Abstract
Background
Liver transplantation for patients with non‐resectable colorectal liver metastases offers increased survival, with median overall survival of more than 5 years. The aim of this study was to compare quality of life before and up to 3 years after liver transplantation for colorectal liver metastases.
Methods
Quality of life was assessed using the European Organisation for Research and Treatment of Cancer QLQ‐C30 questionnaire version 3.0. The patients received the questionnaire before and up to 3 years after liver transplantation.
Results
Some 23 patients were included in the analysis. Three months after liver transplantation they reported reduced quality of life (global health status scale), physical function and role function, and increased dyspnoea. At 6 months, global health status, physical function and role function had returned to pretransplant values. Three years after liver transplantation all symptom and function scores were comparable to baseline values. Patients with high scores for fatigue, pain and appetite loss at baseline had reduced 3‐year overall survival.
Conclusion
Patients with non‐resectable colorectal liver‐only metastases receiving liver transplantation had good long‐term quality of life. Patients with high symptom scores before transplantation had reduced 3‐year overall survival.
Introduction
Colorectal cancer is one of the most common malignancies in Western countries and a leading cause of cancer‐related death1. Many of these patients present with or develop metastases, most commonly affecting the liver2. Hepatic resection is considered the only curative treatment, with a reported 5‐year overall survival (OS) rate after resection of about 40 per cent. Only about 20 per cent of patients with colorectal liver metastases (CRLM), however, are candidates for liver surgery and the majority will develop further recurrences3. The standard treatment option for most patients with metastatic disease from colorectal cancer is palliative chemotherapy, with median OS of about 2 years from the start of chemotherapy and a 5‐year OS rate of about 10 per cent4. Progression‐free survival after the start of first‐line chemotherapy is less than 12 months5. Patients with liver metastases that become resectable after chemotherapy have increased survival compared with those who have non‐resectable disease6. Improved response rates to chemotherapy regimens have been associated with increased resection rates, and better progression‐free survival and OS. This may be further enhanced by the use of antiepidermal growth factor receptor antibodies7 8.
Liver transplantation (LT) is the standard treatment in patients with end‐stage liver failure, and is offered widely to selected patients with primary liver cancers and liver metastasis from neuroendocrine tumours9, 10, 11, 12, 13, 14. LT for malignant tumours accounts for about 16 per cent of all LTs in the European Liver Transplant Registry15. The shortage of donor livers led to the abandonment of LT for CRLM owing to poor survival16 17. The present authors18 have previously reported a 5‐year OS rate of 56 per cent in patients with non‐resectable colorectal metastases confined to the liver receiving LT, compared with 9 per cent in patients treated with chemotherapy. LT is a major surgical procedure and major postoperative complications have been described after transplantation in patients with colorectal cancer19. Whether LT has a negative impact on quality of life (QoL) has not been determined.
Liver resection and treatment of peritoneal metastases with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy result in reduced QoL lasting 3–6 months after treatment20 21. Before LT is offered to selected patients with non‐resectable colorectal cancer it is important to document that LT does not result in long‐term reduction in QoL. Short‐term QoL results for ten patients followed for up to 12 months after LT have been described22. The present study sought to describe long‐term QoL after LT in patients with non‐resectable CRLM without extrahepatic disease, based on assessments at inclusion and up to 3 years after LT of all 23 patients included in the LT trial (SECA‐I study).
Methods
The SECA‐I study was an open prospective pilot study of LT in patients with non‐resectable liver‐only metastases from colorectal cancer. The study obtained approval from the Regional Ethics Committee and Institutional Review Board, and was registered in ClinicalTrials.gov (NCT01311453) before inclusion of patients. The primary endpoint of the study was OS at 2 years after LT; secondary endpoints included disease‐free survival and QoL evaluation. The first patient was transplanted in November 2006 and the last included patient in April 2012. The inclusion criteria have been described previously23. The main inclusion criteria were patients with non‐resectable CRLM without extrahepatic disease and good performance status (Eastern Cooperative Oncology Group grade 0–1). The immunosuppressive treatment used in the study comprised induction with basiliximab (interleukin 2 receptor antibody) and thereafter patients were maintained on an immunosuppressive regimen containing sirolimus (mTOR inhibitor), mycophenolate mofetil (inosine monophosphate dehydrogenase inhibitor) and corticosteroids. Corticosteroid treatment was tapered to zero in the course of the first 6 months after surgery.
QoL was assessed at baseline, and 3, 6, 12, 18, 24, 30 and 36 months after LT, using the European Organisation for Research and Treatment of Cancer (EORTC) QLQ‐C30 questionnaire version 3.0. The results obtained at the different time points were compared with baseline values. EORTC QLQ‐C30 is a self‐administered and multidimensional questionnaire that contains 30 items covering health issues relevant to patients with cancer; it includes a two‐item global health status scale (GHS), five function scales (physical, cognitive, emotional, social and role), three symptom scales (fatigue, pain and nausea/vomiting) and six single items (dyspnoea, sleep disturbance, appetite loss, constipation, diarrhoea and financial impact). The responses were transformed linearly to range from 0 to 100, and related items were transformed to function or symptom scales according to the manual24. A high function score indicates good function, whereas a high symptom score indicates more symptoms. A change in 10 points or more on the 0–100 scale was considered clinically significant24. The EORTC QLQ‐C30 has been translated into several languages and tested for psychometric properties in a number of countries, including Norway24. Questionnaires were completed by the patients before surgery (baseline) and were responded to by mail at the time points after transplantation. Patients who did not return questionnaires were reminded by a telephone call.
Statistical analysis
Data were registered continuously in case report forms. QoL data are presented as mean values, and differences over time were evaluated by non‐parametric related‐samples Wilcoxon signed‐rank test. Between‐group differences were determined by independent‐samples t test. Survival data were estimated using the Kaplan–Meier method, and outcomes between groups compared using log rank tests. For all tests, two‐sided P < 0·050 was considered statistically significant. Analyses were performed using SPSS® version 21 (IBM, Armonk, New York, USA).
Results
A total of 23 patients received LT according to the study protocol in the SECA‐I study. Patient characteristics are shown Table 1. In general, at inclusion the patients had good GHS and function scores with low symptom scores, although they had considerable liver metastases (Table 1).
Table 1.
Baseline characteristics of patients in SECA‐1 trial
| No. of patients* (n = 23) | |
|---|---|
| Age (years)† | 54·7 (44·5–64·7) |
| Sex ratio (F : M) | 10 : 13 |
| Tumour site | |
| Colon | 13 |
| Rectum | 10 |
| Timing of metastasis | |
| Synchronous | 19 |
| Metachronous | 4 |
| Liver resection before LT | 4 |
| Chemotherapy before LT (no. of lines) | |
| 1 | 10 |
| 2 | 9 |
| 3 | 4 |
| > 10 liver metastases | 8 |
| Largest lesion > 5 cm | 10 |
| CEA > 5 μg/l | 14 |
Unless indicated otherwise.
Values are median (range). LT, liver transplantation; CEA, carcinoembryonic antigen.
Quality of life
The numbers of patients responding to the questionnaire of patients alive at baseline, 3, 6, 12, 18, 24, 30 and 36 months were 23 of 23, 22 of 23, 21 of 23, 22 of 22, 21 of 21, 19 of 21, 15 of 18 and 16 of 16 respectively.
At 3 months after LT, the patients had a significant and at least 10‐point decrease in mean GHS, physical function and role function scores (Fig. 1). Of the 23 patients, ten and 12 had decreases of 10 points or more in GHS and physical function scores respectively. Among the ten patients with such decreases in GHS score, four had Clavien–Dindo complication grades III–IV, whereas seven of 12 patients with decreased physical function scores had grades III–IV. Patients also reported significant and clinically relevant worsening in the symptom score for dyspnoea (Fig. 2).
Figure 1.
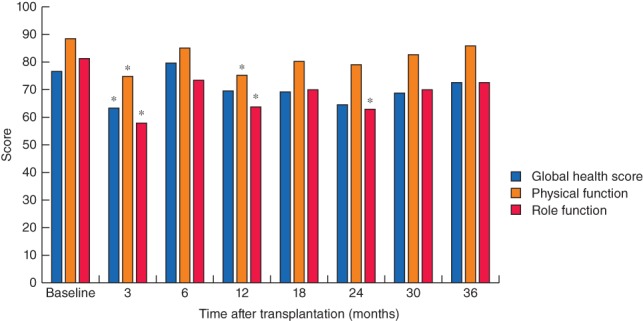
Mean global health, physical function and role function scores at baseline and up to 3 years after liver transplantation. Number of patients responding to the quality‐of‐life questionnaire at each time point: baseline, 23 of 23; 3 months, 22 of 23; 6 months, 21 of 23; 12 months, 22 of 22; 18 months, 21 of 21; 24 months, 19 of 21; 30 months, 15 of 18; and 36 months, 16 of 16. *P < 0·050 versus baseline (Wilcoxon signed‐rank test)
Figure 2.
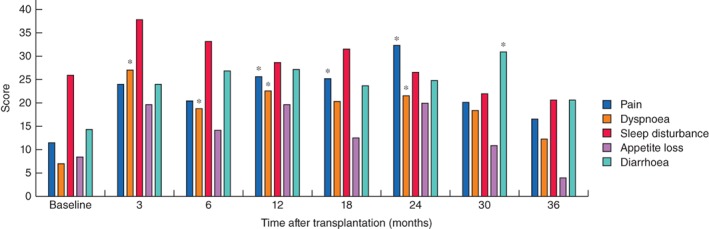
Symptom scores for pain, dyspnoea, sleep disturbance, appetite loss and diarrhoea at baseline and up to 3 years after liver transplantation. Number of patients responding to the quality‐of‐life questionnaire at each time point: baseline, 23 of 23; 3 months, 22 of 23; 6 months, 21 of 23; 12 months, 22 of 22; 18 months, 21 of 21; 24 months, 19 of 21; 30 months, 15 of 18; and 36 months, 16 of 16. *P < 0·050 versus baseline (Wilcoxon signed‐rank test)
GHS, physical function and role function scores were comparable to baseline scores (difference less than ± 10 points) at 6 months after LT (Fig. 1). Although GHS and role function scores were reduced by more than 10 points at some times during follow‐up, by 3 years the reported scores on the GHS and different function scales were similar to baseline values (Fig. 1). Cognitive, emotional and social function scores were also similar to baseline values at all time points after LT (data not shown).
At 6 months there was no clinically relevant and statistical difference compared with baseline in sleep disturbance, appetite loss, pain and diarrhoea, but the symptom score for dyspnoea was still worse than baseline values (Fig. 2). The mean values for pain, dyspnoea and diarrhoea were increased by more than 10 points at some time points from 12 to 30 months after LT. At 3 years after transplantation, these symptom scores were similar to baseline values. Symptom scores for fatigue, nausea/vomiting, constipation and financial impact were no different from baseline scores at any time point following LT (data not shown).
Quality of life and survival
Seven patients died within 3 years of LT. Symptom scores for fatigue, pain and appetite loss at baseline were all significantly related to OS at 3 years after LT. Patients who died within 3 years after transplantation had significantly higher scores for all these symptoms at baseline. For patients who had died or were alive 3 years after LT, baseline scores were 39·7 and 21·5 respectively for fatigue (P = 0·033), 23·8 and 6·3 for pain (P = 0·032), and 23·8 and 2·1 for appetite loss (P = 0·005). OS was significantly reduced in patients with appetite loss (P = 0·002) (Fig. 3) and patients with a fatigue score of 30 or more (P = 0·023) (Fig. 4) at baseline.
Figure 3.
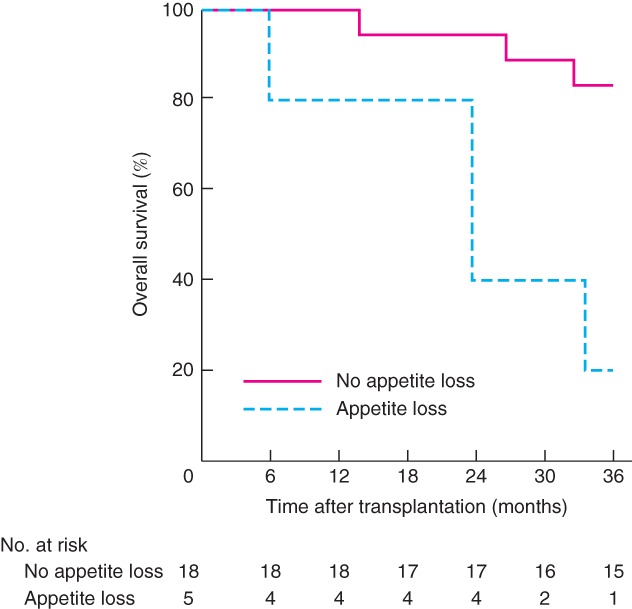
Kaplan–Meier estimated overall survival up to 3 years after liver transplantation related to symptom scores for appetite loss at baseline. P = 0·002 (log rank test)
Figure 4.
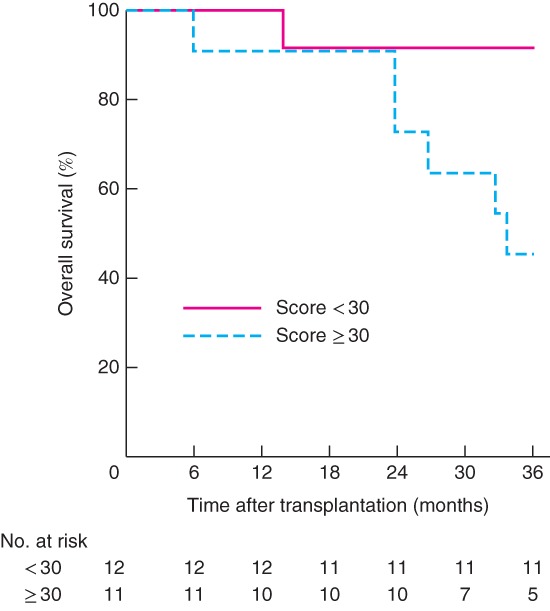
Kaplan–Meier estimated overall survival up to 3 years after liver transplantation related to fatigue symptom scores at baseline. Patients were divided into two groups with fatigue score of less than 30, and 30 or above. P = 0·023 (log rank test)
Five patients had appetite loss and also had a pain score above 17 (mean 30) and fatigue score greater than 33 (mean 54). The patients with appetite loss at inclusion all had progressive disease on chemotherapy at the time of LT. In these five patients, the median size of the largest liver lesion was 90 mm and median carcinoembryonic antigen (CEA) level was 104 μg/l, compared with 46 mm and 8·5 μg/l respectively among patients who did not report appetite loss at inclusion.
Patients who died within 3 years after LT had a GHS score at inclusion in the study of 67·9 compared with 81·3 in patients who were alive at 3 years after LT, although this difference was not significant (P = 0·273). None of the function scores were significantly related to OS at 3 years and no items in the QoL questionnaire were significantly related to disease‐free survival at 12 months.
Discussion
At 3 months after LT, patients had significantly worse GHS and physical function scores, along with worse symptom score for dyspnoea. These changes were all considered to be of clinical relevance. Function and symptom scores except those for dyspnoea had returned to baseline values 6 months after LT suggesting relatively rapid recovery from LT‐related problems. Decrease in health‐related QoL and physical function with full recovery within 6 months has also been reported after resection of CRLM20 25. Short‐term reduced physical function and increased symptom scores have also been reported in patients with colorectal cancer treated by pelvic radiation therapy and abdominal surgery combined with intraperitoneal chemotherapy21 26.
There was no significant and clinically important change from baseline in any function or symptom scale 3 years after LT, suggesting that the patients maintained good QoL for a long period of time and that the immunosuppressive treatment used in this study did not have a negative impact on reported QoL. In contrast, patients undergoing LT for chronic liver failure have reported reduced GHS and physical function scores compared with the general population. It has been suggested that this may be due to immunosuppressive therapies27.
Although the reported symptom scores in the whole cohort were low, patients who died within 3 years of transplant reported significantly higher scores for fatigue, pain and appetite loss at baseline. Those with any appetite loss or a fatigue score of at least 30 had significantly lower 3‐year survival rates than those without appetite loss or with fatigue score below 30. These observations may suggest that patients with general symptoms related to the malignant disease have reduced OS after LT. It has been shown previously that patients with larger colorectal tumours (exceeding 5·5 cm) or CEA levels over 80 μg/l at time of LT have reduced OS19. In the present study, patients with general symptoms also had a bigger largest lesion and higher CEA levels suggesting more advanced disease at the time of LT. This suggests that symptom score may be incorporated into the selection process for LT in patients with colorectal cancer. QoL scoring has also been shown to be related to OS in patients with head and neck cancer receiving curative radiation therapy28, and asymptomatic patients with colorectal cancer have increased OS after starting palliative chemotherapy compared with patients reporting various disease‐related symptoms29.
QoL evaluation in this study was performed using the generic cancer instrument EORTC QLQ‐C30 covering GHS, physical function, social function, cognitive function and role function as well as symptom scores for pain, dyspnoea, sleep disturbance, appetite loss and diarrhoea. The EORTC QLQ‐CR38 colorectal questionnaire covers function scales including body image and sexuality, and symptom scales for micturition problems, gastrointestinal problems, chemotherapy‐related side‐effects, defaecation problems, stoma‐related problems and sexual problems. These functions and symptoms are not covered by the EORTC QLQ‐C30 questionnaire and mainly relate to the primary surgery rather than transplantation. Nevertheless, they may still be relevant issues in these patients.
Acknowledgements
The authors thank M. Hjermstad for converting the questionnaire to the standard 0–100 scale. Supported by Oslo University Hospital, South‐Eastern Norway Regional Health Authority and the Norwegian Cancer Society (182704).
Disclosure: The authors declare no conflict of interest.
References
- 1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M et al Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359–E386. [DOI] [PubMed] [Google Scholar]
- 2. Leporrier J, Maurel J, Chiche L, Bara S, Segol P, Launoy G. A population‐based study of the incidence, management and prognosis of hepatic metastases from colorectal cancer. Br J Surg 2006; 93: 465–474. [DOI] [PubMed] [Google Scholar]
- 3. Butte JM, Gönen M, Allen PJ, Peter Kingham T, Sofocleous CT, DeMatteo RP et al Recurrence after partial hepatectomy for metastatic colorectal cancer: potentially curative role of salvage repeat resection. Ann Surg Oncol 2015; 22: 2761–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Masi G, Vasile E, Loupakis F, Cupini S, Fornaro L, Baldi G et al Randomized trial of two induction chemotherapy regimens in metastatic colorectal cancer: an updated analysis. J Natl Cancer Inst 2011; 103: 21–30. [DOI] [PubMed] [Google Scholar]
- 5. Tveit KM, Guren T, Glimelius B, Pfeiffer P, Sorbye H, Pyrhonen S et al Phase III trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX alone in first‐line treatment of metastatic colorectal cancer: the NORDIC‐VII study. J Clin Oncol 2012; 30: 1755–1762. [DOI] [PubMed] [Google Scholar]
- 6. Imai K, Allard MA, Castro Benitez C, Vibert E, Sa Cunha A, Cherqui D et al Nomogram for prediction of prognosis in patients with initially unresectable colorectal liver metastases. Br J Surg 2016; 103: 590–599. [DOI] [PubMed] [Google Scholar]
- 7. Van Cutsem E, Köhne CH, Láng I, Folprecht G, Nowacki MP, Cascinu S et al Cetuximab plus irinotecan, fluorouracil, and leucovorin as first‐line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 2011; 29: 2011–2019. [DOI] [PubMed] [Google Scholar]
- 8. Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M et al Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first‐line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 2010; 28: 4697–4705. [DOI] [PubMed] [Google Scholar]
- 9. Hoti E, Adam R. Liver transplantation for primary and metastatic liver cancers. Transpl Int 2008; 21: 1107–1117. [DOI] [PubMed] [Google Scholar]
- 10. Le Treut YP, Grégoire E, Klempnauer J, Belghiti J, Jouve E, Lerut J et al; for ELITA . Liver transplantation for neuroendocrine tumors in Europe – results and trends in patient selection: a 213‐case European liver transplant registry study. Ann Surg 2013; 257: 807–815. [DOI] [PubMed] [Google Scholar]
- 11. Masuoka HC, Rosen CB. Transplantation for cholangiocarcinoma. Clin Liver Dis 2011; 15: 699–715. [DOI] [PubMed] [Google Scholar]
- 12. Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L et al; Metroticket Investigator Study Group . Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol 2009; 10: 35–43. [DOI] [PubMed] [Google Scholar]
- 13. Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F et al Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996; 334: 693–699. [DOI] [PubMed] [Google Scholar]
- 14. Lee KK, Kim DG, Moon IS, Lee MD, Park JH. Liver transplantation versus liver resection for the treatment of hepatocellular carcinoma. J Surg Oncol 2010; 101: 47–53. [DOI] [PubMed] [Google Scholar]
- 15. Adam R, Karam V, Delvart V, O'Grady J, Mirza D, Klempnauer J et al; all contributing centers (www.eltr.org); European Liver and Intestine Transplant Association (ELITA). Evolution of indications and results of liver transplantation in Europe. A report from the European liver transplant Registry (ELTR). J Hepatol 2012; 57: 675–688. [DOI] [PubMed] [Google Scholar]
- 16. Kappel S, Kandioler D, Steininger R, Längle F, Wrba F, Ploder M et al Genetic detection of lymph node micrometastases: a selection criterion for liver transplantation in patients with liver metastases after colorectal cancer. Transplantation 2006; 81: 64–70. [DOI] [PubMed] [Google Scholar]
- 17. Mühlbacher F, Huk I, Steininger R, Gnant M, Götzinger P, Wamser P et al Is orthotopic liver transplantation a feasible treatment for secondary cancer of the liver? Transplant Proc 1991; 23: 1567–1568. [PubMed] [Google Scholar]
- 18. Dueland S, Guren TK, Hagness M, Glimelius B, Line PD, Pfeiffer P et al Chemotherapy or liver transplantation for nonresectable liver metastases from colorectal cancer? Ann Surg 2015; 261: 956–960. [DOI] [PubMed] [Google Scholar]
- 19. Hagness M, Foss A, Line PD, Scholz T, Jørgensen PF, Fosby B et al Liver transplantation for nonresectable liver metastases from colorectal cancer. Ann Surg 2013; 257: 800–806. [DOI] [PubMed] [Google Scholar]
- 20. Langenhoff BS, Krabbe PF, Peerenboom L, Wobbes T, Ruers TJ. Quality of life after surgical treatment of colorectal liver metastases. Br J Surg 2006; 93: 1007–1014. [DOI] [PubMed] [Google Scholar]
- 21. Chia CS, Tan GH, Lim C, Soo KC, Teo MC. Prospective quality of life study for colorectal cancer patients with peritoneal carcinomatosis undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol 2016; 23: 2905–2913. [DOI] [PubMed] [Google Scholar]
- 22. Andersen MH, Dueland S, Hagness M, Vidnes T, Finstad ED, Wahl AK et al Quality of life following liver transplantation in patients with liver metastases from colorectal carcinoma. Scand J Caring Sci 2012; 26: 713–719. [DOI] [PubMed] [Google Scholar]
- 23. Dueland S, Foss A, Solheim JM, Hagness M, Line PD. Survival following liver transplantation for liver‐only colorectal metastases compared with hepatocellular carcinoma. Br J Surg 2018; 105: 736–742. [DOI] [PubMed] [Google Scholar]
- 24. Kaasa S, Bjordal K, Aaronson N, Moum T, Wist E, Hagen S et al The EORTC core quality of life questionnaire (QLQ‐C30): validity and reliability when analysed with patients treated with palliative radiotherapy. Eur J Cancer 1995; 31A: 2260–2263. [DOI] [PubMed] [Google Scholar]
- 25. Wiering B, Oyen WJ, Adang EM, van der Sijp JR, Roumen RM, de Jong KP et al Long‐term global quality of life in patients treated for colorectal liver metastases. Br J Surg 2011; 98: 565–571. [DOI] [PubMed] [Google Scholar]
- 26. Guren MG, Dueland S, Skovlund E, Fosså SD, Poulsen JP, Tveit KM. Quality of life during radiotherapy for rectal cancer. Eur J Cancer 2003; 39: 587–594. [DOI] [PubMed] [Google Scholar]
- 27. Yang LS, Shan LL, Saxena A, Morris DL. Liver transplantation: a systematic review of long‐term quality of life. Liver Int 2014; 34: 1298–1313. [DOI] [PubMed] [Google Scholar]
- 28. Fang FM, Tsai WL, Chien CY, Chen HC, Hsu HC, Huang TL et al Pretreatment quality of life as a predictor of distant metastasis and survival for patients with nasopharyngeal carcinoma. J Clin Oncol 2010; 28: 4384–4389. [DOI] [PubMed] [Google Scholar]
- 29. Láng I, Köhne CH, Folprecht G, Rougier P, Curran D, Hitre E et al Quality of life analysis in patients with KRAS wild‐type metastatic colorectal cancer treated first‐line with cetuximab plus irinotecan, fluorouracil and leucovorin. Eur J Cancer 2013; 49: 439–448. [DOI] [PubMed] [Google Scholar]


