To the Editor: Histiocytic sarcoma (HS) is a rare hematopoietic neoplasm consisting of a malignant proliferation of cells that resembles mature tissue histiocytes.1 Prognosis for HS is poor; a recent survey of the Surveillance, Epidemiology, and End Results (SEER) database demonstrated a median overall survival of 6 months among a cohort of 158 patients.2 There is not a universally accepted treatment for HS, though responses have been seen with chemotherapy, surgery, or radiation therapy.3,4 Recent reports have demonstrated a high rate of PD-L1 expression in histiocytic disorders, suggesting a potential therapeutic target.5,6 Here we report a case of HS in a young adult patient who had a favorable response to nivolumab.
A 17-year-old female presented to her primary care provider with intermittent left upper leg pain, fatigue, fevers, unintentional weight loss, and swollen lymph nodes. Initial physical examination was notable for thin body habitus and prominent cervical and supraclavicular adenopathy. She was referred to pediatric hematology-oncology for evaluation. An 18F-fluorodeoxyglucose (FDG) PET-CT revealed multifocal, hypermetabolic axial and appendicular skeletal metastases, extensive hypermetabolic lymphadenopathy, and multiple pulmonary nodules (Supporting Information Figure 1A). No evidence of intracranial disease was noted on brain MRI. Bone marrow biopsy and aspirate were negative.
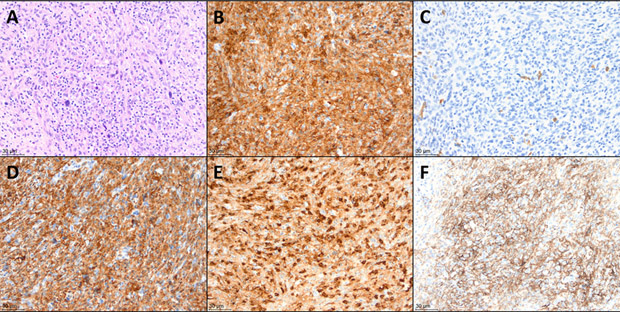
Biopsy of the right iliac mass revealed atypical cell nodules with a histiocytic immunophenotype including CD68+, CD163+, lysozyme+, CD45+ (mod), CD45RO+ (weak), CD31+, Vimentin+, CD33+ (weak), and HLA-DR+ (weak), and CD99 (weak) (Figure 1A-E). Some of the large atypical cells stained weakly for CD30 and PAX5, but not for CD15. CD1a and markers of T-cell (CD3) and B-cell (CD20) lineage were negative. The KI-67 proliferation index in the atypical cells was low (<1% overall). These findings led to a diagnosis of metastatic HS.
FIGURE 1.
Histologic findings in the patient's initial excisional biopsy (A) Hematoxylin and eosin stain. Atypical histiocytic proliferation with scattered large, hyperchromatic cells, along with background small lymphocytes and occasional eosinophils. (B) CD45 immunohistochemical stain, positive in the atypical cells. (C) CD1a immunohistochemical stain, negative in the atypical cells, while positive in rare background dendritic cells. (D) CD163 stain, positive in the atypical cells. (E) Lysozyme stain, variably positive in the atypical cells. (F) PD-L1 stain, positive in approximately 75% of the atypical cells. All panels are 200× magnification.
The patient initially received induction therapy according to Children's Oncology Group protocol AALL0434 with vincristine, daunoru-bicin, PEG-asparaginase, and prednisone.7 Consolidation therapy included cytarabine, mercaptopurine, and cyclophosphamide. A CT scan performed in the setting of persistent fevers revealed interval enlargement of multiple lymph nodes, and a biopsy confirmed disease progression. The patient next received a modified ALCL99 regimen including dexamethasone, vinblastine, ifosfamide, high-dose methotrexate, cytarabine, cyclophosphamide, and etoposide.8 The patient initially had a favorable response to this regimen, however she was found to have progressive disease after eight cycles. She was next treated with cladribine and cytarabine, as described for refractory Langerhans cell histiocytosis9 and in a case report for HS.10 The patient received four cycles of this regimen, but her treatment course was complicated by a prolonged hospitalization for febrile neutropenia and the development of Aspergillus pneumonia.
At the end of this prolonged hospitalization, a PET-CT scan revealed overall improvement in her bone and soft tissue disease. However, significant pulmonary findings were present, which were difficult to characterize in the context of her recent pneumonia (Supporting Information Figure 1B). Given the state of her marked thrombocytopenia and lymphopenia, it was felt that she would not tolerate further myelosuppressive chemotherapy. Prior reports suggested that high levels of PD-L1 expression could be detected in histiocytic neoplasms,5,6 so PD-L1 expression was checked on the primary biopsy sample. Indeed, PD-L1 was found to be expressed in 75% of the tumor cells (Figure 1F). The patient was therefore started on a trial of nivolumab 3 mg/kg every 2 weeks.
After 2 months of nivolumab treatment, a PET-CT scan showed worsening metastatic disease in the left femoral neck and bilateral lungs (Supporting Information Figure 1C). Nivolumab treatment was continued despite apparent progression, since her persistent lymphopenia offered an explanation for her lackluster response.11 Although pseudoprogression may have contributed to the increased size of some preexisting sites of metastatic disease, it was thought to more likely represent true progression based on the presence of new disease and her overall immunosuppressed state. Repeat imaging after 2 months (in the setting of a rising lymphocyte count) revealed significant interval decrease in the size of numerous pulmonary nodules and the lesion in her femoral neck. After 5 months of treatment on nivolumab, she was transitioned to the adult dosing of 480 mg every 4 weeks. Disease evaluation 4 months after her prior imaging revealed no new metastatic disease, absence of previously described pulmonary nodules, and the FDG-avid disease in the left proximal femur showed continued improvement (Supporting Information Figure 1D).
In summary, HS is a rare hematopoietic neoplasm associated with a poor prognosis. Novel therapeutic targets are required to improve patient survival. Genomic profiling of HS has revealed the mitogen-activated protein kinase (MAPK) pathway as one potential target.12,13 There is also a report of a 66-year-old male with refractory HS who was treated with nivolumab after noting PD-L1 expression in 15-20% of the tumor.14 Although this patient did not have a favorable response to nivolumab, we recommend testing for PD-L1 expression in HS based on the treatment response in our young adult patient. If PD-L1 expression is elevated, then immune checkpoint inhibition should be considered as a therapeutic option.
Supplementary Material
Footnotes
CONFLICTS OF INTEREST
None of the authors has any direct or indirect commercial financial incentive associated with publishing this article.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Takahashi E, Nakamura S. Histiocytic sarcoma: an updated literature review based on the 2008 WHO classification. J Clin Exp Hematop. 2013;53:1–8. [DOI] [PubMed] [Google Scholar]
- 2.Kommalapati A, Tella SH, Durkin M, Go RS, Goyal G. Histiocytic sarcoma: a population-based analysis of incidence, demographic disparities, and long-term outcomes. Blood. 2018;131:265–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kommalapati A, Tella SH, Go RS, Goyal G. Predictors of survival, treatment patterns, and outcomes in histiocytic sarcoma. Leuk Lymphoma. 2018:1–3. [DOI] [PubMed] [Google Scholar]
- 4.Shukla N, Kobos R, Renaud T, et al. Successful treatment of refractory metastatic histiocytic sarcoma with alemtuzumab. Cancer. 2012;118:3719–3724. [DOI] [PubMed] [Google Scholar]
- 5.Gatalica Z, Bilalovic N, Palazzo JP, et al. Disseminated histiocytoses biomarkers beyond BRAFV600E: frequent expression of PD-L1. Oncotarget. 2015;6:19819–19825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu J, Sun HH, Fletcher CD, et al. Expression of programmed cell death 1 ligands (PD-L1 and PD-L2) in histiocytic and dendritic cell disorders. Am J Surg Pathol. 2016;40:443–453. [DOI] [PubMed] [Google Scholar]
- 7.Heath JL, Burgett SE, Gaca AM, Jaffe R, Wechsler DS. Successful treatment of pediatric histiocytic sarcoma using abbreviated high-risk leukemia chemotherapy. Pediatr Blood Cancer. 2014;61:1874–1876. [DOI] [PubMed] [Google Scholar]
- 8.Le Deley MC, Rosolen A, Williams DM, et al. Vinblastine in children and adolescents with high-risk anaplastic large-cell lymphoma: results of the randomized ALCL99-vinblastine trial. J Clin Oncol. 2010;28:3987–3993. [DOI] [PubMed] [Google Scholar]
- 9.Donadieu J Bernard F, van Noesel M, et al. Cladribine and cytarabine in refractory multisystem langerhans cell histiocytosis: results of an international phase 2 study. Blood. 2015;126:1415–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwabuchi H, Kawashima H, Umezu H, et al. Successful treatment of histiocytic sarcoma with cladribine and high-dose cytosine arabinoside in a child. Int J Hematol. 2017;106:299–303. [DOI] [PubMed] [Google Scholar]
- 11.Diehl A, Yarchoan M, Hopkins A, Jaffee E, Grossman SA. Relationships between lymphocyte counts and treatment-related toxicities and clinical responses in patients with solid tumors treated with PD-1 checkpoint inhibitors. Oncotarget. 2017;8:114268–114280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gounder MM, Solit DB, Tap WD. Trametinib in histiocytic sarcoma with an activating MAP2K1 (MEK1) mutation. N Engl J Med. 2018;378:1945–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voruz S, Cairoli A, Naveiras O, et al. Response to MEK inhibition with trametinib and tyrosine kinase inhibition with imatinib in multifocal histiocytic sarcoma. Haematologica. 2018;103:e39–e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voruz S, Martins F, Cairoli A, et al. Comment on "MEK inhibition with trametinib and tyrosine kinase inhibition with imatinib in multifocal histiocytic sarcoma." Haematologica. 2018; 103:e130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.