Abstract
Purpose of review
Direct-acting antiviral agents (DAAs) eradicate hepatitis C virus (HCV) infection in the majority of patients. We critically evaluated the impact of DAAs on hepatocellular carcinoma (HCC) risk, a major complication of HCV infection.
Recent findings
Large cohort studies show that patients who achieve sustained virologic response (SVR) with DAAs have a significantly lower risk of developing de novo HCC than patients who fail treatment or remain untreated. Furthermore, reduction in HCC risk is similar whether SVR is achieved with DAAs or interferon (IFN). However, DAA-induced SVR does not eliminate HCC risk entirely. Therefore, patients with pre-existing cirrhosis require ongoing surveillance even after SVR is achieved.
Early, descriptive, uncontrolled reports suggested that DAAs may increase the risk of recurrent HCC. While studying HCC recurrence presents major methodologic challenges, larger studies containing appropriate comparison control groups largely refuted these concerns.
Summary
Recent studies provide evidence that DAA-induced SVR reduces HCC risk.
Keywords: Direct-acting antiviral, hepatitis C, hepatocellular carcinoma, sustained virologic response
Introduction
Direct-acting antivirals (DAA) are highly effective for the treatment of chronic hepatitis C virus (HCV) infection. Rates of sustained virologic response (SVR) in clinical trials exceed 95% and treatment is well tolerated. Viral eradication is now possible in the majority of infected patients including those who were traditionally considered difficult to cure. Current guidelines recommend treatment for all patients with chronic HCV who do not have comorbid conditions that limit life expectancy (1). The goal of antiviral therapy is to reduce transmission and prevent the consequences of chronic HCV infection including cirrhosis, hepatocellular carcinoma (HCC), and death.
Chronic HCV is a major cause of HCC worldwide (2). Approximately 20 to 70% of HCC cases are due to underlying HCV in European countries and in the United States (3). Among patients with HCV, HCC occurs predominantly in those with cirrhosis with an annual incidence of 3.5% per year (2). In addition to cirrhosis, important risk factors for HCC in patients with HCV are older age, male sex, hepatitis B co-infection, alcohol abuse, genotype 3 HCV and diabetes mellitus (3). HCC leads to significant morbidity and mortality among patients with HCV. Curative treatment is available for early stage tumors, but options are limited for disease diagnosed at advanced stages and overall survival is poor (4).
Eradication of HCV might be expected to reduce the risk of HCC by preventing progression to cirrhosis, promoting fibrosis regression or by abrogating direct carcinogenic effects of the virus. Indeed, achieving SVR using interferon (IFN)-based therapies has been shown to reduce the risk of HCV-related HCC compared to treatment failure or no treatment (5, 6). Studies reporting similar outcomes after DAA-induced SVR are emerging (7–12), although reports detailing unexpectedly high rates of HCC following DAA treatment (13–17) have created some uncertainty regarding the impact of DAAs on tumor development.
We aim to summarize the evidence evaluating the effect of DAA therapy on HCC risk in patients with chronic HCV infection. We focus on recent literature examining the association between DAA therapy and both incident and recurrent HCC.
De novo versus Recurrent HCC
Antiviral therapy may impact the development of both de novo HCC and recurrent HCC. De novo HCC is defined as cancer that develops in patients without any prior history of HCC. Existing studies equivalently refer to de novo HCC as “incident HCC” or “HCC occurrence”. In contrast, recurrent HCC is cancer that develops in patients with a history of HCC who have undergone curative treatment and presumably do not have active tumor at the start of antiviral therapy. Studies determining the association between DAAs and the risk of each outcome require different considerations and present different challenges.
Interferon Treatment and HCC
SVR is an appropriate endpoint of HCV treatment because it is strongly correlated with clinically important outcomes. SVR with IFN-based treatment has been shown to reduce the risk of liver-related morbidity (18) and mortality (19). Studies of antiviral therapy in the IFN era demonstrated a clear reduction in HCC risk following SVR. A meta-analysis of 14 studies showed that patients with HCV-related cirrhosis who achieve SVR with IFN have a reduced risk of HCC compared to patients who fail to respond (RR 0.35, 95% CI 0.26 – 0.46) (6). A separate meta-analysis of 12 studies demonstrated that patients with HCV at all stages of fibrosis benefit from SVR and have a lower risk of HCC compared to non-responders (RR 0.24, 95% CI 0.18 – 0.31) (5). In addition to antiviral effects, IFN is also purported to have anti-proliferative and immune modulating activity, which may contribute to an environment that inhibits tumor formation.
While the clinical benefits observed with IFN-induced SVR might be expected to occur with DAA-induced SVR as well, there are important differences between the patients who received antiviral treatment in the IFN and DAA eras that influence observed rates of HCC. Patients eligible for IFN-based therapy were highly selected due to the poor tolerability and safety profile of IFN, especially among patients with cirrhosis. DAAs, on the other hand, may be safely used for patients who have more advanced stages of liver disease and therefore a higher baseline risk of HCC. Epidemiologic trends may also influence HCC incidence among DAA eligible patients. The age group with the highest prevalence of chronic HCV in the United States is the 1945–1965 “baby boomer” birth cohort. As members of this birth cohort age, they contribute to an expanding pool of patients who are at high risk of HCC by virtue of advanced age and cirrhosis (20). Furthermore, metabolic comorbidities such as diabetes (21, 22) and obesity (23), which are increasing in prevalence over time, are additive risk factors for HCC in patients with chronic HCV. A recent study using NHANES data confirmed that HCV-infected patients have a higher prevalence of diabetes than non-infected patients (24). Collectively, the consequence of these factors is that post-SVR HCC incidence should be expected to be higher in patients receiving DAA therapy than was seen after IFN therapy. Therefore, when comparing DAA-treated to IFNtreated patients, these confounders have to be carefully adjusted for in multivariable models.
DAA Treatment and De Novo HCC
With the caveat that follow-up time for DAA-treated patients is still limited, evidence is accumulating that SVR may be associated with fibrosis regression (25), improvement in portal pressures (26, 27), improvement in extrahepatic manifestations of HCV (28, 29), and reduced risk of mortality (30, 31). However, enthusiasm for DAAs was tempered after a series of publications reported potentially higher than expected rates of HCC following DAA treatment. The two scenarios brought to attention were: 1) a higher than expected rate of de novo HCC and 2) a higher than expected rate of recurrent HCC in patients previously treated for HCC.
The concern that DAA therapy may increase the risk of de novo HCC was based on descriptive studies observing a higher than expected proportion of patients who developed HCC after antiviral treatment. Percentages of patients with incident HCC ranged from 3.16% to 9.1% (14–17). These proportions were noted to be higher than expected based on the historic incidence of HCC in untreated or IFN-treated patients. The biologic mechanism that was proposed was that rapid viral clearance with DAAs could lead to reduced cancer immune surveillance and anti-tumor activity (15).
The main limitations of these studies were small sample size and the lack of a control group. Comparison to historic controls is inappropriate because patients in the current antiviral era tend to have characteristics associated a higher baseline risk of HCC. Additionally, follow-up time ranged from 6 months to 1 year after antiviral therapy completion, which was too short to accurately reflect the consequences of viral eradication. The outcome definition was also variable, with some studies including HCCs diagnosed during DAA treatment (17). These most likely represented prevalent tumors that were already present before starting antiviral therapy. Data presented in these studies were therefore insufficient to draw the conclusion that DAAs increase the risk of HCC.
To determine the risk of de novo HCC after DAA therapy, the incidence of HCC must be compared between patients treated with DAAs and a control group. Recent studies have used as controls patients who fail to respond, patients who remain untreated, or patients who received IFN-based therapy.
DAA Treatment with SVR versus Treatment Failure
Studies that compared DAA-treated patients with SVR to non-responders provide strong evidence that DAAinduced SVR is associated with a reduced risk of de novo HCC. A large retrospective cohort study of Veterans Affairs (VA) patients demonstrated that DAA-induced SVR significantly reduces the incidence of HCC. Out of 21,948 DAA-treated patients followed for a mean of approximately 2 years, the incidence of HCC in patients who achieved SVR was 0.92 per 100 patient-years versus 5.19 per 100 patient-years in patients who failed treatment (9). The associated hazard ratio (HR) for HCC was 0.29 (95% CI 0.23–0.37) after adjusting for demographic characteristics, cirrhosis, HCV genotype and viral load, HBV or HIV co-infection, and liver-related laboratory parameters. Similar results were obtained in a separate, independently conducted study of 22,500 DAA-treated VA patients (adjusted HR 0.28, 95% CI 0.22–0.36) (8).
Of note, although antiviral therapy reduces the risk of HCC, the incidence of HCC is not completely eliminated. Patients with cirrhosis in particular remain at high risk of HCC. In both VA studies, patients with HCV-related cirrhosis had a higher incidence of HCC post-SVR than patients without cirrhosis (8, 9). However, the benefit of SVR in terms of HCC risk reduction was similar in patients with and without cirrhosis. Patients with cirrhosis who achieved SVR had a 68% (adjusted HR 0.32, 95% CI 0.23–0.44) (8) and 50% (adjusted HR 0.50, 95% CI 0.43–0.49) (9) lower risk of HCC compared to non-responders.
While a strength of large cohort studies is adequate statistical power, they are often limited by lack of granularity. Ascertainment of and adjustment for all potential confounders is challenging and – consequently – the possibility of residual confounding cannot be excluded. Furthermore, because of the lack of granularity and retrospective nature of these studies, it is not possible to ensure that all patients had imaging to rule out HCC that before starting antiviral therapy. Efforts were made in both VA studies to avoid inclusion of these patients by starting follow-up at the end of antiviral therapy (8) or 180 days from antiviral initiation (9). An additional limitation is that there may be differences in intensity and content of follow-up between DAA-treated patients and controls, leading to differential ascertainment of the outcome.
A prospective Italian cohort study of 2,249 DAA-treated patients with cirrhosis required that all patients have a baseline ultrasound (US) before antiviral therapy and excluded patients who did not have an US after DAA initiation (11). The 1-year cumulative incidence of HCC was 2.6% in patients who achieved SVR versus 8.0% in patients who failed to achieve SVR. Treatment failure was strongly associated with increased HCC risk (adjusted HR 2.88, 95% CI 1.57 – 5.29). These results must be interpreted with caution, however, as the start of follow-up in this cohort was at antiviral initiation, yet exposure was defined by SVR status. As a result, patients could have developed HCC before their SVR status was even determined. It is conceivable that the higher rate of HCC in the non-SVR group was simply because their HCC placed them at higher risk of treatment failure. In addition, the cumulative incidence of HCC among patients with treatment failure in this study was fairly high at nearly 10% at 12 months of follow-up and 25% at 24 months. These values far exceed the historical incidence of HCC among even untreated patients (approximately 3.5% per year). This suggests that the population in this study was inherently different than the typical patient with HCV-related cirrhosis. Patients with treatment failure in the study may have possessed characteristics that placed them at particularly high risk of HCC, limiting the generalizability of the results.
DAA Treatment versus No Treatment
Studies have also compared DAA-treated patients to patients who remain untreated. In the ERCHIVES study of VA patients with chronic HCV, 5,834 DAA-treated patients were compared to 8,468 untreated patients (10). Untreated patients were assigned a “baseline” date based on the average time between HCV diagnosis and treatment initiation in antiviral recipients. Follow-up was calculated from the time of antiviral initiation in DAA recipients or the baseline date in controls. The incidence of HCC among cirrhosis patients with DAA-induced SVR was 2.3/100 person-years (95% CI 1.68–3.3) compared to 4.5/100 person-years (95% CI 3.9–5.2) among untreated cirrhosis patients, although both incidence rates were unadjusted.
A unique challenge presented by using untreated patients as controls is the potential for immortal time bias (32). In studies evaluating de novo HCC as the outcome, patients who receive antivirals necessarily remain HCC free between the time of HCV infection and antiviral initiation, during which they are technically also “untreated”. Failure to count this as “untreated” person-time leads to underestimation of HCC incidence in treated patients and overestimation in untreated patients. A recent retrospective cohort study comparing DAAtreated and untreated patients employed methods to control for immortal time bias. Using a large administrative claims database, 30,183 patients receiving DAAs were compared to 137,502 contemporary untreated patients. After additionally controlling for differences in baseline characteristics between the two groups using propensity score weighted analysis, DAA-treatment was found to be associated with reduced HCC risk with an adjusted HR of 0.84 (95% CI 0.73 – 0.96) (12).
DAA Treatment versus IFN Treatment
Another way to address the association between DAAs and HCC risk is to compare patients treated with DAAs to patients treated with IFN with respect to HCC risk. So far, several cohort studies and a meta-analysis have not corroborated the concern that DAA and IFN-induced SVRs have different effects on HCC risk. A metaanalysis that included 9 studies of 6,002 DAA-treated patients and 17 studies of 5,521 IFN-treated patients found that the incidence of de novo HCC was 2.96 per 100 person-years (95% CI 1.76–4.96) following DAAs and 1.14 per 100 person-years (95% CI 0.86 – 1.52) following IFN (7). The higher incidence of HCC in DAAtreated patients was most likely because DAA treated patients were older and a greater proportion had advanced liver disease. Meta-regression adjusting for age and follow-up time demonstrated that DAA treatment was not associated with increased risk of HCC compared to IFN treatment (adjusted RR 0.68, 95% CI 0.18– 2.55).
Large, retrospective cohort studies published after the meta-analysis confirmed its main findings. A VA cohort study of 21,498 DAA-treated patients demonstrated that the magnitude of HCC risk reduction was similar whether patients received DAAs (adjusted HR 0.29, 95% CI 0.23 – 0.37), IFN (adjusted HR 0.32, 95% CI 0.28 – 0.37), or DAA + IFN (adjusted HR 0.48, 95% CI 0.35 – 0.73) (9). In a direct comparison of DAA- and IFNtreated patients, there was no difference in the likelihood of HCC between the two groups (adjusted HR 1.12, 95% CI 0.95 – 1.32). A recent study using administrative claims data also included an IFN-treated control group. After implementing propensity score weighting to control for differences between DAA and IFN patients, the risk of HCC was lower in DAA-treated patients (adjusted HR 0.69, 95% CI 0.59 – 0.81) (12).
The available follow-up time after DAA therapy is still relatively short compared to follow-up time after IFN therapy. Therefore, current studies will need to be updated over time. A summary of the main findings of these studies is presented in Table 1.
Table 1.
Summary of the main findings of recent studies examining de novo or recurrent HCC risk following DAA therapy
| Outcome | Study designs | Representative studies |
Main Findings |
|---|---|---|---|
| De novo HCC | |||
| DAA with SVR vs. Treatment failure |
(8, 9, 11) | Lower risk of HCC in patients with SVR after DAAs compared to patients without SVR |
|
| DAA vs. No treatment |
(10, 12) | Lower risk of HCC in patients receiving DAAs compared to patients remaining untreated |
|
| DAA vs. IFN |
(7, 9, 12) |
No difference in risk of HCC with DAA treatment compared to IFN treatment |
|
| Recurrent HCC | |||
| DAA treated patients only |
(13, 15) | Higher than expected proportion of patients with recurrent HCC after starting DAA therapy |
|
| DAA treated vs. control |
(38–41) | No difference in risk of recurrent HCC with DAA treatment compared to no treatment or IFN- treated patients |
DAA Treatment and Recurrent HCC
Tumor recurrence after treatment of HCC is common, even after treatments that are considered “curative”. Recurrence rates at 5 years are 80% following ablation, 70% following surgical resection, and 10–20% following liver transplantation (33, 34). Treatment with IFN following curative therapy may provide some benefit in terms of recurrence risk and overall survival, particularly if SVR is achieved (35, 36).
Small descriptive studies raised concern that, unlike IFN, DAA therapy may paradoxically increase or accelerate the risk of HCC recurrence and lead to more aggressive tumors. One study reported HCC recurrence in 16/58 (27.8%) patients previously treated with resection, ablation, or transarterial chemoembolization (TACE) during a median follow-up of ~ 6 months from the start of DAA therapy (13). Another study reported HCC recurrence in 17/59 (28.8%) patients previously treated with resection, ablation, or TACE over a follow-up period of 6 months from the end of DAA therapy (15). Interpretation of these results is challenging due to small sample size, the lack of a comparison group, and differences in what constituted follow-up time. Substantial variation in the design of these and several subsequent studies makes it difficult to estimate the true rate of HCC recurrence following DAA therapy. Indeed, in a recent meta-analysis that included 26 studies, there was significant statistical heterogeneity in reported rates of recurrence (37)The pooled estimate of the proportion of patients with HCC recurrence after DAA therapy was 25.1% (95% CI 19.431.2%) but ranged from 0% to as high as 59%. There was also variability in the duration of follow-up across studies (2.5 to 35.7 months), time between HCC treatment and initiation of DAA therapy, modality of tumor treatment, and the proportion of patients with non-early-stage HCC or multiple prior recurrences.
In contrast to the small, uncontrolled studies that first suggested a higher-than-expected rate of recurrent HCC following DAA treatment, studies that included untreated or IFN-treated comparison groups have come to different conclusions. A French collaborative cohort study of 267 patients with HCV and previously treated HCC showed that DAA therapy was not associated with increased risk of HCC recurrence when compared to no treatment (adjusted HR 1.09, 95% CI 0.55 – 2.16) (38). A recent single-center study of 149 patients with HCV-related HCC on the liver transplant waiting list also found that the risk of HCC recurrence (pre-transplant) was not different between patients treated with DAAs and patients who were untreated (adjusted HR 0.91, 95% CI 0.58 – 1.42) (39). Compared to IFN-treated patients, the incidence of recurrent HCC was not higher in patients treated with DAAs following curative HCC treatment in two Japanese cohort studies (40, 41). In a meta-analysis of studies that compared DAA-treated and untreated patients with respect to HCC recurrence, DAA treatment was associated with a lower risk of recurrence with a pooled odds ratio of 0.55 (95% CI 0.250.85) (37). A meta-analysis and meta-regression of studies comparing HCC recurrence after DAAs versus IFN found no difference after adjusting for age and follow-up time (7), but was limited by lack of individual patient data, inability to properly adjust for confounders and very large differences in follow-up time between the DAA and IFN studies.
While these latter studies benefit from having control groups and slightly larger sample sizes, they share the dilemma of how to appropriately calculate follow-up time. The period defined as follow-up is heterogeneous across studies. Some begin follow-up at the time of curative HCC treatment (39). This is problematic when comparing DAA treatment to no treatment because patients receiving antivirals are – by definition – recurrence-free from the time of HCC cure to DAA treatment. DAA-treated patients will therefore always appear to have a longer time-to-recurrence and lower incidence rate of recurrence than untreated patients. Other studies begin follow-up at the start or end of antiviral therapy in treated patients but without a similar index date in untreated patients (38, 40), which ignores the natural history of HCC recurrence following curative therapy. Furthermore this approach suffers from immortal time bias because the period between HCC cure and antiviral therapy is by definition recurrence free but also technically “untreated” person-time in that DAA exposure has not yet occurred. In the French ANRS study, this “immortal time” was incorporated into the HCC incidence among untreated controls to reduce bias (38). In spite of this correction, bias remains an issue because follow-up starts at the time of antiviral initiation for DAA recipients but at the time of HCC cure for untreated patients. Early recurrences can only occur in the untreated group, which ensures that untreated patients will always appear to have a higher incidence of HCC than treated patients.
We suggest an approach comparing HCC recurrence between DAA-treated and untreated patients shown in Figure 1. Follow-up time should begin at the start of antiviral therapy for treated patients and at the “Index date” for untreated patients. The index date is chosen in untreated controls to match the date of DAA treatment initiation in their matched treated patients, i.e. to be an equal duration of time from the time of HCC treatment. Both treated and untreated patients need to have evidence of la (42)ck of residual tumor prior to the initiation of DAA treatment (in the treated patients) or the index date (in untreated patients). This accounts for immortal time bias, that is, the bias related to the fact that DAA-treated patients cannot possibly have had HCC recurrence prior to DAA treatment and that almost all patients with early recurrence will, necessarily, be in the untreated group unless a matching scheme such as the one shown is used. Additionally, the comparison between treated and untreated patients has to be adjusted for baseline characteristics that are associated with tumor recurrence, especially tumor burden and type of tumor treatment. Finally, treated and untreated patients need to have similar and adequate methods of surveillance for HCC recurrence during the follow-up period. To our knowledge, a study that fulfils these criteria has not yet been completed, although ongoing prospective studies to address this issue are underway
Figure 1.
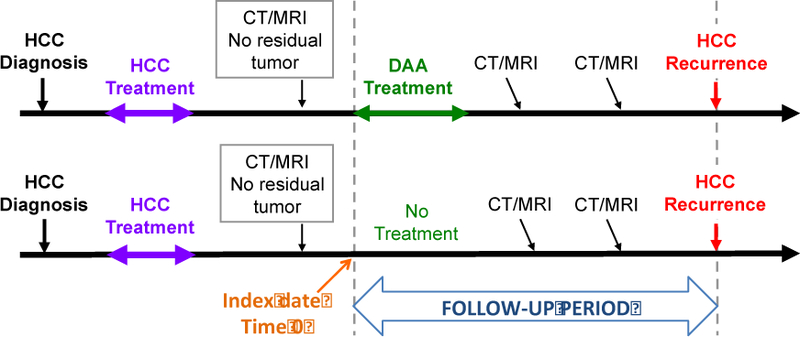
Proposed study design for evaluating the risk of recurrent HCC associated with DAA treatment. Follow-up time begins at the start of antiviral therapy for treated patients and at the “Index date” for untreated patients. The index date is chosen in untreated controls to match the date of DAA treatment initiation in their matched treated patients, i.e. to be an equal duration of time from the time of HCC treatment. Both treated and untreated patients need to have evidence of lack or residual tumor prior to the initiation of DAA treatment (in the treated patients) or the index date (in untreated patients). This accounts for immortal time bias, that is, the bias related to the fact that DAA-treated patients cannot possibly have had HCC recurrence prior to DAA treatment and that almost all patients with early recurrence will, necessarily, be in the untreated group unless a matching scheme such as the one shown is used. Furthermore, the comparison between treated and untreated patients has to be adjusted for baseline characteristics that are associated with tumor recurrence, especially tumor burden and type of tumor treatment. Finally, treated and untreated patients need to have similar and adequate methods of surveillance for HCC recurrence during the follow-up period.
Another method for accounting for immortal time bias involves modeling DAA treatment as a time-dependent covariate and comparing HCC risk in patients receiving treatment and those who remain untreated (43). However, this method requires having a sufficient proportion of patients starting DAA treatment “immediately” after HCC treatment for robust modeling, which does not happen in clinical practice.
Despite some inconsistencies in methodology and the inherent difficulty of evaluating HCC recurrence risk through observational studies, existing studies have mostly offered reassurance that DAA therapy is reasonable in patients who have undergone curative HCC treatment. We recommend that antiviral therapy be pursued after a sufficient period has elapsed following HCC treatment for patients to demonstrate durable complete response. Indeed, a shorter interval between HCC treatment and DAA therapy is independently associated with a higher risk of HCC recurrence (37, 42, 44). This observation is most likely because patients who start antivirals after a longer recurrence-free interval are those who have “proven” through the test of time to have achieved complete tumor response. Therefore, it is reasonable to wait at least 6 months after HCC complete response is first documented before initiating antiviral treatment and to obtain at least two consecutive 4-phase MRI or CT scans during that period to confirm absence of residual or recurrent HCC.
HCC Surveillance after DAA-Induced SVR
DAA-induced SVR reduces HCC risk but does not completely eliminate it. In patients without cirrhosis, the incidence of HCC after DAA-induced SVR is very low (0.24 to 0.34 per 100 patient-years) (8, 9). Given the low incidence of HCC in this population, current guidelines do not recommend HCC surveillance after SVR in patients who have not developed advanced fibrosis by the start of DAA therapy (1, 45). In contrast, patients with pre-existing cirrhosis have a substantial HCC risk even after SVR. Among VA patients with cirrhosis who achieve SVR with DAA regimens, the annual incidence of HCC was 1.82% (95% CI 1.52–2.12) (8). In the metaanalysis of de novo HCC risk in patients with HCV-related cirrhosis, the combined estimate of HCC incidence after SVR was 2.96% per year (95% CI 1.76–4.96) (7). These rates exceed the incidence at which HCC surveillance is considered to be cost-effective (46, 47). Therefore, continued HCC surveillance is recommended for patients with cirrhosis, even after SVR. The high residual incidence of HCC among these patients also emphasizes the importance of early treatment in patients with chronic HCV, before cirrhosis has developed.
It is unclear if the risk of HCC in patients with DAA-induced SVR declines sufficiently over time such that eventually surveillance is no longer warranted. Studies from the IFN-era showed that while HCC risk was reduced with SVR, the incidence of HCC was constant for at least 5 years afterwards (21, 48). On the other hand, studies with long term follow-up in the IFN-era also demonstrate that SVR is associated with durable improvements in laboratory markers of liver disease severity including platelet count and albumin (48) as well as FIB-4 and APRI scores (49). Improvements in these laboratory parameters as well as fibroscan-derived liver stiffness may correlate with reductions in HCC risk, although evidence to support this is still needed. Studies with long term follow-up in patients with DAA-induced SVR will be useful to determine the extent to which HCC risk declines over time, and whether improvements in fibrosis or markers of liver disease severity can aide in identifying patients who have achieved a low-enough risk of HCC to forego surveillance.
In patients who receive DAAs after HCC treatment, HCC surveillance is crucial before, during, and after therapy. An initial 4-phase CT or MRI should be obtained prior to antiviral treatment to confirm lack of residual, recurrent or new HCC. It would also be prudent to perform HCC surveillance with 4-phase CT or MRI during antiviral treatment and for the subsequent 6 months, especially if the interval between first demonstration of HCC complete response and initiation of antiviral treatment is relatively short.
Conclusion
Emerging data strongly suggest that DAA-induced SVR dramatically reduces the risk of de-novo HCC. Studies with even longer follow-up will be important to confirm this finding and to address whether risk continues to decline over time. Patients with cirrhosis still retain a risk of HCC that merits ongoing surveillance. Whether some of these patients can ever safely discontinue surveillance after SVR remains to be determined. This will depend on identifying improvements in liver function or other biomarkers over time that correlate with reduction in HCC risk after SVR. Ultimately, the best strategy is to eradicate HCV before cirrhosis develops in order to avoid residual HCC risk.
The impact of DAAs on HCC recurrence will be harder to characterize definitively due to methodological limitations of observational studies. However, ongoing studies are likely to shed more light. In the meantime, we propose a “middle of the road strategy” whereby patients with HCC who achieve complete response, consider DAA-based antiviral treatment once they have had at least two surveillance 4-phase CTs or MRIs showing no evidence of HCC at least 6 months after the first demonstration of HCC complete response.
Footnotes
Disclaimer
The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Compliance with Ethical Standards
Conflict of Interest
Feng Su and George N. Ioannou each declare no potential conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.AASLD-IDSA. Recommendations for testing, managing, and treating hepatitis C [Available from: http://www.hcvguidelines.org.
- 2.Younossi ZM, Kanwal F, Saab S, Brown KA, El-Serag HB, Kim WR, et al. The impact of hepatitis C burden: an evidence-based approach. Aliment Pharmacol Ther 2014;39 (5):518–31. [DOI] [PubMed] [Google Scholar]
- 3.Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology 2004;127 (5 Suppl 1):S35–50. [DOI] [PubMed] [Google Scholar]
- 4.Momin BR, Pinheiro PS, Carreira H, Li C, Weir HK. Liver cancer survival in the United States by race and stage (2001–2009): Findings from the CONCORD-2 study. Cancer 2017;123 Suppl 24:5059–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med 2013;158 (5 Pt 1):329–37. [DOI] [PubMed] [Google Scholar]
- 6.Singal AK, Singh A, Jaganmohan S, Guturu P, Mummadi R, Kuo YF, et al. Antiviral therapy reduces risk of hepatocellular carcinoma in patients with hepatitis C virus-related cirrhosis. Clin Gastroenterol Hepatol 2010;8 (2):192–9. [DOI] [PubMed] [Google Scholar]
- 7.Waziry R, Hajarizadeh B, Grebely J, Amin J, Law M, Danta M, et al. Hepatocellular carcinoma risk following direct-acting antiviral HCV therapy: A systematic review, meta-analyses, and meta-regression. J Hepatol 2017;67 (6):1204–12. [DOI] [PubMed] [Google Scholar]
- 8.Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of Hepatocellular Cancer in HCV Patients Treated With Direct-Acting Antiviral Agents. Gastroenterology 2017;153 (4):996–1005 e1. ** Retrospective VA cohort study demonstrating that DAA-induced SVR is associated with reduced de novo HCC risk compared to treatment failure. [DOI] [PubMed] [Google Scholar]
- 9.Ioannou GN, Green PK, Berry K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol 2017. ** Separate VA cohort study demonstrating that DAA-induced SVR is associated with reduced risk of de novo HCC compared to treatment failure, and the risk of HCC after DAA therapy is similar to the risk after IFN therapy. [DOI] [PMC free article] [PubMed]
- 10.Li DK, Ren Y, Fierer DS, Rutledge S, Shaikh OS, Lo Re V, et al. The short-term incidence of hepatocellular carcinoma is not increased after hepatitis C treatment with direct-acting antivirals: An ERCHIVES study. Hepatology 2017. [DOI] [PubMed]
- 11.Calvaruso V, Cabibbo G, Cacciola I, Petta S, Madonia S, Bellia A, et al. Incidence of Hepatocellular Carcinoma in Patients with HCV-associated Cirrhosis Treated with Direct-Acting Antiviral Agents. Gastroenterology 2018. [DOI] [PubMed]
- 12.Singer AW, Reddy KR, Telep LE, Osinusi AO, Brainard DM, Buti M, et al. Direct-acting antiviral treatment for hepatitis C virus infection and risk of incident liver cancer: a retrospective cohort study. Aliment Pharmacol Ther 2018. * Large administrative claims-based retrospective cohort study demonstrating that DAA therapy reduces the risk of de novo HCC compared to no treatment, after adjusting for important sources of bias. [DOI] [PubMed]
- 13.Reig M, Marino Z, Perello C, Inarrairaegui M, Ribeiro A, Lens S, et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol 2016;65 (4):719–26. * First publication describing an unexpectedly high proportion of patients who developed recurrent HCC after DAA therapy. [DOI] [PubMed] [Google Scholar]
- 14.Kozbial K, Moser S, Schwarzer R, Laferl H, Al-Zoairy R, Stauber R, et al. Unexpected high incidence of hepatocellular carcinoma in cirrhotic patients with sustained virologic response following interferonfree direct-acting antiviral treatment. J Hepatol 2016;65 (4):856–8. [DOI] [PubMed] [Google Scholar]
- 15.Conti F, Buonfiglioli F, Scuteri A, Crespi C, Bolondi L, Caraceni P, et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol 2016;65 (4):727–33. [DOI] [PubMed] [Google Scholar]
- 16.Cardoso H, Vale AM, Rodrigues S, Gonçalves R, Albuquerque A, Pereira P, et al. High incidence of hepatocellular carcinoma following successful interferon-free antiviral therapy for hepatitis C associated cirrhosis. J Hepatol 2016;65 (5):1070–1. [DOI] [PubMed] [Google Scholar]
- 17.Ravi S, Axley P, Jones D, Kodali S, Simpson H, McGuire BM, et al. Unusually High Rates of Hepatocellular Carcinoma After Treatment With Direct-Acting Antiviral Therapy for Hepatitis C Related Cirrhosis. Gastroenterology 2017;152 (4):911–2. [DOI] [PubMed] [Google Scholar]
- 18.Singal AG, Volk ML, Jensen D, Di Bisceglie AM, Schoenfeld PS. A sustained viral response is associated with reduced liver-related morbidity and mortality in patients with hepatitis C virus. Clin Gastroenterol Hepatol 2010;8 (3):280–8, 8.e1. [DOI] [PubMed] [Google Scholar]
- 19.Simmons B, Saleem J, Heath K, Cooke GS, Hill A. Long-Term Treatment Outcomes of Patients Infected With Hepatitis C Virus: A Systematic Review and Meta-analysis of the Survival Benefit of Achieving a Sustained Virological Response. Clin Infect Dis 2015;61 (5):730–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology 138 United States2010 p. 513–21, 21 e1–6. [DOI] [PubMed] [Google Scholar]
- 21.El-Serag HB, Kanwal F, Richardson P, Kramer J. Risk of Hepatocellular Carcinoma after Sustained Virologic Response in Veterans with HCV-infection. Hepatology 2016. [DOI] [PMC free article] [PubMed]
- 22.van der Meer AJ, Feld JJ, Hofer H, Almasio PL, Calvaruso V, Fernández-Rodríguez CM, et al. Risk of cirrhosis-related complications in patients with advanced fibrosis following hepatitis C virus eradication. J Hepatol 2017;66 (3):485–93. [DOI] [PubMed] [Google Scholar]
- 23.Terrault NA, Hassanein TI. Management of the patient with SVR. J Hepatol 2016;65 (1 Suppl):S120–S9. [DOI] [PubMed] [Google Scholar]
- 24.Lazo M, Nwankwo C, Daya NR, Thomas DL, Mehta SH, Juraschek S, et al. Confluence of Epidemics of Hepatitis C, Diabetes, Obesity, and Chronic Kidney Disease in the United States Population. Clin Gastroenterol Hepatol 2017;15 (12):1957–64.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knop V, Hoppe D, Welzel T, Vermehren J, Herrmann E, Vermehren A, et al. Regression of fibrosis and portal hypertension in HCV-associated cirrhosis and sustained virologic response after interferonfree antiviral therapy. J Viral Hepat 2016;23 (12):994–1002. [DOI] [PubMed] [Google Scholar]
- 26.Lens S, Alvarado-Tapias E, Mariño Z, Londoño MC, LLop E, Martinez J, et al. Effects of All-Oral AntiViral Therapy on HVPG and Systemic Hemodynamics in Patients With Hepatitis C Virus-Associated Cirrhosis. Gastroenterology 2017;153 (5):1273–83.e1. [DOI] [PubMed] [Google Scholar]
- 27.Afdhal N, Everson GT, Calleja JL, McCaughan GW, Bosch J, Brainard DM, et al. Effect of viral suppression on hepatic venous pressure gradient in hepatitis C with cirrhosis and portal hypertension. J Viral Hepat 2017;24 (10):823–31. [DOI] [PubMed] [Google Scholar]
- 28.Comarmond C, Garrido M, Pol S, Desbois AC, Costopoulos M, Le Garff-Tavernier M, et al. DirectActing Antiviral Therapy Restores Immune Tolerance to Patients With Hepatitis C Virus-Induced Cryoglobulinemia Vasculitis. Gastroenterology 2017;152 (8):2052–62.e2. [DOI] [PubMed] [Google Scholar]
- 29.Saadoun D, Pol S, Ferfar Y, Alric L, Hezode C, Si Ahmed SN, et al. Efficacy and Safety of Sofosbuvir Plus Daclatasvir for Treatment of HCV-Associated Cryoglobulinemia Vasculitis. Gastroenterology 2017;153 (1):49–52.e5. [DOI] [PubMed] [Google Scholar]
- 30.Backus LI, Belperio PS, Shahoumian TA, Mole LA. Direct-acting antiviral sustained virologic response: Impact on mortality in patients without advanced liver disease. Hepatology 2018. [DOI] [PubMed]
- 31.Backus LI, Belperio PS, Shahoumian TA, Mole LA. Impact of sustained virologic response with direct-acting antiviral treatment on mortality in patients with advanced liver disease. Hepatology 2017. [DOI] [PubMed]
- 32.Suissa S Immortal time bias in pharmaco-epidemiology. Am J Epidemiol 2008;167 (4):492–9. 33. [DOI] [PubMed] [Google Scholar]
- 33.Hatzaras I, Bischof DA, Fahy B, Cosgrove D, Pawlik TM. Treatment options and surveillance strategies after therapy for hepatocellular carcinoma. Ann Surg Oncol 2014;21 (3):758–66. 34. [DOI] [PubMed] [Google Scholar]
- 34.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet 2018;391 (10127):1301–14. [DOI] [PubMed] [Google Scholar]
- 35.Shinkawa H, Hasegawa K, Arita J, Akamatsu N, Kaneko J, Sakamoto Y, et al. Impact of Sustained Virological Response to Interferon Therapy on Recurrence of Hepatitis C Virus-Related Hepatocellular Carcinoma. Ann Surg Oncol 2017;24 (11):3196–202. [DOI] [PubMed] [Google Scholar]
- 36.Singal AK, Freeman DH, Anand BS. Meta-analysis: interferon improves outcomes following ablation or resection of hepatocellular carcinoma. Aliment Pharmacol Ther 2010;32 (7):851–8. [DOI] [PubMed] [Google Scholar]
- 37.Saraiya N, Yopp AC, Rich NE, Odewole M, Parikh ND, Singal AG. Systematic review with metaanalysis: recurrence of hepatocellular carcinoma following direct-acting antiviral therapy. Aliment Pharmacol Ther 2018;48 (2):127–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.ANRS collaborative study group on hepatocellular carcinoma (ANRS CO22 HEPATHER COCaCCcEaspaf. Lack of evidence of an effect of direct-acting antivirals on the recurrence of hepatocellular carcinoma: Data from three ANRS cohorts. J Hepatol 2016;65 (4):734–40. * First study of the impact of DAA on recurrent HCC risk that contained a control group and found no increase in risk associated with DAA therapy. [DOI] [PubMed] [Google Scholar]
- 39.Huang AC, Mehta N, Dodge JL, Yao FY, Terrault NA. Direct-acting antivirals do not increase the risk of hepatocellular carcinoma recurrence after local-regional therapy or liver transplant waitlist dropout. Hepatology 2018. [DOI] [PMC free article] [PubMed]
- 40.Nagata H, Nakagawa M, Asahina Y, Sato A, Asano Y, Tsunoda T, et al. Effect of interferon-based and -free therapy on early occurrence and recurrence of hepatocellular carcinoma in chronic hepatitis C. J Hepatol 2017;67 (5):933–9. [DOI] [PubMed] [Google Scholar]
- 41.Mashiba T, Joko K, Kurosaki M, Ochi H, Osaki Y, Kojima Y, et al. Does interferon-free direct-acting antiviral therapy for hepatitis C after curative treatment for hepatocellular carcinoma lead to unexpected recurrences of HCC? A multicenter study by the Japanese Red Cross Hospital Liver Study Group. PLoS One 2018;13 (4):e0194704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogawa E, Furusyo N, Nomura H, Dohmen K, Higashi N, Takahashi K, et al. Short-term risk of hepatocellular carcinoma after hepatitis C virus eradication following direct-acting anti-viral treatment. Aliment Pharmacol Ther 2018;47 (1):104–13. [DOI] [PubMed] [Google Scholar]
- 43.Zhou Z, Rahme E, Abrahamowicz M, Pilote L. Survival bias associated with time-to-treatment initiation in drug effectiveness evaluation: a comparison of methods. Am J Epidemiol 2005;162 (10):1016–23. [DOI] [PubMed] [Google Scholar]
- 44.Kolly P, Waidmann O, Vermehren J, Moreno C, Vögeli I, Berg T, et al. Hepatocellular carcinoma recurrence after direct antiviral agent treatment: A European multicentre study. J Hepatol 2017;67 (4):876–8. [DOI] [PubMed] [Google Scholar]
- 45.easloffice@easloffice.eu EAftSotLEa, Liver EAftSot. EASL Recommendations on Treatment of Hepatitis C 2018. J Hepatol 2018. [Google Scholar]
- 46.Bruix J, Sherman M, Practice Guidelines Committee AeAftSoLD. Management of hepatocellular carcinoma. Hepatology 2005;42 (5):1208–36. [DOI] [PubMed] [Google Scholar]
- 47.Bruix J, Sherman M, Diseases AAftSoL. Management of hepatocellular carcinoma: an update. Hepatology 2011;53 (3):1020–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morgan TR, Ghany MG, Kim HY, Snow KK, Shiffman ML, De Santo JL, et al. Outcome of sustained virological responders with histologically advanced chronic hepatitis C. Hepatology 2010;52 (3):833–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu M, Li J, Zhang T, Rupp LB, Trudeau S, Holmberg SD, et al. Serum Biomarkers Indicate Long-term Reduction in Liver Fibrosis in Patients With Sustained Virological Response to Treatment for HCV Infection. Clin Gastroenterol Hepatol 2016;14 (7):1044–55.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]


