Abstract
Many variables can influence animal behavior and physiology, potentially affecting scientific study outcomes. Laboratory and husbandry procedures—including handling, cage cleaning, injections, blood collection, and animal identification—may produce a multitude of effects. Previous studies have examined the effects of such procedures by making behavioral and physiologic measurements at specific time points; this approach can be disruptive and limits the frequency or duration of observations. Because these procedures can have both acute and long-term effects, the behavior and physiology of animals should be monitored continuously. We performed a retrospective data analysis on the effects of 2 routine procedures, animal identification and cage changing, on motion and breathing rates of mice continuously monitored in the home cage. Animal identification, specifically tail tattooing and ear tagging, as well as cage changing, produced distinct and reproducible postprocedural changes in spontaneous motion and breathing rate patterns. Behavioral and physiologic changes lasted approximately 2 d after tattooing or ear tagging and 2 to 4 d for cage changing. Furthermore, cage changes showed strain-, sex-, and time-of-day–dependent responses but not age-dependent differences. Finally, by reviewing data from a rodent model of multiple sclerosis as a retrospective case study, we documented that cage changing inadvertently affected experimental outcomes. In summary, we demonstrate how retrospective analysis of data collected continuously can provide high-throughput, meaningful, and longitudinal insights in to how animals respond to routine procedures.
High-throughput and reproducible phenotypic analysis of laboratory animals is an area of rapidly growing priority, especially with the explosive growth in functional genomics, preclinical evaluation, and drug discovery research.10 The effects of sex, age, genetic background, and environmental enrichment are well-studied and carefully considered in the study design process, but routine laboratory and husbandry procedures are only beginning to receive attention.7,13 Although considered incidental in nature, these procedures may inadvertently and profoundly affect experimental outcomes.7,13,27,28
Numerous laboratory and husbandry procedures—including handling, cage movement and cleaning, injections, blood collection, animal identification, and personnel entry into the housing room—produce a multitude of effects on animal behavior and physiology. These effects include changes in activity patterns, stress and anxiety levels, heart rate, blood pressure, and body temperature.2,7,13,27,28 Current methods to monitor responses, such as behavioral tests (for example, assessments of exploratory behavior, anxiety, stress), telemetry, and blood or tissue collection, may be invasive and as such further alter behavior.4,6 Limitations in current monitoring methods result in studies that are commonly cross-sectional and often restricted to a single age, sex, and strain or are statistically underpowered (for example, small sample size). In addition, the cross-sectional nature of current methods may constrain concrete recommendations for experimental design, including timelines for ample habituation and postprocedural recovery. Ideally, continuous monitoring of behavior and physiology in the home cage would allow the assessment of both acute and long-term effects. The ability to gather longitudinal data on a large sample population of animals would allow researchers to explore how these environmental manipulations interact with age and genetics and to understand the magnitude and duration of procedure-related effects.
We hypothesized that the sensitivity and resolution of continuous monitoring of animal behavior and physiology would provide high-throughput, longitudinal, and physiologically relevant insights into how animals respond to routine laboratory and husbandry procedures. These insights can be used to develop concrete decisions around experiment design, including incorporating appropriate postprocedural recovery times, as well as to understand how other experimental variables (for example, subject strain, sex, age) affects responses. Using a continuous monitoring platform and its automated assessment of motion and breathing rate which were developed inhouse,24 we performed retrospective data analyses on several independent mouse studies to investigate the effects of 2 routine procedures, animal identification and cage changing. Because the repercussions of these procedures on study outcomes are rarely discussed, we provide, as a case study, an example of how the timing of cage changing inadvertently affected experimental outcomes in a rodent model of multiple sclerosis.
Materials and Methods
Our studies can be divided into the following parts: 1, reproducibility studies regarding tail tattooing, ear tagging, and cage changing; 2, strain and sex study; 3, longitudinal study; 4 early and late cage-change study; and 5, case study of the cuprizone mouse model of multiple sclerosis. All procedures were performed during the light cycle (0600 to 1800 PDT). Experiments were conducted in Vium's AAALAC-accredited Digital Vivarium in accordance with the current National Research Council Guide for the Care and Use of Laboratory Animals17 and were IACUC-approved.
Animals and housing.
On arrival, all animals were maintained in SPF facilities and housed in instrumented IVC (Digital Smart House, Vium, San Mateo, CA, and Innovive, San Diego, CA) containing corncob bedding. Animals had unrestricted access to food (Pico Rodent Diet 5053, Lab Diet, St Louis, MO) and acidified, sterile water (Innovive). Depending on the type of study, mice were either group- or pair-housed or single-housed. When group- or pair-housed, male and female mice were kept separated. Single-housing was performed to gather the most accurate motion and breathing rate data because the sensors on the Vium Digital Platform are not currently optimized to distinguish multiple animals in the same cage. Environmental enrichment, including running wheels, ladders, domes, cotton squares (Ancare, Bellmore, NY), and foraging mixes (Veggie Relish, LabDiet), was provided to each cage.
All of the analyses described herein were performed retrospectively on available data sets from completed mouse studies. As much as possible, mice of similar sex, age, and housing condition were compared directly, but conditions across experiments could not be controlled due to the retrospective nature of the analysis method (for example, type of equipment; enrichment or nesting materials used; animal handlers; number of animals in cage; cage size).
Part 1: reproducibility studies of tail tattooing, ear tagging, and cage changing.
Female C57BL/6J mice (age, 8 wk; Jackson Laboratories, Bar Harbor, ME) were pair-housed in small (14.7 × 9.2 × 5.5 in.) Vium Digital Smart Houses. Retrospective data analysis was performed on 2 independent studies (experiments 1 and 2) on data collected during a 14-d acclimation period prior to study start and at least 3 d after arrival. In studies wherein mice were tattooed, tail tattoos were applied by using the ATS3 Rodent Tattoo System (Animal Identification and Marking Systems, Hornell, NY) without anesthesia. Sample sizes were 87 to 117 cages (experiment 1) and 24 to 27 cages (experiment 2). In studies wherein mice were ear tagged, tags (RapID Lab, San Francisco, CA [experiment 1] or Stoelting, Wood Dale, IL [experiment 2]) were applied without the use of anesthesia. Sample sizes were 33 to 36 cages (experiment 1) and 85 to 100 cages (experiment 2). In studies wherein cage changing was investigated, mice were transferred to new IVC containing corncob bedding, 2 cotton squares, food, and water, as well as standard enrichment (foraging enrichment, ladder, and running wheel). Sample sizes were 44 to 61 cages (experiment 1) and 39 to 50 cages (experiment 2).
Part 2: strain and sex study.
Male and female C57BL/6J (n = 15 mice per sex), BALBc/J (n = 15 mice per sex), and C3H/J (n = 14 mice per sex; age, 4 wk; Jackson Laboratories) were group-housed (approximately 5 mice of the same sex per cage) in large (17.0 × 13.4 × 7.8 in.) Vium Digital Smart Houses. Mice were single-housed within 3 d after arrival. Cage changes conducted at 12 and 17 wk of age for single-housed male and female mice were averaged for retrospective data analysis. During each cage change, animals were transferred to a clean IVC containing corncob bedding, brown paper nesting material, food, and water, as well as standard enrichment (foraging enrichment, dome, and running wheel).
Part 3: longitudinal study.
Male (n = 14 or 15 mice) and female (n = 13 or 14 mice) C57BL/6J (Jackson Laboratories) were singly housed in small (14.7 × 9.2 × 5.5 in.) Vium Digital Smart Houses. Data for cage changes conducted from 6 to 25 mo of age were used for retrospective analysis. During each bimonthly cage change, animals were transferred to a clean IVC containing corncob bedding, 2 cotton squares, food, and water, as well as standard enrichment (foraging enrichment, ladder, and running wheel).
Part 4: early and late cage-change study.
Cage changes from the same cohort of male (n = 14) and female (n = 9 to 13) C57BL/6J (Jackson Laboratories) mice involved in the longitudinal study (part 3) were also used for the early and late cage-change study. Given that bimonthly cage changes are normally performed by vivarium staff between 0600 and 1400 PDT, we specifically performed cage changes late in the light cycle, close to the beginning of the dark cycle. Cages of approximately 29-mo-old male and female mice were changed at approximately 1700 PDT (late cage change). Data from the late cage change were compared with previous cage changes performed at approximately 0900 PDT and 1400 PDT (early cage changes) in approximately 29- and 20-mo-old mice, respectively. During each cage change, animals were transferred to a clean IVC containing corncob bedding, 2 cotton squares, food, and water, as well as standard enrichment (foraging enrichment, ladder, and running wheel).
Part 5: case study of cuprizone mouse model of multiple sclerosis.
To provide an example of how cage changing can inadvertently affect in-life experimental outcomes, we identified a previously completed study in the laboratory that involved a rodent model of multiple sclerosis. Briefly, 7-wk-old, female C57BL/6J mice (Jackson Laboratories) were single-housed in small (14.7 × 9.2 × 5.5 in.) Vium Digital Smart Houses. After a 7-d acclimation period, mice were fed either 0.2% cuprizone diet (TD.140804, Envigo-Teklad, Madison, WI; n = 30 mice) or control chow (TD.00588, Envigo-Teklad; n = 18 mice) for 41 d. Cuprizone-containing and control chow were completely replaced twice each week according to the manufacturer's recommendation. Cage changes performed during this 41-d study period and their effects on in-life experimental outcomes were analyzed retrospectively. During a cage change, animals were transferred to a clean IVC containing corncob bedding, 2 cotton squares, food, and water, as well as standard enrichment (foraging enrichment, ladder, and running wheel).
Environmental conditions.
Animal cages were maintained on a 12:12-h light:dark cycle (0600 to 1800 PDT). Individual cages were lit by using LED lights between 85 to 110 lx for a 12:12-h light:dark photoperiod, with illumination levels collected and monitored continuously at the cage level. The animal room environmental controls were maintained at temperatures of 20 to 26 °C and relative humidity of 30% to 70%. The room ventilation rate was set at a minimum of 15 room air changes hourly (100% fresh), and cage ventilation rate was set at a minimum of 40 to 60 cage changes hourly.
The Vium Digital Platform.
Vium Digital Smart Houses consist of standard IVC slotted in Vium's proprietary rack system. Vium Digital Smart Houses are outfitted with sensors and a high-definition camera that enable continuous monitoring of animals and that streams data to a secure cloud-based infrastructure. The Vium Digital Platform obtains and maintains a digital record of the following information: 1) procedures with corresponding event times; 2) data analytics on motion and breathing rate; and 3) verification of illumination. This study used the validated Vium Motion (m/sec)24 and Vium Breathing Rate (breaths per minute) metrics. To compute breathing rate, computer vision algorithms search for greater than 30-s regions of time when animals are stationary and identify periodic motion that falls within a frequency band containing known rodent breathing rates.14 The peak root mean square power is compared with a threshold to determine whether the periodic motion is significant.
Statistical analysis.
Daytime motion (collected from 0600 to 1800 PDT), nighttime motion (collected from 1800 to 0600 PDT), and breathing rate (collected 0600 to 0600 PDT) metrics were averaged for each day or night. Metrics were aligned to the day of procedure (day 0). Therefore, day –1 and night –1 represent the day and night just prior to the procedure, respectively, whereas day 1 and night 1 represent the first day and night postprocedurally, respectively. Data collected on the day or night after the procedure (night 0) as well as on subsequent days or nights were normalized to the day or night prior to procedure (day –1 or night –1, respectively) to produce motion ratios (Figure 1 A). Metrics were normalized to account for broad interanimal variability and to compare the effects and reproducibility of effects across independent experiments. In part 1 (reproducibility studies of tail tattooing, ear tagging, and cage changing), to evaluate the effects of a specified procedure within each experiment or cohort of mice, consecutive days when the specified procedure (for example, animal identification, cage changing) was not performed (that is, no-procedure control days) were used for comparison. Unless otherwise indicated, values for daytime, nighttime, and breathing rate metrics are indicated as ratios, which represent changes from baseline.
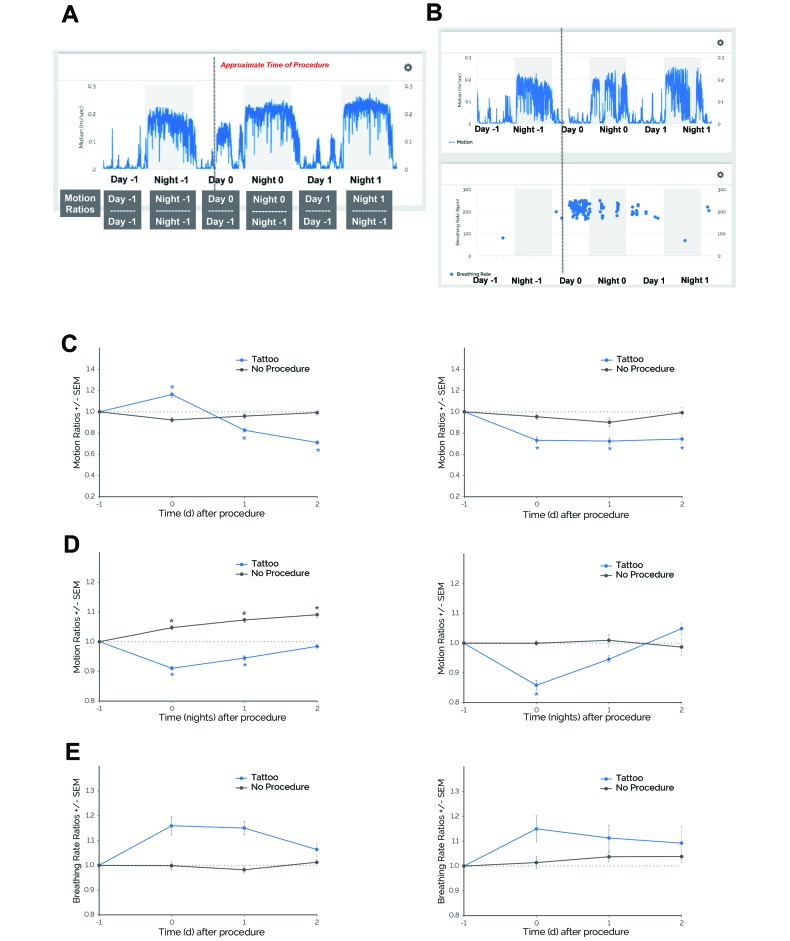
Figure 1.
Mice demonstrated behavioral and physiologic responses to tail tattooing in 2 independent experiments (experiment 1, left panels; experiment 2, right panels). (A) Sample schematic showing how metric ratios were determined. Day 0 is aligned to the day of procedure (for example, day of tattooing, ear tagging, or cage changing); therefore, day –1 and night –1 represent the day and night before the procedure, respectively, whereas day 1 and night 1 represent the day of and night after the procedure, respectively. In this example, daytime and nighttime motion data are averaged for each day or night and then normalized to the averaged motion collected on day –1 or night –1, respectively, to produce the motion ratios. (B) Motion and breathing rate profiles before and after tail tattooing of a representative subject. Gray shaded areas depict the dark phase (1800 to 0600 PDT); the dotted gray line represents the approximate time of tail tattooing. In 2 independent experiments with pair-housed, female C57BL/6J mice, (C) daytime motion ratios and (D) nighttime motion ratios significantly decreased during days when tail tattooing was performed (Tattoo) compared with days when this procedure was not performed (No Procedure). (E) Breathing rate ratios did not differ significantly after compared with before procedures. *, P ≤ 0.05 (Dunnett test) compared with day –1. Data are given as means (error bars, SEM). Experiment 1: daytime and nighttime motion ratios, n = 117 cages (Tattoo) and n = 56 cages (No Procedure); breathing rate ratios, n = 87 cages (Tattoo) and n = 44 cages (No Procedure). Experiment 2: daytime and nighttime motion ratios, n = 27 cages (Tattoo and No Procedure); breathing rate ratios, n = 24 cages (Tattoo) and n = 25 cages (No Procedure).
To calculate the maximal change for each procedure, the highest or lowest daytime motion, nighttime motion, and breathing rate ratios were identified for each subject in each experiment. The time of maximum change was identified as the day or night when the maximal change was observed.
Individual 2-way ANOVA were used to compare the effects of procedure, strain, age, time of procedure, and treatment across time. Follow-up pairwise comparisons were made by using Dunnett tests for comparing within groups and Sidak or Tukey tests for comparing between 2 or more groups, respectively. Unpaired t tests were used to assess differences in maximal change (ratio). Linear regression analyses were used to investigate relationships between age and motion ratios. For all pair-housed mice, the unit of replication was cages, and for all single-housed mice, the unit of replication was individual subjects. P values less than 0.05 were considered significantly different. Prism 7.0 (GraphPad Software, La Jolla, CA) was used for statistical analysis.
Results
Behavioral and physiologic responses to animal identification procedures and cage changing.
We first investigated whether there were detectable behavioral (motion) and physiologic (breathing rate) changes in response to 2 commonly used animal identification procedures (tail tattooing and ear tagging) and to a common husbandry event (cage changing) by performing retrospective data analysis on completed studies.
Tail tattooing.
Female C57BL/6J mice exhibited changes in motion (m/sec) and breathing rate (breaths per minute) data in response to tail tattooing (Figure 1 B). In experiment 1, daytime motion was significantly altered during specific days (procedure×time, F3,684 = 23.67, P ≤ 0.0001; Figure 1 C). Compared with the day before a procedure (day –1), daytime motion increased on day 0 and then decreased on days 1 and 2 postprocedurally (P ≤ 0.0001). Nighttime motion was also significantly altered (procedure×time, F3,616 = 16.61, P ≤ 0.0001; Figure 1 D). Compared with the night before the procedure (night –1), nighttime motion decreased on nights 0 and 1 postprocedurally (P ≤ 0.0001).
We also determined physiologic responses to tail tattooing by examining changes in breathing rate. There was no significant interaction between procedure and time (F3,470 = 2.50; Figure 1 E), although breathing rate trended toward being elevated postprocedurally. During consecutive days or nights when no procedure was performed (no-procedure control), there were no significant changes in daytime motion and breathing rate (Figure 1 C through E), with the exception of nighttime motion, which increased across consecutive nights (P ≤ 0.05 compared with night –1).
Similar results were observed in experiment 2. Daytime motion was significantly altered during specific days (procedure×time, F3,208 = 2.93, P ≤ 0.05; Figure 1 C). However, in contrast to the first experiment, daytime motion during experiment 2 was consistently reduced from days 0 through 2 postprocedurally (P ≤ 0.001 compared with day –1). Nighttime motion was also significantly altered (procedure×time, F3,208 = 7.44, P ≤ 0.0001; Figure 1 D). Compared with night –1, motion decreased on night 0 postprocedurally (P ≤ 0.0001). When breathing rate was examined, there was no significant interaction between procedure and time (F3,208 = 2.93), although breathing rate trended toward being elevated postprocedurally (Figure 1 E). During the no-procedure control days and nights, there were no significant changes in daytime motion, nighttime motion, or breathing rate (Figure 1 C through E).
Table 1 summarizes the results from the retrospective analysis of tail tattooing. For daytime motion, mice in experiments 1 and 2 showed significantly decreased ratios (P ≤ 0.05 compared with the no-procedure control) and, on average, attained their lowest daytime motion ratios 1.57 and 0.96 d postprocedurally, respectively. For nighttime motion, mice in experiments 1 and 2 showed significantly decreased ratios (P ≤ 0.05 compared with the no-procedure control) and, on average, attained their lowest nighttime motion ratios less than 1 d postprocedurally (0.54 and 0.27 night, respectively). For breathing rate, only mice in experiment 1 demonstrated significantly increased ratios (P ≤ 0.05 compared with no-procedure control), although mice from experiment 2 trended toward a similar effect. On average, mice in experiments 1 and 2 attained their highest breathing rate ratios at similar times (0.87 and 1.09 d postprocedurally, respectively).
Table 1.
Summary statistics for 2 independent tail-tattooing experiments using pair-housed, female C57Bl/6J mice
| Experiment 1 |
Experiment 2 |
|||||
| Tattooed (n = 87–117) | No procedure (n = 44–56) | Tattooed (n = 24–27) | No procedure (n = 25–27) | |||
| Daytime motion | ||||||
| Maximal change (ratio) | 0.67 ± 0.23a | 0.84 ± 0.20 | 0.59 ± 0.13a | 0.79 ± 0.15 | ||
| Time (day) of maximal change | 1.57 ± 0.62 | not applicable | 0.96 ± 0.82 | not applicable | ||
| Nighttime motion | ||||||
| Maximal change (ratio) | 0.87 ± 0.10a | 1.02 ± 0.07 | 0.84 ± 0.10a | 0.92 ± 0.13 | ||
| Time (night) of maximal change | 0.54 ± 0.75 | not applicable | 0.27 ± 0.57 | not applicable | ||
| Breathing rate | ||||||
| Maximal change (ratio) | 1.23 ± 0.47a | 1.04 ± 0.14 | 1.18 ± 0.36 | 1.12 ± 0.23 | ||
| Time (day) of maximal change | 0.87 ± 0.71 | not applicable | 1.09 ± 0.87 | not applicable | ||
Data are given as mean ± 1 SD. Experiment 1: daytime and nighttime motion ratios, n = 117 cages (Tattoo) and n = 56 cages (No Procedure); breathing rate ratios, n = 87 cages (Tattoo) and n = 44 cages (No Procedure). Experiment 2: daytime and nighttime motion ratios, n = 27 cages (Tattoo and No Procedure); breathing rate ratios, n = 24 cages (Tattoo) and n = 25 cages (No Procedure).
P ≤ 0.05 (unpaired t test) compared with No Procedure.
Ear tagging.
Female C57BL/6J mice exhibited changes in motion and breathing rate data in response to ear tagging (Figure 2 A). In experiment 1, daytime motion differed significantly during specific days (procedure×time, F3,280 = 22.73, P ≤ 0.0001; Figure 2 B). Compared with day –1, daytime motion decreased on days 0 through 2 postprocedurally (P ≤ 0.01). Nighttime motion was significantly altered also (procedure×time, F3,280 = 6.95, P ≤ 0.001; Figure 2 C). Compared with night –1, motion decreased (P ≤ 0.05) on nights 0 through 2 postprocedurally. Breathing rate showed a significant interaction between procedure and time (F3,247 = 7.85 P ≤ 0.0001; Figure 2 D). Compared with day –1, breathing rate decreased on days 1 through 2 postprocedurally (P ≤ 0.05). With the exception of breathing rate, which increased on day 1 to 2 (P ≤ 0.05 compared with day –1), there were no significant changes in daytime or nighttime motion for the no-procedure control.
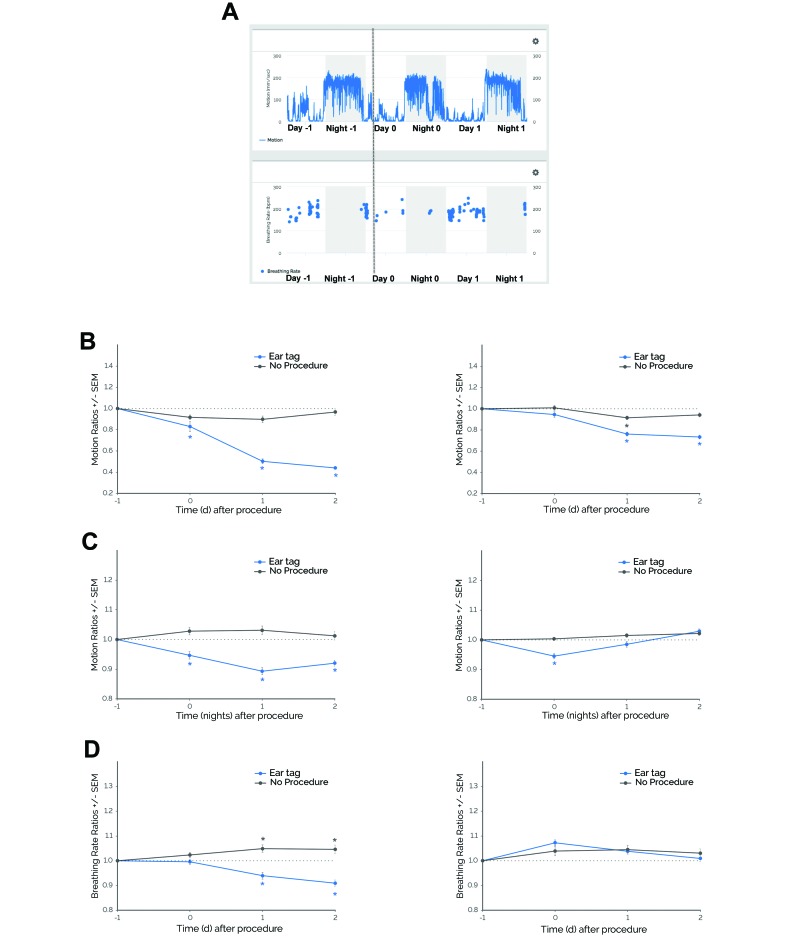
Figure 2.
Mice demonstrated behavioral and physiologic responses to ear tagging in 2 independent experiments (experiment 1, left panels; experiment 2, right panels). (A) Motion and breathing rate profiles before and after ear tagging in a representative subject. Shaded gray areas depict the dark phase (1800–0600 PDT). The dotted gray line represents the approximate time of ear tagging. In 2 independent experiments with pair-housed, female C57Bl/6J mice, (B) daytime motion ratios and (C) nighttime motion ratios significantly decreased during days when or after ear tagging was performed (Ear tag) compared with days when this procedure was not performed (No Procedure). (D) Breathing rate ratios significantly decreased in experiment 1 but did not show significant differences in experiment 2. *, P ≤ 0.05 (Dunnett test) compared with day –1. Data are given as means (error bars, SEM). Experiment 1: daytime and nighttime motion ratios, n = 36 cages (Ear tag and No Procedure); breathing rate ratios, n = 33 cages (Ear tag) and n = 34 cages (No Procedure). Experiment 2: daytime and night-time motion ratios, n = 100 cages (Ear tag and No Procedure); breathing rate ratios, n = 85 cages (Ear tag) and n = 89 cages (No Procedure).
In experiment 2, similar results were found for daytime and nighttime motion but not breathing rate. Daytime motion was significantly altered during specific days (procedure×time, F3,824 = 7.11, P ≤ 0.0001; Figure 2 B). Compared with day –1, daytime motion decreased on days 1 through 2 postprocedurally (P ≤ 0.0001). For the no-procedure control, daytime motion decreased only on day 1 (P ≤ 0.05 compared with day –1). In addition, nighttime motion was significantly altered (procedure×time, F3,800 = 5.22, P ≤ 0.001; Figure 2 C). Compared with night –1, motion decreased on night 0 (P ≤ 0.0001). There were no significant changes in nighttime motion during the no-procedure control nights. In contrast to experiment 1, there was no significant interaction between procedure and time on breathing rate (F3,744 = 1.45; Figure 2 D). Regardless of whether ear tagging was performed, breathing rate was similar across consecutive days.
Table 2 summarizes the results from retrospective analysis of ear tagging. For daytime motion, mice in experiments 1 and 2 demonstrated significantly decreased daytime motion ratios after ear tagging (P ≤ 0.05 compared with no-procedure control) and, on average, attained their lowest daytime motion ratios at 1.61 and 1.38 d postprocedurally, respectively. For nighttime motion, mice in experiments 1 and 2 showed significantly decreased ratios (P ≤ 0.05 compared with no-procedure control) and, on average, attained their lowest nighttime motion ratios at 1.00 and 0.51 nights postprocedurally, respectively. For breathing rate, mice from experiments 1 and 2 demonstrated significantly decreased and increased ratios, respectively (P ≤ 0.05 compared with no-procedure control). Furthermore, they attained their largest changes in breathing rate ratios at 1.42 and 1.12 d, respectively.
Table 2.
Summary statistics for 2 independent ear-tagging experiments using pair-housed, female C57Bl/6J mice
| Experiment 1 |
Experiment 2 |
|||||
| Ear tagged (n = 33–36) | No procedure (n = 34–36) | Ear tagged (n = 85–100) | No procedure (n = 89–100) | |||
| Daytime motion | ||||||
| Maximal change (ratio) | 0.41 ± 0.09a | 0.79 ± 0.15 | 0.68 ± 0.24a | 0.82 ± 0.21 | ||
| Time (day) of maximal change | 1.61 ± 0.49 | not applicable | 1.38 ± 0.71 | not applicable | ||
| Nighttime motion | ||||||
| Maximal change (ratio) | 0.86 ± 0.08a | 0.96 ± 0.13 | 0.92 ± 0.11a | 0.97 ± 0.07 | ||
| Time (night) of maximal change | 1.00 ± 0.75 | not applicable | 0.51 ± 0.72 | not applicable | ||
| Breathing rate | ||||||
| Maximal change (ratio) | 0.88 ± 0.10a | 0.99 ± 0.12 | 1.10 ± 0.16a | 0.97 ± 0.22 | ||
| Time (day) of maximal change | 1.42 ± 0.66 | not applicable | 1.12 ± 0.80 | not applicable | ||
Data are given as mean ± 1 SD.
Experiment 1: daytime and nighttime motion ratios, n = 36 cages (Ear Tagged and No Procedure); breathing rate ratios, n = 33 cages (Ear Tagged) and n = 34 cages (No Procedure). Experiment 2: daytime and nighttime motion ratios, n = 100 cages (Ear Tagged and No Procedure); breathing rate ratios, n = 85 cages (Ear Tagged) and n = 89 cages (No Procedure).
P ≤ 0.05 (unpaired t test) compared with No Procedure.
Cage changing.
Female C57BL/6J mice exhibited changes in motion and breathing rate data in response to cage changing (Figure 3 A). In experiment 1, daytime motion was significantly altered during specific days (procedure×time, F3,384 = 137.80, P ≤ 0.0001; Figure 3 B). Compared with day –1, daytime motion increased on days 0 through 2 postprocedurally (P ≤ 0.0001). In contrast to daytime motion, there was no significant interaction between procedure and time for nighttime motion (F2,345 = 1.00; Figure 3 C). Regardless of whether a cage change was performed, nighttime motion was similar across consecutive nights. When breathing rate was examined, there was a significant interaction between procedure and time (F2,251 = 6.14, P ≤ 0.01; Figure 3 D). Compared with day –1, breathing rate increased on days 0 through 1 postprocedurally (P ≤ 0.001). Daytime motion, nighttime motion, and breathing rate remained similar during the no-procedure control days and nights.
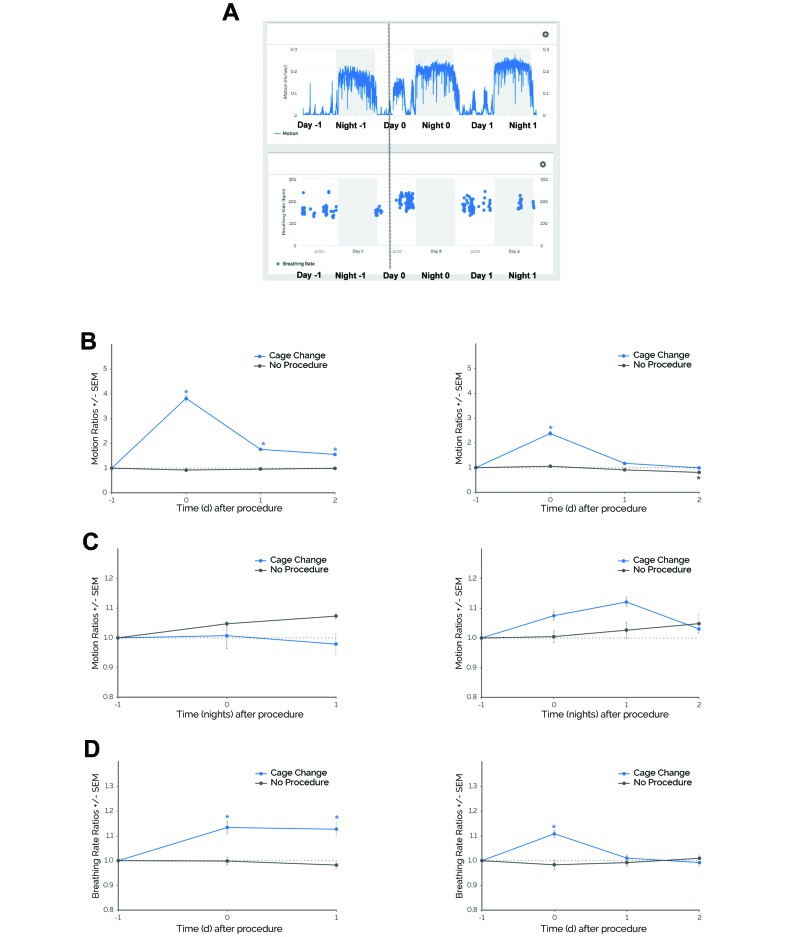
Figure 3.
Mice demonstrated behavioral and physiologic responses to cage changing in 2 independent experiments (experiment 1, left panels; experiment 2, right panels). (A) Motion and breathing rate profiles before and after cage changing in a representative subject. Shaded gray areas depict the dark phase (1800–0600 PDT). The dotted gray line represents the approximate time of cage changing. In 2 independent experiments with pair-housed, female C57BL/6J mice, daytime motion ratios significantly increased during days when cage changing was performed (Cage Change) compared with days when this procedure was not performed (No Procedure). There was no change in nighttime motion ratios. Breathing rate ratios significantly increased. *, P ≤ 0.05 (Dunnett test) compared with day –1. Error bars, SEM. Experiment 1: daytime and nighttime motion ratios, n = 61 cages (Cage Change) and n = 56 cages (No Procedure); breathing rate ratios, n = 44 cages (Cage Change and No Procedure). Experiment 2: daytime and night-time motion ratios, n = 50 cages (Cage Change and No Procedure); breathing rate ratios, n = 39 cages (Cage Change) and n = 44 cages (No Procedure).
Similar results were observed in experiment 2. Daytime motion was significantly altered during specific days (procedure×time, F3,392 = 38.52, P ≤ 0.0001; Figure 3 B). Compared with day –1, daytime motion increased on day 0 postprocedurally (P ≤ 0.0001). There was no significant interaction between procedure and time for nighttime motion (F3,392 = 2.14; Figure 3 C); nighttime motion was similar across consecutive nights regardless of whether a cage change was performed. Breathing rate showed a significant interaction between procedure and time (F3,305 = 5.97, P ≤ 0.001; Figure 3 D). Compared with day –1, breathing rate increased on the day of procedure (day 0, P ≤ 0.0001). During the no-procedure control nights and days, there were no significant changes in daytime motion, nighttime motion, or breathing rate compared with day –1 or night –1.
Table 3 summarizes the results from the retrospective analysis of cage changes. For daytime motion, mice in experiments 1 and 2 demonstrated significantly increased daytime motion ratios after cage changing (P ≤ 0.05 compared with no-procedure control) and, on average, attained their highest daytime motion ratios on the day of the procedure (0.00 and 0.06 d, respectively). For nighttime motion, mice from both experiments showed maximal ratio changes that were similar during cage change procedures and during no-procedure control nights. For breathing rate, mice in experiments 1 and 2 demonstrated significantly increased breathing rate (P ≤ 0.05 compared with no-procedure control) and, on average, attained their highest breathing rate ratios less than 1 d postprocedurally (0.44 and 0.31 d, respectively).
Table 3.
Summary statistics for 2 independent cage-changing experiments using pair-housed, female C57BL/6J mice
| Experiment 1 |
Experiment 2 |
|||||
| Cage changing (n = 44–61) | No procedure (n = 44–56) | Cage changing (n = 39–50) | No procedure (n = 44–50) | |||
| Daytime motion | ||||||
| Maximal change (ratio) | 3.80 ± 1.22a | 1.08 ± 0.28 | 2.39 ± 1.01a | 1.17 ± 0.50 | ||
| Time (day) of maximal change | 0.00 ± 0.00 | not applicable | 0.06 ± 0.31 | not applicable | ||
| Nighttime motion | ||||||
| Maximal change (ratio) | 1.06 ± 0.45 | 1.10 ± 0.09 | 1.16 ± 0.15 | 1.11 ± 0.23 | ||
| Time (night) of maximal change | 0.41 ± 0.49 | not applicable | 0.98 ± 0.68 | not applicable | ||
| Breathing rate | ||||||
| Maximal change (ratio) | 1.17 ± 0.23a | 1.02 ± 0.13 | 1.13 ± 0.12a | 1.06 ± 0.13 | ||
| Time (day) of maximal change | 0.44 ± 0.50 | not applicable | 0.31 ± 0.61 | not applicable | ||
Data are given as mean ± 1 SD.
Experiment 1: daytime and nighttime motion ratios, n = 61 cages (Cage Changing) and n = 56 cages (No Procedure); breathing rate ratios, n = 44 cages (Cage Changing and No Procedure). Experiment 2: daytime and nighttime motion ratios, n = 50 cages (Cage Changing and No Procedure); breathing rate ratios, n = 39 cages (Cage Changing) and n = 44 cages (No Procedure).
P ≤ 0.05 (unpaired t test) compared with No Procedure.
Effects of strain and sex on responses to cage changing.
The data we have presented thus far are all from female C57BL/6J mice. To investigate whether these patterns are more broadly applicable across sex and strain, we retrospectively analyzed cage-change data from male and female mice of 3 commonly used laboratory strains (C57BL/6J, BALBc/J, and C3H/J).
For daytime motion, we found a significant interaction between strain and time for male mice (F8,215 = 2.21, P ≤ 0.05; Figure 4 A). BALBc/J male mice exhibited higher daytime motion on the day of cage changing (day 0; P ≤ 0.001 compared with C57BL/6J male mice). These differences persisted until day 2 postprocedurally, when BALBc/J male mice showed higher daytime motion compared with other strains (P ≤ 0.01). There were also significant differences in the duration of the response. All mice demonstrated elevations in daytime motion on day 0 (P ≤ 0.0001 compared with day –1). However, increased activity was observed only on day 0 for C3H/J male mice but was observed through day 1 for C57BL/6J male mice (P ≤ 0.05 compared with day –1) and through day 2 for BALBc/J male mice (P ≤ 0.0001 compared with day –1). For female mice, although there was no significant interaction between strain and time (F8,205 = 0.83), there were significant main effects of strain (F2,205 = 4.83, P ≤ 0.01) and time (F4,205 = 138.20, P ≤ 0.0001; Figure 4 A). BALBc/J female mice showed higher daytime motion on day 1 (P ≤ 0.05 compared with C3H/J females) and day 2 (P ≤ 0.05 compared with C57BL/6J and C3H/J female mice). Daytime motion was increased on days 0 through 3 (P ≤ 0.001 compared with day –1).
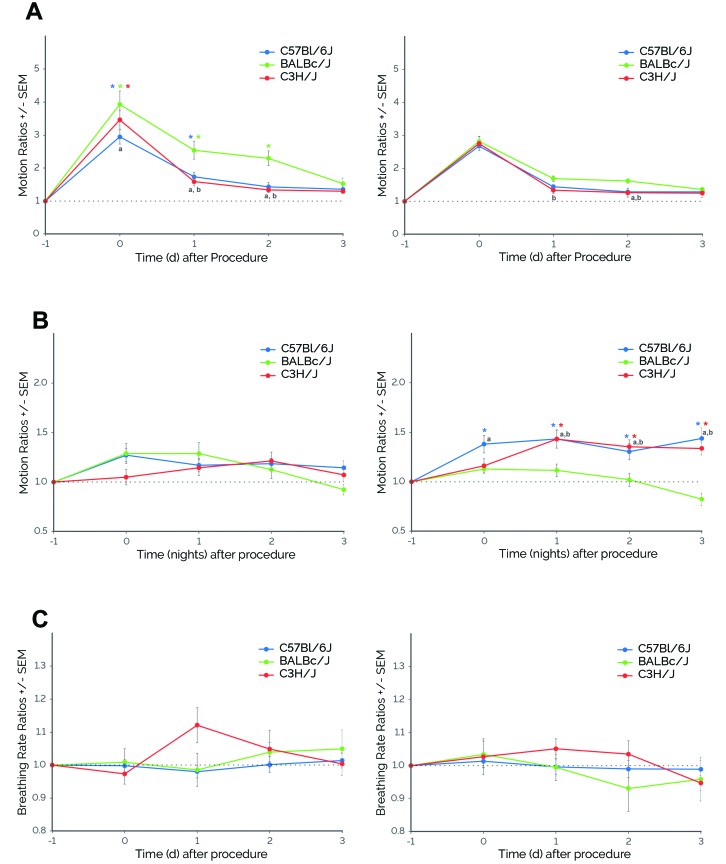
Figure 4.
Mice demonstrated sex- (males, left panels; female, right panels) and strain-dependent responses to cage changing. (A) Daytime motion. All mice exhibited significantly higher daytime motion after caging change. For males, there was a significant interaction between strain and time, with increases in activity on day 0 for C3H/J, through day 1 for C57BL/6J, and through day 2 for BALBc/J. For females, there were significant main effects of strain and time. Daytime motion significantly increased on days 0 through 3 (P ≤ 0.001 [Dunnett test] compared with day –1). For both males and females, BALBc/J mice showed the highest responses and longest periods of elevated daytime motion compared with C57BL/6J and C3H/J mice. (B) Nighttime motion. For males, there was a significant main effect of night but not strain. Nighttime motion significantly increased on nights 0 through 2 (P ≤ 0.01 [Dunnett test] compared with night –1). For females, there was a significant interaction between strain and time, with C57BL/6J and C3H/J mice demonstrating significantly higher nighttime motion after cage changing. (C) Breathing rate. For these experiments, there were no significant differences in breathing rates among strains for both males and females. *, P ≤ 0.05 [Dunnett test] compared with day –1; a, P ≤ 0.05 (Tukey test) BALBc/J compared with C57BL/6J; b, P ≤ 0.05 (Tukey test) BALBc/J compared with C3H/J. Error bars, SEM; C57BL/6J, n = 15; BALBc/J, n = 15; and C3H/J, n = 14.
For nighttime motion, there was no significant interaction between strain and time for male mice (F8,215 = 1.71; Figure 4 B). In addition, there was no significant main effect of strain (F2,215 = 0.86), but there was a significant main effect of time (F4,215 = 5.40, P ≤ 0.001). Nighttime motion increased during nights 0 through 2 (P ≤ 0.01 compared with night –1). For female mice, there was a significant interaction between strain and time (F8,205 = 3.72, P ≤ 0.001; Figure 4 B). Compared with the other strains, BALBc/J female mice showed lower nighttime motion on the night after cage changing (night 0; P ≤ 0.05 compared with C57BL/6J females), which persisted until night 3 postprocedurally (P ≤ 0.01 compared with C57BL/6J and C3H/J females). In addition, there were significant differences between strains in the responses of the female mice. After cage changing, C57BL/6J and C3H/J female mice demonstrated higher nighttime motion during nights 0 through 3 and nights 1 through 3, respectively (P ≤ 0.01 compared with night –1). In contrast to motion, breathing rates did not change significantly across specific days for either males (strain×time, F8,171 = 1.50) or females (strain×time, F8,186 = 0.69; Figure 4 C).
Effects of age on activity in response to cage changing.
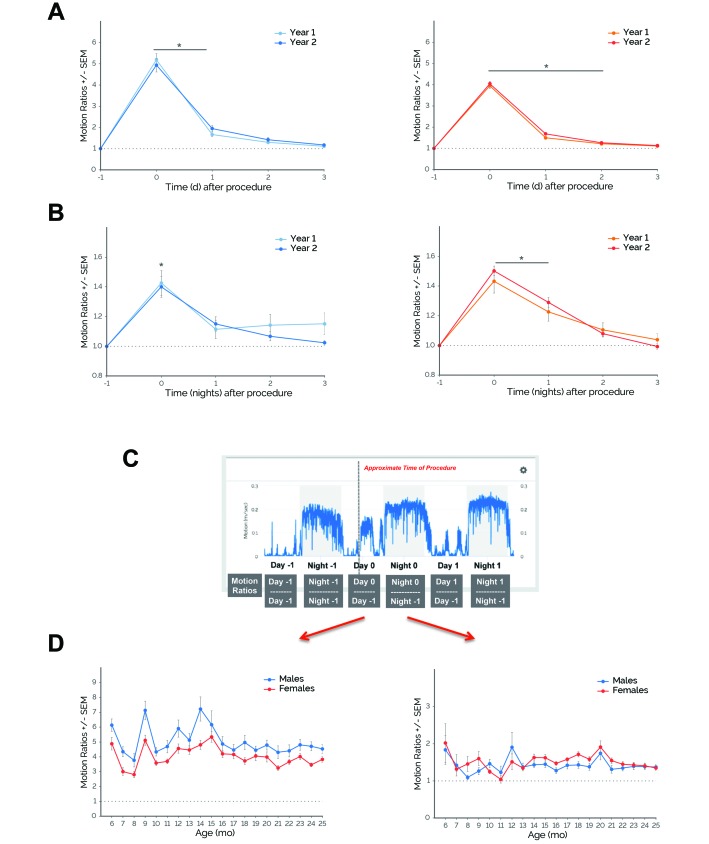
To assess longitudinal effects of routine husbandry procedures, we retrospectively examined the motion responses of male and female C57BL/6J mice to cage changes performed over an approximately 2-y period. When all cage-change responses were averaged by year (year 1, months 6 through 12; year 2, months 13 through 24), there were no significant main effects of age on daytime motion for either males (F1,135 = 0.20) or females (F1,125 = 3.28; Figure 5 A). There was a significant main effect of time on daytime motion for both males (F4,135 = 246.40, P ≤ 0.0001) and females (F4,125 = 851.20, P ≤ 0.0001). For both males and females, daytime motion increased on days 0 through 1 and days 0 through 2 postprocedurally, respectively (P ≤ 0.001 compared with day –1).
Figure 5.
Increased activity in response to cage changing remained consistent over time. (A) During years 1 and 2, both single-housed male (left panels) and female (right panels) C57BL/6J mice exhibited similar increases in daytime motion during days when cage changing was performed. (B) During years 1 and 2, both single-housed male and female C57BL/6J mice demonstrated similar increases in nighttime motion during days when a cage changing was performed. *, P ≤ 0.05 (Dunnett test) compared with day –1. (C) Revised schematic. Red arrows indicate that day 0 (day of a procedure) and night 0 (night after a procedure) motion ratios were used for subsequent analyses. (D) day 0 (left) and night 0 (right) motion ratios plotted during 6 through 25 mo of age for single-housed male and female C57BL/6J mice. Over time, there was a linear trend for day 0 ratios for males only, and there was no linear trend for night 0 ratios for both males and females. Error bars, SEM. For females: year 1, n = 14; year 2, n = 13; for males: year 1, n = 15; year 2, n = 14.
Similar results were observed for nighttime motion. When all cage-change responses were averaged by year, there were no significant main effects of age on nighttime motion for either males (F1,135 = 1.21) or females (F1,125 = 0.22; Figure 5 B). However, there was a significant main effect of time on nighttime motion for both males (F4,135 = 16.05, P ≤ 0.0001) and females (F4,125 42.94, P ≤ 0.0001). For both male and female mice, nighttime motion increased on night 0 and nights 0 through 1 postprocedurally, respectively (P ≤ 0.0001 compared with night –1).
We further dissected the responses of mice to cage changes over a 2-y period by examining the trend of monthly day 0 and night 0 motion ratios (Figure 5 C). For day 0 ratios, there was a linear trend for males (F1,264 = 6.64, P ≤ 0.01; Slope, –0.05) but not for females (F1,240 = 2.87; Figure 5 D). For night 0 motion ratios, there was no linear trend for either males (F1,264 = 0.27) or females (F1,246 = 0.005; Figure 5 D). Linear regression analyses demonstrated that for both males and females, age was neither a significant predictor of day 0 motion ratio (R2 = 0.0895 and R2 = 0.0256, respectively) nor night 0 motion ratios (R2 = 0.01045 and R2 = 0.00013, respectively).
Effects of cage changes performed later in the day on activity patterns of mice.
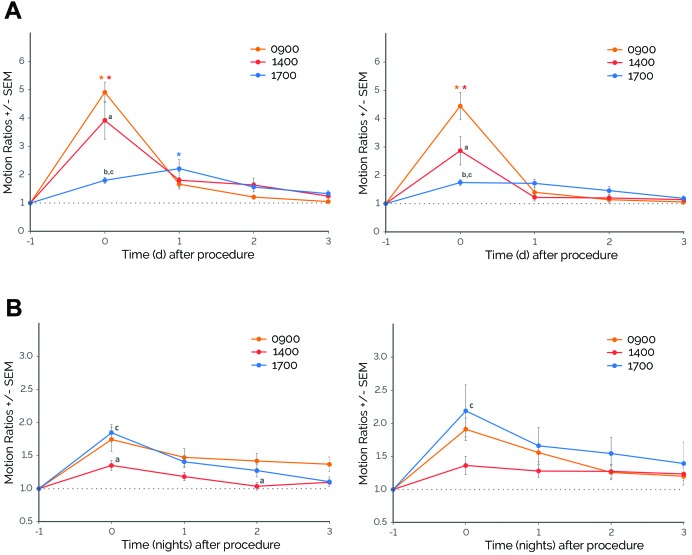
Because routine procedures are typically performed in the middle of the photocycle, when rodents are least active, we determined whether cage changing performed later in the day would attenuate the observed effects. We retrospectively analyzed cage-change data performed at different times during the day, specifically during typical husbandry hours (that is, cage changing at 0900 and 1400) compared with closer to the beginning of the dark cycle (that is, at 1700). We found significant effects of time of procedure on daytime motion during specific days for both males (time of procedure×day, F8,195 = 10.02, P ≤ 0.0001) and females (time of procedure×day, F8,138 = 9.38, P ≤ 0.0001; Figure 6 A). For male mice, cage changing performed at 0900 and 1400 led to increased daytime motion on the day of the procedure (day 0; P ≤ 0.0001 compared with day –1); however, when cage changes were performed immediately before the beginning of the dark cycle (1700), daytime motion remained similar on day 0 but increased on day 1 after cage changing (P ≤ 0.01 compared with day –1; Figure 6 A). There were also significant differences in the magnitude of change among procedure times. Cage changing at 0900 produced the highest daytime activity on day 0 (P ≤ 0.05 compared with cage changing at 1400 and 1700), followed by cage changing at 1400 (P ≤ 0.0001 compared with cage changing at 1700) and then at 1700. Similar results were observed for females: when cage changing was performed at 0900 or 1400, daytime motion increased on day 0 (P ≤ 0.0001 compared with day –1). In contrast, cage changing at 1700 did not result in significant increases in daytime motion (Figure 6 A). There were also significant differences in the magnitude of change among procedure times. Cage changing at 0900 produced the highest daytime activity on day 0 (P ≤ 0.0001 compared with cage changing at 1400 and 1700), followed by cage changing at 1400 (P ≤ 0.01 compared with cage changing at 1700), and then at 1700.
Figure 6.
Cage changes performed later in the day modified activity patterns of mice. (A) Daytime motion. Depending on the time of procedure, there were significant differences in the responses of males and females to cage changing. On the day of cage changing (day 0), cage changing at 0900 showed the highest responses, followed by cage changing at 1400, and then at 1700. For males (left panels), but not females (right panels), cage changing at 1700 resulted in significant increases in daytime motion only at day 1 after cage changing. (B) Nighttime motion. For males and females, there was no significant interaction between time of procedure and night but there were significant main effects of night and time of procedure. Nighttime motion increased on nights 0 through 3 for males (P ≤ 0.05 [Dunnett test] compared with night –1 by) and nights 0 through 2 for females (P ≤ 0.01 [Dunnett test] compared with night –1). Cage changing at 1400 showed significantly lower responses compared with cage changing performed at other times of the day. *, P ≤ 0.05 [Dunnett test] compared with day –1 or night –1; a, P ≤ 0.05 [Tukey test] 0900 compared with 1400 cage changing; b, P ≤ 0.05 [Tukey test] 0900 compared with 1700 cage changing; and c, P ≤ 0.05 [Tukey test] 1400 compared with 1700 cage changing; error bars, SEM. For females, n = 9–13; for males, n = 14.
We also investigated changes in nighttime motion. Although there were no significant effects of time of procedure on nighttime motion during specific nights for either males (time of procedure×night, F8,195 = 1.68) or females (time of procedure×night, F8,137 = 0.87), there were significant main effects of night (males: F4,195 = 20.03, P ≤ 0.0001; females: F4,137 = 8.63, P ≤ 0.0001) and time of procedure (males: F2,195 = 11.18, P ≤ 0.0001; females: F2,137 = 3.69, P ≤ 0.05; Figure 6 B). For males, nighttime motion increased on nights 0 through 3 (P ≤ 0.05 compared with night –1). Cage changing at 1400 produced the smallest increase in nighttime motion (nights 0 and 2, P ≤ 0.05 compared with cage changing at 0900; night 0, P ≤ 0.001 compared with cage changing at 1700; Figure 6 B). Similar results were observed for female mice: nighttime motion increased on nights 0 and 1 (P ≤ 0.01 compared with night –1). Cage changing at 1400 produced the smallest increase in nighttime motion (night 0, P ≤ 0.01 compared with cage changing at 1700). For both males and females, cage changes performed at 0900 and 1400 did not show significant differences from each other.
Case study: effects of cage changing on a cuprizone mouse model of multiple sclerosis.
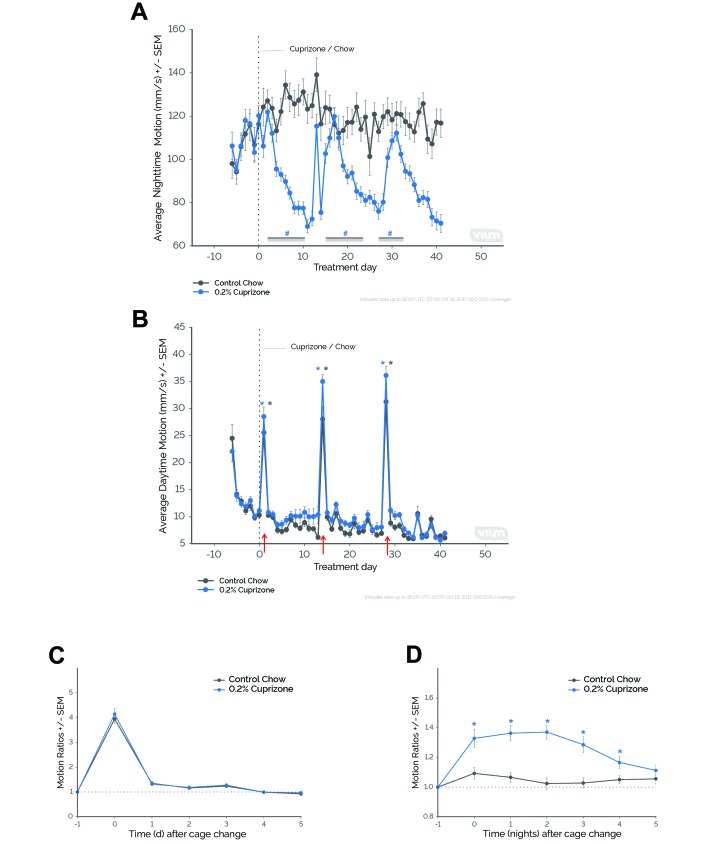
To provide an example of how cage changes might inadvertently affect research outcomes, we retrospectively analyzed a study conducted in our laboratory using a cuprizone mouse model of multiple sclerosis. Mice fed the neurotoxin cuprizone (0.2%) displayed reductions in nighttime motion compared with mice fed control chow (treatment×time, F41,1929 = 5.25, P ≤ 0.0001), specifically on nights 5 through 14, 20 through 28 (with exception of night 25), 33, and 35 through 41 (P ≤ 0.05 compared with night 0). We noticed a distinct pattern throughout the study: nighttime motion in cuprizone-treated mice returned to baseline or control levels during specific nights (that is, nights 15 through 19 and nights 29 through 32) despite maintaining reductions in activity on the nights prior to and after the increases. These periods of time consistently coincided with significant increases in daytime motion for both cuprizone-treated and control mice (main effect of time, F41,1929 = 88.53, P ≤ 0.0001), specifically on days 1, 14, and 28 (P ≤ 0.0001 compared with day 0). Retrospective review of annotated observations revealed that cage changing occurred on these days (red arrows, Figure 7 C). Both cuprizone-treated and control mice demonstrated similar elevations in daytime motion ratios during periods of cage changing (main effect of time only, F6,322 = 252.99, P ≤ 0.0001; Figure 7 C), specifically on days 0, 1, and 3 after cage changing (P ≤ 0.05 compared with day –1). In contrast to daytime motion, nighttime motion showed significant differences between treatment groups in response to cage changing (treatment×time, F6,322 = 4.21, P ≤ 0.001). Nighttime motion of cuprizone-treated—but not control mice—increased on nights 0 through 4 after cage changing (P ≤ 0.05 compared with night –1; Figure 7 D).
Figure 7.
The inadvertent effects of cage changing on a cuprizone mouse model of multiple sclerosis— a retrospective case study. (A) Nighttime motion significantly decreased in single-housed, female C57BL/6J mice fed a diet containing 0.2% cuprizone (0.2% Cuprizone) compared with mice fed a control diet (Control Chow). (B) Daytime motion significantly increased during specific study days for both treated and control mice. Periods when nighttime motion was similar between treated and control mice coincided with increases in daytime motion, which occurred on days of cage changing (that is, days 1, 14, and 28; red arrows). (C and D) Average daytime and nighttime motion ratios aligned to cage changing (day 0 and night 0, respectively). (C) Although there was no significant interaction between time and treatment, there was a significant main effect of time. Daytime motion ratios significantly increased on days 0, 1, and 3 (P ≤ 0.05 compared with day –1). (D) Only treated mice demonstrated significant increases in nighttime motion ratios, and this effect lasted for as long as 4 nights after cage changing. #, P ≤ 0.05 (Sidak test) compared with control chow; *, P ≤ 0.05 (Dunnett test) compared with day 0 or day –1; error bars, SEM. For 0.2% cuprizone mice, n = 30; for control chow mice, n = 18.
Discussion
Using retrospective data analysis, we investigated the behavioral and physiologic profiles of mice in response to common laboratory and husbandry procedures: animal identification (tail tattooing and ear tagging) and cage changing. Across independent studies, we found that these procedures were associated with distinct and reproducible changes in motion and breathing rate patterns (Table 4). Responses to cage changing were strain- and sex-dependent and consistent over an approximately 2-y period in our vivarium. However, the time of day at which cage changing was performed affected the responses of mice. Finally, we described results from a previously completed study showing how failing to consider the experimental coordination of cage changes inadvertently affected results (for example, motion data) in a cuprizone mouse model of multiple sclerosis. Our results confirm and extend previously published studies showing that routine procedures affect rodent behavior and physiology.1,7,13,27,28
Table 4.
Summary of metric changes in response to routine procedures during 2 independent experiments using pair-housed, female C57BL/6J mice
| Tattooing | Ear tagging | Cage changing | |
| Daytime motion | |||
| Change | decrease | decrease | increase |
| Maximal change (average ratio) | 0.63 ± 0.06 | 0.55 ± 0.19 | 3.10 ± 1.00 |
| Time (average day) of maximal change | 1.27 ± 0.43 | 1.50 ± 0.16 | 0.03 ± 0.04 |
| No. of days to return to baseline | 2+ | 2+ | 1–2 |
| Nighttime motion | |||
| Change | decrease | decrease | no change |
| Maximal change (average ratio) | 0.86 ± 0.02 | 0.89 ± 0.04 | 1.11 ± 0.07 |
| Time (average night) of maximal change | 0.41 ± 0.19 | 0.76 ± 0.35 | 0.70 ± 0.40 |
| No. of nights to return to baseline | 0–1 | 0–2 | not applicable |
| Breathing rate | |||
| Change | increase | inconsistent | increase |
| Maximal change (average ratio) | 1.21 ± 0.03 | 0.99 ± 0.16 | 1.15 ± 0.03 |
| Time (average day) of maximal change | 0.98 ± 0.16 | 1.27 ± 0.21 | 0.38 ± 0.09 |
| No. of days to return to baseline | 0–1 | 0–2 | 0–1 |
Data for maximal change and time of maximal change are given as mean ± 1 SD of 2 independent experiments. Data for number of days or nights to return to baseline are given as ranges of the number of days or nights when ratios returned back to baseline.
All of the analyses that we conducted in this report were performed retrospectively on available data from completed studies. This approach has a number of advantages, including the ability to observe reproducible effects across several studies with large sample sizes and with no additional use of animals solely for the purpose of investigating our hypothesis. However, using retrospective studies that have other in-life study measurements had several limitations. First, we were limited by the number of postprocedural days available for assessment. For example, although we were able to assess at least 2 d after animal identification procedures or cage changing for most studies, we were only able to assess nighttime motion and breathing rate metrics for a maximum of 1 d after cage changing in experiment 1. In this case, day 2 was omitted from the analysis due to in-life study measurements, which confounded data interpretation for nighttime motion and breathing rate. Second, conditions across several experiments could not be completely controlled due to the nature of the analysis method. For example, ear tagging in experiment 1 differed from that of experiment 2, in that different brands of ear tags were used in each experiment. Variations between and among experiments may account for some of the observed differences and inconsistencies in results (for example, breathing rate data from ear tagging during experiment 1 compared with experiment 2). Third, we were unable to investigate the differential effects of single- compared with group-housing conditions and analgesia use. With the exception of all the animal identification studies and cage changing experiments 1 and 2, several of our studies used individually housed mice because our continuous monitoring platform is currently optimized to track a single animal in each cage. Whether the responses observed after animal identification procedures or cage changing are modified due to housing condition (that is, social buffering)18 or the use of analgesia—which might be used concurrently during invasive animal identification procedures—remains to be examined. We are in the process of optimizing our platform to track group-housed mice and will reexamine these questions. Finally, we were unable to collect blood or tissue samples for further biochemical analyses or perform conventional behavioral assays to correlate with the automated motion and breathing rate metrics. These endpoints may provide further insight regarding the mechanisms underlying behavioral and physiologic changes (for example, stress, pain, hyperactivity). Despite these limitations, the most robust behavioral and physiologic patterns were generally similar across different experiments and conditions, thus allaying these concerns.
Although widely used and relatively safe, animal identification procedures—such as tail tattooing, ear tagging, ear notching, toe or tail clipping, and microchip or transponder insertion—are associated with various adverse side effects, including infection, inflammation, and pain.6,8,9 In adult rodents, previous research using cardiovascular telemetry reported increases in heart rate and mean arterial pressure in rats undergoing paw microtattooing, ear tattooing, and ear notching; these measures returned to baseline by 24 h postprocedurally.20 In addition, neonatal mice undergoing various animal identification procedures demonstrated increased vocalization.6
We compared motion and breathing rate after 2 commonly used animal identification procedures—tail tattooing and ear tagging—and found that both procedures led to similar decreases in spontaneous daytime and nighttime activity (decreases of less than 50% and less than 15%, respectively). Changes in motion were observed for 2 d (or potentially more than 2 d, in the case of daytime motion) postprocedurally. We also found elevations in breathing rate (approximately 20% increase), which lasted for as long as 1 d after tattooing.
Decreases in motion and increases in breathing rates resulting from animal identification procedures may be suggestive of pain and discomfort in these animals.3,15,22 In addition, changes in breathing rate distinguished animals’ responses to tail tattooing and ear tagging. Modest elevations in breathing rate after tattooing compared with ear tagging may suggest that tattooing is the more invasive of these 2 animal identification methods. Further investigation is required to correlate these behavioral and physiologic changes with standard behavioral and biochemical measurements. In summary, our data suggest that animal identification methods can lead to short-term alterations in activity and breathing rate and that tail tattooing may be more invasive than ear tagging. Data from both methods indicate that, with the exception of daytime motion, it takes approximately 2 d for mouse behavior and physiology to return to preprocedural levels.
Although routine cage changing (that is, approximately every 14 d for mouse cages) is important for maintaining the health and hygiene of animals, this husbandry procedure is associated with a number of behavioral and physiologic effects in rodents. Previous research is rich with examples, including: 1) increases in heart rate and mean arterial pressure;1,11,25 2) aggression among male cage mates;5 3) elevations in locomotion and other spontaneous activities;28 4) disruptions in sleep and circadian rhythms;12,27 and 5) elevated stress responses.26,29 Therefore, there is growing interest not only to understand the effects and repercussions of animal responses to cage changes but also to reduce them.
We compared motion and breathing rate after cage changing. Compared with animal identification procedures, cage changing led to a larger increase in daytime motion (approximately 3fold increase from baseline). Although activity changes were observed for nighttime motion also (Figures 4 B, 5 B, 6 B, and 7 D), increases in daytime motion were more consistent and dramatic than for nighttime motion (3fold compared with 1.5fold increase from baseline, respectively). Peak changes in daytime motion occurred during the day of cage change and lasted as long as 2 d postprocedurally. However, for some studies, changes in motion (daytime or nighttime) were observed for as long 4 d after cage changing (Figure 7 D). We also found that cage changing resulted in an approximately 15% increase in breathing rate, which was observed as long as 1 d postprocedurally.
Increases in motion and breathing rate after cage changing may be indicative of hyperactivity, increased energy expenditure, or acute stress resulting from exposure to a novel environment (that is, neophobia). Cage changing results in a new environment for rodents (that is, fresh bedding, cotton squares, enrichment) and thus can subsequently affect animal behavior and physiology. Animals rely heavily on olfactory cues for establishing territory as well as for recognizing and communicating with cage mates.13 Therefore, completely removing all prior olfactory cues by providing a clean cage can provide a strong aversive stimulus.
Our current study also demonstrated that responses to cage changing may or may not differ depending on the sex, strain, and age of the animal. BALBc/J mice showed heightened responses compared with 2 other commonly used mouse strains (C57BL/6J and C3H/J). Phenotyping studies have documented that the BALBc/J strain shows higher levels of emotional reactivity and anxiety compared with other strains.10 The increased magnitude and duration of the responses of BALBc/J mice, specifically males, may reflect the stress-inducing nature of cage changing.26,29 We also found that, regardless of sex, animals’ responses to cage changing remained remarkably consistent over time even as animals aged and became less active.2,16 How responses to routine procedures interact with age-related changes in activity and metabolic patterns, habituation, cognition, or stress responses requires further investigation. In summary, cage changing in mice: 1) results in more dramatic behavioral (motion) responses than animal identification procedures; 2) elicits sex- and strain-dependent differences; and 3) maintains remarkably consistent responses even as animal age. Furthermore, our data suggest that it takes approximately 2 to 4 d for mouse behavior and physiology to return to levels before cage changing.
Several studies have explored various methods to reduce the behavioral and physiologic effects of routine procedures, including identifying methods that provide the least disturbance (for example, different animal handling procedures), manipulating environmental factors such as bedding or nesting material, and adjusting the scheduling of the procedure within the facility.27,28 Experiments in rodents are often conducted during the middle of the light cycle, when rodents are least active, instead of during the dark cycle to coincide with periods of increased activity. We found that cage changing conducted late during the day or near the beginning of the dark cycle attenuated daytime motion on the day of the procedure compared with cage changing performed early during the day. However, the effects of late cage changing (1700) on daytime motion appeared to be prolonged for male mice, as demonstrated by the significantly higher daytime motion ratios on day 1 compared with the other procedure times. Furthermore, for both male and female mice, cage changing at 1700 did not reduce nighttime motion. These results concur with previously published literature27 and highlight the robust effect of cage changing on animal behavior. Regardless of the time of the procedure, mice modify their behavior patterns in response to a cage change, suggestive of an immediate requirement for animals to explore, build nests, and mark their new surroundings. Minor disruptions to an animal's circadian rhythm or sleep patterns can have long-term physiologic, metabolic, and behavioral consequences, thereby negatively affecting animal health and confounding interpretation of scientific data.19
Behavioral and physiologic effects of routine husbandry and laboratory procedures have been reported, yet the practical physiologic relevance and repercussions have rarely been discussed. By performing retrospective analysis on a previously completed study involving a cuprizone mouse model of multiple sclerosis, we found that cage changing conducted during experiments may inadvertently affect data collection and interpretation. As expected, mice fed a diet containing 0.2% cuprizone showed decreases in spontaneous activity compared with mice fed control chow; these effects may be interpreted as reductions in gross motor function due to demyelination.23 However, there were periods of apparent ‘recovery,’ when diseased animals displayed similar levels of activity compared with controls, and these periods coincided with increases in daytime motion due to cage changing. First, these results demonstrate that activity deficits in this mouse model can be uncovered by examining changes in nighttime motion by using a continuous monitoring platform. Second, these results reveal the inadvertent effects of cage changes on the collection and interpretation of study data. From these observations, we cannot determine whether this mouse model might have shown relapsing and remitting disease symptoms in the absence of cage changing or whether cage changing might have approximated standard assays that invoke behaviors. Because the cuprizone mouse model is not commonly used to study the relapsing–remitting clinical symptoms of multiple sclerosis and because remyelination is not normally observed until cuprizone is withdrawn,21 the observed effects are likely associated with cage changing. Although further investigation is required to assess the effects of cage changing on disease severity and pathology, this case study example underscores the importance of understanding the effects of routine procedures and of planning these procedures appropriately.
By using a continuous monitoring platform, the goal of the current research was to provide meaningful insights into the effects of routine laboratory and husbandry procedures and to direct recommendations to guide researchers when planning procedures. Our data suggest that approximately 2 d after animal identification methods and 2 to 4 d after cage changing may be sufficient for the return of mouse behavior and physiology to preprocedural levels. These insights highlight the importance of considering how responses to common procedures are affected by the strain, sex, and age of study subjects; the type of study; and its relevant endpoints. Understanding and considering the effects of these procedures not only enhances animal welfare by providing ample recovery or habituation time prior to performing in-life data collection but also refines scientific work practices that may ultimately improve quality control and study reproducibility.
Acknowledgments
We acknowledge M Van Hoy and A Battiwala for assisting with experiments used for retrospective analysis; M Rabe, C Ichim, and J Do for their insightful comments during manuscript preparation; and S Bengali for assistance with figures. All authors are or were employed by Vium, which developed the continuing monitoring platform and accompanying metrics used in the study.
References
- 1.Balcombe JP, Barnard ND, Sandusky C. 2004. Laboratory routines cause animal stress. Contemp Top Lab Anim Sci 43:42–51. [PubMed] [Google Scholar]
- 2.Bartling B, Al-Robaiy S, Lehnich H, Binder L, Hiebl B, Simm A. 2017. Sex-related differences in the wheel-running activity of mice decline with increasing age. Exp Gerontol 87:139–147. 10.1016/j.exger.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 3.Baumans V, Brain PF, Brugere H, Clausing P, Jeneskog T, Perretta G.1994. Pain and distress in laboratory rodents and lagomorphs. Report of the Federation of European Laboratory Animal Science Associations (FELASA) Working Group on Pain and Distress accepted by the FELASA Board of Management November 1992. Lab Anim 28:97–112. 10.1258/002367794780745308. [DOI] [PubMed] [Google Scholar]
- 4.Brenneis C, Westhof A, Holschbach J, Michaelis M, Guehring H, Kleinschmidt-Doerr K. 2017. Automated tracking of motion and body weight for objective monitoring of rats in colony housing. J Am Assoc Lab Anim Sci 56:18–31. [PMC free article] [PubMed] [Google Scholar]
- 5.Burn CC, Peters A, Day MJ, Mason GJ. 2006. Long-term effects of cage-cleaning frequency and bedding type on laboratory rat health, welfare, and handleability: a cross-laboratory study. Lab Anim 40:353–370. 10.1258/002367706778476460. [DOI] [PubMed] [Google Scholar]
- 6.Castelhano-Carlos MJ, Sousa N, Ohl F, Baumans V. 2010. Identification methods in newborn C57BL/6 mice: a developmental and behavioural evaluation. Lab Anim 44:88–103. 10.1258/la.2009.009044. [DOI] [PubMed] [Google Scholar]
- 7.Champy MF, Selloum M, Piard L, Zeitler V, Caradec C, Chambon P, Auwerx J. 2004. Mouse functional genomics requires standardization of mouse handling and housing conditions. Mamm Genome 15:768–783. 10.1007/s00335-004-2393-1. [DOI] [PubMed] [Google Scholar]
- 8.Chen M, Kan L, Ledford BT, He JQ. 2016. Tattooing various combinations of ears, tail, and toes to identify mice reliably and permanently. J Am Assoc Lab Anim Sci 55:189–198. [PMC free article] [PubMed] [Google Scholar]
- 9.Cover CE, Keenan CM, Bettinger GE. 1989. Ear-tag–induced Staphylococcus infection in mice. Lab Anim 23:229–233. 10.1258/002367789780810482. [DOI] [PubMed] [Google Scholar]
- 10.Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. 1997. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl) 132:107–124. 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- 11.Duke JL, Zammit TG, Lawson DM. 2001. The effects of routine cage-changing on cardiovascular and behavioral parameters in male Sprague-Dawley rats. Contemp Top Lab Anim Sci 40:17–20. [PubMed] [Google Scholar]
- 12.Febinger HY, George A, Priestley J, Toth LA, Opp MR. 2014. Effects of housing condition and cage change on characteristics of sleep in mice. J Am Assoc Lab Anim Sci 53:29–37. [PMC free article] [PubMed] [Google Scholar]
- 13.Gerdin AK, Igosheva N, Roberson LA, Ismail O, Karp N, Sanderson M, Cambridge E, Shannon C, Sunter D, Ramirez-Solis R, Bussell J, White JK. 2012. Experimental and husbandry procedures as potential modifiers of the results of phenotyping tests. Physiol Behav 106:602–611. 10.1016/j.physbeh.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Groeben H, Meier S, Tankersley CG, Mitzner W, Brown RH. 2003. Heritable differences in respiratory drive and breathing pattern in mice during anaesthesia and emergence. Br J Anaesth 91:541–545. 10.1093/bja/aeg222. [DOI] [PubMed] [Google Scholar]
- 15.Hawkins P. 2002. Recognizing and assessing pain, suffering and distress in laboratory animals: a survey of current practice in the UK with recommendations. Lab Anim 36:378–395. 10.1258/002367702320389044. [DOI] [PubMed] [Google Scholar]
- 16.Houtkooper RH, Argmann C, Houten SM, Canto C, Jeninga EH, Andreux PA, Thomas C, Doenlen R, Schoonjans K, Auwerx J. 2011. The metabolic footprint of aging in mice. Sci Rep 1:1–11. 10.1038/srep00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 18.Kappel S, Hawkins P, Mendl MT. 2017. To group or not to group? Good practice for housing male laboratory mice. Animals (Basel) 7:1–25. 10.3390/ani7120088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karatsoreos IN, Bhagat S, Bloss EB, Morrison JH, McEwen BS. 2011. Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc Natl Acad Sci USA 108:1657–1662. 10.1073/pnas.1018375108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kasanen IH, Voipio HM, Leskinen H, Luodonpaa M, Nevalainen TO. 2011. Comparison of ear tattoo, ear notching and microtattoo in rats undergoing cardiovascular telemetry. Lab Anim 45:154–159. 10.1258/la.2011.010113. [DOI] [PubMed] [Google Scholar]
- 21.Kipp M, Nyamoya S, Hochstrasser T, Amor S. 2017. Multiple sclerosis animal models: a clinical and histopathological perspective. Brain Pathol 27:123–137. 10.1111/bpa.12454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirsch JH, Klaus JA, Blizzard KK, Hurn PD, Murphy SJ. 2002. Pain evaluation and response to buprenorphine in rats subjected to sham middle cerebral artery occlusion. Contemp Top Lab Anim Sci 41:9–14. [PubMed] [Google Scholar]
- 23.Liebetanz D, Merkler D. 2006. Effects of commissural de- and remyelination on motor skill behaviour in the cuprizone mouse model of multiple sclerosis. Exp Neurol 202:217–224. 10.1016/j.expneurol.2006.05.032. [DOI] [PubMed] [Google Scholar]
- 24.Lim MA, Louie B, Ford D, Heath K, Cha P, Betts-Lacroix J, Lum PY, Robertson TL, Schaevitz L. 2017. Development of the digital arthritis index, a novel metric to measure disease parameters in a rat model of rheumatoid arthritis. Front Pharmacol 8:1–18. 10.3389/fphar.2017.00818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meller A, Kasanen I, Ruksenas O, Apanaviciene N, Baturaite Z, Voipio HM, Nevalainen T. 2011. Refining cage change routines: comparison of cardiovascular responses to 3 different ways of cage change in rats. Lab Anim 45:167–173. 10.1258/la.2011.010134. [DOI] [PubMed] [Google Scholar]
- 26.Rasmussen S, Miller MM, Filipski SB, Tolwani RJ. 2011. Cage change influences serum corticosterone and anxiety-like behaviors in the mouse. J Am Assoc Lab Anim Sci 50:479–483. [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson-Junker AL, O'Hara BF, Gaskill BN. 2018. Out like a light? The effects of a diurnal husbandry schedule on mouse sleep and behavior. J Am Assoc Lab Anim Sci 57:124–133. [PMC free article] [PubMed] [Google Scholar]
- 28.Saibaba P, Sales GD, Stodulski G, Hau J. 1996. Behaviour of rats in their home cages: daytime variations and effects of routine husbandry procedures analysed by time sampling techniques. Lab Anim 30:13–21. 10.1258/002367796780744875. [DOI] [PubMed] [Google Scholar]
- 29.Van Loo PL, Van der Meer E, Kruitwagen CL, Koolhaas JM, Van Zutphen LF, Baumans V. 2004. Long-term effects of husbandry procedures on stress-related parameters in male mice of two strains. Lab Anim 38:169–177. https://doi.org/10.1258/ 002367704322968858. [DOI] [PubMed] [Google Scholar]