Abstract
It has been postulated that glia play a critical role in modifying neuronal activity, mediating neurovascular coupling, and in seizure initiation. We investigated the role of glia in ictogenesis and neurovascular coupling through wide-field multicell and 2-photon single cell imaging of calcium and intrinsic signal imaging of cerebral blood volume in an in vivo rat model of focal neocortical seizures. Ictal events triggered a slowly propagating glial calcium wave that was markedly delayed after both neuronal and hemodynamic onset. Glial calcium waves exhibited a stereotypical spread that terminated prior to seizure offset and propagated to an area ~60% greater than the propagation area of neural and vascular signals. Complete blockage of glial activity with fluoroacetate resulted in no change in either neuronal or hemodynamic activity. These ictal glial waves were blocked by carbenoxolone, a gap junction blocker. Our in vivo data reveal that ictal events trigger a slowly propagating, stereotypical glial calcium wave, mediated by gap junctions, that is spatially and temporally independent of neuronal and hemodynamic activities. We introduce a novel ictally triggered propagating glial calcium wave calling into question the criticality of glial calcium wave in both ictal onset and neurovascular coupling.
Keywords: epilepsy, glia, ictogenesis, neurovascular coupling, optical imaging
Introduction
Epilepsy is a disease marked by hyperexcitability and hypersynchrony in a population of neurons that cause a massive increase in cerebral blood flow (CBF) to meet the resulting supranormal metabolic demand. The cellular mechanisms underlying these events are not well understood. In recent years, the role of glia in both the initiation of seizures and neurovascular coupling has been investigated, partly with conflicting results. While increasing evidence suggests that the chronic process of astroglial scarring, which is typically seen in human epilepsies, may impair astrocytes’ ability to maintain electrolyte and neurotransmitter homeostasis (Devinsky et al. 2013; Robel and Sontheimer 2016), healthy glia have also been proposed to play a role in mediating excitation and synchrony to promote acute ictogenesis (Kang et al. 2005; Tian et al. 2005; Gomez-Gonzalo et al. 2010). However, the possible role of glia in seizure initiation has been called into question by other studies in which the blockage of astrocytic glutamate release did not impair ictogenesis (Fellin et al. 2006). Part of the reason for the discrepancy of results may be that many of these studies have been performed in disconnected in vitro brain slices involving various experimental approaches such as bath application of chemoconvulsants or perfusion solutions devoid of magnesium. Despite the importance of this issue, the fine scale interactions between glial and neuronal networks during epilepsy have remained poorly understood, especially in the intact brain.
Neurovascular coupling or “functional hyperemia” is the process whereby the brain autoregulates CBF to meet metabolic demand. This process is extremely important in epilepsy where a massive increase in neuronal activity during seizures, has been shown to drive a parallel increase in the supply of CBF and oxygen (Schwartz and Bonhoeffer 2001; Suh et al. 2005; Zhao et al. 2007, 2009; Ma et al. 2009a, 2009b, 2013). In recent years, several investigators have provided evidence that glia may play a critical role in the coupling of hemodynamic and neuronal activities (Nedergaard et al. 2003; Iadecola 2004; Girouard and Iadecola 2006; Metea and Newman 2006; McCaslin et al. 2011; Hillman 2014). However, more recent in vitro and in vivo data has cast doubt on the role of astrocytes in neurovascular coupling (Wang et al. 2006; Petzold et al. 2008; Schummers et al. 2008; Nizar et al. 2013; Takata et al. 2013; Bonder and McCarthy 2014; Jego et al. 2014).
In this paper, we report the use of in vivo wide-field calcium imaging of glial activity in conjunction with calcium imaging of neuronal activity and intrinsic signal imaging of the hemodynamic response during focal seizures. Focal ictal activity is elicited with injection of 4-Aminopyridine (4-AP), a K+-channel blocker that induces ictal-like events in rodent neocortex whose electrographic patterns mimic the low voltage fast (LVF) activity seizure pattern found in human epilepsy (Pongracz and Szente 1979; Avoli et al. 2016). An advantage of the 4-AP model is that inhibitory mechanisms remain intact unlike other models such as tetanus toxin, picrotoxin, penicillin, or bicuculline. Recent studies have demonstrated the importance of inhibition in the initiation and synchronization of ictal events (de Curtis and Avoli 2016). Moreover, interictal events also occur between ictal events, thus providing a model to study both interictal and ictal activities.
Our data show that a stereotypical glial calcium wave occurs after, and not prior to, both ictal onset and the hemodynamic response. Moreover, the resulting glial wave is uncoupled in its evolution from the seizure, and its blockade has no impact on the ictal neurovascular event. Glial calcium waves were not noted after interictal events. These results raise substantial doubts about the criticality of glial calcium waves in both ictogenesis and ictal neurovascular coupling.
Materials and Methods
Animal Preparation
All experimental procedures were approved by the Weill Cornell Medical College Animal Care and Use Committee following the National Institutes of Health guidelines. Adult male Sprague–Dawley rats (200–350 g) were anesthetized with isoflurane in 70% N2:30% O2, 4% induction, and 1.5–2% maintenance. Body temperature was maintained at 37 °C with a regulated heating blanket (Harvard Apparatus). The heart rate, pO2, and the end tidal carbon dioxide (ETCO2) were carefully monitored with a small animal capnography (Surgivet) and were sustained throughout the experiment (heart rate: 250–300 pulse/min, pO2 >90%, ETCO2 ca. 25–28 mmHg). The head was fixed in a stereotaxic frame.
Dye Staining
Two different calcium dyes were employed. The calcium indicator Oregon Green 488 BAPTA-1 AM (OGB-1, Life Technologies) was employed for recording of neuronal activity. Convection enhanced delivery (CED) was employed to bulk load the entire neocortex with OGB-1 (Ma et al. 2014a, 2014b). In brief, 50 µg of OGB-1 was diluted in 5 µl of DMSO-F127 then in 50 µl of artificial cerebrospinal fluid (ACSF). In total, 8 µl of OGB-1 solution was injected in the neocortex via a glass electrode (50–100 µm opening) placed ~1 mm below the brain surface at the speed of 100 nL/min, using a micro-pump (WPI). A ~5 × 8 mm craniotomy window was then opened over the stained hemisphere between bregma and lambda, from 1 to 6 mm to the central line. The exposed brain was covered with silicon oil (12 500 centistoke) to preserve cortical moisture. In a separate group, Calcium indicator Rhod-2 AM was employed to record the superficial astrocytes activity only (Wang et al. 2006; Ghosh et al. 2013). In total, 50 µg of Rhod-2 was diluted in 5 µl of DMSO-F127, then in 50 µl of ACSF. First a ~5 × 8 mm craniotomy window was drilled open. A temporal well was created surrounding the craniotomy window with silicon glue to hold the dye solution. The dura was removed and the Rhod-2 was topically applied for 90 min. After staining, the cortex was washed with ACSF to remove the unbound dye.
Epilepsy Model and Electrophysiology
To induce epileptiform events, 4-AP (Sigma-Aldrich, 15 mM, 0.5 μL) was injected 300–500 μm below the somatosensory cortical surface through a glass microelectrode using a Nanoject II injector (Drummond Scientific). The local field potential (LFP) was also recorded through the 4-AP electrode. The LFP was amplified and band-pass filtered (1–500 Hz) using a Grass amplifier, digitized using CED Power 1401 and recorded via a personal computer running Spike2 software (Cambridge Electronic Design).
Optical Recording
A “temporal separation” technique was employed to simultaneously image wide-field calcium and intrinsic optical signals (IOS) (Bouchard et al. 2009; Ma et al. 2014a, 2014b). A CCD camera (Dalsa camera in Imager 3001, Optical Imaging, Rehovot, Israel) using a tandem lens (50 × 50 mm) arrangement was focused 300–400 μm below the cortical surface. Two LEDs were employed as the illumination source for calcium-sensitive dye and IOS, respectively (for OGB-1, 470 and 530 nm; for Rhod-2, 530 and 570 nm). The LEDs were coupled with a dichroic mirror (for OGB-1: 490 nm, for Rhod-2: 560 nm) and illumination directed using fiber-optic light guides. A long-pass filter (for OGB-1: 510 nm; for Rhod-2:560 nm), was placed before the camera to prevent the calcium illumination contamination, but permitting both calcium dye fluorescence and IOS signal. The ultra-fast multispectral switching between the 2 LEDs was time-locked to camera frames. A Master-9 (A.M.P.I.) was used to couple the frame indicator from the camera and the LEDs. The wavelength we used for IOS mainly reflect total hemoglobin change, which is a surrogate for cerebral blood volume (CBV) change given a steady hematocrit. Throughout the paper, the word “hemodynamic” is used to refer to IOS changes of CBV.
Pharmacology Study
In a subset of experiments, glial activity was blocked with fluoroacetate (FA), a toxin that blocks the tricarboxylic acid cycle and is a well-established astrocyte-specific inhibitor (Fonnum et al. 1997; Martin et al. 2007; Peña-Ortega et al. 2016). After calcium dye staining, the exposed cortex received bath application FA solution (10 mM) for 90 min before 4-AP injection. To investigate the role of gap junctions in glial activity, carbenoxolone (CBX) (Sigma-Aldrich, 100 µM) was bath applied to the exposed cortex for 90 min prior to 4-AP injection. To investigate if neuronal activity is necessary for the glial activity, tetrodotoxin (TTX) (Sigma-Aldrich, ~2 µM), a sodium channel blocker, was bath applied to the exposed cortex for 10 min before 4-AP injection.
Two-Photon Imaging
To verify the wide-field calcium signals at the single cell level, activity of cortical neurons and glial cells was recorded with a 2-photon microscope (Bruker) and a Ti:Sapphire laser (Chameleon Ultra II; Coherent) using a 25× objective (water immersion, N.A. 1,05, Olympus). One hour prior to imaging, OGB-1 was dissolved in 4 μL of freshly prepared DMSO containing 20% Pluronic F-127 (Molecular Probes) and further diluted in 35 μL of dye buffer (150 mM NaCl, 2.5 mM KCl, and 10 mM HEPES [pH 7.4]). Sulforhodamine 101 (SR101), a selective astrocyte marker, was added to the solution (0.25 mg/mL) (Nimmerjahn et al. 2004). A glass capillary pulled to a micropipette was carefully lowered into left somatosensory cortex at a 30° angle to a depth of 100–150 µm below the pial surface using an ROE-200 manipulator (Sutter Instruments) under visual guidance by 2-photon imaging (×20 water immersion objective, N.A. 0.5, Olympus). Using our previously described convection enhanced loading technique (Ma et al. 2014a, 2014b), the dye was slowly pressure-injected (10 psi, 8 min) using a picospritzer III system (Parker). For imaging, OGB-1 was excited with a laser wavelength of 940 nm and SR101 with a wavelength of 1000 nm. Fluorescence emission was collected through 535 nm (green, for OGB-1) or 645 nm (red, for SR101) filters (Chroma). Resonant galvanometer scanning and image acquisition (frame rate 30 fps, 512 × 512 pixels, 100–170 µm below the pial surface) were controlled by Prairie View Imaging software.
Data Analysis
Custom written MATLAB programs were used for data processing and statistical analysis. To increase the signal-to-noise ratio of the imaging data, spatial averaging was achieved by convolution with a Gaussian kernel (σ = 3 pixels). A first order Butterworth filter was employed to separate the neuronal and astrocytic activities. Neuronal activity was extracted with 1-Hz high-pass filter, which showed similar waveforms to the simultaneously recorded LFP. Glial activity was recorded using Rhod-2. For imaging data, the signal changes were calculated as dI/I, where I was the baseline illumination when no epileptiform activity was occurring and dI was the signal change during epileptiform activity as identified by the LFP.
The seizure onset and offset times were defined similarly regardless of the origin of the signal. For LFP and calcium imaging of neuronal activity (high-frequency component), the onset was defined as the time-point when the signal power increased >3 SD above the baseline. The offset was defined when the signal power decreased <3 SD above the baseline. For the slow-wave glial and CBV signals, an amplitude increase >3 SD above baseline also served to define the seizure onset. Ictal termination was selected as the moment the signals returned within 3 SD of baseline.
To quantify the spatial spread of the seizure from each signal source, we employed a seed trace initiated correlation method (White et al. 2011). For each seizure, a seed trace was selected from a small region of interest (ROI), in which the waveform best correlated with the LFP (usually an ROI containing the 4-AP electrode). The correlation coefficients (CCs) between the seed trace and trace from every individual pixel in the surrounding image were calculated. A heat map was generated using the CC at each pixel. A threshold at 50% of the maximal CC was used to determine the spatial extent of the imaged signals.
For 2-photon image analysis, ROIs were registered in a semi-automated manner by using custom written software in MATLAB followed by manual confirmation. To minimize cell signal contamination by surround neuropil fluorescence changes, we applied ROI shrinkage (radial subtraction of 1.5 pixels from somatic ROI). This approach has been proven successful in limiting bleed-in of the surrounding neuropil fluorescence (Hofer et al. 2011). Cells with low signal-to-noise ratio or no apparent calcium transients as well as cells whose somata could not be reliably followed over the course of an experiment were excluded from further analysis.
Results
Simultaneous Calcium and Intrinsic Imaging of 4-AP Seizures
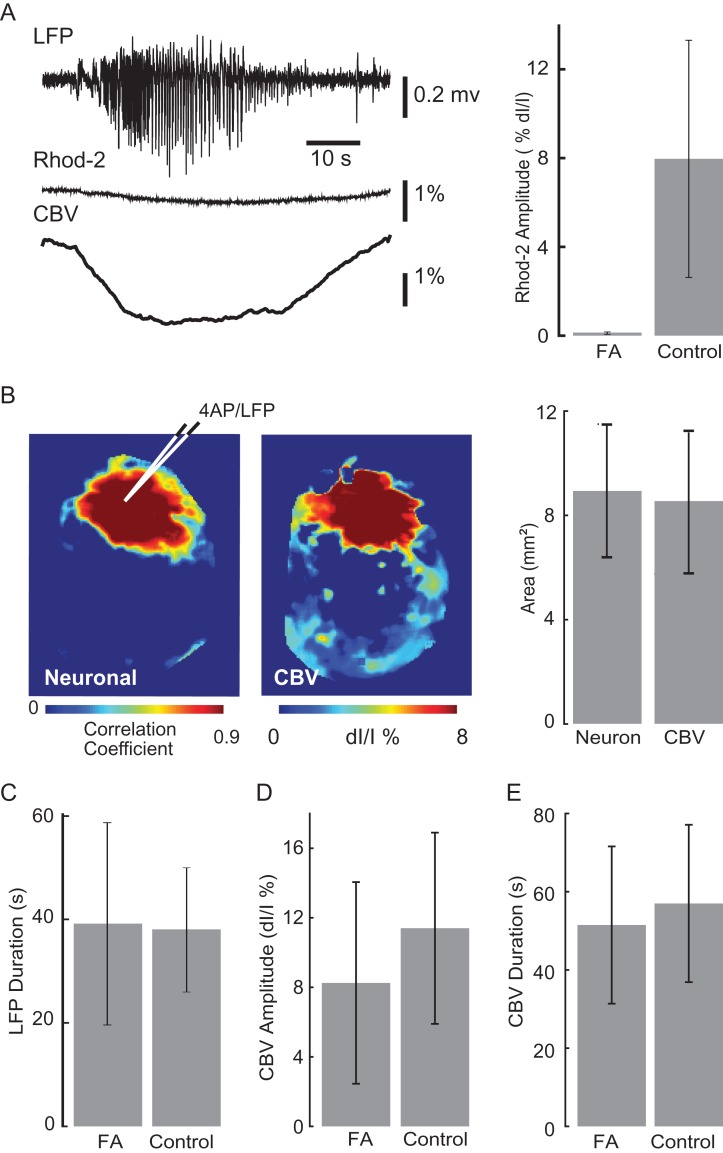
Local injection of 4-AP resulted in epileptiform events discernible via the 4-AP/LFP electrode, calcium, and IOS imaging. After a few minutes, recurrent spontaneous ictal-like events occurred with an electrographic LVF pattern. As demonstrated previously, these events all arose from the same location, namely the injection site, and as such represent a model of recurrent focal epilepsy (Zhao et al. 2009; Ma et al. 2009b, 2013). In spite of their spatially stereotypical onset, seizure duration, and power were extremely variable. Average seizure duration was 39.18 ± 19.59 s with a range from 5.03 to 98.35 s (n = 116 seizures). This variability provided a model for examining the relationship between neuronal, glial, and hemodynamic compartments through a range of ictal powers and durations. Figure 1 shows a typical example of simultaneous calcium and IOS imaging data. The OGB-1 seizure signal demonstrates fast activity riding on a wave of slower activity. The high-frequency component (>1 Hz) of the calcium signal closely reflected the LFP waveform and represents neuronal input and output activities in populations of neurons (Kerr et al. 2005; Bouchard et al. 2009; Grewe and Helmchen 2009; Schulz et al. 2012). The calcium influx in glia was recorded with astrocyte-specific Rhod-2 imaging (Ghosh et al. 2013) that showed a slow wave without higher frequency activity (Fig. 1B). Interictal spikes, on the other hand, elicited no glial calcium influx (see Supplementary Fig. 1). The IOS signal from CBV changes also demonstrated a slow waveform that is characteristic of hemodynamic signals. Using these optical techniques combined with the LFP signal, we were able to examine the spatiotemporal relationship of neuronal and glial dynamics and the concomitant hemodynamic changes.
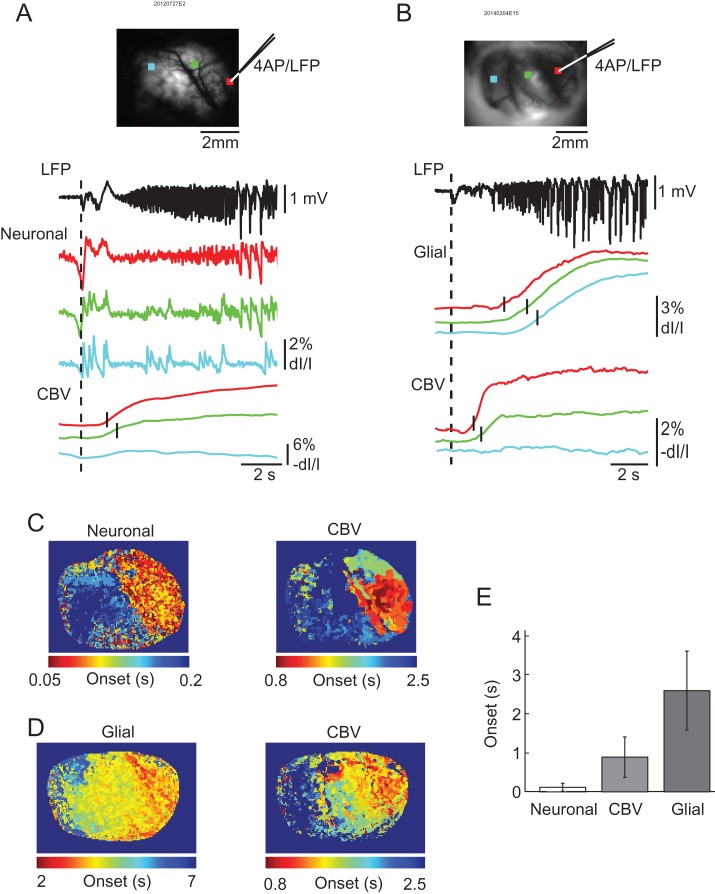
Figure 1.
Simultaneous LFP, calcium, and IOS imaging of ictal events. (A) Wide-field imaging of neuronal and hemodynamic activities during an ictal event. On the left is the field of view of the imaged cortex with a white ROI indicating the 4-AP injection and LFP recording site where the traces on the right were obtained. OGB-1 signal contains both high- and low-frequency components. IOS imaging shows the relatively slow and delayed increase of CBV during the event. (B) Wide-field imaging of glial and hemodynamic activities during an ictal event. On left is field of view of the imaged cortex with white ROI indicating 4-AP injection and LFP recording site. Rhod-2 (glial) and IOS imaging both result in slow waves that appear to be triggered by the seizure. CBV appears to last longer than both the LFP neuronal and glial components with glial activation terminating first.
Onset Times for Neuronal, Glial, and Hemodynamic Activity
To investigate the role of glial waves in ictal initiation and neurovascular coupling, we used wide-field imaging to examine the timing of the onset and propagation speed and extent of each signal (Fig. 2). Neuronal calcium activity, derived from the OGB-1 high-frequency component, showed the earliest onset time with respect to the electrographic seizure onset (top red trace). The neuronal calcium signal began on average 0.12 ± 0.10 s after LFP onset (n = 20 seizures from 7 rats). This was slightly ahead of the hemodynamic signal, which started at 0.89 ± 0.52 s (n = 44 seizures from 9 rats) after LFP onset (lower red trace). The onset of both neuronal and hemodynamic activity changes significantly preceded glial signal onset (recorded with Rhod-2) which began at 2.60 ± 1.02 s (n = 24 seizures from 5 rats). One way ANOVA test indicated that the start time for the 3 groups was significantly different; (P < 0.001, F = 89.26, degree of freedom (DF) = 87). Tukey–Kramer post hoc analysis indicated that the glial activation was significantly delayed with respect to both neuronal (P < 0.001) and hemodynamic activity (P < 0.001). The hemodynamic activity was also significantly delayed with respect to neuronal activity (P < 0.001).
Figure 2.
The onset latency in the neuronal, glial, and hemodynamic signals. (A) A comparison of the neuronal and hemodynamic onsets of an ictal event. The top figure shows the field of view of the imaged cortex with a red box indicating the 4-AP injection and LFP recording. The green and blue boxes indicate 2 other ROIs at differing distances from the 4-AP injection site. Below, traces correspond to ROI of same color and vertical dashed line indicates the ictal onset time of the LFP. The neuronal and LFP traces show similar onset times for the first spike, however, subsequent activity captured by calcium imaging of neuronal revealed spatially dependent propagation patterns. The red and green CBV traces indicate a delay in local blood volume increase due to seizure activity. The short vertical black lines represent the onset time of CBV change in that pixel. No significant blood volume changes were detected from the blue ROI. (B) A comparison between the glial and hemodynamic onsets of a different ictal event. The top figure shows the field of view with 3 ROIs located at different distances to the 4-AP and LFP electrode. Below, traces correspond to ROI of same color. The dashed line indicates the ictal onset time of the LFP. Glial activation was observed in all regions observed and were significantly delayed compared to both LFP and CBV. The delay was also spatially dependent as regions further away from the injection site were more delayed. The black lines represent the onset time of the recorded signal from each ROI. (C) Onset time mapping revealed the spatiotemporal dependence of neuronal and hemodynamic activation during ictal events. The neuronal and CBV figures represent the onset times of ictal activation for individual pixels taken from the seizure shown in (A). Note: neuronal activation occurs earlier than CBV and both signals propagate to similar area. (D) Onset time mapping of the simultaneously recorded glial and CBV change. Note: glial activation was delayed in comparison to that of CBV change but propagated to a larger area than CBV change. (E) Average ictal onset times. The glial onset time is much later than both the neuronal and hemodynamic signals with neuronal activation being very similar to LFP onset. Error bars: SD.
The map of the early seizure initiation time-period showed that the neuronal activity arose rapidly from the whole seizure focus during the initiation process. The CBV change also showed a similar but slightly slower onset time within the seizure focus (Fig. 2C). The glial activity, exhibiting the greatest temporal delay, showed a gradual propagation to a much larger area than the seizure focus. The propagation speed of the glial wave was 1.10 ± 0.58 mm/s (n = 26 seizures from 5 rats).
Together, our results show that glial activation during seizure initiation is remarkably delayed suggesting that glia waves neither play a critical role in seizure initiation nor neurovascular coupling.
Duration of Neuronal, Glial, and Hemodynamic activity
To further investigate the role of glia in supporting the evolution of seizures, we examined the relative duration of each signal. As we have shown previously, the hemodynamic signal had a much longer duration (54.49 ± 17.70 s, n = 13 seizures from 5 rats), and was uncoupled from seizure termination recorded with LFP (43.54 ± 17.63 s, n = 25 seizures from 5 rats) (Fig. 3A,B). The duration of glial activity (23.62 ± 6.47 s, n = 25 seizures from 5 rats) was also uncoupled from seizure termination, however, unlike the hemodynamic signal, the glial signal generally ended prior to the LFP and hemodynamic signal, except when seizures were of short duration (Fig. 3A,B). One way ANOVA test showed a significant difference between 3 signals (P < 0.001, F = 23.26, DF = 62). The Turkey–Kramer post hoc analysis indicated that the glial duration was significantly shorter than LFP duration (P < 0.001) and the hemodynamic durations (P < 0.001). The LFP duration was also shorter than hemodynamic durations (P = 0.0721).
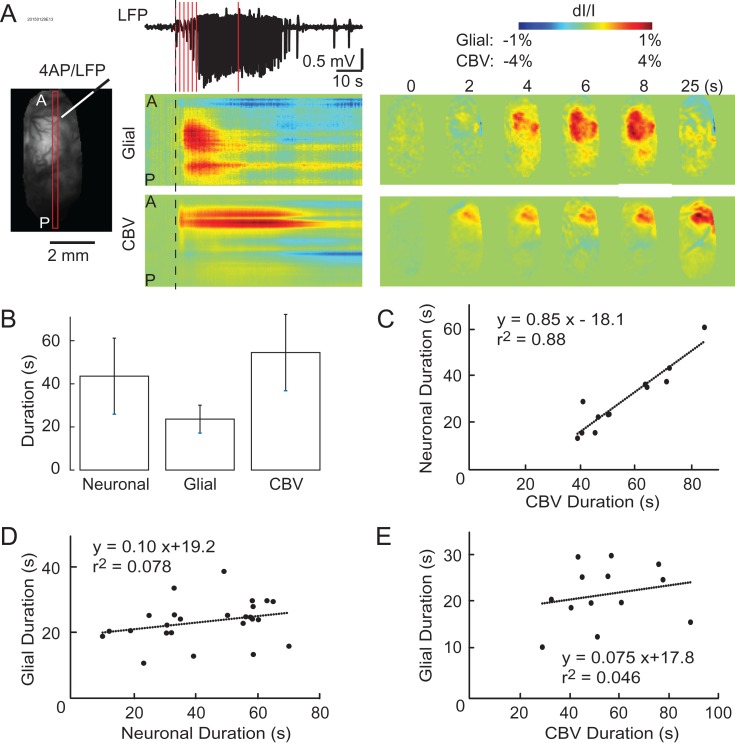
Figure 3.
Simultaneous imaging demonstrates the temporal variability of the neurovascular components during ictal events. (A) Left: linear ROIs allow for the observation of neuronal, glial, and hemodynamic ictal activities over time. A = anterior, P = posterior. Middle: LFP, glial, and CBV activities during an ictal event. The dashed lines indicate the LFP seizure onset time. Vertical red lines indicate time points at which the activity maps are showed in the right. Right: The spatial propagation of glial and CBV signals at certain time points. Note: CBV change is localized with a more prolonged duration than LFP seizure activity. Glial activation has a shorter duration and further spatial spread than both the LFP and CBV signals. (B) Average normalized ictal duration of neurovascular components. Error bars: SD. (C) The plot of neuronal versus CBV durations with linear correlation. (D and E) The plot of glial duration versus neuronal and CBV durations, respectively.
We further determined the relative duration of each signal using a linear regression model. A highly positive correlation was found between neuronal and hemodynamic durations, with the latter lasting roughly 20 s longer than the former (r2 = 0.88, Fig. 3C). The slope of 0.85 indicated that an increase in the duration of the seizure was mirrored by a similar increase in the duration of the hemodynamic signal. However, the relationship between glial and neuronal or glial and hemodynamic duration had a much poorer correlation, r2 = 0.078 and 0.046, respectively, (Fig. 3D,E). Despite varying seizure durations, ranging from approximately 10–70 s measured by LFP and 30–90 s measured by IOS, the duration of glial activity was fairly constant, with a range from 10 to 30 s. The slopes of these relationships were small, 0.1 (hemodynamic) and 0.075 (neuronal), indicating that the duration of the glial wave was stereotypical and did not increase proportionally with an increase of the electrographic seizure duration. Overall, these data show that the duration of the glial wave triggered by the seizure was a constant, stereotypical event that was generally unrelated to the duration of the electrographic event.
Spatial Propagation of Ictal Signals
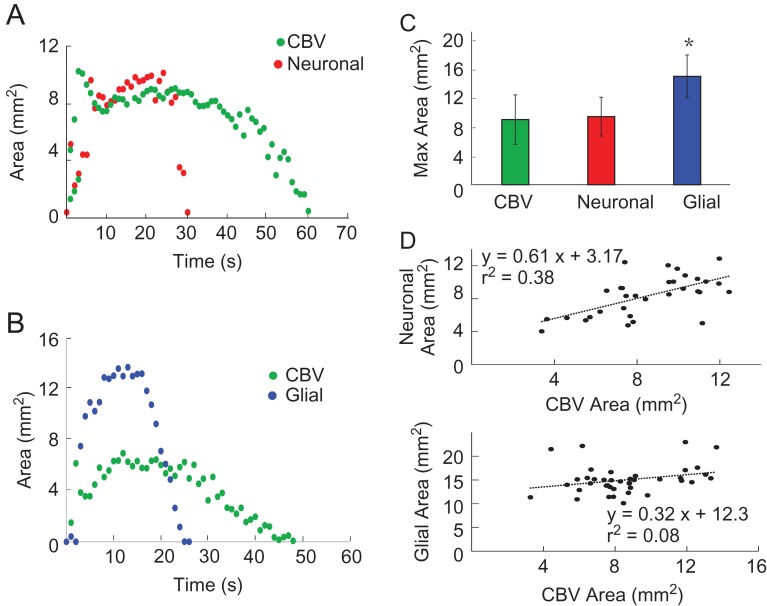
After determining that the onset of glial waves was delayed and glial wave duration was independent of the neuronal and hemodynamic signal duration, we investigated whether the maximal area of spread during seizure evolution was correlated to the area of spread of the neuronal and hemodynamic events. With simultaneous OGB-1 and IOS imaging, we first compared the spatial propagation of the neuronal and hemodynamic compartment. As previously described in this paper, the hemodynamic signal lasted longer than the neuronal signal, returning to baseline ~30 s after ictal termination, but the maximal areas of spread were similar (Fig. 4A,C). Simultaneous Rhod-2 and IOS imaging showed that while the glial signal was shorter in duration than the neuronal signal, it propagated to a much larger area (Fig. 4B,C). One way ANOVA test showed that there were significant differences among the averaged maximal area of spread of the 3 signals (P < 0.001, F = 58.420, DF = 147). The Tukey–Kramer post hoc analysis showed that the glial area (15.04 ± 2.96 mm2, n = 40 seizures from 7 rats) was roughly 60% larger than neuronal area (9.44 ± 2.69 mm2, n = 32 seizures from 9 rats) and hemodynamic area (9.04 ± 3.43 mm2, n = 76 seizures from 16 rats; P < 0.001 for both). Conversely, there was no significant difference between neuronal area and hemodynamic area (P = 0.5434, Fig. 4C). Linear correlation between neuronal area and hemodynamic area showed a strong linear relationship (Fig. 4D, top). The glial area of propagation was stereotypical regardless of the area of spread of the hemodynamic signal with a poor linear correlation (Fig. 4D, bottom).
Figure 4.
Spatial distribution of neuronal, glial, and CBV changes during seizure evolution. (A) Spatial evolution of neuronal and CBV change during an ictal event over time. (B) Spatial evolution of glial and CBV changes during an ictal event over time. (C) Average of maximum area of ictal activity shows the significant difference between CBV, glial, and neuronal signals. Top: the average of original data. Error bars: SD. (D) Discerning neuronal, glial, and hemodynamic relationships of area of ictal activation. The top figure exhibits the linear relationship between neuronal and CBV maximum area. The lower figure shows the spatial independence between the maximal area of glial activity and CBV spread, with the glial signal spreading to a relatively similar area regardless of the CBV spread.
Two-Photon Validation of Wide-Field Imaging
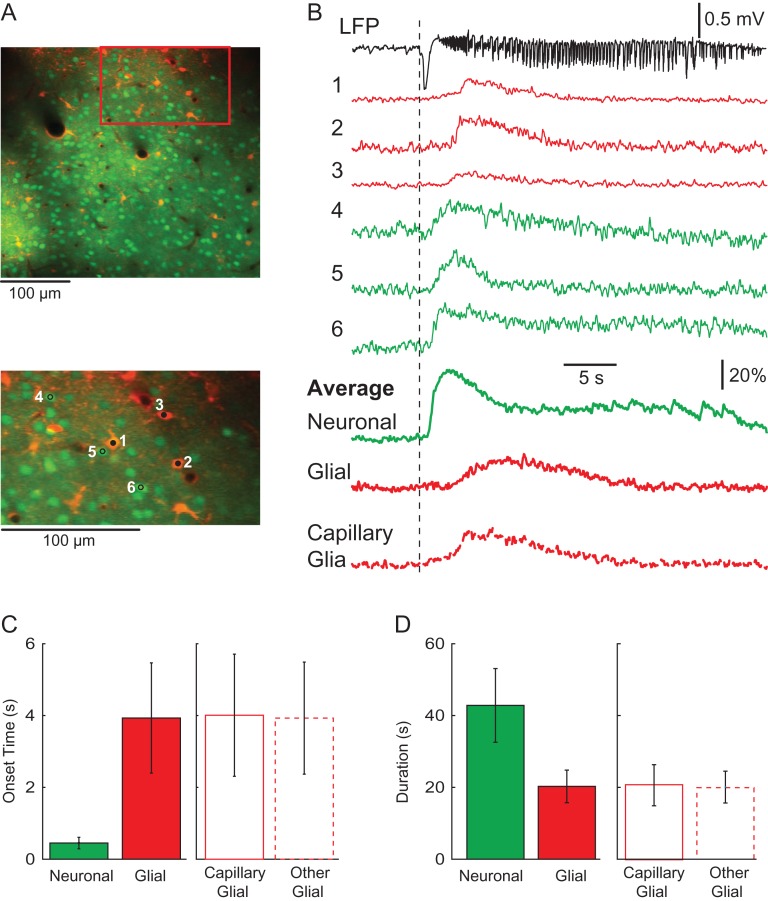
In order to determine if individual astrocytes were active prior to seizure onset or the hemodynamic response, we performed 2-photon single cell imaging with use of OGB-1 and SR101 staining to enable the distinction between neuronal and astrocytic activities (Fig. 5A). Consistent with our wide-field imaging results, neuronal calcium dynamics differed considerably from astrocytic signals (Fig. 5B). Neurons showed a relatively early onset time, immediately following the electrographic onset of the seizure with a duration of activity that correlated with the length of the seizure while the glia activity showed both a delayed onset and a shorter duration. Relative to the LFP, averaged onset of neuronal calcium change was 0.45 ± 0.16 s (450 cells, 5 animals) compared with the average glial onset time of 3.93 ± 1.51 s (123 cells, 5 animals; unpaired t-test, P < 0.001). Moreover, the duration of glial activity (20.28 ± 8.54 s) was significantly shorter than that of neuronal activity (42.82 ± 10.26 s, unpaired t-test, P < 0.001).
Figure 5.
Two-photon imaging of glial and neuronal activities during ictal events. (A) The field of view of the cell population. The orange cells are glia and the green cells are neurons. Note, that blood vessels can be detected and some glia are immediately adjacent to the blood vessels. A red box indicates a ROI that is zoomed in below. Three glia (1–3) and 3 neurons (4–6) were arbitrarily selected to demonstrate the single-cell activity during an ictal event. (B) Activity of individual glia and neurons during an ictal event. Top: the LFP of the ictal event. Traces 1–6 show the calcium signal from glia and neurons during the ictal event. The averaged neuronal and glial activities from the field of view are shown as the thick solid lines below. The averaged signal of glia that are adjacent to blood vessels is shown as the thick dotted line in the bottom. Note the different onset time and duration between glia and neurons. (C) Bar graph depicting the onset times for neuronal and glial signals in respect to LFP. Glial signals are separated into capillary and other. Note the longer onset delay of all glial signals as compared to the neuronal calcium signal. Furthermore, there is no significant difference between the 2 glial types shown here. (D) Bar graph depicting the duration of neuronal and glial signals in seconds. Note the significantly longer duration of the neuronal signal in comparison to the average glial signal. Glial signals are also separated into capillary and other cell types, none of which differ significantly in duration.
We further examined the possibility that glia in the immediate vicinity of blood vessels may be more closely related to neurovascular coupling and could, therefore, exhibit a different activity time course. After selecting glia exclusively located within 50 µm of a capillary (capillary-glia), we found that the averaged waveform of capillary-glia was similar to the average waveform of all glia cells. (Fig. 5B). The averaged onset time of capillary-glia was 4.01 ± 1.71 s (45 cells from 5 animals), which was not significant different than that of the other glia (3.93 ± 1.57 s; 78 cells from 5 animals; unpaired t-test, P = 0.783; Fig. 5C). Moreover, the duration of the capillary-glia (20.88 ± 5.71 s) did not differ significantly from other glia (19.93 ± 4.43 s; unpaired t-test, P = 0.551; Fig. 5D). These imaging experiments at cellular resolution strongly support our wide-field recordings, providing further evidence that glial activation, even at the single cell level, was delayed and not critical for either ictogenesis or neurovascular coupling.
Blockade of Glial Activation
Since we were not able to image endfeet in vivo, which may exhibit independent activity from glial somata (Bazargani and Attwell 2016), we examined the necessity of glial calcium waves for either ictogenesis or neurovascular coupling, by completely blocking glial activity with the application of FA 90 min prior to 4-AP injection. To confirm that FA was effectively blocking glial activity, we performed Rhod-2 imaging during ictal events and recorded negligible glial signal (Fig. 6A). Nevertheless, ictal events persisted in LFP recording; moreover, simultaneous OGB-1 calcium and IOS imaging revealed that neuronal and hemodynamic activities were not only present, but similarly coupled with similar areas of spatial propagation at 8.93 ± 2.56 mm2 and 8.54 ± 2.71 mm2, respectively (n = 14 seizures from 6 rats; paired t-test, P = 0.737) (Fig. 6B). The absence of glial activity also had no impact on the duration of the seizures as measured by the LFP (39.18 ± 19.60 s, n = 116 seizures, 8 rats) compared with ictal event duration recorded with functionally intact astrocytes (38.07 ± 11.90 s, n = 116 seizures from 6 rats) (unpaired t-test, P = 0.613) (Fig. 6C). Likewise, the amplitude and the duration of the hemodynamic response in the presence of FA (8.254 ± 5.8% dI/I, n = 39 seizures 8 rats and 51.48 ± 20.10 s, n = 21 seizures, 8 rats, respectively) was not significantly different than control condition (11.4 ± 5.451% dI/I, n = 36 seizures 6 rats, unpaired t-test, P = 0.256 and 56.99 ± 20.12 s, n = 16 seizures from 5 rats, unpaired t-test, P = 0.462) (Fig. 6D,E). These data indicate that the glial inactivation had no impact on either seizure initiation, duration, and propagation or the hemodynamic response.
Figure 6.
Simultaneous imaging of CBV and glial activities during ictal events after FA application. (A) LFP, Rhod-2, and IOS imaging of glial and CBV signals, respectively, during an ictal event after FA bath application. The glial signal is abolished after exposure to FA while CBV signal does not differ significantly from control. At right, the averaged amplitude of Rhod-2 signal during FA application and control condition are shown. (B) Spatial spread of neuronal activity and hemodynamic activity with FA application. Left, an example of the spatial spread of neuronal and CBV signals after FA application in one animal. The averaged neuronal and CBV area are shown on the right. (C–E) The comparison of averaged values during FA application versus control condition. (C) The seizure duration recorded with LFP. (D) The amplitude of hemodynamic changes. (E) The duration of hemodynamic changes. Error bar: SD.
Mechanism of Glial Propagation and Initiation
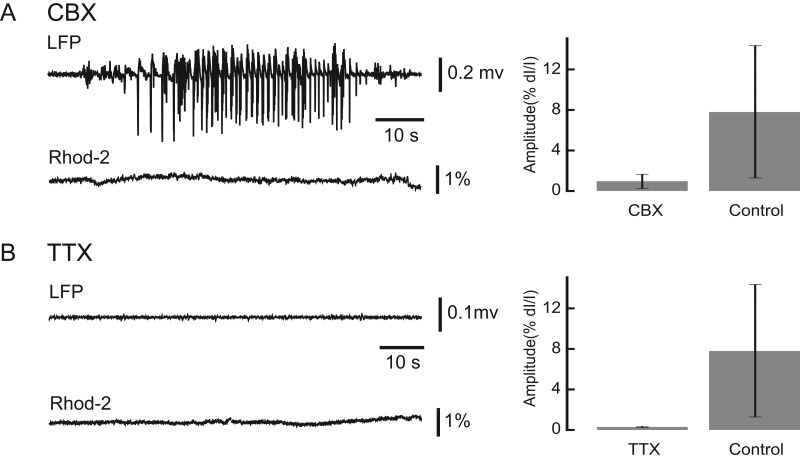
To probe the mechanism of glial wave propagation during ictal events, we introduced a bath application of 100 µM CBX, a known gap junction blocker, to the exposed cortex. In the presence of CBX, ictal events persisted as recorded with the LFP but the glial wave amplitude recorded with Rhod-2 imaging was only 0.99 ± 0.65% (n = 23 seizures, Fig. 7A). Under control conditions without CBX, ictal events induced a glial wave with an amplitude of 7.96 ± 6.70% (n = 14 seizures unpaired t-test, P < 0.001). (Fig. 7A). This finding suggests that gap junctions are important for astrocytic recruitment during ictal events but not critical for the underlying neuronal event.
Figure 7.
The neuronal and glia activities after drug application. (A) The effect of CBX. Left, the LFP and Rhod-2 traces recorded after CBX application. Note, seizures occur after CBX application, but negligible Rhod-2 deflection was observed. Right: averaged Rhod-2 amplitude under CBX and control condition. (B) The effect of TTX. Left, the LFP and Rhod-2 traces recorded with TTX application. After TTX application, neither seizures nor glial activity were observed. Right: averaged Rhod-2 amplitude under TTX and control condition. Error bars: SD.
We next examined the glial dependence on neuronal activity during seizures with the use of bath application ~2 µM TTX, a sodium channel blocker. The inhibition of neural activity was confirmed through LFP and OGB-1 recording. Rhod-2 imaging showed the near abolishment of glial activity with only small deviations (dI/I, 0.40 ± 0.07%, n = 7) from the baseline (Fig. 7B). This amplitude is significantly lower than the control condition (unpaired t-test, P = 0.008). This finding suggests that the initiation of the glial wave described here is dependent on neural activity, is not independently triggered by 4-AP, and does not exist in the absence of neuronal activity.
Discussion
In this article, we describe the existence of a widely propagating glial calcium wave that is triggered by neuronal seizure activity and appears to have no role in ictogenesis nor relationship with the accompanying hemodynamic response. The evidence for this conclusion rests upon several lines of evidence. First, the glial signal onset was relatively delayed, occurring after both neuronal and hemodynamic onset. Second, the glial wave had little spatial or temporal correlation with the ictal event and terminated well before the termination of both neuronal and hemodynamic activities. Finally, blockade of glial activity had no impact on the either the neuronal or the hemodynamic event suggesting that seizure initiation, propagation, and neurovascular coupling during ictal events are not critically dependent on the glial syncytium.
A Novel Ictal Glial Wave
Glial waves have been previously described in cultured neurons (Cornell-Bell et al. 1990), during spreading depression (Peters et al. 2003), and in the hippocampus (Kuga et al. 2011). Ictally triggered glial calcium influx has also been described, particularly in slice, with limited 2-photon imaging, or in perfused brain, but never with wide-field imaging in a focal in vivo model (Tashiro et al. 2002; Tian et al. 2005; Gomez-Gonzalo et al. 2010). The widely propagating, temporally stereotypical glial waves we describe differ in several key aspects from previous descriptions. First, cortical spreading depression and hippocampal glial waves are both accompanied by slowing of the LFPs and electroencephalograms rather than increases in power and frequency as occur during seizures. Second, previously described events are both characterized by decreases in CBV, rather than the massive increases in our experiments. Third, the previously described ictal glial calcium responses were not found to propagate concentrically. Furthermore, they were not imaged more than a few hundred microns from the focus and thus data on spatial spread, propagation speed, mechanism, and relation to hemodynamics have never been reported at the mesoscale level (Tian et al. 2005; Gomez-Gonzalo et al. 2010). Finally, the speed of propagation in cultured astrocytes (10–20 µm/s) (Scemes and Giaume 2006) or during cortical spreading depression (30–40 µm/s), or hippocampal waves (61 µm/s), differ from our waves that propagate much more rapidly at 1.10 ± 0.58 mm/s. Hence, the ictal glial waves described here are a novel, previously unrecognized phenomenon, although like these other glial waves, appear to be mediated by gap junctions.
Glia and Seizure Initiation
Our results contradict prior findings that point to a critical role for astrocytes, and more specifically to glial calcium waves, in ictogenesis. This theory first arose from data in support of a nonsynaptic mechanism for increased excitability (Konnerth et al. 1986). Glial release of extrasynaptic glutamate was shown to trigger both action potentials (Fellin et al. 2004) and paroxysmal depolarizing shifts, the hallmark of an epileptic event (Kang et al. 2005; Tian et al. 2005). On the other hand, other studies have found that astrocyte blockade did not eliminate epileptiform events (Fellin et al. 2006; Gomez-Gonzalo et al. 2010). Gomez-Gonzalo et al. (2010) then showed that glial inactivation impaired ictogenesis and that glial activation could trigger ictogenesis through extracellular NMDA. While compelling, these studies were all performed in vitro with bath application of chemoconvulsants and their findings may not be relevant in vivo.
One prior in vivo study reported pre-ictal astrocyte activation almost 5 s before focal ictal onsets, in direct contradiction with our findings (Tian et al. 2005). However, in vivo blockade of glial activity was not performed in that study to determine the impact on ictogenesis. One possible explanation for these contradictory findings is the recent evidence that glia have compartments with spatiotemporally diverse calcium dynamics and events occurring in the endfeet may transpire in isolation from the soma (Bazargani and Attwell 2016). Moreover, calcium dynamics in the cytoplasm may be quite different than activity in the membrane. Hence, subtle small scale calcium fluctuations may go unrecognized in our bulk loaded wide-field experiments. However, fluoracetate would be expected to block carbon flux through the Krebs cycle in endfeet as well as elsewhere in glia and such blockade failed to impact ictogenesis (Hassel et al. 1992).
Glia and Ictal Neurovascular Coupling
The hemodynamic response to ictal activity is robust with focal onset and subsequent propagation of increases in CBF that is spatiotemporally linked to the onset and evolution of the ictal event (Schwartz and Bonhoeffer 2001; Suh et al. 2005; Zhao et al. 2007, 2009; Ma et al. 2009a, 2009b, 2013). The mechanisms underlying the coupling between neuronal activity and the hemodynamic response is widely debated but astrocytes have been thought to play a critical role. Glia are known to have contact with vessels, both diving arterioles and capillaries below the pial surface (McCaslin et al. 2011). Furthermore, astrocytes respond to neuronal activity by taking up neurotransmitters released during synaptic transmission and, in turn, releasing gliotransmitters that can influence vasodilation or vasoconstriction (Attwell et al. 2010). These findings would support the hypothesis that glia also impact neurovascular coupling during ictal events (Howarth 2014). However, glial calcium influx tends to occur on a slower timescale than hemodynamic reactivity, which has raised serious questions about their role in neurovascular coupling (Wang et al. 2006; Petzold et al. 2008; Schummers et al. 2008; Nizar et al. 2013). Even the most “rapid” glial response has a ~1 s delay (Lind et al. 2013), which is too slow to mediate the ictal hemodynamic response we recorded, which began ~0.8 s after the ictal onset.
Indeed, other recent studies support our hypothesis arguing against a glial role in neurovascular coupling. The blockade of astrocyte calcium failed to impact stimulus-induced vasodilation (Nizar et al. 2013; Takata et al. 2013; Jego et al. 2014). Moreover, in a recent in vivo study calcium signaling in astrocytes was neither necessary nor sufficient to generate functional hyperemia (Bonder and McCarthy 2014). Our results further extend this concept to the field of epilepsy, where vascular signals are often used clinically for brain mapping in preparation for surgery. Our results suggest that the glial network may not offer an explanation of this signal source. Nevertheless, as mentioned above, we cannot rule out the possibility that astrocyte endfeet may have independent calcium dynamics mediating some aspect of neurovascular coupling below the resolution of our imaging. However, as mentioned above, FA would be expected to impact this functionality, which it did not in our experiments.
The Role of the Glial Wave
The glial waves described in this paper spread throughout the entire cortex in a slow, stereotypical concentric fashion, seemingly independent of the ictal event. While the ictal event and vascular response remain localized, the glial wave spreads across the whole cortex and terminates prior to the end of the seizure and well before the hemodynamic response disappears. It is well known that astrocytes in the adult brain form functional networks connected through gap-junctions (Nagy and Rash 2000). One of the presumed critical functions of this astroglial network is the buffering [K+], glutamate, GABA, and ATP (Olsen and Sontheimer 2008; Holmseth et al. 2009). However, if the ictal event remains focal then the elevations in ions and neurotransmitters should also be relatively focal and thus one would not expect the glial wave to spread further than the seizure nor to terminate before the seizure is over. Likewise, if the glial activity is important for maintaining the seizure activity, then one would expect the seizure to spread as far as the glial wave. However, if glial waves transport K+ away from the focus, waves may need to propagate to distant regions beyond the ictal zone. Another possible explanation is that once the glia become activated, they respond in a passive, all-or-none, stereotypical wave of activity. While 2 mechanisms for wave propagation have been described, either through a diffusible messenger (e.g., ATP or glutamate) or through gap-junctions, the waves we describe triggered in vivo by ictal events appear to be mediated by gap junctions. Moreover, while other studies have shown the existence of rhythmic activity in isolated glial networks even in the presence of TTX (Verkhratsky and Kettenmann 1996; Aguado et al. 2002; Giaume et al. 2010), the wave we described is clearly dependent on neuronal activation and thus blocked by TTX. Our data undermine the concept of glia as active participants in ictal activity and introduces the concept of the ictal glial wave as a reactive, “reflexive” event.
Another clue to the etiology of the ictal glial calcium wave is that lack of glial activity associated with interictal spikes. This phenomenon was first identified in a picrotoxin/zero Mg2+ entorhinal cortex slice model (Gomez-Gonzalo et al. 2010). Our data confirms this finding in an in vivo focal epilepsy model and provides further evidence that glutamate release triggered by calcium elevation in astrocytes is not critical for interictal spike generation, as previously hypothesized (Tian et al. 2005). Interictal discharges may also be insufficient in duration or intensity to elevate glial calcium to detectable levels. The ictal glial wave may be triggered specifically by the LVF activity which occurs only for a limited time at seizure onset. This would explain why the duration and propagation of the glial calcium wave have little relationship with the duration and spatial spread of the ictal event as it evolves into intermittent spike-and-wave activity. Hence, the glial wave may only be related to the ictal event at its focal initiation point, where ions and neurotransmitters require buffering and distant transport, but the spatial extent and propagation speed are an independent stereotypical event. Our findings are also consistent with studies by Lux and Heinemann (1977) showing decreases in extracellular calcium associated with ictal events.
In summary, we show that in an in vivo focal epilepsy model, glial activity is not necessary for ictogenesis or ictal neurovascular coupling. Moreover, ictal but not interictal events trigger delayed stereotypical concentrically spreading glial calcium waves that have little relationship with the duration or spatial spread of the underlying electrophysiological event or the accompanying neurovascular response.
Supplementary Material
Notes
Conflict of Interest: The authors declare no competing financial interests.
Supplementary Material
Supplementary material is available at Cerebral Cortex online.
Funding
The National Science Foundation (NSF-1264948), CURE: Citizens United for Research in Epilepsy, Department of Science and Technology of Jilin Province, China (20160414006GH), and the Deutsche Forschungsgemeinschaft (DFG, grant WE 5517/1-1). M.W. and R.Y. are supported by the NEI (DP1EY024503, R01EY011787); NIMH (R01MH101218, R01 MH100561) and DARPA SIMPLEX N66001-15-C-4032. This material is based upon work supported by, or in part by, the USA.
References
- Aguado F, Espinosa-Parrilla JF, Carmona MA, Soriano E. 2002. Neuronal activity regulates correlated network properties of spontaneous calcium transients in astrocytes in situ. J Neurosci. 22:9430–9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. 2010. Glial and neuronal control of brain blood flow. Nature. 468:232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avoli M, de Curtis M, Gnatkovsky V, Gotman J, Kohling R, Levesque M, Manseau F, Shiri Z, Williams S. 2016. Specific imbalance of excitatory/inhibitory signaling establishes seizure onset pattern in temporal lobe epilepsy. J Neurophysiol. 115:3229–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazargani N, Attwell D. 2016. Astrocyte calcium signaling: the third wave. Nat Neurosci. 19:182–189. [DOI] [PubMed] [Google Scholar]
- Bonder DE, McCarthy KD. 2014. Astrocytic Gq-GPCR-linked IP3R-dependent Ca2+ signaling does not mediate neurovascular coupling in mouse visual cortex in vivo. J Neurosci. 34:13139–13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard MB, Chen BR, Burgess SA, Hillman EM. 2009. Ultra-fast multispectral optical imaging of cortical oxygenation, blood flow, and intracellular calcium dynamics. Opt Express. 17:15670–15678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. 1990. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 247:470–473. [DOI] [PubMed] [Google Scholar]
- de Curtis M, Avoli M. 2016. GABAergic networks jump-start focal seizures. Epilepsia. 57:679–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Vezzani A, Najjar S, De Lanerolle NC, Rogawski MA. 2013. Glia and epilepsy: excitability and inflammation. Trends Neurosci. 36:174–184. [DOI] [PubMed] [Google Scholar]
- Fellin T, Gomez-Gonzalo M, Gobbo S, Carmignoto G, Haydon PG. 2006. Astrocytic glutamate is not necessary for the generation of epileptiform neuronal activity in hippocampal slices. J Neurosci. 26:9312–9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellin T, Pascual O, Gobbo S, Pozzan T, Haydon PG, Carmignoto G. 2004. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron. 43:729–743. [DOI] [PubMed] [Google Scholar]
- Fonnum F, Johnsen A, Hassel B. 1997. Use of fluorocitrate and fluoroacetate in the study of brain metabolism. Glia. 21:106–113. [PubMed] [Google Scholar]
- Ghosh A, Wyss MT, Weber B. 2013. Somatotopic astrocytic activity in the somatosensory cortex. Glia. 61:601–610. [DOI] [PubMed] [Google Scholar]
- Giaume C, Koulakoff A, Roux L, Holcman D, Rouach N. 2010. Astroglial networks: a step further in neuroglial and gliovascular interactions. Nat Rev Neurosci. 11:87–99. [DOI] [PubMed] [Google Scholar]
- Girouard H, Iadecola C. 2006. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol. 100:328–335. [DOI] [PubMed] [Google Scholar]
- Gomez-Gonzalo M, Losi G, Chiavegato A, Zonta M, Cammarota M, Brondi M, Vetri F, Uva L, Pozzan T, de Curtis M, et al. 2010. An excitatory loop with astrocytes contributes to drive neurons to seizure threshold. PLoS Biol. 8:e1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewe BF, Helmchen F. 2009. Optical probing of neuronal ensemble activity. Curr Opin Neurobiol. 19:520–529. [DOI] [PubMed] [Google Scholar]
- Hassel B, Paulsen RE, Johnsen A, Fonnum F. 1992. Selective inhibition of glial cell metabolism in vivo by fluorocitrate. Brain Res. 576:120–124. [DOI] [PubMed] [Google Scholar]
- Hillman EM. 2014. Coupling mechanism and significance of the BOLD signal: a status report. Annu Rev Neurosci. 37:161–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer SB, Ko H, Pichler B, Vogelstein J, Ros H, Zeng H, Lein E, Lesica NA, Mrsic-Flogel TD. 2011. Differential connectivity and response dynamics of excitatory and inhibitory neurons in visual cortex. Nat Neurosci. 14:1045–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmseth S, Scott HA, Real K, Lehre KP, Leergaard TB, Bjaalie JG, Danbolt NC. 2009. The concentrations and distributions of three C-terminal variants of the GLT1 (EAAT2; slc1a2) glutamate transporter protein in rat brain tissue suggest differential regulation. Neuroscience. 162:1055–1071. [DOI] [PubMed] [Google Scholar]
- Howarth C. 2014. The contribution of astrocytes to the regulation of cerebral blood flow. Front Neurosci. 8:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C. 2004. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci. 5:347–360. [DOI] [PubMed] [Google Scholar]
- Jego P, Pacheco-Torres J, Araque A, Canals S. 2014. Functional MRI in mice lacking IP3-dependent calcium signaling in astrocytes. J Cereb Blood Flow Metab. 34:1599–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang N, Xu J, Xu Q, Nedergaard M, Kang J. 2005. Astrocytic glutamate release-induced transient depolarization and epileptiform discharges in hippocampal CA1 pyramidal neurons. J Neurophysiol. 94:4121–4130. [DOI] [PubMed] [Google Scholar]
- Kerr JN, Greenberg D, Helmchen F. 2005. Imaging input and output of neocortical networks in vivo. Proc Natl Acad Sci USA. 102:14063–14068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konnerth A, Heinemann U, Yaari Y. 1986. Nonsynaptic epileptogenesis in the mammalian hippocampus in vitro. I. Development of seizurelike activity in low extracellular calcium. J Neurophysiol. 56:409–423. [DOI] [PubMed] [Google Scholar]
- Kuga N, Sasaki T, Takahara Y, Matsuki N, Ikegaya Y. 2011. Large-scale calcium waves traveling through astrocytic networks in vivo. J Neurosci. 31:2607–2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind BL, Brazhe AR, Jessen SB, Tan FC, Lauritzen MJ. 2013. Rapid stimulus-evoked astrocyte Ca2+ elevations and hemodynamic responses in mouse somatosensory cortex in vivo. Proc Natl Acad Sci USA. 110:E4678–E4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux HD, Heinemann U. 1977. Ionic changes during experimentally induced seizure activity. Electroencephalogr Clin Neurophysiol Suppl. 34:289–297. [PubMed] [Google Scholar]
- Ma H, Geneslaw A, Zhao M, Suh M, Perry C, Schwartz TH. 2009. a. The importance of latency in the focality of perfusion and oxygenation changes associated with triggered afterdischarges in human cortex. J Cereb Blood Flow Metab. 29:1003–1014. [DOI] [PubMed] [Google Scholar]
- Ma H, Harris S, Rahmani R, Lacefield CO, Zhao M, Daniel AG, Zhou Z, Bruno RM, Berwick J, Schwartz TH. 2014. a. Wide-field neocortical calcium dye imaging using a convection-enhanced loading technique combined with simultaneous multiwavelength imaging of voltage-sensitive dyes and hemodynamic signals. Neurophotonics. 1:015003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Zhao M, Harris S, Schwartz TH. 2014. b. Simultaneous multi-wavelength optical imaging of neuronal and hemodynamic activity. In. Neurovascular Coupling Methods p. 237–249.
- Ma H, Zhao M, Schwartz TH. 2013. Dynamic neurovascular coupling and uncoupling during ictal onset, propagation, and termination revealed by simultaneous in vivo optical imaging of neural activity and local blood volume. Cereb Cortex. 23:885–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Zhao M, Suh M, Schwartz TH. 2009. b. Hemodynamic surrogates for excitatory membrane potential change during interictal epileptiform events in rat neocortex. J Neurophysiol. 101:2550–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin ED, Fernandez M, Perea G, Pascual O, Haydon PG, Araque A, Cena V. 2007. Adenosine released by astrocytes contributes to hypoxia-induced modulation of synaptic transmission. Glia. 55:36–45. [DOI] [PubMed] [Google Scholar]
- McCaslin AF, Chen BR, Radosevich AJ, Cauli B, Hillman EM. 2011. In vivo 3D morphology of astrocyte-vasculature interactions in the somatosensory cortex: implications for neurovascular coupling. J Cereb Blood Flow Metab. 31:795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metea MR, Newman EA. 2006. Glial cells dilate and constrict blood vessels: a mechanism of neurovascular coupling. J Neurosci. 26:2862–2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy JI, Rash JE. 2000. Connexins and gap junctions of astrocytes and oligodendrocytes in the CNS. Brain Res Brain Res Rev. 32:29–44. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Ransom B, Goldman SA. 2003. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 26:523–530. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Kerr JN, Helmchen F. 2004. Sulforhodamine 101 as a specific marker of astroglia in the neocortex in vivo. Nat Methods. 1:31–37. [DOI] [PubMed] [Google Scholar]
- Nizar K, Uhlirova H, Tian P, Saisan PA, Cheng Q, Reznichenko L, Weldy KL, Steed TC, Sridhar VB, MacDonald CL, et al. 2013. In vivo stimulus-induced vasodilation occurs without IP3 receptor activation and may precede astrocytic calcium increase. J Neurosci. 33:8411–8422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen ML, Sontheimer H. 2008. Functional implications for Kir4.1 channels in glial biology: from K+ buffering to cell differentiation. J Neurochem. 107:589–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña-Ortega F, Rivera-Angulo AJ, Lorea-Hernández JJ. 2016. Pharmacological Tools to Study the Role of Astrocytes in Neural Network Functions. In: Glial Cells in Health and Disease of the CNS. Springer. p 47–66. [DOI] [PubMed]
- Peters O, Schipke CG, Hashimoto Y, Kettenmann H. 2003. Different mechanisms promote astrocyte Ca2+ waves and spreading depression in the mouse neocortex. J Neurosci. 23:9888–9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold GC, Albeanu DF, Sato TF, Murthy VN. 2008. Coupling of neural activity to blood flow in olfactory glomeruli is mediated by astrocytic pathways. Neuron. 58:897–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongracz F, Szente M. 1979. Simulation of the ionic mechanisms of molluscan neurons under pentylenetetrazol-induced effects. Acta Physiol Acad Sci Hung. 53:327–336. [PubMed] [Google Scholar]
- Robel S, Sontheimer H. 2016. Glia as drivers of abnormal neuronal activity. Nat Neurosci. 19:28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scemes E, Giaume C. 2006. Astrocyte calcium waves: what they are and what they do. Glia. 54:716–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz K, Sydekum E, Krueppel R, Engelbrecht CJ, Schlegel F, Schroter A, Rudin M, Helmchen F. 2012. Simultaneous BOLD fMRI and fiber-optic calcium recording in rat neocortex. Nat Methods. 9:597–602. [DOI] [PubMed] [Google Scholar]
- Schummers J, Yu H, Sur M. 2008. Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science. 320:1638–1643. [DOI] [PubMed] [Google Scholar]
- Schwartz TH, Bonhoeffer T. 2001. In vivo optical mapping of epileptic foci and surround inhibition in ferret cerebral cortex. Nat Med. 7:1063–1067. [DOI] [PubMed] [Google Scholar]
- Suh M, Bahar S, Mehta AD, Schwartz TH. 2005. Temporal dependence in uncoupling of blood volume and oxygenation during interictal epileptiform events in rat neocortex. J Neurosci. 25:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata N, Nagai T, Ozawa K, Oe Y, Mikoshiba K, Hirase H. 2013. Cerebral blood flow modulation by basal forebrain or whisker stimulation can occur independently of large cytosolic Ca2+ signaling in astrocytes. PloS one. 8:e66525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A, Goldberg J, Yuste R. 2002. Calcium oscillations in neocortical astrocytes under epileptiform conditions. J Neurobiol. 50:45–55. [DOI] [PubMed] [Google Scholar]
- Tian GF, Azmi H, Takano T, Xu Q, Peng W, Lin J, Oberheim N, Lou N, Wang X, Zielke HR, et al. 2005. An astrocytic basis of epilepsy. Nat Med. 11:973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, Kettenmann H. 1996. Calcium signalling in glial cells. Trends Neurosci. 19:346–352. [DOI] [PubMed] [Google Scholar]
- Wang X, Lou N, Xu Q, Tian GF, Peng WG, Han X, Kang J, Takano T, Nedergaard M. 2006. Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat Neurosci. 9:816–823. [DOI] [PubMed] [Google Scholar]
- White BR, Bauer AQ, Snyder AZ, Schlaggar BL, Lee JM, Culver JP. 2011. Imaging of functional connectivity in the mouse brain. PloS one. 6:e16322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Ma H, Suh M, Schwartz TH. 2009. Spatiotemporal dynamics of perfusion and oximetry during ictal discharges in the rat neocortex. J Neurosci. 29:2814–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Suh M, Ma H, Perry C, Geneslaw A, Schwartz TH. 2007. Focal increases in perfusion and decreases in hemoglobin oxygenation precede seizure onset in spontaneous human epilepsy. Epilepsia. 48:2059–2067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.