Abstract
This article provides an overview of the integration of biomarkers and biological mechanisms in social science models of stratification and health. The goal in reviewing this literature is to highlight research that identifies the social forces that drive inequalities over the life course and across generations. The article is structured in the following way. First, descriptive background information on biomarkers is presented, followed secondly by a review of the general theoretical paradigms that lend themselves to an integrative approach. Third, the biomarkers used to capture several biological systems that are most responsive to social conditions are described. Fourth, research that explicates how social exposures “get under the skin” to affect physiological functioning and downstream health is discussed, using socioeconomic disadvantage as an illustrative social exposure. The review ends with emerging directions in the use of biomarkers in social science research. This article endeavors to encourage sociologists to embrace biosocial approaches in order to elevate the importance of social factors in biomedical processes and to intervene on the social conditions that create inequities.
Keywords: biomarkers, health, social stratification, life course, stress, biosocial
Introduction
In the last few decades, the field of sociology has progressed from one that approached the role of biology in social and behavioral research with trepidation and hostility (see Duster 1990, Freese et al. 2003, Massey 2004) to one in which leading social science studies and sources of data explicitly incorporate biology in their research designs, theoretical models, measurement strategies and analysis of social and behavioral phenomena (Harris 2010, McDade 2008, Singer & Ryff 2001).
This development is due to several recent trends. The study of human behavior and social interactions has historically held the view that humans are biological beings embedded within social contexts, including families, peer networks, communities, and institutions. While social scientists recognize biological components in the social and behavioral processes that they study, much of their focus is on the role of the social world, leaving biology in a “tightly closed ‘black box’” (Bury 1997, Timmermans & Haas 2008). This focus was reinforced by a uni-disciplinary approach and available data that was primarily social in nature—coming from large social surveys of individuals or organizations, government and federal databases, and ethnographic studies (Levine 1995).
The push for multi- and inter-disciplinary research—both from individual researchers seeking to address complex societal problems with expertise outside their disciplinary training and from university and government funding sources—changed the landscape of scholarship for social scientists (Enhancing the Effectiveness of Team Science 2015, Jacobs & Frickel 2009, Massey 2004, Porter & Rafols 2009, Singer & Ryff 2001, Wuchty et al. 2007). The more we learned how context mattered for such outcomes as educational attainment, relationships and health, the more we realized how context mattered for the human biology underlying these outcomes. Ignoring the black box of biology meant that social scientists were missing the interplay of social conditions and underlying physiological processes related to health inequalities. For example, the social gradient in health and longevity has been documented across both time and place since the 17th century (Graunt 1662), but only recently have social scientists begun to make headway in explaining the social pathways that create this gradient (Adler & Ostrove 1999, Adler et al. 1994, Marmot et al. 1997, Wolfe et al. 2012). To better understand the development of social stratification processes and health outcomes, there was increasing recognition of the need to uncover the social and biological linkages involved in these processes. Furthermore, the potential ability to intervene on social factors that could alter biological mechanisms to reduce social stratification and improve the health and wellbeing of individuals and societies was especially compelling motivation to integrate biology in social science research.
Around the same time that theoretical and conceptual interest was growing in the role of biology in social stratification and health research, technological advances in survey field methods for the collection of biological data began to rapidly develop (Harris & McDade 2018, Karlamangla et al. 2012). Historically, community- and population-based research in the social sciences has relied on vital records or self-reported, survey-based measures of health and well-being (Crimmins & Vasunilashorn 2016, Timmermans & Haas 2008). This information is readily assessable, but insight into biological processes is limited. Moreover, self-reported health measures are inherently subjective, confounding physiological with psychological and emotional well-being (Altman et al. 2016, Franks et al. 2003), and the subjectivity of self-rated health varies by characteristics of the individual (Ferraro & Farmer 1999). In contrast, biomedical research employs in-depth biological measures collected in controlled clinical or laboratory settings, but typically relies on smaller, select groups of participants based on pre-existing criteria. Generalizability and external validity are limited, and social factors are generally not considered, beyond standard measures of socioeconomic status or self-reported health behaviors. Methodological options for collecting and generating biological data have expanded greatly over the past 15 years, allowing us to bridge this gap (Weinstein et al., 2007). Low cost, noninvasive, “field-friendly” options for collecting blood, saliva, urine and other specimens in the home or local community have allowed investigators to gain access to physiological information from large numbers of survey participants in naturalistic settings (Adam & Kumari 2009, Finch 2010, McDade et al. 2007). Measures based on such biological specimens are typically referred to as “biomarkers.”
Today, social scientists need not fear the integration of biology, biomarkers, and biological mechanisms in social science models of stratification and health. As social stratification and health researchers, our goal remains the same—to identify the social forces that drive inequalities over the life course and across generations. The social researcher holds the ability to allay previous concerns that including biology in social science models will privilege biological explanations or foster a determinism that is not receptive to intervention or change. By including biological data that accurately measure biological phenomena and modeling social-biological linkages grounded in biological theory, we inform the biomedical field and the public of the important role that social factors play in health and wellbeing. Ultimately, attention to biology enables social scientists to illuminate the mechanisms through which social, economic, and psychological factors shape human development and wellbeing within the contexts of everyday life.
This literature review focuses on the integration of biological data, measures, and mechanisms in research on social stratification and health. We have chosen this focus because of the long tradition and rich history of social science research on the social determinants of health and behavior, and, at the same time, the rising importance of health as a factor in social stratification (Adler et al. 1994, House 2002, House et al. 1994, Link 2008, Link & Phelan 1995, Marmot et al. 1991, Palloni 2006, Smith 1999, Williams 2003). Moreover, this area of research is especially illustrative of an integrative approach with cogent theoretical pathways for how the social environment influences biological systems, which in turn, influence health, social status, and well-being. While the incorporation of biological measures in social science research has included promising developments in understanding the interplay of genetic and environmental factors in shaping health (American Journal of Public Health 2013, Conley 2016), this review focuses on physiological outcomes across immune, cardiovascular, and metabolic systems. We first provide some descriptive background information on biomarkers, including the benefits of using biomarkers, their history, and current state in social stratification and health research. We discuss some practical considerations in the collection of biomarkers by social science field studies and their use in research. We review the general theoretical paradigms that provide effective frameworks for integrating biological mechanisms measured by biomarkers, including life course and stress response models. We then discuss the main biological systems inside the body that are most responsive to social conditions outside the body and the biomarkers typically used to measure system functioning (and dysregulation). We then review the literature for how social exposures “get under the skin” to affect physiological functioning in fundamental biological systems, using socioeconomic disadvantage as an illustration of the social exposure. We conclude our review with emerging directions in the use of biomarkers in social science research.
Biomarkers in Social Stratification and Health Research
In a social survey field setting, objective health measures are those derived from the collection of biospecimens or through physical measurement by trained and certified personnel (e.g., interviewers or phlebotomists) following standardized protocols. We refer to these objective measures as “biomarkers” or “biological markers” of (ab)normal biological states resulting from underlying (patho)physiological processes (Halpern et al. 2014, Piazza et al. 2010). Of importance for social scientists, biomarkers represent biological indicators of health risks that are known to be associated with current or future disease (Crimmins & Seeman 2004). By identifying risk, biomarker measures enable researchers to identify the causes of health risk before permanent biological damage is done and point to social or medical interventions to prevent future disease, promote wellbeing and reduce disparities.
Integration of biomarkers in social science research has theoretical, methodological, and measurement benefits. Social stratification and health processes occur both outside the body through the social and physical environment and life experience, and inside the body through neurological and physiological function. Use of biomarker data enables researchers to therefore model what happens to individuals both outside and inside the body as they experience social relationships, life events, and diverse environments throughout all developmental stages of the life course. Decades of research show that social circumstances affect health (Adler et al. 1994, Crimmins & Seeman 2004, House et al. 1988, Marmot et al. 1991, Timmermans & Haas 2008, Umberson et al. 2010, Wolfe et al. 2012), implying a social pathway that involves biological systems inside the body. Biomarkers enable social researchers to map the pathways by which the social world comes to be embodied within the biological being to influence physiological functioning. For example, if living in poverty is associated with poor health, understanding how and when exposure to poverty operates to “get under the skin” and disrupt normal functioning of biological systems will elucidate the biological mechanisms linking poverty experiences with a poor health outcome.
Importantly, identifying health risks through the use of biomarker measures provides the opportunity to intervene on the upstream social causes of health risk before chronic illness and frank disease are manifest. In addition, the incorporation of biomarker data in sociological research helps to illuminate the ways in which the timing, duration, and magnitude of particular social exposures such as poverty or social isolation uniquely shape physiological states and health trajectories. For example, exposure to poverty in childhood may have unique physiological costs during certain stages of physical development that would not be observed among those who experienced poverty during a different life stage. Many of our sociological theories for the development of social stratification and health inequities can be better tested with the integration of biomarkers as physiological indicators of health risk in response to social conditions (reviewed further below).
There are several measurement and methodological benefits to the use of biomarkers. First, biomarkers provide objective measures of health, devoid of subjective feelings of well-being. To understand what’s going on “under the skin,” you need “under the skin” measures, available through the collection of biological specimens or physical measurements (e.g., anthropometric, blood pressure). Second, although self-reported health measures vary in quality, they generally underestimate health risks that go undetected or for which symptoms do not appear early or consistently; this is especially true among young, otherwise healthy populations (Harris 2010, Kehoe et al. 1994, Timmermans & Haas 2008). For example, when participants in the Add Health cohort1 were aged 24–32, 19% were hypertensive according to their measured blood pressure, but only three-fourths knew of their condition through a self-report of diagnosed hypertension (Nguyen et al. 2011). Third, socioeconomic status, race, ethnicity, nativity, and language proficiency influence knowledge of health, symptoms and disease, access to diagnostic services, and understanding of health questionnaires. Consequently, self-reported health survey data can vary in ways unrelated to the underlying physiological functioning (Crimmins & Seeman 2004, Dowd & Zajacova 2010). Disparities based on biomarker measures of health risk by these characteristics provide a more objective comparison of underlying physiological functioning and furthermore serve to identify at risk populations. Fourth, biomarkers can be used to validate self-reports of health conditions or calibrate how well self-assessed health matches objective health (Mcdade et al. 2007). Overall, as objective measures of health, biomarkers are more valid measures of physiological function “under the skin” and therefore physical health (McDade et al. 2007, Piazza et al. 2010).
History and current state of biomarkers in social stratification and health research
Although biomarkers have been the staple of clinical trials and biomedical research, their use in the social sciences is relatively recent. Calls for the integration of biology within social sciences have occurred sporadically since the 1990s, but became more frequent at the turn of the 21st century (e.g., (Freese et al. 2003, Fremont & Bird 1999, Harris 2010, Levine 1995, Singer & Ryff 2001, Timmermans & Haas 2008, Zerhouni 2003). Until biological data were widely available in social science surveys, however, these calls were primarily theoretical. Researchers in medical sociology were most prominent advocates for a greater understanding of biological systems and function in social studies of health and disease (Levine 1995). Public health research, probably the closest scholarship to medical sociology, provided the impetus for the integration of biomarkers within social science studies. Borrowing from the biomedical approach, public health studies often collected biological data in clinical settings, typically at several selected sites, and then supplemented these data with survey and other observational data gathered in a community setting (Wallace 2008). The survey data are often referenced as “population-based” data: that is, data on a population in which collection occurs “outside the clinic.” Most public health and epidemiological studies do not use probability sampling or representative samples (e.g., Women’s Health Initiative [WHI], Reasons for Geographic and Racial Differences in Stroke [REGARDS], Atherosclerosis Risk in Communities Study [ARIC], the Coronary Artery Risk Development in Young Adults [CARDIA]), although some samples are representative of local homogeneous populations (e.g., Framingham Heart Study).
The integration of biomarkers in social science studies has a different set of design priorities. The study design and sampling strategy for the population of substantive interest and to which findings will be generalized is the primary driver of social science studies and surveys. The acquisition of relevant biological data that can subsequently augment the social data on survey participants is then a secondary design feature that enables the integration of biomarkers in social science research.
Perhaps the most influential study that led to the promotion of biomarker data collections in representative social science studies was the National Health and Nutrition Examination Study (NHANES), which merged these two contrasting approaches. NHANES is conducted under the auspices of the National Center for Health Statistics, the federal agency for the collection and reporting of population health data and statistics. NHANES uses a clustered probability sampling design to select a cross-sectional, nationally representative sample of the civilian non-institutionalized population of all possible ages beginning in 1971. Following the survey administration, adult participants then receive a detailed health examination, which includes a wide battery of biological and physical measurements. The health exam is conducted by medical professionals in a traveling mobile exam van (called a mobile examination center, see https://www.cdc.gov/nchs/newsletter/2013_January/a2.htm) fully equipped for the collection of clinic-standard measures. The mobile examination vans travel around the country so that survey participants have access to the exam center. However, travel to the exam center can be burdensome for those with financial, work or disability constraints, leading to potential attrition bias in the biomarker subsample.
NHANES therefore merged the two different approaches of public health and social science by achieving a probability sample of the national population of all ages with clinical biological measures for statistical and research purposes. NHANES is considered the gold standard for population health data in the U.S., documenting key trends in health status by age and other demographic characteristics for federal reporting and programmatic purposes. The limited but valuable socioeconomic variables, however, opened the door for rising social scientific interest, especially in the social determinants of health (Dowd et al. 2009, Geronimus et al. 2006, Kanjilal S et al. 2006, Loucks et al. 2007). NHANES was furthermore responsive to this interest and has continued to add survey items on social factors hypothesized to affect health such as social support and social isolation (Ford et al. 2006, Yang et al. 2013).
It has only been in the last few decades that research incorporating biological data appeared in research articles within social sciences (Brunner et al. 1997, James et al. 1992, Marmot et al. 1991, Winkleby et al. 1992). Scholars studying aging were some of the first to collect biomarkers in their surveys to strengthen and complement self-reported data on health and aging (see National Research Council 2000, 2008). Still, many of these studies were either international, confined to one state or large city, or collected biomarkers in an adjacent clinical setting rather than in the field.
Biomarkers did not enter mainstream national social surveys until the 2000s when the National Longitudinal Study of Adolescent to Adult Health (Add Health) along with the aging studies of National Survey of Midlife Development in the United States (MIDUS), the Health and Retirement Survey (HRS), and the National Social Life, Health, and Aging Project (NSHAP) launched broad-based biomarker collections. In 2001, Add Health collected urine and saliva from its 15,000+ participants across the U.S. to test for sexually transmitted infections and HIV, and also collected buccal cell DNA on its embedded genetic sample (Add Health also collected DNA in 1996 to test for the zygosity of some of its twins). Add Health, HRS and NSHAP collected blood pressure and blood spots from a finger prick in the mid- to late-2000s for biomarkers of diabetes, inflammation, cholesterol, and immune function. Methodological developments for the collection of biospecimens in a field setting that were robust to temperature and time constraints for shipping and processing in a lab made this nationwide biomarker collection possible (Adam & Kumari 2009, Crimmins et al. 2010, McDade et al. 2007, Weinstein et al. 2007). Developments in assay technology facilitated the measurement of proteins, gene transcripts, epigenetic marks, and DNA sequences with higher resolution in smaller quantities of specimens, at increasingly lower costs (Dedeurwaerder et al. 2011, McDade et al. 2016). Portable devices and low-cost monitors facilitate assessment of sleep, physical function and activity, blood pressure, and body size and composition (Lindau & McDade 2008, Marino et al. 2013, Piazza et al. 2010).
Practical considerations of biomarker collection and use
The principal investigators and directors of major social and demographic field studies that collect and disseminate biomarkers face numerous costly and logistical challenges. The integration of biomarker data in national studies has many financial costs. Equipment and supplies are needed for measuring, collecting, storing, shipping, and processing. For example, scales with acceptable levels of accuracy and upper range bounds are needed to measure weight. Tubes and syringes are needed for venous blood collection, and materials to keep specimens cold may be needed for packaging and shipment. To obtain clinical-like measures, specific and complex protocols are often needed to be carried out by specialized field personnel (e.g., nurses or phlebotomists to collect venous blood) or lay interviewers with extensive training (e.g., to conduct skinfold measurements or collect blood spots), increasing costs of measurement. There are laboratory costs to receive, store, and process specimens and to measure the marker of interest. These costs can vary substantially, depending on the biomarker and technology to be used.
The standardization of equipment and protocols can be logistically challenging in a natural field setting where specimens and measurements are being collected in individuals’ home or places of work, study, and leisure. For example, the Add Health protocol for finger prick whole blood spot collection required the interviewer to: clean the respondent’s middle or ring finger with an alcohol prep pad; apply a tourniquet to the arm; prick the finger with a lancet and wipe away the 1st drop of blood; drop (ideally) 7 blood spots onto a special collection card, ensuring that the finger did not touch the card and that the blood spot saturated the collection circle on the card; air dry the sample over desiccant for three hours; then package and FedEx the sample to the lab on the same day as collection with a secure biospecimen ID that differs from the field interviewer ID (Whitsel et al. 2012a,b).
Training and re-training certified interviewers or field examiners to follow standardized collection protocols is costly yet vital to the validity and reliability of biomarker measures (Halpern et al. 2014). Studies that use different methods or equipment to collect the same measure, such as blood pressure, height or weight, will not provide reliable and valid measures that allow for within or across-study comparisons. Many of the leading national surveys conduct extensive quality control and quality assurance procedures to examine and document the quality of the biomarker data they collect and produce, including calibration analysis, external and internal validation studies, and intra-individual reliability analysis (e.g., collect repeat biomarker measures on the same subset of respondent, see Halpern et al. 2014) (see (Crimmins et al. 2013, Entzel et al. 2009, Love et al. 2010, Nguyen et al. 2011, Whitsel et al. 2011, 2012b). As users of biomarker data, researchers must familiarize themselves with study documentation on the quality of biomarker data and for guidance in the appropriate use and interpretation of biomarker measures in specific studies. We now move to the substantive motivations for use of biomarkers in social stratification and health research in the next section.
Life Course Perspective
Human development is a process that has social and biological determinants and intergenerational linkages beginning in utero and continuing throughout all stages of the human life span (Hertzman & Boyce 2010). There is a general consensus that early life conditions and childhood experiences matter for subsequent social and biological development in adolescence, early adulthood, mid adulthood and old age (Gluckman et al. 2008, Hayward & Gorman 2004). Yet, most social and biomedical research does not capture the ways in which developmental processes are linked and interrelated across phases of human life, nor does it capture the dynamic interactions of social and biological forces that underlie development across time and space. This research gap is due to a lack of longitudinal, multilevel life course data and intergenerational study designs. In addition, disciplinary approaches are typically designed to identify disciplinary-specific determinants of social, behavioral, or health outcomes at a point in time.
The life course framework posits that the timing and succession of social roles and circumstances experienced within and across multilevel contexts over time shapes life chances in social, economic, psychological, and health domains (Elder 1998, Elder et al. 2004, Elder Jr. & Shanahan 2007). A life course perspective is essential in integrative research because outcomes at any point in time reflect the product of prior interactions between social and biological forces that occur across human development (Shanahan et al. 2003). Along these lines, research on the developmental origins of health and disease (DOHaD) has exploded, following early biomedical research by Barker and colleagues documenting significant links between birth weight and later cardiovascular disease risk within cohorts (Barker 1997, 1998, 2006). The life course approach has had a major impact on epidemiologic research on the determinants of adult disease risk, with a particular emphasis on cardiovascular diseases and the physiological processes through which they are influenced by early life nutritional environments (Almond & Currie 2011, Ben-Shlomo & Kuh 2002, Gluckman et al. 2008, Kuh & Ben-Shlomo 1997, Smith & Ryckman 2015, Wadhwa et al. (2009). Numerous studies have linked uterine, birth, and childhood exposures to adult physical health and disease (Bengtsson & Broström 2009, Cameron & Demerath 2002, Crimmins & Finch 2006, Smith & Ryckman 2015). Demographic and social research on the “long arm of childhood” has also demonstrated the value of a life course perspective where early life circumstances are both directly and indirectly associated with health outcomes that emerge decades later in adulthood (Anne Case & Christina Paxson 2010, Blackwell et al. 2001, Elo & Preston 1992, Hayward & Gorman 2004, Preston et al. 1998).
Most of this research, however, links early life conditions with chronic disease outcomes in later adulthood within cross-sectional research designs, with limited attention to what happens in between: during the majority of the early life course from later childhood to adulthood. From a life course perspective, this means we are missing the social processes and contexts that structure, mediate, and moderate biology over the life course. All life course stages have unique social and biological forces that determine life-long human development and that operate independently and jointly to influence physical and social wellbeing in that life stage and beyond. There are immense data demands to study how social and biological phenomena operate in distinct life stages and are linked to health and social inequities in subsequent stages across the life course. In spite of these demands, there is emerging research that is beginning to examine how social conditions and health in early life course stages are related to later adult socioeconomic attainments and physical health using biomarkers.
The life course paradigm is an effective framework for studying how social exposures get “under the skin” to affect physiological function depending on the intensity of the exposure, the life stage in which the exposure occurs, and the duration of exposure (as well as the life stage in which physiology is measured). There are four general life course models that have been used in the literature integrating biomarkers in social stratification and health research. The “sensitive period” model examines timing effects in which exposures during sensitive periods of development have stronger effects on health outcomes than they would at other life stages (Cohen et al. 2010, Gluckman et al. 2008, Hayward & Gorman 2004). Sensitive period effects operate through a “biological embedding” mechanism whereby social exposures during sensitive windows of development have the potential to induce structural and functional changes to the developing individual through biological programming that cannot be reversed irrespective of intervening experience. This life course model posits that the effect of the sensitive period exposure is typically “latent” in that its impact on health outcomes may not appear until later life stages, often decades later.
Second, duration effects of social stratification processes can be explored through the accumulation life course model, which emphasizes the role of persistent advantage or disadvantage over time—in both specific life stages and over life stages—on health and development. The effects of multiple exposures over the life course are both additive and interactive and combine in synergistic ways to influence biological mechanisms and, in turn, health and development outcomes. Cumulative effects can either be multiple exposures to a recurrent stressor (e.g., chronic poverty) or a series of exposures to different social environments or life experiences. For example, poverty experienced only during childhood is not as detrimental on subsequent adult health as poverty during childhood, adolescence, and the transition to adulthood.
A third life course model that might explain how social stratification processes are related to health outcomes is the pathway model, which tracks how social exposures in one life stage influence the probability of related social exposures in subsequent life stages. Also known as the “chains of risk” model, it emphasizes pathway effects whereby early experiences set in motion a chain of events that put individuals on paths differentiated by types and levels of stress exposures to social and biological factors (Marmot et al. 2001, Pudrovska & Anikputa 2014). This model elaborates on the ways in which inter- and intra-generational social stratification pathways are linked across the life course. For example, the connection between early life conditions and adult health and disease may be explained by the SES pathway where early life SES determines adult SES, which in turn, is a more proximate and important predictor of adult health and disease (Yang et al. 2017).
A fourth life course model considers the unique importance of social mobility in explaining the early-life and later-life SES and health link. This model posits that social and economic mobility may affect health risk, and predicts that the health effects of early-life exposures can be modified by later-life SES such that upward mobility may mitigate negative impacts of early-life adversity, while downward mobility is detrimental to health (Hallqvist et al. 2004, Luo & Waite 2005). While this model offers a distinct conceptual framework in which to consider the role of social mobility in shaping physical health, distinguishing between the unique impacts of social mobility and the impacts of the other life course models described above remains an empirical challenge (Hallqvist et al. 2004).
Social Exposures, Stress, and Physiological Indicators of Health
A vast literature has established significant links between social conditions and markers of physical health. Social exposures in the form of socioeconomic status (House 2002, Link & Phelan 1995, Poulton et al. 2002, Turrell et al. 2007), the quality of social relationships (Berkman & Syme 1979, Berkman et al. 2000, Yang et al. 2016), and surrounding school, work, and neighborhood contexts (Crosnoe & Muller 2004, Toker et al. 2005, Wight et al. 2008) are thought to promote or damage health through multiple social, psychological, and behavioral mechanisms that affect underlying physiology. While positive social exposures such as affluence, strong social ties, or cohesive environments have been found to benefit health through access to material and social resources (Browning & Cagney 2003, House et al. 1988, Wen et al. 2003), most biosocial research explores the damaging health effects of adverse social conditions, such as poverty, social strain, or social isolation (e.g., Yang et al., 2013; Yang, Schorpp and Harris, 2014; Jensen, Berens and Nelson, 2017).
The stress paradigm is the most common mechanism used to frame the influence of negative social exposures on physiological processes (Pearlin et al. 1981). Since Selye’s seminal work that identified stress as a significant contributor to physical health among rats (Selye 1956), a growing literature in the social sciences has examined the role of adverse social experiences in shaping exposure to stress and ultimately physical health in humans. According to McEwen and colleagues, repeated exposure to adverse social experiences leads to the perception of stress and ultimately affects the ability of the body to maintain “allostasis,” or physiological stability through changing environments (McEwen 1998, McEwen & Seeman 1999, Sterling & Eyer 1981). The gradual dysregulation between the autonomic nervous system, the hypothalamic-pituitary-adrenal (HPA) axis, and the metabolic, cardiovascular, and immune systems due to chronic exposure to social stressors is termed allostatic load. McEwen and colleagues ultimately conclude that allostatic load contributes to the development of chronic diseases such as diabetes, coronary heart disease, and dementia.
Figure 1 displays the general stress process model most relevant for social science. There are individual differences in the perception of stress related to individuals’ genetic makeup, attributes and life experiences. The human body reacts to stress by activating stress management systems in the brain known as the “fight-or-flight” response. The autonomic nervous system and the HPA axis ready the body for fight or flight by releasing stress hormones into the bloodstream. The autonomic nervous system triggers release of the hormone epinephrine, which increases heart rate and, in turn, blood pressure and pulse rate. Epinephrine also triggers the release of blood sugar (glucose) and fats from temporary storage sites in the body for immediate energy. The HPA axis releases the stress hormone cortisol, which increases glucose and inflammatory protein levels and inhibits insulin production to prevent glucose from being stored.
Figure 1: Physiological and Behavioral Response to Stressors.
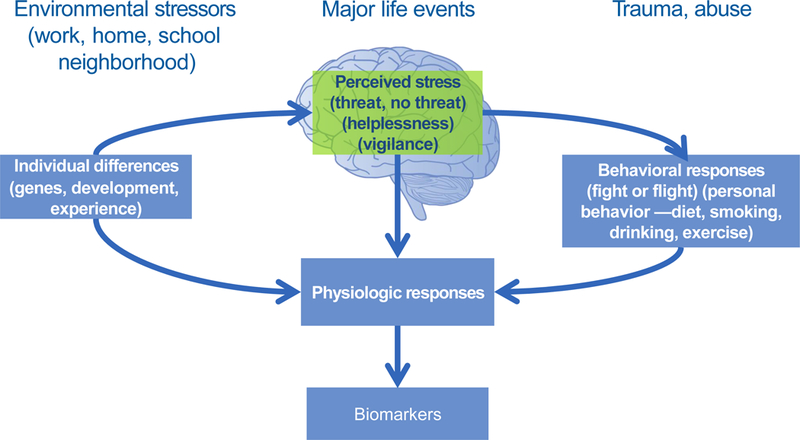
Adaptation of model in McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179 (permissions obtained).
This physiological reaction is normal in response to an unanticipated, temporary stressor, and once the threat passes, epinephrine and cortisol levels fall. However, chronic low-level stress keeps the SNS and HPA axis activated, which takes a toll on the body. Elevated cortisol and epinephrine levels contribute to the buildup of fat tissue and to weight gain, high levels of glucose in the blood, increased glucose intolerance and the risk of diabetes with deleterious effects on weight, immune function, and chronic disease risk (Lupein et al. 2006, Piazza et al. 2010). Stress is a natural occurrence in most individuals’ lives, but it is the mundane, cumulative stressors that accompany daily life in the contexts of work, home, and the larger community (e.g., poverty, bullying, social isolation) rather than acute or exceptional stressful events such as the death of a parent that social scientists typically study (Hertzman & Boyce 2010). Daily cumulative conditions of these low-level stressors operate to break down the body’s physiological equilibrium (Almeida & Wong 2009, McEwen & Seeman 1999). These physiological responses to cumulative and chronic stress can be measured by biomarkers.
Adverse social environments are also thought to shape health behaviors and access to health-related resources that affect physiological functioning. This is shown in Figure 1, where behavioral responses to stress can include overeating, substance use or inactivity. However, it is important to note that these mechanisms are not mutually exclusive; for example, health risk behaviors such as cigarette smoking or binge drinking are often considered coping behaviors in the face of exposure to stress (Grzywacz & Almeida 2008, Westman et al. 1985). Several studies have found that associations between socioeconomic status and physiological markers of health were largely attenuated after adjustment for health behaviors (Mackenbach et al. 2000, Pollitt et al. 2007). Thus, stressful social exposures not only have a direct influence on physiological processes, but also have an indirect influence via engagement in health-risk behaviors used to manage stress.
Biological Systems in Social Science Research
The majority of social stratification and health research that incorporates biomarkers focuses on markers of metabolic, cardiovascular, and immune function. These biological systems are most responsive to the physiological effects of chronic stressors in the social environments of individuals (Ferraro & Shippee 2009, Hertzman & Boyce 2010, Piazza et al. 2010).
While the term metabolic function describes many essential, life-sustaining chemical processes, much of the social science and public health literature focuses on specific biological indicators related to food metabolism, including obesity, cholesterol, and blood sugar. Obesity and body mass index (BMI) are among the earliest and most widely studied biomarkers of metabolic function in the social sciences because of the relative ease of collecting height and weight measurements in the field. Further, as rates of obesity have steeply risen over the past several decades, gaining a better understanding of the social contributors to obesity is of great importance to identify public health interventions. Other measures of metabolic function increasingly incorporated into health disparities research include cholesterol (Kanjilal S et al. 2006, Winkleby et al. 1992), glycosylated hemoglobin (Feldman & Steptoe 2003, Tsenkova et al. 2007), and metabolic syndrome as a composite measure of metabolic function (Brunner et al. 1997, Chichlowska et al. 2008, Danese et al. 2009, Park et al. 2003).
Biomarkers of cardiovascular function capture several domains of heart health, including blood pressure and hypertension (Brummett et al. 2012, Colhoun et al. 1998, Poulton et al. 2002, Winkleby et al. 1992), heart rate (McGrath et al. 2006, Shishehbor et al. 2006), and cardiovascular reactivity (Chen & Matthews 2001, Kapuku et al. 2002, Wilson et al. 2000). In addition, because metabolic and immune function also impact cardiovascular functioning, composite measures of cardiovascular risk often incorporate indicators of cholesterol levels, glycosylated hemoglobin, waist circumference or body mass, and inflammation (Hatzenbuehler et al. 2014, Non et al. 2014, Slopen et al. 2013).
Social science studies that test immune function typically incorporate indicators of inflammation and/or infection burden and recovery. Inflammation, measured through biomarkers such as C-reactive protein (CRP), fibrinogen, and interleukin-6 (IL-6), is thought to be a significant risk factor in the development of cardiovascular disease (Ridker 2003), and has also been found to be a mediator in the links between social conditions and mortality (Yang et al. 2013). In addition, evidence suggests that exposure to chronic stress contributes to increased inflammation burden due to impaired immune system response to the anti-inflammatory signaling of glucocorticoids (Miller et al. 2002, 2009). While most social science research relies on measurement of a single inflammatory marker to measure inflammation, the availability of a more extensive array of inflammatory markers in studies such as MIDUS allows for the creation of composite scores that capture broader inflammation burden (Yang et al. 2014, 2017). In addition to inflammation, several studies have incorporated measures of infection burden and susceptibility to examine how social conditions shape immune function (i.e., Cohen et al. 2004, Dowd et al. 2009).
Composite biomarker measures integrate physiological markers from multiple biological systems. While grouping together multiple complex physiological systems inevitably leads to a loss of biological specificity, these measures are useful for identifying the capacity for social exposures to have widespread physiological impacts that in turn shape health outcomes. In addition to metabolic syndrome, cardiovascular risk, and inflammation burden described above, a number of studies have considered the impacts of social conditions on allostatic load. Allostatic load is especially useful in stress research, as it captures dysregulation across neuroendocrine, immune, metabolic, and cardiovascular systems that is thought to be caused by chronic stress (McEwen 1998, McEwen & Seeman 1999). Allostatic load is typically measured by using a composite score that is some combination of blood pressure/hypertension, body mass, waist circumference, cholesterol, glucose metabolism, inflammation, symbpathetic and parasympathetic nervous system activity, and hypothalamic-pituitary-adrenal axis activity (Geronimus et al. 2006, Gruenewald et al. 2012, Gustafsson et al. 2014, Upchurch et al. 2015).
There are several excellent reference lists and inventories of potential biomarkers used in social science and public health research that are indexed by major physiological systems (Karlamangla et al. 2012), stress response systems (Piazza et al. 2010), and common markers in social science studies (Crimmins & Vasunilashorn 2016, Crimmins et al. 2008).
Socioeconomic Disadvantage as an Illustration of the Physiological Effects of Social Exposures
To illustrate the influence of social exposures on physiological markers of health, we consider the example of socioeconomic disadvantage. Socioeconomic disadvantage, typically defined by low educational attainment (i.e., less that a high school degree), low household income, unemployment or low occupational status, neighborhood-level poverty, or some combination of these measures, is persistently associated with poorer physical health (Galea et al. 2011, House 2002, Luo & Waite 2005, Ross & Mirowsky 2001). Aligned with fundamental cause theory, socioeconomic disadvantage is a quintessential negative exposure that influences multiple downstream mechanisms important for health (Link & Phelan 1995). A number of mechanisms tie socioeconomic disadvantage to physical health. First, the social and financial strains regularly experienced by the poor, such as increased job insecurity, difficulty paying bills, and exposure to unsafe neighborhoods greatly increase exposure to chronic stress (Baum et al. 1999, Turner et al. 1995). In addition, disadvantaged individuals are significantly less likely to have the social support systems that aid in coping with stressful life experiences (Aneshensel 1992, Kessler 1979), are less likely to access health-promoting resources such as health insurance or recreational facilities (Feinstein 1993, Giles-Corti 2002, Moore et al. 2008, Newacheck et al. 2003), and are more likely to engage in health-risk behaviors such as cigarette smoking, unhealthy diet, and physical inactivity (Feinstein 1993, Hanson & Chen 2007, Lantz et al. 1998). Thus, adverse socioeconomic conditions influence many of the daily experiences, social resources, and individual behaviors that are instrumental for physical health.
The specific health impacts of socioeconomic disadvantage have been extensively studied, and such research increasingly includes the use of biomarkers as indicators of physiological functioning. This research has also increasingly applied advanced theoretical and life course frameworks that consider how the timing, duration, and magnitude of exposure to disadvantage is related to physiological health outcomes. Studies find that socioeconomic conditions are associated with adverse metabolic (McLaren 2007, Ogden et al. 2010, Park et al. 2003, Sundquist & Johansson 1998), cardiovascular (Kanjilal S et al. 2006, Winkleby et al. 1992), and immune (Aiello et al. 2016; Cohen et al. 2004, 2010; Dowd et al. 2009, Piazza et al. 2010) outcomes. A more detailed discussion of the links between exposure to socioeconomic disadvantage and each of these health outcomes is below.
Metabolic Function
Socioeconomic disadvantage has significant links to metabolic function. Within developed countries, socioeconomic disadvantage is associated with elevated BMI and higher risk of obesity, a pattern that is beginning to emerge in developing countries as well (McLaren 2007, Popkin et al. 2012). Within the U.S., the association of socioeconomic conditions with body mass is already apparent in childhood (Ogden et al. 2010, Shrewsbury & Wardle 2008) and persists into adulthood (Stunkard & Sorensen 1993). In addition, a growing body of research applies a life course framework to identify the importance of timing and duration of exposure to socioeconomic conditions for body mass and obesity outcomes. This research finds evidence for the importance of both early life and adult socioeconomic conditions for adult body mass and obesity (Langenberg et al. 2003, Power et al. 2005).
Because body mass is one of the few biomarkers in which extensive longitudinal data is available, studies incorporating body mass or obesity have increasingly applied a life course perspective to understand how the timing and duration of exposure to disadvantage shaped trajectories of change in body mass across the life span (Ball & Crawford 2005, Giskes et al. 2008, Lee et al. 2009, Scharoun-Lee et al. 2009). This approach is distinct from other work applying the life course perspective because it simultaneously incorporates longitudinal perspectives and measurements to both socioeconomic conditions and body mass, thus providing further insight into life course processes and strengthening causal inferences.
Socioeconomic status is also associated with other indicators of metabolic function, including cholesterol (Kanjilal S et al. 2006, Winkleby et al. 1992), glycosylated hemoglobin (Feldman & Steptoe 2003, Kanjilal S et al. 2006), and composite measures of metabolic syndrome (Brunner et al. 1997, Chichlowska et al. 2008, Danese et al. 2009, Park et al. 2003). Because measurement of biomarkers such as cholesterol or glycosylated hemoglobin requires collection of blood samples, these studies often rely on single, point-in-time measures of metabolic function. However, these cross-sectional measures can still be applied to longitudinal or retrospective data on social conditions to allow for identification of how life course socioeconomic status is associated with metabolic outcomes in adulthood. For example, several studies have found evidence that childhood and adolescence is a sensitive period for metabolic syndrome in adulthood (Chichlowska et al. 2008, Danese et al. 2009, Gustafsson et al. 2011b, Hostinar et al. 2017). Other studies have found evidence for a pathway model in the association between early life socioeconomic conditions and adult metabolic syndrome, whereby the link between early life SES and adult metabolic syndrome is mediated by adult SES (Yang et al. 2017). These studies offer support for the importance of timing and duration of exposure to socioeconomic conditions for future metabolic function. Further examination of these associations has recently become possible by capturing trajectories of change in overall metabolic outcomes with the availability of longitudinal biomarker data available in national studies such as HRS, MIDUS, and Add Health. We expect that future studies using this new information will build on findings in the existing literature.
Cardiovascular Function
Research across sociology, epidemiology, and public health relate socioeconomic disadvantage with elevated blood pressure (Brummett et al. 2012, Colhoun et al. 1998, Poulton et al. 2002, Winkleby et al. 1992), increased risk of hypertension (Kanjilal S et al. 2006, Leng et al. 2015), and increased risk of cardiovascular disease (Cooper et al. 2000, Lazzarino et al. 2013). Aligned with the research on metabolic function, a growing number of studies apply a life course framework to identify how socioeconomic conditions across the life span shape adult cardiovascular outcomes. These studies find evidence for the unique importance of early life SES on adult cardiovascular outcomes (Galobardes et al. 2006, Gliksman et al. 1995, Pollitt et al. 2005), as well as the detrimental impacts of cumulative exposure to socioeconomic disadvantage (O’Rand & Hamil-Luker 2005, Pollitt et al. 2005). Emerging research has also identified how change in socioeconomic status affects change in markers of cardiovascular health; for example, Boen & Yang (2016) found that wealth shocks during the Great Recession had a detrimental impact on both systolic blood pressure and inflammation.
Evidence also suggests that socioeconomic conditions influence cardiovascular reactivity to stress, particularly among African Americans and males (Chen & Matthews 2001, Kapuku et al. 2002, Wilson et al. 2000). Socioeconomic differences in cardiovascular reactivity not only implicate exposure to stress in cardiovascular function, but also have important implications for inequalities in the emergence of cardiovascular disease across the life span (Treiber et al. 2003).
Immune Function
A growing body of research ties socioeconomic conditions to indicators of immune function, such as chronic inflammation, immunological aging, and infection burden and recovery. Socioeconomic conditions are associated with several inflammatory markers, including C- reactive protein (Brummett et al. 2013, Gruenewald et al. 2009, Liu et al. 2017, Toker et al. 2005, Yang et al. 2017), fibrinogen (Pollitt et al. 2007, Toker et al. 2005), and interleukin-6 (Carroll et al. 2011, Gruenewald et al. 2009). Inflammatory markers have also been applied in life course research examining the early life influences of adult immune function. For example, several studies find that early life socioeconomic conditions are associated with adult inflammation independently of adult SES (Carroll et al. 2011, Danese et al. 2009, Liu et al. 2017), implicating early life as a sensitive period that contributes to the emergence of inflammation burden in adulthood. In contrast, Yang and colleagues (2017) study early life SES influences on inflammation across adulthood—from young adulthood to old age. They find that while early life SES is directly associated with inflammation in young adulthood, it is explained by adult SES in latter stages of mid- and late-adulthood, suggesting that life course socioeconomic pathways explain the influence of early life socioeconomic conditions on adult inflammation burden.
Socioeconomic conditions are also thought to shape vulnerability to and recovery from infection. Socioeconomic conditions during early childhood may be especially influential for future infection burden because children in disadvantaged contexts are more likely to be exposed to infections during a critical period for the development of immune function (McDade 2005). Indeed, early life socioeconomic disadvantage is associated with higher burden of chronic infections in adulthood, such as Helicobacter pylori, cytomegalovirus, herpes simplex virus-1, hepatitis A and B, and chronic respiratory conditions (Dowd et al. 2009). In addition, adults who report lower childhood SES display lower host resistance to the common cold (Cohen et al. 2004), suggesting that childhood socioeconomic conditions have a lasting effect on immune function.
Composite Measures
Composite measures of physiological functioning have been used in a number of studies examining the health impacts of socioeconomic disadvantage. In addition to the studies described above that consider metabolic syndrome (e.g., (Danese et al. 2009, Hostinar et al. 2017) and inflammation burden (Yang et al. 2017), research examining socioeconomic health disparities employs composite measures that capture overall cardiovascular risk (Doom et al. 2017, Non et al. 2014) and allostatic load (Friedman et al. 2015, Gruenewald et al. 2012, Gustafsson et al. 2011a). Collectively, these studies implicate early life disadvantage and cumulative exposure to disadvantage as significant determinants of physiological health risk across multiple biological systems. Emerging research has also begun to identify the links between life course SES and patterns of change in allostatic load across time (Merkin et al. 2014), providing additional evidence for the longitudinal ties between socioeconomic conditions and physiological trajectories.
Mechanisms Underlying Socioeconomic Disparities in Biomarkers of Physiological Function
While the mechanisms underlying the association of socioeconomic conditions with metabolic, cardiovascular, and immune function continue to be explored, prior research implicates several interrelated mechanisms in explaining socioeconomic disparities in physiological outcomes. Ultimately, through observing these underlying mechanisms, we see that upstream socioeconomic conditions shape a number of downstream processes that have more direct influences on underlying physiology.
Exposure to stress is among the most studied underlying mechanisms tying socioeconomic conditions to biomarkers of health. Applying a sociological lens to the study of stress and health, the stress process model emphasizes the role of contextual factors such as socioeconomic disadvantage in shaping exposure to stress (Pearlin 1989), which in turn is thought to affect underlying physiological function across multiple systems (Piazza et al. 2010). Indeed, several studies have found partial support for the mediating role of stress in the relationship between socioeconomic conditions and physiological markers of health (Adler & Snibbe 2003, Chen & Matthews 2001, Chen et al. 2003, Lazzarino et al. 2013, Upchurch et al. 2015). Future research incorporating more advanced life course conceptualizations and measurements is needed to better elucidate the role of stress in mediating the links between socioeconomic conditions and underlying physiology.
Additional mechanisms linking socioeconomic conditions to physiological functioning include social integration and support (John-Henderson et al. 2015, Yang et al. 2013), health behaviors such as diet, alcohol consumption, and cigarette smoking (Colhoun et al. 1998, Lawlor et al. 2004), and physical resources and environment (Dowd et al. 2009, Rundle et al. 2008). While these mechanisms are thought to independently contribute to physical health outcomes, it is important to note that they also have the potential to affect exposure and vulnerability to stress. In other words, the availability of social support, engagement in health-risk behaviors, and exposure to environmental risk are all likely to be tied to the stress process (Pearlin 1989).
While we present how biomarkers have been incorporated in the literature on socioeconomic conditions and health, this approach can also be applied to understanding how other stress-related social exposures are related to underlying physiological processes, such as neighborhood conditions (Chichlowska et al. 2008, Gustafsson et al. 2014), family distress (Danese et al. 2007, Yang et al. 2014), discrimination (Cunningham et al. 2012, Lewis et al. 2010), and social isolation (Shankar et al. 2011; Yang et al. 2013, 2016). Exploration of a spectrum of social experiences will elucidate whether such experiences have unique or overlapping effects on underlying physiological processes. Just as socioeconomic conditions vary over the life course and across generations, so do other stress-related social exposures for which uncovering their time-varying influences is imperative.
Emerging and Future Directions in the Use of Biomarkers in Social Science Research
Interdisciplinary research teams
The development of interdisciplinary research teams has been instrumental for the integration of biomarkers in social stratification and health research. Interdisciplinary research integrates “information, data, techniques, tools, perspectives, concepts, and/or theories from two or more disciplines” (Enhancing the Effectiveness of Team Science 2015), representing the integrative approach embraced by large-scale social and demographic studies that have collected and disseminated biological and social data to the scientific community (Harris 2010). The multidisciplinary Add Health Study, a national longitudinal study of adolescent health and health behavior was a pioneer of this interdisciplinary approach in the national context, including study investigators from sociology, demography, public health, genetics, psychology, biology, medicine, cardiology, survey methodology, economics, geography, and nutrition who designed the study in the early 1990s. Although an individual investigator can master and integrate knowledge from diverse disciplines, this process has become more difficult with the rapid growth of specialized knowledge, technological change in data collection methods and measures, and increasing time investment needed to gain mastery in other disciplines (National Research Council 2015). Researchers using biomarkers for secondary data analysis are often required to add a study principal investigator as a co-author for access to the data in biomedical studies (e.g., ARIC, CARDIA, REGARDS) to ensure appropriate disciplinary application of the biomarker measures. Researchers using other publically available data (e.g., Add Health, HRS, MIDUS, NSHAP) should consult with interdisciplinary scholars on the disciplinary topics for which they have less knowledge and training, and reference specific study documentation on use of the biological data.
Longitudinal data to establish causality
The challenge of identifying causal effects remains omnipresent in most all social science research, including social stratification and health research that integrates biomarkers. The theoretical motivation for integrating biomarkers in social science research is to elaborate how social factors “gets under the skin” to affect biological mechanisms within the body that, in turn, influence social and health outcomes outside the body. By definition, social factors must precede change in biological processes that directly impact health outcomes. Thus, biomarkers enable us to articulate the pathways by which social factors influence health and wellbeing. Evidence on the basis of which to identify causal effects of social factors is therefore subject to the same standards as any social science question (Gangl 2010, Morgan & Winship 2015). In this regard, longitudinal data are especially useful for sorting out cause and effect. For example, having baseline biomarker measures, prior to some social exposure or experience, enables researchers to identify change in that biomarker as a measure of biological response to that exposure.
Identifying a causal relationship requires consideration of the degree to which the social exposure is exogenous or endogenous to the biological stress process measured by the biomarker (Elwert & Winship 2014, Wagner et al. 2013). An endogenous social exposure is one in which some observed or unobserved third factor influences both the social exposure and the biomarker, making their relationship spurious. For example, the social exposures of child neglect or abuse may be caused by family poverty, typically observed, or parental mental health sometimes unobserved, which also affect stress biomarkers. An exogenous social factor is best thought of as a random event such as a natural disaster or policy change, which may have potential causal impacts on biomarker outcomes by directly affecting stress processes of individuals subject to the event. Thus, the degree to which the social exposure of interest is either exogenous or endogenous to biological function informs the appropriate design, statistical approach, and interpretation of causal social effects (Morgan & Winship 2015).
One particular advantage of biomarkers however, with regard to causality, is that there is substantial biomedical research that can causally link health risk, as measured by biomarkers (e.g., high cholesterol, hypertension) with subsequent chronic disease and increased mortality risk (e.g., heart disease, stroke) (Loucks et al. 2008, Xu & Zeger 2001, Zeger 2000). Much of the evidence on causality of these links comes from animal studies with controlled randomization designs. Because we cannot randomly assign human subjects to have higher cholesterol or hypertension, and then observe their disease outcomes at some later point, we cannot definitively determine the direct causal effect of such health risk as measured by biomarkers. For example, diet may be causally linked to both cholesterol and heart disease; without measuring and controlling for diet, some part of the relationship between cholesterol and heart disease is spurious.
Emerging Biological Systems of Interest
Beyond the biomarkers we discuss here, advances in the understanding and measurement of additional and novel biological indicators of health have provided opportunity for new integration of biosocial perspectives in sociological research. These advances include exploration of how the social environment shapes gene regulation (Needham et al. 2015, Stringhini et al. 2015), neurobiological functioning (Brito & Noble 2014, Hackman & Farah 2009, Tomalski et al. 2013), immunological aging (Aiello et al. 2016), and the gut microbiome (Kau et al. 2011, Maier & Al’Absi 2017, NIH HMP Working Group et al. 2009). New quantitative measures of biological age (versus chronological age) have been developed based on a wide range of physical, biological, and genetic markers to represent the progressive deterioration of integrity in bodily systems with advancing age (Belsky et al. 2015, 2016; Levine 2013). In addition, advances in the non-invasive collection of markers related to the stress response will provide additional insight into the physiological mechanisms underlying the social determinants of health. For example, measurement of hair cortisol levels has the potential to provide social scientists with a longitudinal view of the physiological response to stress (Bosma et al. 2015, Vliegenthart et al. 2016).
Conclusion
Given the conceptual shift over the past several decades that underscores the importance of the environment in shaping underlying physiological processes important for health, sociologists need not fear biosocial approaches to understanding complex health outcomes. Rather, examining the physiological impacts of social conditions allows us to document the biological consequences of persistent social inequalities, further justifying the need to intervene on the social conditions that lead to physiological health disparities. The study of health in the social sciences enables identification of biological processes that have important demographic, social, and environmental sources. Indeed, health is a critical element of social stratification research, as health is both a determinant of the processes by which individuals are stratified into subgroups with differential access to resources and mobility, as well as an outcome that is shaped by differential access to resources and mobility (Harris 2010, Harris & McDade 2018). In this review, we have focused mainly on the latter where the bulk of the integrative sociological research has been.
Incorporating biology into models of social stratification and health positions social scientists to explicate the critically important role of social factors in biological function and response that affect health, a universal goal of human life. This provides social scientists with immense power. By convincing biomedical researchers that we have successfully measured and modeled a key aspect of their domain, we can then elevate the role of the social world in the biomedical processes and health outcomes highly valued by biomedical researchers, policymakers, partners, insurance companies, and individuals across all social classes, races, ethnicities and genders. We hold the power to point to the social levers for direct intervention to reduce health and social disparities and promote the wellbeing of individuals, families and society as a whole.
Footnotes
National Longitudinal Study of Adolescent to Adult Health, see www.cpc.unc.edu/addhealth.
Contributor Information
Kathleen Mullan Harris, University of North Carolina at Chapel Hill.
Kristen M. Schorpp, Roanoke College
References
- Adam EK, Kumari M. 2009. Assessing salivary cortisol in large-scale, epidemiological research. Psychoneuroendocrinology. 34(10): 1423–36 [DOI] [PubMed] [Google Scholar]
- Adler NE, Boyce T, Chesney MA, Cohen S, Folkman S, et al. 1994. Socioeconomic status and health: The challenge of the gradient. Am. Psychol. 49(1):15–24 [DOI] [PubMed] [Google Scholar]
- Adler NE, Ostrove JM. 1999. Socioeconomic Status and Health: What We Know and What We Don’t. Ann. N. Y. Acad. Sci. 896(1):3–15 [DOI] [PubMed] [Google Scholar]
- Adler NE, Snibbe AC. 2003. The Role of Psychosocial Processes in Explaining the Gradient Between Socioeconomic Status and Health. Curr. Dir. Psychol. Sci. 12(4): 119–23 [Google Scholar]
- Aiello AE, Feinstein L, Dowd JB, Pawelec G, Derhovanessian E, et al. 2016. Income and Markers of Immunological Cellular Aging. Psychosom. Med. 78(6):657–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida DM, Wong JD. 2009. Life transition and stress: A life course perspective on daily stress processes In The Craft of Life Course Research, eds. Elder GH, Giele JZ, pp. 141–62. New York: Guilford Press [Google Scholar]
- Almond D, Currie J. 2011. Killing Me Softly: The Fetal Origins Hypothesis. J. Econ. Perspect. 25(3): 153–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman CE, Van Hook J, Hillemeier M. 2016. What Does Self-rated Health Mean? Changes and Variations in the Association of Obesity with Objective and Subjective Components Of Self-rated Health. J. Health Soc. Behav. 57(1):39–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Journal of Public Health. 2013. Am. J. Public Health. 103(S1): [Google Scholar]
- Aneshensel CS. 1992. Social Stress - Theory and Research. Annu. Rev. Sociol. 18(1): 15–38 [Google Scholar]
- Anne Case A, Christina Paxson C. 2010. Causes and Consequences of Early-Life Health. Demography. 47(S):S65–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball K, Crawford D. 2005. Socioeconomic status and weight change in adults: A review [DOI] [PubMed] [Google Scholar]
- Barker DJ. 1998. In utero programming of chronic disease. Clin. Sci. 95(2): 115–28 [PubMed] [Google Scholar]
- Barker DJ. 2006. Adult Consequences of Fetal Growth Restriction. Clin. Obstet. Gynecol. 49(2):270–83 [DOI] [PubMed] [Google Scholar]
- Barker DJP. 1997. Maternal nutrition, fetal nutrition, and disease in later life. Nutrition. 13(9):807–13 [DOI] [PubMed] [Google Scholar]
- Baum A, Garofalo JP, Yali AM. 1999. Socioeconomic Status and Chronic Stress: Does Stress Account for SES Effects on Health? Ann. N. Y. Acad. Sci. 896(1): 131–44 [DOI] [PubMed] [Google Scholar]
- Belsky DW, Caspi A, Houts R, Cohen HJ, Corcoran DL, et al. 2015. Quantification of biological aging in young adults. Proc. Natl. Acad. Sci. U. S. A. 112(30):E4104–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky DW, Moffitt TE, Cohen AA, Corcoran DL, Levine ME, et al. 2016. Telomere, epigenetic clock, and biomarker-composite quantifications of biological aging: Do they measure the same thing? bioRxiv. 71373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shlomo Y, Kuh D. 2002. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. Int J Epidemiol. 31(2):285–93 [PubMed] [Google Scholar]
- Bengtsson T, Broström G. 2009. Do conditions in early life affect old-age mortality directly and indirectly? Evidence from 19th-century rural Sweden. Soc. Sci. Med. 68(9):1583–90 [DOI] [PubMed] [Google Scholar]
- Berkman LF, Glass T, Brissette I, Seeman TE. 2000. From social integration to health: Durkheim in the new millennium. Soc. Sci. Med. 51:843–57 [DOI] [PubMed] [Google Scholar]
- Berkman LF, Syme LS. 1979. Social networks, host resitance, and mortality: a nine year follow- up study of alameda country residents. Am. J. Epidemiol. 109(186–204):186–204 [DOI] [PubMed] [Google Scholar]
- Blackwell DL, Hayward MD, Crimmins EM. 2001. Does childhood health affect chronic morbidity in later life? Soc. Sci. Med. 52(8):1269–84 [DOI] [PubMed] [Google Scholar]
- Boen C, Yang YC. 2016. The physiological impacts of wealth shocks in late life: Evidence from the Great Recession. Soc. Sci. Med. 150:221–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma H, Golsteyn B, Groffen D, Schils T, Stalder T, et al. 2015. The Socioeconomic Patterning of Perceived Stress and Hair Cortisol in Dutch 10–12 Year Olds. Int. J. Public Heal. Epidemiol. 4(8):195–97 [Google Scholar]
- Breitling LP, Saum K-U, Perna L, Schöttker B, Holleczek B, Brenner H. 2016. Frailty is associated with the epigenetic clock but not with telomere length in a German cohort. Clin. Epigenetics. 8:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito NH, Noble KG. 2014. Socioeconomic status and structural brain development. Front. Neurosci. 8:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning CR, Cagney KA. 2003. Moving beyond poverty: Neighborhood structure, social processes, and health. J. Health Soc. Behav. 44(4): 552–71 [PubMed] [Google Scholar]
- Brummett BH, Babyak MA, Siegler IC, Harris KM, Elder GH, et al. 2012. Systolic blood pressure, socioeconomic status, and biobehavioral risk factors in a nationally representative US youg dult sample. Hypertension. 58(2):161–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummett BH, Babyak MA, Singh A, Jiang R, Williams RB, et al. 2013. Socioeconomic indices as independent correlates of C-reactive protein in the National Longitudinal Study of Adolescent Health. Psychosom. Med. 75(9):882–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner E, Marmot MG, Nanchahal K, Shipley MJ, Stansfeld SA, et al. 1997. Social inequality in coronary risk: central obesity and the metabolic syndrome. Evidence from the Whitehall II study. Diabetologia. 40(11): 1341–49 [DOI] [PubMed] [Google Scholar]
- Bury M 1997. Health and Illness in a Changing Society. London: Routledge [Google Scholar]
- Cameron N, Demerath EW. 2002. Critical periods in human growth and their relationship to diseases of aging. Yearb. Phys. Anthropol. 45(S35):159–84 [DOI] [PubMed] [Google Scholar]
- Carroll JE, Cohen S, Marsland AL. 2011. Early childhood socioeconomic status is associated with circulating interleukin-6 among mid-life adults. Brain. Behav. Immun. 25(7):1468–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BH, Marioni RE, Colicino E, Peters MJ, Ward-Caviness CK, et al. 2016. DNA methylation-based measures of biological age: meta-analysis predicting time to death. Aging (Albany. NY). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Fisher EB, Bacharier LB, Strunk RC. 2003. Socioeconomic Status, Stress, and Immune Markers in Adolesce... : Psychosomatic Medicine. Psychosom. Med. 65(6):984–92 [DOI] [PubMed] [Google Scholar]
- Chen E, Matthews K. 2001. Cognitive appraisal bias: An approach to understanding the relation between socioeconomic status and cardiovascular reactivity in children. Ann Behav Med. 23(2): 101–11 [DOI] [PubMed] [Google Scholar]
- Chichlowska KL, Rose KM, Diez-Roux AV, Golden SH, McNeill AM, et al. 2008. Individual and Neighborhood Socioeconomic Status Characteristics and Prevalence of Metabolic Syndrome. The Atherosclerosis Risk in Communities (ARIC) Study. Psychosom Med. 70(9):986–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AA, Milot E, Yong J, Seplaki CL, Fülöp T, et al. 2013. A novel statistical approach shows evidence for multi-system physiological dysregulation during aging. Mech. Ageing Dev. 134(3—4): 110–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Turner RB, Alper CM, Skoner DP. 2004. Childhood Socioeconomic Status and Host Resistance to Infectious Illness in Adulthood. Psychosom. Med. 66(4):553–58 [DOI] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Chen E, Matthews KA. 2010. Childhood socioeconomic status and adult health [DOI] [PubMed] [Google Scholar]
- Colhoun HM, Hemingway H, Poulter NR. 1998. Socio-economic status and blood pressure: an overview analysis. J. Hum. Hypertens. 12:91–110 [DOI] [PubMed] [Google Scholar]
- Conley D 2016. Socio-Genomic Research Using Genome-Wide Molecular Data. Annu. Rev. Sociol. 42(1):275–99 [Google Scholar]
- Cooper R, Cutler J, Desvigne-Nickens P, Fortmann SP, Friedman L, et al. 2000. Trends and Disparities in Coronary Heart Disease, Stroke, and Other Cardiovascular Diseases in the United States. Circulation. 102(25): [DOI] [PubMed] [Google Scholar]
- Crimmins E, Kim JKI, Vasunilashorn S. 2010. Biodemography: New Approaches to Understanding Trends and Differences in Population Health and Mortality*. Demography. 47(Supplement):S41–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins E, Vasunilashorn S, Kim JK, Alley D. 2008. Chapter 5 Biomarkers Related To Aging In Human Populations [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins EM, Faul J, Kim JK, Guyer H, Langa K, et al. 2013. Documentation of Biomarkers in the 2006 and 2008 Health and Retirement Study [Google Scholar]
- Crimmins EM, Finch CE. 2006. Infection, inflammation, height, and longevity. Proc. Natl. Acad. Sci. U. S. A. 103(2):498–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins EM, Seeman TE. 2004. Integrating Biology into the Study of Health Disparities. Popul. Dev. Rev. 30:89–107 [Google Scholar]
- Crimmins EM, Vasunilashorn S. 2016. Biodemography: Adding Biological Insight into Social, Economic, and Psychological Models of Population and Individual Health Change with Age In Handbook of Aging and the Social Sciences, eds. L George KF Ferraro, pp. 55–72. Elsevier Science; 8th Editio ed. [Google Scholar]
- Crosnoe R, Muller C. 2004. Body Mass Index, Academic Achievement, and School Context: Examining the Educational Experiences of Adolescents at Risk of Obesity. J. Health Soc. Behav. 45(4):393–407 [DOI] [PubMed] [Google Scholar]
- Cunningham TJ, Seeman TE, Kawachi I, Gortmaker SL, Jacobs DR, et al. 2012. Racial/ethnic and gender differences in the association between self-reported experiences of racial/ethnic discrimination and inflammation in the CARDIA cohort of 4 US communities. Soc. Sci. Med. 75(5):922–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Moffitt TE, Harrington H, Milne BJ, Polanczyk G, et al. 2009. Adverse Childhood Experiences and Adult Risk Factors for Age-Related Disease. Arch. Pediatr. Adolesc. Med. 163(12): 1090–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. 2007. Childhood maltreatment predicts adult inflammation in a life-course study. Proc. Natl. Acad. Sci. U. S. A. 104(4):1319–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedeurwaerder S, Defrance M, Calonne E, Denis H, Sotiriou C, Fuks F. 2011. Evaluation of the Infinium Methylation 450K technology. Epigenomics. 3(6):771–84 [DOI] [PubMed] [Google Scholar]
- Doom JR, Mason SM, Suglia SF, Clark CJ. 2017. Pathways between childhood/adolescent adversity, adolescent socioeconomic status, and long-term cardiovascular disease risk in young adulthood. Soc. Sci. Med. 188:166–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd JB, Zajacova A. 2010. Does self-rated health mean the same thing across socioeconomic groups? Evidence from biomarker data. Ann. Epidemiol. 20(10):743–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd JB, Zajacova A, Aiello A. 2009. Early origins of health disparities: Burden of infection, health, and socioeconomic status in U.S. children. Soc. Sci. Med. 68(4):699–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duster T 1990. Backdoor to Eugenics. New York: Routledge [Google Scholar]
- Elder GH. 1998. The Life Course as Developmental Theory. Child Dev. 69(1): 1–12 [PubMed] [Google Scholar]
- Elder GHJ, Johnson MK, Crosnoe R. 2004. The emergence and development of life course theory In Handbook of the Life Course, pp. 3–19. Boston, MA: Springer US [Google Scholar]
- Jr Elder GH., Shanahan MJ 2007. The Life Course and Human Development. Handb. Child Psychol. 665–715 [Google Scholar]
- Elo IT, Preston SH. 1992. Effects of Early-Life Conditions on Adult Mortality: A Review. Popul. Index. 58(2):186. [PubMed] [Google Scholar]
- Elwert F, Winship C. 2014. Endogenous Selection Bias: The Problem of Conditioning on a Collider Variable. Annu. Rev. Sociol. 40(1):31–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enhancing the Effectiveness of Team Science. 2015 [PubMed]
- Entzel P, Whitsel E, Richardson A, Tabor J, Hallquist S, et al. 2009. Add Health Wave IV Documentation: Cardiovascular and anthropometric measures. See http//www. ….1–16
- Feinstein JS. 1993. The relationship between socio-economic status and health: a review of the literature. Milbank Q. 71(2):279–322 [PubMed] [Google Scholar]
- Feldman PJ, Steptoe A. 2003. Psychosocial and socioeconomic factors associated with glycated hemoglobin in nondiabetic middle-aged men and women. Heal. Psychol. 22(4):398–405 [DOI] [PubMed] [Google Scholar]
- Ferraro KF, Farmer MM. 1999. Utility of Health Data from Social Surveys: Is There a Gold Standard for Measuring Morbidity? Am. Sociol. Rev. 64(2):303 [Google Scholar]
- Ferraro KF, Shippee TP. 2009. Aging and cumulative inequality: How does inequality get under the skin? Gerontologist. 49(3):333–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch C 2010. The Biology of Human Longevity: Inflammation, Nutrition, and Aging in the Evolution of Lifespans. Academic Press [Google Scholar]
- Ford ES, Loucks EB, Berkman LF. 2006. Social integration and concentrations of C-reactive protein among US adults. Ann. Epidemiol. 16(2):78–84 [DOI] [PubMed] [Google Scholar]
- Franks P, Gold MR, Fiscella K. 2003. Sociodemographics, self-rated health, and mortality in the US. Soc. Sci. Med. 56(12):2505–14 [DOI] [PubMed] [Google Scholar]
- Freese J, Li J-CA, Wade LD. 2003. The Potential Relevances of Biology to Social Inquiry. Annu. Rev. Sociol. 29(1):233–56 [Google Scholar]
- Fremont AM, Bird CE. 1999. Integrating Sociological and Biological Models: An Editorial. J. Health Soc. Behav. 40(2):126. [PubMed] [Google Scholar]
- Friedman EM, Karlamangla AS, Gruenewald TL, Koretz B, Seeman TE. 2015. Early Life Adversity and Adult Biological Risk Profiles. Psychosom. Med. 77(2):176–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea S, Tracy M, Hoggatt KJ, DiMaggio C, Karpati A. 2011. Estimated Deaths Attributable to Social Factors in the United States. Am. J. Public Health. 101(8): 1456–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galobardes B, Smith GD, Lynch JW. 2006. Systematic review of the influence of childhood socioeconomic circumstances on risk for cardiovascular disease in adulthood [DOI] [PubMed] [Google Scholar]
- Gangl M 2010. Causal Inference in Sociological Research. Annu. Rev. Sociol. 36(1):21–47 [Google Scholar]
- Geronimus AT, Hicken M, Keene D, Bound J. 2006. “Weathering” and Age Patterns of Allostatic Load Scores Among Blacks and Whites in the United States. Am. J. Public Health. 96(5):826–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles-Corti B 2002. Socioeconomic Status Differences in Recreational Physical Activity Levels and Real and Perceived Access to a Supportive Physical Environment. Prev. Med. (Baltim). 35(6):601–11 [DOI] [PubMed] [Google Scholar]
- Giskes K, van Lenthe FJ, Turrell G, Kamphuis CBM, Brug J, Mackenbach JP. 2008. Socioeconomic Position at Different Stages of the Life Course and Its Influence on Body Weight and Weight Gain in Adulthood: A Longitudinal Study With 13-Year Follow-up. Obesity. 16(6):1377–81 [DOI] [PubMed] [Google Scholar]
- Gliksman MD, Kawachi I, Hunter D, Colditz GA, Manson JE, et al. 1995. Childhood socioeconomic status and risk of cardiovascular disease in middle aged US women: a prospective study. J. Epidemiol. Community Health. 49(1): 10–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Cooper C, Thornburg KL. 2008. Effect of in utero and early-life conditions and adult health and disease. N. Engl. J. Med. 359(1): 1523–1524; author reply 1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graunt J. 1662. Natural and Political Observations Mentioned in a Following Index, and Made upon the Bills of Mortality. London
- Gruenewald TL, Cohen S, Matthews KA, Tracy R, Seeman TE. 2009. Association of socioeconomic status with inflammation markers in black and white men and women in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Soc. Sci. Med 69(3):451–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenewald TL, Karlamangla AS, Hu P, Stein-Merkin S, Crandall C, et al. 2012. History of socioeconomic disadvantage and allostatic load in later life. Soc. Sci. Med. 74(1):75–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzywacz JG, Almeida DM. 2008. Stress and Binge Drinking: A Daily Process Examination of Stressor Pile-up and Socioeconomic Status in Affect Regulation. Int. J. Stress Manag. 15(4):364–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson PE, Janlert U, Theorell T, Westerlund H, Hammarstrom A. 2011a. Socioeconomic status over the life course and allostatic load in adulthood: results from the Northern Swedish Cohort. J. Epidemiol. Community Heal. 65(11):986–92 [DOI] [PubMed] [Google Scholar]
- Gustafsson PE, Miguel SS, Janlert U, Theorell T, Westerlund H, Hammarstrom A. 2014. Life-Course accumulation of neighborhood disadvantage and allostatic load: Empirical integration of three social determinants of health frameworks. Am. J. Public Health. 104(5):904–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson PE, Persson M, Hammarstrom A. 2011b. Life Course Origins of the Metabolic Syndrome in Middle-Aged Women and Men: The Role of Socioeconomic Status and Metabolic Risk Factors in Adolescence and Early Adulthood. Ann. Epidemiol. 21(2):103–10 [DOI] [PubMed] [Google Scholar]
- Hackman DA, Farah MJ. 2009. Socioeconomic status and the developing brain [DOI] [PMC free article] [PubMed]
- Hallqvist J, Lynch J, Bartley M, Lang T, Blane D. 2004. Can we disentangle life course processes of accumulation, critical period and social mobility? An analysis of disadvantaged socio-economic positions and myocardial infarction in the Stockholm Heart Epidemiology Program. Soc. Sci. Med. 58(8):1555–62 [DOI] [PubMed] [Google Scholar]
- Halpern CT, Harris KM, Whitsel EA. 2014. Studying Family Transitions from a Systems Perspective: The Role of Biomarkers In Emerging Methods in Family Research, eds. McHale SM, Amato PR, Booth A, pp. 127–44. Springer International Publishing [Google Scholar]
- Hanson MD, Chen E. 2007. Socioeconomic status and health behaviors in adolescence: A review of the literature [DOI] [PubMed] [Google Scholar]
- Harris KM. 2010. An integrative approach to health. Demography. 47(1):1–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, McDade TW. 2018. The biosocial approach to human development, behavior, and health across the life course. Russell Sage Found. J. Soc. Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzenbuehler ML, Slopen N, McLaughlin KA. 2014. Stressful life events, sexual orientation, and cardiometabolic risk among young adults in the United States. Heal. Psychol. 33(10): 1185–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward MD, Gorman BK. 2004. The Long Arm of Childhood: The Influence of Early-Life Social Conditions on Men’s Mortality. Demography. 41(1):87–107 [DOI] [PubMed] [Google Scholar]
- Hertzman C, Boyce T. 2010. How Experience Gets Under the Skin to Create Gradients in Developmental Health. Annu. Rev. Public Heal. 31:329–47 [DOI] [PubMed] [Google Scholar]
- Horvath S 2013. DNA methylation age of human tissues and cell types. Genome Biol. 14(10):R115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Ross KM, Chen E, Miller GE. 2017. Early-Life Socioeconomic Disadvantage and Metabolic Health Disparities. Psychosom. Med. 79(5):514–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- House JS. 2002. Understanding social factors and inequalities in health: 20th century progress and 21st century prospects. JHeal. Soc Behav. 43(43):125–42 [PubMed] [Google Scholar]
- House JS, Landis KR, Umberson D. 1988. Social relationships and health. Science (80-.). 241:540–45 [DOI] [PubMed] [Google Scholar]
- House JS, Lepkowski JM, Kinney AM, Mero RP, Kessler RC, Herzog AR. 1994. The social stratification of aging and health. J.Health Soc.Behav.1994.Sep. 35(3):213. [PubMed] [Google Scholar]
- Jacobs JA, Frickel S. 2009. Interdisciplinarity: A Critical Assessment. Annu. Rev. Sociol. 35(1):43–65 [Google Scholar]
- James SA, Keenan NL, Strogatz DS, Browning SR, Garrett JM. 1992. Socioeconomic Status, John Henryism, and Blood Pressure in Black Adults. Am. J. Epidemiol. 135(1):59–67 [DOI] [PubMed] [Google Scholar]
- Jensen SKG, Berens AE, Nelson CA. 2017. Review Effects of poverty on interacting biological systems underlying child development. Lancet ChildAdolesc. Heal. [DOI] [PubMed] [Google Scholar]
- John-Henderson NA, Stellar JE, Mendoza-Denton R, Francis DD. 2015. Socioeconomic Status and Social Support. Psychol. Sci. 26(10):1620–29 [DOI] [PubMed] [Google Scholar]
- Kanjilal S, Gregg EW, Cheng YJ, et al. 2006. Socioeconomic status and trends in disparities in 4 major risk factors for cardiovascular disease among us adults, 1971–2002. Arch. Intern. Med. 166(21):2348–55 [DOI] [PubMed] [Google Scholar]
- Kapuku GK, Treiber FA, Davis HC. 2002. Relationships among socioeconomic status, stress induced changes in cortisol, and blood pressure in african american males. Ann. Behav. Med. 24(4):320–25 [DOI] [PubMed] [Google Scholar]
- Karlamangla AS, Gruenewald TL, Seeman TE. 2012. Promise of Biomarkers in Assessing and Predicting Health In The Biological Consequences of Socioeconomic Inequalities, pp. 38–62. New York: Russell Sage Foundation [Google Scholar]
- Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. 2011. Human nutrition, the gut microbiome and the immune system. Nature. 474(7351):327–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe R, Wu S-Y, Leske MC, Chylack LT. 1994. Comparing Self-reported and Physician- reported Medical History. Am. J. Epidemiol. 139(8):813–18 [DOI] [PubMed] [Google Scholar]
- Kessler RC. 1979. Stress, Social Status, and Psychological Distress. J. Health Soc. Behav. 20(3):259–72 [PubMed] [Google Scholar]
- Kuh D, Ben-Shlomo Y. 1997. A Life Course Approach to Chronic Disease Epidemiology. Oxford: Oxford University Press; [PubMed] [Google Scholar]
- Langenberg C, Hardy R, Kuh D, Brunner E, Wadsworth M. 2003. Central and total obesity in middle aged men and women in relation to lifetime socioeconomic status: evidence from a national birth cohort. J Epidemiol Community Heal. 57:816–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantz PM, House JS, Lepkowski JM, Williams DR, Mero RP, Chen J. 1998. Socioeconomic Factors, Health Behaviors, and Mortality. JAMA. 279(21):1703. [DOI] [PubMed] [Google Scholar]
- Lawlor DA, Davey Smith G, Ebrahim S. 2004. Association between childhood socioeconomic status and coronary heart disease risk among postmenopausal women. Findings from the British Women’s, Heart and Health Study. Am. J. Public Health. 94(8):1386–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzarino AI, Hamer M, Stamatakis E, Steptoe A. 2013. Low Socioeconomic Status and Psychological Distress as Synergistic Predictors of Mortality From Stroke and Coronary Heart Disease. Psychosom. Med. 75(3):311–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Harris KM, Gordon-Larsen P. 2009. Life Course Perspectives on the Links between Poverty and Obesity during the Transition to Young Adulthood. Popul. Res. Policy Rev. 28(4):505–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng B, Jin Y, Li G, Chen L, Jin N. 2015. Socioeconomic status and hypertension. J. Hypertens. 33(2):221–29 [DOI] [PubMed] [Google Scholar]
- Levine ME. 2013. Modeling the rate of senescence: Can estimated biological age predict mortality more accurately than chronological age? Journals Gerontol. Ser. A Biol. Sci. Med. Sci. 68(6):667–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S 1995. Time for creative integration in medical sociology. J. Health Soc. Behav. Spec No(1995):1–4 [PubMed] [Google Scholar]
- Lewis TT, Aiello AE, Leurgans S, Kelly J, Barnes LL. 2010. Self-reported experiences of everyday discrimination are associated with elevated C-reactive protein levels in older African-American adults. Brain. Behav. Immun. 24(3):438–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindau ST, McDade TW. 2008. Minimally Invasive and Innovative Methods for Biomeasure Collection in Population-Based Research In Surveys Biosocial, eds. Weinstein M, Vaupel JW, Wachter KW, pp. 251–77. Washington, DC: The National Academies Press [Google Scholar]
- Link BG. 2008. Epidemiological Sociology and the Social Shaping of Population Health. J. Health Soc. Behav. 49(4):367–84 [DOI] [PubMed] [Google Scholar]
- Link BG, Phelan J. 1995. Social Conditions As Fundamental Causes of Disease. J Heal. Soc Behav. 80–94 [PubMed] [Google Scholar]
- Liu RS, Aiello AE, Mensah FK, Gasser CE, Rueb K, et al. 2017. Socioeconomic status in childhood and C reactive protein in adulthood: a systematic review and meta-analysis. J. Epidemiol. Community Health. 71(8): 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loucks EB, Juster RP, Pruessner JC. 2008. Neuroendocrine biomarkers, allostatic load, and the challenge of measurement: A commentary on Gersten. Soc. Sci. Med. 66(3):525–30 [Google Scholar]
- Loucks EB, Magnusson KT, Cook S, Rehkopf DH, Ford ES, Berkman LF. 2007. Socioeconomic Position and the Metabolic Syndrome in Early, Middle, and Late Life: Evidence from NHANES 1999–2002. Ann. Epidemiol. 17(10):782–90 [DOI] [PubMed] [Google Scholar]
- Love GD, Seeman TE, Weinstein M, Ryff CD. 2010. Bioindicators in the MIDUS National Study: Protocol, Measures, Sample, and Comparative Context. J. Aging Health. 22(8): 1059–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Waite LJ. 2005. The impact of childhood and adult SES on physical, mental, and cognitive well-being in later life. J. Gerontol. B. Psychol. Sci. Soc. Sci. 60(2):S93–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupein SJ, Ouellet-Morin I, Hupback A, Tu MT, Buss C, et al. 2006. Beyond the Stress Concept: Allostatic Load--A Developmental, Biological, and Cognitive Perspective. In Developmental Psychopathology: Developmental Neuroscience, pp. 578–628 [Google Scholar]
- Mackenbach JP, Cavelaars AEJM, Kunst AE, Groenhof, Andersen O, et al. 2000. Socioeconomic inequalities in cardiovascular disease mortality. An international study. Eur. Heart J. 21(14):1141–51 [DOI] [PubMed] [Google Scholar]
- Maier KJ, Al’Absi M. 2017. Toward a Biopsychosocial Ecology of the Human Microbiome, Brain-Gut Axis, and Health. Psychosom. Med. 79(8):1. [DOI] [PubMed] [Google Scholar]
- Marino M, Li Y, Rueschman MN, Winkelman JW, Ellenbogen JM, et al. 2013. Measuring Sleep: Accuracy, Sensitivity, and Specificity of Wrist Actigraphy Compared to Polysomnography. Sleep. 36(11): 1747–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmot M, Shipley M, Brunner E, Hemingway H. 2001. Relative contribution of early life and adult socioeconomic factors to adult morbidity in the Whitehall II study. J. Epidemiol. Community Health. 55(5):301–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmot MG, Bosma H, Hemingway H, Brunner E, Stansfeld S. 1997. Contribution of job control and other risk factors to social variations in coronary heart disease incidence. Lancet. 350(9073):235–39 [DOI] [PubMed] [Google Scholar]
- Marmot MG, Stansfeld S, Patel C, North F, Head J, et al. 1991. Health inequalities among British civil servants: the Whitehall II study. Lancet. 337(8754): 1387–93 [DOI] [PubMed] [Google Scholar]
- Massey DS. 2004. Segregation and Stratification: A Biosocial Perspective. Du Bois Rev. 1(1):7–25 [Google Scholar]
- McDade TW, Williams S, Snodgrass JJ. 2007. What a Drop Can Do : Dried Blood Spots As a minimally invasive method for integrating biomarkers into population-based research. Demography. 44(4):899–925 [DOI] [PubMed] [Google Scholar]
- McDade TW. 2005. Life history, maintenance, and the early origins of immune function. Am. J. Hum. Biol. 17(1):81–94 [DOI] [PubMed] [Google Scholar]
- McDade TW. 2008. Challenges and opportunities for integrative health research in the context of culture: A commentary on Gersten. Soc. Sci. Med. 66(3):520–24 [Google Scholar]
- M. McDade TW L. Ross K Fried R, Arevalo JMG, Ma J, et al. 2016. Genome-Wide Profiling of RNA from Dried Blood Spots: Convergence with Bioinformatic Results Derived from Whole Venous Blood and Peripheral Blood Mononuclear Cells. Biodemography Soc. Biol. 62(2): 182–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. 1998. Protective and Damaging Effects of Stress Mediators. Library (Lond). 338(3): 171–79 [DOI] [PubMed] [Google Scholar]
- McEwen BS, Seeman T. 1999. Protective and Damaging Effects of Mediators of Stress: Elaborating and Testing the Concepts of Allostasis and Allostatic Load. Ann. N. Y. Acad. Sci. 896(1):30–47 [DOI] [PubMed] [Google Scholar]
- McGrath JJ, Matthews KA, Brady SS. 2006. Individual versus neighborhood socioeconomic status and race as predictors of adolescent ambulatory blood pressure and heart rate. Soc. Sci. Med. 63(6):1442–53 [DOI] [PubMed] [Google Scholar]
- McLaren L. Socioeconomic status and obesity. 2007 doi: 10.1093/epirev/mxm001. [DOI] [PubMed] [Google Scholar]
- Merkin SS, Karlamangla A, Roux AVD, Shrager S, Seeman TE. 2014. Life course socioeconomic status and longitudinal accumulation of allostatic load in adulthood: Multi-Ethnic study of atherosclerosis. Am. J. Public Health. 104(4):e48–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Fok AK, Walker H, Lim A, et al. 2009. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc. Natl. Acad. Sci. U. S. A. 106(34):14716–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Cohen S, Ritchey AK. 2002. Chronic psychological stress and the regulation of proinflammatory cytokines: A glucocorticoid-resistance model. Heal. Psychol. 21(6):531–41 [DOI] [PubMed] [Google Scholar]
- Moore LV, Diez Roux A V, Evenson KR, McGinn AP, Brines SJ. 2008. Availability of Recreational Resources in Minority and Low Socioeconomic Status Areas. Am. J. Prev. Med. 34(1):16–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan SL, Winship C. 2015. Counterfactuals and Causal Inference: Methods and Principles for Social Research. Cambridge University Press. Second Edi ed. [Google Scholar]
- Needham BL, Smith JA, Zhao W, Wang X, Mukherjee B, et al. 2015. Life course socioeconomic status and DNA methylation in genes related to stress reactivity and inflammation: The multi-ethnic study of atherosclerosis. Epigenetics. 10(10):958–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newacheck PW, Hung YY, Park MJ, Brindis CD, Irwin CE. 2003. Disparities in Adolescent Health and Health Care: Does Socioeconomic Status Matter? [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen QC, Tabor JW, Entzel PP, Lau Y, Suchindran C, et al. 2011. Discordance in national estimates of hypertension among young adults. Epidemiology. 22(4):532–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH HMP Working Group TNHW, Peterson J, Garges S, Giovanni M, McInnes P, et al. 2009. The NIH Human Microbiome Project. Genome Res. 19(12):2317–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Non AL, Rewak M, Kawachi I, Gilman SE, Loucks EB, et al. 2014. Childhood Social Disadvantage, Cardiometabolic Risk, and Chronic Disease in Adulthood. Am. J. Epidemiol. 180(3):263–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rand AM, Hamil-Luker J. 2005. Processes of Cumulative Adversity: Childhood Disadvantage and Increased Risk of Heart Attack Across the Life Course. Journals Gerontol. Ser. B 60(Special_Issue_2):S117–24 [DOI] [PubMed] [Google Scholar]
- Ogden CL, Lamb MM, Carroll MD, Flegal KM. 2010. Obesity and socioeconomic status in children and adolescents: United States, 2005–2008. NCHSData Brief. 127(51): 1–8 [PubMed] [Google Scholar]
- Palloni A 2006. Reproducing Inequalities: Luck, Wallets, and the Enduring Effects of Childhood Health. Demography. 43(4):587–615 [DOI] [PubMed] [Google Scholar]
- Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. 2003. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, 1988–1994. Arch. Intern. Med. 163(4):427–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlin LI. 1989. The Sociological Study of Stress. J. Health Soc. Behav. 30(3):241. [PubMed] [Google Scholar]
- Pearlin LI, Menaghan EG, Lieberman MA, Mullan JT. 1981. The Stress Process. J. Health Soc. Behav. 22(4):337. [PubMed] [Google Scholar]
- Piazza JR, Almeida DM, Dmitrieva NO, Klein LC. 2010. Frontiers in the use of biomarkers of health in research on stress and aging. J. Gerontol. Psychol. Sci. 65(5):513–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollitt RA, Kaufman JS, Rose KM, Diez-Roux AV., Zeng D, Heiss G. 2007. Early-life and adult socioeconomic status and inflammatory risk markers in adulthood. Eur. J. Epidemiol. 22(1):55–66 [DOI] [PubMed] [Google Scholar]
- Pollitt RA, Rose KM, Kaufman JS. 2005. Evaluating the evidence for models of life course socioeconomic factors and cardiovascular outcomes: a systematic review. BMC Public Health. 5(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popkin BM, Adair LS, Ng SW. 2012. Global nutrition transition and the pandemic of obesity in developing countries. Nutr. Rev. 70(1):3–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter AL, Rafols I. 2009. Is science becoming more interdisciplinary? Measuring and mapping six research fields over time. Scientometrics. 81(3):719–45 [Google Scholar]
- Poulton R, Caspi A, Milne BJ, Thomson WM, Taylor A, et al. 2002. Association between children’s experience of socioeconomic disadvantage and adult health: A life-course study. Lancet. 360(9346):1640–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power C, Graham H, Due P, Hallqvist J, Joung I, et al. 2005. The contribution of childhood and adult socioeconomic position to adult obesity and smoking behaviour: an international comparison. Int. J. Epidemiol. 34(2):335–44 [DOI] [PubMed] [Google Scholar]
- Preston SH, Hill ME, Drevenstedt GL. 1998. Childhood conditions that predict survival to advanced ages among African-Americans. Soc. Sci. Med. 47(9):1231–46 [DOI] [PubMed] [Google Scholar]
- Pudrovska T, Anikputa B. 2014. Early-life socioeconomic status and mortality in later life: An integration of four life-course mechanisms [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM. 2003. C-Reactive Protein. Circulation. 108(12): [DOI] [PubMed] [Google Scholar]
- Ross CE, Mirowsky J. 2001. Neighborhood Disadvantage, Disorder, and Health*. J. Heal. Soc. Behav. J. Heal. Soc. J. Heal. Soc. Behav. 4221924733(42):258–76 [PubMed] [Google Scholar]
- Rundle A, Field S, Park Y, Freeman L, Weiss CC, Neckerman K. 2008. Personal and neighborhood socioeconomic status and indices of neighborhood walk-ability predict body mass index in New York City. Soc. Sci. Med. 67(12): 1951–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharoun-Lee M, Kaufman JS, Popkin BM, Gordon-Larsen P. 2009. Obesity, race/ethnicity and life course socioeconomic status across the transition from adolescence to adulthood. J. Epidemiol. Community Health. 63(2):133–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selye H 1956. The Stress of Life. New York: McGraw Hill Publishing [Google Scholar]
- Shanahan MJ, Hofer SM, Shanahan L. 2003. Biological Models of Behavior and the Life Course In Handbook of Sociology and Social Research, pp. 597–622. Springer Nature [Google Scholar]
- Shankar A, McMunn A, Banks J, Steptoe A. 2011. Loneliness, social isolation, and behavioral and biological health indicators in older adults. Heal. Psychol. 30(4):377–85 [DOI] [PubMed] [Google Scholar]
- Shishehbor MH, Litaker D, Pothier CE, Lauer MS. 2006. Association of Socioeconomic Status With Functional Capacity, Heart Rate Recovery, and All-Cause Mortality. JAMA. 295(7):784. [DOI] [PubMed] [Google Scholar]
- Shrewsbury V, Wardle J. 2008. Socioeconomic Status and Adiposity in Childhood: A Systematic Review of Cross-sectional Studies 1990–2005. Obesity. 16(2):275–84 [DOI] [PubMed] [Google Scholar]
- Singer BH, Ryff CD. 2001. New Horizons in Health: An Integrative Approach. Washington, DC: National Academy Press; [PubMed] [Google Scholar]
- Slopen N, Goodman E, Koenen KC, Kubzansky LD, Ford E. 2013. Socioeconomic and Other Social Stressors and Biomarkers of Cardiometabolic Risk in Youth: A Systematic Review of Less Studied Risk Factors. PLoS One. 8(5):e64418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CJ, Ryckman KK. 2015. Epigenetic and developmental influences on the risk of obesity, diabetes, and metabolic syndrome. Diabetes. Metab. Syndr. Obes. 8:295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JP. 1999. Healthy bodies and thick wallets: the dual relation between health and economic status. J. Econ. Perspect. 13(2):144–66 [PMC free article] [PubMed] [Google Scholar]
- Sterling P, Eyer J. 1981. Biological basis of stress-related mortality. Soc. Sci. Med. Part EMed. Psychol. 15(1):3–42 [DOI] [PubMed] [Google Scholar]
- Stringhini S, Polidoro S, Sacerdote C, Kelly RS, van Veldhoven K, et al. 2015. Life-course socioeconomic status and DNA methylation of genes regulating inflammation. Int. J. Epidemiol. 44(4):1320–30 [DOI] [PubMed] [Google Scholar]
- Stunkard AJ, Sorensen TIA. 1993. Obesity and Socioeconomic Status -- A Complex Relation. N. Engl. J. Med. 329(14):1036–37 [DOI] [PubMed] [Google Scholar]
- Sundquist J, Johansson SE. 1998. The influence of socioeconomic status, ethnicity and lifestyle on body mass index in a longitudinal study. Int. J. Epidemiol. 27(1):57–63 [DOI] [PubMed] [Google Scholar]
- Timmermans S, Haas S. 2008. Towards a sociology of disease. Sociol. Heal. Illn. 30(5):659–76 [DOI] [PubMed] [Google Scholar]
- Toker S, Shirom A, Shapira I, Berliner S, Melamed S. 2005. The association between burnout, depression, anxiety, and inflammation biomarkers: C-reactive protein and fibrinogen in men and women. J. Occup. Health Psychol. 10(4):344–62 [DOI] [PubMed] [Google Scholar]
- Tomalski P, Moore DG, Ribeiro H, Axelsson EL, Murphy E, et al. 2013. Socioeconomic status and functional brain development - associations in early infancy. Dev. Sci. 16(5):676–87 [DOI] [PubMed] [Google Scholar]
- Treiber FA, Kamarck T, Schneiderman N, Sheffield D, Kapuku G, Taylor T. 2003. Cardiovascular reactivity and development of preclinical and clinical disease states. Psychosom Med. 65(1):46–62 [DOI] [PubMed] [Google Scholar]
- Tsenkova VK, Love GD, Singer BH, Ryff CD. 2007. Socioeconomic Status and Psychological Well-Being Predict Cross-Time Change in Glycosylated Hemoglobin in Older Women Without Diabetes. Psychosom. Med. 69(8):777–84 [DOI] [PubMed] [Google Scholar]
- Turner RJ, Wheaton B, Lloyd DA. 1995. The epidemiology of social stress. Am. Sociol. Rev. 60(1): 104–25 [Google Scholar]
- Turrell G, Lynch JW, Leite C, Raghunathan T, Kaplan GA. 2007. Socioeconomic disadvantage in childhood and across the life course and all-cause mortality and physical function in adulthood: Evidence from the Alameda County Study. J. Epidemiol. Community Health. 61(8):723–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umberson D, Crosnoe R, Reczek C. 2010. Social Relationships and Health Behavior Across the Life Course. Annu. Rev. Sociol. 36(1): 139–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upchurch DM, Stein J, Greendale GA, Chyu L, Tseng C-H, et al. 2015. A Longitudinal Investigation of Race, Socioeconomic Status, and Psychosocial Mediators of Allostatic Load in Midlife Women: Findings From the Study of Women’s Health Across the Nation. Psychosom. Med. 77(4):402–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vliegenthart J, Noppe G, van Rossum EFC, Koper JW, Raat H, van den Akker ELT. 2016. Socioeconomic status in children is associated with hair cortisol levels as a biological measure of chronic stress. Psychoneuroendocrinology. 65:9–14 [DOI] [PubMed] [Google Scholar]
- Wadhwa P, Buss C, Entringer S, Swanson J. 2009. Developmental Origins of Health and Disease: Brief History of the Approach and Current Focus on Epigenetic Mechanisms. Semin. Reprod. Med. 27(5):358–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner B, Li J, Liu H, Guo G. 2013. Gene-environment correlation: difficulties and a natural experiment-based strategy. Am. J. Public Health. 103 Suppl 1(S 1):S 167–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace RB. 2008. The Women’s Health Initiative: Lessons for the Population Study of Biomarkers In Surveys Biosocial, eds. M Weinstein, JW Vaupel, KW Wachter, pp. 136–48. Washington, DC: The National Academies Press [Google Scholar]
- Weinstein M, Vaupel JW, Wachter KW, eds. 2007. Biosocial Surveys. Washington, DC: National Academies Press; [PubMed] [Google Scholar]
- Wen M, Browning CR, Cagney KA. 2003. Poverty, affluence, and income inequality: Neighborhood economic structure and its implications for health. Soc. Sci. Med. 57(5):843–60 [DOI] [PubMed] [Google Scholar]
- Westman M, Eden D, Shirom A. 1985. Job stress, cigarette smoking and cessation: the conditioning effects of peer support. Soc. Sci. Med. 20(6):637–44 [DOI] [PubMed] [Google Scholar]
- Whitsel EA, Cuthbertson C, Tabor JW, Potter A, Wener M, et al. 2012a. Add Health Wave IV Documentation: Measures of inflammation and immune function. UNC Chapel Hill: Carolina Population Center [Google Scholar]
- Whitsel EA, Nguyen QC, Suchindran C, Hussey JM, Killeya-Jones LA, et al. 2011. Value Added: Quality, Quantity, and Diversity of National Blood Pressure Data on Young Adults. Epidemiology. 22(4):544–45 [Google Scholar]
- Whitsel EA, Tabor JW, Nguyen QC, Cuthbertson C, Wener M, et al. 2012b. Add Health Wave IV Documentation: Measures of glucose homeostasis. UNC Chapel Hill: Carolina Population Center. www.cpc.unc.edu/projects/addhealth/data/guides/Glucose_HbA1c.pdf [Google Scholar]
- Wight RG, Cummings JR, Miller-Martinez D, Karlamangla AS, Seeman TE, Aneshensel CS. 2008. A multilevel analysis of urban neighborhood socioeconomic disadvantage and health in late life. Soc. Sci. Med. 66(4):862–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GH. 2003. The determinants of health: structure, context and agency. Sociol. Health Illn. 25(3): 131–54 [PubMed] [Google Scholar]
- Wilson DK, Kliewer W, Plybon L, Sica DA. 2000. Socioeconomic Status and Blood Pressure Reactivity in Healthy Black Adolescents. Hypertension. 35(1): [DOI] [PubMed] [Google Scholar]
- Winkleby MA, Jatulis DE, Frank E, Fortmann SP. 1992. Socioeconomic status and health: How education, income, and occupation contribute to risk factors for cardiovascular disease. Am. J. Public Health. 82(6):816–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe B, Evans W, Seeman TE, eds. 2012. The Biological Consequences of Socioeconomic Inequalities. New York: Russell Sage Foundation [Google Scholar]
- Wuchty S, Jones BF, Uzzi B. 2007. The Increasing Dominance of Teams in Production of Knowledge. Science (80-.). 316(5827): [DOI] [PubMed] [Google Scholar]
- Xu J, Zeger SL. 2001. The Evaluation of Multiple Surrogate Endpoints. Biometrics. 57(1):81–87 [DOI] [PubMed] [Google Scholar]
- Yang YC, Boen C, Gerken K, Li T, Schorpp K, Harris KM. 2016. Social relationships and physiological determinants of longevity across the human life span. Proc. Natl. Acad. Sci. U. S. A. 113(3):578–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YC, Gerken K, Schorpp K, Boen C, Harris KM. 2017. Early-Life Socioeconomic Status and Adult Physiological Functioning: A Life Course Examination of Biosocial Mechanisms. Biodemography Soc. Biol. 63(2):87–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YC, McClintock MK, Kozloski M, Li T. 2013. Social isolation and adult mortality: the role of chronic inflammation and sex differences. J. Health Soc. Behav. 54(2):183–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YC, Schorpp K, Harris KM. 2014. Social support, social strain and inflammation: evidence from a national longitudinal study of U.S. adults. Soc. Sci. Med. 107:124–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger SL. 2000. Biomarkers as surrogates in clinical research In Biomarkers and Surrogate Endpoints: Clinical Research and Applications, ed. Downing G, pp. 22–26. New York: Elsevier [Google Scholar]
- Zerhouni E 2003. Medicine. The NIH Roadmap. Science. 302(5642):63–72 [DOI] [PubMed] [Google Scholar]


