Abstract
Although social housing of mice generally is preferred, mice must be individually housed in some situations. In these cases, enhanced attention to environmental enrichment is encouraged, but few studies assess the wellbeing of mice provided various enrichments. In this study, we used female ICR mice to evaluate enrichment strategies that encouraged natural behaviors including foraging, exercise, sheltering, and socialization. After 3 mo of exposure to the assigned enrichment strategy, wellbeing was assessed by evaluating behavioral and physiologic differences between groups. The results suggested that the use of red-tinted igloos may decrease markers of mouse wellbeing. However, none of the selected strategies yielded measures of wellbeing indicating improvement as compared to individually housed mice with no enrichment (negative control). Furthermore, measures were not significantly different between paired mice and individually housed mice with no enrichment.
The Guide for the Care and Use of Laboratory Animals emphasizes the importance of social housing for many species, including rodents, because social isolation is generally considered to be detrimental psychologically and physiologically.17,18,31 Barren environments have been suggested to decrease wellbeing, immune function, and neurologic development.21 However, sometimes rodents must be housed individually because of injury, attrition, incompatibility, or study protocol. In these situations, the Guide recommends provision of enrichment to decrease stress and to improve the wellbeing of the individual animal.17
Although completely eliminating stress is impossible and arguably undesirable, ideal environmental enrichment provides coping mechanisms for laboratory animals through a variety of mechanisms. Cage structures (such as nesting material, boxes, and tubes) compartmentalize cage space and provide mice some measure of control over light, temperature, and air exposure.4,29 Other enrichment strategies provide animals the means to practice behaviors they normally would use in the wild (or when otherwise given liberty to choose their behaviors). For example, in the wild, most of a mouse's time is spent foraging,4 so finding ways to encourage this activity mimics their natural environment.
From an evolutionary standpoint, these innate behaviors presumably serve to enhance animals’ survivability and ability to pass their genes to future generations. For example, opportunities for exercise enhance cardiovascular and skeletal health; successful social participation increases likelihood of offspring; chewing and foraging promotes dental and gastrointestinal health, and suitable hiding and nesting areas provide security and warmth.23,27,32 The more animals can perform these natural behaviors, the healthier they will be and the better they will model humans, who are able to choose their own behaviors.3
In this study, we examined a variety of enrichment strategies to assess which might be most beneficial for mice. To this end, we provided groups of female ICR mice with 1 of 11 different environmental enrichment strategies that encouraged natural behaviors such as foraging, exercise, sheltering, and socialization. We then assessed physiologic and behavioral measurements of wellbeing.
For this study, we hypothesized that singly housed, unenriched mice would demonstrate behavioral and physiologic evidence of being stressed (negative control); those pair-housed would be the least stressed (positive control); and singly housed, enriched animals would fall in the middle, with little difference as to which enrichment device was used. Within the treatment groups and in light of current literature,23,27,32 we predicted that nesting materials would show the most evidence of use and improved animal wellbeing. We also expected that rodents would become acclimated to their enrichment device and hypothesized that a rotation of devices would be more novel and stimulating. Recognizing that environmental enrichment is best evaluated through multiple measures of behavior and physiology,4 we measured body weight, CBC, organ weights, and behavior.
Materials and Methods
Experimental design.
Through peer-sharing groups, marketing materials, and research papers, we compiled a list of potential enrichment devices for our institution. From this list, we selected 11 treatments which included foraging, exercise, sheltering, and socialization behaviors (Figure 1) for evaluation in this study.
Figure 1.
Description of enrichment treatments. All treatments were provided 7 d each week. For analysis, treatment groups were subdivided into subgroups according to enrichment type. These subgroups were compared with singly housed (negative control) and paired (positive control) mice.
Animals.
With regard to the principle of reduction, this study was part of our existing indirect sentinel program for one quarter. We used female ICR mice (Taconic, Cambridge City, IN) purchased at 4 to 6 wk of age. With the exception of the mice in the paired animal group, which were pair-housed upon arrival, the mice were single-housed and allowed to acclimate to the facility for 1 wk. The singly housed mice were randomly assigned to their treatment group (Figure 1) and balanced across caging type and facility, by calculating the number of sentinel cages required per animal facility and determining the proportion of cages in each treatment to be assigned to each facility. Facility 1 had 16 rooms, Facility 2 had 11 rooms, Facility 3 had 7 rooms, Facility 4 had 6 rooms, and Facility 5 had 3 rooms. We were unable to ensure that each room had each treatment, due to the design of the sentinel program with which this project was associated. Because room could be a potential confounder for the experimental design, we strove to mitigate this effect by spreading treatments across all facilities. The range of treatments per room was dependent on the room population size and was 0 to 2 per room, with an average of 1 per room.
General husbandry.
This study was conducted across 5 animal facilities at the Laboratory Animal Resource Center (LARC) of the Indiana University School of Medicine, which is AAALAC-accredited. All parts of this study were performed under an IACUC-approved protocol. Traffic flow was from clean to dirty with regard to biosecurity and rodent husbandry room disease status. Access was restricted at facility and room levels to investigator personnel. All husbandry and veterinary staff wore facility-laundered scrub uniforms when in animal rooms. Personal protective equipment, including disposable gowns, bonnets, face masks, and gloves, was required for everyone when handling animals. Cages were opened only within a laminar flow workstation.
Mouse colonies at this institution were screened quarterly for the following pathogens by using indirect sentinel exposure: Sendai virus, mousepox virus, minute virus of mice, reovirus 3, pneumonia virus of mice, mouse hepatitis virus, Mycoplasma pulmonis, epizootic diarrhea of infant mice virus, murine encephalitis virus, and rodent pinworms and mites. In addition to the quarterly panel, mouse colonies were screened (SMART-SPOT 9-Analyte ELISA Panel, Biotech Trading Partners, Dublin, OH) semiannually for Clostridium piliforme, ectromelia virus, lymphocytic choriocytic meningitis virus, hantavirus, mouse adenovirus, and murine cytomegalovirus. The facilities do not regularly screen mouse colonies for Helicobacter spp. or norovirus, except for colonies where investigators have requested this additional screening. At the time of study, 2 of the 43 rooms of mice were positive for fur mites and pinworms (both in Facility 1).
Microenvironment.
Mice were housed in IVC (Alternative Design, Siloam Springs, AK, or Lab Products, Seaford, DE, depending on the facility) with the air handler set to 50 air changes per hour. Either corncob (Teklad 7097, Harlan, Madison, WI) or pelleted paper (Teklad 7084, Harlan) bedding were used, depending on the facility. Standard chow (Teklad 2018, Harlan) and reverse-osmosis–filtered water were provided without restriction through water bottles or an automatic watering system. As described earlier, cages were randomized to ensure balance across these facility-level variations.
Cage sanitation.
Cages were changed every other week in a laminar flow workstation (NuAire, Plymouth, MN). Animal caretakers wore gloves while changing cages and sprayed gloved hands with disinfectant (either 10% bleach solution or MB10, Quip Labs, Wilmington, DE) between cages, to control opportunistic pathogens. Soiled cages and reusable enrichment devices were sanitized in a mechanical cage washer with a final rinse temperature of 180 °F (82 °C). All caging equipment (including reusable and paper or cardboard enrichment devices) were autoclaved prior to use. Because animals in this study were sentinels, at the time of cage change, all cages of mice were exposed to approximately 1 teaspoon of dirty bedding from each cage on a rack. Facility standard operating procedures call for one sentinel cage to every 70 colony cages, so choosing large population rooms (that is, those with at least 100 cages of colony animals) kept sentinel exposure rates similar.
Macroenvironment.
Rooms were maintained on a 12:12-h light:dark cycle. Temperature was maintained at 72 ± 1 °F (22.2 ± 0.5 °C), and humidity was 30% to 70%.
Staff training.
Prior to the start of the study, one author (JP) met in-person with husbandry staff and management at each facility to explain the study purpose, process, and expectations. A 1-page hard-copy summary was provided for future reference.
Staff changed and exposed sentinel cages to dirty bedding as scheduled for the facility sentinel program and moved enrichment items from the old to the new cage. For mice in the dietary enrichment group, husbandry staff was directed to scatter or hide 5 seeds in each cage daily. Research staff provided new enrichment every other week when they collected data (enrichment use, handling scores, and body weights). Labels on each cage card indicated the enrichment item that belonged in the cage.
Experimental procedures.
After a 1-wk acclimation period, rodents were moved to our surgery suite area in preparation for their baseline blood sample collection. They were given 1 h to recover from the potential stress of cage transportation before collection began.5
For blood collection, mice were anesthetized by using isoflurane (Piramal Enterprises, Andhra Pradesh, India). Samples were collected from either the medial saphenous or superficial facial vein, according to technician preference. Although the use of anesthesia can result in additional stress, we wanted baseline samples comparable to the final blood collection (exsanguination under isoflurane anesthesia). Next, each mouse was weighed, one was ear tagged (when housed in the paired experimental set), and the cage was assigned a unique identifier. Enrichment devices were added to the cage according to assigned treatment group. After return of the mice to their housing rooms, husbandry staff were instructed to begin dirty-bedding sentinel exposure. Every other week, research staff replaced or renewed enrichment (as appropriate), weighed the sentinel, and scored handling tolerance and enrichment use behaviors. All scoring was done within a 4-h window in the afternoon.
Enrichment use was scored manually cageside, before the cage was disturbed in any way. The scale criteria were: 0, enrichment item shows no indication of use; 1, enrichment item shows sign of use; and 2, mouse is currently using enrichment item.
After scoring of enrichment use, the cage was brought to the change station, where body weight was measured and a handling tolerance score was assigned on a scale of 1 to 3: 1, not agreeable to handling (aggressive, elusive, and so forth); 2, tolerates handling as expected; and 3, seems content or friendlier than expected. New enrichment then was provided, and the cage was closed and returned to the ventilation rack.
At the end of the 3-mo data collection period, mice were processed as sentinels, with terminal cardiac blood collection after isoflurane anesthesia. The collected sample was split between an EDTA-treated tube and a tube with no additive. The serum was processed as part of the sentinel program (see General Husbandry section for sentinel pathogen screening performed), whereas the EDTA-treated blood was used for CBC analysis (Hemavet, Drew Scientific, Miami Lakes, FL). The neutrophil:lymphocyte ratio, a measurement of chronic stress in mice,10,16 was calculated by dividing the total number of neutrophils by the total number of lymphocytes.
Final body weights were recorded for comparison between groups. Gross necropsy was performed for signs of abnormal clinical conditions, and adrenal glands, spleen, thymus, and heart were collected. The proportional weight of each organ to total body weight was calculated, because organ weights (especially of adrenal glands and thymus) are relatively reliable reflections of chronic stress.1,6,12
Statistical analysis.
All statistical analyses were performed by using JMP 10.0.0 software (SAS Institute, Cary, NC). Data were tested for normal distribution by using the Shapiro–Wilk test. The measured parameters were analyzed by using one-way ANOVA, with Tukey posthoc tests applied as needed. Treatment groups were subdivided according to category of enrichment item for analysis. These subgroups were compared with singly housed (negative control) and paired (positive control) mice (Figure 1). Summary data are expressed as mean ± SE, and a P value of less than 0.05 was considered to be statistically significant.
Results
CBC results.
There were no significant intergroup differences in baseline whole blood cell count (F11,197 = 0.8961, P = 0.5452), total neutrophils (F11,197 = 1.0348, P = 0.4171), total lymphocytes (F11,197 = 0.8643, P = 0.5763), or the neutrophil:lymphocyte ratio (F11,197 = 0.5508, P = 0.8664). All values (Table 1) were within published normal ranges.24
Table 1.
Baseline and final mean leukocyte values
| Baseline (x109 cells) | Final (x109 cells) | |||||
| Treatment | WBC (2–10) | Neutrophils (0.4–3) | Lymphocytes (1.4–8) | WBC (2–10) | Neutrophils (0.4–3) | Lymphocytes (1.4–8) |
| Singly housed | 3.69 | 0.78 | 2.66 | 3.60 | 0.68 | 2.67 |
| Paired | 4.71 | 1.11 | 3.30 | 3.80 | 0.71 | 2.82 |
| Rotated enrichment | 4.37 | 1.01 | 3.09 | 3.73 | 0.63 | 2.82 |
| Disposable hut | 4.15 | 0.89 | 2.97 | 3.77 | 0.71 | 2.77 |
| Igloo | 4.82 | 1.16 | 3.38 | 3.04 | 0.71 | 2.10a |
| Drinking bottle | 4.74 | 1. 00 | 3.47 | 3.98 | 0.71 | 3.04 |
| Tissue | 4.67 | 1.12 | 3.24 | 4.58 | 0.90 | 3.36 |
| Cotton square | 4.15 | 0.90 | 2.98 | 3.89 | 0.64 | 3.02 |
| Cardboard tube | 4.29 | 0.91 | 3.03 | 4.59 | 0.84 | 3.40 |
| Sunflower seeds | 4.15 | 0.84 | 2.99 | 3.72 | 0.83 | 2.64 |
| Shower curtain hook | 5.02 | 1.13 | 3.55 | 4.28 | 0.96a | 2.99 |
| Running track | 4.17 | 0.93 | 2.90 | 4.00 | 0.76 | 2.97 |
Normal range (from reference 24) is given in parentheses.
P < 0.05 compared with baseline value for enrichment treatment.
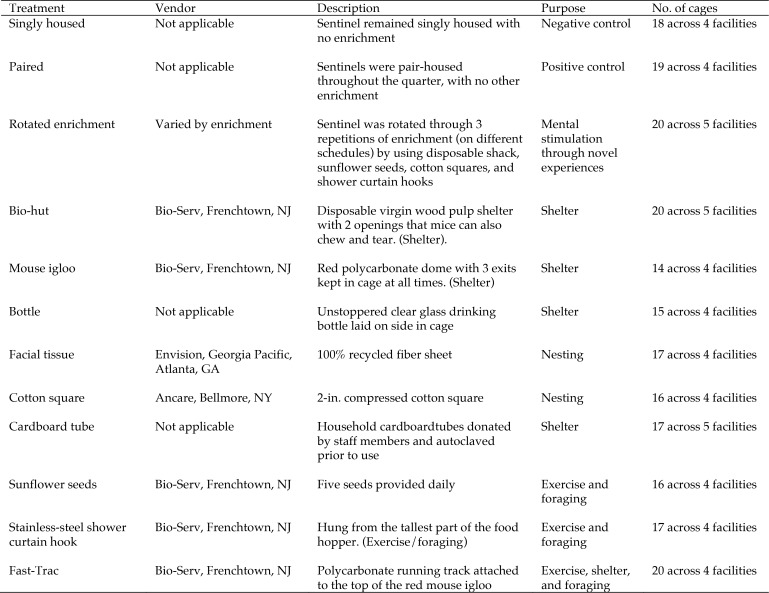
There were no significant intergroup differences in the final whole blood cell count (F11,196 = 1.0724, P = 0.3854) or total neutrophils (F11,196 = 1.0678, P = 0.3892), but the final total neutrophil count was significantly (P = 0.0298) increased compared with baseline in the mice provided with shower curtain hooks. Although final total lymphocyte count did not differ between groups (F11,196 = 1.1878, P = 0.2976), final total lymphocytes were lower (P = 0.0192) than baseline in the mice provided the red igloo. There were no significant intergroup differences in the final neutrophil:lymphocyte ratio (F11,196 = 1.1675, P = 0.3119), but the final neutrophil:lymphocyte ratio was increased (P = 0.0352) compared with baseline in the mice provided the red igloo (Figure 2).
Figure 2.
The neutrophil:lymphocyte ratio (mean ± SE) was calculated by dividing the total number of neutrophils by the total number of lymphocytes. The ratio at the end of the study was increased (*, P = 0.0352) in the mice provided with the red igloo compared with baseline.
Final body and organ weights.
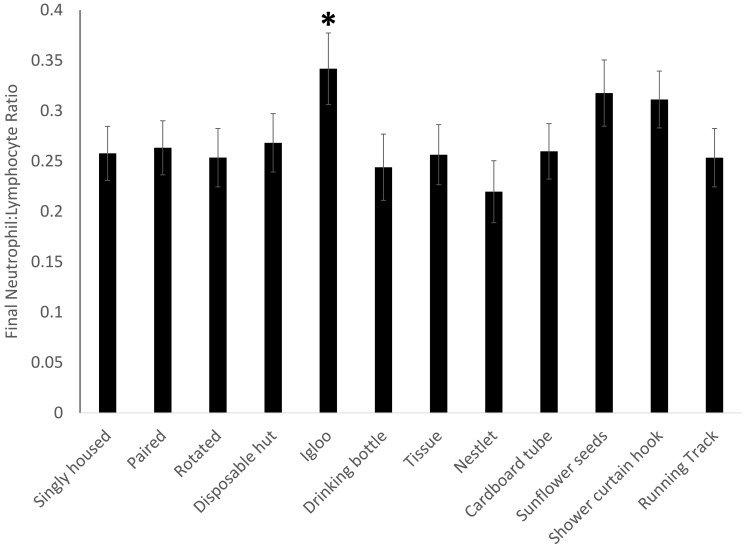
Body weight (Figure 3) showed a significant effect of treatment (F11,213 = 2.4379, P = 0.0070). Compared with baseline values, final body weight was significantly higher in the paired mice (P = 0.0342) and in mice provided cotton squares (P = 0.0451) but significantly lower in the mice provided running tracks (P = 0.0002).
Figure 3.
Compared with corresponding baseline values, body weight was increased in paired mice (P = 0.0342) and mice provided cotton squares (P = 0.0451) but decreased in those provided the running track (P = 0.0002).*, P < 0.05.
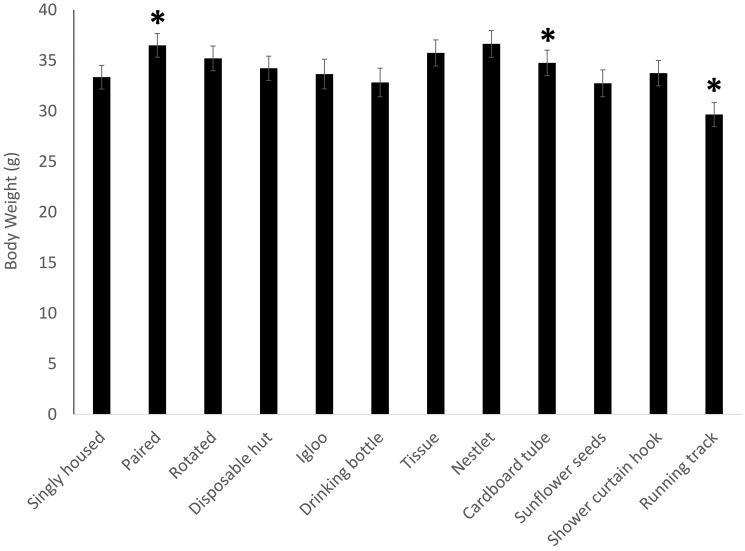
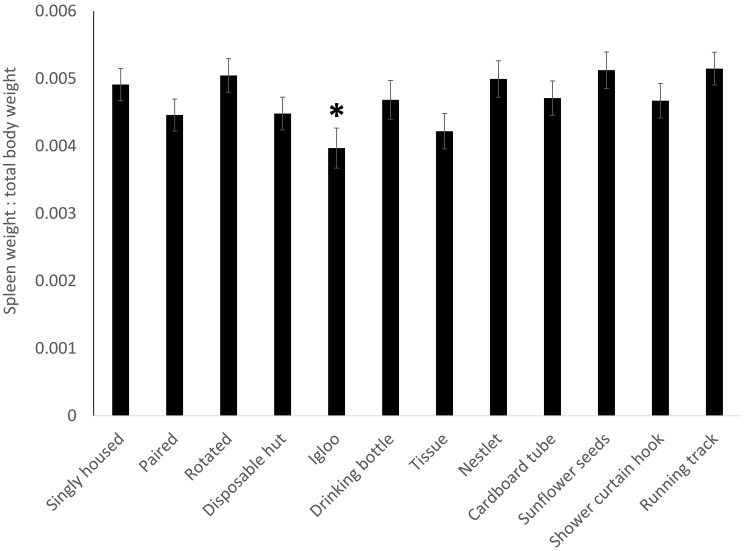
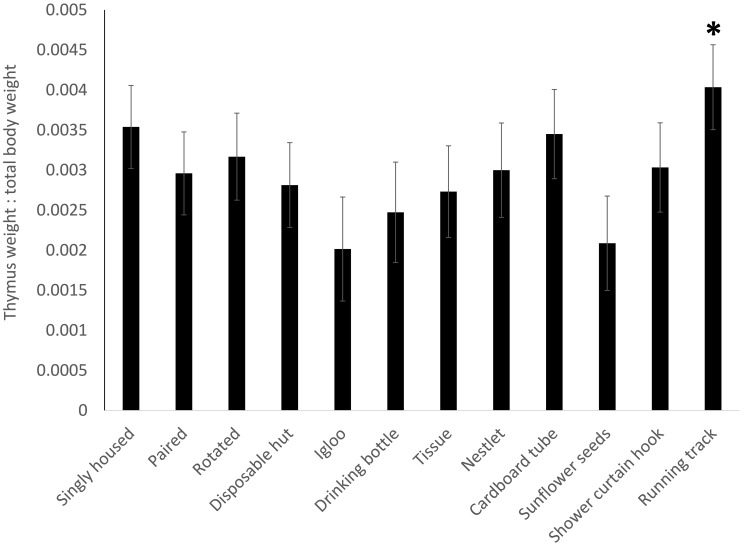
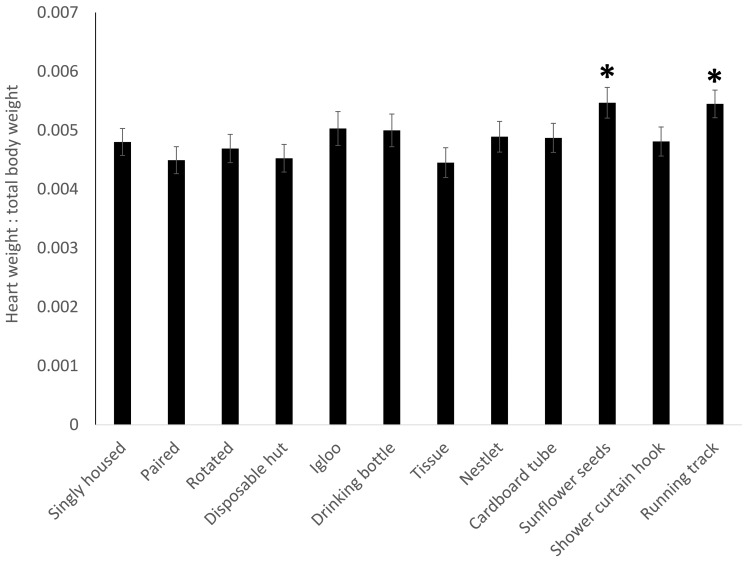
Proportional adrenal weights (Figure 4) showed no significance in the overall model (F11,213 = 1.4168, P = 0.1668) but a treatment-associated increase (P = 0.0210) in mice provided a red igloo. For proportional spleen weight (Figure 5), the overall model was significant (F11,213 = 1.9112, P = 0.0393), with a decrease (P = 0.0099) in the spleen weight of mice given a red igloo. Proportional thymus weights (Figure 6) demonstrated no significance in the overall model (F11,213 = 1.0379, P = 0.4141) but a significant (P = 0.0334) increase in mice provided a running track. Proportional heart weight (Figure 7) lacked significance in the overall model (F11,213 =1.8302, P = 0.0506) but was significantly higher in the mice given sunflower seeds (P = 0.0176) or a running track (P = 0.0118).
Figure 4.
Adrenal weight as a proportion of total body weight shows a treatment-associated effect, with a significant (P = 0.0210) increase in adrenal weight in the mice provided a red igloo. Data are presented as mean ± SE, and significance is set at P < 0.05.
Figure 5.
Spleen weight as a proportion of total body weight shows a significant (P = 0.0099) decrease in the spleen weight of mice given a red igloo. Data are presented as mean ± SE, and significance is set at P < 0.05.
Figure 6.
Thymus weight as a proportion of total body weight shows a significant (P = 0.0334) increase in the thymus weight of the mice provided a running track. Data presented with SE and significance is set at P < 0.05.
Figure 7.
Heart weight as a proportion of total body weight showed significant increases in the mice receiving sunflower seeds (P = 0.0176) or enriched with the running (P = 0.0118). Data are presented as mean ± SE and significance is set at P < 0.05.
Handling and use scores.
All enrichment items were observed to be used. However, the shower curtain hook showed the least use, in part because there was no evidence of its use except for direct observation. The few mice that were observed using the hook during the day spun and flipped through it repeatedly, as would usually be associated with stereotypies.
Handling scores showed a significant effect of treatment (F12,1330 = 4.6533, P < 0.001), with the pair-housed and drinking bottle groups exhibiting the highest final handling tolerance scores (P < 0.001 and P = 0.192, respectively). The mice given the sunflower seeds showed a significant (P = 0.0006) decrease in their handling score. Final handling tolerance scores ranged from 2.0 (sunflower seeds) to 2.6 (pair-housed).
Discussion
In this study, we had hypothesized that singly housed, unenriched mice would serve as a control for high stress levels, and we expected pair-housed mice to be validated as the least-stressful situation. Furthermore, we expected that single-housed enriched mice would demonstrate little difference in stress levels between treatment groups but perhaps show a measurable preference of use for one or more devices over others.
However, none of our measures revealed a significant benefit to the singly housed unenriched mice, even when compared with pair-housed mice (which we viewed as the preferred situation). This study ran 12 wk, and perhaps mice became habituated to their situation. Other studies have demonstrated that a long-term isolated and unenriched environment causes chronic stress in mice,2,7,19 but those studies did not use the ICR strain, as we did. Similarly, we expected pair housing to provide a positive control as the least-stressed group; however, the only significant finding for pair-housed mice in this study was an increase in total body weight as compared with all other treatment groups.
However, individually housed animals are still exposed through sight, smell, and sound to other animals in the same room.1 This situation is especially true for our current study because we used sentinel mice; the exposure of dirty bedding from other animals may itself be enriching or distressing. In the current study, we controlled for this effect by exposing all mice, albeit at different levels, depending on the cage census in each room. Repeating this study with nonsentinel animals might be valuable, to compare physiologic responses with these dirty-bedding exposures.
In addition, we were unable to detect significant differences in enrichment preference. Although this study was not a formal preference test (with comparison between 2 objects), we did hope to find some significance of duration of time used, indicating preference. All enrichment items were seen in use, at least periodically. However, when examining use data, we determined our scoring system was not designed to identify differences in use, so we excluded these data. For example, because we observed during the light cycle, shelters were used extensively, but mice were only rarely observed eating sunflower seeds. Although we suspect that these groups did not differ due to habituation of the animals to the enrichment (or lack thereof), we found no significant benefit to mice provided rotating enrichment. However, we discovered some differences in stress parameters among various enrichment devices, as described following.
Our biggest challenge in this study was consistency in the provision of the enrichment items, because this study relied on a large husbandry staff to comply with the prescribed enrichments. The most common error encountered was that singly housed sentinels received our standard enrichment of tissue when they should not have. We did not record the occasions of this error, but it occurred infrequently, and the tissue was always removed within 24 h
The nesting options we used in this study were cotton squares and facial tissue. Facial tissue was chosen for this study because it was the standard cage enrichment used at our institution. Nesting material is repeatedly recommended as the single most important commodity in mice, with even poor nest-building strains showing some preference and attempts at nest building behaviors.14
Mice provided cotton squares and paired mice both showed significant increases in total body weight compared with nonenriched mice. These data support recent findings of the importance of thermoregulation on metabolism.9,32 The lack of significant findings from mice provided tissues or cardboard tubes likely testifies to the inadequacies of these items for use as nesting materials. Indeed, in the current study, as well as our institutional standard, mice were provided only one tissue, which weighs only about 1 g, whereas a cotton square averages a little over 2.5 g22 of material. Others recommend that mice in laboratory conditions should be provided 6 to 10 g of nesting material that can be woven into a complex nest.13,15
Cardboard tubes, plastic igloos, water bottles, and disposable huts were chosen for study because they increase the complexity of an otherwise barren cage, provide additional wall space for naturally thigmotactic creatures, and provide a visual or physical barrier.1,20,28 Data from the current study suggest that red within-cage shelters may increase stress in mice, as evidenced by increased neutrophil:lymphocyte ratios, increased adrenal weights, and decreased spleen weights.16,30 These outcomes were unexpected considering that the mice chose to spend most of their time inside the provided shelters. Although the animals may prefer the dim light and thigmotactic properties of the shelter, this may effectively limit the useable cage space for that animal to the size of the shelter, leading to chronic stress. Other laboratories have reported that translucent red shelters partially rescued mice from effects of cold with a resulting decrease in adrenal weight9 however, we found the opposite adrenal effect. We did not find similar effects in mice provided the disposable huts or cardboard tubes. However, mice can alter disposable huts and cardboard tubes as they choose. Individual mice varied in this regard: some mice left these items intact, whereas others destroyed the structure.
Benefits of the bottle include visibility for husbandry staff and low to no up-front cost. Many mice in our study designated it as a latrine site, which could improve overall cage condition for fastidious animals. Unfortunately, the design of the bottle, with its single entrance–exit, could create escape problems for mice in a group housing environment.
To encourage exercise and foraging behavior, we chose sunflower seeds, a shower curtain hook, and a running wheel (Fast-Trac, Bio-Serv) for this study. Despite their size, mice in the wild cover a lot of ground when searching for food and mates and in territory defense8 and will do so in captivity if provided the opportunity.4 Captive mice will work for access to an exercise wheel.28 In addition, foraging can help mice cope with thermal stress by selecting higher energy parts of food.23
Our finding of a significant decrease in the overall body weight of mice provided a running track was unsurprising, given that it provided an exercise opportunity. Also unsurprising was the accompanying increase in heart weight, presumably due to muscle gained through voluntary exercise.26 The significant increase in thymus weight is in direct opposition to studies demonstrating that exercise results in a decrease in thymus weight.11,25 The running tracks in this study were mounted on top of the red igloo; however, if the igloo is in fact a source of increased stress, we would also expect a decrease in thymus weight.33 Perhaps the dirty-bedding exposure and exercise in combination caused these results.
Mice given 5 seeds daily did not show increases in overall body weight; however, they did demonstrate a significant increase in proportional heart weight. We did not further analyze the composition of heart size to determine whether it resulted from an increase in muscle or fat. The increased cardiac size could be due to the exercise in search for seeds or an effect of the diet on heart composition.
Cages in the sunflower seed group were opened daily to provide enrichment. This quick treatment involved taking the cage to a biosafety cabinet, opening the lid, and scattering seeds within; it did not result in additional handling of the mice themselves. However, this enrichment resulted in a decreased handling score in this study. This decreased score was not accompanied by a physiologic indication of increased distress (neutrophil:lymphocyte ratio, organ weight change). Because they housed sentinel animals, all cages in this study were likely to be opened more than once weekly for the addition of dirty bedding, although cages housing the sunflower seed group were opened the most often.
The use of a shower curtain hook as a viable enrichment device warrants further investigation. If viable, it is inexpensive and easy to use and demands little cage space. Our research staff found it difficult to determine whether the hook was being used regularly; husbandry staff reported that the mice that did use it did so almost compulsively, similar to a stereotypic flipping behavior. In addition, we are unsure of the relevance of the isolated increase in neutrophils (which is potentially indicative of chronic stress) in mice provided the shower curtain hook.16
In conclusion, our study did not reveal a clearly superior enrichment device, although we did identify some potential concerns associated with the use of the red igloo. We allayed concern that the use of sunflower seeds for enrichment would result in obese mice. We found no benefit from our selected nesting materials; however, other materials may be superior options. Finally, although investigations have been made about the ability of dirty-bedding sentinels to reveal specific pathogens, the physiologic and psychologic changes caused by exposure to dirty bedding, outside of effects due to an infectious agent, warrant investigation.
References
- 1.Abou-Ismail UA, Mahboub HD. 2011. The effects of enriching laboratory cages using various physical structures on multiple measures of welfare in singly housed rats. Lab Anim 45:145–153. 10.1258/la.2011.010149. [DOI] [PubMed] [Google Scholar]
- 2.An D, Chen W, Yu DQ, Wang SW, Yu WZ, Xu H, Wang DM, Zhao D, Sun YP, Wu JC, Tang YY, Yin SM. 2016. Effects of social isolation, resocialization, and age on cognitive and aggressive behaviors of Kunming mice and BALB/c mice. Anim Sci J 88:798–806. doi: 10.1111/asj.12688. [DOI] [PubMed] [Google Scholar]
- 3.Arranz L, De Castro NM, Baeza I, Mate I, Viveros MP, De la Fuente M. 2010. Environmental enrichment improves age-related immune system impairment: long-term exposure since adulthood increases life span in mice. Rejuvenation Res 13:415–428. 10.1089/rej.2009.0989. [DOI] [PubMed] [Google Scholar]
- 4.Baumans V. 2005. Environmental enrichment for laboratory rodents and rabbits: requirements of rodents, rabbits, and research. ILAR J 46:162–170. 10.1093/ilar.46.2.162. [DOI] [PubMed] [Google Scholar]
- 5.Boivin GP, Bottomley MA, Grobe N. 2016. Responses of male C57BL/6N mice to observing the euthanasia of other mice. J Am Assoc Lab Anim Sci 55:406–411. [PMC free article] [PubMed] [Google Scholar]
- 6.Broom DM, Fraser AF. 2010. Domestic animal behaviour and welfare, 4th ed Cambridge (MA): CAB International. [Google Scholar]
- 7.Chen W, An D, Xu H, Cheng X, Wang S, Yu W, Yu D, Zhao D, Sun Y, Deng W, Tang Y, Yin S. 2016. Effects of social isolation and re-socialization on cognition and ADAR1 (p110) expression in mice. PeerJ 4:e2306 10.7717/peerj.2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crowcroft P. 1955. Territoriality in wild house mice, Mus musculus L. J Mammal 36:299–301. 10.2307/1375908. [DOI] [Google Scholar]
- 9.David JM, Knowles S, Lamkin DM, Stout DB. 2013. Individually ventilated cages impose cold stress on laboratory mice: a source of systemic experimental variability. J Am Assoc Lab Anim Sci 52:738–744. [PMC free article] [PubMed] [Google Scholar]
- 10.Davis AK, Maney DL, Maerz JC. 2008. The use of leukocyte profiles to measure stress in vertebrates: a review for ecologists. Funct Ecol 22:760–772. 10.1111/j.1365-2435.2008.01467.x. [DOI] [Google Scholar]
- 11.Droste SK, Gesing A, Ulbricht S, Muller MB, Linthorst AC, Reul JM. 2003. Effects of long-term voluntary exercise on the mouse hypothalamic–pituitary–adrenocortical axis. Endocrinology 144:3012–3023. 10.1210/en.2003-0097. [DOI] [PubMed] [Google Scholar]
- 12.Everds NE, Snyder PW, Bailey KL, Bolon B, Creasy DM, Foley GL, Rosol TJ, Sellers T. 2013. Interpreting stress responses during routine toxicity studies: a review of the biology, impact, and assessment. Toxicol Pathol 41:560–614. 10.1177/0192623312466452. [DOI] [PubMed] [Google Scholar]
- 13.Gaskill BN, Gordon CJ, Pajor EA, Lucas JR, Davis JK, Garner JP. 2012. Heat or insulation: behavioral titration of mouse preference for warmth or access to a nest. PLoS One 7:1–11. 10.1371/journal.pone.0032799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goto T, Okayama T, Toyoda A. 2015. Strain differences in temporal changes of nesting behaviors in C57BL/6N, DBA/2N, and their F1 hybrid mice assessed by a 3-dimensional monitoring system. Behav Processes 119:86–92. 10.1016/j.beproc.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Hess SE, Rohr S, Dufour BD, Gaskill BN, Pajor EA, Garner JP. 2008. Home improvement: C57BL/6J mice given more naturalistic nesting materials build better nests. J Am Assoc Lab Anim Sci 47:25–31. [PMC free article] [PubMed] [Google Scholar]
- 16.Hickman DL. 2017. Evaluation of the neutrophil:lymphocyte ratio as an indicator of chronic distress in the laboratory mouse. Lab Anim (NY) 46:303–307. 10.1038/laban.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 18.Konkle AT, Kentner AC, Baker SL, Stewart A, Bielajew C. 2010. Environmental-enrichment–related variations in behavioral, biochemical, and physiologic responses of Sprague–Dawley and Long Evans rats. J Am Assoc Lab Anim Sci 49:427–436. [PMC free article] [PubMed] [Google Scholar]
- 19.Liu F, Liu YP, Lei G, Liu P, Chu Z, Gao CG, Dang YH. 2016. Antidepressant effect of recombinant NT4-NAP/AAV on social isolated mice through intranasal route. Oncotarget 8:10103 –10113. doi: 10.18632/oncotarget.14356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Connor AM, Burton TJ, Leamey CA, Sawatari A. 2014. The use of the puzzle box as a means of assessing the efficacy of environmental enrichment. J Vis Exp 94:1–8. 10.3791/52225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patterson-Kane EP. [Internet] 2011. Horizons of enrichment: the history, accomplishments, obstacles, and aspirations of environmental enrichment, p 12–14. [Cited 05 December 2018]. Available at: http://enrichmentrecord.com/wp-content/uploads/2012/07/er7-0411.pdf.
- 22.Peveler J. [Internet]. 2011. Comparison of mouse nesting materials: comparing apples to oranges, p 16–17. [Cited 05 December 2018]. Available at: http://enrichmentrecord.com/wp-content/uploads/2014/07/ER20-0714.pdf.
- 23.Pritchett-Corning KR, Keefe R, Garner JP, Gaskill BN. 2013. Can seeds help mice with the daily grind? Lab Anim 47:312–315. 10.1177/0023677213491403. [DOI] [PubMed] [Google Scholar]
- 24.Provencher Bollinger A, Everds N. 2012. Part 2: Anatomy and normative biology. In: Hedrich HJ. The laboratory mouse. San Diego (CA):Academic Press. [Google Scholar]
- 25.Rosa EF, Alves GA, Luz J, Silva SM, Suchecki D, Pesquero JB, Aboulafia J, Nouailhetas VL. 2014. Activation of HPA axis and remodeling of body chemical composition in response to an intense and exhaustive exercise in C57BL/6 mice. Physiol Res 63:605 –613. [DOI] [PubMed] [Google Scholar]
- 26.Sharma S, Merghani A, Mont L. 2015. Exercise and the heart: the good, the bad, and the ugly. Eur Heart J 36:1445–1453. 10.1093/eurheartj/ehv090. [DOI] [PubMed] [Google Scholar]
- 27.Sherwin CM. 1996. Laboratory mice persist in gaining access to resources: a method of assessing the importance of environmental features. Appl Anim Behav Sci 48:203–213. [Google Scholar]
- 28.Sherwin CM. 2002. Comfortable quarters for mice in research institutions, p 6–17. In: Reinhardt V. Comfortable quarters for laboratory animals, 9th ed Washington (DC): Animal Welfare Institute. [Google Scholar]
- 29.Smith AL, Corrow DJ. 2005. Modifications to husbandry and housing conditions of laboratory rodents for improved wellbeing. ILAR J 46:140–147. 10.1093/ilar.46.2.140. [DOI] [PubMed] [Google Scholar]
- 30.Swan M, Hickman D. 2013. Evaluation of the well-being of rats housed in multilevel caging with red tinted polysulfone, p. 91. In: Hotzel MJ, Filho L. Proceedings of the 47th Congress of the International Society for Applied Ethology. Florianopolis, Brazil: 2–6 June 2013. Wageningen (Netherlands): Wageningen Academic Publishers. [Google Scholar]
- 31.Varty GB, Paulus MP, Braff DL, Geyer MA. 2000. Environmental enrichment and isolation rearing in the rat: effects on locomotor behavior and startle response plasticity. Biol Psychiatry 47:864–873. 10.1016/S0006-3223(99)00269-3. [DOI] [PubMed] [Google Scholar]
- 32.Winnicker C. [Internet]. 2012. Mice no longer out in the cold. [Cited 5 December 2018]. Available at: http://eureka.criver.com/mice-no-longer-out-in-the-cold/.
- 33.Živković I, Rakin A, Petrovic-Djergovic D, Miljkovic B, Micic M. 2005. The effects of chronic stress on thymus innervation in the adult rat. Acta Histochem 106:449–458. 10.1016/j.acthis.2004.11.002. [DOI] [PubMed] [Google Scholar]