Abstract
Purpose
Circadian rhythm affects learning process, memory consolidation, and long-term memory. In this study, the alleviating effect of exercise on circadian rhythm disruption-induced memory deficits was investigated.
Methods
BMAL1 knockdown transgenic mice (BMAL1 TG) were used as the BMAL1-TG group and the BMAL1-TG with treadmill exercise group. Female C57BL/6J mice of the same age were used as the wildtype group and the wildtype with treadmill exercise group. The mice in the treadmill exercise groups performed running on a motorized treadmill under the dark-dark conditions for 8 weeks. Short-term memory, nonspatial object memory, and spatial learning memory were determined using stepdown avoidance test, novel object-recognition test, and radial 8-arm maze test. Immunohistochemistry for doublecortin and 5-bromo-2’-deoxyuridine was conducted for the determination of hippocampal neurogenesis. Using the western blot analysis, we determined the expressions of glucocorticoid receptor (GR) and factors related to the neurogenesis and memory consolidation, such as brain-derived neurotrophic factor, tyrosine kinase B, p44/42 mitogen-activated protein kinase, cyclic AMP-responsive element binding protein, phosphatidylinositol 3-kinase, protein kinas B, protein kinase C alpha, early-growth-response gene 1.
Results
Circadian rhythm disruption impaired memory function through inhibiting the expressions of GR and the factors related to neurogenesis and memory consolidation. Treadmill exercise improved memory function via enhancing the expressions of GR and above-mentioned factors.
Conclusions
Treadmill exercise acts as the zeitgeber that improves memory function under the circadian rhythm disrupted conditions.
Keywords: BMAL1, Circadian rhythm, Memory impairment, Treadmill exercise
• HIGHLIGHTS
- Circadian rhythm disruption impairs memory function.
- Circadian rhythm disruption inhibits the factors related to neurogenesis and memory consolidation.
- Treadmill exercise improves memory function via enhancing these factors under the circadian rhythm disrupted conditions.
INTRODUCTION
In mammals, circadian rhythm depends on the integrity of the master circadian oscillator placed in the suprachiasmatic nucleus (SCN) of hypothalamus, which synchronizes peripheral oscillators present in the cells of most organs and tissues [1]. Circadian rhythm affects various physiological functions, such as feeding behavior, regulation of body temperature, and secretion of hormone, thus disruption of circadian rhythm is related to many diseases [1].
Circadian rhythm affects learning process, memory consolidation, and long-term potentiation (LTP) at CA1 in the hippocampal slices [2,3]. Hippocampus exhibits rhythms in clock genes affecting and being affected by central cell signaling pathways, such as mitogen-activated protein kinase (MAPK) and cyclic AMP (cAMP) [4]. The hippocampus gains circadian information via adrenal glucocorticoid [4].
Glucocorticoid is biosynthesized in the adrenal gland and regulates homeostasis, ion transport, glycogenolysis, and immune response [5]. Glucocorticoid modulates synaptic plasticity, neurogenesis, and memory formation in the hippocampus [3,6]. Glucocorticoid binds to following 2 main receptors: one is glucocorticoid receptor (GR) and the other is mineralocorticoid receptor (MR). These MR and GR are expressed throughout the brain, at highest level in the hippocampus, however, the functions of these receptors are different. MR modulates the initial phase of memory encoding, whereas, GR is involved in the memory consolidation [5].
Memory consolidation is regulated by a brain-derived neurotrophic factor (BDNF)-dependent autoregulatory loop [7]. BDNF plays an important regulatory role in the survival and differentiation of neurons, and then BDNF is an ideal candidate mediating the process of learning and memory [8]. Aging and exercise affect the expression of BDNF in the hippocampus, leading to promotion or impairment of learning and memory function [9,10]. In particular, exercise is known to improve learning and memory function by enhancing BDNF expression in the hippocampus [11,12]. CLOCK mutant mice exhibited exercise intolerance and reduced mitochondrial content in skeletal muscle, whereas, endurance exercise increased exercise tolerance and mitochondrial level, suggesting that exercise controls circadian rhythm [13].
Circadian rhythm influences memory acquisition and recall [4]. In the present study, we investigated the alleviating effect of exercise on circadian rhythm disruption-induced memory deficits using BMAL1 knockdown transgenic mice.
MATERIALS AND METHODS
Animals and Treatments
BMAL1 knockdown transgenic mice (BMAL1 TG, 9–12 weeks of age) strains were used as the BMAL1-TG group (TG, n=10) [14] and the BMAL1-TG with treadmill exercise group (TG-EX, n=10). Other female C57BL/6J mice of the same age were used as the wildtype group (WT, n=10) and the wildtype with treadmill exercise group (WT-EX, n=10). The mice were kept in constant darkness for 8 weeks. The mice in the treadmill exercise groups performed running on a motorized treadmill for 30 minutes daily, 5 days per a week, for 8 consecutive weeks, under the dark-dark conditions. The exercise load was running at a 3 m/min for 5 minutes, 5 m/min for 5 minutes, and 8 m/min for 20 minutes, with 0o inclination.
Step-Down Avoidance Test
According to the previously described method [15], the latency of the step-down avoidance test was measured for the evaluation of short-term memory. The mice were trained in a stepdown avoidance test, and then, the latency (second) was measured at 2 hours after training session. The mice were placed on a platform with width, length, and height of 25 cm×7 cm×2.5 cm. The platform has a parallel stainless steel bars measuring 25 cm×42 cm with a diameter of 0.1 cm and a spacing of 1 cm. During the training session, 0.5 mA scramble foot shock was applied to the mice for 2 seconds immediately after stepping down. We defined the time interval as the latency until the mouse came down and reached four feet on the grid. The latency of 300 seconds or longer was calculated as 300 seconds.
Novel Object Recognition Test
For the assessment of non-spatial object memory, we conducted the novel object-recognition test using an open field box with width, length, and height of 45 cm×45 cm×30 cm, as previously described method [16]. Before to test, the mice were accustomed to the box for 15 minutes without objects. On the object trial, the mice were placed into the test box with 2 identical objects (approximately 30 cm apart from each other), and then allowed to explore for 10 minutes. After 1 hour waiting time, the mice were put back into the test box and allowed to explore the objects. One object was the same as that used in the previous training session, and the other one was a novel object. We measured the time spent for exploring novel object and familiar object. They were considered to be exploring when they were facing, sniffing, or biting the object within a 1.5-cm radius. This test was conducted under the dark conditions, and the test box and objects were washed using 70% ethanol between tests. The percentage of novel object recognition time and the discrimination ratio were obtained by the following formula. Novel object recognition time (%)=(novel object time/[novel object time+familiar object time])×100. Discrimination ratio=(novel object time–familiar object time)/(novel object time+familiar object time).
Radial 8-Arm Maze Test
As the previously described method [17], we performed radial 8-arm maze test for the determination of spatial learning memory, using a radial 8-arm maze apparatus. This apparatus is seated 1 m above the floor and it has a central octagonal plate (30 cm in diameter) with 8 radiating arms (50 cm in length, 10 cm in width). At the end of the arm, there was a small dish filled with water (3 cm in diameter, 1 cm in depth). Before the spatial learning memory test, the mice were trained 3 times. For training sessions, the water supply to the mice was stopped for 24 hours. After each training session, water was supplied to the mice for 5 minutes. During the radial 8-arm maze test, the spending time for seeking water was calculated as the latency. This test was stopped when the mice drink water in all 8 arms or experiment time was over 8 minutes. When the mice entered arm that was already visited, it was counted as the error number. The continuous number of correct choices before the first error occurred was counted as the correct number.
Tissue Preparation for Immunohistochemistry
Immediately after finishing the behavior testing, the mice were fully anesthetized by Zoletil 50 (10 mg/kg, intraperitoneally; Vibac Laboratories, Carros, France). Then, 50mM phosphate-buffered saline (PBS) was transcardially perfused, and the mice were fixed using 4% paraformaldehyde in 100mM phosphate buffer (pH, 7.4). After dissecting the brains, we made 5-μmthick coronal sections using a freezing microtome (Leica, Nussloch, Germany).
Doublecortin Immunohistochemistry
We conducted doublecortin (DCX) immunohistochemistry, as the previously described method [18]. After washing the sections by 0.1M PBS (pH, 7.4), the sections were treated with a 3% H2O2 and 20% methanol. The sections were treated with anti-α-doublecortin antibody (1 μg/mL; Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4°C for 48 hours, then the sections were incubated with donkey α-goat biotinylated antibody for 1 hour at room temperature (Chemicon Inc., Temecula, CA, USA). The sections were treated with ABC Elite kit (Vector Laboratories, Burlingame, CA, USA) for 1 hour. After that, 0.02% diaminobenzidine (DAB) with 0.03% H2O2 was treated to the sections for 5 minutes. And then, gelatin-coated slides were used for the mounting of the sections.
5-Bromo-2’-Deoxyuridine Immunohistochemistry
The mice received 50 mg/kg 5-Bromo-2’-Deoxyuridine (BrdU) (Sigma Chemical Co., St. Louis, MO, USA) intraperitoneally at the third, fourth, tenth, and eleventh day after onset of exercise. We conducted BrdU-specific immunohistochemistry, as the previously described method [18]. After permeabiliztion of the sections with 0.5% Triton X-100 in PBS for 20 minutes, the section was treated with 50% formamide-2 x standard saline citrate at 65°C for 2 hours. The sections were denatured using 2 N HCl at 37°C for 30 minutes, and then washed 2 times by 100mM sodium borate (pH, 8.5). BrdU-specific mouse monoclonal antibody (1:600) (Roche, Mannheim, Germany) was treated to the sections during overnight at 4°C. After incubating the sections with biotinylated mouse secondary antibody (1:200) (Vector Laboratories) for 1 hour, an avidin-peroxidase complex (1:100) (Vector Laboratories) was treated to the sections for next 1 hour. After that, the sections were treated with 50mM Tris-HCl (pH, 7.6) including 0.03% DAB, 40-mg/mL nickel chloride, and 0.03% H2O2 for 5 minutes.
Mouse antineuronal nuclei antibody (Chemicon Inc.) was used to detect the differentiation of BrdU-positive cells in the same sections. After incubation of the sections with a biotinylated anti-mouse secondary antibody for 1 hour, reaction mixture composing of 0.03% DAB and 0.03% H2O2 was treated to the sections for 5 minutes. And then, gelatin-coated slides were used for the mounting of the sections.
Western Blot Analysis
We performed western blot analysis, as the previously described method [15,17]. After homogenizing hippocampal tissues on ice, hippocampal tissues were lysed using a lysis buffer including 1mM sodium orthovanadate, 50mM HEPES (pH, 7.5), 10% glycerol, 1% Triton X-100, 100mM sodium fluoride, 150mM NaCl, 1.5mM MgCl26H2O, 1mM phenylmethylsulfonyl fluoride, and 1mM ethylene glycol tetraacetic acid. After separating protein (20 μg) from the sodium dodecyl sulfate-polyacrylamide gels, the protein was transferred to the nitrocellulose membrane. For the primary antibodies, we used rabbit GAPDH antibody (AbFrontier, Seoul, Korea; 1:5,000), rabbit GR antibody (Santa Cruz Biotechnology; 1:1,000), rabbit BDNF antibody (Santa Cruz Biotechnology; 1:1,000), rabbit tyrosine kinase B (TrkB) antibody (Santa Cruz Biotechnology; 1:1,000), rabbit cAMP-responsive element binding protein (CREB) antibody (Santa Cruz Biotechnology; 1:1,000), rabbit phospho-CREB (p-CREB) (Ser133) antibody (Santa Cruz Biotechnology; 1:1,000), mouse phosphatidylinositol 3-kinase (PI 3-kinase) p85α (Santa Cruz Biotechnology; 1:1,000), rabbit phospho-PI 3-kinase p85α (p-PI 3-kinase p85α) (Tyr467) antibody (Santa Cruz Biotechnology; 1:1,000), rabbit protein kinase C alpha (PKCα) antibody (Santa Cruz Biotechnology; 1:1,000), rabbit phospho-PKCα (p-PKCα) (Ser657) antibody (Merck Millipore Corp., Darmstadt, Germany; 1:1,000), rabbit protein kinas B (Akt) antibody (Cell Signaling Technology, Beverly, MA, USA; 1:1,000), rabbit phospho-Akt (p-Akt) (Ser473) antibody (Cell Signaling Technology; 1:1,000), rabbit early-growth-response gene 1 (Egr-1) antibody (Santa Cruz Biotechnology; 1:1,000), rabbit total p44/42 MAPK (Erk1/2) antibody (Cell Signaling Technology; 1:1,000), rabbit phospho-p44/42 MAPK (p-Erk1/2) antibody (Cell Signaling Technology; 1:1,000). For the secondary antibodies, we used horseradish peroxidase-conjugated anti-rabbit antibody (Vector Laboratories; 1:2,000) for GAPDH, GR, BDNF, TrkB, Akt, p-Akt, CREB, p-CREB, p-PI 3-kinase p85α, PKCα, p-PKCα, Egr-1, ERK1/2, p-ERK1/2, and horseradish peroxidaseconjugated anti-mouse antibody for PI 3-kinase p85α (Amersham Pharmacia Biothech GmbH, Freiburg, Germany; 1:2,000). An enhanced chemiluminescence detection kit (Santa Cruz Biotechnology) was used to calculate the detected bands.
Data Analysis
Molecular AnalystTM, version 1.4.1 (Bio-Rad, Hercules, CA, USA) was used for the densitometrical calculation of these bands. The numbers of DCX-positive and BrdU-positive cells in the hippocampal dentate gyrus were hemilaterally counted, and the results were expressed as the number of DCX-positive or BrdU-positive cells per mm2 in the hippocampal dentate gyrus. We used the Image-Pro Plus image analysis system (Media Cyberbetics Inc., Silver Spring, MD, USA) to measure the total area of the hippocampal dentate gyrus.
The results were statistically analyzed by Duncan post hoc test following 1-way analysis of variance. Data are presented as the mean±standard error of the mean. Statistical significance was considered when P-value was less than 0.05.
RESULTS
Memory Function in Circadian Rhythm Disturbed Mice
For the step-down avoidance test, disruption of circadian rhythm showed shorter latency (P<0.05). On the contrary, treadmill exercise lengthened this latency in TG mice (P<0.05) (Fig. 1A). For the novel object recognition test, disruption of circadian rhythm showed lower recognition time percentage and discrimination ratio (P<0.05). On the contrary, treadmill exercise increased the percentage of recognition time in TG mice (P<0.05) (Fig. 1B). For the radial arm maze test, disruption of circadian rhythm showed longer time, more error, and less correct during the task (P<0.05). However, treadmill exercise showed no significant effect on latency, number of error, and number of correct (Fig. 1C).
Fig. 1.
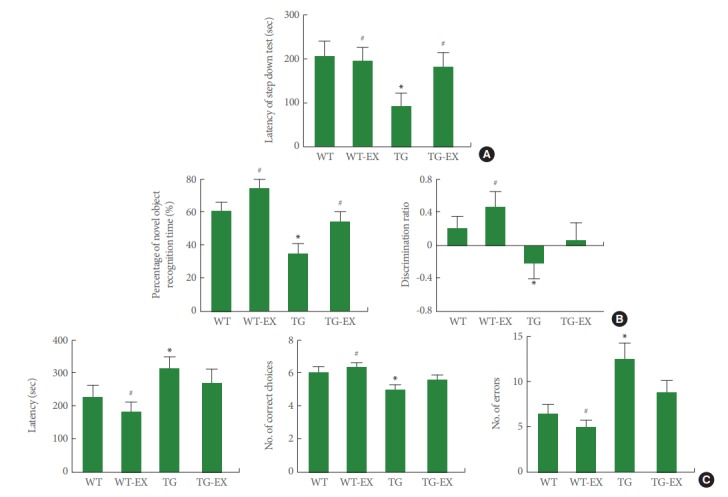
Effects of treadmill exercise on the performance of behavior tests. (A) Step-down avoidance test. (B) Novel object recognition test. (C) Radial 8-arm maze test. WT, wildtype group; WT-EX, wildtype with treadmill exercise group; TG, BMAL1-transgenic group; TG-EX, BMAL1-transgenic with treadmill exercise group. Data are represented as mean±standard error of the mean. *P<0.05 compared to the WT. #P<0.05 compared to the TG.
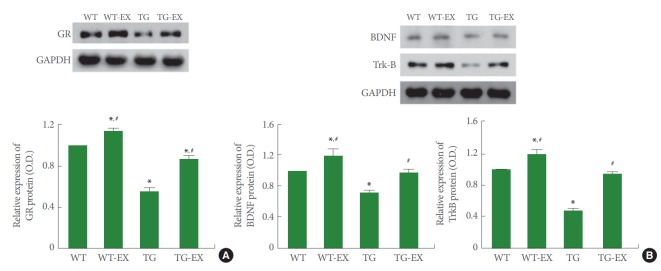
GR Expression in Circadian Rhythm Disturbed Mice
Disruption of circadian rhythm inhibited the expression of GR in the hippocampus (P<0.05). On the contrary, treadmill exercise improved the expression of GR both in TG mice and WT mice (P<0.05) (Fig. 2A).
Fig. 2.
Effects of treadmill exercise on the expressions of glucocorticoid receptor (GR), brain-derived neurotrophic factor (BDNF), and tyrosine kinase B (TrkB). (A) GR expression in the hippocampus. (B) BDNF and TrkB expressions in the hippocampus. WT, wildtype group; WT-EX, wildtype with treadmill exercise group; TG, BMAL1-transgenic group; TG-EX, BMAL1-transgenic with treadmill exercise group. Data are represented as mean±standard error of the mean. *P<0.05 compared to the WT. #P<0.05 compared to the TG.
BDNF and TrkB Expressions in Circadian Disturbed Mice
Disruption of circadian rhythm reduced the expressions of BDNF and TrkB in the hippocampus (P<0.05). On the contrary, treadmill exercise improved the expressions of BDNF and TrkB both in TG mice and WT mice (P<0.05) (Fig. 2B).
p-Erk1/2 Expression in Circadian Rhythm Disturbed Mice
Disruption of circadian rhythm reduced the ratio of p-Erk1/2 to Erk1/2 in the hippocampus (P<0.05). On the contrary, treadmill exercise improved the expression of p-Erk1/2 adjusted by Erk1/2 both in TG mice and WT mice (P<0.05) (Fig. 3A).
Fig. 3.
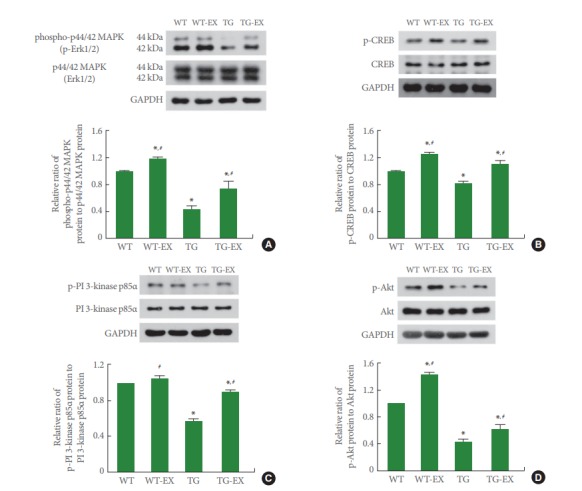
Effects of treadmill exercise on the expressions of mitogen-activated protein kinase (MAPK), cAMP-responsive element binding protein (CREB), phosphatidylinositol 3-kinase (PI 3-kinase), and protein kinas B (Akt). (A) p-p44/42 MAPK (p-Erk1/2) expression in the hippocampus. (B) p-CREB expression in the hippocampus. (C) p-PI 3-kinase p85α expression in the hippocampus. (D) p-Akt expression in the hippocampus. WT, wildtype group; WT-EX, wildtype with treadmill exercise group; TG, BMAL1-transgenic group; TG-EX, BMAL1-transgenic with treadmill exercise group. Data are represented as mean ±standard error of the mean. *P<0.05 compared to the WT. #P<0.05 compared to the TG.
p-CREB Expression in Circadian Rhythm Disturbed Mice
Disruption of circadian rhythm reduced the ratio of p-CREB to CREB in the hippocampus (P<0.05). On the contrary, treadmill exercise improved the expression of p-CREB adjusted by CREB both in TG mice and WT mice (P<0.05) (Fig. 3B).
p-PI 3-Kinase p85α Expression in Circadian Rhythm Disturbed Mice
Disruption of circadian rhythm reduced the ratio of p-PI 3 kinase p85α to PI 3 kinase p85α in the hippocampus (P<0.05). On the contrary, treadmill exercise improved the expression of p-PI 3 kinase p85α adjusted by PI 3 kinase p85α in TG mice (P<0.05) (Fig. 3C).
p-Akt Expression in Circadian Rhythm Disturbed Mice
Disruption of circadian rhythm reduced the ratio of p-Akt to Akt in the hippocampus (P<0.05). On the contrary, treadmill exercise improved the expression of p-Akt adjusted by Akt both in TG mice and WT mice (P<0.05) (Fig. 3D).
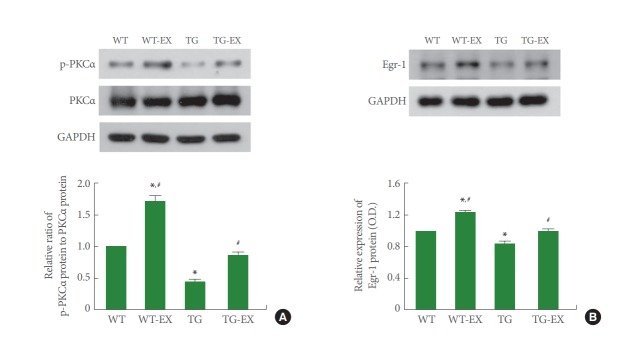
p-PKCα Expression in Circadian Rhythm Disturbed Mice
Disruption of circadian rhythm reduced the ratio of p-PKCα to PKCα in the hippocampus (P<0.05). On the contrary, treadmill exercise improved the expression of p-PKCα adjusted by PKCα both in TG mice and WT mice (P<0.05) (Fig. 4A).
Fig. 4.
Effects of treadmill exercise on the expressions of protein kinase C (PKC) and Egr-1. (A) p-PKCα expression in the hippocampus. (B) Egr-1 expression in the hippocampus. WT, wildtype group; WT-EX, wildtype with treadmill exercise group; TG, BMAL1-transgenic group; TG-EX, BMAL1-transgenic with treadmill exercise group. Data are represented as mean±standard error of the mean. *P<0.05 compared to the WT. #P<0.05 compared to the TG.
Egr-1 Expression in Circadian Rhythm Disturbed Mice
Disruption of circadian rhythm inhibited the expression of Egr-1 in the hippocampus (P<0.05). On the contrary, treadmill exercise improved Egr-1 expression both in TG mice and WT mice (P<0.05) (Fig. 4B).
Neurogenesis in Circadian Rhythm Disturbed Mice
Disruption of circadian rhythm reduced the number of DCX-positive cells in the hippocampal dentate gyrus (P<0.05). On the contrary, treadmill exercise enhanced the number of DCX-positive cells both in TG mice and WT mice (P<0.05) (Fig. 5A).
Fig. 5.
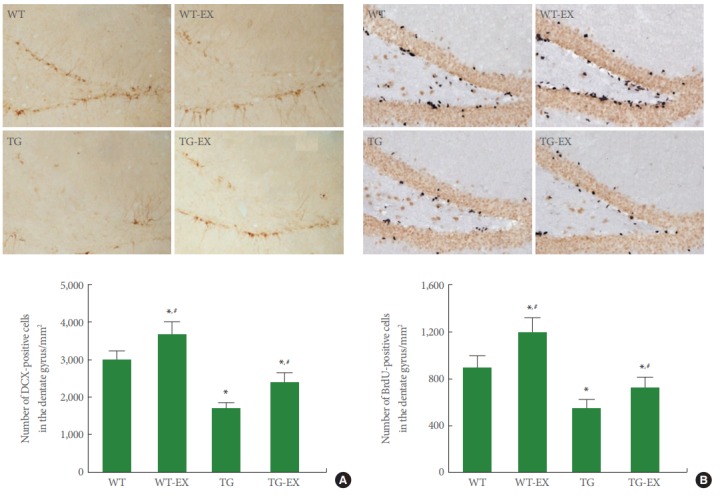
Effects of treadmill exercise on cell proliferation and neurogenesis in the hippocampal dentate gyrus. (A) Doublecortin (DCX)-positive cells in the hippocampal dentate gyrus. Upper panel: Photomicrographs of DCX-positive cells. The scale bar represents 50 μm. Lower panel: The number of DCX-positive cells in each group. (B) 5-Bromo-2’-deoxyuridine (BrdU)-positive cells in the hippocampal dentate gyrus. Upper panel: Photomicrographs of BrdU-positive cells. The scale bar represents 50 μm. Lower panel: The number of BrdU-positive cells in each group. WT, wildtype group; WT-EX, wildtype with treadmill exercise group; TG, BMAL1-transgenic group; TG-EX, BMAL1-transgenic with treadmill exercise group. The data are represented as mean±standard error of the mean. *P<0.05 compared to the WT. #P<0.05 compared to the TG.
Disruption of circadian rhythm reduced the number of BrdU-positive cells in the hippocampal dentate gyrus (P<0.05). On the contrary, treadmill exercise enhanced the number of BrdU-positive cells both in TG mice and WT mice (P<0.05) (Fig. 5B).
DISCUSSION
As SCN is the anatomical center for generation of circadian rhythm, SCN lesions interfere with hippocampus-dependent memory [19]. Mice lacking forebrain BMAL1 showed deficits in the spatial and novel object location memory [20]. In this study, BMAL1-knockdown mice displayed deficits of shortterm, non-spatial object, and spatial learning memory. In contrast, treadmill exercise showed alleviating effect on deficits of short-term and non-spatial object memory (Fig. 1).
BMAL1-knockout mice exhibited impairment of spatial learning memory with suppressed MAPK activation [21]. MAPK activation stimulates CREB pathway necessary for spatial and social learning [19]. CREB plays a critical role in memory formation and integration through modulating adult hippocampal neurogenesis [22]. In this study, Erk1/2 phosphorylation in the hippocampus of circadian rhythm disturbed mice was weakened compared with wildtype mice (Fig. 3A). CREB activation induced by phosphorylation at a serine residue (Ser 133) was also decreased in the hippocampus of circadian rhythm disturbed mice (Fig. 3B).
BMAL1-/- mice show reduced glucocorticoid level under the both light-dark and dark-dark conditions without change of ACTH secretion [23]. GR expression in BMAL1-/- mouse embryonic fibroblasts was reduced compared to the wildtype mouse embryonic fibroblasts [1]. In this study, GR expression in the hippocampus of BMAL1-knockdown mice under the constant darkness was decreased compared with WT mice. Treadmill exercise enhanced the expression of GR in the hippocampus of circadian rhythm disturbed mice (Fig. 2A).
GR is activated by binding to glucocorticoid hormone, and translocated to cell nuclei, then affects transcription through binding to glucocorticoid responsive element (GRE) in the promoter region of the target genes. During period of inactivity of the circadian cycle, low level of glucocorticoid results in a marked decrease in nuclear GR and GRE binding level [24]. Injection of GR antagonist into the dorsal hippocampus impaired long-term avoidance memory in rats through suppressing phospho-calcium calmodulin kinase II α, p-Akt, p-CREB, p-TrkB, p-ERK, and phospho-phospholipase Cγ [7]. For the memory consolidation, BDNF-dependent pathway is an important downstream effector of GR activation [5]. In this study, circadian rhythm disturbed mice showed reduced expressions of p-CREB, BDNF, TrkB, p-PI3 kinase p-85α, p-Akt, and p-Erk1/2 in the hippocampus with impaired memory function. Treadmill exercise increased the expressions of BDNF, TrkB, and facilitated the phosphorylation of CREB, PI3 kinase p85α, Akt, Erk1/2 in the hippocampus of circadian rhythm disturbed mice, contributing to improvement of memory function (Figs. 1-3).
Hippocampal BDNF mediates the exercise-induced enhancing effect on synaptic plasticity and cognition [11,12]. CREB potentiates consolidation and performance of memory through regulation of BDNF expression [25]. The beneficial effect of exercise on learning ability and memory function is due to activation of CREB and Akt in the hippocampus by exercise, which in turn culminates in the increase of BDNF [9]. Treadmill exercise improves spatial learning memory via increased phosphorylation of PI3K, Akt, and Erk1/2 in the hippocampus of autistic rats [26].
TrkB activates the PLCγ, which in turn, produces diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3) [27]. In particular, DAG activates PKC, and PKC activity plays a crucial role in diverse memories and cognitive function [27]. Especially, PKCα modulates the process of differentiation and regeneration of neurons, and PKCα is associated with the acquisition of learning [28]. Lin et al. [29] also suggested that running exercise improves learning ability and memory function against Alzheimer disease through enhancing expressions of p-PKC, p-TrkB, and p-Akt. In this study, treadmill exercise activated PKCα in the hippocampus of circadian rhythm disturbed mice, showing that decreased PKCα activity is associated with impaired memory function (Figs. 1, 4A).
Activation of hippocampal GR increases Egr-1 expression through enhancing enzymatic activity in the MAPK signaling pathway including phosphorylation of CREB [30]. Since Egr-1 is required for late LTP and long-term memory [31], Egr-1-knockout mice showed impairment of long-term memory [32]. In this study, treadmill exercise enhanced Egr-1 expression in the hippocampus of both TG mice and wildtype mice (Fig. 4B), which is consistent with increased expressions of BDNF, p-CREB, and p-Erk1/2 (Figs. 2B, 3A, and 3B).
BDNF and phosphorylated CREB promote new cell formation in the hippocampal dentate gyrus, and then contribute to memory consolidation and memory improvement [22,33]. BDNF promotes birth, survival, and maturation of new neurons in the hippocampus [33]. Phosphorylated CREB is associated with newly born neurons, such areas as hippocampal dentate gyrus or subventricular zone [22]. Treadmill exercise is known to increase the production of new neurons by increasing BDNF expression in the hippocampus, as a result, it achieves learning and memory enhancement [10,11]. In this study, consistent with reduced expressions of BDNF, TrkB, p-CREB, and p-Erk1/2 in the hippocampus of BMAL1-knockdown mice, circadian rhythm disturbed mice showed decreased neurogenesis. Treadmill exercise enhanced neurogenesis in the circadian rhythm disturbed mice (Fig. 5).
In the present results, circadian rhythm disruption impaired memory function through inhibiting the expressions of GR and various factors related to neurogenesis and memory consolidation, such as BDNF, p-CREB, p-Erk1/2, PI3K, p-Akt, p-PKC, and Egr-1in the hippocampus. Treadmill exercise effectively improved memory function via enhancing the expressions of GR and above-mentioned factors. Zeitgeber is a cue to reset the internal body clock, such as a change in light or temperature, given by the environment. Treadmill exercise acts as the zeitgeber that improves memory function under the circadian rhythm disrupted conditions.
Footnotes
Research Ethics
The procedure of this study followed the guidelines of Korean Academy of Medical Science and the regulations of National Institutes of Health (NIH) and. We obtained the approval from the Kyung Hee University Institutional Animal Care and Use Committee (Seoul, Korea) (KHUASP [SE]-18-096).
Conflict of Interest
KHK, an associate editor of INJ, is the corresponding author of this article. However, he played no role whatsoever in the editorial evaluation of this article or the decision to publish it. Except for that, no potential conflict of interest relevant to this article was reported.
Authors’ contribution
·Full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis: SEK, CJK
·Study concept and design: SEK, KHK
·Acquisition of data: IGK, ESJ, JJJ, LH, SHK
·Analysis and interpretation of data: IGK, ESJ, JJJ, LH, SHK
·Drafting of the manuscript: SEK
·Critical revision of the manuscript for important intellectual content: CJK
·Statistical analysis: IGK, ESJ, JJJ, LH, SHK
·Administrative, technical, or material support: KK, SC
·Study supervision: KHK
REFERENCES
- 1.Han DH, Lee YJ, Kim K, Kim CJ, Cho S. Modulation of glucocorticoid receptor induction properties by core circadian clock proteins. Mol Cell Endocrinol. 2014;383:170–80. doi: 10.1016/j.mce.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 2.Gerstner JR, Yin JC. Circadian rhythms and memory formation. Nat Rev Neurosci. 2010;11:577–88. doi: 10.1038/nrn2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hwang DS, Kwak HB, Ko IG, Kim SE, Jin JJ, Ji ES, et al. Treadmill exercise improves memory function depending on circadian rhythm changes in mice. Int Neurourol J. 2016;20(Suppl 2):S141–9. doi: 10.5213/inj.1632738.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smarr BL, Jennings KJ, Driscoll JR, Kriegsfeld LJ. A time to remember: the role of circadian clocks in learning and memory. Behav Neurosci. 2014;128:283–303. doi: 10.1037/a0035963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finsterwald C, Alberini CM. Stress and glucocorticoid receptor-dependent mechanisms in long-term memory: from adaptive responses to psychopathologies. Neurobiol Learn Mem. 2014;112:17–29. doi: 10.1016/j.nlm.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schoenfeld TJ, Gould E. Stress, stress hormones, and adult neurogenesis. Exp Neurol. 2012;233:12–21. doi: 10.1016/j.expneurol.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen DY, Bambah-Mukku D, Pollonini G, Alberini CM. Glucocorticoid receptors recruit the CaMKIIα-BDNF-CREB pathways to mediate memory consolidation. Nat Neurosci. 2012;15:1707–14. doi: 10.1038/nn.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tyler WJ, Alonso M, Bramham CR, Pozzo-Miller LD. From acquisition to consolidation: on the role of brain-derived neurotrophic factor signaling in hippocampal-dependent learning. Learn Mem. 2002;9:224–37. doi: 10.1101/lm.51202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aguiar AS, Jr, Castro AA, Moreira EL, Glaser V, Santos AR, Tasca CI, et al. Short bouts of mild-intensity physical exercise improve spatial learning and memory in aging rats: involvement of hippocampal plasticity via AKT, CREB and BDNF signaling. Mech Ageing Dev. 2011;132:560–7. doi: 10.1016/j.mad.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Cho JW, Jung SY, Lee SW, Lee SJ, Seo TB, Kim YP, et al. Treadmill exercise ameliorates social isolation-induced depression through neuronal generation in rat pups. J Exerc Rehabil. 2017;13:627–33. doi: 10.12965/jer.1735180.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SE, Ko IG, Shin MS, Kim CJ, Jin BK, Hong HP, et al. Treadmill exercise and wheel exercise enhance expressions of neutrophic factors in the hippocampus of lipopolysaccharide-injected rats. Neurosci Lett. 2013;538:54–9. doi: 10.1016/j.neulet.2013.01.039. [DOI] [PubMed] [Google Scholar]
- 12.Vivar C, Potter MC, van Praag H. All about running: synaptic plasticity, growth factors and adult hippocampal neurogenesis. Curr Top Behav Neurosci. 2013;15:189–210. doi: 10.1007/7854_2012_220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pastore S, Hood DA. Endurance training ameliorates the metabolic and performance characteristics of circadian Clock mutant mice. J Appl Physiol. 2013;114:1076–84. doi: 10.1152/japplphysiol.01505.2012. [DOI] [PubMed] [Google Scholar]
- 14.Son GH, Chung S, Choe HK, Kim HD, Baik SM, Lee H, et al. Adrenal peripheral clock controls the autonomous circadian rhythm of glucocorticoid by causing rhythmic steroid production. Proc Natl Acad Sci U S A. 2008;105:20970–5. doi: 10.1073/pnas.0806962106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung SY, Kim DY. Treadmill exercise improves motor and memory functions in cerebral palsy rats through activation of PI3K-Akt pathway. J Exerc Rehabil. 2017;13:136–42. doi: 10.12965/jer.1734964.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sungur AÖ, Stemmler L, Wöhr M, Rust MB. Impaired object recognition but normal social behavior and ultrasonic communication in cofilin1 mutant mice. Front Behav Neurosci. 2018;12:25. doi: 10.3389/fnbeh.2018.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin JJ, Ko IG, Kim SE, Hwang L, Lee MG, Kim DY, et al. Age-dependent differences of treadmill exercise on spatial learning ability between young- and adult-age rats. J Exerc Rehabil. 2017;13:381–6. doi: 10.12965/jer.1735070.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park HS, Kim TW. Paternal physical exercise improves spatial learning ability by enhancing hippocampal neuroplasticity in male pups born from obese maternal rats. J Exerc Rehabil. 2017;13:266–72. doi: 10.12965/jer.1734998.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eckel-Mahan KL, Phan T, Han S, Wang H, Chan GC, Scheiner ZS, et al. Circadian oscillation of hippocampal MAPK activity and cAMP: implications for memory persistence. Nat Neurosci. 2008;11:1074–82. doi: 10.1038/nn.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snider KH, Dziema H, Aten S, Loeser J, Norona FE, Hoyt K, et al. Modulation of learning and memory by the targeted deletion of the circadian clock gene Bmal1 in forebrain circuits. Behav Brain Res. 2016;308:222–35. doi: 10.1016/j.bbr.2016.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wardlaw SM, Phan TX, Saraf A, Chen X, Storm DR. Genetic disruption of the core circadian clock impairs hippocampus-dependent memory. Learn Mem. 2014;21:417–23. doi: 10.1101/lm.035451.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ortega-Martínez S. A new perspective on the role of the CREB family of transcription factors in memory consolidation via adult hippocampal neurogenesis. Front Mol Neurosci. 2015;8:46. doi: 10.3389/fnmol.2015.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leliavski A, Shostak A, Husse J, Oster H. Impaired glucocorticoid production and response to stress in Arntl-deficient male mice. Endocrinology. 2014;155:133–42. doi: 10.1210/en.2013-1531. [DOI] [PubMed] [Google Scholar]
- 24.Kitchener P, Di Blasi F, Borrelli E, Piazza PV. Differences between brain structures in nuclear translocation and DNA binding of the glucocorticoid receptor during stress and the circadian cycle. Eur J Neurosci. 2004;19:1837–46. doi: 10.1111/j.1460-9568.2004.03267.x. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki A, Fukushima H, Mukawa T, Toyoda H, Wu LJ, Zhao MG, et al. Upregulation of CREB-mediated transcription enhances both short- and long-term memory. J Neurosci. 2011;31:8786–802. doi: 10.1523/JNEUROSCI.3257-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seo TB, Cho HS, Shin MS, Kim CJ, Ji ES, Baek SS. Treadmill exercise improves behavioral outcomes and spatial learning memory through up-regulation of reelin signaling pathway in autistic rats. J Exerc Rehabil. 2013;9:220–9. doi: 10.12965/jer.130003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshii A, Constantine-Paton M. Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease. Dev Neurobiol. 2010;70:304–22. doi: 10.1002/dneu.20765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pascale A, Amadio M, Scapagnini G, Lanni C, Racchi M, Provenzani A, et al. Neuronal ELAV proteins enhance mRNA stability by a PKCα-dependent pathway. Proc Natl Acad Sci U S A. 2005;102:12065–70. doi: 10.1073/pnas.0504702102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin TW, Shih YH, Chen SJ, Lien CH, Chang CY, Huang TY, et al. Running exercise delays neurodegeneration in amygdala and hippocampus of Alzheimer’s disease (APP/PS1) transgenic mice. Neurobiol Learn Mem. 2015;118:189–97. doi: 10.1016/j.nlm.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Revest JM, Di Blasi F, Kitchener P, Rougé-Pont F, Desmedt A, Turiault M, et al. The MAPK pathway and Egr-1 mediate stress-related behavioral effects of glucocorticoids. Nat Neurosci. 2005;8:664–72. doi: 10.1038/nn1441. [DOI] [PubMed] [Google Scholar]
- 31.Jones MW, Errington ML, French PJ, Fine A, Bliss TV, Garel S, et al. A requirement for the immediate early gene Zif268 in the expression of late LTP and long-term memories. Nat Neurosci. 2001;4:289–96. doi: 10.1038/85138. [DOI] [PubMed] [Google Scholar]
- 32.Bozon B, Davis S, Laroche S. Regulated transcription of the immediate-early gene Zif268: mechanisms and gene dosage-dependent function in synaptic plasticity and memory formation. Hippocampus. 2002;12:570–7. doi: 10.1002/hipo.10100. [DOI] [PubMed] [Google Scholar]
- 33.Suri D, Vaidya VA. Glucocorticoid regulation of brain-derived neurotrophic factor: relevance to hippocampal structural and functional plasticity. Neuroscience. 2013;239:196–213. doi: 10.1016/j.neuroscience.2012.08.065. [DOI] [PubMed] [Google Scholar]