Abstract
Objective:
We aimed to investigate the musculoskeletal and pulmonary outcomes of patients with osseous sarcoidosis.
Methods:
We identified 24 patients with osseous sarcoidosis and at least one year of follow-up after diagnosis (baseline). We collected outcome data at 1-year follow-up and last follow-up. We defined a composite outcome measure; worsening considered as worsening in any of the following 4 components compared to baseline: 1) osseous sarcoidosis symptoms, 2) musculoskeletal imaging of affected bone, 3) chest imaging, or 4) pulmonary function testing (PFT).
Results:
A minority of patients had a worsening composite outcome at 1-year (9/24, 38%) and last follow-up (5/24, 21%). When only considering musculoskeletal symptoms and imaging, only 25% (6/24) and 13% (3/24) of patients worsened compared to baseline at 1-year and last follow-up, respectively. Patients with a worsening composite overall outcome tended to be older at baseline than those without the outcome for both 1-year (54.3 years vs. 47.5 years, p=0.11) and last follow-up (55.0 years vs. 48.7 years; p=0.23), although these differences were non-significant. Treatment was not associated with worsening composite overall outcome at 1-year follow-up (p=0.40), but was significantly associated with decreased risk for worsening at last follow-up (p=0.05).
Conclusions:
In this retrospective cohort study of osseous sarcoidosis, most patients had a favorable outcome according to symptoms, musculoskeletal/chest imaging, and PFTs, even though only a minority were treated with glucocorticoids or DMARDs. These results suggest that the natural history of osseous sarcoidosis is often benign, although some patients experience clinical progression.
Key words: sarcoidosis, health outcomes, pulmonary disease, autoimmune disease, bone tissue, pulmonary function test
Introduction
Sarcoidosis is a systemic inflammatory disease characterized by accumulation of non-caseating granulomas in affected organs (1). Sarcoidosis primarily affects the lungs and lymph nodes resulting in varying degrees of severity ranging from asymptomatic involvement to life-threatening manifestations such as pulmonary fibrosis (2–4). A less common manifestation of sarcoidosis is involvement of the skeletal system. Osseous sarcoidosis was first described in the late 1800s (5). Since then, multiple case reports and case series have detailed the features of osseous sarcoidosis (6-17), highlighting the challenge of diagnosis and treatment of the disease. Despite an extensive literature regarding the natural history of pulmonary sarcoidosis, little is known about the epidemiology of osseous sarcoidosis and even less about the natural course of this manifestation of sarcoidosis (3, 5, 18). Recent case series have provided detailed information on the underlying characteristics and extent of osseous sarcoidosis at diagnosis, demonstrating that the disease commonly involves multiple bones, including the spine, and is often asymptomatic, with diagnosis made incidentally with imaging (5, 18, 19). However, no previous study has systematically investigated the long-term clinical outcomes of sarcoidosis from imaging, musculoskeletal, and pulmonary perspectives.
Our objective was to investigate osseous sarcoidosis over time using a retrospective cohort of patients with incident osseous sarcoidosis. Given review of previous studies, mostly focused on the presentation of osseous sarcoidosis (5, 6, 12, 20, 21), we expected that most patients would have a relatively stable clinical disease course in terms of stable or improved symptoms, pulmonary function testing (PFT), and musculoskeletal and chest imaging. We also sought to investigate whether the distribution of osseous lesions and other clinical variables at baseline might predict later clinical outcomes.
Methods
Design
We performed a retrospective cohort study investigating the musculoskeletal and pulmonary outcomes of patients following incident diagnosis of osseous sarcoidosis. The Partners HealthCare Institutional Review Board approved all aspects of the study.
Case ascertainment and study sample
Cases were ascertained from a single center (Brigham and Women’s Hospital, Boston, MA) using electronic medical record searches and directed inquiry to rheumatologists in the Division of Rheumatology, Immunology, and Allergy and pulmonologists in the Division of Pulmonary and Critical Care Medicine, as previously described (19). All patients were verified to have osseous sarcoidosis based on either bone biopsy showing non-caseating granulomas or known sarcoidosis who had bone lesions on imaging that were determined to be due to sarcoidosis by the treating clinician and radiologist as well as two medical record reviewers (ERM and JAS). Inclusion criteria for the study sample were: at least one follow-up clinical visit with a pulmonologist or rheumatologist at least one year from the date of osseous sarcoidosis diagnosis (baseline). In addition, we required patients to have PFTs, musculoskeletal/chest imaging, or laboratory measures (at least one of the following: serum angiotensin converting enzyme [ACE] level, erythrocyte sedimentation rate [ESR], C-reactive protein [CRP] level, serum calcium level, or 25-hydroxy vitamin D level), associated with at least one of the follow-up visits. All follow-up visits (inpatient, outpatient, or emergency department) were reviewed retrospectively from date of first sarcoidosis diagnosis (osseous or systemic) to the end of the study on July 1, 2016.
Data acquisition/covariates
We reviewed clinical and imaging data at baseline and all follow-up visits from rheumatologists and pulmonologists as well as any other notes relevant to covariates or outcomes. Clinical radiographic reports on imaging procedures of the chest (including plain radiographs, computed tomography [CT] scans, magnetic resonance imaging [MRI]) as well as musculoskeletal imaging (plain radiographs, CT scans, MRI, bone scans, and positron emission tomography [PET] scans) were reviewed by a single abstractor (ERM), and used to determine progression related to sarcoidosis over time. In addition, clinical data were collected including encounter type, clinician type (pulmonology, rheumatology, other), baseline and follow-up laboratory data (ACE, ESR, CRP, calcium, hemoglobin, and 25-hyrdoxy vitamin D), PFT results, and organ involvement, and treatment. We also collected data on emergency department visits as well as hospital admissions (including intensive care unit admissions), fractures overall and at sites of sarcoidal involvement, and death.
Outcomes
Follow-up notes and objective data were reviewed to determine each patient’s disease status. For every visit, medical records were reviewed to classify improvement, stability, or worsening in osseous sarcoidosis (symptoms and musculoskeletal imaging) and pulmonary sarcoidosis (chest imaging and PFT results). Osseous sarcoidosis symptoms were considered to have worsened if, after reviewing medical record notes, the treating provider documented that patient reported worsening pain or other symptoms attributable to osseous sarcoidosis. Progression in PFTs was determined using the impression of the pulmonologist who interpreted the results, PFTs were considered worsening if there was a significant change (defined as ≥12% and ≥200 ml) in forced expiratory volume in 1 second (FEV1) or forced vital capacity (FVC) typically referring to new or worsened obstruction or restriction respectively, as per current ATS recommendations (22). Imaging was considered worsening, based on review of the clinical radiologist’s interpretation. In cases where comparison either across modality (for imaging), or between time points (for imaging and/or PFTs), was not explicitly interpreted, worsening was decided by the medical record reviewer (ERM) based on clinical reports.
As our primary outcome, we created a 4-component composite measure to capture the overall disease course. A worsening composite outcome was defined as any worsening compared to baseline in one or more of: 1) osseous sarcoidosis symptoms, 2) musculoskeletal imaging, 3) chest imaging, or 4) PFTs. To more specifically evaluate the musculoskeletal disease course, we also investigated a composite osseous outcome, which we defined as any worsening compared to baseline in either osseous sarcoidosis symptoms or musculoskeletal imaging. We also investigated each component of the composite overall measure separately. Since data were obtained from routine clinical care, patients had varying durations of follow-up without fixed intervals. We considered their 1-year and last follow-up time points, since all patients had these time points for outcomes available. We also analyzed other follow-up time points as a sensitivity analysis (2 years and 5 years), but these analyses had restricted sample size and did not meaningfully change the results. We also performed a sensitivity analysis of patients who received no treatment as a proxy for the natural history of osseous sarcoidosis.
Statistical analysis
We report descriptive statistics of patients at baseline and follow-up visits using frequencies and proportions for categorical variables, mean and standard deviation for continuous normally distributed variables, and median, range, and interquartile range for continuous non-normally distributed variables. We report the number and proportion of the worsening composite outcomes as well as the components classified as improved, stable, or worsening at 1-year and last follow-up.
We investigated whether baseline factors were associated with worsening composite overall and osseous outcomes at 1-year and last follow-up in separate analyses. In these analyses, the composite outcomes were binary variables indicating that there was or was not worsening in any of the components at that follow-up time point compared to baseline. We used Fisher’s exact tests for categorical variables given cells with low frequencies, t-tests for normally distributed continuous variables, and Wilcoxon rank-sum tests for non-normally distributed continuous variables. We did not include multivariable regression models due to the limited numbers of outcomes and few statistically significant results in univariate models. Patients with missing data were not included in analyses pertaining to that variable.
We considered a two-sided p value <0.05 as statistically significant in all analyses. Analyses were performed using SAS v9.4 (Cary, NC).
Results
Description of cohort
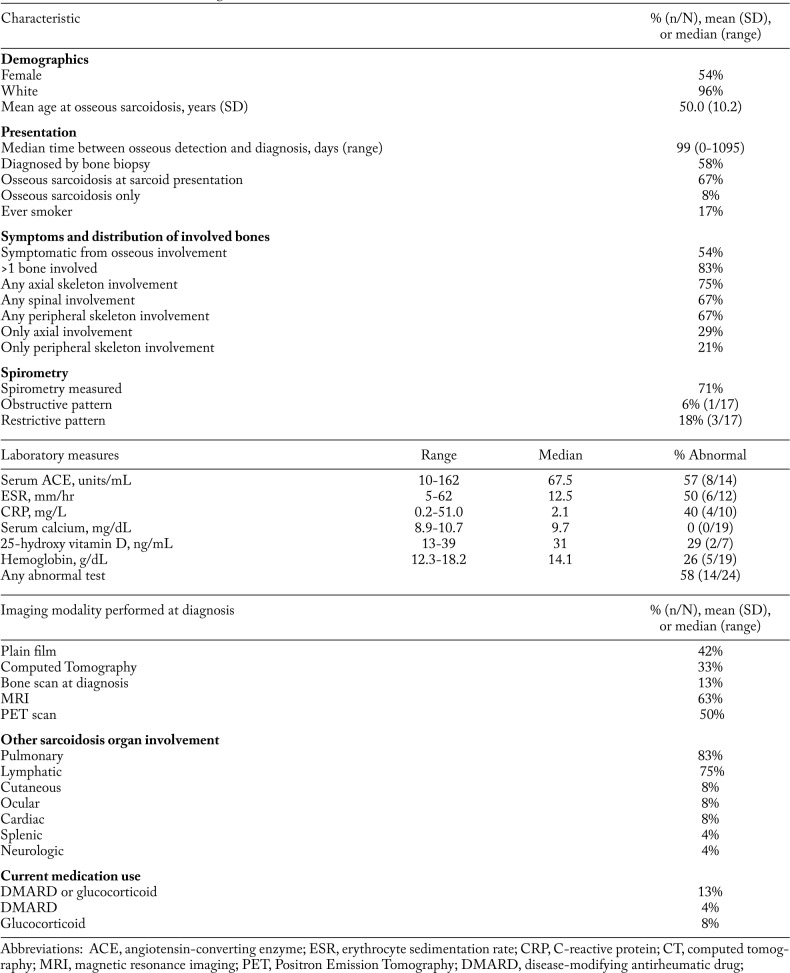
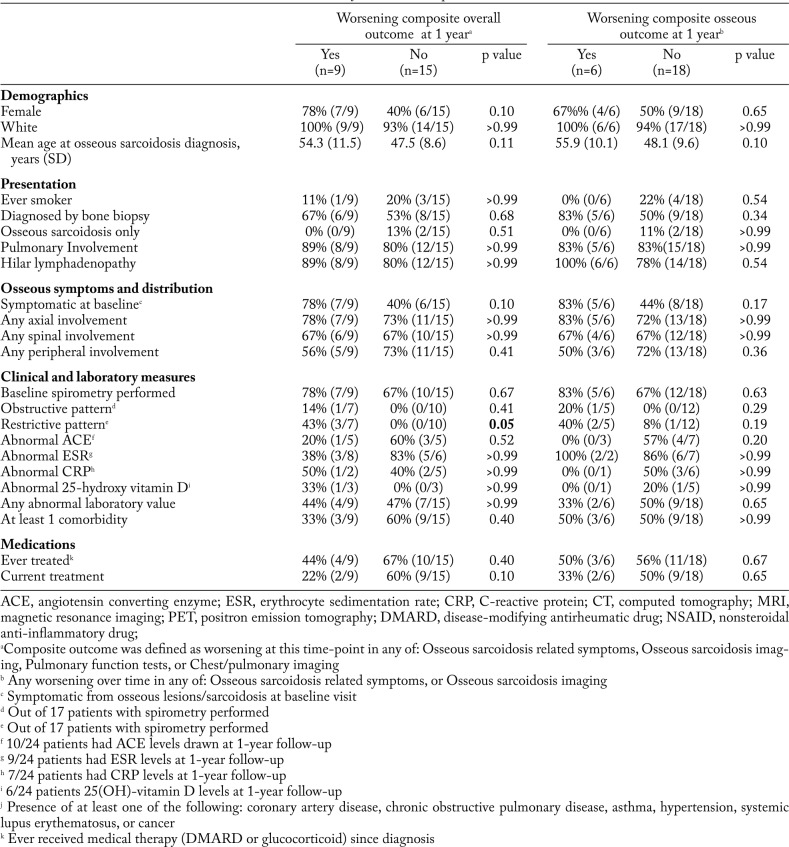
We identified 24 patients with osseous sarcoidosis and at least one year of follow-up. A summary of their baseline characteristics at the time of osseous sarcoidosis diagnosis is presented in Table 1. Patients were predominately white (96%); 54% were female; and mean age at diagnosis was 50.0 years (SD 10.2). Fifty-eight percent of patients were diagnosed by bone biopsy revealing non-caseating granulomas, and 67% of patients had osseous sarcoidosis as part of their initial presentation for sarcoidosis. Most (75%) had hilar lymphadenopathy, with pulmonary parenchymal involvement in 83%, and nearly all (83%) had evidence of involvement in more than one bone. A minority (21%) had involvement only in the peripheral skeleton while most had axial involvement (75%).
Table 1.
Baseline characteristics at diagnosis of osseous sarcoidosis (n=24)
Of the 17 (71%) patients with spirometry measured at the time of initial diagnosis, 3 (18%) had a restrictive pattern, and 1 (6%) had an obstructive pattern. Laboratory values available at time of diagnosis revealed 14 (58%) patients with at least one abnormal laboratory test in serum ACE, ESR, CRP, calcium, hemoglobin, or 25-hydroxy vitamin D levels. Half of the patients had at least one comorbidity (hypertension, cancer, coronary artery disease, asthma, or chronic obstructive pulmonary disease). Three patients (13%) were already being treated with medications (DMARDs or glucocorticoids) for other sarcoidosis manifestations at the time of osseous diagnosis.
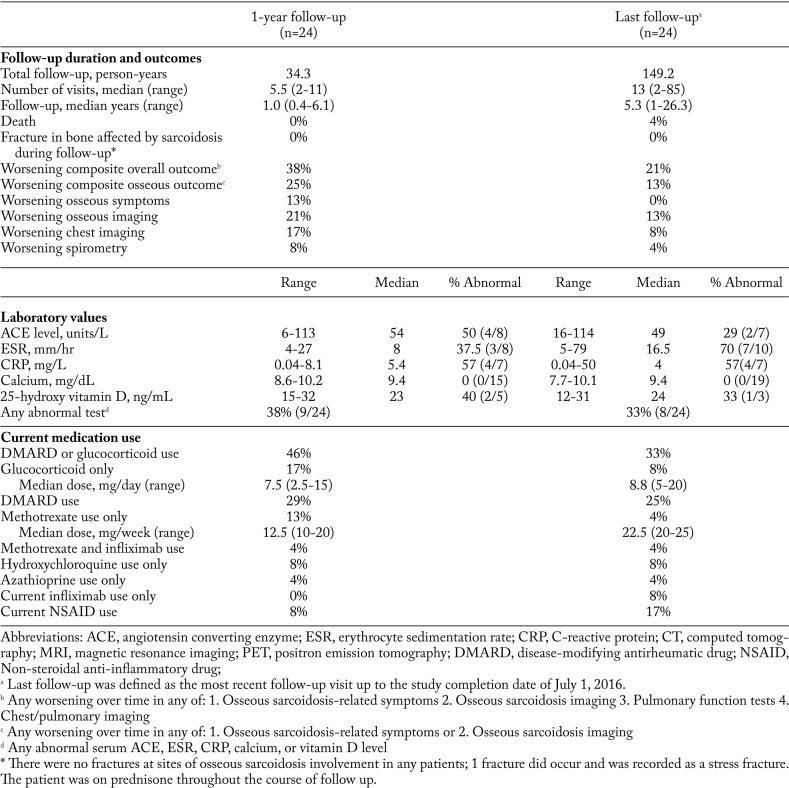
Table 2 shows the distribution of outcomes, including clinical and laboratory findings, at the 1-year and last follow-up time points. There were a total of 149.2 person-years of follow-up for all patients, with a median follow-up time of 5.3 years per patient. One patient died due to massive hemoptysis in the setting of pulmonary hypertension and diffuse pulmonary involvement with sarcoidosis. There was one bone fracture during follow-up. This was a stress fracture of the foot which had no prior evidence of sarcoid involvement. The median total number of clinic visits to rheumatology or pulmonary providers was 5.5 and 13 at the 1-year and last follow-up, respectively. Rates of treatment for systemic sarcoidosis increased from 13% at baseline to 46% at 1-year follow-up, and 29% at last follow-up. Methotrexate and hydroxychloroquine were the most commonly prescribed DMARDs at all time points.
Table 2.
Characteristics of patients with osseous sarcoidosis at each specified follow-up time point
Composite overall and osseous outcomes
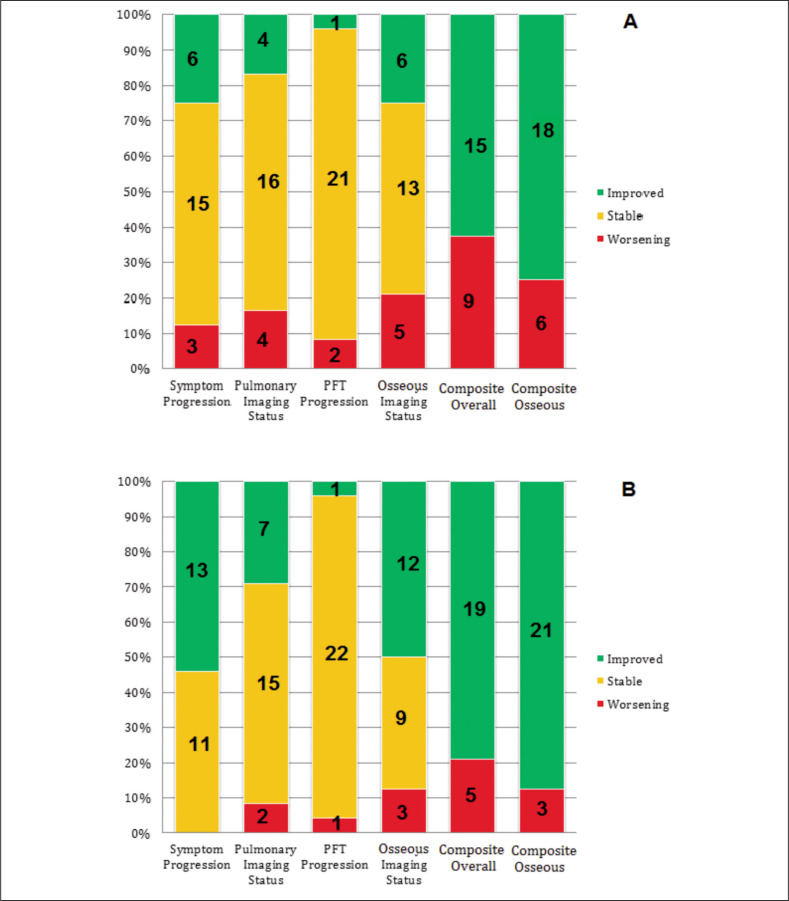
At 1-year follow-up, most patients had either stability or improvement compared to baseline in the individual components of the composite overall outcome (Figure 1A). At 1-year follow-up, 38% and 25% of patients had worsening composite and composite osseous outcomes, compared to 21% and 13% at last follow-up. Only three (13%) patients had worsening symptoms attributable to osseous sarcoidosis at 1-year follow-up. Osseous sarcoidosis lesions were evaluated by imaging for all 24 patients at least once in the first year of follow up. Six (25%) patients had objective radiographic improvement in osseous sarcoidosis lesions, 13 (54%) had stable osseous lesions and 5 (21%) had worsening in imaging of osseous lesions at 1-year follow-up. Pulmonary imaging remained stable for 16 (67%) patients, worsened in 4 (17%), and improved in 4 (17%). PFTs remained stable for 16 (88%) patients at 1 year, with only 1 (4%) and 2 (8%) patients in the group either having improved or worsened pulmonary function tests, respectively.
Fig. 1.
Clinical outcomes at (A) 1-year follow-up and (B) last follow-up compared to baseline. For Symptom Progression, Pulmonary Imaging Status, PFT Progression, and Osseous Imaging Status, the numbers in green refer to absolute number of patients with improvement, numbers in yellow refer to absolute number of patients with stability, and numbers in red refer to absolute number of patients with worsening. The Y-axis shows percentage for each category. For Composite Overall, numbers in red refer to a positive composite outcome of any worsening in Symptom Progression, Pulmonary Imaging, PFT Progression, or Osseous Imaging. Numbers in green refer to negative Composite outcome. For Composite Osseous, numbers in red refer to a positive composite osseous outcome of any worsening in Symptom Progression or Osseous Imaging
The descriptive clinical outcomes at last follow-up were similar to the 1-year follow-up (Figure 1B). Thirteen (52%) patients had improvements in symptoms related to osseous sarcoidosis and the remaining 11 (48%) had unchanged symptoms. Most patients had improvement or stability of osseous lesions on musculoskeletal imaging at last follow-up compared to baseline, with 12 (50%) improving, 9 (38%) remaining stable, and 3 (13%) worsening at time of last follow-up. Pulmonary imaging revealed improvement in pulmonary sarcoidosis in 7 (28%) patients, stability in 15 (60%) and worsening in 2 (8%) patients at last follow up. There were few PFT changes at last follow-up, with 22 (88%) remaining unchanged, 1 (4%) showing improvement and 1 (4%) worsening.
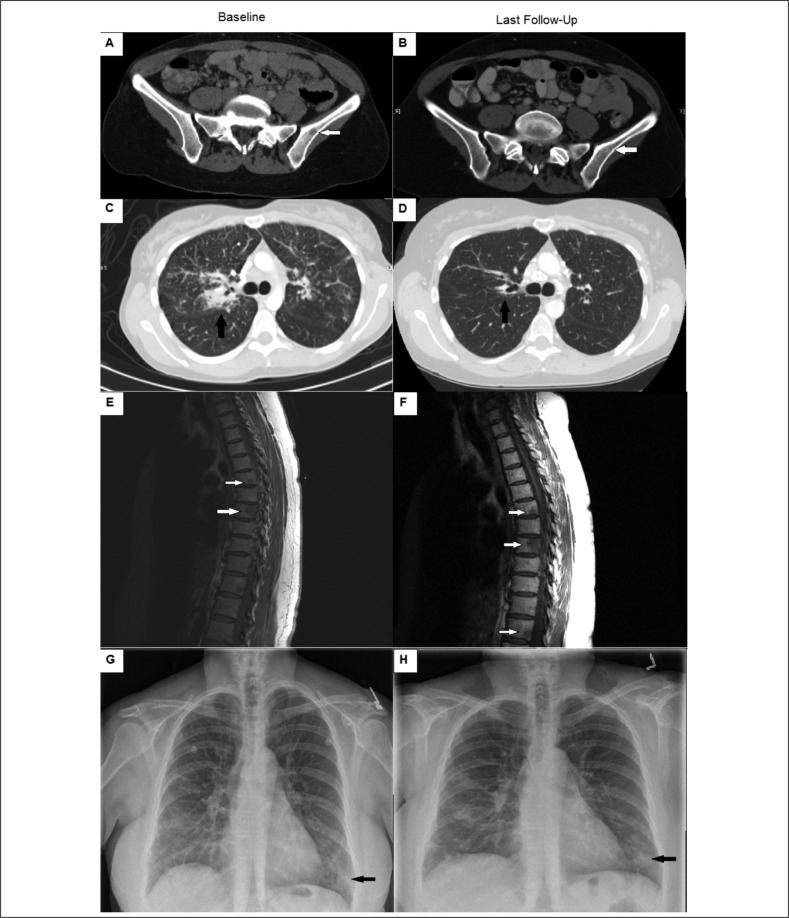
Figure 2 panels A-D shows a representative patient with improved osseous sarcoidosis on musculoskeletal and chest imaging over a 3-year period compared to baseline. The patient was treated initially with prednisone, methotrexate, and infliximab and was gradually weaned off all medications at the follow-up time point. Figure 2 panels E-H shows a representative patient with worsening osseous lesions on musculoskeletal imaging and pulmonary infiltrates that were read as stable over 1.5 years compared to baseline. This patient was treated with azathioprine and rituximab for nearly 1 year due to intolerance of corticosteroids.
Fig. 2.
Panels A-D show representative images of radiographic features of a patient with improving osseous sarcoidosis. Panel A (baseline) and panel B (follow-up) show osseous sarcoidosis improvement on pelvic computed tomography imaging of the left iliac bone (white arrows). Panel C (baseline) and panel D (follow-up) show improvement in chest imaging, particularly in peribronchiovascular consolidation (black arrows) on chest computed tomography imaging. Panels E-H show a patient with worsening osseous sarcoidosis. Panel E (baseline) and panel F (follow-up) show larger osseous lesions in the thoracic spine (white arrows) on T1-weighted magnetic resonance imaging. Panels G (baseline) and panel H (follow-up) show stable lower lobe nodular opacities (black arrows) on chest plain film.
Associations of baseline factors with worsening outcomes at follow-up
At 1-year follow-up, 9 (38%) patients had worsening composite overall outcome, and 6 (25%) had worsening osseous outcome. Few baseline characteristics were statistically associated with worsening outcome at 1 year (Table 3). While these results did not reach statistical significance (likely due to small sample size), female sex, older age at osseous diagnosis, symptoms related to osseous sarcoidosis at time of diagnosis, and treatment for sarcoidosis all showed a trend toward an association with worsening composite overall outcome. Patients tended to be older at baseline (mean 54.3 vs. 47.5 years, p=0.11) for those with a worsening composite outcome at 1 year. Current DMARD or glucocorticoid use at baseline was associated with a lower proportion of patients with positive worsening composite outcome (22% vs. 60%, p=0.10). A worsening composite overall outcome was also more common in patients with a restrictive pattern on PFTs (43% vs. 0%), p=0.05). In addition, having a worsening composite osseous outcome at 1 year showed a trend toward association with older age at baseline (p=0.10), and the presence of musculoskeletal symptoms at time of diagnosis (p=0.17).
Table 3.
Clinical characteristics at baseline and outcomes at 1 year of follow-up (n=24)
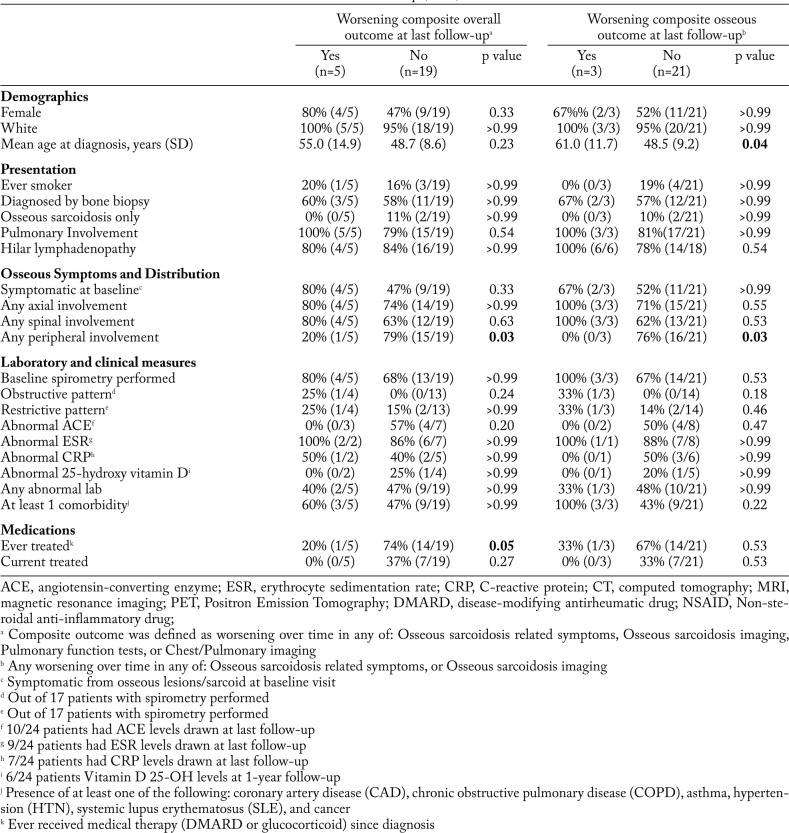
Table 4 shows the associations of baseline factors with outcomes at the last follow-up visit, similar to the results at 1-year follow-up. Patients with peripheral skeletal involvement were significantly less likely to have worsening composite or osseous outcome compared to those with no peripheral involvement (20% vs. 79%, p=0.03, and 0% and 76%, p=0.03). Worsening osseous composite overall outcome was associated with older age at baseline (mean 61.0 years vs. 48.5, p=0.04). Patients who had ever been treated for sarcoidosis at any time between baseline and follow-up were significantly less likely (20% vs. 74%, p=0.05) to have a worsening composite outcome at last follow-up.
Table 4.
Clinical characteristics at baseline and outcomes at last follow-up (n=24)
In sensitivity analyses, we found similar results in subgroups restricted to no treatment and at 2-year and 5-year follow-up time points (data not shown).
Discussion
We evaluated the baseline characteristics, clinical features, and outcomes of 24 patients with osseous sarcoidosis followed for at least one year. This study represents a relatively large clinical cohort whose disease outcomes were investigated over time. Unique to our study was the use of a composite clinical outcome metric to assess the clinically relevant progression of this rare manifestation of an uncommon disease. We found that a majority of patients with osseous sarcoidosis had a stable or improved course of disease during follow-up. Regardless of the distribution of bones involved, patients had mostly favorable outcomes. However, a minority of patients did experience clinical deterioration in terms of symptoms and musculoskeletal and chest imaging.
Unlike previous studies focusing on clinical characteristics of patients with osseous sarcoidosis at diagnosis, we designed our study to investigate relevant subsequent disease outcomes after diagnosis to assess long-term clinical outcomes. We created a composite outcome incorporating both subjective and objective measures of osseous and pulmonary disease to represent clinically relevant outcomes for patients with osseous involvement in sarcoidosis. Similar to the World Association of Sarcoidosis and Other Granulomatous diseases (WASOG) (23) classifications, we incorporated clinician evaluation of symptoms and objective reports of imaging modalities in order to determine worsening, stability, or improvement. At baseline, just over half of the patients in our cohort were symptomatic from their osseous sarcoidosis based on review of provider notes. This is similar to rates of symptoms in larger series (5, 18). At 1-year and last follow-up, most patients remained with stable or improved composite disease or osseous-specific disease outcomes. This finding is consistent with our previous descriptive study (19), and also consistent with other case series of osseous sarcoidosis (9, 10). Our study provides reassurance to both patients and clinicians that osseous involvement of sarcoidosis may have a favorable prognosis in terms of imaging and symptoms. However, given the small number of patients in our series and relatively limited follow-up, we cannot comment on risks that may develop in the longer term or that may have been detected with a larger sample size.
In our cohort, we found few baseline demographic or clinical features associated with the composite or osseous outcomes. At 1-year follow-up, only the baseline presence of restrictive spirometry was statistically significantly associated with progression of osseous sarcoidosis by the composite overall outcome. This association disappeared at last follow-up, suggesting that abnormal spirometry may not be associated with worsening osseous sarcoidosis. However, we cannot definitively determine whether or not pulmonary sarcoidosis was the driver of either clinical evaluation or improvement. Ours is the largest study to investigate the pulmonary function of patients with osseous sarcoidosis. A smaller case series involving seven patients with osseous involvement also noted no clear association between spirometry patterns and osseous involvement (12). While we had few statistically significant associations, the detailed description of patients at baseline, 1-year, and last follow-up will be useful to provide counseling to patients who present with osseous sarcoidosis.
The distribution of affected bones did not appear to correlate with long-term outcomes. At 1-year follow-up, there were more patients who either improved or did not worsen in both composite outcomes and osseous outcomes with any peripheral involvement. At last follow-up, peripheral involvement was statistically significantly associated with stability or improvement in composite or osseous outcomes. However, other patterns were not associated with differences in outcomes. Axial or spinal involvement was not associated with worsening osseous sarcoidosis outcomes in our study.
We previously reported on the clinical characteristics of patients at our center with osseous sarcoidosis at diagnosis and this current report adds more cases (19). Our results are also similar to another case series (18) finding that a majority of osseous sarcoid patients were middle-aged, and have involvement of the axial skeleton with multiple bone lesions. Another case series reported a majority of patients were African American and diagnosed at a younger age than our cohort (5). Our results likely reflect the local selection of our patient population, rather than a true difference in prevalence of osseous sarcoidosis across different populations.
Most of our patients had involvement of the axial skeleton, including 67% with spinal involvement, including the vertebrae. There are conflicting data on the typical distribution of skeletal involvement in osseous sarcoidosis. Larger case series describe axial involvement ranging from 51%-88% (5, 6, 18, 24). In one of these series, none of the 17 patients identified with osseous sarcoidosis by MRI had involvement of the vertebrae (24). Involvement of the axial skeleton is of particular clinical concern as it can be difficult to distinguish radiographically from metastatic cancer (9).
In our cohort, laboratory measurements were available for most patients. Just over half had at least one abnormal laboratory test, and approximately half had elevated serum inflammatory markers. None of the patients had an abnormal serum calcium level. This finding suggests that, unlike the lytic lesions of metastatic cancer (25), the osseous lesions in sarcoidosis generally present without hypercalcemia. We found that baseline laboratory abnormalities were not associated with worsening composite or osseous outcomes over time, suggesting that abnormal serum levels of ACE and inflammatory markers are not reliable predictors for worsening clinical outcomes for patients with osseous sarcoidosis.
Osseous sarcoidosis can mimic metastatic bone lesions (9, 13) and for this reason is often heavily evaluated by physicians. Patients in our cohort had a median of 5.5 and 13 visits at 1 year and last follow-up, respectively. This high level of resource utilization in a cohort with, on average, favorable outcomes, may suggest that less aggressive follow-up may be warranted for some patients with osseous sarcoidosis. However, the timing and frequency of clinical follow-up should be determined based on clinical expertise since it is unclear that osseous involvement alone was responsible for this utilization of health care.
We found that treatment at baseline and during follow-up was associated with favorable clinical outcomes. Patients who were undergoing treatment with DMARDs or glucocorticoids at baseline were more likely to have stability or improvement than those patients who were never treated for osseous sarcoidosis during follow-up. Despite reports suggesting that osseous sarcoidosis is resistant to treatment (26), our data, along with other case reports and case series, (5, 6, 11, 18, 19, 27) demonstrate that patients with osseous sarcoidosis requiring treatment do respond favorably to systemic anti-inflammatory drugs. It is unclear whether the natural history of osseous sarcoidosis portends a relatively benign clinical course or whether some of the favorable clinical outcomes observed were related to treatment. Our study provides a rationale for future, prospective studies to further investigate the role of treatment in osseous sarcoidosis.
There are several limitations to our study. While ours is one of the largest studies of patients with osseous sarcoidosis and the first to concentrate on outcomes after initial presentation, our sample size was small and the length of follow-up was relatively limited. Therefore, we may have been unable to detect true associations and may not have observed clinically relevant outcomes that require larger sample size and longer follow-up, such as bone fractures or death. We created a composite measure to detect any worsening on three objective tests (musculoskeletal/chest imaging and PFTs) and one subjective measure (symptoms). However, this composite measure has not been validated, and it may not fully capture the burden of disease. We created this measure since no other outcome measures currently exist, and we utilized criteria that clinicians and patients are likely to consider important metrics for success and/or initiating treatment. In our analysis, an abnormality in any one of four outcome measures resulted in a positive composite outcome, indicating overall deterioration. We find the relatively small number of patients who experienced this broad definition of a worsening composite outcome, to be reassuring information for providers and patients.
Although we collected detailed data on a variety of clinically relevant characteristics, our study was retrospective, and so not all data were available at all time points. We included patients through electronic medical record search and case specialist case referral. This process may bias our sample, and may account for the rate of biopsies performed to diagnose osseous sarcoidosis. The frequency of follow-up visits was determined by routine clinical care and was inevitably variable. It is possible that clinically meaningful outcomes may have occurred and were not noted in our medical record. However, most patients received all their care at our institution. While we collected a large list of laboratory, imaging, and clinical data, there was a relatively large amount of missing data since not all of these measurements were clinically indicated for all patients. Also, patients with active disease requiring close clinical follow-up may have been seen more often and received more clinical measures than patients with more stable disease. However, since we found that most patients had a relatively stable course during follow-up, we find this unlikely to explain our results. While our intent was to describe the natural history of patients with osseous sarcoidosis, some patients were treated with DMARDs and glucocorticoids. When we restricted the sample size to patients who received no treatment, we found overall similar results.
Those who were treated with glucocorticoids or DMARDs were slightly more likely to have improvement at last follow-up compared to those who did not receive treatment. However, we found no association at the 1-year follow-up time point, so this result may be due to chance. Larger, prospective observational and interventional studies are needed to further describe the natural history of osseous sarcoidosis and to determine the role of treatment. Finally, we only included patients at a large tertiary care hospital so our results may not be generalizable to other patients owing to the care setting and demographics of our population. We encourage collaborative efforts to further investigate rheumatic manifestations of sarcoidosis on a larger scale across institutions.
Conclusion
In this study of patients with 5-year median follow-up, we found that most patients with osseous sarcoidosis have a favorable clinical course. After initial diagnosis, most patients with osseous sarcoidosis were either stable or improved as measured by symptoms, musculoskeletal/chest imaging, and PFTs. Notably, the distribution and number of osseous sarcoidal lesions was not associated with worsened clinical outcomes, including among those presenting with widespread axial involvement. We found no association between pulmonary burden at baseline and subsequent osseous sarcoidosis outcomes. While many patients did not require treatment with DMARDs or glucocorticoids, those who were treated also had favorable response. Our findings suggest that osseous sarcoidosis often has a favorable clinical course, though further prospective research is needed for definitive conclusions.
Support: Dr. Miller is supported by NIH Grant Number T32 HL007633. Dr. Sparks and this work were supported by NIH Grant Numbers: K23 AR069688, L30 AR066953, P30 AR070253, and P30 AR072577. The funders had no role in study design, data collection, analysis, decision to publish, or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contributions: All authors were involved in drafting the article or revising it critically for important intellectual contact, and all authors approved the final version to be published. Dr. Sparks had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study Design: Miller, Fanta, McSparron, Coblyn, Sparks
Acquisition, analysis or interpretation of the data: Miller, Fanta, McSparron, Pan, Coblyn, Sparks.
Critical revision of the manuscript for important intellectual content: Miller, Fanta, McSparron, Pan, Coblyn, Sparks.
Statistical analysis: Miller, Pan, Sparks
Obtained funding: Sparks
This paper is subject to the NIH public access policy: http://www.nih.gov/about/publicaccess/Finalpublicaccessimplementation031505.html
References
- 1.Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357:2153–2165. doi: 10.1056/NEJMra071714. [DOI] [PubMed] [Google Scholar]
- 2.Judson MA. The Clinical Features of Sarcoidosis: A Comprehensive Review. Clin Rev Allergy Immunol. 2015;49:63–78. doi: 10.1007/s12016-014-8450-y. [DOI] [PubMed] [Google Scholar]
- 3.Hunninghake GW, Thomas KW, Hunninghake GW. Sarcoidosis. JAMA. 2003;289:3300. doi: 10.1001/jama.289.24.3300. [DOI] [PubMed] [Google Scholar]
- 4.Shlobin OA, Nathan SD. Management of end-stage sarcoidosis: Pulmonary hypertension and lung transplantation. Eur Respir J. 2012;39:1520–1533. doi: 10.1183/09031936.00175511. [DOI] [PubMed] [Google Scholar]
- 5.Gowani ZS, Sathiyakumar V, Holt GE. Osseous Sarcoidosis. JBJS Rev. 2015;3:1. doi: 10.2106/JBJS.RVW.N.00082. [DOI] [PubMed] [Google Scholar]
- 6.Kuzyshyn H, Feinstein D, Kolasinski SL, Eid H. Osseous sarcoidosis: a case series. Rheumatol Int. 2015;35:925–933. doi: 10.1007/s00296-014-3170-4. [DOI] [PubMed] [Google Scholar]
- 7.Yang H, Numani S, Liu S. Monitoring the Therapy of Extensive Osseous Sarcoidosis With FDG PET/CT. Clin Nucl Med. 2017;42:337–339. doi: 10.1097/RLU.0000000000001598. [DOI] [PubMed] [Google Scholar]
- 8.Brandy-García AM, Cabezas-Rodriguez I, Caminal-Montero L, Suarez-Cuervo C, Redondo-Buil P. Sarcoidosis mimicking lytic osseous metastases. Cleve Clin J Med. 2017;84:753–754. doi: 10.3949/ccjm.84a.16108. [DOI] [PubMed] [Google Scholar]
- 9.Packer CD, Mileti LM. Vertebral Sarcoidosis Mimicking Lytic Osseous Metastases. JCR J Clin Rheumatol. 2005;11:105–108. doi: 10.1097/01.rhu.0000158538.29753.b8. [DOI] [PubMed] [Google Scholar]
- 10.Johnson AK, Johnson JM, Ames E, Filippi C. Spontaneous Clinical and Radiological Resolution of Vertebral Sarcoidosis. Spine (Phila Pa 1976) 2012;37:E414–E416. doi: 10.1097/BRS.0b013e31822f30fd. [DOI] [PubMed] [Google Scholar]
- 11.Hasni SA, Kunz D, Finzel K, Gruber BL. Osseous Sarcoidosis Treated With Tumor Necrosis Case Report and Review of the Literature. 2010;35:904–907. doi: 10.1097/brs.0b013e3181dc9a54. [DOI] [PubMed] [Google Scholar]
- 12.Horr AFS, Urphy FTM, Illiland WRG, Natiuk OWH. Osseous disease in patients with pulmonary sarcoidosis and musculoskeletal symptoms. 2000:228–232. doi: 10.1053/rmed.1999.0709. doi:10.1053/rmed.1999.0709. [DOI] [PubMed] [Google Scholar]
- 13.Moore SL, Kransdorf MJ, Schweitzer ME, Murphey MD, Babb JS. Can sarcoidosis and metastatic bone lesions be reliably differentiated on routine MRI. Am J Roentgenol. 2012;198:1387–1393. doi: 10.2214/AJR.11.7498. [DOI] [PubMed] [Google Scholar]
- 14.Binicier O, Sari I, Sen G, Onen F, Akkoc N, Manisali M, Akar S. Axial sarcoidosis mimicking radiographic sacroiliitis. Rheumatol Int. 2009;29:343–345. doi: 10.1007/s00296-008-0677-6. [DOI] [PubMed] [Google Scholar]
- 15.Fisher AJ, Gilula LA, Kyriakos M, Holzaepfel CD. MR imaging changes of lumbar vertebral sarcoidosis. AJR Am J Roentgenol. 1999;173:354–6. doi: 10.2214/ajr.173.2.10430135. [DOI] [PubMed] [Google Scholar]
- 16.Silver HM, Shirkhoda A, Simon DB. Symptomatic osseous sarcoidosis with findings on bone scan. Chest. 1978;73:238–241. doi: 10.1378/chest.73.2.238. [DOI] [PubMed] [Google Scholar]
- 17.Vu K, Atkinson J, Ranganathan P. Bone Lesions, Lymphadenopathy, and Hepatic Granulomas in a Patient with Psoriasis. Arthritis Care Res. 2016;68:394–399. doi: 10.1002/acr.22607. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Y, Lower EE, Li H, Farhey Y, Baughman RP. Clinical characteristics of patients with bone sarcoidosis. Semin Arthritis Rheum. 2017;47:143–148. doi: 10.1016/j.semarthrit.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Sparks JA, McSparron JI, Shah N, Aliabadi P, Paulson V, Fanta CH, Coblyn JS. Osseous sarcoidosis: Clinical characteristics, treatment, and outcomes-Experience from a large, academic hospital. Semin Arthritis Rheum. 2014 doi: 10.1016/j.semarthrit.2014.07.003. doi:10.1016/j.semarthrit.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Gottlieb JE, Israel HL, Steiner RM, Triolo J, Patrick H. Outcome in sarcoidosis: The relationship of relapse to corticosteroid therapy. Chest. 1997;111:623–631. doi: 10.1378/chest.111.3.623. [DOI] [PubMed] [Google Scholar]
- 21.Thelier N, Assous N, Job-Deslandre C, Meyer O, Bardin T, Orcel P, Lioté F, Dougados M, Kahan A, Allanore Y. Osteoarticular involvement in a series of 100 patients with sarcoidosis referred to rheumatology departments. J Rheumatol. 2008;35:1622–1628. [PubMed] [Google Scholar]
- 22.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CPM, Gustafsson P, Hankinson J, Jensen R, Johnson DC, MacIntyre N, McKay R, Miller MR, Navajas D, Pedersen OF, Wanger J. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 23.Baughman RP, Nagai S, Balter M, Costabel U, Drent M, Du Bois R, Grutters JC, Judson MA, Lambin I, Lower EE, Muller-Quernheim J, Prasse A, Rizzato G, Rottoli P, Spagnolo P, Teirstein A. Defining the clinical outcome status (COS) in sarcoidosis: Results of WASOG Task Force. Sarcoidosis Vasc Diffus Lung Dis. 2011;28:56–64. [PubMed] [Google Scholar]
- 24.Moore SL, Teirstein A, Golimbu C, Sl M, Teirstein A, Golimbu C. MRI of Sarcoidosis Patients with Musculoskeletal Symptoms. 2005:154–159. doi: 10.2214/ajr.185.1.01850154. [DOI] [PubMed] [Google Scholar]
- 25.Coleman RE. Clinical Features of Metastatic Bone Disease and Risk of Skeletal Morbidity. 2006;12:6243–6250. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 26.Wilcox A, Bharadwaj P, Sharma OP. Bone sarcoidosis. Curr Opin Rheumatol. 2000;12:321–330. doi: 10.1097/00002281-200007000-00016. [DOI] [PubMed] [Google Scholar]
- 27.Judson MA, Baughman RP, Costabel U, Flavin S, Lo KH, Kavuru MS, Drent M, Culver DA, Davis GS, Fogarty CM, Hunninghake GW, Teirstein AS, Mandel M, McNally D, Tanoue L, Newman L, Wasfi Y, Patrick H, Rossman MD, Raghu G, Sharma O, Wilkes D, Yeager H, Donahue JF, Kaye M, Sweiss N, Vetter N, Thomeer M, Brutsche M, et al. Efficacy of infliximab in extrapulmonary sarcoidosis: Results from a randomised trial. Eur Respir J. 2008;31:1189–1196. doi: 10.1183/09031936.00051907. [DOI] [PubMed] [Google Scholar]