Abstract
Aseptic technique, including the use of sterile drapes, is essential to reduce microbial contamination to the lowest practical level and improve surgical outcomes. Recently, some institutions have approved the use of Press'n Seal cling film (CF; Glad Products, Oakland, CA) as a practical, cost-effective alternative to sterile drapes for rodent surgeries. The purpose of this study was to evaluate the sterility of CF by using ATP and replicate organism detection and counting (RODAC) plates. We tested 10 boxes of CF at days 0, 14, and 28 after opening the box and compared the results with traditional packaged sterile drapes. Our data indicated that CF ATP bioluminescence remained at or below 10 relative light units for 28 d after opening the box. In addition, RODAC plates had no growth for 70% of CF boxes at day 0, 100% at day 14, and 90% at day 28. The mean growth for the positive plates was 0.024 cfu/cm2 sampled after contacting locations on the front and back of the CF. The results of this study support the use of CF as an acceptable alternative to traditional sterile drapes during rodent aseptic surgery.
Abbreviations: CF, Glad Press’n Seal cling film; RLU, relative light units; RODAC, replicate organism detection and counting
The use of aseptic technique is essential to prevent transmission of microbial organisms into sterile tissues below the skin surface, thus minimizing the risk of infection during rodent surgeries.3 Many components of aseptic technique—including preparation of the patient and surgeon, appropriate personal protective equipment, sterilization of equipment, and correct surgical technique—work together to reduce microbial contamination to the lowest practical level. The Guide for the Care and Use of Laboratory Animals states that principles of aseptic technique should be followed for all survival surgeries and that failure to do so may have negative consequences on surgical success, animal welfare, and research results.9 Accordingly, most animal care and use programs have developed guidelines to help researchers meet requirements for asepsis during rodent survival surgery activities.
The use of sterile drapes to create a barrier between the surgical site and potential sources of contamination is a critical element of aseptic technique for many procedures. Current options for rodent sterile draping include purchasing prepackaged sterile drape or autoclaving inhouse bulk drape or other material such as stockinette. More recently, some institutions have allowed the use of commercial cling film (CF; Press'n Seal cling film, Glad Products, Oakland, CA) as rodent draping material. Benefits to using CF include that it is widely available, inexpensive, allows for visualization of the patient, adheres to diverse surfaces, and traps heat to aid in thermoregulation. Importantly, in our experience, the ease of its use increases investigator compliance to rodent surgical requirements. In addition, rodent surgeries are commonly performed alone; therefore, application of CF to equipment such as anesthesia vaporizer dials, stereotaxic knobs, and light handles may minimize breaks in sterility during surgery.
Despite the many benefits to using CF as draping material, no peer-reviewed data have been published regarding its sterility. In a 2017 CompMed listserv survey, 11 of 14 respondents reported using CF for rodent surgery at their institution; 4 of these 11 respondents described no microbial growth during inhouse analysis, and the remaining did not report testing.2 In addition to anecdotal information, very few data have been published describing the bacterial culture of the product.17,20 Correspondence with the manufacturer revealed that very high temperatures are used during the extrusion process, but sterility of the final product cannot be guaranteed. Of late, ethylene oxide-sterilized boxes of CF have become available for purchase through laboratory supply vendors at markedly increased cost, and no published data have confirmed the sterility of the CF roll after this sterilization process.
The purpose of this study was to use ATP swabs and replicate organism detection and counting (RODAC) plates to evaluate the sterility of CF sold for food preparation, to help institutions make informed decisions regarding its use as a rodent surgical drape. We hypothesized that commercial CF would yield minimal to no microbial growth on opening of the box and for 28 d thereafter.
Materials and Methods
Drape materials.
This study tested 10 boxes of commercial CF (100 ft2 roll, Press'n Seal, Glad Products) representing several manufacturer lot numbers. The boxes were stored on a shelf or countertop in areas of an animal research facility with regular human and laboratory animal activity. Between sampling time points, the tops of the boxes were left ajar, according to the natural conformation of the box. The boxes were handled without gloves when moved to accommodate room activities or for sampling purposes. Daily conventional rodent work and well as occasional large animal activities occurred near the boxes. Sections of CF were removed only during sampling. In addition, 5 individually wrapped sterile drapes (catalog no. 89534, Sterile Half Drape, Halyard Health, Alpharetta, GA) were tested in this study and are referred to as ‘traditional drapes’.
RODAC testing.
RODAC plates (BBL Trypticase Soy Agar with Lecithin and Polysorbate 80, Becton Dickson, Franklin Lakes, NJ) were used to detect bacterial growth (Figure 1 A). After sampling, plates were incubated at 35 °C for 72 h. Plates were checked for growth at 24, 48, and 72 h after sampling, and plates with any observable growth at the 72-h time point were submitted to Ohio Department of Agriculture Animal Disease Diagnostic Laboratory (Reynoldsburg, OH) for bacterial identification.
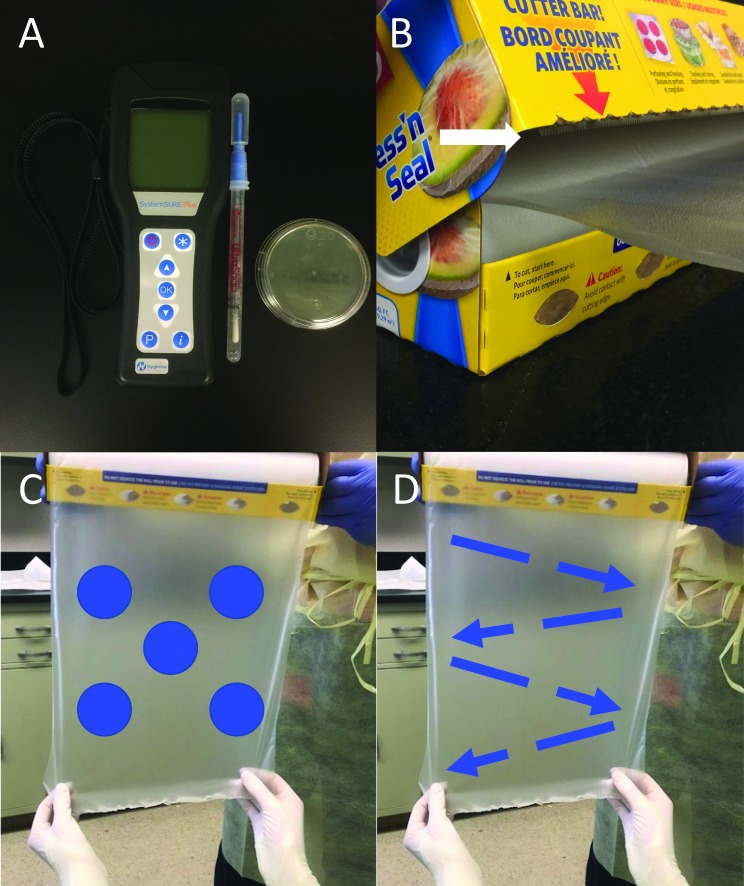
Figure 1.
(A) ATP luminometer, ATP swab, and RODAC plate used for microbiologic testing. (B) Close up of the box, demonstrating avoidance of the cutting surface (white arrow) with any other box surfaces while unrolling the product. (C) The RODAC plate was applied for 5 s of contact to 5 positions on the front (blue dots) and then 5 positions on the back of the CF sampling area. (D) After RODAC testing, the same sampling area was swabbed with an ATP swab in a zigzag pattern on the front and back of the material. For both RODAC and ATP testing, an approximately 2-cm margin around the testing region was avoided.
ATP testing.
ATP swabs (UltraSnap ATP Surface Test Swab, Hygiena, Camarillo, CA) and a luminometer (SystemSURE PLUS ATP Measurement System, Hygiena) were used to measure bioluminescence from the reaction of ATP with luciferase, which is reported in relative light units (RLU; Figure 1 A). The ATP swabs were analyzed according to the manufacturer's recommendations and within 30 min of completing microbial sampling.
Microbiologic sampling procedure.
Microbiologic testing (ATP and RODAC) was performed when the box (CF) or package (traditional drape) was opened (day 0). Additional testing was performed on the CF boxes at 14 and 28 d after opening. All CF testing results (days 0, 14, and 28) were compared with the day 0 results from the traditional drapes.
The boxes were held open while a person wearing sterile gloves (Criterion Powder-Free Latex Surgical Gloves, Henry Schein, Melville, NY) pulled out and cut a 25-cm segment of CF by using sterile scissors, carefully avoiding contact of the CF with the box or cutting surface (Figure 1 B); the circumference of an unused CF roll was 25 cm, and this first cut section was discarded. Next, another 25-cm segment was sterilely pulled from the roll and held exposed for sampling. A RODAC plate was applied for 5 s of contact time at 5 locations on the front and 5 locations on the back of CF; a 2-cm margin around the edges of the sampling area was avoided (Figure 1 C). Then, the same segment of CF was sampled by using an ATP swab in a zigzag pattern on the front and then back of the CF (Figure 1 D). Once sampling was complete, the exposed segment was removed from the roll, and each box was returned to its storage location. Sterile gloves were changed between samples. Negative control testing was executed by mimicking the actions and duration of CF testing but not touching the ATP swab or RODAC plate to the CF surface (n = 5).
Microbiologic sampling of the traditional drapes (n = 5) was accomplished in a similar fashion. Each drape was sterilely removed from the package and held open by using sterile gloves. A 25 cm × 25 cm region in the center of the drape was tested on both sides by using an ATP swab and RODAC plate, as described earlier.
Statistical analysis.
All data were analyzed by using Prism 7 statistical software (GraphPad Software, La Jolla, CA). ATP levels are reported as group means ± SEM. Standard one-way ANOVA was used for statistical comparison of ATP testing between groups. Where appropriate, post hoc analysis was performed by using the Tukey multiple comparisons test. For all analyses, a P value of 0.05 or less was considered statistically significant.
Results
ATP analysis.
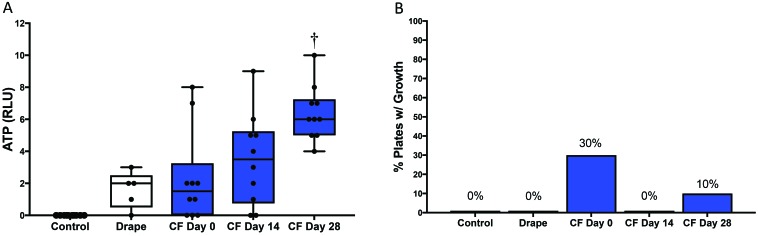
Traditional drapes (n = 5) had a mean ATP bioluminescence of 1.6 ± 0.51 RLU. ATP levels from CF (n = 10) were comparable to those of the traditional drapes on days 0 (2.3 ± 0.91 RLU) and 14 (3.5 ± 0.91 RLU) but higher (P = 0.007) on day 28 (6.4 ± 0.54 RLU; Figure 2 A. ATP bioluminescence was at or below 10 RLU for all samples tested during this study. Negative control testing detected 0 RLU for all samples (n = 5). ATP testing of a RODAC plate (n = 1) detected 29 RLU after passing the swab once over the plate surface.
Figure 2.
(A) ATP bioluminescence (in RLU) for control (n = 5), traditional drapes (n = 5, white), and CF on days 0, 14, and 28 (n = 10, blue). The middle line represents the median, the bounds of the box represent the upper and lower quartiles, and the whiskers represent the lowest and highest ATP detection values for each group. †, P < 0.01 (one-way ANOVA) compared with traditional drape. (B) RODAC plate testing for control (n = 5), traditional drapes (n = 5), and CF (n = 10) on days 0, 14, and 28. Bars indicate the percentage of plates with growth after 72 h of incubation.
RODAC plate analysis.
All traditional drapes (n = 5) had no growth after 72 h of incubation. However, some of the CF samples yielded bacterial growth (Figure 2 B, Table 1). On day 0, 3 of the 10 CF boxes had visible growth on RODAC plates, with 1, 5, and 6 cfu (0.004, 0.024, and 0.020 cfu/cm2 sampled); organisms were identified as Kocuria rhizophila, Neisseria spp., Streptococcus parasanguinis, Streptococcus spp., and Rothia mucilaginosa. On day 14, none of the 10 CF boxes had visible growth, and on day 28, 1 of the 10 CF boxes yielded 12 cfu (0.048 cfu/cm2 sampled); these organisms were identified as Staphylococcus epidermidis and Staphylococcus pasteuri. Negative control samples (n = 5) had no growth after 72 h of incubation.
Table 1.
ATP (no. of RLU) and RODAC (no. of cfu per plate, no. of cfu/cm2) testing results
| Day 0 | Day 14 | Day 28 | ||||
| CF box no. | ATP | RODAC | ATP | RODAC | ATP | RODAC |
| 1 | 1 | 0, 0 | 5 | 0, 0 | 7 | 12, 0.048d |
| 2 | 0 | 1, 0.004a | 6 | 0, 0 | 8 | 0, 0 |
| 3 | 2 | 0, 0 | 2 | 0, 0 | 4 | 0, 0 |
| 4 | 8 | 0, 0 | 5 | 0, 0 | 7 | 0, 0 |
| 5 | 1 | 6, 0.024b | 9 | 0, 0 | 5 | 0, 0 |
| 6 | 2 | 0, 0 | 1 | 0, 0 | 6 | 0, 0 |
| 7 | 7 | 5, 0.02c | 4 | 0, 0 | 6 | 0, 0 |
| 8 | 0 | 0, 0 | 3 | 0, 0 | 6 | 0, 0 |
| 9 | 0 | 0, 0 | 0 | 0, 0 | 5 | 0, 0 |
| 10 | 2 | 0, 0 | 0 | 0, 0 | 10 | 0, 0 |
Organism identified as Kocuria rhizophila.
Organisms identified as Neisseria spp., Streptococcus parasanguinis, and Streptococcus species (mitis group).
Organisms identified as Neisseria spp. and Rothia mucilaginosa.
Organisms identified as Staphylococcus epidermidis and Staphylococcus pasteuri.
Discussion
This study investigated the sterility of commercial CF (Press'n Seal, Glad Products) to critically evaluate its use as rodent surgical draping material. Here we demonstrated that ATP detection from all 10 boxes of CF tested was at or below 10 RLU for as long as 28 d after opening. ATP detection is fast and easy to perform, but there are several limitations to consider when interpreting the results. Notably, ATP bioluminescence systems measure cellular material, specifically ATP, regardless of viability.5,18 Thus, sources of extracellular ATP such as dead cells, organic material, and debris from the packaging or environment can generate positive ATP readings but lack infectious potential. In the current study, we performed ATP testing after RODAC sampling and, consequently, residue from the plates could have been detected. ATP swabbing the surface of a RODAC plate in a single pass produced 29 RLU, indicating that positive ATP results due to RODAC plate residue is a valid concern. These additional sources of ATP or variation in sampling conditions may account for why the mean ATP reading of CF at day 28 was significantly higher than from traditional drapes without a corresponding increase in colony counts. Nonetheless, ATP detection was at or below 10 RLU for all CF samples, a common threshold for acceptable ATP levels on sterilized surfaces.14 Other benchmarks created by using the same testing system in hospital settings recommend higher thresholds, such as 50 to 100 RLU, to prevent nosocomial infections.4,13,21
Tryptic Soy Agar RODAC plates are designed to aid in quantification of live microorganisms on a surface. These plates identified only minimal growth on several of the CF boxes (3 of the 10 boxes on day 0 and 1 on day 28). Importantly, each plate made contact with 10 discrete locations, to test a larger surface area than the conventional sampling method. Even so, growth on the positive plates from day 0 was below the threshold of 10 cfu per plate (0.4 cfu/cm2) that is used at our institutions to delineate adequate sterilization. This threshold is adapted from APHA Guidelines, literature from hospital settings, and inhouse testing.11,13,19 Notably, the 12 colonies on the day 28 plate were arranged in a ring around the edge of the plate. Due to the raised agar conformation and parallel sampling technique, the side of the agar was unlikely to directly contact the CF. We are highly suspicious that this bacterial growth was contamination from contact with the lid during the recapping process. Overall, microbial growth declined sharply after the initial testing on day 0, suggesting that discarding a larger piece of CF initially may greatly reduce the risk of microbial contamination of the material; further research is needed to confirm this recommendation.
Streptococcus spp., Neisseria spp., and Rothia mucilaginosa were identified from the CF samples on day 0 and are commensal flora of human mucosal surfaces. These ubiquitous organisms could have been introduced during any point during manufacturing, storage, or sampling. Moreover, the identification of Kocuria rhizophila on day 0 was particularly interesting. This soil-dwelling bacterium is commonly used in the food industry for quality control and antimicrobial testing.16 Accordingly, this organism likely originated from the manufacturing facility. On day 28, Staphylococcus epidermidis and Staphylococcus pasteuri— commensal flora of human skin— were identified on the single positive plate. Due to potential contact of the RODAC plate lid with skin, this identification supports our suspicion that lid contamination was responsible for the growth. Although most of these bacteria are either not reported or known to be pathogenic in rodents, their pathogenic potential in immunocompromised animals should be considered.1,8,10,12,15
Negative control sampling (air sampling) encompassed performing the complete CF sampling method but avoiding contact of the ATP swab or RODAC plate with the material. This was implemented to identify whether contamination occurred during the sampling process. All of the control ATP results were 0 RLU, and all of the control RODAC plates were negative for growth. However, further studies performing RODAC sampling in a biosafety cabinet would provide evidence that the bacterial growth we observed was truly due to sample on the CF surface rather than aerosolized contaminants in the room.
The use of traditional sterilized draping material is the ‘gold standard’ for animal surgery; however, modifications in aseptic technique for rodent surgery may be permissible when they meet performance indices. Achieving true asepsis—the elimination of all bacteria, viruses, and other microorganisms—is essentially impossible during any surgery. Instead, the goal is to limit microbial contamination to the lowest possible levels, to minimize effects on the immune systems and the risk of postsurgical infection.3,6,7 Experimental surgery on rodents is often performed by biomedical researchers of various backgrounds and is rarely done by veterinarians or physicians with formal aseptic surgical training. Indeed, creating attainable standards is essential for compliance with rodent surgical requirements.
Our results demonstrate that minimal ATP detection and bacterial growth occurred on a small percentage of CF boxes after opening. Because microbial detection was highest at the start of the roll, discarding a piece longer than 25 cm before using the roll may reduce risk of contamination. These results—coupled with many benefits including cost, availability, visualization, and thermoregulation—support the continued use of CF as rodent surgical drape and likely meet performance standards for rodent aseptic surgery as recommended in the Guide. Our institutions have begun using CF for rodent surgeries, and we have not appreciated any postsurgical complications. However, future studies evaluating surgical outcomes might be explored.
Acknowledgments
Natalie Celeste was funded by the Jules and Ruth Cass Clerkship Award in Comparative Laboratory Animal Medicine, Science, and Technology. We gratefully acknowledge Dana LeMoine, Toi Collins, Curtis Rheingold, Bridget Clancey, and Daniel MacKessy for their technical assistance with sampling. Special thanks to Valerie Bergdall and Judy Hickman-Davis for supporting this research project.
References
- 1.Akinkunmi EO, Adeyemi OI, Igbeneghu OA, Olaniyan EO, Omonisi AE, Lamikanra A. 2014. The pathogenicity of Staphylococcus epidermidis on the intestinal organs of rats and mice: an experimental investigation. BMC Gastroenterol 14:1–8. 10.1186/1471-230X-14-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Association for Laboratory Animal Science. [Internet]. 2017. Summary on Glad Press n’ Seal. CompMed listserv [14 July 2017]. Available at: https://www.aalas.org/get-involved/listservs/compmed. [Google Scholar]
- 3.American College of Laboratory Animal Medicine. 2016. ACLAM position statement on rodent surgery. J Am Assoc Lab Anim Sci 55:822–823. [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson RE, Young V, Stewart M, Robertson C, Dancer SJ. 2011. Cleanliness audit of clinical surfaces and equipment: who cleans what? J Hosp Infect 78:178–181. 10.1016/j.jhin.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 5.Arroyo MG, Ferreira AM, Fronta OP, Rigotti MA, de Andrade D, Brizzotti NS, Peresi JTM, Castilho EM, de Almeida MTG. 2017. Effectiveness of ATP bioluminescence assay for presumptive identification of microorganisms in hospital water sources. BMC Infect Dis 17:1–5. 10.1186/s12879-017-2562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernal J, Baldwin M, Gleason T, Kuhlman S, Moore G, Talcott M. 2009. Guidelines for rodent survival surgery. J Invest Surg 22:445–451. 10.3109/08941930903396412. [DOI] [PubMed] [Google Scholar]
- 7.Berríos-Torres SI, Umscheid CA, Bratzler DW, Leas B, Stone EC, Kelz RR, Reinke CE, Morgan S, Solomkin JS, Mazuski JE, Dellinger EP, Itani KMF, Berbari EF, Segreti J, Parvizi J, Blanchard J, Allen G, Kluytmans JAJW, Donlan R, Schecter WP, Healthcare Infection Control Practices Advisory Committee 2017. Centers for Disease Control and Prevention guideline for the prevention of surgical site infection. JAMA Surg 152:784–791. 10.1001/jamasurg.2017.0904. [DOI] [PubMed] [Google Scholar]
- 8.Deighton MA, Borland R, Capstick JA. 1996. Virulence of Staphylococcus epidermidis in a mouse model: significance of extracellular slime. Epidemiol Infect 117:267–280. 10.1017/S0950268800001448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 10.Ma M, Powell DA, Weyand NJ, Rhodes KA, Rendon MA, Frelinger JA, So M. 2018. A natural mouse model of Neisseria colonization. Infect Immun 86:1–13. 10.1128/IAI.00839-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malik RE, Cooper RA, Griffith CJ. 2003. Use of audit tools to evaluate the efficacy of cleaning systems in hospitals. Am J Infect Control 31:181–187. 10.1067/mic.2003.34. [DOI] [PubMed] [Google Scholar]
- 12.Maraki S, Papadakis IS. 2015. Rothia mucilaginosa pneumonia: a literature review. Infect Dis (Lond) 47:125–129. 10.3109/00365548.2014.980843. [DOI] [PubMed] [Google Scholar]
- 13.Mulvey D, Redding P, Robertson C, Woodall C, Kingsmore P, Bedwell D, Dancer SJ. 2011. Finding a benchmark for monitoring hospital cleanliness. J Hosp Infect 77:25–30. 10.1016/j.jhin.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Scigiene Corporation. [Internet]. 2018. Setting ATP threshold levels for EnSURE, SystemSURE Plus, and SystemSUREII luminometers. [Cited 15 August 2018]. Available at: https://www.scigiene.com/. [Google Scholar]
- 15.Shelburne SA, Sahasrabhojane P, Saldana M, Yao H, Su X, Horstmann N, Thompson E, Flores AR. 2014. Streptococcus mitis strains causing severe clinical disease in cancer patients. Emerg Infect Dis 20:762–771. 10.3201/eid2005.130953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takarada H, Sekine M, Kosugi H, Matsuo Y, Fujisawa T, Omata S, Kishi E, Shimizu A, Tsukatani S, Fujita N, Harayama S. 2008. Complete genome sequence of the soil actinomycete Kocuria rhizophila. J Bacteriol 190:4139–4146. 10.1128/JB.01853-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinkos L, Olson D, Perret-Gentil M. [Internet]. 2016. Rodent drape that allows for perioperative observation. Surgical Savvy 2:2 [Cited 25 February 2018]. Available at: www.surgicalresearch.org. [Google Scholar]
- 18.Venkateswaran K, Hattori N, La Duc MT, Kern R. 2003. ATP as a biomarker of viable microorganisms in cleanroom facilities. J Microbiol Methods 52:367–377. 10.1016/S0167-7012(02)00192-6. [DOI] [PubMed] [Google Scholar]
- 19.Vesley D, Pryor AK, Walter WG, Shaffer JG. 1970. A cooperative microbiological evaluation of floor-cleaning procedures in hospital patient rooms. Health Lab Sci 7:256–264. [PubMed] [Google Scholar]
- 20.Voronin GO. 2013. Microbiological evaluation of an alternative surgical draping material. Abstract presented at the 29th Annual Meeting of the Academy of Surgical Research, Clearwater Beach, Florida, 26–28 September 2013. J Invest Surg 26:107 10.3109/08941939.2013.775860 [DOI] [Google Scholar]
- 21.Whiteley GS, Glasbey TO, Fahey PP. 2016. A suggested sampling algorithm for use with ATP testing in cleanliness measurement. Infect Dis Health 21:169–175. 10.1016/j.idh.2016.11.003. [DOI] [Google Scholar]