Abstract
Candida is an opportunistic pathogen and the most commonly isolated fungal genus in humans. Though Candida is often detected in respiratory specimens from humans with and without lung disease, its significance remains undetermined. While historically considered a commensal organism with low virulence potential, the status of Candida as an innocent bystander has recently been called into question by both clinical observations and animal experimentation. We here review what is currently known and yet to be determined about the clinical, microbiological and pathophysiological significance of the detection of Candida spp. in the human respiratory tract.
Keywords: Candida, colonization, respiratory tract
This is a review of what is currently known and yet to be determined about the clinical, microbiological and pathophysiological significance of the detection of Candida spp. in the human respiratory tract.
INTRODUCTION
Candida species (spp.) are, by far, the most common fungal pathogens in humans. Of the 8%–10% of all nosocomial infections caused by fungal pathogens, 80% are attributable to Candida spp. (Edwards 1991). Of the nearly 200 identified species of Candida, only 20 have been implicated as a cause of disease in humans (Williams et al. 2013). Despite a steady increase in the relative frequencies of infections attributed to non-Candida albicans species, C. albicans remains the most commonly identified pathogenic member of the genus. Despite its impressive potential for virulence, Candida spp. colonize the respiratory tract of half of all healthy individuals, the vast majority of whom are asymptomatic (Baum 1960). Candida spp. have historically been considered commensal constituents of normal human oral microbiota, with little significance attached to their detection in respiratory specimens. Yet in recent years, both animal experimentation and human observations have provided provocative evidence that Candida spp. may represent more than an innocent bystander, both in infectious and non-infectious disease states. The clinical significance of the detection of Candida spp. in the respiratory tract is increasingly uncertain. In this review, we summarize what is currently known—microbiologically, pathophysiologically and clinically—regarding Candida's significance in the respiratory tract, and highlight key areas in need of further study.
BLURRED LINES: CONTAMINATION, COMMENSALISM, COLONIZATION AND CANDIDIASIS
Candida is commonly identified as a constituent of normal human microbiota throughout the body. Frequently detected living on the human epidermis, it can also inhabit the gastrointestinal tract, the genitourinary tract of women and the respiratory tract (Kumamoto and Vinces 2005). Regardless of sampling site, no single test or threshold distinguishes whether the yeast is an artifact of sampling (contamination), benign and native to the patient's microbiota (commensalism), residing in a body site without causing active infection (colonization) or etiologic in an acute infection (candidasis). Instead, the detection of Candida must always be interpreted within its clinical and microbiological context.
Candida albicans—the most abundant and clinically significant representative of the Candida genus—has a variety of microbiological traits that equip it with adaptability to colonize the mucosa alongside the bacteria and exist in a ‘commensal’ or mutualistic state or to become pathogenic and invasive during disease. The presence and behavior of bacterial microbiota are believed to be key in determining Candida's relative virulence. Mucosal-associated bacteria prevent overgrowth of Candida via competition for epithelial cell adhesion sites, metabolic inference of hyphal transformation and induction of antimicrobial mechanisms (Huffnagle and Noverr 2013; Hofs, Mogavero and Hube 2016). In the absence of indigenous bacterial microbiota, Candida is able to increase in number, infect and invade epithelial surfaces.
Adherence of Candida to host surfaces is necessary for initial colonization, contributes to persistence within the host, and may also play an important role during the progression from colonization to infection. Changes in the expression of adherence ligands and receptors may play an important role in the shift from commensalism towards colonization or infection. While initial adhesion likely occurs between Candida in its yeast state and epithelial cells, adhesins expressed exclusively on hyphal cells are of great significance for ongoing adhesion (Zhu and Filler 2010; Moyes, Richardson and Naglik 2015). The ability of Candida to switch morphologies between a budding yeast and a filamentous hyphal form is its most important virulence trait, and is known to be regulated both by a variety of environmental stimuli as well as the presence of quorum-sensing molecules (Biswas, Van Dijck and Datta 2007; Han, Cannon and Villas-Boas 2011). Compared with its yeast state, the hyphal form of Candida exhibits increased adherence properties, invasiveness and greater pathogenicity (Kimura and Pearsall 1980; Odds 1988). The ability of Candida to secrete hydrolytic enzymes that digest molecules for nutrient acquisition and cause tissue damage also contributes to its virulence. These secreted enzymes contribute to host invasion by digesting host surface molecules to enhance adhesion or to distort host cell membranes allowing for deeper invasion (Cannon et al. 1995). Finally, loss of epithelial integrity or deficiencies in innate host immune mechanisms may allow for Candida colonization or infection to prevail. Prominent risk factors for acquisition of Candida in the respiratory tract include exposure to antibiotics, critical illness, immune compromised status, use of mechanical ventilation and hospital or intensive care unit stay (Table 1) (Fidel 2002; Perlroth, Choi and Spellberg 2007; Lalla et al. 2010; Moore et al. 2010; van de Veerdonk et al. 2011; Hofs, Mogavero and Hube 2016; Krause et al. 2016).
Table 1.
Major risk factors for acquisition of Candida in the respiratory tract.
| Host factors | Latrogenic causes | Immunosuppression | Extraneous |
|---|---|---|---|
| Genetic factors including (STAT1 and dectin-1 mutations) | Broad spectrum antibiotics | Neutropenia | Prolonged hospital stay |
| Mechanical ventilation | Steroid use | ICU stay | |
| HIV | Burns | ||
| Radiation therapy | Diabetes mellitus | ||
| Bone marrow or solid organ transplant | |||
| Use of systemic immunosuppression |
Though we have known for decades that Candida colonizes the mouth of even healthy individuals, both its temporal persistence within individuals and the extent of its presence in the lower respiratory tract remain unsettled questions. When Candida is isolated on a single occasion from a given host reservoir, clinicians cannot know with confidence if this represents transient presence or persistent colonization. Older studies attempted to address this question in specific regard to oral colonization of Candida and concluded that carriage was continuous in the overwhelming majority of subjects with Candida detected (Lilienthal 1950; Williamson 1972). In most current studies and practice, colonization is pragmatically defined as detection of Candida from one or more samples from a surveillance site; however, no consensus definition exists at this time. Rates of Candida carriage in the human mouth vary widely by study, ranging from 20% to 50% among asymptomatic healthy individuals, with even higher rate of carriage in hospitalized patients (Baum 1960; Morales and Hogan 2010). In an early and influential study by Baum (1960), the majority of hospitalized patients had Candida detectable in their sputum. The authors arrived at two key conclusions that have shaped the body of Candida literature since their time. First, since the healthy tracheobronchial tree was considered a sterile environment, they inferred that Candida detected in the sputum of healthy individuals must reflect contamination of sputum as it passes through the mouth during expectoration. Second, they concluded that due to the frequency of isolation of Candida albicans in both healthy and diseased subjects, little clinical significance could be attached to the finding of Candida in the sputum.
Regarding this first conclusion (the absence of Candida in the lungs of healthy individuals), our understanding of the sterility of the lower respiratory tract has been revolutionized in the past decade by the rise of culture-independent microbiology and the dawn of the lung microbiome field. Using culture-independent techniques, numerous groups have detected diverse communities of microbes in the lungs of healthy individuals, and features of the lung microbiome have been correlated with the presence and prognosis of numerous respiratory diseases (Dickson et al. 2016). These studies, largely focused on bacteria, have prompted reconsideration of the assumption that Candida and other fungi are absent from healthy lungs. Recent studies have used molecular techniques to re-address whether the finding of Candida in the oral cavity reflects Candida in the lower respiratory tract. In a recent study by Krause et al. (2016), detection of Candida in the oral cavity correlated poorly with its detection in the lower respiratory tract as sampled directly from either endobronchial tubes or bronchoscopically. Despite detection of Candida from the oral specimens of 29% of healthy volunteers, Candida was essentially absent from the lower respiratory tract of healthy subjects, whether tested using conventional culture (87 subjects) or culture-independent means (four subjects). Thus, despite the increased sensitivity of modern culture-independent techniques, we still have no direct evidence that Candida is present in the lower respiratory tract of healthy individuals.
METHODS USED IN ISOLATION AND IDENTIFICATION OF CANDIDA
Currently, clinical laboratory identification of Candida in the respiratory tract relies upon the successful recovery of species from culture. No culture-based or molecular test of respiratory specimens can distinguish between contamination, colonization and invasive disease. Despite ongoing advances in culture-independent techniques for identification of other respiratory pathogens, fungal culture remains the ‘gold standard’ for the identification of Candida spp., regardless of sampling site. Candida spp. grow well on a variety of commonly used culture media, but most frequently Sabouraud's glucose agar and broth are used. Colonies of Candida generally grow between 25°C and 37°C and have a smooth to wrinkled texture and a white to beige color on Sabouraud's agar (Calderone 2002). Media containing chromogenic compounds may be useful in the detection of clinically important Candida spp. including C. albicans, C. tropicalis, C. glabrata, C. krusei and C. dubliniensis as colonies grown on such agars may be distinguished according to color (Odds and Bernaerts 1994). While culture can provide both definitive identification and speciation of a fungus, it lacks sensitivity, and time to fungal growth is lengthy. Additional factors affecting isolation of Candida spp. from respiratory specimens include previous exposure to antimicrobial therapy as well as technical factors including volume of sample, culture media used, incubation time and temperature (World Health Organization 2000). Direct microscopic examination, another method of detection for samples such as bronchoalveolar lavage (BAL) fluid, is more sensitive than culture, but utilization is limited as it requires expertise for interpretations and distinguishes poorly among fungi with similar morphology.
Given the poor sensitivity of culture, studies have begun to investigate non-culture-based modalities for identification of Candida in the respiratory tract. The 1,3-ß-D-glucan assay detects soluble fungal wall components released during fungal growth and division (Bowman and Free 2006). It has demonstrated decent performance as a screening test for invasive fungal infections within the bloodstream, but its utility in BAL fluid is unproven. The largest relevant study to date, a retrospective review, demonstrated poor consistency and specificity using 1,3-ß-D-glucan for the detection of invasive fungal infections of the lung in BAL specimens (Rose et al. 2014). Furthermore, the 1,3-ß-D-glucan assay is unable to distinguish between Candida and other fungi, further limiting its clinical utility as a diagnostic test. Additional studies have examined the use of latex agglutination in BAL fluid to test for the presence of Candida antigens as well as the use of polymer-based chain reaction (PCR) for molecular identification of Candida isolates with mixed results (Ness et al. 1988; Zarrinfar et al. 2016). Currently, no methods for the identification of Candida in the respiratory tract, aside from standard culture, have garnered enough evidence or momentum to be adopted into clinical practice. Further investigations into methods of reliably identifying Candida within a timely fashion, or distinguishing between colonization or invasive Candidiasis, would be a welcome contribution to the field.
DOES CANDIDA CAUSE PNEUMONIA?
True invasive Candida pneumonia is so rare in immunocompetent patients that its very existence is debated. When it occurs, it is attributed either to seeding of the lungs from hematogenous dissemination or (less likely) to aspiration of colonized oropharyngeal or gastric contents (Meersseman et al. 2009). Definitive diagnosis of pulmonary Candida infection depends on histologic demonstration of yeast as well as inflammatory cells in lung tissue. The diagnosis of Candida pneumonia is challenging, as histology is rarely available clinically, and less invasive means fail to distinguish infection (a rarity) from colonization (which is common). Numerous studies have demonstrated that the incidence of Candida isolation from pulmonary biopsies or BAL fluid in critically ill mechanically ventilated patients is around 40%–50%, whereas the incidence of true Candida pneumonia is considerably lower (el-Ebiary et al. 1997; Meersseman et al. 2009; Hamet et al. 2012). An informative study by el-Ebiary et al. (1997) demonstrated that Candida colonization of the lungs occurs in roughly 40% of immunocompetent critically ill patients, based on rigorous post-mortem histologic examination. Comparison of tissue diagnoses with pre-mortem, clinically available information was revealing: only 9% of BAL cultures from the same patients grew Candida, and only 2 patients out of 25 in the study had definitive Candida pneumonia confirmed by histology. Two other prospective studies have confirmed the frequency of Candida spp. colonization in mechanically ventilated critically ill patients (present in more than half (53%–56%) of patients (Meersseman et al. 2009; Hamet et al. 2012) while showing that true Candida pneumonia is rarely—if ever—present histologically.
CANDIDA: MERELY AN INNOCENT BYSTANDER?
Based on these clinical observations, and aligned with the second conclusion of Baum et al. (that little significance can be attached to the finding of Candida in the sputum of healthy or diseased patients), Candida pneumonia is rare if ever present, and when Candida is detected in respiratory specimens it is generally considered an ‘innocent bystander’ (Ricard and Roux 2012). Yet recent experimental and clinical observations have suggested that even if not directly pathogenic, Candida may still contribute indirectly to respiratory disease. In their study of 803 patients across six ICUs, Azoulay et al. (2006) studied the significance of Candida isolation from respiratory specimens in critically ill patients. Respiratory tract Candida colonization was associated with a longer duration of mechanical ventilation and increased length of ICU and hospital stay. Provocatively, they found a significant, independent association between respiratory tract Candida colonization and ventilator-associated pneumonia due to Pseudomonas aeruginosa. Interestingly, no association was found between Candida colonization and other bacterial pathogens that commonly cause ventilator-associated pneumonias, a finding which may be attributed to the small number of patients included in that analysis. A subsequent retrospective analysis performed on 639 patients with suspected VAP revealed a significant increase in median hospital stay and mortality in patients who harbored Candida in their respiratory tract when compared to those that did not (Delisle et al. 2008). Detection of Candida in respiratory specimens was independently associated with a 2.5-fold increase in mortality. A similar retrospective analysis again confirmed that among patients with suspected VAP, detection of Candida in the absence of bacterial pathogens was predictive of increased ICU and hospital mortality, longer duration of mechanical ventilation and increased hospital length of stay (Delisle et al. 2011). The same association between Candida isolation and mortality was found by Hamet et al. (2012); furthermore, Candida colonization was an independent predictor for co-isolation of multidrug-resistant bacteria. These effects could not be ascribed to invasive Candida infection, as none were observed during the study periods.
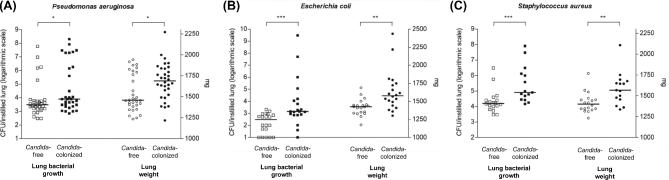
Why then, if Candida does not cause pneumonia, does detection of Candida in the respiratory tract of critically ill patients consistently portent increased morbidity and mortality? An intuitive explanation is that Candida colonization is the proverbial canary in the coal mine: a marker of disease severity and dysregulation of the host immune system. Yet a myriad of animal studies examining fungal–bacterial mixed inoculation suggest a more direct role in disease pathogenesis. The findings of a representative study by Roux et al. are shown in Fig. 1. In rodent models of pneumonia, a given inoculum of P. aeruginosa in the respiratory tract unable to cause bacterial pneumonia on its own can indeed provoke infection when co-instilled with C. albicans (Roux et al. 2009). This effect is not observed when ethanol-killed C. albicans is inoculated, indicating that Candida must be alive to potentiate P. aeruginosa's virulence. This observation has held true across bacterial pathogens, including Staphylococcus aureus and Escherichia coli (Roux et al. 2013) (Fig. 1).
Figure 1.
Candida colonization of the airway potentiates bacterial pneumonia in a rat model. Pseudomonas aeruginosa (A), E. coli (B) and S. aureus (C) were instilled into the tracheas of rats with and without prior instillation of C. albicans. With all three pathogens, C. albicans significantly worsened both bacterial burden and lung injury. Figure reproduced from Roux et al.
The notion of a synergistic effect between Candida species and bacterial pathogens has been explored previously. While both in vivo and in vitro studies have lent plausibility to a synergistic relationship between C. albicans and P. aeruginosa, the relationship is not limited to these two pathogens (Hogan and Kolter 2002; Peleg, Hogan and Mylonakis 2010). Early studies in mice found that dual infection with C. ablicans and S. aureus led to overall increased mortality when compared to infection with either pathogen in isolation (Carlson 1982). More recent work has examined the synergistic relationship between S. aureus and C. albicans as it pertains biofilm formation. Staphylococcus aureus more readily forms biofilms in the presence of C. albicans than in its absence, and more readily demonstrates antibiotic resistance within these polymicrobial biofilms (Harriott and Noverr 2009).
The mechanisms behind Candida's potentiation of bacterial pneumonia in animal models remain incompletely understood despite burgeoning research into the effects of Candida colonization on host immunity. Airway colonization with Candida alone is associated with increased levels of TNF-alpha and IFN-gamma within the lung, even in the histological absence of acute infection (Roux et al. 2009). Co-inoculation with Candida and P. aeruginosa further elevate alveolar concentrations of TNF-alpha, IFN-gamma and IL-6. As IFN-gamma is capable of impairing function of alveolar macrophages (Sun and Metzger 2008), some have hypothesized that the presence of C. albicans in the airways can induce an immune response that inhibits the normal antibacterial function of host immune cells, allowing bacterial pathogens to evade clearance and initiate infection.
The interaction between Candida and bacterial respiratory infection is not limited to mechanically ventilated, critically ill patients nor animal models of pneumonia. A growing body of literature in cystic fibrosis (CF) patients has shown that Candida is the dominant fungal genus comprising the respiratory microbiome of these patients, and that of this genus C. albicans is the dominant species. To date, two longitudinal studies examining the effects of respiratory colonization with Candida in CF patients have been conducted with similar results. Colonization with C. albicans occurs commonly (∼50%) in CF patients and is associated with the presence of pancreatic insufficiency, osteopenia or co-colonization with P. aeruginosa (Chotirmall et al. 2010). Chronic respiratory colonization with C. albicans is associated with impaired lung function, the trajectory of worsening lung function, unintentional weight loss and increased frequency of hospital-treated exacerbations (Gileles-Hillel et al. 2015).
TO TREAT OR NOT TO TREAT?
Despite this considerable literature demonstrating that detection of Candida in the respiratory tract is associated with increased risk of bacterial pneumonia, multidrug-resistant pathogens, and morbidity and mortality, the clinical utility of eradication of Candida with antifungal drugs remains a matter of debate. Animal studies have demonstrated that antifungal treatment in the setting of respiratory colonization with Candida significantly decreased susceptibility to bacterial pneumonia with attenuated lung inflammation (Venkatesh et al. 2007; Roux et al. 2013). A second study, in neonatal rats septic from co-infection with Candida and Staphylococcus epidermidis, showed enhanced survival in the setting of fluconazole prophylaxis (Venkatesh et al. 2007). These promising results from experimental models of pneumonia have prompted retrospective and prospective human studies to determine the clinical utility of treating respiratory Candida with antifungal therapy.
Retrospective data on the question have proven provocative but conflicting. A French study of 102 mechanically ventilated patients with tracheobronchial Candida colonization found that antifungal therapy was the only variable independently associated with significantly reduced risk of development of Pseudomonas aeruginosa pneumonia (Nseir et al. 2007). Yet a larger German study instead found that patients treated with antifungal therapy had higher in-hospital mortality and pneumonia rates than those who did not (Lindau et al. 2015). A recent third study found no association between antifungal therapy and favorable clinical outcome (Terraneo et al. 2016). These retrospective and non-interventional studies are inescapably limited by potential confounding: patients who receive antifungal therapy are quite certainly subject to treatment bias, as patients harboring respiratory tract Candida disproportionately have risk factors for invasive fungal infection, immunosuppression or higher severity of illness. In the setting of these conflicting and confounded results, no firm treatment guidelines have been established, and clinical practice varies widely.
In light of the discrepancy between animal and clinical studies assessing the utility of antifungal drug use in the setting Candida airway colonization in ICU patients, a feasibility study was recently undertaken. The CANTREAT trial is the largest randomized, placebo-controlled trial to date aimed at evaluating inflammatory profiles and clinical outcomes in Candida colonized patients with clinically suspected VAP (Albert and Heyland 2014). It enrolled a total of 89 patients and was designed to determine the feasibility of a large-scale, randomized controlled trial. At baseline, TNF-alpha levels were higher in patients with suspected VAP in the setting of Candida colonization compared to those with clinically suspected VAP in the absence of Candida airway colonization. Following at least 72 h of antifungal therapy or placebo, no differences were found in either inflammatory marker profiles or hospital mortality between the groups. Unfortunately, the study was discontinued prior to reaching target enrollment due to slow recruitment, and the authors concluded that a larger randomized controlled trial was likely not feasible. As outlined by other critics of the study, the CANTREAT trial had many limitations (Roux and Ricard 2014). First, the study was designed for feasibility, not to definitively answer this clinical question, and was thus underpowered for this purpose. Furthermore, patients enrolled already had presumed VAP in the setting of Candida airway colonization; thus, its results tell us nothing regarding the effects of antifungal therapy in preventing or attenuating early VAP in at-risk patients. At this time, no prospective studies have been undertaken to evaluate this question.
CONCLUSIONS
Though provocative animal experimentation suggests that Candida is more than an innocent bystander in the respiratory tract, no clinical data to date provides support for its treatment in colonized patients. Further study into fungal–bacterial interactions and the potentiation of bacterial pneumonia are needed, and prospective human studies into the efficacy of treating colonization are justified. The final word on Candida's significance in the respiratory tract has yet to be spoken.
Conflict of interest. None declared.
REFERENCES
- Albert M, Heyland D. Candida in the respiratory tract secretions of critically ill patients and the impact of antifungal treatment: reply to Roux and Ricard. Intensive Care Med 2014;40:1613. [DOI] [PubMed] [Google Scholar]
- Azoulay E, Timsit JF, Tafflet M et al. Candida colonization of the respiratory tract and subsequent pseudomonas ventilator-associated pneumonia. Chest 2006;129:110–7. [DOI] [PubMed] [Google Scholar]
- Baum GL. The significance of Candida albicans in human sputum. New Engl J Med 1960;263:70–3. [DOI] [PubMed] [Google Scholar]
- Biswas S, Van Dijck P, Datta A. Environmental sensing and signal transduction pathways regulating morphopathogenic determinants of Candida albicans. Microbiol Mol Biol R 2007;71:348–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman SM, Free SJ. The structure and synthesis of the fungal cell wall. Bioessays 2006;28:799–808. [DOI] [PubMed] [Google Scholar]
- Calderone RA. Candida and Candidiasis. Washington D.C.: ASM Press, 2002. [Google Scholar]
- Cannon RD, Holmes AR, Mason AB et al. Oral Candida: clearance, colonization, or candidiasis? J Dent Res 1995;74:1152–61. [DOI] [PubMed] [Google Scholar]
- Carlson E. Synergistic effect of Candida albicans and Staphylococcus aureus on mouse mortality. Infect Immun 1982;38:921–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chotirmall SH, O'Donoghue E, Bennett K et al. Sputum Candida albicans presages FEV(1) decline and hospital-treated exacerbations in cystic fibrosis. Chest 2010;138:1186–95. [DOI] [PubMed] [Google Scholar]
- Delisle MS, Williamson DR, Perreault MM et al. The clinical significance of Candida colonization of respiratory tract secretions in critically ill patients. J Crit Care 2008;23:11–7. [DOI] [PubMed] [Google Scholar]
- Delisle MS, Williamson DR, Albert M et al. Impact of Candida species on clinical outcomes in patients with suspected ventilator-associated pneumonia. Can Respir J 2011;18:131–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson RP, Erb-Downward JR, Martinez FJ et al. The Microbiome and the Respiratory Tract. Annu Rev Physiol 2016;78:481–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards JE Jr. Invasive candida infections–evolution of a fungal pathogen. New Engl J Med 1991;324:1060–2. [DOI] [PubMed] [Google Scholar]
- el-Ebiary M, Torres A, Fabregas N et al. Significance of the isolation of Candida species from respiratory samples in critically ill, non-neutropenic patients. An immediate postmortem histologic study. Am J Resp Crit Care 1997;156(2 Pt 1):583–90. [DOI] [PubMed] [Google Scholar]
- Fidel PL Jr. Distinct protective host defenses against oral and vaginal candidiasis. Med Mycol 2002;40:359–75. [PubMed] [Google Scholar]
- Gileles-Hillel A, Shoseyov D, Polacheck I et al. Association of chronic Candida albicans respiratory infection with a more severe lung disease in patients with cystic fibrosis. Pediatr Pulmonol 2015;50:1082–9. [DOI] [PubMed] [Google Scholar]
- Hamet M, Pavon A, Dalle F et al. Candida spp. airway colonization could promote antibiotic-resistant bacteria selection in patients with suspected ventilator-associated pneumonia. Intensive Care Med 2012;38:1272–9. [DOI] [PubMed] [Google Scholar]
- Han TL, Cannon RD, Villas-Boas SG. The metabolic basis of Candida albicans morphogenesis and quorum sensing. Fungal Genet Biol 2011;48:747–63. [DOI] [PubMed] [Google Scholar]
- Harriott MM, Noverr MC. Candida albicans and Staphylococcus aureus form polymicrobial biofilms: effects on antimicrobial resistance. Antimicrob Agents Ch 2009;53:3914–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofs S, Mogavero S, Hube B. Interaction of Candida albicans with host cells: virulence factors, host defense, escape strategies, and the microbiota. J Microbiol 2016;54:149–69. [DOI] [PubMed] [Google Scholar]
- Hogan DA, Kolter R. Pseudomonas-Candida interactions: an ecological role for virulence factors. Science 2002;296:2229–32. [DOI] [PubMed] [Google Scholar]
- Huffnagle GB, Noverr MC. The emerging world of the fungal microbiome. Trends Microbiol 2013;21:334–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura LH, Pearsall NN. Relationship between germination of Candida albicans and increased adherence to human buccal epithelial cells. Infect Immun 1980;28:464–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause R, Halwachs B, Thallinger GG et al. Characterisation of Candida within the mycobiome/microbiome of the lower respiratory tract of ICU patients. PLoS One 2016;11:e0155033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto CA, Vinces MD. Alternative Candida albicans lifestyles: growth on surfaces. Annu Rev Microbiol 2005;59:113–33. [DOI] [PubMed] [Google Scholar]
- Lalla RV, Latortue MC, Hong CH et al. A systematic review of oral fungal infections in patients receiving cancer therapy. Support Care Cancer 2010;18:985–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilienthal B. Studies of the flora of the mouth. III. Yeast-like organisms: some observations on their incidence in the mouth. Aust J Exp Biol Med 1950;28:279–86. [PubMed] [Google Scholar]
- Lindau S, Nadermann M, Ackermann H et al. Antifungal therapy in patients with pulmonary Candida spp. colonization may have no beneficial effects. J Intensive Care 2015;3:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meersseman W, Lagrou K, Spriet I et al. Significance of the isolation of Candida species from airway samples in critically ill patients: a prospective, autopsy study. Intensive Care Med 2009;35:1526–31. [DOI] [PubMed] [Google Scholar]
- Moore EC, Padiglione AA, Wasiak J et al. Candida in burns: risk factors and outcomes. J Burn Care Res 2010;31:257–63. [DOI] [PubMed] [Google Scholar]
- Morales DK, Hogan DA. Candida albicans interactions with bacteria in the context of human health and disease. PLoS Pathog 2010;6:e1000886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyes DL, Richardson JP, Naglik JR. Candida albicans-epithelial interactions and pathogenicity mechanisms: scratching the surface. Virulence 2015;6:338–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ness MJ, Rennard SI, Vaughn WP et al. Detection of Candida antigen in bronchoalveolar lavage fluid. Acta Cytol 1988;32:347–52. [PubMed] [Google Scholar]
- Nseir S, Jozefowicz E, Cavestri B et al. Impact of antifungal treatment on Candida-Pseudomonas interaction: a preliminary retrospective case-control study. Intensive Care Med 2007;33:137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odds FC. Candida and Candidosis. Bailliere Tindall, London: Elsevier Science Health Science Division; 1988. [Google Scholar]
- Odds FC, Bernaerts R. CHROMagar Candida, a new differential isolation medium for presumptive identification of clinically important Candida species. J Clin Microbiol 1994;32:1923–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg AY, Hogan DA, Mylonakis E. Medically important bacterial-fungal interactions. Nat Rev Microbiol 2010;8:340–9. [DOI] [PubMed] [Google Scholar]
- Perlroth J, Choi B, Spellberg B. Nosocomial fungal infections: epidemiology, diagnosis, and treatment. Med Mycol 2007;45:321–46. [DOI] [PubMed] [Google Scholar]
- Ricard JD, Roux D. Candida colonization in ventilated ICU patients: no longer a bystander! Intensive Care Med 2012;38:1243–5. [DOI] [PubMed] [Google Scholar]
- Rose SR, Vallabhajosyula S, Velez MG et al. The utility of bronchoalveolar lavage beta-D-glucan testing for the diagnosis of invasive fungal infections. J Infect 2014;69:278–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux D, Gaudry S, Dreyfuss D et al. Candida albicans impairs macrophage function and facilitates Pseudomonas aeruginosa pneumonia in rat. Crit Care Med 2009;37:1062–7. [DOI] [PubMed] [Google Scholar]
- Roux D, Gaudry S, Khoy-Ear L et al. Airway fungal colonization compromises the immune system allowing bacterial pneumonia to prevail. Crit Care Med 2013;41:e191–9. [DOI] [PubMed] [Google Scholar]
- Roux D, Ricard JD. Ask the wrong question, you’ll the get the wrong answer. Intensive Care Med 2014;40:1611–2. [DOI] [PubMed] [Google Scholar]
- Sun K, Metzger DW. Inhibition of pulmonary antibacterial defense by interferon-gamma during recovery from influenza infection. Nat Med 2008;14:558–64. [DOI] [PubMed] [Google Scholar]
- Terraneo S, Ferrer M, Martin-Loeches I et al. Impact of Candida spp. isolation in the respiratory tract in patients with intensive care unit-acquired pneumonia. Clin Microbiol Infect 2016;22:94 e91–8. [DOI] [PubMed] [Google Scholar]
- van de Veerdonk FL, Plantinga TS, Hoischen A et al. STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. New Engl J Med 2011;365:54–61. [DOI] [PubMed] [Google Scholar]
- Venkatesh MP, Pham D, Fein M et al. Neonatal coinfection model of coagulase-negative Staphylococcus (Staphylococcus epidermidis) and Candida albicans: fluconazole prophylaxis enhances survival and growth. Antimicrob Agents Ch 2007;51:1240–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DW, Jordan RP, Wei XQ et al. Interactions of Candida albicans with host epithelial surfaces. J Oral Microbiol 2013;5, 10.3402/jom.v5i0.22434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson JJ. A study of extent of variation in daily counts of Candida albicans in saliva. Aust Dent J 1972;17:106–9. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Guidelines on Standard Operating Procedures for Microbiology, Geneva: 2000. [Google Scholar]
- Zarrinfar H, Kaboli S, Dolatabadi S et al. Rapid detection of Candida species in bronchoalveolar lavage fluid from patients with pulmonary symptoms. Braz J Microbiol 2016;47:172–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Filler SG. Interactions of Candida albicans with epithelial cells. Cell Microbiol 2010;12:273–82. [DOI] [PMC free article] [PubMed] [Google Scholar]