Abstract
Chimpanzees demand specialized housing and care and the highest degree of attention to animal welfare. The current project used a survey method to collate information on chimpanzee housing and behavioral indices of welfare across all 6 of the chimpanzee research facilities in the United States. Data were compiled on 701 chimpanzees ranging from 2 to 62 y old (mean age, 26.0 y). All chimpanzees except for one were socially housed; the median group size was 7 animals, and group sizes ranged from 1 to 14. All of the subjects had access to outdoor spaces each day. Daily access to a natural substrate in the chimpanzee's enclosure was available for 63.8% of the subjects. Overall, 94.1% of the chimpanzees used tools to acquire food, 48.1% built nests, 75.8% copulated, and 83.3% initiated grooming bouts. The following atypical behaviors were reported most often: rocking (13.0%), coprophagy (10.0%), and stereotyped behaviors other than rocking (9.4%). There was widespread evidence of positive animal training techniques, with nearly all (97.7%) subjects reported to generally voluntarily cooperate with shifting in their enclosure, and 72.2% were reported to present for an injection of anesthetic. We include some comparison between these findings and data describing zoo-housed chimpanzees. In addition, we discuss survey findings in reference to recommendations made by the NIH Working Group on the Use of Chimpanzees in NIH-supported Research. The current survey assessed a larger sample of chimpanzees living under human care than has been published previously. This broad analysis can help to guide future improvements in behavioral management to address behavioral problems or deficits.
Abbreviations: HL, Hosmer and Lemeshow; MR, mother-reared; PRT, positive reinforcement training; WB, wild-born
Chimpanzees living under human care in the United States live in 4 major settings: research centers, sanctuaries, zoos, and privately-owned facilities. For many years, the largest proportion of this chimpanzee population has lived in research centers, but with the on-going retirement of NIH-owned chimpanzees, there is a population shift toward sanctuaries.32 It is important that the welfare of chimpanzees is assessed objectively through behavioral science. For more than 3 decades, empirical research has been published regarding the welfare and psychologic wellbeing of captive chimpanzees. This research has focused on social housing, environmental enrichment, facility design, and animal training methods.17 Notably, the majority of these studies has been conducted in research facilities. It is laudable that similar research is becoming more widespread in zoos48,51,89 and has recently been published by some sanctuaries.45,55,56
Here we provide information regarding the current state of chimpanzee care and management in research facilities in the United States, including assessment of some aspects of their care (for example social group size, outdoor access) as it relates to recently proposed recommendations.75 In addition, some data for zoo-living chimpanzees in the United States are provided as a point of reference for interpreting the information about research chimpanzees. Chimpanzees require specialized housing and care and the highest degree of attention to animal welfare. In addition, chimpanzees engender high degrees of public and political interest. For example, at the request of the NIH and in response to congressional inquiry, the Institute of Medicine conducted an analysis of the ongoing and future scientific necessity of chimpanzees for NIH-funded biomedical and behavioral research. In 2011, the Institute of Medicine committee issued a report that established criteria to guide current and future research use of chimpanzees; the Working Group on the Use of Chimpanzees in NIH-Supported Research (hereafter referred to as the Working Group) was then established to advise NIH on implementing recommendations from the Institute of Medicine report. The Working Group75 defined a number of objectives for chimpanzee care and management. Because these objectives represent an expert consensus on issues important to chimpanzee welfare, they are useful benchmarks against which to compare the current status of research chimpanzees.
The current project used a survey method to collate fundamental information on chimpanzee housing and behavioral indices of welfare across all of the chimpanzee research facilities in the United States and encompasses a larger number of chimpanzees than has previously been published. The survey focused on topics considered central to chimpanzee welfare; most of these topics have broad support by experts in chimpanzee care as well as support by scientific data.
The survey included questions addressing outdoor access and enclosure substrate because the physical environment in which chimpanzees live influences their behavior. For example, providing chimpanzees with access to outside spaces leads to behavioral benefits, including less abnormal behavior, less tension-related behavior, more physical activity, and perhaps more use of enrichment materials.6,99 Providing chimpanzees with natural substrates such as grass, hay, and woodchips has been demonstrated to increase natural foraging behavior, activity, and play and to decrease abnormal behavior.3,26
In addition, the survey contained questions on current social group size and early social environment. Scientists who study chimpanzee behavior in field, zoo, and research environments agree that social housing is the most critical aspect of captive chimpanzee welfare.2,14,15,42,84 Chimpanzees housed alone show increased levels of anxiety, aggression,4 and abnormal behavior as compared with those socially housed.29 The early social environment is particularly influential, and rearing away from the mother (for example, nursery setting) leads to elevated levels of abnormal behavior compared with mother-reared counterparts66,93,94 as well as impaired sexual and maternal abilities15,30,57 and reduced problem-solving skills.28 Indeed, even different methods of rearing by humans can have different, long-term effects on chimpanzee health and behavior measures.33
Species-typical behaviors were assessed by the survey. Questions regarding chimpanzees’ tool use, nesting, copulation, and grooming behavior were included. The presence of species-typical behaviors is one of the most commonly used metrics of welfare, and “an aim of many welfare assessments is to increase natural behaviors.”67 Although some natural behaviors do not indicate positive welfare (fear-related behaviors, for example), those we chose for this survey fall within the realm of positive natural behaviors, which are behaviors that animals tend to exhibit under natural conditions and because they are pleasurable or promote biologic functioning.25 Behaviors with negative welfare value, in contrast, relate to stress, frustration, abnormal behavior, aggression, and reduced fitness.25 Wild chimpanzees show many types of feeding-related tool use, including nut cracking, termite fishing, honey fishing, and spearing of mammalian prey.23,71,83,90 Because tool use is ubiquitous among wild chimpanzees,61,83 this is a desirable behavior to encourage in captive conditions.65,92
Wild chimpanzees build nests made from branches and leaves and spend about 12 of every 24 h in their nests.84 Some scientists have argued that nesting opportunities should be viewed as an important component of captive chimpanzee environments,84 and the NIH Working Group likewise recommended daily provision of materials for nest construction.75 Intense social interactions are a hallmark of chimpanzee life and thus were important to measure; grooming behavior is relatively easy to observe and to identify, as is copulation. Copulation has an obvious relationship to individual fitness, is typically shown by all adult chimpanzees, and is sensitive to perturbation by poor social conditions.57,86 Social grooming is important to chimpanzees as a primary mechanism for maintaining social relationships,46 and active participation in grooming bouts is important for the formation and maintenance of close bonds.42 Grooming is negatively influenced by poor welfare conditions, such as early social and perceptual deprivation.41,97 Therefore, questions that addressed grooming and copulation were included in the survey.
A wide diversity of abnormal behaviors is well-documented among captive chimpanzees,100 including stereotypical behaviors, abnormal appetitive behaviors, self-directed abnormal behaviors, and potentially self-injurious behaviors. Abnormal behaviors are those that are not typical of the species in the wild or that occur at very different frequencies in captivity than in the wild.38 Abnormal behaviors are generally regarded as indicators of poor welfare because of their relationship to restrictive environments, deficiencies in the social environment, and insufficient management practices; notably, however, some experts have argued recently that coprophagy in chimpanzees should not be classified as an indicator of poor welfare because it is associated with desirable conditions, such as mother rearing.51,54 The survey included questions regarding the occurrence several types of abnormal behaviors in individual chimpanzees.
Positive reinforcement techniques (PRT) have been used to train chimpanzees to cooperate with a variety of husbandry, veterinary and research procedures.20,34,58,59 PRT relies on the voluntary participation of the animals, and they receive rewards, such as food, for offering a particular behavior. For example, if the desired behavior is for the chimpanzee to open his or her mouth when a cue is given, then the animal will receive a small piece of food after opening his or her mouth after the appropriate cue is presented.81 PRT reduces distress experienced by chimpanzees during veterinary-related procedures58 and can have positive, generalized, and prolonged effects on behavior beyond the training sessions.82 Questions concerning the use of PRT were included in the survey because of these beneficial effects on welfare.
Because sex and early social rearing environments are known to affect behavioral measures of chimpanzee welfare,15,27,33,36 these factors were used as independent variables in some of the analyses. In addition, the age of the subjects was assessed as a possible factor, because age alone can affect some welfare-related behaviors.66 Age also was considered relevant for this survey, because older research chimpanzees lived in conditions quite different from current conditions, and their prior environments might have altered their behavior long-term. The current size of each chimpanzee's social group was included as an independent variable to determine whether larger groups are associated with beneficial behavioral outcomes.
Materials and Methods
A written questionnaire was sent to each of the 6 research facilities in the United States that house chimpanzees (Yerkes National Primate Research Center, the Southwest National Primate Research Center, the Keeling Center, the New Iberia Research Center, the Alamogordo Primate Facility, and the Language Research Center at Georgia State University). All are AAALAC-accredited facilities. This survey-based research project complied with the appropriate IACUC approval requirements, the Guide for the Care and Use of Laboratory Animals,52 and the USDA Animal Welfare Regulations1 assuring humane care and use of the animals. Questionnaires were sent between 2015 and 2017 in a staggered manner, and each facility completed the information for each chimpanzee in their care at that time (see Figure 1 for the survey questions). Individual chimpanzees were indicated as displaying a particular abnormal behavior when the behavior had been observed or recorded within the 2-y period prior to survey completion, thus giving a current assessment of the abnormal behavior profile of each subject.
Figure 1.
Survey questions.
The following predictors were entered into a logistic regression analysis by using a forward Wald stepwise procedure: sex (reference, female), age (reference, adult), rearing (reference, mother-reared or wild-born), and group size (reference, small groups). We also included all possible interactions in the analyses: sex×rearing, sex×age, sex×group size, rearing×group size, age×rearing, and age×group size. Animals were categorized as immature when they were 11 y or younger, adult when they were 12 to 39 y old, and elderly when they were 40 y or older. Small groups contained 3 to 6 animals; large groups contained 7 or more animals. The rest of the chimpanzees lived in pairs, with one singly housed animal who was included in the ‘pair’ category for analyses. Animals whose rearing history was unknown were not included in these analyses; this criterion resulted in the removal of 83 chimpanzees (n = 618). Each of the species-typical behaviors and each abnormal behavior was analyzed individually, as were 2 summary measures (presence of all 4 species-typical behaviors and presence of 3 or more abnormal behaviors). Of the models reported by each analysis, only models that were significant and passed a goodness-of-fit (Hosmer and Lemeshow [HL]) test were considered; we report the model that retained the most predictors or that accounted for the most variance in the data (according to Cox and Snell and Nagelkerke R2 analysis). All predictors retained by the selected models are listed, but β values and associated statistics are reported for significant predictors only. Although some of our analyses required the removal of subjects, this study includes every chimpanzee living in a research facility in the United States and thus does not need many generalizations from sample to population.
Early rearing was defined in the survey as the predominant type of social setting during the subject's first 12 mo of life. For example, those animals identified as nursery-reared spent more than 6 of their first 12 mo in a nursery and being raised by humans; they may or may not have also lived with other infant chimpanzees. For many of the analyses, wild-born subjects were categorized with mother-reared subjects because chimpanzees that were captured in the wild and survived transport to the United States were typically older than 1 y (younger infants tended to not survive the experience) and therefore would have met the minimum requirement of at least 6-mo of mother-rearing while living in the wild. This category thus comprises mother-reared or wild-born (MR–WB) subjects. The category of ‘not MR–WB’ (NOTMR/WB) comprised nursery-reared subjects predominantly but also included a few animals with other, known, rearing backgrounds (for example, in a human home or in the entertainment industry). Many of the NOTMR/WB chimpanzees were nursery-reared because of maternal incompetence that would have led to the death of the infant had there been no intervention by those caring for the animals.
Because the type and amount of observation time was not specified for this project, it undoubtedly varied across facilities, thus potentially leading to variation in the identification of behaviors (that is, observing more would lead to the detection of more infrequent behaviors). Some of the behaviors evaluated required that the subjects had appropriate opportunities to display that behavior. For example, to assess whether a chimpanzee makes a nest, s/he must have been provided with appropriate materials. To assess whether a chimpanzee copulates, we determined that s/he must be at least 5 y old and housed with appropriate social partners. The assessment of tool use excluded those who were not observed with tools or did not have access to tools. Questions regarding PRT did not ask respondents to indicate whether each subject who did or did not perform the behavior had been trained, because this information would be impractical to complete for large numbers of subjects. Instead, the questions focused only on the outcome.
Results
The survey return rate was 100%. Each facility used archival animal records data, observational data, and animal training records to complete the survey. Methods of behavioral assessment varied across facilities (for example, 1–0 sampling of behavioral measures performed quarterly), but similar types of information were collected at all facilities. Data were compiled on 701 chimpanzees ranging from 2 to 62 y old, with a mean age of 26.0 y. Overall, 11.3% of the sample were immature animals, 78.2% were adult, and 10.6% were elderly (Table 1). In addition, 4 of the 49 wild-born animals were adults (3 female, 1 male), and the remaining 45 were elderly (27 female, 18 male); WB, and thus chiefly elderly, animals made up about 16% of the MR–WB group (Table 1).
Table 1.
Sex and early rearing status of chimpanzee subjects
| Mother-reared | Wild-born | Not mother-reared or wild-born | Other rearing or unknown | Total | |
| Females | 139 | 30 | 164 | 45 | 378 |
| Males | 118 | 19 | 148 | 38 | 323 |
| Total | 257 (36.7%) | 49 (7%) | 312 (44.6%) | 83 (11.9%) | 701 |
Housing.
Of 701 subjects, 11.1% lived in pairs, 51.9% in small groups, and 36.5% lived in large groups. The median group size was 7 chimpanzees, and the mean group size was 4.6 animals. Group size ranged from 1 to 14; one subject was housed individually. All of the subjects had daily access to outdoor spaces throughout the year (weather conditions permitting). Daily access to a natural substrate in the chimpanzee's enclosure was reported for 63.8% of the subjects. Grass was reported most commonly (43.8%), with soil (24.1%) and pea gravel (14.7%) reported also (some facilities reported multiple substrates). Postsurvey communication with each reporting facility confirmed that all chimpanzees lived in primary housing that was larger than the minimal allowable size.1
Species-typical behaviors.
Tool use.
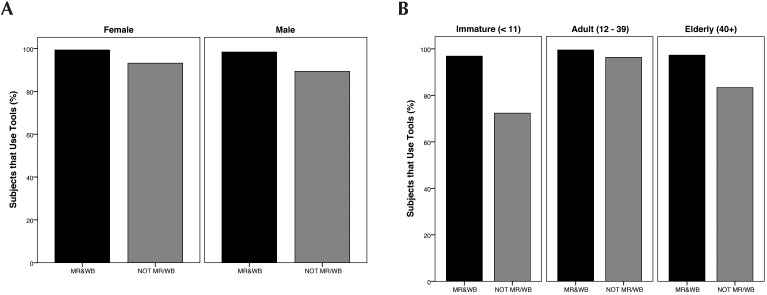
Data regarding tool use was not provided for 95 subjects, but among those for which data were provided (n = 561), 94.1% were reported to use tools to acquire food (Table 2). Regression analysis was conducted based on data from subjects with known rearing history and for which data were provided (n = 523; Figure 2). The selected model retained the predictors of sex×rearing and age×rearing. This model was significant (χ2[3] = 43.94, P < 0.001) and passed the goodness-of-fit test (HL χ2[1] = 0.86, P = 0.354). The model had a log likelihood of 150.85 and R2 range of 8.1% (Cox and Snell) to 25.9% (Nagelkerke). According to the model, immature (β = –2.77, SE = 0.47, Wald(1) = 35.26, P < 0.001) and elderly (β = -2.33, SE = 1.17, Wald(1) = 4.00, P < 0.05) animals had a lower likelihood of exhibiting tool use than adults if they were also NOTMR/WB, and males also had a lower likelihood of exhibiting tool use than females if also NOTMR/WB (β = –1.22, SE = 0.46, Wald(1) = 7.03, P < 0.01).
Table 2.
Occurrence of species-typical behaviors
| Group | Uses tools to acquire food | Makes nests | Copulates | Initiates grooming | Shows all 4 species-typical behaviors |
| Sex | |||||
| Female | 291/301 (96.7%) | 173/301 (57.5%) | 227/269 (84.4%) | 335/378 (88.6%) | 150/268 (56.0%) |
| Male | 237/260 (91.2%) | 97/260 (37.3%) | 149/227 (65.6%) | 249/323 (77.1%) | 75/226 (33.2%) |
| Early rearing | |||||
| Mother-reared and wild-born | 275/278 (98.9%) | 189/278 (68.0%) | 229/263 (87.1%) | 292/306 (95.4%) | 171/263 (65.0%) |
| Nonmother- reared/wild-born | 224/245 (91.4%) | 73/245 (29.8%) | 127/193 (65.8%) | 241/312 (77.2%) | 50/193 (25.9%) |
Data are given as no. of animals showing behavior / no. of animals evaluated (percentage showing behavior)
Figure 2.
Percentage of subjects that use tools, categorized (A) according to early rearing conditions by sex and (B) according to early rearing conditions by age. Categories graphed are those retained by a forward Wald regression.
Nesting.
The 95 subjects that did not have data provided on nesting were removed from this analysis; of the remaining subjects (n = 561), about half (48.1%) were reported to build nests (Table 2). Regression analysis was conducted on all subjects for which data on nesting were provided and rearing history was known (n = 523; Figure 3). The selected model retained the predictors of age, sex, rearing, group size, age×sex, and group size×sex. This model was significant (χ2[10] = 293.63, P < 0.001) and passed the goodness-of-fit test (HL χ2[8] = 5.83, P = 0.666). The model had a log likelihood of 431.40 and R2 range of 43.0% (Cox and Snell) to 57.3% (Nagelkerke). According to the model, immature animals had a lower likelihood of exhibiting nest-building than adults (β = –3.73, SE = 0.76, Wald[1] = 24.39, P < 0.001), male chimpanzees had a lower likelihood than female (β = –1.71, SE = 0.30, Wald[1] = 32.14, P < 0.001), NOTMR/WB subjects had a lower likelihood than MR–WB (β = –1.65, SE = 0.25, Wald[1] = 44.24, P < 0.001), and animals living in large groups were more likely to exhibit nest-building than those living in small groups (β = 1.09, SE = 0.39, Wald[1] = 7.89, P < 0.01). The retained interactions did not have any significant Wald values associated with predictors.
Figure 3.
Percentage of subjects that build nests, categorized (A) according to early rearing conditions, (B) to group size, C) to age group and D) to sex. Categories graphed are those retained by a forward Wald regression.
Initiating grooming.
No subjects were missing data regarding grooming, so the prevalence of grooming was assessed for all 701 subjects. Grooming was initiated by 83.3% of the chimpanzees (Table 2). Logistic regression was conducted on the subset for which rearing history was known (n = 618; Figure 4). The selected model retained the predictors of sex, rearing, age×sex, and group size×sex. This model was significant (χ2[6] = 94.14, P < 0.001) and passed the goodness-of-fit test (HL χ2[5] = 5.37, P = 0.372). The model had a log likelihood of 400.85 and R2 range of 14.1% (Cox and Snell) to 25.6% (Nagelkerke). According to the model, male chimpanzees had a lower likelihood than female to initiate grooming (β = –0.96, SE = 0.28, Wald[1] = 12.15, P < 0.001), and NOTMR/WB subjects had a lower likelihood than MR–WB (β = –1.99, SE = 0.35, Wald[1] = 32.94, P < 0.001); for elderly animals, being male further reduced the likelihood of grooming (β = –1.35, SE = 0.54, Wald[1] = 6.21, P < 0.05).
Figure 4.
Percentage of subjects that initiate social grooming bouts, categorized according to (A) sex, (B) early rearing conditions, (C) sex by age and (D) sex by group size. Categories graphed are those retained by a forward Wald regression.
Copulating.
Animals that had not had the opportunity to copulate (for example, those in same-sex groups) or who were young (5 y or younger) were not assessed for copulation, resulting in the removal of 162 animals from this analysis. Overall, 75.8% of the remaining chimpanzees (n = 496) copulated (Table 2). Regression was conducted on the subset of these animals for which rearing history was known, resulting in a sample size of 456 chimpanzees (Figure 5). The selected model retained the predictors of age, sex, rearing, group size, sex×rearing, and age×group size. This model was significant (χ2[10] = 115.22, P < 0.001) and passed the goodness-of-fit test (HL χ2[7] = 7.63, P = 0.367). The model had a log likelihood of 364.51 and R2 range of 22.3% (Cox and Snell) to 34.3% (Nagelkerke). According to the model, immature animals had a lower likelihood of copulating than adults (β = –1.66, SE = 0.42, Wald[1] = 15.39, P < 0.001), NOTMR/WB animals had a lower likelihood than MR–WB (β = –0.88, SE = 0.41, Wald[1] = 4.63, P < 0.05), chimpanzees living in large groups had a higher likelihood of copulating than those in small groups (β = 3.19, SE = 1.02, Wald(1) = 9.68, P < 0.01), but immature animals living in large groups had a lower likelihood than adults (β = –3.57, SE = 1.33, Wald[1] = 7.23, P < 0.01).
Figure 5.
Percentage of subjects that copulate, categorized according to (A) sex, (B) age, (C) sex by early rearing conditions and (D) age by group size. Categories graphed are those retained by a forward Wald regression.
All 4 species-typical behaviors.
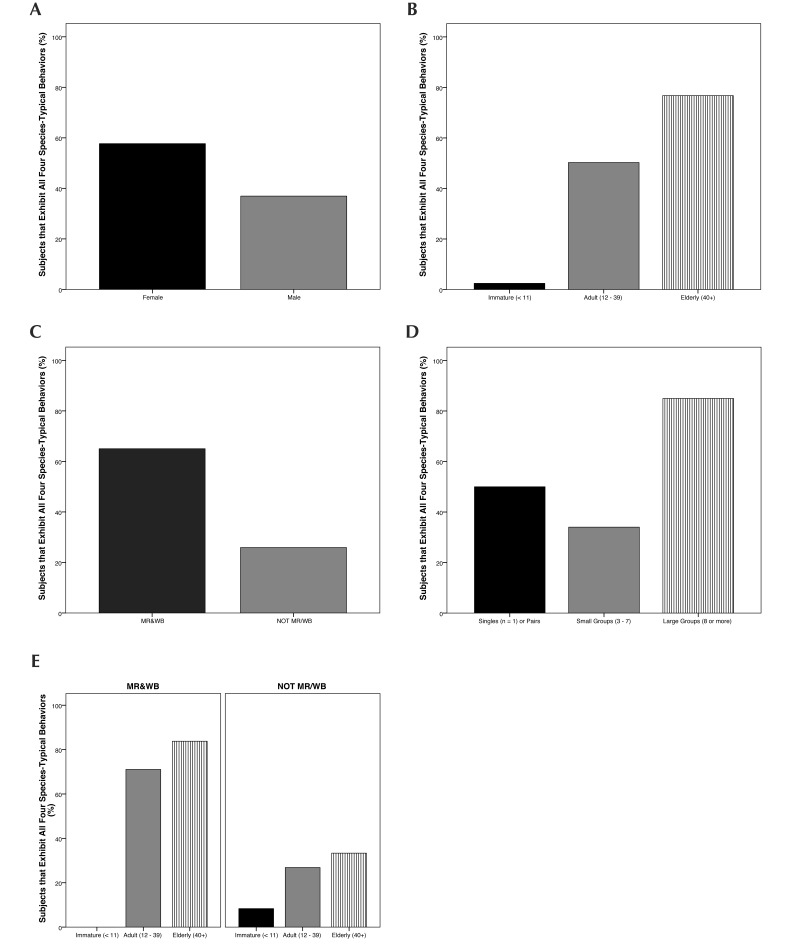
Only subjects that had data on all 4 species-typical behaviors were assessed for this summary measure. All 4 of the species-typical behaviors were shown by 45.5% of the 494 chimpanzees with appropriate data. Regression was conducted on the 456 subjects with appropriate data and known rearing history (Figure 6). The selected model retained the predictors of age, sex, rearing, group size, and age×rearing. This model was significant (χ2[8] = 242.62, P < 0.001) and passed the goodness-of-fit test (HL χ2[8] = 12.67, P = 0.124). The model had a log likelihood of 389.10 and R2 range of 41.3% (Cox and Snell) to 55.0% (Nagelkerke). According to the model, elderly animals had a higher likelihood of exhibiting all 4 species-typical behaviors than adults (β = 1.19, SE = 0.50, Wald[1] = 5.73, P < 0.05), male animals had a lower likelihood than female (β = –1.12, SE = 0.26, Wald[1] = 17.99, P < 0.001), NOTMR/WB animals had a lower likelihood than MR–WB (β = –1.84, SE = 0.27, Wald[1] = 45.92, P < 0.001), and animals living in large groups had a higher likelihood than those living in small groups (β = 2.88, SE = 0.39, Wald[1] = 54.53, P < 0.001).
Figure 6.
Percentage of subjects that show all four STBs, categorized according to (A) sex, (B) age, (C) early rearing conditions, (D) group size, and (E) age by early rearing conditions. Categories graphed are those retained by a forward Wald regression.
Abnormal behaviors.
General information.
Overall, 37.1% of the chimpanzees (n = 701) showed one or more abnormal behaviors, and 5% exhibited 3 or more abnormal behaviors. Across the complete sample (n = 701), the prevalence of individual abnormal behaviors was: rocking, 13.0%; coprophagy, 10.0%; stereotyped behaviors other than rocking, 9.4%; hair plucking, 8.8%; regurgitation and reingestion, 7.7%; self-directed abnormal behavior, 7.3%; and self-injurious behavior with wounding, 1.4% (Table 3).
Table 3.
Occurrence of abnormal behaviors (no. showing behavior [%])
| Group | Rocking | Coprophagy | Stereotypy other than rocking | Hair plucking | Regurgitation and reingestion | Self- directed | Self-injurious behavior with wounding | At least 1 behavior | 3 or more behaviors |
| Sex | |||||||||
| Female (n = 378) | 48 (12.7%) | 61 (16.1%) | 33 (8.7%) | 38 (10.1%) | 30 (7.9%) | 27 (7.1%) | 2 (0.5%) | 148 (39.2%) | 20 (5.3%) |
| Male (n = 323) | 43 (13.3%) | 9 (2.8%) | 33 (10.2%) | 24 (7.4%) | 24 (7.4%) | 24 (7.4%) | 8 (2.5%) | 112 (34.7%) | 15 (4.6%) |
| Rearing | |||||||||
| Mother-reared and wild-born (n = 306) | 14 (4.6%) | 50 (16.3%) | 12 (3.9%) | 36 (11.8%) | 19 (6.2%) | 8 (2.6%) | 1 (0.3%) | 96 (31.4%) | 10 (3.3%) |
| Nonmother-reared/ wild-born (n = 312) | 74 (23.7%) | 20 (6.4%) | 43 (13.8%) | 21 (6.7%) | 28 (9.0%) | 37 (11.9%) | 7 (2.2%) | 141 (45.2%) | 22 (7.1%) |
Coprophagy.
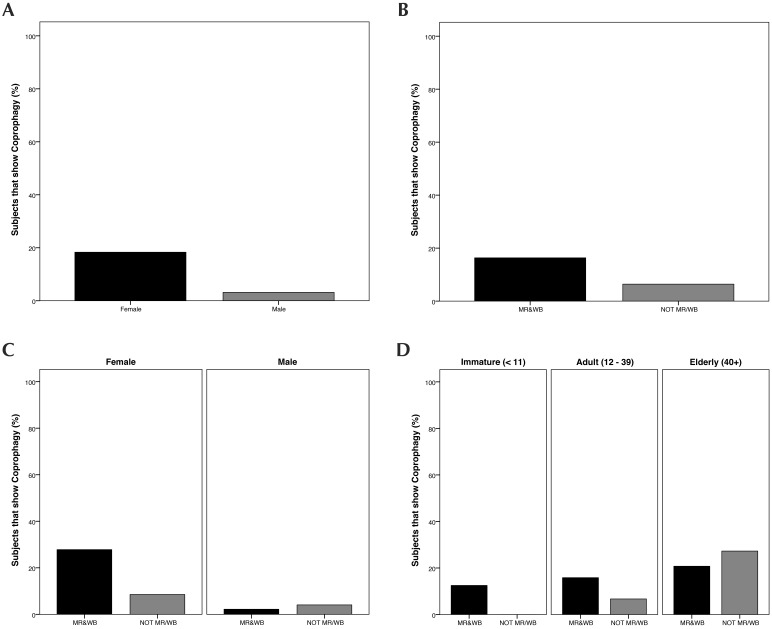
All subjects with rearing history provided (n = 618) were assessed for coprophagy. The selected model retained the predictors of sex, rearing, sex×rearing, and age×rearing (Figure 7). This model was significant (χ2[5] = 73.73, P < 0.001) and passed the goodness-of-fit test (HL χ2[4] = 1.36, P = 0.852). The model had a log likelihood of 362.94 and R2 range of 11.2% (Cox and Snell) to 22.2% (Nagelkerke). According to the model, male chimpanzees were less likely to engage in coprophagy than female (β = –2.85, SE = 0.61, Wald[1] = 21.87, P < 0.001) and NOTMR/WB were less likely than MR–WB (β = –1.32, SE = 0.34, Wald[1] = 14.59, P < 0.001). However, NOTMR/WB male chimpanzees were more likely to show coprophagy than MR–WB males (β = 1.94, SE = 0.79, Wald(1) = 5.98, P < 0.05) and NOTMR/WB elderly animals were more likely than MR–WB elderly animals (β = 1.65, SE = 0.74, Wald[1] = 5.06, P < 0.05).
Figure 7.
Percentage of subjects that show coprophagy, categorized according to (A) sex, (B) early rearing conditions, (C) sex by early rearing and (D) age by early rearing. Categories graphed are those retained by a forward Wald regression.
Regurgitation and reingestion.
All subjects with rearing history provided (n = 618) were assessed for regurgitation and reingestion. The selected model retained the predictors of age and rearing×group size. This model was significant (χ2[4] = 35.53, P < 0.001) and passed the goodness-of-fit test (HL χ2[5] = 0.212, P = 0.900). The model had a log likelihood of 296.98 and R2 range of 5.6% (Cox and Snell) to 13.4% (Nagelkerke). According to the model, elderly animals had a higher likelihood of exhibiting regurgitation and reingestion than adults (β = 1.21, SE = 0.37, Wald[1] = 10.64, P < 0.005), and NOTMR/WB animals living in pairs had a higher likelihood than MR–WB animals living in pairs (β = 1.68, SE = 0.45, Wald[1] = 14.12, P < 0.001).
Stereotypy other than rocking.
All subjects with rearing history provided (n = 618) were assessed for stereotypy other than rocking. The selected model retained the predictors of rearing and age×rearing. This model was significant (χ2[3] = 36.87, P < 0.001) and passed the goodness-of-fit test (HL χ2[1] = 0.00, P = 1.00). The model had a log likelihood of 334.19 and R2 range of 5.8% (Cox and Snell) to 12.8% (Nagelkerke). According to the model, NOTMR/WB animals had a higher likelihood than MR–WB animals (β = 1.30, SE = 0.35, Wald[1] = 13.84, P < 0.001), and elderly NOTMR/WB subjects had a further increased likelihood of engaging in stereotypy as compared with MR–WB elderly chimpanzees (β = 2.46, SE = 0.65, Wald[1] = 14.16, P < 0.001).
Stereotyped rocking.
All subjects with rearing history provided (n = 618) were assessed for stereotyped rocking. The selected model retained the predictors of rearing, age by rearing, and age by group size. This model was significant (χ2[7] = 88.17, P < 0.001) and passed the goodness-of-fit test (HL χ2[3] = 0.223, P = 0.974). The model had a log likelihood of 417.71 and R2 range of 13.3% (Cox and Snell) to 23.8% (Nagelkerke). According to the model, NOTMR/WB chimpanzees had a higher likelihood of rocking than MR–WB (β = 1.59, SE = 0.34, Wald[1] = 22.14, P < 0.001), and this likelihood was further increased for NOTMR/WB immature animals (β = 2.06, SE = 0.38, Wald[1] = 30.01, P < 0.001). Immature chimpanzees in large groups, however, had a reduced likelihood as compared with immature animals in small groups (β = –1.78, SE = 0.86, Wald[1] = 4.27, P < 0.05).
Hair plucking.
All subjects with rearing history provided (n = 618) were assessed for hair plucking. The selected model retained only the predictor of sex×rearing. This model was significant (χ2[1] = 5.35, P < 0.05), but evaluation using the HL test for goodness of fit was not possible. The model had a log likelihood of 374.93 and R2 range of 0.9% (Cox and Snell) to 1.9% (Nagelkerke). According to the model, NOTMR/WB male chimpanzees had a lower likelihood of engaging in hair plucking than MR–WB males (β = –0.88, SE = 0.42, Wald[1] = 4.44, P < 0.05).
Self-directed abnormal behavior.
All subjects with rearing history provided (n = 618) were assessed for self-directed abnormal behavior. The selected model retained the predictors of age, rearing, and rearing×group size. This model was significant (χ2[5] = 47.27, P < 0.001) and passed the goodness-of-fit test (HL χ2[5] = 0.43, P = 0.994). The model had a log likelihood of 275.15 and R2 range of 7.4% (Cox and Snell) to 18.1% (Nagelkerke). According to the model, NOTMR/WB animals had a higher likelihood of exhibiting self-directed abnormal behavior than MR–WB (β = 1.41, SE = 0.45, Wald[1] = 9.94, P < 0.005), and this likelihood was further increased for NOTMR/WB animals living in pairs as compared with NOTMR/WB chimpanzees in small groups (β = 1.56, SE = 0.45, Wald[1] = 11.74, P < 0.005).
Self-injurious behavior with injury.
All subjects with rearing history provided (n = 618) were assessed for this measure. The selected model retained only the predictor of sex×rearing. This model was significant (χ2[1] = 9.40, P < 0.005) but it was not possible to test for goodness of fit. The model had a log likelihood of 76.05 and R2 range of 1.5% (Cox and Snell) to 11.7% (Nagelkerke). According to the model, NOTMR/WB animals had a higher likelihood than MR–WB of engaging in self-injurious behavior with injury if they were also male (β = 2.29, SE = 0.82, Wald[1] = 7.77, P < 0.01). Of the 10 cases during the 2-y period assessed for this study, only one required veterinary intervention (for example, sutures, surgical intervention, surgical debridement).
One or more abnormal behaviors.
Overall, 37.1% of the 701 subjects were reported to engage in at least one of the abnormal behaviors surveyed. All subjects with rearing history provided (n = 618) were assessed further for this summary measure (Figure 8). The selected regression model retained the predictors of sex, sex×rearing, group size×rearing, and age×rearing. This model was significant (χ2[6] = 52.73, P < 0.001) and passed a goodness-of-fit test (HL χ2[4] = 1.07, P = 0.900). The model had a log likelihood of 770.13 and R2 range of 8.2% (Cox and Snell) to 11.1% (Nagelkerke). According to the model, male animals had a lower likelihood of exhibiting an abnormal behavior than female (β = –1.11, SE = 0.25, Wald[1] = 19.92, P < 0.001), NOTMR/WB males were more likely than MR–WB males (β = 1.17, SE = 0.28. Wald(1) = 17.35, P < 0.001), NOTMR/WB chimpanzees in large groups were less likely than NOTMR/WB animals in small groups (β = –0.97, SE = 0.31, Wald[1] = 10.21, P < 0.005), and NOTMR/WB immature chimpanzees were more likely than MR–WB immature animals (β = 0.75, SE = 0.32, Wald[1] = 5.56, P < 0.05).
Figure 8.
Percentage of subjects that show some type of abnormal behavior, categorized according to (A) sex, (B) early rearing conditions by sex, (C) early rearing conditions by group size, and (D) early rearing by age. Categories graphed are those retained by a forward Wald regression.
Three or more abnormal behaviors.
Only 5.0% of the subjects (n = 701) showed 3 or more abnormal behaviors. All subjects with rearing history provided (n = 618) were assessed through a regression procedure for this summary measure. The selected model retained the predictors of rearing×age and rearing×group size. This model was significant (χ2[4] = 27.74, P < 0.001) and passed the goodness-of-fit test (HL χ2[2] = 0.006, P = 0.997). The model had a log likelihood of 224.06 and R2 range of 4.4% (Cox and Snell) to 13.1% (Nagelkerke). According to the model, elderly animals had a higher likelihood of exhibiting 3 or more abnormal behaviors than adult animals if they were also NOTMR/WB (β = 1.77, SE = 0.72, Wald[1] = 6.08, P < 0.05), and NOTMR/WB animals had a higher likelihood of exhibiting 3 or more abnormal behaviors if they were in either pairs (β = 1.18, SE = 0.48, Wald[1] = 6.19, P < 0.05) or large groups (β = 1.79, SE = 0.53, Wald[1] = 11.54, P < 0.005) rather than in small groups.
Positive reinforcement training.
Nearly all (97.7%) of the 701 subjects generally voluntarily cooperate with shifting within their enclosure, and 95.4% cooperate with separating from their group members for brief periods (n = 586; data were not provided for some subjects). Cooperation with receiving an injection of anesthetic was reported for 72.2% (n = 701), and 69.5% (n = 701) have been trained to offer at least one body part for examination (that is, show hand or foot when asked). About 1/5 (19.5%, n = 701) of the chimpanzees were trained to provide a urine sample, 9.1% (n = 701) to cooperate with a capillary blood draw, 7.7% (n = 561; data were not provided for some subjects) to cooperate with an injected vaccination or other medication, and 1.7% (n = 701) to cooperate with blood collection by using a syringe.
Discussion
The current study collated information on a variety of measures relevant to chimpanzee welfare and included all of the chimpanzees living in research laboratories in the United States. The survey assessed a larger sample of chimpanzees living under human care than has been published previously. Because of this comprehensive subject pool, many of the chimpanzees were undoubtedly subjects in published studies that were conducted at United States research facilities that we cited here as comparisons, so it is important to note that the subject pools are not independent.
Nearly every chimpanzee living in a research facility is socially housed. This finding is significant because social housing is such an important component of supporting chimpanzee wellbeing.14,74 There was a single exception to this rule, so 0.14% of the United States research chimpanzee population was individually housed at the time of survey completion. This figure represents a notable reduction in single housing since a previous survey, when 7.6% (n = 93) of the chimpanzees at research facilities were individually housed.17 One reason for this reduction is the cessation of biomedical research with chimpanzees, 53 which sometimes required periods of single housing. Other reasons may be an increase in research conducted on socially housed subjects, the retirement of singly housed chimpanzees to sanctuaries, and the development of methods to treat illnesses in chimpanzees while they are socially housed (for example, by training them to cooperate with treatment). However, it is worth noting that biomedical research was being conducted in 2004 when the previous survey was performed, and even then, more than 90% of the chimpanzees were socially housed.17 The NIH's Working Group Report recommended that “[u]nless dictated by clearly documented medical or social circumstances, no chimpanzee should be required to live alone for extended periods of time” (p 21).75 With more than 99.0% of the research chimpanzee population currently socially housed, this objective seems to have been met.
Chimpanzees in United States research facilities are socially housed, but the size of their social groups is smaller than might be recommended. The mean group size was 4.6 animals, and the median was 7, with the largest group having 14 members. The Working Group Report (2013) recommended at least 7 animals per group. Only 36.5% of chimpanzees lived in groups of 7 or more, and the largest portion (51.9%) lived in groups of 3 to 6 animals. For comparison, in 2017, the 260 chimpanzees living in all 32 of the United States zoos accredited by the American Zoological Association87 had a mean group size of 6.8 animals, a median group size of 6, and a range of 2 to 15 group members. Only 2 of these zoo chimpanzees were housed in a pair. These group sizes are influenced by several zoos that used a ‘fission–fusion’ management approach, with fluid group membership that varied from day to day. These groups were coded as being 2 smaller groups rather than a single, larger one. The most notable difference between zoos and research facilities in terms of group size is the higher proportion of pairs housed in research facilities (11.1% compared with 0.8%). The median group sizes were quite similar across the 2 types of facilities, as was the range of group sizes. The mean group size in the research setting was smaller than in the zoo setting (4.6 animals compared with 6.8, respectively).
Wild chimpanzees live in large communities with 40 to more than 100 members.22,46 With a fission–fusion social structure, they spend most of their time in much smaller traveling parties with fluid membership, typically with 4 to 8 chimpanzees per party.22 Some authors have suggested that captive chimpanzee groups should mimic these traveling parties given that large, static groups are not species-typical for wild chimpanzees.84 However, captive groups of 20 or more chimpanzees have successfully been managed in research facilities, zoos, and sanctuaries. The Chimpanzee Care Manual2 recommends a group size of at least 3 adult male animals and 5 adult females and dependent offspring. The Working Group recommendation was that “[c]himpanzees must have the opportunity to live in sufficiently large, complex, multi-male, multi-female social groupings, ideally consisting of at least 7 individuals” (p 21).75 These recommendations for group size are not based on published literature, because the relevant research projects have not been conducted. A recent conference presentation reported on behavioral differences between groups of different sizes (more or fewer than 7 members) and found that both affiliative and abnormal behaviors were higher in the larger groups and that other behaviors were unaffected by group size.85 More data are needed to fully identify optimal or acceptable group sizes for chimpanzees and the needed characteristics of available space to support those groupings.
Research chimpanzees in the United States all have access to the outdoors on a daily basis. Our findings reflect an increase in the percentage of chimpanzees at research facilities housed with access to the outdoors since an earlier survey, which found that 7.6% of them lived indoors only.17 Some evidence suggests—albeit not strongly—that outdoor housing improves chimpanzee wellbeing.6,99 In general, it is difficult to unambiguously evaluate the effect of living outdoors on primate behavior, given that outdoor access is typically confounded by size and complexity of the enclosure, complexity of the social group, and by husbandry routines and research procedures that may affect behavior.40 One group6 assessed the behavior of 25 chimpanzees, only 13 of which were living indoors, so the sample size is modest, and the information is only available as a published abstract. Regardless, they found that chimpanzees with outdoor access showed less abnormal behavior, less yawning, more self-grooming, and more activity than those living indoors. These preliminary findings are promising, but a complete manuscript has not been published, and the type of enclosure and social group varied between the indoor and indoor–outdoor settings. Another study99 found that chimpanzees housed indoors used enrichment less than those housed in indoor–outdoor enclosures. However, there was an age confound such that more of the chimpanzees housed indoors were older, and it has been demonstrated that age influences enrichment use.18,65 Studies of other NHP have found increases in species-typical behavior31,78 and reductions in abnormal behavior7,40 associated with outdoor housing, but again, confounding factors do not allow strict conclusions regarding the role that outdoor housing plays in promoting welfare. Despite the lack of compelling scientific data, there is a common perception that outdoor housing is beneficial, and the Working Group Report indicates that “[c]himpanzees must be housed in environments that provide outdoor access year round” (p 22).75 This recommendation is clearly met.
The Working Group further recommended that chimpanzees “should have access to natural substrates, such as grass, dirt, and mulch, to enhance environmental complexity” (p 22).75 Approximately 2/3 of the research chimpanzees lived on such surfaces on a daily basis. This situation is beneficial, because these substrates provide environmental complexity; encourage foraging, activity, and play; and reduce abnormal behavior.3,26 Although the majority of research chimpanzees have this opportunity, not all do, and this provision should be encouraged as a future enhancement. It is notable that all of the research chimpanzees lived in primary housing that was larger than the minimal allowable size.
Two of the species-typical behaviors surveyed were tool use and nest building. Nearly all (94.1%) of the chimpanzees surveyed used tools to acquire food. Staff at chimpanzee research facilities have developed and used enrichment opportunities that facilitate tool use for many years,65 and considerable research in tool use has been conducted with these chimpanzees.50 Both of these activities have probably contributed to the widespread tool capabilities in this population. Some rearing-associated effects were found for tool use, such that male chimpanzees were less likely than female animals to use tools when they were NOTMR/WB, and younger and elderly chimpanzees were less likely to use tools than adults when they were NOTMR/WB. These negative effects of nonmother rearing on tool use are consistent with other published literature.28 Since tool use involves social learning or social facilitation,62,96 it is unsurprising that MR and WB subjects would be more likely to acquire this skill. Half (48.1%) of the research chimpanzees make nests, and females were more likely to make nests than males. This sex-associated difference is not species-appropriate, because essentially all wild adult chimpanzees make nests.84 MR–WB subjects were more likely than NOTMR/WB to make nests, and immature chimpanzees were less likely to do so than adults. Finally, chimpanzees living in large groups were more likely to construct nests than those living in small groups. Previous studies found that adult chimpanzees who do not make nests and are then housed with or near others who do make nests still did not readily learn to do so themselves.9,10 Even when nesting materials were provided 25 times over several months, none of the nonnest-builders learned to produce a nest.10 In one study, wild-born chimpanzees built and used nests significantly more than captive-born, and captive-born, mother-reared subjects spent more time building and using nests than nursery-reared animals.98 Because experience with bedding materials as adults did not change this finding, the author concluded that nest building requires early experience and practice.98 With less than half (44.5%) of the research chimpanzee population being mother-reared or wild-born, it is possible that NOTMR/WB animals will simply not learn to build nests, at least not through mere exposure to successful nest-builders and appropriate materials. Although the possibility has not been empirically tested, PRT techniques might be helpful, reinforcing chimpanzees as they manipulate nesting materials and thus shaping the behaviors required to successfully build a nest. Scaffolding the chimpanzees’ behavior in this way could make it possible to ‘teach’ adult animals to successfully engage in some species-typical behaviors, assuming they are not entirely instinctive behaviors (see the discussion following). The Working Group Report recommendation that “[c]himpanzees must be provided with materials to construct new nests on a daily basis”75 (p 23) cannot be fully evaluated through the current study because the survey focused on individual behavior and did not ask about the provision of nesting materials.
The species-typical social behaviors that were queried were copulating and initiating grooming. A vast majority of the research subjects (83.3%) initiate grooming bouts with conspecifics. About 3/4 of the subjects older than 5 y and with access to an opposite sex partner (n = 496) copulate. In an archival study of a much smaller sample of zoo-housed chimpanzees, 75% of the subjects exhibited functional copulatory behavior,57 mirroring the result in the research population. In one study,57 93% of zoo chimpanzees that lived with their mothers for at least their first year did copulate as adults, but only 50% of those separated from their mothers before 1 y of age copulated as adults. Of research chimpanzees that were mother-reared for at least 6 mo of their first year, 87.1% copulated compared with 65.8% who were not mother-reared. Again, research chimpanzees’ copulation behavior shows a similar pattern to zoo-living chimpanzees.
MR–WB chimpanzees were more likely to initiate grooming and to copulate than NOTMR/WB animals. In addition, males were less likely than females to initiate grooming, and within males, age had an additional effect, such that elderly male chimpanzees were less likely than adult males to initiate grooming. Young animals were less likely to copulate than adults, but for immature chimpanzees, living in large social groups increased the likelihood of copulation over that of immature animals in small groups. Results of the current survey therefore parallel earlier findings,41 in which chimpanzees with less exposure to other chimpanzees during infancy showed a lower frequency of sexual behavior and grooming later in life. It is encouraging that the vast majority of research chimpanzees express these important social behaviors. However, given the substantial minority that does not, it is important to continue efforts to elicit these behaviors.
Ideally, all captive chimpanzees would show all 4 of the species-typical behaviors surveyed, but in the current population, only 45% (n = 494) did. The presence of all 4 behaviors was more likely for females (compared with males), elderly chimpanzees (compared with adults), MR–WB animals (compared with NOTMR/WB), and chimpanzees living in large groups (compared with small groups). Male animals, particularly those not reared by their mothers, are more negatively affected than females: only 14% of NOTMR/WB male chimpanzees for whom we had appropriate data (n = 90) exhibited all 4 behaviors, while 35.9% of NOTMR/WB females did. It is possible that this sex-associated difference is a result of differential social learning tendencies, given that females have been reported to develop socially learned skills at younger age than do males,61 to watch behavior being modeled more than males, and to copy models’ techniques more conservatively,61,63 implying a less goal-directed style of learning. The mechanisms of social learning in chimpanzees, perhaps particularly for male animals, may be based on a more functional understanding of a demonstrated task's goal.73 When the goal of a behavior is not obvious or not clearly desirable, this situation could interfere with learning behaviors such as copulation or nest-building. In other words, perhaps it is not that male, nursery-reared chimpanzees cannot learn to make nests or to copulate but rather that they are not sufficiently motivated to do so. PRT could perhaps address this gap by providing a clear, valued reward for engaging in the appropriate behavior (for example, nesting) until, ideally, the chimpanzee finds the well-built nest to be its own reward. Using PRT in this novel manner could contribute to improving welfare by facilitating the expression of a natural and positive behavioral pattern. This possibility may be worth investigation, because the potential benefits are of high value and because prior research has shown that PRT can increase natural behaviors, such as social grooming, even outside of training sessions.91 Once the behavior is ‘trained,’ social grooming seems to be reinforced without external reward (such as food treats).
Identifying factors that underlie abnormal behaviors can improve our understanding of primate welfare and our ability to distinguish between abnormal behaviors that reflect poor welfare and those that may not.51 The measures of prevalence of abnormal behavior gathered in the current study do not reflect the frequency, duration, or intensity of these behaviors, and all of these features are important to consider when evaluating welfare.77 In the current survey, about 1/3 (Table 3) of the research center chimpanzees showed some type of abnormal behavior in the 2 y prior to survey completion, and a small portion (5%) showed 3 or more abnormal behaviors.
The most common abnormal behavior was stereotyped rocking (13%), and early rearing effects were pronounced: only 4.6% of the MR–WB subjects rocked, whereas 23.7% of the NOTMR/WB subjects did. An effect of age was also evident, given that young chimpanzees who were NOTMR/WB were more likely to rock than adult NOTMR/WB animals, and young chimpanzees living in large groups were less likely to rock than young chimpanzees living in small groups. Stereotyped rocking has often been found to be associated with socially restrictive early rearing,8,36,68,69 although other factors also influence its expression.80 Although stereotyped rocking typically develops at a young age, it may persist in adolescent and adult chimpanzees.35,37 Stereotypies such as rocking may serve to help an animal cope with environmental conditions that are suboptimal,70 such as nursery-rearing and social restriction,68 and once they have developed, they will persist as a response to encountered stressors.93,97
In contrast to our findings, other studies identified coprophagy as the most common abnormal behavior in zoo-housed and research-facility-housed chimpanzees,12,54,68,74 and this behavior was much more prevalent in those samples than in the current sample (Table 4). Some of this difference is undoubtedly accounted for by the greater proportion of mother-reared animals in zoo populations12,54 (see following discussion), but a 1999 study of research chimpanzees also revealed higher rates.74 The lower rate in the current study may be due to improved enrichment practices that reduce coprophagy.13,21,43 Although coprophagy may not be associated with poor welfare conditions such as nonmother-rearing, and thus is argued to be less reliable as a general welfare indicator than abnormal behaviors such as rocking or self-directed behaviors,51 it is still not a desirable behavior, particularly at high frequencies. Coprophagy or seed reingestion from feces has been reported in multiple wild communities of chimpanzees, although at much lower frequencies than are observed in captive chimpanzees. Coprophagy has been posited to be an adaptive strategy to maximize nutritional intake in certain conditions,11,79 but in captivity, where it is much more pervasive than in wild chimpanzee populations, it may be more likely to contribute to parasitic and bacterial disease transmission through a fecal–oral route of exposure with less benefit as from a nutritional deficiency.
Table 4.
Prevalence of various abnormal behaviors in chimpanzees in other published studies
| 1 or more abnormal behaviors (including coprophagy) | 1 or more abnormal behaviors (excluding coprophagy) | Coprophagy | Hair plucking (self or other) | Regurgitation and reingestion | |
| Current data (research facilities) | 37.1% | 32.5% | 10.0% | 8.8% | 7.7% |
| Reference 4 (research facilities) | 33.2% | 22.4% (maximum possible) | 5.6% | ||
| Reference 12 (zoos) | 100.0% | 83.0% | 78.0% (maximum possible) | 30.0% | |
| Reference 54 (zoos) | 64.0% | 48.0% | 41.0% | 32.0% | 7.0% |
| Reference 56 (former research animals) | 72.0% | ||||
| Reference 68 (former research animals) | 37.7% |
The prevalence of hair plucking in the current study is lower than in others (Table 4), including a 2016 study54 that used the same methodology. Other colleagues reported that 16.4% of the research chimpanzees they studied pulled their own hair, and 6.0% pulled from another animal.74 Assuming none of the previous subjects did both, the sum would be 22.4%, showing a hair-pulling rate which is still lower than the rates reported in zoos. In addition, the previously cited study74 reports higher numbers for this behavior than in the current study, and both studies defined hair-plucking in the same way. It is possible that, like coprophagy, the lower rates in the current study are due to improved enrichment and management practices, some of which have been shown to reduce hair plucking.13 Hair plucking directed toward oneself and toward others has been observed in many primate species across different settings.60,64
The prevalence of regurgitation–reingestion was similar across several studies, including the current one, although higher rates were reported elsewhere12 (Table 4). This finding may indicate that regurgitation–reingestion is not socially influenced, given that populations with more mother-reared subjects do not have higher prevalence rates of regurgitation. This contrasts with coprophagy which is affected by the social setting, as described earlier. Regurgitation–reingestion is related to feeding practices5,95 and animal training techniques have been applied to reduce it.72
Self-directed abnormal behaviors (for example, bizarre posturing, self-clasping) did not vary with sex, but NOTMR/WB subjects were more likely to show these behaviors than MR–WB chimpanzees. This rearing effect is consistent with literature on other NHP.64,77 Previous research has found that self-directed abnormal behaviors were more widespread among chimpanzees that had been individually housed earlier in life as compared with those that were individually housed at later stages in their development,56 generally corroborating our finding that early social environment affects self-directed behaviors.
Self-injurious behavior is a very concerning behavioral problem.76 It has a strong relationship to early life stressors (for example, early removal from mother, indoor rearing), individual housing, and more chronic, stressful experiences, such as repeated room moves and blood withdrawals.47,76 With only 10 of 701 subjects showing this behavior, our findings are tentative, but did corroborate prior research. Across our subjects, we found that NOTMR/WB animals were more likely to show this behavior, but only if they were also male. This possible male bias had been noted previously.42 Unfortunately, self-injurious behavior may function to reduce arousal and is often resistant to treatment,76 although comprehensive and intensive treatment may be successful.24
Zoo-housed chimpanzees may have a higher prevalence of abnormal behavior than chimpanzees in United States research facilities. Higher rates of coprophagy in zoo populations account for some of this difference, but when those chimpanzees that showed only coprophagy were removed from the sample (reference 54 values in Table 4), there were still proportionally more zoo subjects showing abnormal behavior than in the current dataset. The “hypothetical average individual” in a 2011 zoo-based study12 showed 5 different abnormal behaviors, and all subjects had at least 2, which again are higher values than the current findings (although see reference 88 for a critique of the methods in reference 12 study). Another possible explanation for reported differences between zoo and research facility behavioral patterns is that the facilities use different observational methods. Survey methods like the current study are susceptible to bias relating to the amount of behavioral observation that is done: facilities that conduct more observation of their chimpanzees would report more of the infrequently occurring behaviors. Although we are doubtful there is a systematic bias between the 2 facility types, it would be valuable to have more precise information regarding observational methods, the amount of time observing each individual, and staff training in behavior at each facility. The somewhat surprising result that more zoo chimpanzees show abnormal behavior than research chimpanzees will be addressed in an upcoming analysis (Bloomsmith and colleagues).16
Many abnormal behaviors in chimpanzees are influenced by early rearing history,41,55,74 and this was confirmed by the current analysis. In some cases, nonmother rearing seemed to have impact even within a particular subset of individuals, so, for example, males who were also NOTMR/WB might be more likely to engage in a particular behavior than males who were MR and WB, even if generally males were already more likely to engage in that behavior than females. Nonmother rearing was associated with more showing any abnormal behavior if subjects were males, with more stereotyped rocking, more nonrocking forms of stereotypy, more regurgitation/reingestion, more self-directed behaviors and more self-injurious behavior. Coprophagy was generally more common among MR and WB subjects, but the effects varied with sex, because NOTMR/WB male chimpanzees were more likely to engage in coprophagy than were MR–WB males, and with age, because elderly NOTMR/WB animals were more likely than elderly MR and WB to do so. Other studies have likewise found that MR–WB chimpanzees are more likely to engage in coprophagy than NOTMR/WB,16,51,54,74 but the reverse effect of rearing on males’ coprophagy has not been revealed in smaller samples. Hair-plucking was higher among MR and WB male chimpanzees than NOTMR/WB males, although the lack of goodness of fit of our model indicates that unmeasured influences may be present. One previous study74 found that self-hairplucking was more prevalent in mother-reared subjects than nonmother-reared, which is somewhat consistent with our findings. When an abnormal behavior seems to be more prevalent in mother-reared animals, one possible explanation is that mother-reared chimpanzees have greater exposure to other chimpanzees that engage in coprophagy than nonmother reared chimpanzees and that the behavior is socially learned. Social learning has been implicated in the development of abnormal behavior in chimpanzees.49 However, socially learned abnormal behaviors may not be indicators of negative welfare,51 thus complicating the usual assumption that when abnormal behavior of any kind is observed, it signals negative welfare, past or present.51,74 Environmental factors related to welfare, however, may be important in the origin of a potentially socially learned and socially transmitted abnormal behavior like coprophagy. These factors need to be understood, both to reduce currently expressed abnormal behavior and to prevent undesirable behaviors from appearing in the first place, from which point they may be socially transmitted. For example, methods of feeding captive apes have been shown to influence coprophagy and regurgitation–reingestion, and can be modified to reduce these behaviors.13,21,43,72
Although male chimpanzees were more likely to show abnormal behavior than female animals, this effect appears to be driven only by females being more likely to show coprophagy than males. This female bias in coprophagy corroborates other findings54,56,74 and may be related to a female bias for social learning.61 Although male biases may have been reported for abnormal behavior in other primates,47 this situation may not be the case for chimpanzees. For example, earlier studies80,93 found no sex-associated difference in stereotyped rocking in chimpanzees, and the current finding confirms that outcome. It is also possible that sex alone is insufficient to significantly affect abnormal behaviors except when combined with an additional factor, such as early rearing, and we did find such effects for hair-plucking and self-injurious behavior. However, in both of these cases, we were unable to find a good-fitting model that was based on our set of predictors, indicating that some mechanism other than sex, rearing, age, and group size influenced the behavior, or that the behavior itself may have more than one etiology and form.
Age affected the prevalence of abnormal behavior in a few situations. Elderly subjects were more likely to show regurgitation–reingestion than adult subjects, and early rearing had a significant effect within the elderly subset, such that NOTMR/WB elderly chimpanzees were more likely to show stereotypical behavior and coprophagy than MR and WB elderly animals. In addition, early rearing had a significant effect on immature subjects, such that NOTMR/WB immature subjects were more likely to exhibit any abnormal behavior and to exhibit rocking behavior than immature chimpanzees that were mother reared. Furthermore group size affected abnormal behavior in some cases. For example, within the category of immature animals, stereotyped rocking was more likely for those that also lived in small groups rather than large groups. Moreover, group size interacted with early rearing to influence abnormal behavior, such that regurgitation–reingestion and self-directed abnormal behavior were more prevalent among pair-housed chimpanzees when they were also NOTMR/WB.
The voluntary cooperation of chimpanzees with routine husbandry activities, veterinary procedures, and research procedures has been greatly enhanced since the early 1990s,17 when the use of PRT techniques was implemented more widely. PRT is now a well-established refinement in chimpanzee care, and it has improved the ease and efficiency of captive chimpanzee management,20 reduced chimpanzee distress related to research and veterinary procedures,58 decreased abnormal and stress-related behaviors in captive chimpanzees,82 and, lastly, reduced aggressive behavior in captive chimpanzees.19 Findings from the current survey indicate that PRT is well-established at research facilities in the United States. The high percentage of chimpanzees that cooperate with receiving an injection of anesthetic (72.2%) and with presentation of body parts for examination (69.5%) is evidence that these approaches can be effective on a widespread basis. Biologic sample collection behaviors (for example, urine and blood collection) had been trained in far fewer subjects, perhaps reflecting an infrequent need for those samples. An earlier survey of 6 chimpanzee research facilities found that 5 of the 6 facilities had formal chimpanzee training programs that relied primarily on PRT and which had been established prior to 2004.17 Evidence from the current survey indicates that this sustained effort has been successful in enhancing chimpanzee welfare, given that the vast majority of research chimpanzees cooperate with receiving injections for veterinary care; with shifting, separating from group members, and other movements required to facilitate husbandry-related procedures; and with at least some body examination behaviors that facilitate veterinary care. This progress is relevant to the Working Group Report recommendation that “[p]ositive reinforcement training is the only acceptable method of modifying behaviors to facilitate animal care and fulfillment of management needs [...] and progress toward achieving established benchmarks should be documented” (p 24).75 Although the current survey did not address this recommendation in detail (that is, did not ask about each subject's training history regarding queried behaviors), it is clear that PRT techniques are widely used and that a major effort has been directed toward gaining cooperation with a variety of husbandry and research-related procedures. It is probable that some of the chimpanzees that currently present for injection learned to do so prior to the establishment of PRT based programs and that they learned to present through negative reinforcement or coercion (for example, being ‘squeezed’ in a transfer box or shown a dart gun, respectively). However, it is highly unlikely that this situation accounts for the high percentage of animals (72.2%) reported to voluntarily present for anesthetic injection in this survey. Similarly, although it is possible that animals complying with requests to shift, separate, or present body parts have not been formally trained to do so, it is highly unlikely that they would comply with these requests at such high rates without positive reinforcement for doing so. So, although the data presented here may not reflect the results of only formal, PRT-based training, the high levels of compliant animals indicate that PRT is being widely used with chimpanzees in research facilities.
In conclusion, chimpanzees at research facilities in the United States are socially housed, have daily access to outdoor spaces, and have been trained using positive techniques to cooperate with a variety of procedures. Just under half of these chimpanzees show all 4 key species-typical behaviors recorded (tool use, nest building, copulating, initiating grooming bouts), and about one-third of them show some type of abnormal behavior. This broad, survey-based analysis can help to guide future improvements in chimpanzee behavioral management to address behavioral problems or deficits. For instance, based on the finding that only half of the chimpanzees make nests, strategies should be developed to facilitate this behavior. Providing the remaining one-third of the population who does not have daily access to a natural substrate with this opportunity would be advantageous to their welfare. The mean group size was 4.6. Such small social groups are not species-typical and are not recommended by some experts because these group sizes do not facilitate important complexities in chimpanzee social interactions. Others have suggested that there may be a threshold for the number of chimpanzees that can live constantly together in compatible captive groups. Further study of group size and welfare is warranted, but reducing the number of research chimpanzees living in pairs or trios is a laudable goal for research chimpanzees. Each of the additional improvements suggested here will require additional resources and personnel effort. Although it is not possible to change the early rearing histories of the current population, the findings underscore the wide-ranging importance of providing early mother rearing for chimpanzees. Less than half of the population of research chimpanzees is mother-reared, and this characteristic affected the display of many behaviors. It is notable that early rearing had such substantial effects given that it was operationalized within the first year of life. That first year had profound and lasting influences on multiple behavioral measures of welfare. Comparing this type of welfare-related information for chimpanzees across settings (research centers, sanctuaries, zoos, privately owned facilities) will provide a more in-depth understanding of captive chimpanzee welfare in the United States, and we are working toward that goal.
Acknowledgments
This project was supported by the NIH/OD Cooperative Agreement U42-OD 011197 to the Michale E Keeling Center for Comparative Medicine and Research. The Southwest National Primate Research Center resources are supported by NIH grant P51-OD011133 from the Office of Research Infrastructure Programs, Office of the Director. We thank the husbandry, veterinary, and behavioral management staff for the care of the chimpanzees at each center.
References
- 1.Animal and Plant Health Inspection Service. [Internet]. 2007. Animal Welfare Act and Animal Welfare Regulations. [Cited 1 May 2018]. Available at: https://www.aphis.usda.gov/animal_welfare/downloads/AC_BlueBook_AWA_FINAL_2017_508comp.pdf
- 2.AZA Ape TAG. 2010. Chimpanzee (Pan troglodytes) care manual. Silver Spring (MD): Association of Zoos and Aquariums. [Google Scholar]
- 3.Baker KC. 1997. Straw and forage material ameliorate abnormal behaviors in adult chimpanzees. Zoo Biol 16:225–236. . [DOI] [Google Scholar]
- 4.Baker KC, Aureli F. 1997. Behavioural indicators of anxiety: an empirical test in chimpanzees. Behaviour 134:1031–1050. 10.1163/156853997X00386. [DOI] [Google Scholar]
- 5.Baker KC, Easley SP. 1996. An analysis of regurgitation and reingestion in captive chimpanzees. Appl Anim Behav Sci 49:403–415. 10.1016/0168-1591(96)01061-1. [DOI] [Google Scholar]
- 6.Baker KC, Ross SK. 1998. Outdoor access: The behavioral benefits to chimpanzees. Abstracts presented at the American Society of Primatologists annual meeting, Georgetown, Texas 28 June–1 July. Am J Primatol 45:166. [Google Scholar]
- 7.Bayne KS, Dexter S, Suomi S. 1992. A preliminary survey of the incidence of abnormal behavior in rhesus monkeys (Macaca mulatta) relative to housing condition. Lab Anim 21:38–46. [Google Scholar]
- 8.Berkson G. 1968. Development of abnormal stereotyped behaviors. Dev Psychobiol 1:118–132. 10.1002/dev.420010210. [DOI] [Google Scholar]
- 9.Bernstein IS. 1962. Response to nesting materials of wild-born and captive-born chimpanzees. Anim Behav 10: 1–2, 3–6. 10.1016/0003-3472(62)90123-9 [DOI] [Google Scholar]
- 10.Bernstein IS. 1967. Age and experience in chimpanzee nest building. Psychol Rep 20 Suppl:1106 10.2466/pr0.1967.20.3c.1106. [DOI] [PubMed] [Google Scholar]
- 11.Bertolani P, Pruetz JD. 2011. Seed reingestion in savannah chimpanzees (Pan troglodytes verus) at Fongoli, Senegal. Int J Primatol 32:1123–1132. 10.1007/s10764-011-9528-5. [DOI] [Google Scholar]
- 12.Birkett LP, Newton-Fisher NE. 2011. How abnormal is the behaviour of captive, zoo-living chimpanzees? PLoS One 6:1–7. 10.1371/journal.pone.0020101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bloomsmith MA, Alford PL, Maple TL. 1988. Successful feeding enrichment for captive chimpanzees. Am J Primatol 16:155–164. 10.1002/ajp.1350160206. [DOI] [PubMed] [Google Scholar]
- 14.Bloomsmith M, Baker K. 2001. Social management of captive chimpanzees, p 205–241. In: Brent L. Special topics in primatology: the care and management of captive chimpanzees, vol 2. San Antonio (TX): American Society of Primatologists. [Google Scholar]
- 15.Bloomsmith MA, Baker KC, Ross SR, Lambeth SP. 2006. Early rearing conditions and captive chimpanzee behavior: Some surprising findings, p 289–312. In: Sackett GP, Ruppenthal GC, Elias K. Nursery rearing of nonhuman primates in the 21st century; New York (NY): Springer. [Google Scholar]
- 16.Bloomsmith M, Clay A, Ross SR, Lambeth S, Lutz C, Breaux S, Pietsch R, Fultz A, Lammey M, Jacobson SL, Perlman J. 2019Chimpanzees in United States zoos, sanctuaries, and research facilities: A survey-based comparison of atypical behaviors. In: Hopper L, Ross SR. Chimpanzees in context. Chicago (IL): University of Chicago Press. In press. [Google Scholar]
- 17.Bloomsmith MA, Else JG. 2005. Behavioral management of chimpanzees in biomedical research facilities: the state of the science. ILAR J 46:192–201. 10.1093/ilar.46.2.192. [DOI] [PubMed] [Google Scholar]
- 18.Bloomsmith MA, Finlay TW, Merhalski JJ, Maple TL. 1990. Rigid plastic balls as enrichment device for captive chimpanzees. Lab Anim Sci 40:319–322. [PubMed] [Google Scholar]
- 19.Bloomsmith MA, Laule GE, Alford PL, Thurston RH. 1994. Using training to moderate chimpanzee aggression during feedings. Zoo Biol 13:557–566. 10.1002/zoo.1430130605. [DOI] [Google Scholar]
- 20.Bloomsmith MA, Stone AM, Laule GE. 1998. Positive reinforcement training to enhance the voluntary movement of group-housed chimpanzees within their enclosures. Zoo Biol 17:333–341. . [DOI] [Google Scholar]
- 21.Bloomstrand M, Riddle K, Alford P, Maple TL. 1986. Objective evaluation of a behavioral enrichment device for captive chimpanzees (Pan troglodytes). Zoo Biol 5:293–300. 10.1002/zoo.1430050307. [DOI] [Google Scholar]
- 22.Boesch C. 1996. Social grouping in Tai chimpanzees, p 101–113. In: McGrew WC, Marchant LF, Nishida T. Great ape societies. Cambridge (United Kingdom): Cambridge University Press. [Google Scholar]
- 23.Boesch C, Boesch-Achermann H. 2000. The chimpanzees of the Taï Forest: behavioural ecology and evolution. Oxford (NY): Oxford University Press. [Google Scholar]
- 24.Bourgeois SR, Vazquez M, Brasky K. 2007. Combination therapy reduces self-injurious behavior in a chimpanzee (Pan troglodytes): a case report. J Appl Anim Welf Sci 10:123–140. 10.1080/10888700701313454. [DOI] [PubMed] [Google Scholar]
- 25.Bracke MBM, Hopster H. 2006. Assessing the importance of natural behavior for animal welfare. J Agric Environ Ethics 19:77–89. 10.1007/s10806-005-4493-7. [DOI] [Google Scholar]
- 26.Brent L. 1992. Woodchip bedding as enrichment for captive chimpanzees in an outdoor enclosure. Anim Welf 1:161–170. [Google Scholar]
- 27.Brent L. 2001. The influence of rearing condition on chimpanzee introductions, p 103–104. In: The apes: challenges for the 21st century. Chicago (IL): Chicago Zoological Society. [Google Scholar]
- 28.Brent L, Bloomsmith MA, Fisher SD. 1995. Factors determining tool-using ability in 2 captive chimpanzee (Pan troglodytes) colonies. Primates 36:265–274. 10.1007/BF02381352. [DOI] [Google Scholar]
- 29.Brent L, Lee DR, Eichberg JW. 1989. The effects of single caging on chimpanzee behavior. Lab Anim Sci 39:345–346. [PubMed] [Google Scholar]
- 30.Brent L, Williams-Blangero S, Stone AM. 1996. Evaluation of the chimpanzee breeding program at the Southwest foundation for biomedical research. Lab Anim Sci 46:405–409. [PubMed] [Google Scholar]
- 31.Chamove AS, Rohrhuber B. 1989. Moving callitrichid monkeys from cages to outside areas. Zoo Biol 8:151–163. 10.1002/zoo.1430080206. [DOI] [Google Scholar]
- 32.Chimp Care. [Internet]. 2018. Chimpanzees in the US. [Cited 1 May 2018]. Available at: http://chimpcare.org/map
- 33.Clay AW, Bard KA, Bloomsmith MA. 2017. Effects of sex and early rearing condition on adult behavior, health, and well-being in captive chimpanzees (Pan troglodytes). Behav Processes 156:58–76. 10.1016/j.beproc.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 34.Coleman K, Pranger L, Maier A, Lambeth SP, Perlman JE, Thiele E, Schapiro SJ. 2008. Training rhesus macaques for venipuncture using positive reinforcement training techniques: a comparison with chimpanzees. J Am Assoc Lab Anim Sci 47:37–41. [PMC free article] [PubMed] [Google Scholar]
- 35.Davenport RK, Jr, Menzel EW., Jr 1963. Stereotyped behavior of the infant chimpanzee. Arch Gen Psychiatry 8:99–104. 10.1001/archpsyc.1963.01720070101013. [DOI] [PubMed] [Google Scholar]
- 36.Davenport RK, Jr, Menzel EW, Jr, Rogers CM. 1966. Effects of severe isolation on ‘normal’ juvenile chimpanzees. Arch Gen Psychiatry 14:134–138. 10.1001/archpsyc.1966.01730080022004. [DOI] [PubMed] [Google Scholar]
- 37.Davenport RK, Rogers CM. 1970. Differential rearing of the chimpanzee. A project survey p 337–360. In: Bourne GH. The chimpanzee volume 3: Immunology, infections, hormones, anatomy, and behavior of chimpanzees. Baltimore and Manchester: University Park Press. [Google Scholar]
- 38.Erwin J, Deni R. 1979. Strangers in a strange land: Abnormal behaviors or abnormal environments? p 1–28. In: Erwin J, Maple TL, Mitchell G. Captivity and behavior: primates in breeding colonies, laboratories, and zoos. New York (NY): Van Nostrand Reinhold. [Google Scholar]
- 39.Fedurek P, Dunbar RIM. 2009. What does mutual grooming tell us about why chimpanzees groom? Ethology 115:566–575. 10.1111/j.1439-0310.2009.01637.x. [DOI] [Google Scholar]
- 40.Fontenot MB, Wilkes MN, Lynch CS. 2006. Effects of outdoor housing on self-injurious and stereotypic behavior in adult male rhesus macaques (Macaca mulatta). J Am Assoc Lab Anim Sci 45:35–43. [PubMed] [Google Scholar]
- 41.Freeman HD, Ross SR. 2014. The impact of atypical early histories on pet or performer chimpanzees. PeerJ 2:1–16. 10.7717/peerj.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fritz J. 1986. Resocialization of asocial chimpanzees, p 351–360. In: Benirschke K. Primates: the road to self-sustaining populations. New York (NY):Springer-Verlag. [Google Scholar]
- 43.Fritz J, Maki S, Nash LT, Martin T, Matevia M. 1992. The relationship between forage material and levels of coprophagy in captive chimpanzees (Pan troglodytes). Zoo Biol 11:313–318. 10.1002/zoo.1430110503. [DOI] [Google Scholar]
- 44.Fruth B, McGrew WC. 1998. Resting and nesting in primates: behavioral ecology of inactivity. Am J Primatol 46:3–5. . [DOI] [PubMed] [Google Scholar]
- 45.Fultz A, Brent L, Breaux SD, Grand AP. 2013. An evaluation of nest-building behavior by sanctuary chimpanzees with access to forested habitats. Folia Primatol (Basel) 84:405–420. 10.1159/000353900. [DOI] [PubMed] [Google Scholar]
- 46.Goodall J. 1986. Chimpanzees of Gombe: patterns of behavior. Cambridge (MA): Belknap Press of Harvard University Press. [Google Scholar]
- 47.Gottlieb DH, Capitanio JP, McCowan B. 2013. Risk factors for stereotypic behavior and self-biting in rhesus macaques (Macaca mulatta): Animal's history, current environment, and personality. Am J Primatol 75:995–1008. 10.1002/ajp.22161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herrelko ES, Buchanan-Smith HM, Vick SJ. 2015. Perception of available space during chimpanzee introductions: number of accessible areas is more important than enclosure size. Zoo Biol 34:397–405. 10.1002/zoo.21234. [DOI] [PubMed] [Google Scholar]
- 49.Hook MA, Lambeth SP, Perlman JE, Stavisky R, Bloomsmith MA, Schapiro SJ. 2002. Intergroup variation in abnormal behavior in chimpanzees (Pan troglodytes) and rhesus macaques (Macaca mulatta). Appl Anim Behav Sci 76:165–176. 10.1016/S0168-1591(02)00005-9. [DOI] [Google Scholar]
- 50.Hopkins WD, Rabinowitz DM. 1997. Manual specialisation and tool use in captive chimpanzees (Pan troglodytes): the effect of unimanual and bimanual strategies on hand preference. Laterality 2:267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hopper LM, Freeman HD, Ross SR. 2016. Reconsidering coprophagy as an indicator of negative welfare for captive chimpanzees. Appl Anim Behav Sci 176:112–119. 10.1016/j.applanim.2016.01.002. [DOI] [Google Scholar]
- 52.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 53.Institute of Medicine of the National Academies. [Internet]. 2011. Chimpanzees in biomedical and behavioral research: assessing the necessity. [Cited 1 May 2018]. Available at: http://www.nationalacademies.org/hmd/~/media/Files/Report%20Files/2011/Chimpanzees/chimpanzeereportbrief.pdf [PubMed]
- 54.Jacobson SL, Ross SR, Bloomsmith MA. 2016. Characterizing abnormal behavior in a large population of zoo-housed chimpanzees: prevalence and potential influencing factors. PeerJ 4:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kalcher E, Franzi C, Crailsheim K, Preuschoft S. 2008. Differential onset of infantile deprivation produces distinctive long-term effects in adult ex-laboratory chimpanzees (Pan troglodytes). Dev Psychobiol 50:777–788. 10.1002/dev.20330. [DOI] [PubMed] [Google Scholar]
- 56.Kalcher-Sommersguter E, Franz-Schaider C, Preuschoft S, Crailsheim K. 2013. Long-term evaluation of abnormal behavior in adult ex-laboratory chimpanzees (Pan troglodytes) following resocialization. Behav Sci (Basel) 3:99–119. 10.3390/bs3010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.King NE, Mellen JD. 1994. The effects of early experience on adult copulatory behavior in zoo-born chimpanzees (Pan troglodytes). Zoo Biol 13:51–59. 10.1002/zoo.1430130107. [DOI] [Google Scholar]
- 58.Lambeth SP, Hau J, Perlman JE, Martino M, Schapiro SJ. 2006. Positive reinforcement training affects hematologic and serum chemistry values in captive chimpanzees (Pan troglodytes). Am J Primatol 68:245–256. 10.1002/ajp.20148. [DOI] [PubMed] [Google Scholar]
- 59.Laule GE, Bloomsmith MA, Schapiro SJ. 2003. The use of positive reinforcement training techniques to enhance the care, management, and welfare of primates in the laboratory. J Appl Anim Welf Sci 6:163–173. 10.1207/S15327604JAWS0603_02. [DOI] [PubMed] [Google Scholar]
- 60.Less EH, Kuhar CW, Lukas KE. 2013. Assessing the prevalence and characteristics of hair-plucking behaviour in captive Western lowland gorillas (Gorilla gorilla gorilla). Anim Welf 22:175–183. 10.7120/09627286.22.2.175. [DOI] [Google Scholar]
- 61.Lonsdorf EV. 2005. Sex differences in the development of termite-fishing skills in the wild chimpanzees, Pan troglodytes schweinfurthii, of Gombe National Park, Tanzania. Anim Behav 70:673–683. 10.1016/j.anbehav.2004.12.014. [DOI] [Google Scholar]
- 62.Lonsdorf EV. 2005. What is the role of mothers in the acquisition of termite-fishing behaviors in wild chimpanzees (Pan troglodytes schweinfurthii)? Anim Cogn 9:36–46. 10.1007/s10071-005-0002-7. [DOI] [PubMed] [Google Scholar]
- 63.Lonsdorf EV, Eberly LE, Pusey AE. 2004. Sex differences in learning in chimpanzees. Nature 428:715–716. 10.1038/428715a. [DOI] [PubMed] [Google Scholar]
- 64.Lutz C, Well A, Novak M. 2003. Stereotypic and self-injurious behavior in rhesus macaques: a survey and retrospective analysis of environment and early experience. Am J Primatol 60:1–15. 10.1002/ajp.10075. [DOI] [PubMed] [Google Scholar]
- 65.Maki S, Alford PL, Bloomsmith MA, Franklin J. 1989. Food puzzle device simulating termite-fishing for captive chimpanzees (Pan troglodytes). Am J Primatol 1:71–78. [Google Scholar]
- 66.Maki S, Fritz J, England N. 1993. An assessment of early differential rearing conditions on later behavioral development in captive chimpanzees. Infant Behav Dev 16:373–381. 10.1016/0163-6383(93)80042-7. [DOI] [Google Scholar]
- 67.Maple TL, Perdue BM. 2013. Zoo animal welfare. New York (NY): Springer Verlag; 10.1007/978-3-642-35955-2 [DOI] [Google Scholar]
- 68.Martin JE. 2002. Early life experiences: activity levels and abnormal behaviours in resocialised chimpanzees. Anim Welf 11:419–436. [Google Scholar]
- 69.Mason WA. 1968. Early social deprivation in the nonhuman primates: implications for human behavior. p 70–100. In: Glass DS. Environmental influences. New York (NY): Rockefeller University Press. [Google Scholar]
- 70.Mason GJ, Latham NR. 2004. Can't stop, won't stop: is stereotypy a reliable animal welfare indicator? Anim Welf 13:S57–S69. [Google Scholar]
- 71.McGrew WC. 2013. Is primate tool use special? Chimpanzee and New Caledonian crow compared. Philos Trans R Soc Lond β Biol Sci 368:1–8. 10.1098/rstb.2012.0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morgan L, Howell SM, Fritz J. 1993. Regurgitation and reingestion in a captive chimpanzee (Pan troglodytes). Lab Anim (NY) 22:42–45. [Google Scholar]
- 73.Nagell K, Olguin RS, Tomasello M. 1993. Processes of social learning in the tool use of chimpanzees (Pan troglodytes) and human children (Homo sapiens). J Comp Psychol 107:174–186. 10.1037/0735-7036.107.2.174. [DOI] [PubMed] [Google Scholar]
- 74.Nash LT, Fritz J, Alford PA, Brent L. 1999. Variables influencing the origins of diverse abnormal behaviors in a large sample of captive chimpanzees (Pan troglodytes). Am J Primatol 48:15–29. . [DOI] [PubMed] [Google Scholar]
- 75.National Institutes of Health Working Group Report. [Internet]. 2013. Council of Councils Working Group on the Use of Chimpanzees in NIH-supported Research Report. [Cited 22 January 2013] Available at: https://dpcpsi.nih.gov/sites/default/files/FNL_Report_WG_Chimpanzees_0.pdf
- 76.Novak MA. 2003. Self-injurious behavior in rhesus monkeys: new insights into its etiology, physiology, and treatment. Am J Primatol 59:3–19. 10.1002/ajp.10063. [DOI] [PubMed] [Google Scholar]
- 77.Novak MA, Kelly BJ, Bayne K, Meyer JS. 2012. Behavioral disorders of nonhuman primates, p 177–196. In: Abee CR, Mansfield K, Tardif S, Morris T. Nonhuman primates in biomedical research: biology and management. Oxford (United Kingdom): Academic Press. [Google Scholar]
- 78.O'Neill PL, Novak MA, Suomi SJ. 1991. Normalizing laboratory-reared rhesus macaque (Macaca mulutta) behavior with exposure to complex outdoor enclosures. Zoo Biol 10:237–245. 10.1002/zoo.1430100307. [DOI] [Google Scholar]
- 79.Payne CLR, Webster TH, Hunt KD. 2008. Coprophagy by the semihabituated chimpanzees of Semliki, Uganda. Pan Africa News 15:29–32. 10.5134/143493. [DOI] [Google Scholar]
- 80.Pazol KA, Bloomsmith MA. 1993. The development of stereotyped body rocking in chimpanzees (Pan troglodytes) reared in a variety of nursery settings. Anim Welf 2:113–129. [Google Scholar]
- 81.Perlman JE, Bloomsmith MA, Whittaker MA, McMillan JL, Minier DE, McCowan B. 2012. Implementing positive reinforcement animal training programs at primate laboratories. Appl Anim Behav Sci 137:114–126. 10.1016/j.applanim.2011.11.003. [DOI] [Google Scholar]
- 82.Pomerantz O, Terkel J. 2009. Effects of positive reinforcement training techniques on the psychological welfare of zoo-housed chimpanzees (Pan troglodytes). Am J Primatol 71:687–695. 10.1002/ajp.20703. [DOI] [PubMed] [Google Scholar]
- 83.Pruetz JD, Bertolani P. 2007. Savanna chimpanzees (Pan troglodytes verus) hunt with tools. Curr Biol 17:412–417. 10.1016/j.cub.2006.12.042. [DOI] [PubMed] [Google Scholar]
- 84.Pruetz JDE, McGrew WC. 2001. What does a chimpanzee need? Using behavior to guide the care and management of captive populations, p 17–37. In: Brent L. Special topics in primatology: the care and management of captive chimpanzees, vol 2. San Antonio (TX): American Society of Primatologists. [Google Scholar]
- 85.Reamer LA, Talbot CF, Hopper LM, Mareno MC, Hall K, Brosnan SF, Lambeth SP, Schapiro SJ. [Internet] 2016. The effects of group size on the behavior of captive chimpanzee (Pan troglodytes). Abstract presented at the Joint Meeting of the International Primate Society and American Society of Primatologists, Chicago, Illinois, 21–27 August 2016. Available at: https://www.asp.org/IPS/meetings/2016abstracts.pdf
- 86.Rogers CM, Davenport RK. 1969. Effects of restricted rearing on sexual behavior of chimpanzees. Dev Psychol 1:200–204. 10.1037/h0027319. [DOI] [Google Scholar]
- 87.Ross SR. 2019Chimpanzee welfare in the context of science, policy, and practice. In: Hopper LM, Ross SR. Chimpanzees in context. Chicago (IL): In press. [Google Scholar]
- 88.Ross SR Bloomsmith MA. [Internet]. 2011. A comment on Birkett and Newton-Fisher (2011). [Cited 14 January 2019]. Available at: https://journals.plos.org/plosone/article/comment?id=info%3Adoi%2F10.1371%2Fannotation%2Fa1d8feb2-979b-4563-a321-31a11108a204.
- 89.Ross SR, Lukas KE. 2006. Use of space in a nonnaturalistic environment by chimpanzees (Pan troglodytes) and lowland gorillas (Gorilla gorilla gorilla). Appl Anim Behav Sci 96:143–152. 10.1016/j.applanim.2005.06.005. [DOI] [Google Scholar]
- 90.Sanz CM, Schöning C, Morgan DB. 2010. Chimpanzees prey on army ants with specialized tool set. Am J Primatol 72:17–24. 10.1002/ajp.20744. [DOI] [PubMed] [Google Scholar]
- 91.Schapiro SJ, Perlman JE, Boudreau BA. 2001. Manipulating the affiliative interactions of group-housed rhesus macaques using positive reinforcement training techniques. Am J Primatol 55:137–149. 10.1002/ajp.1047. [DOI] [PubMed] [Google Scholar]
- 92.Snowdon CT, Savage A. 1989. Psychological wellbeing of captive primates: general considerations and examples from callitrichids, p 75–88. In: Segal EF. Housing, care, and psychological wellbeing of captive and laboratory primates. New Jersey (NJ): Noyes Publications. [Google Scholar]
- 93.Spijkerman RP, Dienske H, Vanhooff JARAM, Jens W. 1994. Causes of body rocking in chimpanzees (Pan troglodytes). Anim Welf 3:193–211. [Google Scholar]
- 94.Spijkerman RP, vanHooff JARAM, Dienske H, Jens W. 1997. Differences in subadult behaviors of chimpanzees living in peer groups and in a family group. Int J Primatol 18:439–454. 10.1023/A:1026342602018. [DOI] [Google Scholar]
- 95.Struck K, Videan EN, Fritz J, Murphy J. 2007. Attempting to reduce regurgitation and reingestion in a captive chimpanzee through increased feeding opportunities: a case study. Lab Anim (NY) 36:35–38. 10.1038/laban0107-35. [DOI] [PubMed] [Google Scholar]
- 96.Tomasello M, Davis-Dasilva M, Camak L, Bard K. 1987. Observational learning of tool use by young chimpanzees. Hum Evol 2:175–183. 10.1007/BF02436405. [DOI] [Google Scholar]
- 97.Turner CH, Davenport RK, Rogers CM. 1969. The effect of early deprivation on the social behavior of adolescent chimpanzees. Am J Psychiatry 125:1531–1536. 10.1176/ajp.125.11.1531. [DOI] [PubMed] [Google Scholar]
- 98.Videan EN. 2006. Bed-building in captive chimpanzees (Pan troglodytes): the importance of early rearing. Am J Primatol 68:745–751. 10.1002/ajp.20265. [DOI] [PubMed] [Google Scholar]
- 99.Videan EN, Fritz J, Schwandr ML, Smith HF, Howell S. 2005. Controllability in environmental enrichment for captive chimpanzees (Pan troglodytes). J Appl Anim Welf Sci 8:117–130. 10.1207/s15327604jaws0802_4. [DOI] [PubMed] [Google Scholar]
- 100.Walsh S, Bramblett CA, Alford PL. 1982. A vocabulary of abnormal behaviors in restrictively reared chimpanzees. Am J Primatol 3:315–319. 10.1002/ajp.1350030131. [DOI] [PubMed] [Google Scholar]