Abstract
Dilated cardiomyopathy (DCM) is a leading cause of morbidity and mortality worldwide; yet how genetic variation and environmental factors impact DCM heritability remains unclear. Here, we report that compound genetic interactions between DNA sequence variants contribute to the complex heritability of DCM. By using genetic data from a large family with a history of DCM, we discovered that heterozygous sequence variants in the TROPOMYOSIN 1 (TPM1) and VINCULIN (VCL) genes cose-gregate in individuals affected by DCM. In vitro studies of patient-derived and isogenic human-pluripotent-stem-cell-derived cardio-myocytes that were genome-edited via CRISPR to create an allelic series of TPM1 and VCL variants revealed that cardiomyocytes with both TPM1 and VCL variants display reduced contractility and sarcomeres that are less organized. Analyses of mice genetically engineered to harbour these human TPM1 and VCL variants show that stress on the heart may also influence the variable penetrance and expressivity of DCM-associated genetic variants in vivo. We conclude that compound genetic variants can interact combinatorially to induce DCM, particularly when influenced by other disease-provoking stressors.
Dilated cardiomyopathy (DCM), due to multifactorial aetiologies including environmental or genetic causes, affects as many as 1 in 250 individuals and is a leading cause of morbidity and mortality worldwide1,2. Although more than 1,000 disease-causing variants in over 40 cardiomyopathy-associated genes have been identified, they only account for ~30–60% of the basis for familial cardiomyopathies and frequently have variable penetrance and expressivity1,3–5. As a result, recent human genetic studies have suggested that a substantial fraction of unaccounted cardiomyopathy heritability may be due to a combination of multigenic causes and gene–environment interactions1,6–10; yet, the functional evidence for these possible aetiologies remains incompletely understood.
Results
Human genetic studies reveal complex inheritance of DCM.
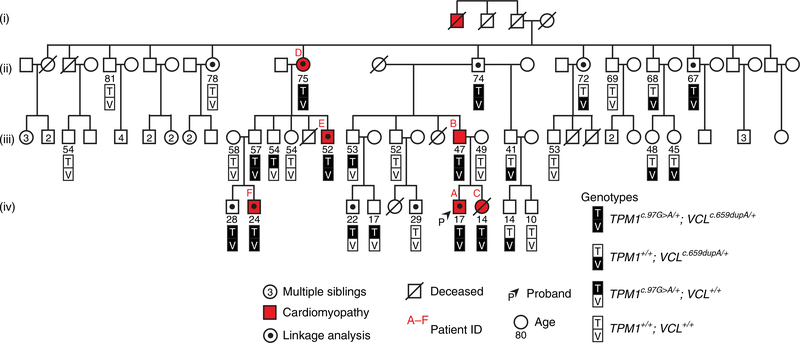
To explore how cardiomyopathy-associated genetic variants may act combinatorially to promote DCM, we identified a family with multiple generations of DCM harbouring novel variants in the sarcomeric gene TROPOMYOSIN 1 (TPM1) and the costameric gene VINCULIN (VCL; Fig. 1). The proband initially presented with severe heart failure and required heart transplantation at age 14 (Fig. 1, arrowhead and Table 1, Patient A). Further examination of an extended pedigree revealed five additional family members who developed variable presentations of cardiomyopathy across a wide age range (Fig. 1 and Table 1), including the proband’s father, who was diagnosed with DCM at age 35 and his sister, who later died from sudden cardiac death at age 15 and displayed early signs of cardiomyopathy on autopsy (Supplementary Fig. 1 and Table 1). Genetic testing of the proband, his sister and his parents identified the TPM1 and VCL heterozygous variants TPM1 c.97G>A (p.Glu33Lys, p.E33K) and VCL c.659dupA (p.Asn220fs, p.N220fs) hereafter referred to as TEK (TPM1 p.E33K) and VFS (VCL frame shift), respectively. Although other TPM1 and VCL variants have been associated with DCM, hypertrophic cardiomyopathy and left-ventricular non-compaction cardiomyopathy3,11, these novel TEK and VFS variants have not been previously analyzed (Table 2). Thus, we confirmed that the VFS variant insertion results in a translational frame shift of VCL and a predicted premature stop at codon 240, which leads to nonsense-mediated messenger RNA decay of the VCL transcript and reduced VCL protein expression without evidence of truncated VCL peptide expression in skin fibroblasts harbouring both heterozygous VFS and TEK variants (Supplementary Fig. 2a–e). On the other hand, the TEK variant results in a change from a negatively charged glutamic acid residue to a positively charged lysine residue at codon 33 of TPM1 (Supplementary Fig. 2f). Notably, this TPM1 E33 residue is highly conserved and located in the first of three alpha helical turns that also contain the highly conserved amino acids E40 and E54, which exhibit similar variant changes (E40K and E54K) that have been associated with DCM (Supplementary Fig. 2h–j)12,13.
Fig. 1 |. Novel TPM1 and VCL variants together cosegregate with family members exhibiting cardiomyopathy.
The pedigree of a large family exhibiting DCM reveals that TPM1 and VCL variants cosegregate with disease (red). Age at the time of genetic testing is shown, for four generations (labelled (i)–(iv)).
Table 1 |.
The onset and severity of cardiomyopathy are variable in TV-Dhet patients
| Patient | Sex | Diagnosis | Age of onset (yr)a | Left ventricular ejection fraction (%) | Left ventricular D/S diameter (cm) | Therapy/intervention |
|---|---|---|---|---|---|---|
| A | M | DCM | 14 | 25 | 8.6/7.6 | Left ventricular assist device/ orthotopic heart transplant |
| B | M | DCM | 35 | 40 | 6.9/5.4 | Automatic implantable cardioverter-defibrillator |
| C | F | Sudden cardiac death, mild hypertrophy consistent with early DCM | 15 | 61 | 4.46/3.0 | N/A |
| D | F | DCM | 60 | 45 | 5.9/3.5 | Medical therapy |
| E | M | DCM | 50 | 46 | 6.1/4.5 | Medical therapy |
| F | M | DCM | 0.5 | 29 | 6.4/5.5 | Mitral valve annuloplasty |
The clinical characteristics of affected individuals marked in Fig. 1 with a red patient identifier (A–F) are described. The mean age of onset ± s.d. was calculated. Cardiac function is represented as left ventricular ejection fraction. The left ventricular diameter was measured during the diastole (D) and systole (S) phases. M, male; F, female; N/A, not applicable.
Mean = 29.1±23.1.
Table 2 |.
Cardiomyopathy associates most significantly with the TV-Dhet genotype in this family
| Genotype summary | Abbreviation | Cardiomyopathy | Unaffected | Odds ratio (95% CI) | Association beta±s.e. | Corrected P value | Fisher’s exact P value | Frequency |
|---|---|---|---|---|---|---|---|---|
| TPM1c.97G>A/+; VCLc.659dupA/+ | TV-Dhet | 6 | 4 | 375 (97.8–1,436.0) | 0.5711±0.1084 | 2.40×10−5 | 7.7268×10−5 | N/A |
| TPM1c.97G>A/+; VCL+/+ | TEK | 0 | 4 | N/A | N/A | N/A | N/A | N/A |
| TPM1+/+; VCLc.659dupA/+ | VFS | 0 | 6 | N/A | N/A | N/A | N/A | N/A |
| TPM1+/+; VCL+/+ | WT | 0 | 11 | N/A | N/A | N/A | N/A | N/A |
| Variant summaryTPM1c.97G>A | TEK | 6 | 8 | 187.5 (59.36–592.2) | 0.4340±0.1199 | 2.22×10−3 | 2.65×10−3 | Not observeda |
| VCLc.659dupA | VFS | 6 | 10 | 150 (49.5–454.0) | 0.4036±0.1156 | 3.13×10−3 | 8.27×10−3 | 1/120,760 alleles |
Genotype analysis shows that all individuals diagnosed with cardiomyopathy harbour the TV-Dhet genotype, which is significantly associated with disease by both mixed linear model association analysis (P=2.40×10−5) and two-sided Fisher’s exact test (P=7.7268×10−5). In addition, the odds ratio for the TV-Dhet genotype is 375 (95% CI: 97.8–1,436). Because neither the TPM1c.97G>A/+ ; VCL+/+ nor the TPM1+/+; VCLc.659dupA/+ genotype was observed in affected individuals, the association beta values, Fisher’s exact test values and odds ratio could not be statistically determined (N/A, not applicable). Analysis of TPM1 c.97G>A and VCL c.659dupA variants independent of genotype revealed that each variant is not only rare (frequency) but also segregates with disease by association beta and Fisher’s exact tests. However, these individual variants associate with disease to a lesser degree than the TV-Dhet genotype combination because each variant is observed not only together in affected individuals but also alone in unaffected individuals.
TPM1 c.97G>A was not observed in exome data with coverage of flanking variants between 49,966–69,152 alleles.
Further analyses of these TEK and VFS variants in 27 additional family members revealed that affected individuals carry both of these variants (TV-Dhet: TEK-, VFS-double heterozygote) but never either variant alone (Fig. 1 and Table 2). However, because TV-Dhet variants were detected in four other family members without obvious clinical evidence of cardiomyopathy, we evaluated whether these variants, in combination or alone, were statistically correlated with disease. Evaluations using both mixed linear model association and Fisher’s exact analyses between genotype and disease showed that the TV-Dhet genotype associates significantly with cardiomyopathy but the TPM1TEK/+; VCL+/+ and TPM1+/+; VCLVFS/+ genotypes do not because there were no individuals with these single variant genotypes that exhibited cardiomyopathy in our pedigree (Table 2, Genotype summary). Examination of the TEK and VFS variants independent of genotype revealed that each variant segregates with disease by association beta and Fisher’s exact test values, but to a lesser degree than the TV-Dhet genotype variant combination because a substantial number of unaffected individuals harboured either variant alone (Table 2, Variant summary). Moreover, assuming a population prevalence of DCM at 1:250, the odds ratio for the TV-Dhet genotype was 375 (95% confidence interval (CI): 97.8–1,436.0), whereas the odds ratios for the TPM1TEK/+; VCL+/+ and TPM1+/+; VCLVFS/+ genotypes could not be determined due to the absence of affected individuals with these genotypes (Table 2). However, when each variant was analysed independently of genotype (Table 2), the odds ratios for the TEK and VFS variants were 187.5 (95% CI: 59.36–592.2) and 150 (95% CI: 49.5–454.0), respectively. Finally, to determine whether the heritability of DCM might extend beyond the TPM1 and VCL genes, we used single-nucleotide-variant microarrays to generate a high-resolution linkage map between common variants and disease using the individuals marked in Fig. 1. This analysis revealed that no single chromosomal region is shared exclusively by the affected family members and that no large structural variation exists in the genomes of selected members of this cohort (Supplementary Figs. 3 and.4). These statistical analyses therefore provide strong evidence that the TEK and VFS variants together are more closely linked to DCM than either variant alone in our pedigree, thus supporting the combinatorial role of these variants in DCM.
Human pluripotent stem cell (hPSC) cardiomyocyte modelling of DCM.
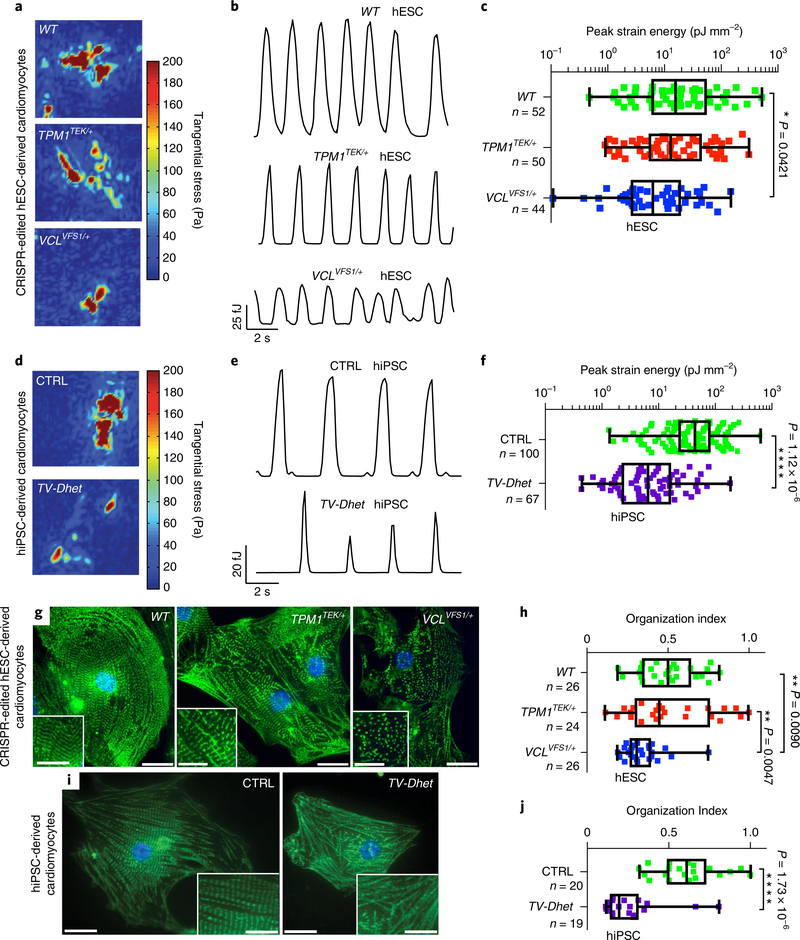
To investigate the impact of these TPM1 and VCL variants on human DCM pathogenesis, we generated cardiomyocytes from patient-derived human induced pluripotent stem cells (hiPSC) and clustered regularly interspaced short palindromic repeats (CRISPR–CRISPR associated protein 9 (Cas9) genome-edited isogenic H9 human embryonic stem cells (hESC). Skin fibroblasts from the DCM proband and his unaffected mother (control, CTRL), who does not carry either TEK or VFS variants, were successfully reprogrammed into hiPSCs that express pluripotency markers (Supplementary Fig. 5c)14. Using CRISPR–Cas9 technology, heterozygous TEK and VCL c.74del7 (VFS1) variants were individually introduced into H9 hESCs and confirmed for targeting specificity as previously reported (Supplementary Fig. 5a,b,d and Supplementary Table 1)15. Similar to the VFS variant, the VCL c.74del7 allele also results in an early VCL frame shift and subsequent decreased VCL protein levels due to nonsense-mediated mRNA decay (Supplementary Fig. 6). Employing a highly efficient cardiac differentiation protocol16,17, these hiPSC and genome-edited hESC lines were successfully differentiated into cardiomyocytes of comparable maturity to investigate whether these variants influence cardiomyocyte function (Supplementary Fig. 5e–h). Traction force microscopy revealed a significant decrease in VCLVFS1/+ hESC- and TV-Dhet hiPSC-cardiomyocyte contractility, as well as a trend towards reduced contractility in TPM1TEK/+ hESC cardiomyocytes when compared with control hiPSC (CTRL) or hESC (WT) cardiomyocytes; however, the greatest reduction was observed in TV-Dhet hiPSC cardiomyocytes (Fig. 2a–f). Furthermore, α-actinin immunostaining of these hPSC cardiomyocytes revealed that sarcomeric organization is reduced in TV-Dhet and VCLVFS1/+ cardiomyocytes but not in TPM1TEK/+ or control cardiomyocytes (Fig. 2g–j). Overall, these functional data support that TPM1 and VCL variants act synergistically to impact cardiomyocyte contractility and sarcomeric organization and suggest that together these variants may perturb sarcomeric–costameric interactions.
Fig. 2 |. hPSC-derived cardiomyocytes harbouring TEK; VCL genetic variants exhibit functional and sarcomeric organization defects.
a–f, Single-cardiomyocyte traction force microscopy studies show that TV-Dhet and VCLVFS1/+ hPSC-derived cardiomyocytes exhibit reduced contractility as detected by tangential stress heat maps (a,d), strain energy time courses (b,e) and peak strain energy analyses (c,f). g–j, Alpha-actinin immunostaining (green; g,i) and quantitation of the sarcomeric organization (h,j) reveal that sarcomeres of TV-Dhet and VCLVFS1/+ hPSC-derived cardiomyocytes are more disorganized than that of WT and CTRL cardiomyocytes. Control hESC-derived (WT) and CRISPR-edited-hESC-derived cardiomyocytes are represented in a–c,g,h. Control hiPSC-derived (CTRL) and cardiomyopathy-affected patient (TV-Dhet) hiPSC-derived cardiomyocytes are represented in d–f,i,j. Data are presented as interquartile range (box) around the mean (horizontal line), whiskers represent the minimum and maximum of the dataset, with the individual data points superimposed. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001; two-sided Student’s t-test (f,j) or one-sided ANOVA with Tukey multiple correction test alpha (c,h). g,i, The representative images shown were obtained from experiments independently repeated three times with similar results; Hoechst nuclear labelling is shown in blue. Scale bars, 20 μm. Inset scale bars, 10 μm. n, number of biologically independent cardiomyocytes of each genotype used for each study.
Gene expression analysis in DCM cardiomyocytes.
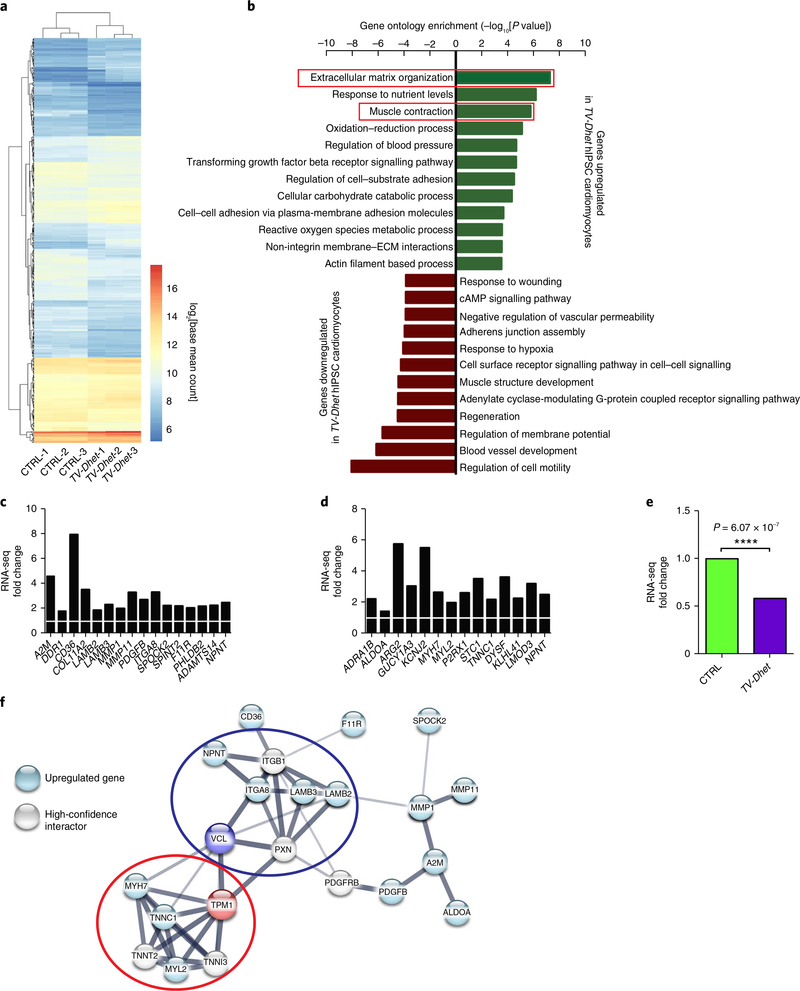
To further explore the mechanistic underpinnings of these morphological and functional defects in TV-Dhet hiPSC cardiomyocytes, RNA-sequencing (RNA-seq) analyses were performed on biological replicates of proband (TV-Dhet) and unaffected (familial control) hiPSC cardiomyocytes. These RNA-seq replicates not only clustered by genotype when comparing differentially expressed genes (Fig. 3a) but also revealed 434 genes that are upregulated (n = 210) or downregulated (n = 224) in TV-Dhet hiPSC cardiomyocytes (Supplementary Table 2), including VCL (Fig. 3e), which was also reduced in the fibroblasts of the proband (Supplementary Fig. 2b). Supporting that the VFS variant is sufficiently expressed to result in this decreased VCL mRNA level in TV-Dhet hiPSC cardiomyocytes, percentage spliced-in analysis revealed complete inclusion of the exon (exon 6) containing the VFS variant in TV-Dhet hiPSC cardiomyocytes (Supplementary Table 3). Furthermore, analysis of the upregulated genes in TV-Dhet hiPSC cardiomyocytes showed significant enrichment for gene ontology terms related to both costameric (VCL-related) function—including extracellular matrix (ECM) organization, regulation of cell–substrate adhesion, cell–cell adhesion via plasma-membrane adhesion molecules and non-integrin membrane–ECM interaction—as well as sarcomeric (TPM1-related) function, such as muscle contraction, regulation of blood pressure and actin filament based process (Fig. 3b). In addition, biological pathways that are activated during the pathogenesis of heart failure, including oxidative stress (gene ontology term: oxidation–reduction process, reactive oxygen species metabolic process)18, cardiac fibrosis and remodelling (gene ontology term: transforming growth factor-β receptor signalling pathway)19 and glycolysis (gene ontology term: response to nutrient levels and cellular carbohydrate catabolic processes), are also increased in TV-Dhet hiPSC cardiomyocytes20. Conversely, genes related to signalling pathways downstream of the G-protein coupled receptor β-adrenergic receptor (gene ontology term: cAMP signalling, adenylate cyclase-modulating G-protein coupled receptor signalling pathway) are downregulated, as is typically observed during human heart failure21. Moreover, genes related to biological pathways that regulate blood-vessel development and response to hypoxia were also notably reduced, which is consistent with similar findings that were recently reported in TITIN mutation hiPSC cardiomyocytes from DCM patients (Fig. 3b)22.
Fig. 3 |. ECM and muscle contraction genes are coordinately upregulated in TV-Dhet hiPSC cardiomyocytes.
a, Hierarchical clustering of differentially expressed genes between control (CTRL) and cardiomyopathy-affected patient (TV-Dhet) hiPSC-derived cardiomyocytes (n = 3 biological replicates per genotype) reveals clusters of genes that are coordinately regulated in TV-Dhet cardiomyocytes. Gene expression data from these replicates were combined by genotype in b–f to further compare relative gene expression changes. b, ECM organization and muscle contraction gene ontology terms are significantly enriched among genes upregulated in TV-Dhet cardiomyocytes whereas various gene ontology terms affecting biological processes that are regulated in heart failure are enriched among downregulated genes when using one-sided accumulative hypergeometric distribution analysis without multiple correction adjustment. c,d, Expression of genes that comprise ECM organization (c) and muscle contraction (d) gene ontology terms upregulated in TV-Dhet cardiomyocytes are normalized to CTRL cardiomyocytes (white bar). e, VCL expression levels are decreased in TV-Dhet cardiomyocytes compared to CTRL cardiomyocytes. DEseq2-adjusted P values displayed for TV-Dhet gene expression relative to CTRL cardiomyocytes. Gene expression P values were calculated using the Wald test with a Benjamini–Hochberg procedure false discovery rate adjustment. ****P < 0.0001. f, STRING database analysis reveals protein–protein interactions between the proteins encoded by upregulated genes, TPM1, VCL and limited high-confidence interactors. TPM1 and VCL are predicted to interact with upregulated components of the sarcomere (red oval) and costamere–ECM complex (blue oval), respectively. The darker edges represent increased STRING database interaction confidence.
To illuminate cardiac-protein interaction complexes that may become activated due to the TV-Dhet variants, we performed STRING database interaction mapping23 of differentially regulated genes associated with VCL (ECM organization genes) and TPM1 (muscle contraction genes; Fig. 3c,d). Upregulation of sarcomere (Fig. 3f, red oval—MYH7, TNNC1 and MYL2) and costamere–ECM bridging (Fig. 3f, blue oval—LAMB2, LAMB3, ITGA8 and NPNT) protein network complexes was observed when plotting the proteins of these altered genes with TPM1, VCL and limited first-degree interacting proteins. Together, these data suggest that costameric and sarcomeric protein networks may be coordinately regulated in TV-Dhet cardiomyocytes as compensatory mechanisms in response to the combinatorial effects of TPM1 and VCL dysfunction.
Mouse complex genetic model phenocopies human DCM family.
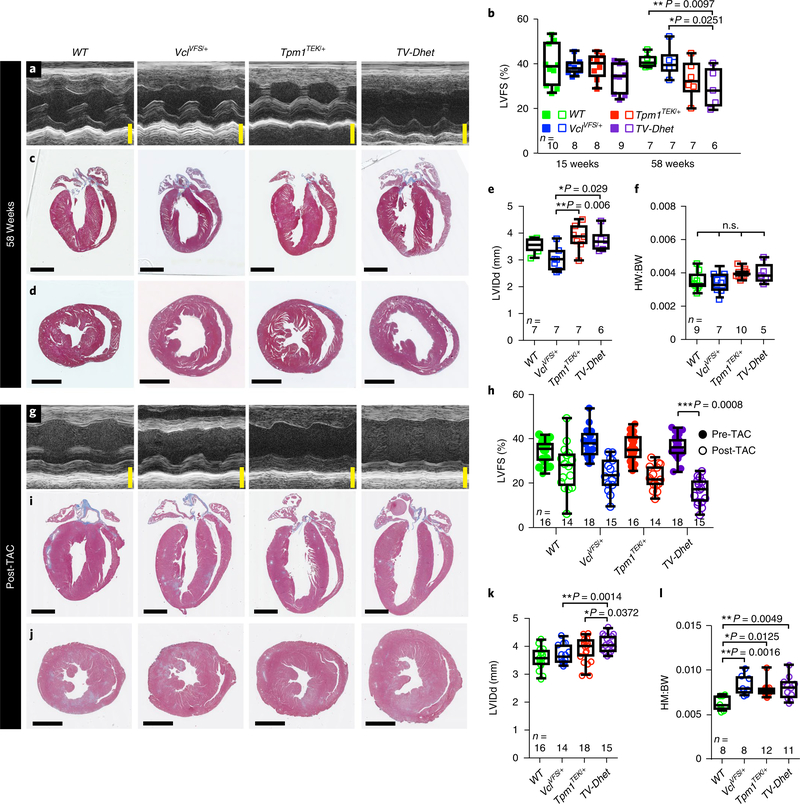
Finally, on the basis of the variable penetrance and expressivity of DCM in family members harbouring TV-Dhet variants (Fig. 1 and Table 1), we further investigated the function of these TEK and VFS variants in mouse models where DCM can be examined at the organ level. To this end, we employed CRISPR genome-editing strategies to create isogenic C57BL/6J mice carrying these patient-specific TEK and VFS variants (Supplementary Fig. 7), which were confirmed for targeting specificity as previously reported (Supplementary Table 4)15. Similar to our findings in human TV-Dhet fibroblasts (Supplementary Fig. 2), VclVFS/+ and TV-Dhet mouse myocardium exhibited reduced Vcl protein levels, predominantly of the Vcl rather than meta-vinculin isoform, with no evidence of truncated Vcl peptide translation (Supplementary Fig. 8). Furthermore, VclVFS/+ and TV-Dhet mice were viable and without any obvious baseline phenotypes. Supporting that the VFS variant produces a true loss-of-function allele, VclVFS/VFS mice were embryonically lethal, phenocopying homozygous Vcl knockout mice as previously reported (Supplementary Fig. 9)24. On the other hand, both Tpm1TEK/+ and Tpm1TEK/TEK mice were viable, but at 58 weeks of age Tpm1TEK/TEK mice exhibited significantly decreased cardiac contractility, thus indicating that the TEK variant is also pathologically relevant (Supplementary Fig. 10 and Supplementary Table 5).
To examine the in vivo effects of heterozygous TEK and VFS variants on mouse cardiac function, we analysed left ventricular contractility, chamber dimension and wall thickness in Tpm1TEK/+, VclVFS/+ and TV-Dhet mouse hearts by echocardiography and histology (Fig. 4a–e and Supplementary Table 6). Consistent with data from our family cohort, TV-Dhet mice, but not Tpm1TEK/+ or VclVFS/+ mice, exhibited significantly decreased left ventricular contractility compared to wild-type (WT) mice at 58 weeks (Fig. 4b); however, these TV-Dhet mice did not display ventricular dilation, increased diastolic wall thickness or significant change in heart-to-bodyweight ratios (Fig. 4c–f and Supplementary Table 6), which suggests that additional factors may synergize with TV-Dhet variants to contribute to the severity of the DCM phenotype. Thus, given that TV-Dhet variants exhibit not only variable DCM penetrance and expressivity in our human family cohort (Fig. 1 and Table 1), but also modest pathologic cardiac effects in isogenic mouse models, we further investigated whether haemodynamic stress may mediate disease susceptibility in this complex genetic background by subjecting mouse hearts to transverse aortic constriction (TAC). This form of haemodynamic stress can increase cardiac workload similar to that observed in hypertension or other cardiac diseases, such as valvular stenosis25. To this end, TV-Dhet, Tpm1TEK/+, VclVFS/+ and WT mice were subjected to TAC or sham surgery (control) and then examined by echocardiography after one week. No significant change in contractility was detected in any of the shamoperated mice (Supplementary Fig. 11 and Supplementary Table 7), but hearts exposed to TAC for one week (post-TAC) displayed decreased cardiac contractility when compared to left ventricular function before TAC (pre-TAC; Fig. 4g,h and Supplementary Table 7). Most importantly, TV-Dhet post-TAC hearts displayed significantly worse contractility than WT post-TAC hearts and, notably, the Tpm1TEK/+ and VclVFS/+ hearts also showed a decrease in contractility that was intermediate between TV-Dhet and WT hearts (Fig. 4h and Supplementary Movies 1–8). Supporting the notion that haemo-dynamic stress affects the impact of TEK and VFS variants on the DCM phenotype, we observed that post-TAC TV-Dhet hearts also exhibited left-ventricular dilation and cardiac hypertrophy (Fig. 4i–l and Supplementary Table 7), in contrast to the unstressed TV-Dhet hearts that only displayed reduced contractility with ageing. Despite these functional differences, histopathologic studies revealed no significant difference in cardiomyocyte size or myocardial fibrosis between genotypes at 58 weeks of age or subsequent to TAC or sham surgery (Supplementary Fig. 12). Overall, these results not only confirm the pathogenicity of the TEK and VFS variants in vivo but also support the cooperative interactions of potentially deleterious genetic variants with disease stressors, which together may influence the penetrance and expressivity of DCM.
Fig. 4 |. TV-Dhet mouse hearts exhibit reduced contractility and respond worse than WT control mouse hearts to TAC.
a,b, M-mode echocardiography (a) reveals that left-ventricular fractional shortening (LVFS) percentage (b), a measure of cardiac function, is decreased in TV-Dhet mice compared to WT and VclVFS/+ mice at 58 weeks. c–f, Trichrome sections of mouse hearts (c,d) and echocardiographic measurements of left ventricular internal dimension in diastole (LVIDd; e) reveal that TV-Dhet mouse hearts are not significantly dilated compared to WT; in addition, the heart-to-body-weight ratios (HW:BW; f) do not differ significantly between genotypes at 58 weeks. In b,e and f, filled and open squares indicate 15 and 58 week timepoints, respectively. g,h, M-mode echocardiography of pre-TAC (14–16 weeks) and (g) post-TAC (one week following TAC surgery) mouse hearts reveals that the LVFS (h) is significantly reduced in TV-Dhet post-TAC mice when compared with WT post-TAC mice. i–k, Trichrome sections and LVIDd measurements of post-TAC mouse hearts show increased chamber size in TV-Dhet mice compared with WT mice. l, Post-TAC heart-to-body-weight ratios are also increased in mice with TEK and/or VFS variants compared to WT mice. Data are presented as interquartile range (box), around the mean (horizontal line), with whiskers representing the minimum and maximum of the dataset, with the individual data points superimposed. *P < 0.05; **P < 0.01; ***P < 0.001; n.s., no statistically significant comparisons; one-sided ANOVA with Tukey multiple correction test alpha. n, number of biologically independent mice of each genotype used for each study. Scale bars, 2 mm. The complete echocardiography data are available in Supplementary Tables 6 and 7.
Discussion
Utilizing a combination of human genetic studies, patient-derived hiPSCs, and genome-edited hESC and mouse models, we provide evidence supporting the interplay between genetic variants and disease stressors in the aetiology of cardiac disease. These results may account for elements of the missing heritability of DCM observed in studies of population genetics1,26,27. Although previous studies have reported individual TPM1 and VCL genetic variants as associated with various cardiomyopathies3,11, we provide functional evidence that genetic variants in these sarcomeric and costameric genes may act synergistically to lead to DCM. Experimental interrogation of these TPM1 and VCL alleles in both hPSC cardiomyocyte and mouse models supports the idea that these alleles may exhibit gene dosage effects whereby homozygous alleles display more severe phenotypes, including embryonic lethality, but heterozygous alleles alone have more modest effects, which may only manifest as pathological when present in combination with other genetic variants and/or disease stressors. These findings may explain why homozygous alleles of these variants have not been observed in exome databases28 or in our pedigree.
This work further emphasizes how genetic variants can have compound effects that may be further influenced by other disease stressors and may explain the variable penetrance and expressivity in many cases of DCM. Thus, combinatorial genetic alterations in cardiac proteins may not only cooperate to regulate cardiomyocyte force generation and transmission, such as those caused by alterations in the sarcomeric protein TPM1 and the costameric protein VCL, but also increase the susceptibility to overt cardiomyopathies when an additional insult, whether genetic and/or environmental, is superimposed on defects in these pathways. As more genetic variants are discovered through whole-exomic and genomic sequencing of large numbers of patients, interactome pathway models may be valuable in ascertaining whether sequence variants in various cardiac genes may synergistically interact to lead to disease (Fig. 3). Furthermore, information on how biophysical forces impact these interactomes may provide insight into how stresses, such as hypertension or other cardiovascular disorders, can interact with genetic variants to cause cardiomyopathies1,29. Overall, these results illuminate how compound genetic interactions can synergistically result in cardiomyopathies and, moreover, corroborate that modifying disease or environmental factors may notably alter genetic risk to cardiomyopathies as was recently suggested for coronary artery disease30.
Methods
Patient sequencing and clinical data collection.
Informed written consent was obtained from all family members who were genetically tested. Permission to release protected health information from clinical records was obtained from all patients or their legal representatives. Disease status assignment and phenotyping was determined by patient self-reporting and clinical diagnoses made by echocardiography or coroner reports. Saliva samples were collected and genomic DNA (gDNA) was purified using the QIAamp DNA Blood Mini Kit (Qiagen, 51104). PCR amplification (Supplementary Table 8) of a targeted region surrounding the TPM1 c.97G>A and VCL c.659dupA alleles was performed using LongAmp Taq DNA Polymerase (NEB, M0323S) per the manufacturer’s instructions. Amplicons were gel extracted (Qiagen, 28706) and Sanger sequenced. The ages reported in the pedigree were the ages of individuals at the time of genetic sequencing in this study.
Human cardiac histology.
Patient and control left-ventricular free wall samples were fixed with 4 or 37% formaldehyde and paraffin embedded for sectioning. Sections were stained with haematoxylin and eosin or trichrome stains by the University of California San Diego (UCSD) Histology and Immunohistochemistry Core facility and were imaged using a Hamamatsu Nanozoomer 2.0HT Slide Scanner (Hamamatsu Photonics).
Statistical genetic analyses.
A kinship matrix (directly related genetic relationship matrix) was derived from the pedigree using the kinship2 R package31. Mixed linear model association analysis was then performed by incorporating the kinship matrix using functions from the genetic analysis and the generalized linear model R packages32. The effect size of the association between genotype and phenotype is reported as beta ± s.e. The associated P values were corrected for the number of genes analysed. Fisher’s exact test P values and odds ratios with 95% CIs were calculated using the phenotype (clinical diagnosis of cardiomyopathy or not) from all genotyped family members.
Linkage analysis was performed using gDNA isolated from selected individuals from whom sufficient, high-quality gDNA was available. Illumina HumanCoreExome-12 v1–1 BeadChip arrays were used to determine DNA sequence at >500k single nucleotide variants in these samples as per the manufacturer’s instructions. The MERLIN v1.1.2 software package was used to calculate non-parametric ExLOD scores that were plotted in R as previously described33,34. If any variants in the cohort subset were shared by all obligate carriers and affected subjects but no unaffected individuals (Theta = 0), a significant non-parametric logarithm of the odds (LOD score) of 3.3 would be calculated. Power analysis was calculated using the Genetic Power Calculator module for case-control for discrete traits35 with the following settings: high risk allele frequency, 0.3636; prevalence, 0.3636; genotype relative risk, 150; D-prime, 0.9; marker allele frequency, 0.32258; cases, 3; control:case ratio, 2.667 and type one error rate, 0.05, yielding a type two error rate (power) of 0.8. This analysis correlates to an 80% power to detect linkage disequilibrium with significance of P < 0.05. B allele frequency (BAF) and log R ratio (LRR) statistics were also calculated using MERLIN v1.1.2 analysis of single nucleotide variant arrays and were plotted using custom R scripts to display diploid genome heterozygosity across the autosomes of analysed family members.
Fibroblast cell culture and qRT–PCR.
Human fibroblasts were obtained from a punch biopsy and cultured in Dulbecco’s Modified Eagle’s medium supplemented with 10% fetal bovine serum as previously described36. Cycloheximide was administered for 3 h at 100 μg ml−1 as previously described36,37. RNA was isolated with TRIzol Reagent (Life Technologies, 15596–018). Complementary DNA was generated using iScript Reverse Transcription Supermix for RT-qPCR (Bio-Rad, 170–8840). Quantitative PCR with reverse transcription (qRT–PCR) was performed using iTaq Universal SYBR Green Supermix (Bio-Rad, 172–5121) and target genes were normalized against the average of three reference genes: ACTB, GAPDH and PPIG (Supplementary Table 8). Relative mRNA levels were calculated using the delta-delta CT quantification method with the Bio-Rad CFX 3.1 software. The fibroblast cell lines were morphologically confirmed as fibroblasts and confirmed by sequence analysis to be both human and reference sequenced at VCL and TPM1 variant loci. Fibroblast lines were regularly tested for mycoplasma contamination using mycoplasma specific PCR primers (Supplementary Table 8).
Protein conservation and molecular modelling.
Conservation alignment was performed using Clustal Omega (http://www.ebi.ac.uk/Tools/msa/clustalo/). Molecular structure representations of the surface charge of TPM1 were generated with PyMOL v1.8 (www.pymol.com) using the structure of TPM1 published by Whitby and colleagues (Protein Data Bank/PDB ID number for TPM1: 1C1G)13.
CRISPR–Cas9 genomic editing of hESCs, hiPSC generation and hESC/hiPSC pluripotency characterization.
Human embryonic stem cells (WiCell, WA09) and hiPSCs were maintained in a feeder-free system on Matrigel-coated tissue culture plates (BD Biosciences) in mTeSR medium (Stem Cell Technologies). To analyse human variants in an isogenic background, hESCs were edited using the CRISPR–Cas9 system as previously described38,39. Targeting gRNA sequences (Supplementary Table 8) were selected to minimize potential off-target binding15 and inserted into an empty gRNA cloning vector (Addgene, plasmid no. 41824). The gRNA plasmid (50 μg ml−1) and human-codon-optimized Cas9 nuclease (hCas9) plasmid (25 μg ml−1; Addgene, plasmid no. 41815) were introduced into hESCs by electroporation via an Amaxa Nucleofector (program B16) using a human Stem Cell Nucleofector Kit 2 (Lonza). To introduce the patient-specific TEK mutation into the cells, a single-stranded DNA oligonucleotide (1 nmol) containing the TEK substitution (Supplementary Table 8) was also included during the nucleofection reaction. Single cells were sorted into 96-well plates using a FACSAria II (BD Biosciences), expanded and genotyped by Sanger sequencing of genomic DNA and PCR clones. Computationally predicted putative off-target CRISPR-target gRNA sites were PCR amplified from gDNA of hESC lines and Sanger sequenced to verify homology with unedited lines as previously described15. VCLVFS1/+ hESCs were tested for evidence of VCL mRNA nonsense-mediated decay using cycloheximide treatment and qRT–PCR protocols as described above for human fibroblasts.
Sendai virus reprogramming of human fibroblasts was used to generate hiPSC lines as previously described as part of the iPSCORE project14. The hiPSC and CRISPR-edited hESC lines were fixed with 3.7% formaldehyde, nuclear stained with Hoechst 33352 (1:2,000; ThermoFisher Scientific) and analysed by immunofluorescence for pluripotency markers including POU5F1 (OCT4; 1:100; Abcam, ab27985), NANOG (1:20; Abgent, AP1486c) and SOX2 (1:100; Abcam, ab171380). The following conjugated secondary antibodies were used (all 1:1,000, ThermoFisher Scientific): anti-mouse IgG Alexa Fluor 488 (A21202), anti-rabbit IgG Alexa Fluor 568 (A10042) and anti-goat IgG Alexa Fluor 647 (A21447). Cardiomyocytes (hiPSC and hESC) were analysed for maturity levels by qRT–PCR analysis of MYH7 and MYH6 as previously described40. The primer sequences are presented in Supplementary Table 8. The hESC and hiPSC lines were regularly tested for mycoplasma contamination using mycoplasma specific PCR primers (Supplementary Table 8).
Differentiation of hPSCs into cardiomyocytes.
Directed differentiation of hPSCs to cardiomyocytes was carried out using established protocols16, followed by metabolic purification for cardiomyocyte enrichment17. At differentiation day 50, cells were dissociated with TrypLE Express (Gibco) and fixed with 1% formaldehyde followed by 90% cold methanol. Cells were washed with PBS containing 2.5% goat serum and 1% Triton-X100 (Flow Buffer) and incubated in primary antibodies against α-muscle actin (1:100; Abcam, ab32575) and cardiac troponin T (1:100; ThermoFisher Scientific, MS-295-P). Cells were washed, incubated with Alexa Fluor 488 and Alexa Fluor 633 (ThermoFisher Scientific, A21070) antibodies and washed again in Flow Buffer. Cell protein expression was analysed using an LSRFortessa flow cytometer (BD Biosciences). Directed cardiac differentiation efficiency was calculated as the percentage of cells expressing both smooth muscle actin and cardiac troponin T, as previously described16.
Traction force microscopy.
The contractility of hPSC cardiomyocytes was assessed using traction force microscopy according to established protocols41. Compliant polyacrylamide culture substrates embedded with fluorescent microbeads were polymerized onto 35-mm-glass-bottom culture dishes (Matek). In brief, the glass surface was activated with ultraviolet/ozone and functionalized with 20 mM 3-(trimethoxysilyl)-propylmethacrylate (Sigma-Aldrich) to enable covalent attachment of the polyacrylamide hydrogel. Polymerization was carried out with a solution of 10% acrylamide, 0.1% N,N′-methylenebisacrylamide, 1% ammonium persulfate, 0.1% N,N,N′,N′-tetramethylethylenediamine and 2% 1.0 μm microspheres (580/605 nm; Invitrogen, F13083) to result in a compliant hydrogel with an elastic modulus of 12 kPa42. Polyacrylamide substrates were then functionalized with N-sulphosuccinimidyl-6-(4′-azido-2′-nitrophenylamino) hexanoate to enable covalent attachment of collagen type I (100 μg ml−1; BD Biosciences).
At differentiation day 50, cardiomyocytes were dissociated with TrypLE Express and re-plated onto polyacrylamide hydrogels at a density of 25,000 cells cm−2. After a five-day recovery period, traction force microscopy was performed using a Nikon Eclipse Ti-S inverted fluorescence microscope with a BD Carv II camera and a stage-top incubator system (Pathology Devices). With a ×60 objective, videos of the fluorescent bead movement were collected at 9.5 frames s−1 for a total of 120 frames. The bead movement during cardiomyocyte contraction was analysed using particle image velocimetry as previously described43 and cardiomyocyte contractility was reported as the mean peak strain energy averaged across all beats recorded in each video. For each cell line, between 44 and 51 cells across at least five different cardiomyocyte differentiations were analysed.
Sarcomere organization analysis.
At differentiation day 50, hESC and hiPSC cardiomyocytes were dissociated with TrypLE Express and re-plated at a density of 80,000 cells cm−2 on gelatin-coated glass coverslips. After 5 d (differentiation day 55), cells were fixed with 3.7% formaldehyde solution, blocked and permeabilized in PBS containing 5% goat serum and 0.1% Triton-X100, and incubated in primary antibody against α-actinin (1:800; Sigma-Aldrich, A7811). The cells were then washed and incubated with Alexa Fluor 488 (1:1,000) and Hoechst 33352 (1:2,000). For each cell line, 19–26 randomly selected cardiomyocytes were imaged with a ×60 objective. Using a custom ImageJ routine, images were background subtracted, binarized and ten random regions in the cell were assigned for analysis. Fast Fourier transform (FFT) analysis was employed to analyse sarcomere organization as previously described22. Briefly, a fast Fourier transform was performed on intensity values from each region and the resulting power spectra were normalized to a value of one. The height of the first non-zero frequency term was used to estimate the sarcomere organization index. All sarcomere organization indices were normalized to the highest recorded value, such that the reported organization index is a value that falls between 0 and 1.
RNA-seq data analysis.
At differentiation day 55, hiPSC cardiomyocytes (n = 3 biological replicates of independent differentiations from both familial control and TV-Dhet iPSCs) were lysed in TRIzol and RNA was extracted according to the manufacturer’s instructions. RNA libraries were prepared using the Illumina TruSeq RNA Library Prep Kit and sequenced using the Illumina HiSeq 4000 system to generate 50 bp single-end reads. FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc) and dupRadar tools were used to assess read quality44. Reads were aligned using Kallisto45 to transcripts from GENCODE v.25 excluding transcripts from the Y chromosome46. Gene counts were generated from transcript counts using the tximport R package47. Genes with low expression counts (<100) were excluded and differential expression levels among the remaining genes were calculated using the DESeq2 R package48 with the following significance criteria: expression fold change <0.8 or >1.2 and P < 0.01, calculated using a Wald test with a Benjamini–Hochberg procedure false discovery rate adjustment22. Clustergram analysis was performed with differentially expressed genes using the pheatmap R package. Gene ontology term analysis was performed on differentially expressed genes using metascape (http://metascape.org)49. Proteins encoded by genes comprising selected gene ontology terms were investigated using STRING network (v10.5) analysis with TPM1, VCL and limited (n = 5) highconfidence interactors to create a predicted protein–protein interactome network23. TITIN N2BA and VCL exon splice isoform inclusion ratios were calculated from RNA-seq transcripts that were mapped using STAR v2.5.2b50 to the hg19 reference genome. Alternative splicing events for mRNA expression in familial control (CTRL) and TV-Dhet hiPSC cardiomyocytes were quantified using rMATS v3.2.5 and the default parameters51 with a false discovery rate of less than 0.05 considered significant.
Mouse CRISPR-mediated genomic editing, genotyping and colony maintenance.
CRISPR-targeting constructs were injected into C57BL/6NHsd single cell zygotes (Harlan Sprague Dawley Inc.) by the UCSD Transgenic Mouse Core Facility as previously described52,53. The injection mix contained 20 ng μl−1 guide RNA (gRNA), 50 ng μl−1 Cas9 mRNA and 100 ng μl−1 single-stranded DNA repair oligonucleotide. Sequences targeting gRNA (Supplementary Table 8) were selected to minimize potential off-target nuclease activity15. Tail clips were taken to isolate gDNA for genotyping. The top ten predicted off-target sites in F0 founder mouse genomes were analysed for evidence of non-specific nuclease activity by targeted amplification and Sanger sequencing15. Mice were outcrossed to WT C57BL/6 mice for at least six generations and were genotyped by either Sanger sequencing of the target loci or by allele-specific PCR. All analysed mice were male and between 15 and 59 weeks of age.
Mouse embryonic lethality studies.
Heterozygous Tpm1TEK/+ or VclVFS/+ mice were incrossed and the resultant litters were genotyped. Because no VclVFS/VFS mice were identified at birth across multiple litters, timed VclVFS/+ sibling matings were performed and pregnant mice were dissected between embyonic day 9.5 and 13.5 (E9.5 and E13.5) as previously described24. The resultant embryos were assigned a unique identifier, photographed, collected and genotyped.
Echocardiography and transverse aortic constriction.
Mouse echocardiography was performed as previously described54. Briefly, hair removal cream was used to expose the anterior chest wall and mice were anaesthetized with 5% isoflurane induction and 0.5% isoflurane maintenance. Transthoracic echocardiography (M-mode and two-dimensional) was performed using a Vevo 2100 (VisualSonics Inc.) high-frequency ultrasound instrument with simultaneous electrocardiogram acquisition via small-needle electrodes. The heart rate was maintained above 500 beats per minute and body temperature was maintained using a warming station. Researchers blinded to mouse genotypes measured the heart rate, left ventricular internal dimension during diastole and systole (LVIDd/s), end-diastolic interventricular septal thickness in diastole and left ventricle posterior wall thickness in diastole from echocardiographic recordings. Left ventricular fractional shortening percentage, a measure of cardiac contractility, was calculated as the average of three (LVIDd−LVIDs)/LVIDd measurements per animal; n = 6–14 mice per group in ageing studies.
TAC was performed as previously described25,54. Briefly, 12- to 16-week-old male mice were anaesthetized with an intraperitoneal injection of ketamine (100 mg kg−1) and xylazine (10 mg kg−1), intubated and connected to a mouse ventilator (Harvard). Following a small incision in the second intercostal space at the left upper sternal border, a double blunted 27-gauge (for mice over 25 g) or 27.5-gauge needle (for mice below 25 g) was ligated to the aorta between the innominate and left common carotid arteries using a 7–0 silk suture. The needle was then removed and the chest wall and skin were sutured closed. Sham surgery mice underwent all components of the TAC surgery, including anaesthesia, intubation, chest wall incision and incision closure, except for aortic suture ligation. Repeat transthoracic echocardiography was performed one week after surgery.
At study termination, systolic pressure gradients were measured by selective cannulation of the left and right carotid arteries; the pressure wave form was recorded (average pressure gradients greater than 70 mm Hg per genotype) and heart tissue was collected for histological and protein analyses; n = 14–18 mice per genotype in the TAC studies, n = 5 or 6 mice per genotype in the sham control surgeries.
Morphometric analyses and histology.
Animals were anaesthetized with ketamine/xylazine and euthanized at 58 weeks of age or one week following TAC or sham surgery in accordance with IACUC protocols. Mice and collected hearts were weighed to calculate heart-weight-to-body-weight ratios (mg mg−1—arbitrary units). In the ageing studies, 5–10 animals were analysed per genotype; 8–12 animals per genotype were analysed in TAC surgery conditions and five or six animals per genotype were analysed in sham surgery conditions. Hearts were fixed with 4% paraformaldehyde, dehydrated in 70% ethanol and embedded in paraffin. Paraffin-embedded cardiac sections (8-μm thick) were trichrome stained at the UCSD Histology and Immunohistochemistry Core facility and were imaged using a Hamamatsu Nanozoomer 2.0HT Slide Scanner. Trichrome-positive regions of mouse heart sections were quantified as a percentage of total cardiac cross-sectional area using ImageJ software (n = 3 or 4 in 58 week mice, n = 4 in post-sham mice, n = 7–10 in post-TAC mice). Mouse cardiac-papillary-muscle sections, fixed in 4% paraformaldehyde and embedded in optimal cutting temperature compound, were cryosectioned at a thickness of 7 μm, washed in PBS and stained with Alexa Fluor 488 conjugated wheat germ agglutinin (ThermoFisher, W11261) at 5 μg ml−1 and DAPI at 0.1 μg ml−1. Cell outlines were measured using Image Pro Plus 6.0 software (Media Cybernetics) and averaged to determine the average cardiomyocyte cross-sectional area (μm2; n = 3 mice and 100 cardiomyocytes per genotype, for each condition).
Protein preparation and western blot analysis.
Fibroblast and hESC/hiPSC pellets were harvested and lysed in protein lysis buffer (50 mM Tris–HCl pH 7.4, 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, 1% NP-40 and protease inhibitor (Amresco)). Total protein was isolated from mouse hearts in heart lysis buffer (10 mM Tris–HCl, pH 8, 100 mM NaCl, 1% NP-40, 2 mM sodium orthovanadate and protease inhibitor (Roche Applied Science)). Protein lysates were quantitated using a Qubit Protein Assay (Life Technologies, Q33211). Lysates underwent gel electrophoresis on a 10–20% Tris glycine SDS–PAGE gel (Novex; EC6135), which was then transferred to polyvinylidene difluoride membrane (Immobilon; IPVH0010) for immunoblotting. Blots were blocked in 5% milk in Tris-buffered saline with 1% Tween (TBST), incubated with primary antibody in 5% milk in TBST, washed in TBST and imaged using Clarity Western ECL substrate (Bio-Rad, 170–5060). The antibodies used include: anti-vinculin (Sigma, V9131), anti-N-terminal vinculin (Santa Cruz Biotechnology, sc-5573), anti-tropomyosin 1 (Abcam, EPR5159), anti-tubulin (Sigma, T5168) and anti-GAPDH (GeneTex, GTX100118 or Santa Cruz Biotechnology; sc-32233). Densitometric protein quantitation was performed using Bio-Rad ImageLab 5.0 or ImageJ software. At least three replicates were performed per quantitation and average density was compared by Student’s t-test or ANOVA as appropriate.
Statistical analysis.
All data compared in statistical analyses were collected from experiments conducted at least three times with reproducible results. All statistical analyses were performed on measurements of at least three independent biologic replicates. Protein quantitation was performed on a minimum of three technical and three biological replicates per sample. Data were expressed as mean ± s.e.m., except where otherwise noted. Comparison of data sets was performed using Student’s t-tests with two-sided analysis or ordinary one-way ANOVA analysis with Tukey multiple correction tests, as appropriate for data type. Differences between mean values were considered to be significant when P < 0.05. Gene-expression value comparisons were performed using the Wald test with Benjamini–Hochberg procedure false discovery rate adjustment. Previous experimental results in comparable systems were used to determine sample sizes for cell culture and mouse experiments. The analysis of traction force microscopy and echocardiography measurements was performed by researchers blinded to sample genotype. No data were excluded, except as described in the RNA-seq analysis methods.
Study approval.
ll human study protocols were approved by the UCSD Institutional Review Board and Human Research Protections Program and written informed consent was obtained from all study participants (Protocol no. 111523). Collection of skin fibroblasts was performed with IRB approval (Protocol nos 110776, 111475 and 090243). The use of human-derived pluripotent cells in this study was approved by the UCSD Institutional Review Board and Embryonic Stem Cell Research Oversight Committee (Protocol nos 141315 and 111475). All animal procedures were performed in accordance with the National Institute of Health guidelines and were approved by the UCSD Institutional Animal Care and Use Committee (Protocol nos S13138 and S00138). UCSD has an Animal Welfare Assurance (no. A3033–01) on file with the Office of Laboratory Animal Welfare and is fully accredited by AAALAC International. All studies reported in this manuscript were performed in compliance with all relevant ethical regulations.
Reporting Summary.
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary Material
Acknowledgements
We thank the patients who participated in this study. K. DeMali (Univ. Iowa) provided the truncated Gallus gallus VCL peptide. Various experiments were conducted with the assistance, expertise and support of the following UCSD core facilities: Institute for Genomic Medicine Core, Mouse Transgenic Core, Histology and Immunohistochemistry Core, Seaweed Canyon Cardiovascular Physiology Laboratory, Microscopy Core and Human Embryonic Stem Cell Core facilities. We also thank P. Mali and H. Taylor-Weiner for assistance with hPSC culture, members of the Bruce Hamilton laboratory for helpful discussions and experimental design and members of the Chi lab for comments on the manuscript. This work was supported in part by grants from the NIH to N.C.C., J.C., R.S.R., E.D.A. and grant no. R01AG045428 to A.J.E. D.C.D. was supported by a CIRM pre-doctoral fellowship (grant no. TG201154) and an NIH pre-doctoral training grant (grant no. T32 GM008666). C.L.H. was supported by post-doctoral fellowships from the American Heart Association (grant no. 15POST25720070) and NIH (grant no. F32HL131424). J.C. is an American Heart Association Endowed Chair. E.N.F. was supported by a NIH pre-doctoral training grant (grant no. 4T32HL007444–34).
Footnotes
Competing interests
The authors declare no competing interests.
Code availability
All custom code used in this study can be found at http://github.com/englea52/Englerlab or https://github.com/enfarah/digital_karyotype.
Data availability
The authors declare that all data supporting the findings of this study are available within the paper and its Supplementary Information. The materials and data of this study are available from the corresponding author on reasonable request, with the exception of patient DNA and tissue, which is limited and protected by local and federal privacy regulations. Microarray study and RNA-seq data are available via dbGaP with GEO accession numbers GSE121844 and GSE121559.
Additional information
Supplementary information is available for this paper at https://doi.org/10.1038/s41551-019-0348-9.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hershberger RE, Hedges DJ & Morales A Dilated cardiomyopathy: the complexity of a diverse genetic architecture. Nat. Rev. Cardiol 10, 531–547 (2013). [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ et al. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 135, e146–e603 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stenson PD et al. The Human Gene Mutation Database: towards a comprehensive repository of inherited mutation data for medical research, genetic diagnosis and next-generation sequencing studies. Hum. Genet 136, 665–677 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villard E et al. A genome-wide association study identifies two loci associated with heart failure due to dilated cardiomyopathy. Eur. Heart J 32, 1065–1076 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meder B et al. A genome-wide association study identifies 6p21 as novel risk locus for dilated cardiomyopathy. Eur. Heart J 35, 1069–1077 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Li L, Bainbridge MN, Tan Y, Willerson JT & Marian AJ A potential oligogenic etiology of hypertrophic cardiomyopathy: a classic single gene disorder. Circ. Res 126, 1084–1090 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roncarati R et al. Doubly heterozygous LMNA and TTN mutations revealed by exome sequencing in a severe form of dilated cardiomyopathy. Eur. J. Hum. Genet 21, 1105–1111 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maron BJ, Maron MS & Semsarian C Double or compound sarcomere mutations in hypertrophic cardiomyopathy: a potential link to sudden death in the absence of conventional risk factors. Heart Rhythm 9, 57–63 (2012). [DOI] [PubMed] [Google Scholar]
- 9.Petropoulou E et al. Digenic inheritance of mutations in the cardiac troponin (TNNT2) and cardiac beta myosin heavy chain (MYH7) as the cause of severe dilated cardiomyopathy. Eur. J. Med. Genet 60, 485–488 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Haas J et al. Atlas of the clinical genetics of human dilated cardiomyopathy. Eur. Heart J 36, 1123–1135 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Kimura A Molecular genetics and pathogenesis of cardiomyopathy. J. Hum. Genet 61, 41–50 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Olson TM, Kishimoto NY, Whitby FG & Michels VV Mutations that alter the surface charge of alpha-tropomyosin are associated with dilated cardiomyopathy. J. Mol. Cell. Cardiol 33, 723–732 (2001). [DOI] [PubMed] [Google Scholar]
- 13.Whitby FG & Phillips GN Crystal structure of tropomyosin at 7 Angstroms resolution. Proteins 38, 49–59 (2000). [PubMed] [Google Scholar]
- 14.Panopoulos AD et al. iPSCORE: a resource of 222 iPSC lines enabling functional characterization of genetic variation across a variety of cell types. Stem Cell Rep. 8, 1086–1100 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu PD et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat. Biotechnol 31, 827–832 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lian X et al. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat. Protoc 8, 162–175 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tohyama S et al. Distinct metabolic flow enables large-scale purification of mouse and human pluripotent stem cell-derived cardiomyocytes. Cell Stem Cell 12, 127–137 (2012). [DOI] [PubMed] [Google Scholar]
- 18.Dhalla AK, Hill MF & Singal PK Role of oxidative stress in transition of hypertrophy to heart failure. J. Am. Coll. Cardiol 28, 506–514 (1996). [DOI] [PubMed] [Google Scholar]
- 19.Rosenkranz S et al. Alterations of β-adrenergic signaling and cardiac hypertrophy in transgenic mice overexpressing TGF-β1. Am. J. Physiol. Heart Circ. Physiol 283, H1253–H1262 (2002). [DOI] [PubMed] [Google Scholar]
- 20.Razeghi P et al. Metabolic gene expression in fetal and failing human heart. Circulation 104, 2923–2931 (2001). [DOI] [PubMed] [Google Scholar]
- 21.Bristow MR et al. β1- and β2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective β1-receptor downregulation in heart failure. Circ. Res 59, 297–309 (1986). [DOI] [PubMed] [Google Scholar]
- 22.Hinson JT et al. HEART DISEASE. Titin mutations in iPS cells define sarcomere insufficiency as a cause of dilated cardiomyopathy. Science 349, 982–986 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szklarczyk D et al. STRINGv10: protein–protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 43, D447–D452 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu W, Baribault H & Adamson ED Vinculin knockout results in heart and brain defects during embryonic development. Development 125, 327–337 (1998). [DOI] [PubMed] [Google Scholar]
- 25.Rockman HA et al. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc. Natl Acad. Sci. USA 88, 8277–8281 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Golbus JR et al. Population-based variation in cardiomyopathy genes. Circ. Cardiovasc. Genet 5, 391–399 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McNally EM & Puckelwartz MJ Genetic variation in cardiomyopathy and cardiovascular disorders. Circ. J 79, 1409–1415 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lek M et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature 536, 285–291 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Happe CL & Engler AJ Mechanical forces reshape differentiation cues that guide cardiomyogenesis. Circ. Res 118, 296–310 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khera AV et al. Genetic risk, adherence to a healthy lifestyle, and coronary disease. N. Engl. J. Med 375, 2349–2358 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Therneau TM et al. kinship2: pedigree functions v.1.6.4 (CRAN, 2015); https://cran.r-project.org/package=kinship2 [Google Scholar]
- 32.Marschner IC & Donoghoe MW glm2: fitting generalized linear models v.1.2.1 (CRAN, 2018); https://CRAN.R-project.org/package=glm2 [Google Scholar]
- 33.Abecasis GR, Cherny SS, Cookson WO & Cardon LR Merlin—rapid analysis of dense genetic maps using sparse gene flow trees. Nat. Genet 30, 97–101 (2002). [DOI] [PubMed] [Google Scholar]
- 34.McGregor TL et al. Consanguinity mapping of congenital heart disease in a South Indian population. PLoS ONE 5, e10286 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Purcell S, Cherny SS & Sham PC Genetic Power Calculator: design of linkage and association genetic mapping studies of complex traits. Bioinformatics 19, 149–150 (2003). [DOI] [PubMed] [Google Scholar]
- 36.Hashem SI et al. Oxidative stress mediates cardiomyocyte apoptosis in a human model of Danon disease and heart failure. Stem Cells 33, 2343–2350 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carter MS et al. A regulatory mechanism that detects premature nonsense codons in T-cell receptor transcripts in vivo is reversed by protein synthesis inhibitors in vitro. J. Biol. Chem 270, 28995–29003 (1995). [DOI] [PubMed] [Google Scholar]
- 38.Byrne SM, Mali P & Church GM in Methods in Enzymolology, Vol. 546 (eds Doudna JA & Sontheimer EJ) 119–138 (Elsevier, Amsterdam, 2014). [Google Scholar]
- 39.Yang L, Mali P, Kim-Kiselak C & Church G CRISPR–Cas-mediated targeted genome editing in human cells. Methods Mol. Biol 1114, 245–267 (2014). [DOI] [PubMed] [Google Scholar]
- 40.Giacomelli E et al. Three-dimensional cardiac microtissues composed of cardiomyocytes and endothelial cells co-differentiated from human pluripotent stem cells. Development 144, 1008–1017 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu H et al. Epigenetic regulation of phosphodiesterases 2A and 3A underlies compromised β-adrenergic signaling in an iPSC model of dilated cardiomyopathy. Cell Stem Cell 17, 89–100 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tse JR & Engler AJ Preparation of hydrogel substrates with tunable mechanical properties. Curr. Protoc. Cell BioI 47, 10.16.1–10.16.16 (2010). [DOI] [PubMed] [Google Scholar]
- 43.del Alamo JC et al. Three-dimensional quantification of cellular traction forces and mechanosensing of thin substrata by Fourier traction force microscopy. PLoS ONE 8, e69850 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sayols S, Scherzinger D & Klein H dupRadar: a Bioconductor package for the assessment of PCR artifacts in RNA-Seq data. BMC Bioinform. 17, 428 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bray NL, Pimentel H, Melsted P & Pachter L Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol 34, 525–527 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Harrow J et al. GENCODE: the reference human genome annotation for the ENCODE Project. Genome Res. 22, 1760–1774 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soneson C, Love MI & Robinson MD Differential analyses for RNA-seq: transcript-level estimates improve gene-level inferences. F1000Research 4, 1521 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Love MI, Huber W & Anders S Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tripathi S et al. Meta- and orthogonal integration of influenza “OMICs” data defines a role for UBR4 in virus budding. Cell Host Microbe 18, 723–735 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dobin A et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shen S et al. rMATS: robust and flexible detection of differential alternative splicing from replicate RNA-Seq data. Proc. Natl Acad. Sci. USA 111, E5593–E5601 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang H, Wang H & Jaenisch R Generating genetically modified mice using CRISPR/Cas-mediated genome engineering. Nat. Protoc 9, 1956–1968 (2014). [DOI] [PubMed] [Google Scholar]
- 53.Wang H et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 153, 910–918 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li R et al. β1 integrin gene excision in the adult murine cardiac myocyte causes defective mechanical and signaling responses. Am. J. Pathol 180, 952–962 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.