Abstract
This study compared a synthetic bedding substrate (SBS), which has the potential to be a particulate-free animal bedding system, with the standard woodchip bedding. The objective was to demonstrate that the SBS is habitable for mice and reduces particulates to levels that would not contaminate the eye or potentially induce ocular (corneal) injury. Newly weaned mice were placed in either standard woodchip bedding or SBS. All mice were monitored regarding overall health (appearance, food and water intake, natural behavior, clinical signs, and provoked behavior) to verify their ability to adjust to the bedding. At 8 to 10 wk of age, the mice underwent slit-lamp evaluation for ocular (corneal) abnormalities. Results showed significant differences in body weight and overall health between bedding groups. The incidence of ocular abnormalities did not differ significantly between groups. We conclude that, without modifications and more testing, SBS is not a favorable bedding for mice, and results were inconclusive regarding its use as a bedding to preclude ocular contamination.
Abbreviation: SBS, synthetic bedding substrate
Bedding material is one of the most important factors in a laboratory animal's environment and can affect results of an experiment.3,9,12 Bedding should be suitable for burrowing, foraging, and building nests; it also should be absorbent, widely available, and relatively cost-effective and keep toxic gases at low levels for as long as possible.7 The Guide for the Care and Use of Laboratory Animals states that bedding should be removed and replaced as often as necessary to keep the animals clean and dry and to maintain acceptable concentrations of pollutants.7 In addition, dust content should be kept low to prevent irreversible damage to the respiratory system of both animals and animal technicians.12 Several bedding products have been developed in recent years to minimize dust for occupational health risks for personnel with allergies and asthma, with varied success.6,8 Many of the common bedding products available today still create a substantial amount of dust.8 The types of bedding currently available for mice include wood chips, corncob bedding, cellulose, newspaper, and similar products.4,8,10 We and others have observed that these products contain dust and other particulates that can contaminate the eyes of mice.1,10 The nature of these materials is such that the dust or other small particulates cannot be entirely removed because the substance itself breaks down into smaller pieces. Even larger pieces of the bedding can be problematic, potentially causing corneal epithelial damage when they come in contact with the eye. Bedding contamination or damage of the eye is particularly a problem in ocular research. We have observed that typically 10% of mice received from vendors have some type of background corneal injury that precludes their use in ocular studies. The cause of this background corneal injury is not entirely clear, but we have ruled out infectious agents, and particulate from the bedding coming in contact with the eye is the leading suspected cause. Perhaps more importantly is that bedding contamination of the eye confounds ocular research studies by potentially affecting the progression of healing and by making it more difficult to study induced ocular injury.
Even though mice are widely used for ocular research and although bedding effects on the eye present a confounding factor, currently there is no commercially available solution for the problem. A synthetic bedding substrate (SBS; 2–3 mm × 4–5 mm; Rio Ammunition, McEwen, TN) was developed to be a particulate and contamination-free bedding system that potentially could eliminate or dramatically decrease this problem. The SBS system consists of an absorbent pad (cellulose–iso pad) that is overlaid with a fine stainless-steel mesh, so the mice are never exposed to the pad, which is then covered with irregularly shaped biocompatible polyethylene shot (beads; Figure 1). These beads are 2 to 3 mm in height and 4 to 5 mm in width. The steel mesh is resistant to corrosion and bordered with a protective edge to provide for smooth edges. The beads function as animal bedding substrate and were designed to permit percolation of liquid waste. The beads are large enough to prevent accidental ingestion but small enough to provide a comfortable bedding material. The beads in combination with the fine stainless-steel mesh and absorbent pad are thought to allow for the drainage and absorption of liquid waste. The fine stainless-steel mesh prevents the animals from reaching the absorbent pad, which, if they gained access to it, could be shredded and adulterate the system. The whole system has been designed to fit into the bottom of a large standard mouse cage. The absorbent pad is disposable; the fine stainless-steel mesh is durable, washable, and replaceable; and the polyethylene beads are disposable, recyclable, or reusable. Unlike traditional bedding, the polyethylene bedding is unlikely to cause corneal injury because the individual beads are smooth.
Figure 1.
The synthetic bedding substrate (SBS) consists of (a) an absorbent pad below (b) a metal mesh screen that is custom-made to fit a large static cage, (c) polyurethane synthetic beads, and (d) a standard large mouse static cage. The concept is that moisture wicks over the beads, through the wire mesh, and absorbed by the underlying pad, thus keeping mice dry.
This study was designed to compare the living conditions and ocular effects associated with SBS with those of standard woodchip bedding. Our hypotheses were that the SBS would adequately support normal mouse activity and homeostasis (including species-typical behavior and thermal protection), would eliminate or reduce particulates to clinically insignificant levels, and might lower the incidence of background corneal injury.
Materials and Methods
Animals, housing conditions, and diet.
Newly weaned female BALB/cAnNCrl mice (age, 3 wk; n = 100) were obtained from Charles River Laboratories (Frederick, MD) and were certified by the vendor to be pathogen-free for all known rodent bacterial, viral, and parasitic pathogens. All mice received tail tattoos (Lab Stamp, SOMARK Innovations,) of 001 to 100 prior to shipment at 3 wk of age. When the mice were received, they were randomly assigned to be socially housed (n = 10) on SBS (50 mice) or standard bedding (50 mice; β Chips, Animal Specialties and Provisions, Quakertown, PA) in solid-bottom polycarbonate cages (Ancare, Bellmore, NY) with static filter tops (Ancare) until 8 to 10 wk old. All the mice were housed in animal holding rooms at our AAALAC-accredited facility, which were maintained on a 12:12-h, dark:light cycle with light intensity between 130 to 325 lx, at a temperature of 68 to 79 °F (20.0 to 26.1 °C), 30% to 70% relative humidity, and 10 to 15 fresh air changes hourly. Each mouse received were fed one cup (2 oz) of DietGel (catalog no. 31M or 76A, Clear H2O, Westbrook, ME) daily, beginning at receiving (3 wk old) and fed until the end of the study (10 wk). All mice received particulate-free enrichment (2 huts and 1 nylon bone per cage; BioServ, Flemington, NJ). Only cages containing the standard bedding received 2 cotton squares (Ancare, Bellmore, NY) because this addition is considered part of our standard bedding for mice. All animals were USDA category C for this project. All procedures were performed in accordance with protocols approved by the United States Army Medical Research Institute of Chemical Defense IACUC, and animals were treated in accordance with the Guide for the Care and Use of Laboratory Animals.7
Health and distress scoring.
Twice each week, both groups of mice were visually evaluated by using health and distress scoring sheets for overall health (appearance, food and water intake, species-typical behavior [that is, nesting, burrowing, congregating]), clinical signs, and provoked behavior (that is, responses to stimuli). Each category of the score sheet had scores ranging from 0 to 4, with 0 being normal and 4 being extremely abnormal. A total score (combining all 5 categories) of 4 or greater was considered abnormal, and treatment or removal from the study was considered. In addition all mice were weighed each week. For consistency, weights and health scoring were measured and recorded by the same person. Most visual observations were completed during the morning hours; due to authors’ schedules, some of the observations were made in the afternoon. After the first cohort of 100 mice, we noticed the significant effect of the SBS bedding on the animals and decided not to finish the study until changes could be made to the SBS design.
Bedding.
The SBS comprised irregularly shaped food-grade polyethylene beads (2–3 mm × 4–5 mm; Rio Ammunition, McEwen, TN) which were laid over the stainless-steel mesh in a layer approximately 1/4 to 1/2 in. deep. Enrichment comprised huts, nylon bones, and cotton squares (controls only). Initially cage changes were planned to be performed at 1-wk intervals for both group, but due to unsanitary conditions, the SBS cages had to be changed every other day. A cage change for SBS included a new cage, new absorbent pad, sanitized stainless-steel mesh bottom, and sanitized–sterilized polyethylene beads. The wire tops, water bottles, and sipper tubes were changed weekly. The polyethylene beads were cleaned by placing them into a polyethylene mesh bag (the mesh size of the polyethylene bag was small enough to retain the bedding but large enough to promote any contaminating particulates [for example, feces] to fall out), immersing the bag into hot soapy water, and manually agitating it until the majority of the urine and feces were removed. Then the beads were rinsed with warm tap water, immersed in sanitizing solution (PREempt, Contec, Spartanburg, SC, or SaniPlex, Quip Laboratories, Wilmington, DE) for at least 20 min, rinsed again with warm tap water, and laid out to dry overnight. After drying, the beads were placed into self-sealing sterilization pouches and sterilized by using an autoclave. Before being returned to a cage, the bedding was inspected for signs of chewing, broken pieces, and contaminating particles.
Slit-lamp examination.
When mice were 8 to 10 wk of age, cages were randomly chosen and all mice were anesthetized with isoflurane for a single ocular evaluation by using a slit-lamp microscope (model SL-D7, Topcon, Paramus, NJ) equipped with a 40× objective, digital camera system (model D200, Nikon, Tokyo, Japan), and integrated through-the-slit flash. Mice were then transferred to a stage equipped with an anesthesia nose cone, and isoflurane anesthesia was maintained for a routine examination. Slight pressure was applied above and below the eye to gently protrude the globe for a thorough examination. Both eyes were evaluated for signs of any abnormalities (for example, keratitis, neovascularization). The eyes were categorized as either positive (corneal abnormalities) or negative (no corneal abnormalities), and observations were photodocumented. Mice anesthetized for slit-lamp examination were placed in a warmed recovery chamber or cage until fully awake and able to right themselves and then were returned to their home cages.
Data analysis.
The incidence of corneal abnormalities at 8 to 10 wk of age was compared between bedding groups by using a Fisher exact test. Two approaches were used. In the first, we counted the number of corneas with injuries; therefore total number of corneas is 100 (2 per mouse) in each bedding group. In the second, we counted the number of animals with at least one injured cornea as presence of corneal injury; therefore the total is 50 animals per group. A sample size of 150 mice per bedding group was determined to be adequate to detect a difference in the presence of ocular injury from 0% to 5%, with an α level of 5% and power of 80%. Because there was a possibility that not all 3 cohorts of 100 mice (300 animals in total) would be used, the groups were sequentially tested in 3 groups of 50 per bedding system. A significant difference between the 2 beddings after any cohort concluded the study. Baseline weights (week 0) were compared by using a 2-sample t test to determine whether normalization to baseline would be needed to adjust for differences in the weights of the bedding groups at the start of the study. Although there was a slight but nonsignificant difference between bedding groups at baseline, percentage change from baseline was calculated for each animal and used in the comparison of bedding groups through repeated-measures (time) ANOVA. Health monitoring data were summed over all categories for a total score on each observation day. A comparison of bedding groups by using a χ2 test at each study week, either by comparing total scores or the total number of animals with a score, was planned. Statistical significance was defined as a P value less than 0.05 for all tests. All analyses were performed by using JMP 13.1.0 (SAS Institute, Cary, NC).
Results
Weight.
Analysis of the weights as percentage change from baseline showed that mice on the standard woodchip bedding mice had a slight but significantly (P = 0.053) higher weight gain, on average, than the SBS group. The standard bedding group had a larger weight increase during the first week of the study. After the first week, the SBS mice gained weight at a similar rate to the standard bedding group but continued to weigh less throughout the study (Figure 2).
Figure 2.
Body weight as the percentage change from the baseline weight, showing that the standard bedding mice had a larger weight increase than the SBS group during the first week of the study. After the first week, the SBS mice gained weight at a similar rate to the standard bedding animals but continued to weigh less throughout the study.
Health monitoring and behavior.
All of the standard bedding mice received a total score of 0 throughout the study and had no clinical issues (that is, had normal appearance, food and water intake, and natural and provoked behaviors), whereas all of the SBS bedding mice had total scores from 1 to 4 throughout the study (Figure 3). On each observation day, the number of mice with observable health and behavior effects differed significantly (P < 0.05) between the SBS and standard bedding groups. Early we noticed that the mice in the SBS bedding were wet and unkempt, and therefore SBS mice received a score of 2 in the first week. Because of these findings, the cage change frequency was adjusted to every other day. This improved health scores by 1 point because the mice became more mobile but continued to be wet and unkempt. As the study progressed the mice's health status worsened. By the time of slit-lamp examinations in week 8 to 10, the SBS mice were at a health score of 4, due to scores of 1 (unkempt) in appearance, 1 (less mobile and no nesting) in natural behavior, and 2 (moderate change in reaction to stimuli) in provoked behavior. The mice did not want to move or sleep on the bedding, and when handled they had a dramatically decreased response and were easily caught. At the completion of the slit-lamp examinations, the SBS mice were placed into standard bedding and followed for an additional week. After 24 h in the standard bedding, these mice were less wet and unkempt, began to elicit more normal behaviors (nesting), and exhibited the expected aversion to handling. In contrast, the standard bedding mice were well groomed, spent much of their time in and on the bedding, and remained difficult to catch throughout the study (Figure 4).
Figure 3.
Clinical health and behavior scores for all mice in each bedding group for the days on which health assessment was completed throughout the study. SBS mice (blue) quickly became wet and unkempt. By day 5, when cage-change interval was decreased to every other day, the mice initially responded positively but soon regressed and began to decline for the rest of the study. The standard bedding mice (red) stayed the same throughout the study and had no health or behavior issues.
Figure 4.
(A) Mice on standard bedding are clean and comfortable, whereas (B) those on SBS are unkempt and uncomfortable in their environment. The SBS mice continued to sleep in groups but preferred not to sleep on the polyurethane beads, instead sleeping on huts to maintain some comfort while resting.
Slit-lamp examinations.
For 25 of the 100 corneas in the 50 mice in the SBS group and 6 of the 100 corneas for the 50 animals in the standard bedding group, the photographs were not focused enough to determine whether any injury occurred. Of the remaining corneas, 18 of 75 (24%) of the SBS and 30 of 94 (32%) of the standard bedding group had no corneal injury (Table 1). Although the standard bedding group had more corneas without injury than the SBS group, this difference was not significant (P = 0.304). Three mice (6%) in the SBS bedding group and 6 animals (12%) in the standard bedding group had no corneal injury in either eye (Figure 5). Two animals in the SBS group had photographs that were not focused well enough to determine whether any injury had occurred to either eye, thus reducing the group size from 50 to 48. All other mice had an injury in either one eye or both eyes or the corneal photo was not focused enough to determine whether any injury occurred (Figure 5). By using the best eye reading (that is, no corneal injury) for each animal, 15 of the 48 mice (31%) of the SBS bedding group and 24 of the 50 animals (48%) of the standard bedding group had no observable corneal injury in at least one eye (Table 2). This difference was not significant (P = 0.103).
Table 1.
Presence of corneal injury (no. [%]) according to total number of corneas
| Bedding group | No injury | Injured | Unknown | Total corneas |
| SBS | 18 (24%) | 57 (76%) | 25 | 100 |
| Standard | 30 (32%) | 64 (68%) | 6 | 100 |
Figure 5.
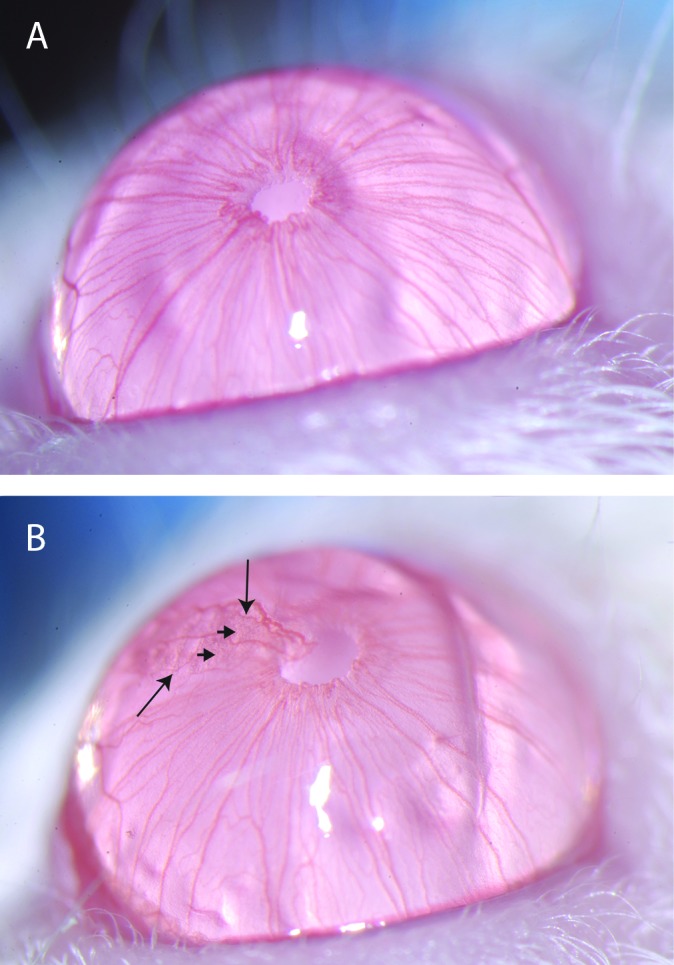
Slit-lamp photograph. (A) A normal eye, without keratitis or neovascularization. (B) An affected eye with evidence of keratitis (arrow heads) and neovascularization (arrows). Mice with affected eyes confound results and typically are removed from study during ocular research. Magnification, 40×.
Table 2.
Presence of corneal injury by animal.
| Bedding Group | No injury | Injured | Total eyes |
| SBS | 15(31%) | 33(69%) | 48 |
| Standard | 24(48%) | 26(52%) | 50 |
For 2 mice in the SBS group, photographs that were not focused well enough to determine whether injury occurred to either eye.
Discussion
Bedding is one of the most important components of the microenvironment for rodents.3,9,12 Bedding material is supposed to be absorbent and cost-effective and to allow for natural behaviors, such as burrowing, foraging, and nesting.7 The type of bedding material used affects not only the microenvironment of the mice but also the macroenvironment and thus personnel.3 In particular, moisture absorbency is one of the most important characteristics of rodent bedding for controlling bacteria and ammonia levels.4 To our knowledge, this study is the first to use SBS for mice, and we did not measure ammonia at this early stage of evaluation. This first attempt was to assess whether living conditions in the beads were hospitable for the mice and to determine whether observable effects on corneal health occurred. Our results showed that the SBS bedding did not control the moisture well. Large urine drops remained on top of the polyethylene beads and not percolate through to the stainless steel mesh screen and absorbent pad. This situation, in turn, did not allow for natural behaviors, such as nesting. Even with bedding changes on alternate days, SBS became very soiled and wet, perhaps leading to frequent contact of urine, feces, or ammonia with the mice's eyes. We also were concerned that prolonged exposure to urine, feces, and wet conditions would lead to pododermatitis in the mice, but these changes were never observed.2 Changing the SBS daily was impractical, nor did we think it would resolve the basic issue of the failure of the SBS to wick away urine. Therefore, we did not evaluate daily SBS cage changes. Moreover, this study had a smaller sample size than planned; we stopped the study early as a result of the SBS bedding being uninhabitable. The original plan was 150 mice per group, in 50-mouse cohorts. After the first cohort, we noticed the significant effect of the SBS bedding on the mice and decided not to finish the study until changes could be made to the SBS design.
Using a slit-lamp to detect corneal abnormalities, we did not find significant differences in corneal damage between the 2 bedding groups, although there was a nonsignificant trend of increased damage in the corneas of mice on SBS. We think that the affected eyes in the SBS were due to urine, feces, or ammonia trapped by the beads, which then came into contact with the eyes. In addition, ammonia levels exceeding the maximal human exposure level, 50 ppm, might affect the animals’ eyes.4,5,11 More research is needed to confirm that the SBS does not accumulate ammonia to noxious levels.
Results also showed that mice in the standard bedding group, on average, weighed significantly more than SBS animals, especially during the first week on the bedding. This difference might have taken place because mice in the SBS group were not familiar with this substrate and very quickly became wet and unkempt, perhaps keeping them from eating well initially. As the mice became more conditioned to the microenvironment and cage changes were completed more often, they stayed drier and slightly more comfortable, allowing their weight gain to mirror the standard bedding group as the study progressed. The results, in addition, showed significant differences in health and distress between the 2 groups, demonstrating at this stage, SBS is uninhabitable. Even though the SBS supported the animals’ eating habits, as evidenced by the mice continuing to gain weight throughout the study, the mice were unkempt, less mobile, and had a moderate change in their provoked behavior. We performed many cage changes, which may have affected the attitudes of the mice, but at the completion of the study the SBS mice were placed on standard woodchip bedding, after which they returned to normal provoked behavior.
Modification of the SBS to allow for increased moisture permeability may render the mice more comfortable and enable them to perform those natural behaviors of burrowing, foraging, and nesting. Modifications may need to include changes to the surface charge of the beads to make them wick moisture and larger holes in the metal mesh to allow the moisture to permeate easier. Another aspect would be to adapt this bedding setup to fit into an IVC, in which the air flow would aid in pushing the moisture down to the absorbent pad, thus keeping the cage somewhat drier and preventing ammonia buildup. In addition, future modifications would need to address cage change frequency. Although it is beneficial that the material is reusable, the resource and time expenditures of frequent cage changes are another reason that the current iteration of the SBS is impractical. Future development of the SBS will need to address these issues and evaluate ammonia levels in the cages.
This study was designed to compare living conditions and ocular effects between SBS and standard woodchip bedding. Our hypotheses were that the SBS would adequately support normal mouse activity and homeostasis (including species typical behavior and thermal protection) and that the SBS would reduce or eliminate particulate contamination of the eye which might, in turn, reduce the incidence of background ocular injuries. However, we determined that SBS does not percolate liquid waste and is therefore inhabitable for mice. Effects on ocular health were statistically nonsignificant but showed a negative trend likely due to increased contact with urine and feces. With major adjustments such as altering the beads so that they wick away urine and support cage change frequencies comparable to standard woodchip bedding, SBS might be a viable reusable bedding option in the future.
Acknowledgments
We thank Alicia Bellin for her assistance; Krinon Moccia and Jessica Connolly for their mentorship and guidance; Cierra Streaker; Katelyn Kelly; and animal care technicians, especially Jon White, Jessie Hughes, and Tiffanie Whitty for their ideas on cleaning beads and assistance with caging.
Amber Gomez was supported in part by an appointment to the Research Participation Program for the US Army Medical Research and Materiel Command administered by the Oak Ridge Institute for Science and Education through an agreement between the US Department of Energy and US Army Medical Research and Materiel Command.
The views expressed in this article are those of the authors and do not reflect the official policy of the Department of Army, Department of Defense, or the US Government.
References
- 1.Bazille PG, Walden SD, Koniar BL, Gunther R. 2001. Commercial cotton nesting material as a predisposing factor for conjunctivitis in athymic nude mice. Lab Anim (NY) 30:40–42. [PubMed] [Google Scholar]
- 2.Blair J. 2013. Bumblefoot: a comparison of clinical presentation and treatment of pododermatitis in rabbits, rodents and birds. Vet Clin North Am Exot Anim Pract 16:715–735. 10.1016/j.cvex.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Blom HJ, Van Tintelen G, Van Vorstenbosch CJ, Baumans V, Beynen AC. 1996. Preferences of mice and rats for types of bedding material. Lab Anim 30:234–244. 10.1258/002367796780684890. [DOI] [PubMed] [Google Scholar]
- 4.Burn CC, Mason GJ. 2005. Absorbencies of 6 different rodent beddings: commercially advertised absorbencies are potentially misleading. Lab Anim 39:68–74. 10.1258/0023677052886592. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. [Internet]. 2016. NIOSH pocket guide to chemical hazards. Washington (DC): Government Printing Office; [Cited 04 February 2018]. Available at: https://www.cdc.gov/niosh/npg/npgd0028.html [Google Scholar]
- 6.Glueck JT, Huneke RB, Perez H, Burstyn I. 2012. Exposure of laboratory animal care workers to airborne mouse and rat allergens. J Am Assoc Lab Anim Sci 51:554–560. [PMC free article] [PubMed] [Google Scholar]
- 7.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 8.Kaliste E, Linnainmaa M, Meklin T, Torvinen E, Nevalainen A. 2004. The bedding of laboratory animals as a source of airborne contaminants. Lab Anim 38:25–37. 10.1258/00236770460734362. [DOI] [PubMed] [Google Scholar]
- 9.Mohamed AS, Fahmy SR, Soliman AM, Gaafar KM. 2018. Effects of 3 rodent beddings on biochemical measures in rats and mice. J Am Assoc Lab Anim Sci 57:443–446. 10.30802/AALAS-JAALAS-18-000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith E, Stockwell JD, Schweitzer I, Langley SH, Smith AL. 2004. Evaluation of cage microenvironment of mice housed on various types of bedding materials. Contemp Top Lab Anim Sci 43: 12–17. [PubMed] [Google Scholar]
- 11.Van Winkle TJ, Balk MW. 1986. Spontaneous corneal opacities in laboratory mice. Lab Anim Sci 36:248–255. [PubMed] [Google Scholar]
- 12.Wirth H. 1983. Criteria for the evaluation of laboratory animal bedding. Lab Anim 17:81–84. 10.1258/002367783780959493. [DOI] [PubMed] [Google Scholar]






