‐1.95) | ||
| Current | 0.86 (0.36‐2.04) | 0.77 (0.32‐1.85) | 0.75 (0.31‐1.80) | ||
| Physical activity (per MET‐hour/week) | 0.97 (0.94‐1.00) | – | 0.99 (0.95‐1.02) | – | – |
| Pulmonary morbidity (vs none)a | 2.68 (1.76‐4.10) | – | 2.27 (1.45‐3.53) | – | 2.26 (1.45‐3.54) |
| Multimorbidity index count | – | – | |||
| 0 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | ||
| 1 | 1.32 (0.85‐2.05) | 1.28 (0.84‐2.37) | 1.23 (0.78‐1.93) | ||
| >1 | 1.64 (1.06‐2.53) | 1.31 (0.81‐2.01) | 1.22 (0.77‐1.94) | ||
| Cough (vs none) | 1.59 (1.10‐2.30) | – | 1.25 (0.85‐1.85) | – | 1.30 (0.88‐1.91) |
| Anxiety (vs none) | 1.49 (1.05‐2.10) | 1.43 (1.00‐2.03) | 1.16 (0.80‐1.68) | ||
| RA characteristics | |||||
| DAS28‐CRP3 continuous (per unit) | 1.13 (1.02‐1.26) | – | – | – | – |
| Moderate/high DAS28‐CRP (vs low/remission) | 1.44 (1.03‐2.02) | – | – | 1.05 (0.72‐1.53) | 1.12 (0.77‐1.61) |
| RA duration (per year) | 1.01 (1.00‐1.03) | – | – | 1.00 (0.98‐1.01) | – |
| Seropositive (vs seronegative) | 0.92 (0.63‐1.33) | – | – | 0.79 (0.52‐1.18) | – |
| MDHAQ (per unit) | 2.36 (1.70‐3.26) | – | – | 2.11 (1.46‐3.05) | 1.94 (1.33‐2.82) |
| Methotrexate use (vs not) | 0.85 (0.60‐1.18) | – | – | 0.85 (0.53‐1.35) | – |
| Non‐biologic DMARD use (vs not) | 0.86 (0.59‐1.24) | – | – | 1.02 (0.60‐1.72) | – |
| Biologic DMARD use (vs not) | 1.16 (0.83‐1.62) | – | – | 1.23 (0.85‐1.78) | – |
| Glucocorticoid use (vs not) | 1.34 (0.91‐1.96) | – | – | 1.02 (0.69‐1.52) | – |
| Erosion present (vs not) | 1.13 (0.81‐1.58) | – | – | 0.86 (0.58‐1.29) | – |
| Rheumatoid nodule present (vs not) | 1.52 (1.07‐2.16) | – | – | 1.36 (0.89‐2.08) | – |
Pulmonary morbidities were asthma, bronchitis, emphysema, pneumonia, or interstitial lung disease. Bold values indicate P < 0.05.
CI = confidence interval; DAS28‐CRP3 = disease activity score with 28 joints and C‐reactive protein; DMARD = disease‐modifying antirheumatic drug; HR = hazard ration; MDHAQ = multi‐dimensional health assessment questionnaire; MRC = Medical Research Council; RA = rheumatoid arthritis.
Discussion
In this large prospective cohort study, we quantified the incidence of clinically significant dyspnea on exertion and identified independent predictors among patients with RA. We found that 13.5% of patients with RA developed clinically significant dyspnea on exertion, an incidence rate of 42.5 per 1000 person‐years. Older age, female sex, mild dyspnea on exertion, and worse functional status by MDHAQ scores were all independent risk factors for developing incident clinically significant dyspnea on exertion.
Previous research has established that patients with RA are at increased risk of developing cardiovascular disease 2. Ongoing research is dissecting the role of a variety of factors for elevated cardiovascular risk, including chronic inflammation, dyslipidemia, autoimmunity, DMARDs, RA disease activity, diminished physical activity, smoking, and presence of multimorbidities, among others 2. One‐third of cardiovascular disease risk may be attributable to these RA characteristics 3. While studies have investigated these risk factors for developing angina, an important symptom heralding significant cardiovascular disease, no previous study has investigated dyspnea on exertion, which may occur for patients with coronary artery disease, pericarditis, or heart failure.
Patients with RA also have greater respiratory morbidity and mortality than do those without RA 1. While ILD is a known extra‐articular RA manifestation associated with poor outcomes after diagnosis 10, other respiratory diseases, such as asthma and chronic obstructive pulmonary disease, may also occur more frequently in RA 1. Pulmonary nodules, pleural effusions, and bronchiectasis are additional RA disease pulmonary manifestations that may present clinically as dyspnea on exertion 14. Patients with RA are also at increased risk for pneumonia and other respiratory infections related to altered immunity and immunosuppressant use 1. Among patients with RA, disease factors such as seropositivity, disease activity, functional status, and nodules/erosions may subset patients at particular risk of developing these respiratory manifestations 1. Thus, the etiology of dyspnea on exertion is complex related to multiple domains (sociodemographics, lifestyle, conditioning, RA disease status, and other clinical factors) and may be multifactorial for many patients or be proximally related to deconditioning or organ dysfunction from complex underlying etiologies resulting in debilitation and functional limitations.
Previous studies have investigated dyspnea on exertion in other patient populations. Among older individuals in the community, those who developed significant dyspnea on exertion had markedly elevated all‐cause mortality 5. Dyspnea on exertion also predicted cardiorespiratory and physical function outcomes 15. Our study highlights patients with RA as a vulnerable population related to this important patient‐reported outcome 7. A recent study of mostly men in the Veterans Affairs RA registry showed that chronic lung disease was associated with increased mortality 16, further underscoring the importance of understanding the effect of respiratory factors on health for patients with RA. In the analysis comparing subjects with prevalent clinically significant dyspnea on exertion to those without, subjects with clinically significant dyspnea on exertion had high rates of smoking, sedentary activity, poor functional status, excess comorbidities, and more severe RA. This suggests that patients with RA who develop clinically significant dyspnea on exertion may be more likely to have adverse health outcomes, both from an overall health standpoint as well as related to RA.
We identified several statistically significant predictors for incident dyspnea on exertion. These were all assessed one year before the dyspnea outcome. We found that poor functional status as measured by MDHAQ was the strongest predictor for dyspnea on exertion, independent of age, sex, mild dyspnea, RA disease activity, smoking, pulmonary morbidities, and other multimorbidities. We found that women were significantly more likely to have dyspnea on exertion than men, even after adjusting for age and other potential confounders. These results were robust in secondary analyses analyzing only subjects who reported no dyspnea and using a lower threshold for the definition of incident dyspnea. A previous study correlated presence of dyspnea with worsened functional status measured by MDHAQ among patients with connective‐tissue‐disease‐associated ILD, but ours is the first to evaluate MDHAQ as a predictor of dyspnea and only in patients with RA 17. However, other functional status measures have been associated with incident dyspnea in other patient populations, including patients with cancer and chronic respiratory diseases, so these findings are unlikely to be specific for RA 18, 19, 20, 21. Similarly, dyspnea commonly affects older people because of many underlying complex etiologies, accounting for a high prevalence in the elderly 7. Still, our findings emphasize the importance of assessing for dyspnea among patients with RA, particularly older women with functional limitations. Further research is needed to clarify potential sex differences for RA patients regarding respiratory and cardiovascular outcomes. Importantly, we found little evidence that DMARDs, including methotrexate, were associated with clinically significant dyspnea on exertion. Other RA characteristics, including seropositivity, RA disease activity, bone erosions, rheumatoid nodules, and glucocorticoid use, were also not associated with dyspnea on exertion after accounting for MDHAQ scores. Our findings highlight the importance of preserving functional status in patients with RA from a cardiopulmonary perspective, particularly among older women.
Our study has some limitations. While we used a validated measure of dyspnea on exertion, this was only administered annually. Therefore, it is possible that we did not capture those patients who intermittently developed dyspnea on exertion and did not report it at the time it was assessed. The MRC dyspnea scale measure may have some inherent inaccuracy because it is patient‐reported. In addition, some of the other predictors such as smoking and physical activity were self‐reported and so may have been misclassified. However, we used a standardized method of data collection that has been previously validated and commonly used in research 4, 6. While our study is the first to investigate dyspnea on exertion within an RA population, a non‐RA control population was not available, so we were unable to study whether there are differential effects for patients with RA. However, we were able to describe the incident rate of clinically significant dyspnea on exertion and also investigate the effect of RA‐specific factors on dyspnea on exertion, both of which have not been previously investigated. While we had detailed data available on many important potential predictors, residual confounding from unmeasured variables is always possible in an observational study. For example, fibromyalgia and other conditions may be nonphysiologic conditions that increase the likelihood of reporting dyspnea 22. We were unable to analyze fibromyalgia because it was not systematically collected in the entire registry. However, anxiety was not associated with dyspnea in the final multivariable model. Data on respiratory morbidities were available, and cardiovascular diseases were part of the multimorbidity index, but these measures may not have captured all clinical reasons a patient may become dyspneic. While our study was relatively large, we may not have been powered to detect all statistical associations with dypnea. For example, while past smoking (HR 1.44, 95% CI, 0.96‐2.17) and cough (HR 1.45, 95% CI, 0.95‐2.20) were not statistically associated with dyspnea, the point estimates and confidence intervals suggest they are important predictors, in agreement with clinical observations. We may not have identified subjects susceptible to dyspnea who had previously developed clinically significant dyspnea on exertion prior to the baseline of BRASS, because this is not an incident RA cohort, and some subjects may have had dyspnea prior to RA onset. However, we were able to compare subjects with prevalent dyspnea to those without severe dyspnea at baseline and found many differences, particularly related to RA disease factors. Finally, subjects in BRASS were recruited from a single center and were predominantly white and well educated, many with longstanding RA. Future studies are needed to investigate the timing and severity of dyspnea related to RA onset and the long‐term impact on clinical and RA outcomes.
Strengths of our study include the large prospective study design with lengthy follow‐up and repeated measures of the validated MRC dyspnea scale. We had detailed data on potential predictors available to identify novel independent predictors of dyspnea on exertion, an important surrogate outcome for poor cardiorespiratory and functional status that is an established risk factor for mortality and other poor outcomes. Our study design assessed potential predictors one year before the dyspnea outcome to maintain the prospective design and minimize the possibility of reverse causality. We had detailed data available on many important RA characteristics including RA duration, serologic status, disease activity, bone erosions, rheumatoid nodules, DMARD/glucocorticoid use, and MDHAQ. Of these, we found that impaired functional status was the most important independent predictor of dyspnea on exertion. We find it reassuring for clinicians that DMARDs, including methotrexate, were not associated with dyspnea on exertion. Methotrexate may have rare but serious respiratory side effects, such as pneumonitis and pulmonary fibrosis, but it appeared well tolerated from a dyspnea perspective in our study. However, we caution that our study was not designed specifically for drug safety, which requires careful attention to confounding by indication, incident user design, timing of drug initiation and the safety outcome, and choice for a clinically appropriate active comparator for observational pharmacoepidemiologic studies or consideration of a randomized clinical trial. Research is ongoing to further clarify the cardiopulmonary safety of methotrexate using a large double‐blind placebo‐controlled randomized trial 23, 24.
In conclusion, we found that dyspnea on exertion occurred relatively frequently in this prospective cohort of patients with RA. Poor functional status, female sex, and older age were significant predictors of dyspnea on exertion. These findings add to the growing literature describing a respiratory and cardiovascular burden of RA.
Author Contributions
All authors were involved in drafting the article or revising it critically for important intellectual contact, and all authors approved the final version to be published. Dr. Sparks had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Sparks, Shadick, Karlson.
Acquisition of data
Iannaccone, Frits, Weinblatt, Shadick.
Analysis and interpretation of data
Sparks, Doyle, He, Pan, Iannaccone, Frits, Dellaripa, Rosas, Lu, Weinblatt, Shadick, Karlson.
Acknowledgments
The authors thank Jie Huang, MSc and Taysir Mahmoud, BA for assistance in data management and statistical analysis. We thank the dedicated RA participants and staff of the Brigham Rheumatoid Arthritis Sequential Study (BRASS) at Brigham and Women's Hospital for their continued participation in this longitudinal research study.
The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard University, its affiliated academic health care centers, or the National Institutes of Health.
This work was supported by the National Institutes of Health (grants K23‐AR069688, L30‐AR066953, P30‐AR070253, P30‐AR072577, R01‐AR049880, and P30‐AR069625). The Brigham Rheumatoid Arthritis Sequential Study is funded by grants from Bristol‐Myers Squibb, Amgen, Crescendo Bioscience, and Sanofi/Regeneron. The funders had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Drs. Shadick and Karlson contributed equally to this work.
Dr. Weinblatt has received research grants from Amgen, Crescendo Bioscience, and Sanofi/Regeneron and consulting fees from Abbvie, Amgen, Bristol‐Myers Squibb, Canfite, Corrona, Crescendo Bioscience, GlaxoSmithKline, Gilead, Lilly, Lycera, Merck, Novartis, Pfizer, Roche, Samsung, Set Point, and Scipher; and owns stock options in Lycera, Canfite, Scipher, Vorso, and Inmedix.
References
- 1. Chatzidionisyou A, Catrina AI. The lung in rheumatoid arthritis, cause or consequence? Curr Opin Rheumatol 2016;28:76–82. [DOI] [PubMed] [Google Scholar]
- 2. England BR, Thiele GM, Anderson DR, Mikuls TR. Increased cardiovascular risk in rheumatoid arthritis: mechanisms and implications. Br Med J 2018;361:k1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Crowson CS, Rollefstad S, Ikdahl E, Kitas GD, van Riel PLCM, Gabriel SE, et al. Impact of risk factors associated with cardiovascular outcomes in patients with rheumatoid arthritis. Ann Rheum Dis 2018;77:48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999;54:581–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ahmed T, Steward JA, O'Mahony MS. Dyspnoea and mortality in older people in the community: a 10‐year follow‐up. Age Ageing 2012;41:545–9. [DOI] [PubMed] [Google Scholar]
- 6. Fletcher CM. Standardised questionnaire on respiratory symptoms: a statement prepared and approved by the MRC Committee on the Aetiology of Chronic Bronchitis (MRC breathlessness score). Br Med J 1969;2:1665. [Google Scholar]
- 7. van Mourik Y, Rutten FH, Moons KG, Bertens LC, Hoes AW, Reitsma JB. Prevalence and underlying causes of dyspnoea in older people: a systematic review [review]. Age Ageing 2014;43:319–26. [DOI] [PubMed] [Google Scholar]
- 8. Quinn T, Bs MF, von Heideken J, Iannaccone C, Shadick NA, Weinblatt M, et al. Validity of the Nurses’ health study physical activity questionnaire in estimating physical activity in adults with rheumatoid arthritis. BMC Musculoskelet Disord 2017;18:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pincus T, Swearingen C, Wolfe F. Toward a multidimensional Health Assessment Questionnaire (MDHAQ): assessment of advanced activities of daily living and psychological status in the patient‐friendly health assessment questionnaire format. Arthritis Rheum 1999;42:2220–30. [DOI] [PubMed] [Google Scholar]
- 10. Doyle TJ, Dellaripa PF, Batra K, Frits ML, Iannaccone CK, Hatabu H, et al. Functional impact of a spectrum of interstitial lung abnormalities in rheumatoid arthritis. Chest 2014;146:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Radner H, Yoshida K, Mjaavatten MD, Aletaha D, Frits M, Lu B, et al. Development of a multimorbidity index: Impact on quality of life using a rheumatoid arthritis cohort. Semin Arthritis Rheum 2015;45:167–73. [DOI] [PubMed] [Google Scholar]
- 12. Radner H, Yoshida K, Frits M, Iannaccone C, Shadick NA, Weinblatt M, et al. The impact of multimorbidity status on treatment response in rheumatoid arthritis patients initiating disease‐modifying anti‐rheumatic drugs. Rheumatology (Oxford) 2015;54:2076–84. [DOI] [PubMed] [Google Scholar]
- 13. Ranganath VK, Yoon J, Khanna D, Park GS, Furst DE, Elashoff DA, et al. Comparison of composite measures of disease activity in an early seropositive rheumatoid arthritis cohort. Ann Rheum Dis 2007;66:1633–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shadick NA, Fanta CH, Weinblatt ME, O'Donnell W, Coblyn JS. Bronchiectasis. A late feature of severe rheumatoid arthritis. Medicine (Baltimore) 1994;73:161–70. [PubMed] [Google Scholar]
- 15. Hegendörfer E, Vaes B, Matheï C, Van Pottelbergh G, Degryse JM. Correlates of dyspnoea and its association with adverse outcomes in a cohort of adults aged 80 and over. Age Ageing 2017;46:994–1000. [DOI] [PubMed] [Google Scholar]
- 16. England BR, Sayles H, Michaud K, Thiele GM, Poole JA, Caplan L, et al. Chronic lung disease in U.S. Veterans with rheumatoid arthritis and the impact on survival. Clin Rheumatol 2018;37:2907–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Swigris JJ, Yorke J, Sprunger DB, Swearingen C, Pincus T, du Bois RM, et al. Assessing dyspnea and its impact on patients with connective tissue disease‐related interstitial lung disease. Respir Med 2010;104:1350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McKenzie E, Hwang MK, Chan S, Zhang L, Zaki P, Tsao M, et al. Predictors of dyspnea in patients with advanced cancer. Ann Palliat Med 2018;7:427–36. [DOI] [PubMed] [Google Scholar]
- 19. Garcia‐Gutierrez S, Quintana JM, Unzurrunzaga A, Esteban C, Baré M, Fernández de Larrea N, et al. Predictors of change in dyspnea level in acute exacerbations of COPD. COPD 2016;13:303–11. [DOI] [PubMed] [Google Scholar]
- 20. Milne KM, Kwan JM, Guler S, Winstone TA, Le A, Khalil N, et al. Frailty is common and strongly associated with dyspnoea severity in fibrotic interstitial lung disease. Respirology 2017;22:728–34. [DOI] [PubMed] [Google Scholar]
- 21. Jiang YQ, Zhu YX, Chen XL, Xu X, Li F, Fu HJ, et al. Impact of adherence to GOLD guidelines on 6‐minute walk distance, MRC dyspnea scale score, lung function decline, quality of life, and quality‐adjusted life years in a Shanghai suburb. Genet Mol Res 2015;14:8861–70. [DOI] [PubMed] [Google Scholar]
- 22. Van Den Houte M, Bogaerts K, Van Diest I, De Bie J, Persoons P, Van Oudenhove L, et al. Perception of induced dyspnea in fibromyalgia and chronic fatigue syndrome. J Psychosom Res 2018;106:49–55. [DOI] [PubMed] [Google Scholar]
- 23. Sparks JA, Barbhaiya M, Karlson EW, Ritter SY, Raychaudhuri S, Corrigan CC, et al. Investigating methotrexate toxicity within a randomized double‐blinded, placebo‐controlled trial: Rationale and design of the Cardiovascular Inflammation Reduction Trial‐Adverse Events (CIRT‐AE) Study. Semin Arthritis Rheum 2017;47:133–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, et al. Low‐dose methotrexate for the prevention of atherosclerotic events. N Engl J Med 2018. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]


