Abstract

Spiropyran is used as a photochromic dye to create colored patterns in highly drawn ultrahigh molecular weight polyethylene (UHMW PE) films. The dye is incorporated in highly crystalline, drawn UHMW PE tapes and fibers and isomerizes to its merocyanine state upon UV light irradiation, resulting in a color change from transparent to purple. The isomerization from merocyanine to spiropyran to erase the color can be simply induced by using heat or a green LED light. The combination of the use of a mask and the reversibility of the isomerization results in colored patterns that can be written, erased, and rewritten using UV light and heat or green LED light.
Keywords: polymer fibers, light-responsive materials, photochromism, rewritable optical materials, spiropyran, ultrahigh molecular weight polyethylene
Introduction
Highly oriented and chain-extended ultrahigh molecular weight polyethylene (UHMW PE) has exceptional mechanical properties. For example UHMW PE fibers reach a maximum Young’s modulus of 100–180 GPa and a maximum tensile strength of 3–5 GPa.1−4 Thanks to these outstanding properties, these fibers are generally employed in very demanding applications, such as bulletproof vests, marine ropes, and cut resistant gloves. The mechanical properties of UHMW PE fibers are essentially the result of an extremely high degree of orientation and chain extension of UHMW PE polymer chains combined with a crystallinity above 90%. To realize such a morphology, the fibers are extended (“drawn”) to a very large deformation (so-called draw ratio) at elevated temperatures in the solid state.
Stimuli-responsive polymers that respond to an external stimulus, such as light or heat, by changing their properties are one of the focal points in materials science. Currently, there is an increased interest in making existing polymers responsive for high-end applications. Despite its remarkable mechanical properties, UHMW PE has not been used to fabricate stimuli-responsive materials as the highly crystalline matrix is believed to restrict motion of embedded, small responsive molecules.5−7 However, if light could be used as a stimulus to develop mechanically robust UHMW PE-based photoresponsive materials, such materials could lead to new applications in the field of optical storage,8,9 sensors,10−14 actuators, artificial muscle, and soft-robotics. The few examples of light-responsive polyethylene that exist in literature do not use UHMW PE, but low density polyethylene (LDPE) matrixes to create actuators15−17 and photopatterns.18
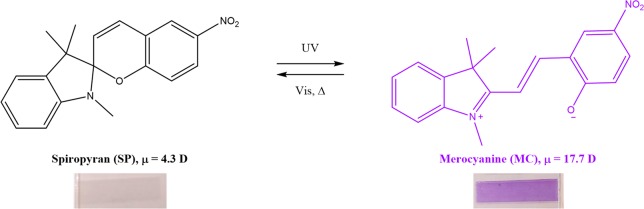
In the past, photochromic dyes such as azobenzene, spirooxazine, and spiropyran (SP) molecules have attracted a lot of interest for the construction of light-responsive materials.19,20 These organic photochromic dyes undergo an isomerization reaction in response to ultraviolet (UV) or visible light.21 In the case of SP, reversible isomerization upon light exposure provides the feature to switch between different functional properties, such as color and polarity. When irradiated with UV light, heterolytic Cspiro–O bond cleavage of the SP occurs and merocyanine (MC) is formed (Figure 1).19 The SP is colorless and apolar having a dipole moment in the range 4–6 D, while the MC form has a deep blue/purple color being more apolar with a dipole moment of 14–18 D.19 By incorporating spiropyran in nonstretched polymers, studies showed the possibility to photocontrol the polymer fluorescence,22−24 solubility,25−27 transport through polymeric systems,28 mechanical properties,29 and metal ion complexation.30,31 In the above-described materials, amorphous or semicrystalline polymers were employed with a low Young’s modulus (<3 GPa) and tensile strength (<0.1 GPa).32,33 Here, so-called gel cast ultradrawn UHMW PE is used with Young’s moduli and tensile strengths above 100 and 3 GPa, respectively. SP dyes have never been incorporated in such polymers to create anisotropic light-responsive materials.
Figure 1.
Photochromism of 1,3-dihydro-1,3,3-trimethyl-6-nitrospiro[2H-1-benzopyran-2,2-(2H)-indole] (SP). Upon exposure to UV, the purple merocyanine (MC) is formed, while upon exposure to visible light and/or heat, the SP spiropyran is obtained again. Top: isomerization of SP and MC molecules.19 Bottom: photographs of drawn UHMW PE tapes containing SP before and after UV light irradiation.
Here, a method to fabricate light-responsive UHMW PE tapes and fibers using spiropyran is presented. Spiropyran is incorporated in the UHMW PE tapes and fibers as a photochromic additive during gel casting or after placing the polymer in spiropyran solution. The polymer irradiated with UV light locally becomes purple and is used to make colored patterns.
Results and Discussion
For the fabrication of the light-responsive UHMW PE tapes, 3 wt % SP is incorporated in UHMW PE during gel casting. The SP and UHMW PE are dissolved in xylene at 135 °C. The solution is then cast in an aluminum tray and after evaporation of the solvent at room temperature (for several days), the UHMW PE films containing SP are obtained. Subsequently, samples are cut from the slightly yellow colored tape and are thereafter drawn on a hot plate to a draw ratio of 30 and 60 (UH_DR30 and UH_DR60). The stretched, anisotropic sample is transparent as the exposure to high temperatures promotes the formation of the transparent SP isomer (Figure 2). After irradiation with UV light (80 mJ cm–2), the drawn tape becomes purple and the UV–vis spectrum exhibits an absorption band centered at 550 nm. The peak at 550 nm is characteristic for the merocyanine (MC) isomer and demonstrates the photoisomerization of the SP to the MC isomer. The maximum conversion is obtained after 5 min of UV light irradiation (Figure 2).34
Figure 2.
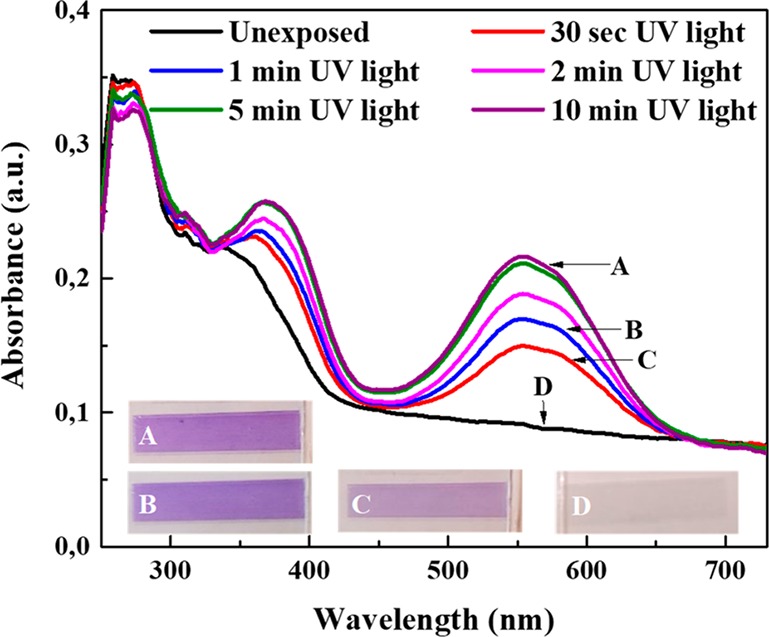
Isomerization from SP to MC in a UH_DR60 by irradiation with UV light (with a dose of 80 mJ cm–2) and corresponding pictures of the UHMW PE tape (0.3 × 3 cm2) becoming purple.
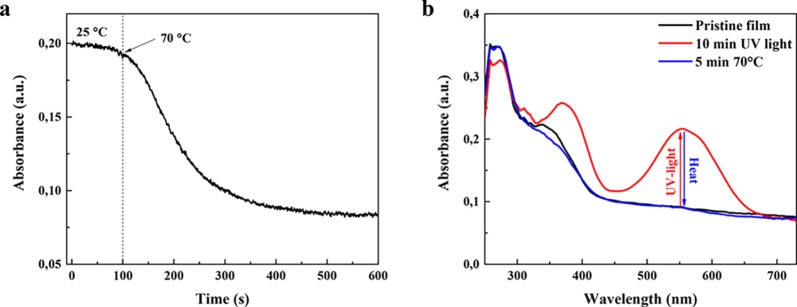
The reversibility of the isomerization is studied using heat and green light (565 nm). After an irradiation of 5 min with UV light, the UH_DR60 tape is heated to 70 °C and the decrease of its absorption at 550 nm is monitored (Figure 3). Heating for 2 min at 70 °C is enough to fully isomerize the MC back to SP. An exposure to green LED light for 30 min at room temperature is also sufficient to fully reverse the system to the SP state. Photoisomerization of the UH_DR60 tape was also examined by cycling repeatedly between the SP and MC isomers using either heat (70 °C) or green light (see Figure S1), revealing that this rewritable process can only be used a couple of times. Such a behavior is typical for nitro-spiropyran derivatives.35
Figure 3.
(a) Isomerization from MC to SP using heat of UH_DR60. Absorbance at 550 nm of the film after an increase of the temperature from 25 to 70 °C. (b) UV–vis spectra of the film showing the reversibility of the patterning during the first cycle using heat.
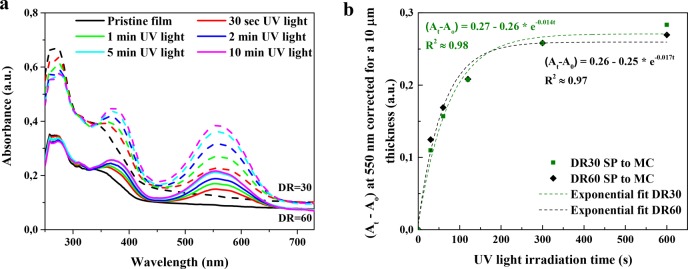
The effect of the draw ratio on the isomerization from SP to MC is also studied (Figure 4a,b). The absorption at 550 nm of UH_DR30 after 5 min of UV light irradiation seems to be almost double the absorption for UH_DR60. However, when the absorption is corrected for the tape thickness by calculating it per 10 μm film thickness, both UH_DR30 and UH_DR60 display similar absorption and isomerization characteristics. Therefore, the draw ratio, thus Young’s modulus of the matrix, does not have a negative impact on the isomerization kinetics. The coloration kinetics of the SP to MC isomerization can be described (Figure 4B) using a first-order exponential fit, having rate constants on the order of ∼−0.015 s–1.36,37 These values are comparable to rate constants found in poly(methyl methacrylate) matrixes.38 In addition, decoloration kinetics in response to green light (λ = 565 nm) or heat (70 °C) can also be fitted using a first order exponential fit, revealing a faster thermal decoloration reaction (Figures S2a,b and S3).
Figure 4.
(a) UV–vis spectra of the films showing the isomerization from SP to MC upon UV irradiation for DR = 30 (dotted lines) and 60 (full lines). (b) SP to MC coloration process fitted using first-order exponential function in a (At – A0) versus time plot at λmax = 550 nm.
With the ability of spiropyran to isomerize and change color, an easy method exists to pattern UHMW PE containing this dye. An image can be created within minutes by using a mask between the oriented UHMW PE material and the UV lamp, as shown in Figure 5. Various patterns can be generated, from image patterns to logos, with a high contrast. Moreover, using heat makes it possible to write, erase, rewrite, and erase again different patterns on the same tape.
Figure 5.
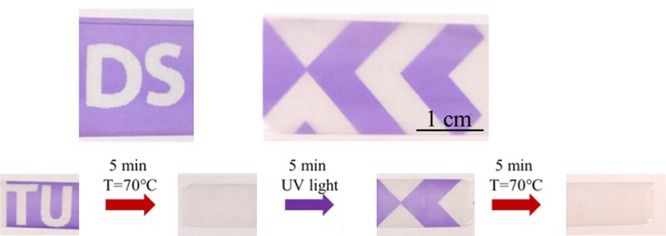
Patterns and images photopatterned into oriented UHMW PE films containing SP drawn 30 times and a demonstration of the reversibility of the patterns using heat.
The photopatterning method can also be used on UHMW PE fibers as shown in Figure 6. The incorporation of spiropyran into the UHMW PE fibers can be performed directly by diffusing the dye into the fiber. First, a solution was prepared by dissolving SP in xylene at room temperature. Then, the UHMW PE fibers were placed in an aluminum tray containing the SP solution. The xylene slowly evaporated and after a day the fibers were dry. After the evaporation of the solvent, the fibers containing SP were collected and photopatterned. The fibers were then irradiated with UV light in combination with a mask following the same method as for the UHMW PE tapes. The photopatterning is also shown to be reversible in the case of the fibers. After 2 days at room temperature, the fibers do not show a pattern anymore and became white again. Ultradrawn, UHMW PE films and fibers are sensitive to counterfeiting, and the above recording and fading can potentially be used for advanced anticounterfeiting features.10,11,39
Figure 6.

Photopatterning of UHMW PE fibers. The fibers are first irradiated with UV light for 5 min using a mask. After 2 days at room temperature, the dye isomerizes back from MC to SP, which results in the pattern disappearing and the fibers becoming white again.
Conclusions
The new facile method presented here opens the possibility to fabricate light-responsive solid state drawn UHMW PE tapes and fibers. Highly crystalline, mechanically robust UHMW PE polymers were embedded with a SP photochromic dye to exploit its erasable and rewritable functional properties as being exposed to UV light, heat, and green light. UV light in combination with a mask can be used to fabricate the colored pattern while heat or green light can be used to erase it. This photopatterning method can simply be extended to other polymers. The reversibility of the spiropyran can most likely be increased by using more stable photochromic dyes.40 The switch between isomers could be further used to change more properties such as polarity, which can lead to new applications. The incorporation of spiropyran can also be performed directly on the finished end products such as fibers. This can be an industrial advantage as it means that no changes are required in the processing of the fibers. Optically responsive UHMW PE fibers are interesting as they are used in a broad range of application where design is important such as clothing.
Experimental Section
Materials
Ultrahigh molecular weight PE was obtained from DSM with a number-average molecular weight Mn of 310 kg/mol and a weight-average molecular weight Mw of 3300 kg/mol. UHMW PE fibers were kindly provided by DSM. 1,3-Dihydro-1,3,3-trimethyl-6-nitrospiro[2H-1-benzopyran-2,2-(2H)-indole] (SP) was purchased from Acros Organics. All reagents were used as received without further purification.
Preparation of Stretched UHMW PE Tapes
Oriented PE films containing spiropyran were produced according to the following procedure. First, the UHMW PE powder was suspended in xylene at room temperature at concentration of 1.5 wt % with 0.05 wt % of antioxidant Irganox 1010 and with 3 wt % of SP added. Air trapped by the powder particles was removed by the application of a vacuum. Then the flask containing the suspension was heated under continuous stirring at 130 °C in an oil bath. Once the Weissenberg effect was observed, the stirring was stopped and the flask was kept in the oil bath for 1 h. The solution was then cast in an aluminum tray at room temperature. Upon cooling, the solution turned opaque and became gel like. Finally, xylene was evaporated for several days in a fume hood and films of uniform thickness were obtained. A piece of the film (1 × 3 cm) was cut and stretched on a hot plate at 120 °C using pliers. The draw ratio was determined by the displacement of two ink marks on the film.
Irradiation with UV Light and Green Light
The UHMW PE tapes containing SP were irradiated with UV and green light. For the UV light irradiation an Exfo lamp was used with a wavelength from 320 to 500 nm and a dose of 80 mJ.cm–2. The green light to reverse the isomerization was generated with an LED with a wavelength of 565 nm and an intensity of roughly 15 mJ.cm–2.
UV–Vis Spectroscopy
The chain-extended tapes were placed between two quartz slides with a drop of silicon oil. The absorption was measured in the range 250–730 nm on a Shimadzu UV-3102 PC spectrophotometer at a 1 nm interval.
Acknowledgments
This work was financially supported by DSM Dyneema BV, The Netherlands. The work of Rob C.P. Verpaalen forms part of the research programme of DPI, programme 731.015.502.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsapm.8b00117.
Photoisomerization cycles, UV–vis spectra, decoloration kinetics, and solvent exposure of the ultradrawn UHMW PE tapes (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Smith P.; Lemstra P. J.; Pijpers J. P. L.; Kiel A. M. Ultra-Drawing of High Molecular Weight Polyethylene Cast from Solution - III. Morphology and Structure. Colloid Polym. Sci. 1981, 259, 1070–1080. 10.1007/BF01524892. [DOI] [Google Scholar]
- Lemstra P. J.; van Aerle N. A. J. M.; Bastiaansen C. W. M. Chain-Extended Polyethylene. Polym. J. 1987, 19, 85–98. 10.1295/polymj.19.85. [DOI] [Google Scholar]
- Yeh J. T.; Chang S. S. Ultradrawing Gel Films of Blends of Ultrahigh Molecular Weight Polyethylene and Low Molecular Weight Polyethylenes with Different Molecular Weights. J. Mater. Sci. 2000, 35, 3227–3236. 10.1023/A:1004858902462. [DOI] [Google Scholar]
- Sawai D.; Nagai K.; Kubota M.; Ohama T.; Kanamoto T. Maximum Tensile Properties of Oriented Polyethylene, Achieved by Uniaxial Drawing of Solution-Grown Crystal Mats: Effects of Molecular Weight and Molecular Weight Distribution. J. Polym. Sci., Part B: Polym. Phys. 2006, 44, 153–161. 10.1002/polb.20682. [DOI] [Google Scholar]
- Ercole F.; Davis T. P.; Evans R. A. Comprehensive Modulation of Naphthopyran Photochromism in a Rigid Host Matrix by Applying Polymer Conjugation. Macromolecules 2009, 42, 1500–1511. 10.1021/ma801947d. [DOI] [Google Scholar]
- Sriprom W.; Néel M.; Gabbutt C. D.; Heron B. M.; Perrier S. Tuning the Color Switching of Naphthopyrans via the Control of Polymeric Architectures. J. Mater. Chem. 2007, 17, 1885–1893. 10.1039/B617865K. [DOI] [Google Scholar]
- Micciche F.; Ramakrishnan V.; Hoeks T. L. The Effect of Molecular Structure on the Secondary Transitions and Their Influence on the Decoloration Kinetics of Photochromic Dyes in Co-Polycarbonates. J. Polym. Sci., Part B: Polym. Phys. 2016, 54, 1593–1601. 10.1002/polb.24062. [DOI] [Google Scholar]
- Abdollahi A.; Alinejad Z.; Mahdavian A. R. Facile and Fast Photosensing of Polarity by Stimuli-Responsive Materials Based on Spiropyran for Reusable Sensors: A Physico-Chemical Study on the Interactions. J. Mater. Chem. C 2017, 5, 6588–6600. 10.1039/C7TC02232H. [DOI] [Google Scholar]
- Weis P.; Wang D.; Wu S. Visible-Light-Responsive Azopolymers with Inhibited π-π Stacking Enable Fully Reversible Photopatterning. Macromolecules 2016, 49, 6368–6373. 10.1021/acs.macromol.6b01367. [DOI] [Google Scholar]
- Abdollahi A.; Sahandi-Zangabad K.; Roghani-Mamaqani H. Light-Induced Aggregation and Disaggregation of Stimuli-Responsive Latex Particles Depending on Spiropyran Concentration: Kinetics of Photochromism and Investigation of Reversible Photopatterning. Langmuir 2018, 34, 13910–13923. 10.1021/acs.langmuir.8b02296. [DOI] [PubMed] [Google Scholar]
- Abdollahi A.; Sahandi-Zangabad K.; Roghani-Mamaqani H. Rewritable Anticounterfeiting Polymer Inks Based on Functionalized Stimuli-Responsive Latex Particles Containing Spiropyran Photoswitches: Reversible Photopatterning and Security Marking. ACS Appl. Mater. Interfaces 2018, 10, 39279–39292. 10.1021/acsami.8b14865. [DOI] [PubMed] [Google Scholar]
- Terpstra A. S.; Hamad W. Y.; Maclachlan M. J. Photopatterning Freestanding Chiral Nematic Mesoporous Organosilica Films. Adv. Funct. Mater. 2017, 27, 1703346. 10.1002/adfm.201703346. [DOI] [Google Scholar]
- Abdollahi A.; Mahdavian A. R.; Salehi-Mobarakeh H. Preparation of Stimuli-Responsive Functionalized Latex Nanoparticles: The Effect of Spiropyran Concentration on Size and Photochromic Properties. Langmuir 2015, 31, 10672–10682. 10.1021/acs.langmuir.5b02612. [DOI] [PubMed] [Google Scholar]
- Abdollahi A.; Rad J. K.; Mahdavian A. R. Stimuli-Responsive Cellulose Modified by Epoxy-Functionalized Polymer Nanoparticles with Photochromic and Solvatochromic Properties. Carbohydr. Polym. 2016, 150, 131–138. 10.1016/j.carbpol.2016.05.009. [DOI] [PubMed] [Google Scholar]
- Uznanski P.; Kryszewski M. Bullelin. Polym. Bull. 1991, 26, 437–443. 10.1007/BF00302612. [DOI] [Google Scholar]
- Bobrovsky A.; Shibaev V.; Elyashevitch G.; Rosova E.; Shimkin A.; Shirinyan V.; Bubnov A.; Kaspar M.; Hamplova V.; Glogarova M. New Photosensitive Polymer Composites Based on Oriented Porous Polyethylene Filled with Azobenzene-Containing LC Mixture: Reversible Photomodulation of Dichroism and Birefringence. Liq. Cryst. 2008, 35, 533–539. 10.1080/02678290802015697. [DOI] [Google Scholar]
- Ryabchun A.; Bobrovsky A.; Stumpe J.; Shibaev V. Novel Generation of Liquid Crystalline Photo-Actuators Based on Stretched Porous Polyethylene Films. Macromol. Rapid Commun. 2012, 33, 991–997. 10.1002/marc.201100837. [DOI] [PubMed] [Google Scholar]
- Varghese S.; Severn J. R.; Schenning A. P. H. J.. Photoresponsive Polyolefins. In Photoactive Functional Soft Materials: Preparation, Properties, and Applications; Li Q., Ed.; John Wiley & Sons, 2019; pp 319–340. [Google Scholar]
- Klajn R. Spiropyran-Based Dynamic Materials. Chem. Soc. Rev. 2014, 43, 148–184. 10.1039/C3CS60181A. [DOI] [PubMed] [Google Scholar]
- Qiao Q.; Zhang X.; Lu Z.; Wang L.; Liu Y.; Zhu X.; Li J. Formation of Holographic Fringes on Photochromic Ag/TiO2nanocomposite Films. Appl. Phys. Lett. 2009, 94, 074104. 10.1063/1.3078232. [DOI] [Google Scholar]
- Fu S.; Liu Y.; Lu Z.; Dong L.; Hu W.; Xie M. Photo-Induced Birefringence and Polarization Holography in Polymer Films Containing Spirooxazine Compounds Pre-Irradiated by UV Light. Opt. Commun. 2004, 242, 115–122. 10.1016/j.optcom.2004.08.022. [DOI] [Google Scholar]
- Zhu M. Q.; Zhu L.; Han J. J.; Wu W.; Hurst J. K.; Li A. D. Q. Spiropyran-Based Photochromic Polymer Nanoparticles with Optically Switchable Luminescence. J. Am. Chem. Soc. 2006, 128, 4303–4309. 10.1021/ja0567642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L.; Wu W.; Zhu M. Q.; Han J. J.; Hurst J. K.; Li A. D. Q. Reversibly Photoswitchable Dual-Color Fluorescent Nanoparticles as New Tools for Live-Cell Imaging. J. Am. Chem. Soc. 2007, 129, 3524–3526. 10.1021/ja068452k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C.; Zhang Y.; Hu J.; Cheng J.; Liu S. Reversible Three-State Switching of Multicolor Fluorescence Emission by Multiple Stimuli Modulated FRET Processes within Thermoresponsive Polymeric Micelles. Angew. Chem., Int. Ed. 2010, 49, 5120–5124. 10.1002/anie.201002203. [DOI] [PubMed] [Google Scholar]
- Koňák Č.; Kopečková P.; Kopeček J. Photoregulated Association of N-(2-Hydroxypropyl)Methacrylamide Copolymers with Azobenzene-Containing Side Chains. Macromolecules 1992, 25, 5451–5456. 10.1021/ma00046a051. [DOI] [Google Scholar]
- Edahiro J. I.; Sumaru K.; Takagi T.; Shinbo T.; Kanamori T. Photoresponse of an Aqueous Two-Phase System Composed of Photochromic Dextran. Langmuir 2006, 22, 5224–5226. 10.1021/la060318q. [DOI] [PubMed] [Google Scholar]
- Lee H. Il; Wu W.; Oh J. K.; Mueller L.; Sherwood G.; Peteanu L.; Kowalewski T.; Matyjaszewski K. Light-Induced Reversible Formation of Polymeric Micelles. Angew. Chem., Int. Ed. 2007, 46, 2453–2457. 10.1002/anie.200604278. [DOI] [PubMed] [Google Scholar]
- Sugiura S.; Sumaru K.; Ohi K.; Hiroki K.; Takagi T.; Kanamori T. Photoresponsive Polymer Gel Microvalves Controlled by Local Light Irradiation. Sens. Actuators, A 2007, 140, 176–184. 10.1016/j.sna.2007.06.024. [DOI] [Google Scholar]
- Davis D. A.; Hamilton A.; Yang J.; Cremar L. D.; Van Gough D.; Potisek S. L.; Ong M. T.; Braun P. V.; Martínez T. J.; White S. R.; Moore J. S.; Sottos N. R. Force-Induced Activation of Covalent Bonds in Mechanoresponsive Polymeric Materials. Nature 2009, 459, 68–72. 10.1038/nature07970. [DOI] [PubMed] [Google Scholar]
- Suzuki T.; Kato T.; Shinozaki H. Photo-Reversible Pb 2 1 -Complexation of Thermosensitive Poly (N -Isopropyl Acrylamide- Co -Spiropyran Acrylate) in Water. Chem. Commun. 2004, 2, 2036–2037. 10.1039/b407342h. [DOI] [PubMed] [Google Scholar]
- Fries K. H.; Driskell J. D.; Samanta S.; Locklin J. Spectroscopic Analysis of Metal Ion Binding in Spiropyran Containing Copolymer Thin Films. Anal. Chem. 2010, 82, 3306–3314. 10.1021/ac1001004. [DOI] [PubMed] [Google Scholar]
- Dixit M.; Mathur V.; Gupta S.; Baboo M.; Sharma K.; Saxena N. S. Morphology, Miscibility and Mechanical Properties of PMMA/PC Blends. Phase Transitions 2009, 82, 866–878. 10.1080/01411590903478304. [DOI] [Google Scholar]
- Gilmour I. W.; Trainor A.; Haward R. N. Elastic Moduli of Glassy Polymers at Low Strains. J. Appl. Polym. Sci. 1979, 23, 3129–3138. 10.1002/app.1979.070231030. [DOI] [Google Scholar]
- Ramos-Garcia R.; Delgado-Macuil R.; Iturbe-Castillo D.; De Los Santos E. G.; Corral F. S. Polarization Dependence on the Holographic Recording in Spiropyran-Doped Polymers. Opt. Quantum Electron. 2003, 35, 641–650. 10.1023/A:1023964804048. [DOI] [Google Scholar]
- Chen J.; Wang D.; Turshatov A.; Muñoz-Espí R.; Ziener U.; Koynov K.; Landfester K. One-Pot Fabrication of Amphiphilic Photoswitchable Thiophene-Based Fluorescent Polymer Dots. Polym. Chem. 2013, 4, 773–781. 10.1039/C2PY20589K. [DOI] [Google Scholar]
- Abdollahi A.; Mouraki A.; Sharifian M. H.; Mahdavian A. R. Photochromic Properties of Stimuli-Responsive Cellulosic Papers Modified by Spiropyran-Acrylic Copolymer in Reusable PH-Sensors. Carbohydr. Polym. 2018, 200, 583–594. 10.1016/j.carbpol.2018.08.042. [DOI] [PubMed] [Google Scholar]
- Lin J. S. Interaction between Dispersed Photochromic Compound and Polymer Matrix. Eur. Polym. J. 2003, 39, 1693–1700. 10.1016/S0014-3057(03)00058-2. [DOI] [Google Scholar]
- Pariani G.; Bianco A.; Castagna R.; Bertarelli C. Kinetics of Photochromic Conversion at the Solid State: Quantum Yield of Dithienylethene-Based Films. J. Phys. Chem. A 2011, 115, 12184–12193. 10.1021/jp207210p. [DOI] [PubMed] [Google Scholar]
- Zhang T.; Fu L.; Chen Z.; Cui Y.; Liu X. Photochromic Properties of Spiropyran in Epoxy Resin as Anti-Counterfeiting Coating on Flexible Materials. Prog. Org. Coat. 2016, 100, 100–104. 10.1016/j.porgcoat.2016.02.001. [DOI] [Google Scholar]
- Ter Schiphorst J.; Coleman S.; Stumpel J. E.; Ben Azouz A.; Diamond D.; Schenning A.; Molecular P. H. J. Design of Light-Responsive Hydrogels, for in Situ Generation of Fast and Reversible Valves for Microfluidic Applications. Chem. Mater. 2015, 27, 5925–5931. 10.1021/acs.chemmater.5b01860. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.