Abstract
Killer cell immunoglobulin-like receptor (KIR) genes encode cell surface molecules that recognize human leukocyte antigen (HLA) molecules and modulate the activity of natural killer (NK) cells. KIR genes exhibit presence and absence polymorphism, which generates a variety of gene-content haplotypes in worldwide populations. KIR gene-content variation is implicated in many diseases and is also important for placentation and transplantation. Due the complexity of KIR polymorphism, variation in this family is still mostly studied at the gene-content level, even with the advent of next generation sequencing methods. Gene-content determination is generally expensive and/or time-consuming. To overcome these difficulties, we developed a method based on multiplex polymerase chain reaction with specific sequence primers (PCR-SSP) followed by melting curve analysis that allows cost-effective, precise and fast generation of results. Our method was 100% concordant with a gel-based method and 99.9% concordant with presence and absence determination by next generation sequencing. The limit of detection for accurate typing was 30 ng of DNA (0.42 μM) with 260/230 and 260/280 ratios as low as 0.19 and of 0.44. In addition, we developed a user-friendly Java-based computational application called killerPeak that interprets the raw data generated by Viia7 or QuantStudio 7 quantitative PCR machines and reliably exports the final genotyping results in spreadsheet file format. The combination of a reliable method that requires low amount of DNA with an automated interpretation of results allows scaling the KIR genotyping in large cohorts with reduced turnaround time.
Keywords: killer-cell immunoglobulin-like receptors, genotyping, PCR-SSP, qPCR, real-time
Introduction
Killer cell immunoglobulin-like receptors (KIR) are transmembrane molecules that modulate the activity of the natural killer cells (NK) by recognizing human leukocyte antigen (HLA) class I molecules as ligands. KIR genes are located on chromosome 19, in the leukocyte receptor complex (LRC)1. Most KIR genes (KIR3DL3, KIR2DS2, KIR2DS5, KIR2DL2/3, KIR2DL5, KIR2DS3, KIR2DL1, KIR2DL4, KIR3DL1/S1, KIR2DS1, KIR2DS4 and KIR3DL2) and pseudogenes (KIR2DP1 and KIR3DP1) exhibit a polymorphism that is not common in the human genome, whereby the composite genes are variably present or absent. This variation generates a vast number of KIR gene content haplotypes across individuals and populations. Additionally, each of the genes has multiple alleles, which results in extreme diversity across human populations2.
Due to their importance for investigations of the immune response, population genetics, transplantation outcome and reproduction3, KIR genotyping is carried out in multiple laboratories from all continents. Genotyping for gene-content is commonly performed by sequence-specific primer polymerase chain reaction (PCR-SSP), followed by gel electrophoresis. This approach was first described by Uhrberg in 19974 and it requires high quality DNA due to the length of the amplicons produced (~1.5 kb). To overcome this limitation, amplification of smaller fragments has been proposed by different authors5–7. Vilches and collaborators in 20076 described a faster method based on smaller amplicons. However, this method had as limitation only one pair of primers for each gene. Considering the high sequence similarity among KIR genes and the extensive allelic polymorphism in each gene, applying a single pair of PCR-SSP primers genotyping KIR increases the chance of obtaining both false negative and false positive results. Kulkarni and collaborators described a multiplex PCR-SSP method with two pairs of primers per gene in 20108 that was faster than most of the previous methods, but required a large amount of DNA in addition to gel analysis and manual inspection.
Alternatively, KIR genes have also been extensively genotyped by polymerase chain reaction-sequence-specific oligonucleotide probe (PCR-SSOP). This method consists of a PCR amplification followed by fixation of the PCR product (usually on nylon membrane) and subsequently hybridizing with a panel of specific biotinylated probes9. Despite the sensitivity of PCR-SSOP, labeled probes are relatively expensive when comparing to PCR-SSP primers. Additionally, this method is time consuming due to extensive post PCR processing.
A KIR genotyping method based on melting curve analysis was first described in 200910. Applying melting curve analysis is advantageous due to the lack of post PCR processing and overall reduced genotyping time. Despite being faster and more precise than gel electrophoresis, this method was based on the same PCR-SSP primers described by Vilches, et al.6, and therefore, had the same limitations in terms of accuracy. In 2016, a high-throughput qPCR method (qKAT) was described for copy number variation and haplotype determination11. Despite offering more information than simply presence and absence, this method relies on the use of dual-labeled probes, which are relatively expensive in comparison to the melting curve approach.
In summary, many different primers and probes have been described for KIR gene-content genotyping. They differ mostly by the strategy (PCR-SSP or PCR-SSOP), length of amplicons and the use of one or two pairs of primers for detecting each gene. What each method has in common are barriers to high-throughput application, with large amounts of data that requires extensive manual inspection and per-individual analysis. To overcome the major limitation of managing large amounts of data, commercial kits have been made available. As example, there is the One Lambda SSO kit (One Lambda, Inc), which requires Luminex® technologies. The major limitation of commercial kits, along with the relatively higher prices, is the lack of flexibility or transparency regarding primer and probe sequences, which limits the use for research and makes difficult to know their empirical limitations.
As a solution to these concerns, we present here a precise, fast and cost-effective method for KIR gene-content genotyping. We selected a set of primers that could provide the most reliable genotyping by extensively analyzing all alleles described in the IPD (immuno polymorphism database) KIR database12,13. We optimized and validated a KIR genotyping method based on conventional multiplex PCR-SSP amplification followed by melting curve analysis in a quantitative PCR machine (real-time PCR or qPCR). The multiplex PCR reduces cost due to a lower number of amplification reactions, while melting curve analysis eliminates post-PCR processing steps such as electrophoresis or specific probe hybridization, reducing considerably the hands-on time. In addition, we developed a Java-based application called killerPEAK, which, as an automated process, reads the raw data from the qPCR instrument, analyzes the dissociation curve peaks based on pre-established parameters and exports the analyzed final genotyping results. The software checks quality by reproducibility of the results among different primers pairs for the same gene, as well as the known linkage disequilibrium patterns within the KIR family. The automatic analysis reduces the chance of errors and allows large scale genotyping.
Material and Methods
PCR-SSP primers
We utilized previously described primers6,8,14 with the inclusion of some novel primers (Supplementary Table 1) and we mapped them to the most updated KIR gene alignments in the Immuno Polymorphism Database (IPD) database (Release 2.7.1, 16 February 2018)12,13. At least two pairs of primers were used to assess presence or absence polymorphism for each KIR gene, except for KIR2DS1, KIR3DL3 and KIR3DP1, for which a single primer set was used each. We created a new set of primers to detect the presence of the 22-base-pair deletion present in some KIR2DS4 alleles. The gene galactosylceramidase (GALC), located in chromosome 14q31.3, was used as an internal amplification control (IC). We assured that all amplicons were suitable for melting curve analysis by checking their sizes, GC content and the melting temperature (Tm) of each amplicon separately. After, we exhaustively combined and tested different amplicons with different Tm for multiplex reactions.
Assay design
The method was designed to evaluate 12 KIR genes, 2 pseudogenes and the presence of the KIR2DS4 allelic group typified by the 22bp deletion. Based on the dissociation temperatures of each amplicon, we assembled a standard layout for 384-well plates (see supplementary material), in which each PCR mix has predetermined and fixed position in each plate column. Each plate allows the analysis of 16 individuals simultaneously. Mix composition and melting temperatures are described in Supplementary Table 2. The method can alternatively be adapted to 96-well qPCR systems (see section III in Supplementary Material).
As mentioned, genotyping is carried out with the amplification of at least two pairs of primers for each gene, with the exception of KIR3DL3 and KIR2DS1 genes, and the pseudogene KIR3DP1. When possible, PCR mixes were designed to amplify two different KIR genes, one being of high frequency to serve as an internal amplification control. Mixes that do not have two different KIR genes amplified simultaneously include the GALC gene as internal amplification control. KIR2DS4 genotyping is performed with three amplifications: a pair of primers that detected all alleles (mix 21); one pair of primers that detected only the 22bp-deleted allelic group (mix 24); and finally, a pair of primers that detected the full-length alleles (mix 5). The detailed protocol is given in Supplementary Material.
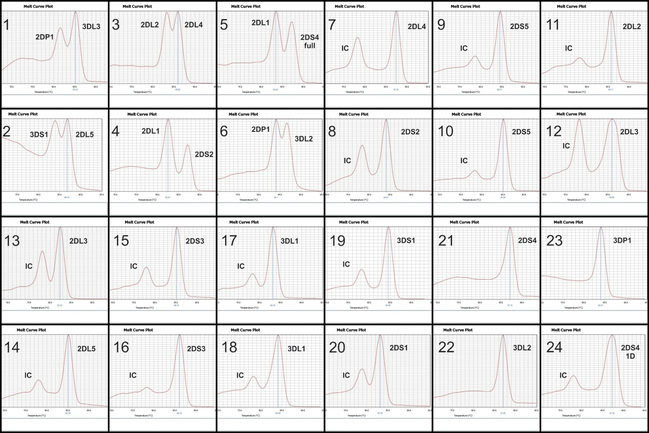
For a total volume of 6 μL, each reaction contained 3.0 μL of 2X GoTaq® RealTime qPCR master mix (Promega), 5 to 20 ng of genomic DNA, 0.25 μM KIR primers and, for some of the reactions, 0.125 μM of GALC primers. For high-throughput, we used a protocol with final volume of 3 μL per reaction (more details in Supplementary Material). The amplification conditions are: 2 min at 94°C; 5 cycles at 94°C for 5 sec, 65°C for 15 sec and 72°C for 30 sec; 21 cycles at 94°C for 5 sec, 60°C for 15 sec and 72°C for 30 sec; 4 cycles at 94°C for 5 sec, 55°C for 15 sec and 72°C for 30 sec; 2 min at 72°C. Amplification can be performed in any regular thermal cycler and the melting curve analysis can be performed in any qPCR system. For automated analysis, the melting curve step has to be performed in a Viia7 Real-Time PCR system or QuantStudio 7 (Thermo Fisher Scientific). The melting curve patterns for the 24 mixes are in Figure 1.
Figure 1. Melting-curve patterns for the 24 amplification reactions.
Numbers 1 to 24 represent the 24 amplification mixes. IC = internal control (GALC gene); 2DS4 1D = 22bp-deleted allelic group; 2DS4 full = alleles without the 22bp deletion
killerPeak - software development for automated analysis
The killerPeak software was written in Java and is freely available at http://lgmh.c3sl.ufpr.br/tools4geniuses. It uses raw .xls file directly exported from QuantStudio software (Thermofisher), automatically interprets the individual KIR presence and absence genotypes from raw data and exports genotypes to spreadsheet file (.xls) format. The software performs quality control by comparing the melting curve peaks from the amplification of different primers for the same KIR gene and checking the internal amplification control. Genes that are amplified with two different primer pairs and exhibit conflicting results between them, or reactions in which neither the internal control nor the KIR gene were amplified fail this first quality control step. The software also examines the results against the known patterns of linkage disequilibrium of some KIR genes. The pairs checked are KIR2DL1 and KIR2DP1; KIR2DL2 and KIR2DL3; KIR3DS1 and KIR3DL1. The software also checks the presence of framework genes, which are expected to be present in all individuals. All situations that do not pass quality control generate a warning message that requires manual inspection before exporting the data.
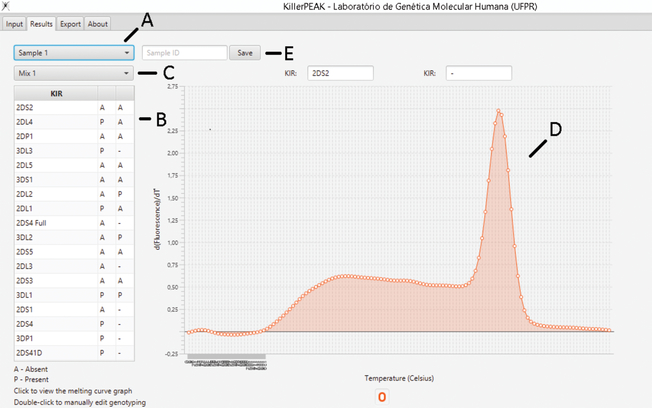
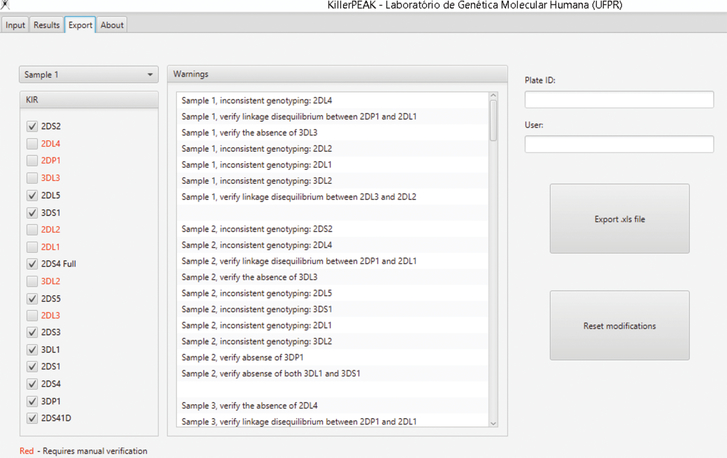
After importing the .xls file generated by the qPCR software, the user can visualize the peaks and the interpreted genotypes for each individual (Figure 2). Before exporting the data, the user can visualize warnings generated by the software in case of inconsistent result (Figure 3) and is able to confirm manually, if needed, before exporting the final result.
Figure 2. KillerPeak results tab.
(A) User can check the results for the 24 amplification reactions for each of the 16 samples from the plate. (B) “P” means presence and “A” means absence. (C) User can choose each one of the 24 mixes to visualize the melting curve for the specific reaction (D). The user may also name the sample (E), if the sample ID file was not pre-imported.
Figure 3. KillerPeak export tab.
User can check for any inconsistencies, e.g. a gene was amplified by one pair of primers and not by the second pair. Genes marked in red mean that genotyping did not pass quality control. Included in the quality control check is the verification of the known KIR linkage disequilibrium patterns. In case of warnings, the user must confirm manually any results before exporting.
The data generated can also be analyzed manually through any software from any qPCR machine. A guide of how to interpret the pattern of each mix is in section VI of the Supplementary Material.
Validation samples
For validation, we used two subsets of DNA samples: 48 from admixed individuals of predominantly European ancestry from Parana State, Brazil; and 48 DNA samples from individuals from the United States with European ancestry collected as healthy controls at the University of California, San Francisco. Samples from Brazil were previously genotyped by PCR-SSP followed by agarose gel electrophoresis analysis15. Samples from the USA had all KIR loci fully sequenced by a previously described next generation sequencing method (NGS)16. After sequencing, presence and absence was determined using the custom PING (pushing immunogenetics to next generation) software pipeline16.
Data analysis
Carrier frequencies for the observed KIR genes were determined by direct counting. Results from the melting curve KIR multiplex assay was directly compared to those from the gel-based and NGS genotyping assays of the same samples using Fisher’s exact test.
Results
Primers specificity analysis
To check whether the primers used missed one or more alleles, KIR alleles from the most recent version of IPD were aligned using BioEdit Sequence Alignment Editor17. Our results showed that the set of primers used in this method amplifies all KIR specifically. Additionally, the primers do not miss any frequent allele of each gene, according to IPD KIR version 2.7.1, 2018 (Supplementary Table 1). The exceptions are the allele KIR3DL2*018, which was missed by both pairs of KIR3DL2 specific primers, and alleles KIR3DL3*00601, KIR3DL3*00602, KIR3DL3*01501, KIR3DL3*01601, KIR 3DL3*02102, missed by the KIR3DL3 specific primers. All other KIR primers complemented each other to avoid false negative results.
Validation of the KIR multiplex melting curve assay
Our method was 100% concordant with the gel-based method and 99.9% concordant with NGS (Table 1). For NGS, the presence of KIR2DL3 was the only one that was not 100% concordant (47/48).
Table 1 –
Validation of KIR multiplex PCR-SSP melting curve assay
| KIR genes | Gel-based PCR-SSP | Melting curve assay | Concordant (%) | Next generation sequencing (NGS) | Melting curve assay | Concordant (%) |
|---|---|---|---|---|---|---|
| 3DL3 | 48/0 | 48/0 | 100 | 48/0 | 48/0 | 100 |
| 2DP1 | 47/1 | 47/1 | 100 | 45/3 | 45/3 | 100 |
| 3DS1 | 25/23 | 25/23 | 100 | 24/24 | 24/24 | 100 |
| 2DL5 | 26/22 | 26/22 | 100 | 29/19 | 29/19 | 100 |
| 2DL2 | 26/22 | 26/22 | 100 | 22/26 | 22/26 | 100 |
| 2DL4 | 48/0 | 48/0 | 100 | 48/0 | 48/0 | 100 |
| 2DL1 | 45/3 | 45/3 | 100 | 45/3 | 45/3 | 100 |
| 2DS2 | 26/22 | 26/22 | 100 | 23/25 | 23/25 | 100 |
| 3DL2 | 48/0 | 48/0 | 100 | 48/0 | 48/0 | 100 |
| 2DS5 | 25/23 | 25/23 | 100 | 23/25 | 23/25 | 100 |
| 2DL3 | 42/6 | 42/6 | 100 | 40/8 | 41/7 | 97.9 |
| 2DS3 | 8/40 | 8/40 | 100 | 17/31 | 17/31 | 100 |
| 3DL1 | 47/1 | 47/1 | 100 | 42/6 | 42/6 | 100 |
| 2DS1 | 28/20 | 28/20 | 100 | 24/23 | 24/23 | 100 |
| 2DS4 | 46/2 | 46/2 | 100 | 42/6 | 42/6 | 100 |
| 2DS4 1D | 36/12 | 36/12 | 100 | 33/15 | 33/15 | 100 |
| 2DS4 full | 20/28 | 20/28 | 100 | 18/30 | 18/30 | 100 |
| 3DP1 | 48/0 | 48/0 | 100 | 48/0 | 48/0 | 100 |
Concordance between assays was established by directly comparing the number of individuals who did/did not carry the KIR gene divided by the KIR genes present/absent x 100. 2DS4 1D = 22bp-deleted allelic group; 2DS4 full = alleles without the 22bp deletion
Limit of detection and precision
We aimed to test the lowest DNA quantity and quality required for genotyping. DNA concentration was measured by Quantus fluorometer (Promega). For each 3 μL reaction, we tested 0.5 μL of DNA samples at 20 μM, 10 μM, 5 μM 2.5 μM and 1 μM. Reactions with final amount as low as 1.25 ng of DNA (0.42 μM) were amplified and genotyped successfully with equivalent results to those obtained with the standard protocol (5 to 20 ng of DNA; 0.83 to 3.33 μM). Reactions with 0.5 ng of DNA (0.167 μM) did not generate consistent results.
To test the limit of detection for DNA quality, we used samples with 260/230 ratio and 260/280 ratio lower than 1, determined by NanoDrop (ThermoFisher). Our method was able to generate reliable results even for samples with 260/230 as low as 0.19, and 260/280 as low as 0.44. Lastly, we used samples that combined low concentration (<10 ng/μL) and low quality (260/230 and 260/280 < 1) of DNA, and observed that our method genotyped those samples successfully. The limit of detection for accurate typing established was 1.25ng of DNA in a 3μl reaction (0.42 μM), with 260/230 and 260/280 ratios 0.19 and of 0.44, respectively. In addition, we genotyped eight random samples five times, in five different runs, to test precision (total of 960 amplification reactions). All the results were repeated every single time.
Discussion
We developed a fast and effective method to determine KIR presence and absence that uses melting curve analysis and Bryt® Green chemistry (Promega, Madison, Wisconsin, United States). This approach is based on melting curve analysis with an intercalant fluorophore having similar properties to Sybr Green®. Characteristics that influence melting temperature are size of the amplicon and GC content. Each amplicon will generate, at the end of the process, a fluorescence peak at its mean dissociation temperature. If the temperature difference of two amplicons is at least approximately 3°C, it was possible to perform a multiplex reaction in which two different amplicons were analyzed simultaneously.
The rationale for choosing qPCR mastermix with Bryt® Green and GoTaq hot start polymerase was that this reagent provided accurate results with the lowest price, in comparison to other qPCR mastermixes. In terms of reagents, the total cost for genotyping one sample for all KIR loci is approximately US$ 1.65 (from US$ 1.50 to 1.80, depending on the kit size, with the current price in the USA). As a comparison, the cost of a commercial KIR SSO Genotyping Test is roughly 15 times higher, at approximately US$ 25 per sample.
The homology of KIR2DS1 with other KIR and the particular sequence similarity with KIR2DL1 make it difficult to design a second set of specific primers. However, the single set of primers that is used for KIR2DS1 is highly specific and amplifies all reported alleles. Absence of KIR3DL3 or KIR3DP1 genes is not frequent and has been observed in only a few individuals from all the worldwide populations listed at Allele Frequency Database18. Therefore, we used one pair of primers for these framework loci.
Reactions 1 to 6 use highly frequent KIR as internal amplification controls, and the remaining mixes include the amplification of GALC, which has a distinctly low-temperature peak. This enables discrimination between the absence of a KIR gene and PCR failure, in combination with the redundancy of the amplifications of the same gene with different primer pairs.
We also developed a Java language computational program for automated analysis. The application uses the values from the output file form the QuantStudio software and recreates the peak of the dissociation temperature (Figure 2). Automatically, the application analyzes whether the gene is present or absent, based on the temperatures of each amplification peak (Supplementary table 2). If there is a divergence between the results of the two set of primers of each gene, the program shows a warning message and prevents to export the result without manual confirmation. Further, the program analyzes the pattern of known linkage disequilibrium among KIR genes and shows warning messages when there is an unusual pattern (indicating a high chance of genotyping error). In all these cases, the user manually checks the warning and identifies the samples that may need to be re-amplified. Finally, the program allows presence or absence genotyping data to be exported in .xls format, which facilitates subsequent analyses, such as calculations of gene and genotype frequencies. The limitation of the software, at this moment, is its capability to analyze only data files exported from QuantStudio (ThermoFisher qPCR instruments). However, new versions may be released in the future, allowing use with different real time machines, such as Eppendorf, Qiagen and BioRad. It is also important to note that the software is not required as this method also enables easy manual analysis of the peaks.
Our method was validated against a gel-based as well as an NGS method that targets and sequences all KIR genes and allows copy number quantification. Impressively, the concordance was 100% for all loci, except KIR2DL3, which presented 98% (47/48) of concordance with NGS. The high concordance with other methods combined with the 100% repeatability (960/960) indicated that our method is consistent and precise.
The melting-curve assay has the advantage of accurately amplifying and genotyping low quantity and quality DNA samples. Only DNA samples with concentration lower than 2.5 μM of DNA were not successfully genotyped. This makes our method suitable even for samples for which DNA is limited.
A limitation of this method and of all other methods based on primers or probes is that they target known KIR alleles. Some populations may potentially have a frequent unknown allele that may not be precisely detected. Another limitation is that none of our primers are able to amplify the alleles KIR3DL2*018, KIR3DL3*00601, KIR3DL3*00602, KIR3DL3*01501, KIR3DL3*01601, KIR3DL3*02102, which would result in a false negative result if two of these alleles were present in each locus. However, these alleles are not frequent in any described populations18, and the chance of homozygosity for these alleles would be remote based on the frequency at which they have been observed. Therefore, this limitation will likely not impact the KIR gene-content genotyping in most populations.
The method we describe here has already been applied for genotyping of a Vietnamese population sample with 140 individuals19. Samples were successfully amplified and genotyped for all KIR loci and provided comparable results with a previous study20, which demonstrates the applicability of our method for genotyping cohorts.
Conventional KIR genotyping techniques usually involve gel-based PCR-SSP and SSOP methods, which are expensive and/or time consuming. We described here an optimized and validated melting-curve based method that shows high concordance with current utilized methods. Additionally, melting curve is more sensitive than gel analysis and provides more precise genotyping even for low concentration or low-quality DNA. In approximately 150 minutes, it is possible to have final analyzed results for 16 samples (60 minutes of hands-on, 60 minutes of amplification, 15 minutes for melting curve, 15 minutes of software analysis). If the assay is designed for large scale, it is possible to prepare the plates with primers in advance and store them in −20 to −80 °C. This way, for each set of 16 samples, only 100 minutes will be needed (only 10 to 15 minutes of hands-on). As the amplification can be performed in a regular thermocycler (only the final step requires a qPCR instrument), it is possible to easily genotype hundreds of samples per day in laboratories with multiple instruments.
Conclusion
The developed melting curve multiplex assay offers a fast, reliable and cost-efficient method for determining KIR presence and absence, which allows fast large scale KIR gene-content genotyping. KIR diversity has been associated with many diseases21–23, pregnancy24, transplantation outcome and is also used for anthropological studies15,25,26. Considering its importance, an affordable and fast method combined with automated analysis enhances the accuracy and contributes to the immunogenetics field.
Supplementary Material
Acknowledgments
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil (CAPES), Finance Code 001, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and by Fundação Araucária. PJN and JAH are supported by U19 NS095774 and R01 AI128775. We thank Professor Jorge Oksenberg, University of California, San Francisco, for providing DNA samples.
References
- 1.Middleton D, Curran M, Maxwell L. Natural killer cells and their receptors. Transpl Immunol. 2002;10(2–3):147–164. http://www.ncbi.nlm.nih.gov/pubmed/12216946. Accessed November 3, 2017. [DOI] [PubMed] [Google Scholar]
- 2.Guethlein LA, Norman PJ, Hilton HG, Parham P. Co-evolution of MHC class I and variable NK cell receptors in placental mammals. Immunol Rev. 2015;267(1):259–282. doi: 10.1111/imr.12326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Augusto DG, Petzl-Erler ML. KIR and HLA under pressure: evidences of coevolution across worldwide populations. Hum Genet. 2015;134(9):929–940. doi: 10.1007/s00439-015-1579-9 [DOI] [PubMed] [Google Scholar]
- 4.Uhrberg M, Valiante NM, Shum BP, et al. Human Diversity in Killer Cell Inhibitory Receptor Genes. Immunity. 1997;7(6):753–763. doi: 10.1016/S1074-7613(00)80394-5 [DOI] [PubMed] [Google Scholar]
- 5.Martin MP, Nelson G, Lee J-H, et al. Cutting edge: susceptibility to psoriatic arthritis: influence of activating killer Ig-like receptor genes in the absence of specific HLA-C alleles. J Immunol. 2002;169(6):2818–2822. http://www.ncbi.nlm.nih.gov/pubmed/12218090. Accessed January 20, 2018. [DOI] [PubMed] [Google Scholar]
- 6.Vilches C, Castaño J, Gómez-Lozano N, Estefanía E. Facilitation of KIR genotyping by a PCR-SSP method that amplifies short DNA fragments. Tissue Antigens. 2007;70(5):415–422. doi: 10.1111/j.1399-0039.2007.00923.x [DOI] [PubMed] [Google Scholar]
- 7.Martin MP, Carrington M. KIR Genotyping and Analysis In: Innate Immunity. Vol 415 Totowa, NJ: Humana Press; 2008:49–64. doi: 10.1007/978-1-59745-570-1_3 [DOI] [Google Scholar]
- 8.Kulkarni S, Martin MP, Carrington M. KIR genotyping by multiplex PCR-SSP. Methods Mol Biol. 2010;612:365–375. doi: 10.1007/978-1-60761-362-6_25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crum KA, Logue SE, Curran MD, Middleton D. Development of a PCR-SSOP approach capable of defining the natural killer cell inhibitory receptor (KIR) gene sequence repertoires. Tissue Antigens. 2000;56(4):313–326. http://www.ncbi.nlm.nih.gov/pubmed/11098931. Accessed September 26, 2016. [DOI] [PubMed] [Google Scholar]
- 10.Alves LGT, Rajalingam R, Canavez F. A novel real-time PCR method for KIR genotyping. Tissue Antigens. 2009;73(2):188–191. doi: 10.1111/j.1399-0039.2008.01184.x [DOI] [PubMed] [Google Scholar]
- 11.Jiang W, Johnson C, Simecek N, et al. qKAT: a high-throughput qPCR method for KIR gene copy number and haplotype determination. Genome Med. 2016;8(1):99. doi: 10.1186/s13073-016-0358-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson J, Waller MJ, Stoehr P, Marsh SGE. IPD--the Immuno Polymorphism Database. Nucleic Acids Res. 2004;33(Database issue):D523–D526. doi: 10.1093/nar/gki032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson J, Halliwell JA, McWilliam H, Lopez R, Marsh SGE. IPD—the Immuno Polymorphism Database. Nucleic Acids Res. 2012;41(D1):D1234–D1240. doi: 10.1093/nar/gks1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Augusto DG, Piovezan BZ, Tsuneto LT, Callegari-Jacques SM, Petzl-Erler ML. KIR Gene Content in Amerindians Indicates Influence of Demographic Factors. Barbour JD, ed. PLoS One 2013;8(2):e56755. doi: 10.1371/journal.pone.0056755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Augusto DG, Zehnder-Alves L, Pincerati MR, Martin MP, Carrington M, Petzl-Erler ML. Diversity of the KIR gene cluster in an urban Brazilian population. Immunogenetics. 2012;64(2):143–152. doi: 10.1007/s00251-011-0565-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norman PJ, Hollenbach JA, Nemat-Gorgani N, et al. Defining KIR and HLA Class I Genotypes at Highest Resolution via High-Throughput Sequencing. Am J Hum Genet. 2016;99(2):375–391. doi: 10.1016/j.ajhg.2016.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41. [Google Scholar]
- 18.Gonzalez-Galarza FF, Takeshita LYC, Santos EJM, et al. Allele frequency net 2015 update: new features for HLA epitopes, KIR and disease and HLA adverse drug reaction associations. Nucleic Acids Res. 2015;43(D1):D784–D788. doi: 10.1093/nar/gku1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amorim LM, van Tong H, Hoan NX, et al. KIR-HLA distribution in a Vietnamese population from Hanoi. Hum Immunol. November 2017. doi: 10.1016/j.humimm.2017.11.011 [DOI] [PubMed] [Google Scholar]
- 20.Toneva M, Lepage V, Lafay G, et al. Genomic diversity of natural killer cell receptor genes in three populations. Tissue Antigens. 2001;57(4):358–362. http://www.ncbi.nlm.nih.gov/pubmed/11380947. Accessed July 6, 2017. [DOI] [PubMed] [Google Scholar]
- 21.Augusto DG, Lobo-Alves SC, Melo MF, Pereira NF, Petzl-Erler ML. Activating KIR and HLA Bw4 Ligands Are Associated to Decreased Susceptibility to Pemphigus Foliaceus, an Autoimmune Blistering Skin Disease. Zimmer J, ed. PLoS One. 2012;7(7):e39991. doi: 10.1371/journal.pone.0039991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jobim MR, Jobim M, Salim PH, et al. Analysis of KIR gene frequencies and HLA class I genotypes in breast cancer and control group. Hum Immunol. 2013;74(9):1130–1133. doi: 10.1016/j.humimm.2013.06.021 [DOI] [PubMed] [Google Scholar]
- 23.Augusto DG, O’Connor GM, Lobo-Alves SC, et al. Pemphigus is associated with KIR3DL2 expression levels and provides evidence that KIR3DL2 may bind HLA-A3 and A11 in vivo. Eur J Immunol. 2015;45(7):2052–2060. doi: 10.1002/eji.201445324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trowsdale J, Moffett A. NK receptor interactions with MHC class I molecules in pregnancy. Semin Immunol. 2008;20(6):317–320. doi: 10.1016/j.smim.2008.06.002 [DOI] [PubMed] [Google Scholar]
- 25.Augusto DG, Amorim LM, Farias TDJ, Petzl-Erler ML. KIR and HLA Genotyping of Japanese Descendants from Curitiba, a City of Predominantly European Ancestry from Southern Brazil. Vol 77; 2016. doi: 10.1016/j.humimm.2016.01.011 [DOI] [PubMed] [Google Scholar]
- 26.Chaisri S, Kitcharoen K, Romphruk AV., Romphruk A, Witt CS, Leelayuwat C Polymorphisms of killer immunoglobulin-like receptors (KIRs) and HLA ligands in northeastern Thais. Immunogenetics. 2013;65(9):645–653. doi: 10.1007/s00251-013-0716-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.